Abstract
Objectives:
Anatomic lung resection provides the best opportunity for long-term survival in the setting of early-stage non-small cell lung cancer (NSCLC). However, 20–30% of patients develop recurrent disease following complete (R0) resection for Stage I disease. In the current study, we analyze the impact of patient, surgical and pathologic variables upon recurrence patterns following anatomic lung resection for clinical stage I NSCLC.
Patients and methods:
A total of 1132 patients (384 segmentectomies, 748 lobectomies) with clinical stage I NSCLC were evaluated. Predictors of recurrence were identified by proportional hazards regression. Differences in recurrence patterns between groups are illustrated by log rank tests applied to Kaplan-Maier estimates.
Results:
A total of 227 recurrences (20.0%) were recorded at a median follow-up of 36.8 months (65 locoregional, 155 distant). There was no significant difference in recurrence patterns when comparing segmentectomy and lobectomy. Multivariate analysis demonstrated that angiolymphatic invasion, tumor size, tumor grade and the presence of only mild-moderate tumor inflammation were independent predictors of recurrence risk.
Conclusions:
Recurrence following anatomic lung resection is influenced predominantly by pathological variables (tumor size, tumor grade, angiolymphatic invasion, tumor inflammation). Optimization of surgical margin in relation to tumor size may improve outcomes. Extent of resection (segmentectomy vs. lobectomy) does not appear to have an impact on recurrence-free survival when adequate margins are obtained.
Keywords: Lobectomy, Multivariate analysis, Recurrence, Segmentectomy, Survival, Wedge resection
1. Introduction
Lung cancer is the most common cause of cancer-related death world-wide, with approximately 1,378,400 deaths reported annually. Deaths due to lung cancer exceed those of breast, prostate, and colorectal carcinoma combined, creating catastrophic health care and economic implications [1,2]. Complete resection, when possible, remains the mainstay of therapy, and affords patients the best chance of long-term survival [3,4]. Not all patients undergoing resection are destined for cure, with recurrence rates in early-stage disease (clinical stage I) ranging from 20 to 40% at five years [5].
Recurrence following “complete resection” for early-stage non-small cell lung cancer remains poorly understood, and is multifactorial in nature. A host of patient, surgical and tumor-specific pathological variables have been shown to contribute to recurrence risk in the setting of resected non-small cell lung cancer. The primary objective of the current analysis was to examine the relative impact of patient, surgical and tumor-related factors on recurrence following resection of clinical stage I NSCLC. We explore whether extent of resection (segmentectomy vs. lobectomy) or surgical margin affect recurrence risk, and elucidate those tumor-specific pathological variables predictive of relapse.
2. Patients and methods
2.1. Patients
Approval for this study was provided by the Institutional Review Board of the University of Pittsburgh, and individual patient consent was waived. We performed a retrospective analysis of 1132 patients who underwent anatomic lung resection (segmentectomy or lobectomy) derived from the Lung Cancer Database of the University of Pittsburgh. Only patients with clinical stage I lung cancer treated by formal anatomic resection (segmentectomy or lobectomy) were included, as defined by the 8th edition of the UICC/AJCC lung cancer staging system [6]. Patient demographics and tumor characteristics are detailed in Table 1.
Table 1.
Patient and Tumor Characteristics.
| Anatomic Resection (n = 1,132) | |
|---|---|
| Age | |
| Mean | 68.1 ± 9.6 |
| Range | 22-91 |
| Gender | 531 M, 601 F |
| Co-Morbidities (%) | |
| Hypertension | 48.7 |
| Coronary Artery Disease | 16.0 |
| Diabetes Mellitus | 16.4 |
| COPD | 27.8 |
| Prior Cancer History | 24.0 |
| Gastroesophageal Reflux | 16.1 |
| PFTs (Pre-Op) | |
| FEV1 (%) | 2.05(79.3%) |
| DLCO (%) | 15.9 (70.0%) |
| Tumor Size (cm) | 2.3 ± 1.0 |
| Clinical Stage | 1A-863 (72.6%) 1B-269 (23.8%) |
| Histology (%) | |
| Adenocarcinoma | 57.9 |
| Squamous Cell CA | 29.2 |
| Other | 12.9 |
| Grade (%) | |
| Well-Differentiated | 12.1 |
| Moderately-Differentiated | 57.4 |
| Poorly-Differentiated | 30.1 |
| Angiolymphatic Invasion (%) | 41.5 |
| Visceral Pleural Invasion (%) | 27.5 |
| Tumor Inflammation (%) | |
| Low | 57.3 |
| Moderate | 34.9 |
| Severe | 7.7 |
| Operation | |
| Segmentectomy | 384 (33.9%) |
| Lobectomy | 748 (66.1%) |
| Approach | |
| VATS | 609 (53.8%) |
| Open | 522 (46.2%) |
| # Lymph Nodes Examined | |
| Median | 9 |
| Range | 0-85 |
| # Lymph Node Stations Sampled | |
| Median | 3 |
| Range | 0-9 |
2.2. Operative technique
Operations performed included 748 lobectomies and 384 anatomic segmentectomies. A VATS approach was utilized in 609 (53.8%) of the patients in this study, with thoracotomy performed in 522 (46.2%) patients. Lobectomy was performed in standard fashion employing a VATS or open approach as described previously [7]. Anatomic segmentectomy is accomplished by the removal of one or more pulmonary parenchymal segments with its corresponding bronchovascular and lymphatic supply [8,9]. In contradistinction to wedge resection (which does not involve anatomic hilar dissection), anatomic segmentectomy is accomplished by individual isolation and division of the targeted segmental bronchial and vascular structures and complete excision of the segmental pedicle.
