ABSTRACT
A 69-year-old male presented with early stage non-small cell lung cancer in 2016. The tumor was resected; however, the patient experienced recurrence 2 years later and subsequently received paclitaxel/carboplatin concurrently with radiotherapy. Within weeks of completing this treatment, he developed a symptomatic pancoast tumor secondary to disease progression and commenced second line nivolumab. Following the second dose of nivolumab, he developed acute unilateral right hearing loss. He commenced intravenous methylprednisolone followed by a slow taper of oral prednisolone. With steroids, he noted a gradual improvement in hearing, confirmed by audiology. Restaging imaging post-nivolumab demonstrated a complete metabolic response. Two prior cases have reported bilateral sensorineural hearing loss post-immune checkpoint inhibitor (ICI). We postulate the hearing impairment relates to the development of autoimmune inner ear disease. To our knowledge, this is the only case of a patient experiencing unilateral loss of hearing following an ICI.
Keywords: non-small cell lung cancer, immune checkpoint inhibitors, autoimmune inner ear disease, nivolumab, sensorineural hearing loss
INTRODUCTION
Lung cancer is among the most frequently diagnosed cancers and the leading cause of cancer-related mortality [1]. The global burden will continue to rise as the world population increases, particularly due to tobacco smoking. Lung cancer can be treated through surgical intervention, radiation therapy, chemotherapy and targeted therapy. The type of treatment will vary depending on the stage, histology and molecular subtype. Immunotherapy targeting the programmed-death receptor 1 (PD1), programmed-death ligand 1 (PD-L1) and cytotoxic T-lymphocyte associated protein 4 (CTLA-4) immune-checkpoint inhibitors (ICI) has transformed the treatment landscape for patients with non-small cell lung cancer (NSCLC) in the first- and second-line settings [2]. PD-L1 is an immune checkpoint protein expressed on the surface of cancer cells, which prevents anticancer immunity through interaction with the PD-1. As a result, cancer cells survive unhindered by the immune system. Nivolumab and pembrolizumab directly target PD-1, while atezolizumab and durvalumab inhibit PD-L1 from interacting with its receptor, with activity in NSCLC in various clinical settings [2–5]. These drugs pose a risk of immune overstimulation which forms the basis of the side effect profile of these drugs.
CASE REPORT
A 69-year-old male was initially diagnosed in Germany with early stage NSCLC of the right upper lobe of the lung in 2016. This lesion was resected. No adjuvant chemotherapy or radiotherapy was offered at that point. In May 2018, the patient developed recurrent disease at the resection site (Fig. 1A). He received carboplatin and paclitaxel concurrently with radiotherapy over 6 weeks from June to July 2018. Within a few weeks of completing concurrent treatment, he developed a symptomatic pancoast tumor, with pain and swelling of the right upper limb. A computed tomography (CT) scan of his chest demonstrated an increase in tumor size. Due to disease progression, a decision was made to commence second line treatment with nivolumab. After the second dose of nivolumab, the patient presented to an ENT clinic with acute onset unilateral right-sided hearing loss. Unilateral sensorineural deafness was diagnosed on audiometry at both high- and low-frequency levels (Fig. 1C). That week he was subsequently reviewed at oncology follow-up. An MRI brain scan revealed no evidence of central nervous system involvement with disease (Fig. 2A and B). As no clear cause was evident on initial assessment to explain the hearing loss, the suspicion of immune related ototoxicity was raised. He commenced high-dose intravenous (IV) methylprednisolone for 3 days, followed by a slow wean of oral prednisolone post-IV treatment. The patient had a gradual improvement in hearing. Repeat audiology confirmed reversal of the sensorineural deafness over time (Fig. 1D).
Figure 1.