2.3. Pathologic evaluation
Pathologic information was derived from the published case synoptic for each patient. Pathologic variables evaluated in this analysis include tumor size, tumor histology, grade, as well as the presence of angiolymphatic invasion, visceral pleural invasion or tumor inflammation (graded as mild, moderate or severe).
2.4. Follow-up
Perioperative data were actively collected from the hospital chart, anesthesia and OR records as well as the electronic medical record and/or office charts for each patient. Complications were documented for each patient based upon standard definitions established for the STS General Thoracic Database [10]. All patients were followed post-operatively at two weeks and at 4–6 month intervals for the first two years, then yearly thereafter with CT scans. Perioperative mortality was defined as any patient who died within the first 30 days after surgery or during the same hospitalization. Ninety-day mortality was also calculated. Locoregional recurrence was defined as evidence of tumor within the same lobe, the hilum or the mediastinal lymph nodes. Distant recurrences were defined as evidence of tumor in another lobe, the pleural space, or elsewhere outside the hemithorax. Median follow-up was 36.8 months for the entire cohort.
2.5. Statistical analysis
Student’s t and Wilcoxon tests were used to compare the distributions of continuous data (age, tumor size, number of lymph nodes removed, operative time, estimated blood loss), and Chi-squared or Fisher’s exact test was used to compare the frequencies of categorical measures (sex, histology, stage, etc.) between lobectomies and segmentectomies. All comparisons were two-tailed. Freedom from recurrence was defined as the time from surgery to the first diagnosis of local, regional or distant disease recurrence, or until last-follow-up; death was considered a censoring event for recurrence. Overall survival was defined as the time from surgery to death or last follow-up. Disease-free survival, freedom from recurrence and overall survival functions were estimated by the Kaplan-Meier method. Survival functions were compared by means of the log-rank test.
Multiple variables were evaluated for their association with time to recurrence risk as follows: Patient Variables - age, gender, co-morbidities (Including chronic obstructive pulmonary disease, hypertension, diabetes mellitus, coronary artery disease, gastroesophageal reflux disease, prior history of cancer other than lung cancer) and pulmonary function (FEV1); Surgical Variables – Operation performed (segmentectomy vs. lobectomy), approach (VATS vs. open), surgical margin (defined as the shortest distance from the tumor to the closest staple line), number of lymph nodes sampled, lymph node stations sampled; Tumor Variables – tumor size, tumor location, histology, lymph node involvement, angiolymphatic invasion, visceral pleural invasion and tumor inflammation. Each variable was assessed for their relationship to recurrence in univariate analysis. Variables demonstrating a significant association with recurrence in univariate analysis (p < 0.05) were then analyzed in a forward proportional hazards (Cox) regression model. Corresponding hazard ratios, confidence intervals and p-values for each variable were determined with the SAS software package (SAS Institute Inc., Cary, NC).
3. Results
3.1. Patient and tumor characteristics
Patient and tumor characteristics are depicted in Table 1. The mean patient age was 68.1 years (Range: 22–91). Female:Male ratio was 601:531. The patient cohort had, on average, a moderate degree of pulmonary impairment with a mean FEV1 of 2.05 (79.3% predicted) and a DLCO of 15.9 (70.0% predicted). The clinical stage distribution was Stage1 A-863 (76.2%) and Stage 1B-269 (23.8%). Mean tumor size was 2.3 cm. Anatomic segmentectomy was performed in 384 patients (VATS = 233, Open = 151). The remainder of patients (n = 748) underwent lobectomy (VATS = 376, Open = 371). Non-small cell lung cancer was associated with upper lobe predominance (n = 698, 61.7%), with the majority of cases encountered in the right upper lobe (n = 378, 33.4%). The most common lobar and segmental resections performed were right upper lobectomy (n = 272, 24.0%) and left upper division segmentectomy (n = 98, 25.5%), respectively.
3.2. Morbidity and mortality
Overall morbidity was 40.3%. Anatomic segmentectomy was associated with a reduced incidence of overall complications (32.9% vs. 44.4%, p = 0.001) when compared to lobectomy. Median length of stay was six days in both groups. The 30-day mortality rate for segmentectomy was 1.0%, compared to 1.9% for lobectomy (p = 0.45). The 90-day mortality rates were 3.0% vs. 4.1%, respectively (p = 0.50).
3.3. Analysis of recurrences
At a median follow-up of 36.8 months, there were 220 recurrences (19.4%) documented within the entire cohort– 65 locoregional, 155 distant. Median time to recurrence has not yet been observed. Actuarial estimates of freedom from recurrence and overall survival were 69.9% [95% CI: (66.3%, 73.6%)] and 56.3% [95% CI: (52.8%, 60.2%)] at five years, respectively. Recurrences were more common among tumors located within the left lower lobe following both lobectomy (22.0%) and segmentectomy (31.6%). No differences were noted in either locoregional (5.7% vs. 5.7%, p = 1.00) or distant (14.6% vs. 13.3%, p = 0.57) recurrence rates when comparing segmentectomy with lobectomy. Actuarial estimates of freedom from recurrence similarly demonstrated no difference between groups at five years (70 vs. 68%, p = 0.33). A significant difference was noted in overall survival at five years when comparing segmentectomy and lobectomy (50% vs. 59%, p = 0.02) due to a higher rate of non-cancer-related death among patients in the segmentectomy group (Table 2).
Table 2.
Recurrence and Survival Patterns by Operation Performed.
| Segmentectomy (n = 384) | Lobectomy (n = 748) | p-value | |
|---|---|---|---|
| Recurrences | |||
| Overall (n) | 78 (20.3%) | 142 (19.0%) | 0.63 |
| Locoregional (n) | 22 (5.7%) | 43 (5.7%) | 0.89 |
| Distant (n) | 56 (14.6%) | 99 (13.2%) | 0.52 |
| Freedom from Recurrence (5 yr) | 69.4% | 70.2% | 0.85 |
| 95% Confidence Interval | (63.4%,76.1%) | (65.9%,74.8%) | |
| Overall Survival (5 yr) | 49.9% | 59.4% | 0.02 |
| 95% Confidence Interval | (43.7%,57.1%) | (55.1%,63.9%) | |
| Median Follow-Up (months) | 34.2 | 38.8 | < 0.01 |
Median follow-up for recurrences is 36.8 months. Estimates and confidence intervals for five-year overall survival and freedom from recurrence are calculated from Kaplan-Meier survival function estimates. P-value for comparing these estimates between segmentectomy and lobectomy are calculated from a χ2 test based on the complementary log-log transformed survival estimates.
3.4. Predictors of recurrence
The first proportional hazards model, on patient variables, demonstrated that none of the patient variables were associated with an increased risk of recurrence. Among the surgical variables, only length of surgical margin was associated with recurrence risk. In univariate analysis, significant tumor variables included tumor size, tumor grade, lymph node involvement, angiolymphatic invasion and tumor inflammation (Table 3). The variables significant in the univariate analysis were combined in a multiple regression model. Tumor size, tumor grade, angiolymphatic invasion and tumor inflammation were all statistically significant predictors of time to recurrence (Table 4, Fig. 1).
Table 3.
Univariate Proportional Hazards Regressions for Time to Recurrence.
| Hazard Ratio (95% Confidence Interval) | (p-value) | |
|---|---|---|
| Patient Variables | ||
| Age | 1.00 (0.99,1.02) | 0.62 |
| Sex | 1.14 (0.88,1.48) | 0.33 |
| Co-Morbidities | ||
| COPD | 1.20 (0.90,1.60) | 0.22 |
| Hypertension | 1.19 (0.92,1.55) | 0.19 |
| Diabetes Mellitus | 1.17 (0.82,1.67) | 0.39 |
| Coronary Artery disease | 1.40 (0.98,2.00) | 0.06 |
| GERD | 0.80 (0.55,1.18) | 0.26 |
| Prior Cancer History | 1.22 (0.91,1.65) | 0.19 |
| Pulmonary Function (FEV1) | 1.00 (0.99,1.00) | 0.23 |
| Pre-Surgical Variables | ||
| Segmentectomy | 1.14 (0.87,1.50) | 0.34 |
| VATS | 0.83 (0.64,1.08) | 0.16 |
| Clinical Stage 1B | 1.41 (1.06,1.89) | 0.02 |
| Location | – | 0.26 |
| Right Upper | 1 | |
| Right Middle | 0.68 (0.36,1.28) | |
| Right Lower | 0.93 (0.63,1.35) | |
| Left Upper | 0.76 (0.54,1.07) | |
| Left Lower | 1.13 (0.77,1.67) | |
| Tumor Variables | ||
| Tumor Size > 2cm | 1.25 (1.10,1.42) | < 0.001* |
| Margin:Tumor Ratio > 1 | 1.49 (1.07,2.08) | 0.02* |
| Surgical Margin (mm) | 0.99 (0.98,0.99) | 0.05* |
| # Lymph Nodes Examined | 0.99 (0.99,1.00) | 0.65 |
| # Lymph Node Stations | 0.96 (0.88,1.04) | 0.30 |
| Histology | – | 0.62 |
| Adenocarcinoma | 1 | |
| Squamous Cell | 1.16 (0.86,1.55) | |
| Large Cell | 0.94 (0.44,2.01) | |
| Adenosquamous | 1.46 (0.79,2.70) | |
| Small Cell | 0.93 (0.23,3.74) | |
| Carcinoid | 0.32 (0.05,2.30) | |
| Other | 0.88 (0.36,2.16) | |
| Lymph Node Involvement | 1.60 (1.07,2.40) | 0.02* |
| Angiolymphatic Invasion | 1.71 (1.31,2.22) | < 0.001* |
| Visceral Pleural Invasion | 1.38 (1.05,1.82) | 0.02* |
| Tumor Inflammation | 0.47 (0.25,0.88) | 0.02* |
| Grade (Poor or Undifferentiated) | 1.48 (1.13,1.94) | 0.005* |
Table 4.
Multivariate Proportional Hazards Regression for Time to Recurrence.
| Hazard Ratio (95% Confidence Interval) | |
|---|---|
| Tumor Size > 2 cm | 1.39 (1.03, 1.87) |
| Angiolymphatic Invasion | 1.55 (1.18, 2.03) |
| Tumor Inflammation (mild-mod) | 2.19 (1.16, 4.17) |
| Grade: Poor, Undifferentiated | 1.37 (1.03,1.82) |
| Number of Above Risk Factors: | |
| 0 | 1 |
| 1 | 1.66 (0.59,4.67) |
| 2 | 2.55 (0.93,6.96) |
| 3 | 3.73 (1.36,10.22) |
| 4 | 5.20 (1.85,14.56) |
Fig. 1.