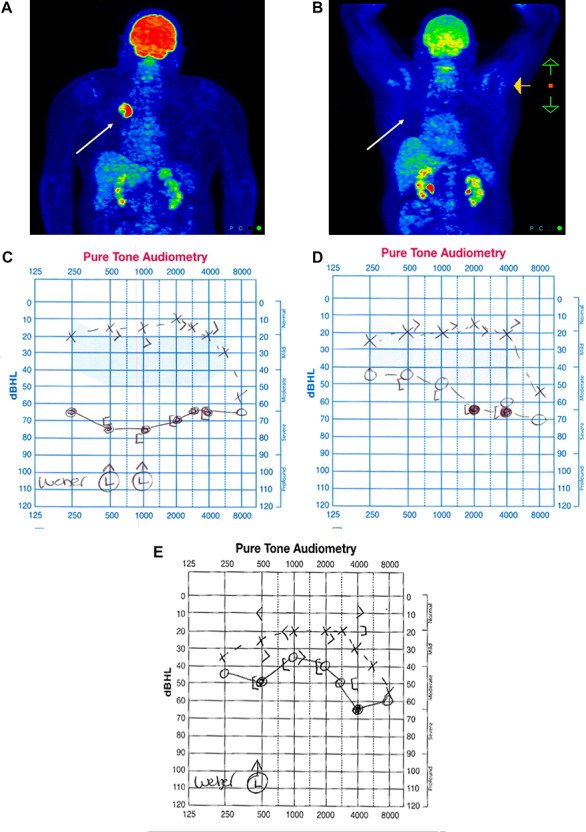
(A) PET/CT at time of recurrence confirming FDG right upper lobe avid lesion. (B) PET/CT post two cycles of nivolumab showing no evidence of FDG avid disease. (C) Baseline audiometry showing complete loss of hearing affecting the right ear. (D) Evidence of initial recovery of low-frequency hearing after 10 days on steroids. (E) Continued improvement in hearing 3 months after discontinuation of nivolumab.
Figure 2.
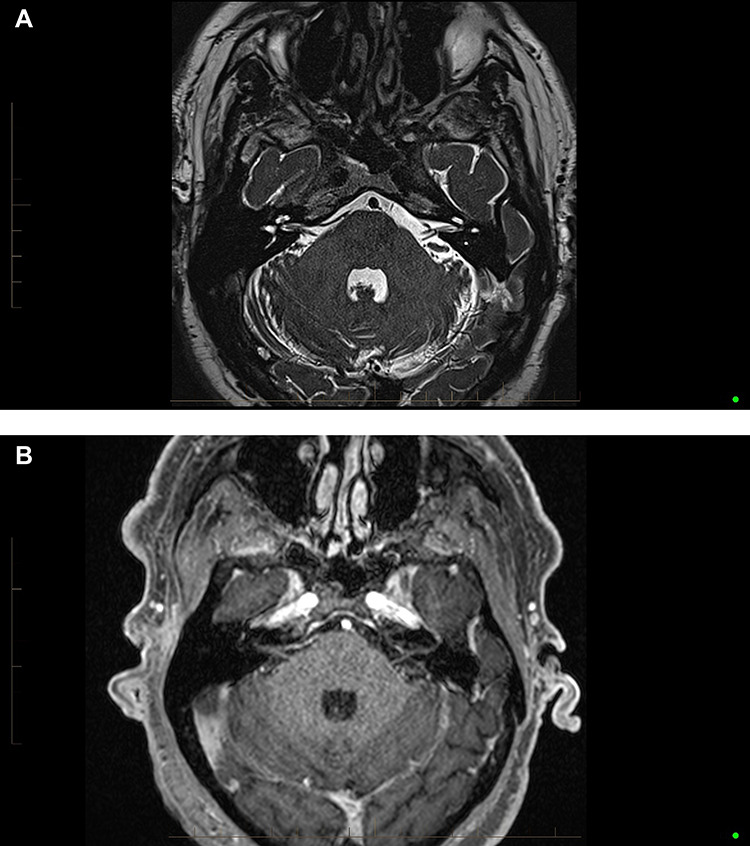
(A) Axial high resolution T2 SPACE sequences demonstrating no structural retrocochlear lesion. (B) Axial T1 fat saturated post-Gadolinium demonstrating no abnormal enhancement of the vestibulocochlear apparatus.
Positron emission tomography (PET)/CT imaging post-nivolumab demonstrated a marked reduction of fluorodeoxyglucose (FDG) avidity, consistent with a complete metabolic response to immunotherapy (Fig. 1B). Based on this and the neurotoxicity, the patient remained off systemic treatment. He remains in follow-up and is clinically well. Sequential scans demonstrate ongoing stability with metabolically inactive disease. Repeated audiology showed further evidence of improvement in the hearing of the right ear (Fig. 1E).