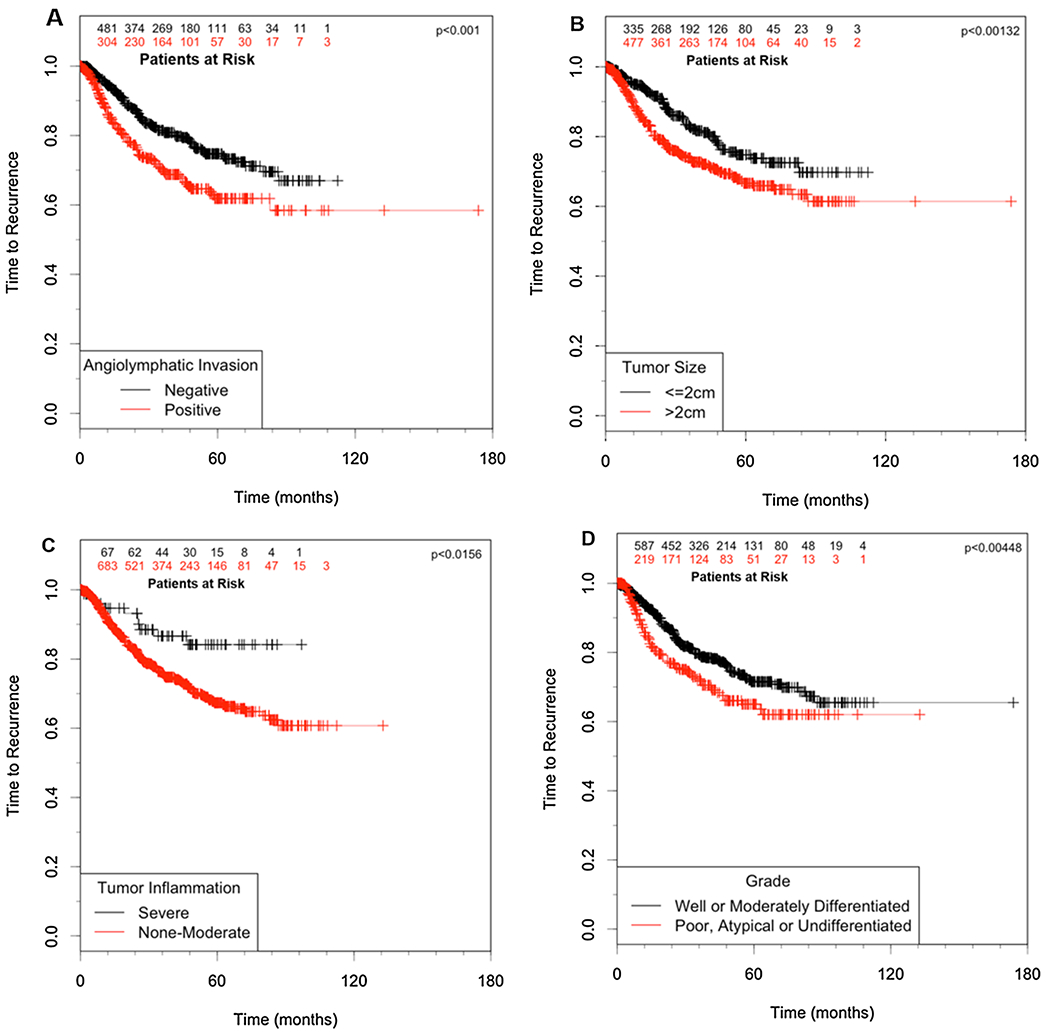
Independent predictors of recurrence in clinical stage I non-small cell lung cancer. A.) Angiolymphatic Invasion, B.) Tumor size, C.) Tumor Inflammation, D.) Tumor Grade.
3.5. Clinical impact of predictors
Based on the results of the multivariate analysis, we constructed a high-risk profile accounting for the significant predictors (angiolymphatic invasion, tumor size > 2 cm, tumor grade and mild-moderate tumor inflammation) by counting the number of factors each patient expressed. The presence of increasing numbers of factors was associated with a significant, step-wise increase in recurrence risk (p < 0.0045, Fig. 2, Table 4). Extent of resection (segmentectomy vs. lobectomy) was again not found to be a significant predictor of recurrence in a proportional hazards model once the high-risk variables were taken into account (p = 0.33).
Fig. 2.
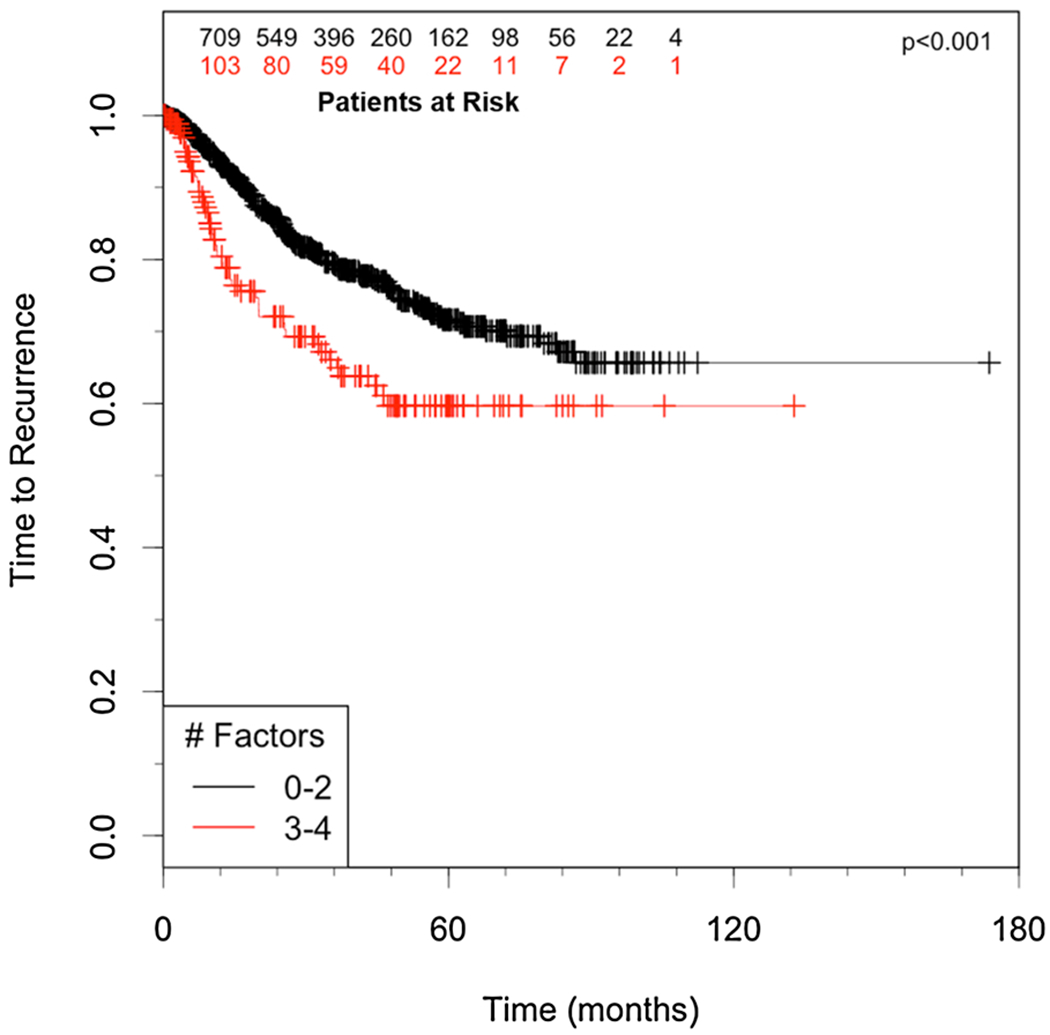
A.) Time to recurrence for high risk patients 3 or 4 of the independent predictors (high risk) compared to those with < 3 predictors (Low-Moderate risk).
3.6. Tumor size and surgical margin
Among all patients with clinical stage I disease, margin data were available in 936 (82.6%) patients, with 186 documented recurrences. Whereas 44.6% of these recurrences had a surgical margin < 1 cm, 68.6% of these patients were found to have a surgical margin:tumor size ratio < 1, suggesting that a ratio of margin:tumor size may be a better indicator of recurrence risk when performing sublobar resection. When analyzing all patients with clinical stage I NSCLC, a margin:tumor ratio of < 1 was associated with a recurrence rate of 22.4%, compared with 16.6% in cases where the margin: tumor ratio was ≥ 1 (p = 0.007). A smaller margin:tumor cut-off of 0.5 was associated with an increased disparity in recurrence risk between patients with a margin:tumor size ratio < 0.5 and those with a ratio of ≥ 0.5 (26.2% vs. 16.6%, p = 0.003). A larger ratio cut-off of 2 did not lead to a significant further reduction in recurrence risk (15.4%, p = 0.14). margin:tumor diameter ratio < 1 was found to be a significant independent predictor of recurrence following resection of clinical Stage I non-small cell lung cancer (Hazard Ratio: 1.57, 95% CI: 1.10, 2.24; p = 0.014). Interestingly, in cases where the margin:tumor ration was < 1, there were no differences in recurrence rates noted between segmentectomy and lobectomy (21.0% vs. 21.5%, respectively; p = 0.92). This finding is corroborated by proportional hazards regression, which similarly demonstrated no significant effect on recurrence based on type of operation performed (Segmentectomy HR = 1.14 (0.87, 1.50, p = 0.34, Table).
4. Discussion
The successful management of early-stage non-small cell lung cancer depends upon a multitude of factors including host anatomy and physiology, the technical ability to achieve complete surgical extirpation of the tumor (R0 resection), as well as underlying tumor biology. Interactions between these factors are complex and frequently unpredictable, and, despite our best efforts, up to 30% of patients with “completely-resected” stage I disease will recur. The most commonly accepted predictors of recurrence and death include the tumor size, nodal involvement and metastasis descriptors employed in the AJCC/UICC International TNM staging system for lung cancer [6]. In the current multivariate analysis, tumor size > 2 cm was again confirmed to be an important predictor of recurrence following resection of clinical stage I non-small cell lung cancer (Table 4, Fig. 1).
Despite complete resection with adequate margins, certain tumors can exhibit biology that increases the likelihood of disease recurrence. Visceral pleural invasion is a recognized prognostic factor in NSCLC, serving to upstage tumors < 3 cm in size. Visceral pleural invasion is the only pathologic variable employed beyond the traditional TNM descriptors, and is associated with increased risk of recurrence and death. Interestingly, in our multivariate analysis visceral pleural invasion was not found to a statistically significant predictor of adverse outcomes (p = 0.46) in this study. Angiolymphatic invasion has also been identified as an important prognostic determinant in many solid tumors including breast [11], colon [12], as well as head and neck [13] cancers. Macchiarini and associates were among the first to demonstrate that blood vessel invasion was an adverse prognostic factor in T1N0 tumors that was associated with increased recurrence risk, especially the development of distant metastases [14]. Several recent studies, however, have suggested that angiolymphatic invasion may represent an important adverse prognostic factor in patients undergoing resection for early-stage lung cancer [15,16], with reduction in recurrence-free and overall survival [17,18]. In the current study, angiolymphatic invasion was again demonstrated as a statistically significant risk factor for the development of recurrent disease in clinical stage I non-small cell lung cancer
In addition to tumor size and angiolymphatic invasion, tumor grade was also identified as an independent predictor of recurrence in this analysis. Though not traditionally recognized by the current staging system for lung cancer, tumor grade has been shown to be an important factor in lung cancer disease recurrence in prior studies whether based on degree of differentiation or mitotic count [19,20]. In the current analysis, poorly-differentiated or undifferentiated tumors were found to be associated with increased recurrence risk when adjusting for other significant clinical and pathological variables (Table 4, Fig. 1).
The presence of a tumor-associated inflammatory infiltrate has been reported as a potentially important prognostic finding in several tumors – including colorectal [21] and esophageal cancer [22]. T cell subsets (CD8+ and CD4+) in particular are felt to represent important constituents of this infiltrate, and have been associated with improved survival [23]. Suppression of inflammatory responses within tumors has been postulated to have an adverse impact on outcomes [24]. Such immune dysregulation may play a particularly important role in the earliest stages of disease [25]. Currently, there is little data evaluating the prognostic impact of tumor-infiltrating lymphocytes in lung cancer. In a study of 219 patients undergoing lobectomy for stage I NSCLC, the presence of moderate-severe tumor inflammatory reaction was associated with reduced risk of recurrence and death in tumors ≥ 5 cm [26]. In the present study examining 1192 cases, increasing degrees of inflammatory reaction correlated with decreased recurrence. Similar to angiolymphatic invasion and increased grade, reduced tumor inflammation was found to be a significant independent predictor of recurrence risk (Table 4, Fig. 1).
The extent of surgical margin was also found to be an important predictor of recurrence in clinical stage I NSCLC. Though this concept makes intuitive sense, what constitutes an adequate margin during anatomic lung resection remains unresolved. Previous recommendations have implied that 1.5–2 cm margins might suffice [27,28]. Some authors have advocated tailoring the surgical margin to the underlying pathologic subtype (1.5 cm for squamous cell carcinoma and 2.0 cm for adenocarcinoma) based upon a differential propensity for peribronchial and submucosal spread [29]. El-Sherif and associates demonstrated that tumor margins ≥ 1 cm were associated with a significantly lower recurrence rate, when compared to margins < 1 cm (8 vs. 19%, p = 0.003) [30]. Sawabata and associates suggested that maintaining margin distance should be greater than the maximum diameter of the tumor (margin:tumor ratio > 1) in an effort to minimize the risk of locoregional recurrence [31]. Schuchert and associates demonstrated that a margin:tumor ratio of less than 1 was associated with a significant increase in recurrence rates compared to ratios ≥1 (25.0% vs. 6.2%; p = 0.0014) [32]. In this updated analysis, surgical margin:tumor diameter ratio > 1 was associated with a significant reduction in the hazard ratio (0.65, p = 0.014). The distance from the tumor to the closest staple line should be assessed at the time of surgery. If the final surgical margin is deemed inadequate following segmentectomy, lobectomy should be performed.
Importantly, extent of resection itself (segmentectomy vs. lobectomy) was not identified as a significant predictor of outcomes in patients with clinical stage I NSCLC. We found no significant difference in risk of locoregional or distant recurrence when comparing the lobectomy and segmentectomy groups (Table 2). This is in accord with a propensity-matched comparison (n = 312 patients per group) of segmentectomy vs. lobectomy in patients with clinical stage I non-small cell lung cancer, which revealed no statistically significant difference in recurrence risk or overall survival [33]. Though not specifically addressed in the current analysis, we and others have previously shown that sublobar wedge resection techniques may be associated with increased locoregional recurrence risk when compared to anatomic segmentectomy techniques (segmentectomy and lobectomy) [5,30,34]. Interestingly, increasing surgical margin does not appear to reduce the risk of locoregional or distant recurrence in this subgroup [34]. These differences seem to disappear for tumors < 1 cm [35]. On the basis of this information, we would recommend anatomic segmentectomy or lobectomy techniques for tumors > 1 cm in size with appropriate attention to surgical margin. Tumors less than 1 cm in size can be adequately managed by wedge resection or segmentectomy, while again ensuring an adequate surgical margin.
Limitations of this study include its retrospective nature, and the inherent introduction of bias in patient selection and surgical approach associated with retrospective analyses. Another potential limitation of the study is the classification of pathological variables based on subjective pathological assessment. Each of the studied variables represent required elements of the pathology synoptic published for each case. Though standard definitions are employed for each discrete variable, the possibility of pathologic sampling error and inter-observer variability exists. These findings will need to be further validated by larger datasets and/or prospective studies.
In conclusion, recurrence in clinical stage I non-small cell lung cancer is predominantly dictated by bad tumor biology (increased tumor size, increased grade, angiolymphatic invasion, decreased tumor inflammation). Complete surgical resection with careful attention to surgical margins is required to optimize outcomes. Extent of anatomic resection (segmentectomy vs. lobectomy) does not appear to impact upon risk of recurrence for clinical stage IA disease. A margin:tumor ratio < 1, angiolymphatic invasion, and decreased tumor inflammation are all statistically significant predictors of recurrence in this setting. These data have implications regarding the potential merits of adjuvant therapy in stage I non-small cell lung cancer. Correlation of these clinical and pathological variables with genetic and proteomic profiles will further our understanding of the molecular mechanisms involved in lung cancer recurrence, and can serve as the basis for future prospective, clinical trials.
Acknowledgements
The authors wish to acknowledge the important contribution of Peg Reamer and Judy Forster in clinical trial enrollment. We would also like to recognize Althea Schneider of the Thoracic Surgery Tumor Registry for her assistance in database management and analysis. Dr. Normolle’s work on this project was supported by NIH/NCI P50 CA090440-11.
Funding support
Dr. Normolle’s work on this project was supported by NIH/NCIP50 CA090440-11.
Abbreviations:
- VATS
video-assisted thoracic surgery
- NSCLC
non-small cell lung cancer
- PFTs
pulmonary function tests
- FEV1
forced expiratory volume in one second
- DLCO
lung diffusion capacity for carbon monoxide
- CT
computed tomography
- PET
positron emission tomography
- COPD
chronic obstructive pulmonary disease
- CA
carcinoma
Footnotes
Disclosure statement
The authors have nothing to disclose in relation to the content of this manuscript.
Conflict of interest statement
The authors have no conflict of interest in relation to the content of this manuscript.
References
- [1].Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D, Global cancer statistics, CA Cancer J. Clin 61 (2) (2011) 69–90, 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- [2].Siegel R, Ward E, Brawley O, Jemal A, Cancer statistics 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths, CA Cancer J. Clin 61 (2011) 212–236, 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- [3].Martini N, Rusch VW, Bains MS, Kris MG, Downey RJ, Flehinger BJ, Ginsberg RJ, Factors influencing ten-year survival in resected stages I-IIIA non-small cell lung cancer, J. Thorac. Cardiovasc. Surg 117 (1) (1999) 32–36. [DOI] [PubMed] [Google Scholar]
- [4].Ginsberg RJ, Lung cancer surgery: acceptable morbidity and mortality, expected results and quality control, Surg. Oncol 11 (2002) 263–266. [DOI] [PubMed] [Google Scholar]
- [5].Ginsberg RJ, Rubinstein LV, Randomized trial of lobectomy vs. limited resection for T1N0 non-small cell lung cancer, Ann. Thorac. Surg 60 (1995) 615–623. [DOI] [PubMed] [Google Scholar]
- [6].Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WE, Nicholson AG, Groome P, Mitchell A, Bolejack V, International Association for the Study of Lung Cancer Staging and Prognostic Factors Committee, Advisory Boards, and Participating Institutions; International Association for the Study of Lung Cancer Staging and Prognostic Factors Committee Advisory Boards and Participating Institutions, The IASLC lung cancer staging project: proposals for the revision of TNM groupings in the forthcoming (8th edition) of the TNM classification for lung cancer, J. Thorac. Oncol 11 (1) (2016) 39–51, 10.1016/j.jtho.2015.09.009. [DOI] [PubMed] [Google Scholar]
- [7].Kirby TJ, Mack MJ, Landreneau RJ, Rice TW, Lobectomy – video-assisted thoracic surgery versus muscle-sparing thoracotomy: a randomized trial, J. Thorac. Cardiovasc. Surg 109 (1995) 997–1001. [DOI] [PubMed] [Google Scholar]
- [8].Churchill ED, Belsey R, Segmental pneumonectomy for bronchiectasis, Ann. Surg 109 (1939) 481–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Schuchert MJ, Pettiford BL, Luketich JD, Landreneau RJ, Parenchymal-sparin resections: why, when and how, Thor Surg Clin. 18 (1) (2008), 10.1016/j.thorsurg.2007.11.007. [DOI] [PubMed] [Google Scholar]
- [10].STS General Thoracic Data Specifications – Version 2.41. (2018) Available at: Updated January 24 https://www.sts.org/sites/default/files/documents/STSThoracicDataSpecsV2_41.pdf.
- [11].Livi L, Paiar F, Simontacchi G, Barca R, Detti B, Fondelli S, Bastiani P, Santini R, Scotti V, Bianchi S, Cataliotti L, Mungai V, Biti G, Locoregional failure pattern after lumpectomy and breast irradiation in 4, 185 patients with T1 and T2 breast cancer, implications for nodal irradiation, Acta Oncol 45 (5) (2006) 564–570. [DOI] [PubMed] [Google Scholar]
- [12].Muller S, Chesner IM, Egan MJ, Rowlands DC, Collard MJ, Swarbrick ET, Newman J, Significance of venous and lymphatic invasion in malignant polyps of the colon and rectum, Gut 30 (10) (1989) 1385–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sparano A, Weinstein G, Chalian A, Yodul M, Weber R, Multivariate predictors of occult neck metastasis in early oral tongue cancer, Otolaryngol. Head Neck Surg 131 (4) (2004) 472–476. [DOI] [PubMed] [Google Scholar]
- [14].Macchiarini P, Fontanini G, Hardin MJ, Chuanchieh H, Bigini D, Vignati S, Pingitore R, Angeletti CA, Blood vessel invasion by tumor cells predicts recurrence in completely resected T1N0M0 non-small cell lung cancer, J. Thorac. Cardiovasc. Surg 106 (1) (1993) 80–89. [PubMed] [Google Scholar]
- [15].Schmid K, Birner P, Gravenhorst V, End A, Geleff S, Prognostic value of lymphatic and blood vessel invasion in neuroendocrine tumors of the lung, Am. J. Surg. Pathol 29 (3) (2005) 324–328. [DOI] [PubMed] [Google Scholar]
- [16].Schuchert MJ, Schumacher L, Kilic A, Close J, Landreneau JR, Pennathur A, Awais O, Yousem SA, Wilson DO, Luketich JD, Landreneau RJ, Impact of angiolymphatic invasion and pleural invasion on surgical outcomes for stage I non-small cell lung cancer, Ann. Thorac. Surg 91 (4) (2011) 1059–1065, 10.1016/j.athoracsur.2010.11.038. [DOI] [PubMed] [Google Scholar]
- [17].Pechet TT, Carr SR, Collins JE, Cohn HE, Farber JL, Arterial invasion predicts early mortality in stage I non-small cell lung cancer, Ann. Thorac. Surg 78 (5) (2004) 1748–1754. [DOI] [PubMed] [Google Scholar]
- [18].Bodendorf MO, Haas V, Laberke HG, Blumenstock G, Wex P, Graeter T, Prognostic value and therapeutic consequences of vascular invasion in non-small cell carcinoma, Lung Cancer 64 (1) (2009) 71–78, 10.1016/j.lungcan.2008.07.011. [DOI] [PubMed] [Google Scholar]
- [19].Shimada Y, Saji H, Yoshida K, Kakihana M, Honda H, Nomura M, Usuda J, Kajiwara N, Ohira T, Ikeda N, Pathological vascular invasion and tumor differentiation predict cancer recurrence in stage 1A non-small cell lung cancer after complete surgical resection, J. Thorac. Oncol 7 (8) (2012) 1263–1270, 10.1097/JTO.0b013e31825cca6e. [DOI] [PubMed] [Google Scholar]
- [20].Kadota K, Suzuki K, Kachala SS, Zabor EC, Sima CS, Moreira AL, Yoshizawa A, Riely GJ, Rusch VW, Adusumilli PS, Travis WD, A grading system combining architectural features and mitotic count predicts recurrence in stage I lung adenocarcinoma, Mod. Pathol 25 (8) (2012) 1117–1127, 10.1038/modpathol.2012.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoué F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pagès F, Type, density and location of immune cells within human colorectal tumors predict clinical outcome, Science 313 (5795) (2006) 1960–1964. [DOI] [PubMed] [Google Scholar]
- [22].Schumacher K, Haensch W, Röefzaad C, Schlag PM, Prognostic significance of activated CD8+ cell infiltrations in esophageal carcinomas, Cancer Res. 61 (10) (2001) 3932–3936. [PubMed] [Google Scholar]
- [23].Hiraoka K, Miyamoto M, Cho Y, Suzuoki M, Oshikiri T, Nakakubo Y, Itoh T, Ohbuchi T, Kondo S, Katoh H, Concurrent infiltration by CD8+ T cells and CD4+ T cells is a favorable prognostic factor in non-small cell lung carcinoma, Br. J. Cancer 94 (2) (2006) 275–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Shimizu K, Nakata M, Hirami Y, et al. , Tumor-infiltrating FoxP3+ regulatory T cells are correlated with cyclooxygenase-2 expression and are associated with recurrence in resected non-small cell lung cancer, J. Thorac. Oncol 5 (5) (2010) 585–590. [DOI] [PubMed] [Google Scholar]
- [25].Ishibashi Y, Tanaka S, Tajima K, Yoshida T, Kuwano H, Expression of FOXp3 in non-small cell lung cancer patients is significantly higher in tumor tissues than normal tissues, especially in tumors smaller than 30 mm, Oncol. Rep 15 (5) (2006) 1315–1319. [PubMed] [Google Scholar]
- [26].Kilic A, Landreneau RJ, Luketich JD, Pennathur A, Schuchert MJ, Density of tumor-infiltrating lymphocytes correlates with disease recurrence and survival in patients with large non-small cell lung cancer tumors, J. Surg. Res 167 (2) (2011) 207–210, 10.1016/j.jss.2009.08.029. [DOI] [PubMed] [Google Scholar]
- [27].Cotton RE, The bronchial spread of lung cancer, Br. J. Dis. Chest 53 (1959) 142–150. [DOI] [PubMed] [Google Scholar]
- [28].Kara M, Sak SD, Orhan D, Yavuzer S, Changing patterns of lung cancer: ¾” (1.9 cm), still a safe length for bronchial resection margin? Lung Cancer 30 (3) (2000) 161–168. [DOI] [PubMed] [Google Scholar]
- [29].Griess DF, McDonald JR, Clagett OT, The proximal extension of carcinoma of the lung in the bronchial wall, J. Thorac. Surg 14 (1945) 362–368. [Google Scholar]
- [30].El-Sherif A, Fernando HC, Santos R, Pettiford B, Luketich JD, Close JM, Landreneau RJ, Margin and local recurrence after sublobar resection of non-small cell lung cancer, Ann. Surg. Oncol 14 (8) (2007) 2400–2405. [DOI] [PubMed] [Google Scholar]
- [31].Sawabata N, Ohta M, Matsumura A, Nakagawa K, Hirano H, Maeda H, Matsuda H, Thoracic Surgery Study Group of Osaka University, Optimal distance of malignant negative margin in excision of non-small cell lung cancer: a multicenter prospective study, Ann. Thorac. Surg 77 (2) (2004) 415–420. [DOI] [PubMed] [Google Scholar]
- [32].Schuchert MJ, Pettiford BL, Keeley S, D’Amato TA, Kilic A, Close J, Pennathur A, Santos R, Fernando HC, Landreneau JR, Luketich JD, Landreneau RJ, Anatomic segmentectomy in the treatment of stage I non-small cell lung cancer, Ann. Thorac. Surg 84 (3) (2007) 926–932. [DOI] [PubMed] [Google Scholar]
- [33].Landreneau RJ, Normolle DP, Christie NA, Awais O, Wizorek JJ, Abbas G, Pennathur A, Shende M, Weksler B, Luketich JD, Schuchert MJ, Recurrence and survival outcomes after anatomic segmentectomy versus lobectomy for clinical stage I non-small cell lung cancer: a propensity-matched analysis, J. Clin. Oncol 32 (23) (2014) 2449–2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Maurizi G, D’Andrilli A, Ciccone AM, Ibrahim M, Andreetti C, Tierno S, Poggi C, Menna C, Venuta F, Rendina EA, Margin distance does not influence recurrence and survival after wedge resection for lung cancer, Ann. Thorac. Surg 100 (3) (2015) 918–925. [DOI] [PubMed] [Google Scholar]
- [35].Schuchert MJ, Kilic A, Pennathur A, Nason KS, Wilson DO, Luketich JD, Landreneau JR, Oncological outcomes after surgical resection of sub-centimeter non-small cell lung cancer, Ann. Thorac. Surg 91 (6) (2011) 1681–1687. [DOI] [PubMed] [Google Scholar]