DISCUSSION
Immune checkpoint inhibitors have revolutionized the treatment of lung cancer, however, modulating the immune system can lead to immune-related adverse events [4]. A myriad of side effects have been observed in clinical trials, including dermatitis, colitis, hepatitis and myositis among others [4]. Grade 1–2 adverse events can usually be treated with oral steroids; however, grade 3–4 events require discontinuation of the ICI in most cases [6].
The first known case of autoimmune inner ear disease, reported an 82-year-old male who presented with metastatic mucosal melanoma and was initially treated with ipilimumab [5]. The patient was later commenced on pembrolizumab and developed acute hearing loss. He was diagnosed with bilateral sensorineural hearing loss. He received bilateral intratympanic dexamethasone injections. Otology confirmed an improvement in the patients’ hearing as well as a decrease in tumor size, 12 weeks later. The authors postulated that the cause was autoimmune in nature as a result of the pembrolizumab.
The second case, described a 67-year-old male who presented to the clinic with an invasive melanoma of the toe [3]. Following amputation of his toe, he was treated with pembrolizumab, however, developed severe bilateral sensorineural hearing loss after his first dose of therapy. The patient received intratympanic steroid injections and had a subjective return to baseline hearing. According to the authors, the FDA has already reported 22 cases of pembrolizumab-induced autoimmune inner ear disease with this case being 1 of 14 related to metastatic melanoma.
A clear similarity among the cases is a sudden onset of loss in auditory function upon treatment with an ICI, with a notable, sometimes subjective improvement in hearing with treatment. In our case, the patient’s auditory function was measured after hearing loss occurred and twice thereafter during recovery. A limitation to this case report is the lack of audiometry measurements pre-nivolumab. Therefore, we are unable to confirm whether the patient had prior hearing difficulties at baseline that may have contributed to his acute hearing loss. Baseline audiometry tests and ENT review should be considered in patients presenting for treatment with evidence of hearing loss prior to commencing ICI. Furthermore, this finding re-iterates the importance of advising the patient to contact their oncologist to discuss any acute toxicity that emerges upon ICI therapy.
Here, we described a case where a patient developed acute unilateral hearing loss of the right ear, after receiving a PD-1 inhibitor, nivolumab. Reversibility of the hearing impairment was apparent on treatment with steroids. Two prior case reports have described a similar phenomenon [3, 5]. Our case is the first to report unilateral sensorineural hearing loss in the lung cancer setting. We postulate the likely cause of the hearing impairment relates to autoimmune inner ear disease secondary to T-cell over activation affecting the vestibulocochlear nerve of the right ear. Given the clinical significance and potential reversibility, oncologists should be aware of this potential rare side effect. In the occurrence of ICI-induced hearing loss, we suggest early treatment with high dose steroids followed by a slow wean of oral steroids.
ACKNOWLEDGEMENTS
None.
FUNDING
This research did not receive any funding.
Conflict of interest statement. No conflicts of interest.
ETHICAL APPROVAL
Not applicable.
CONSENT
The patient has reviewed the case content and has given written consent for his case to be published with this journal.
GUARANTOR
Aleksandra Rajapakse, Queensland University of Technology, Faculty of Health, School of Biomedical Sciences, Institute of Health and Biomedical Innovation, Cancer and Ageing Research Program, Brisbane, QLD. Australia; Translational Research Institute, Brisbane, QLD, Australia.
REFERENCES
- 1. Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer 2019;144:1941–53. doi: 10.1002/ijc.31937. [DOI] [PubMed] [Google Scholar]
- 2. Reck M, Borghaei H, O'Byrne KJ. Nivolumab plus ipilimumab in non-small-cell lung cancer. Future Oncol 2019;15:2287–302. doi: 10.2217/fon-2019-0031. [DOI] [PubMed] [Google Scholar]
- 3. Hobelmann K, Fitzgerald D. A case of pembrolizumab induced autoimmune sensorineural hearing loss. J Otol Rhinol 2019;8:1. doi: 10.4172/2324-8785.1000365. [DOI] [Google Scholar]
- 4. Roberts K, Culleton V, Lwin Z, O'Byrne K, Hughes BG. Immune checkpoint inhibitors: navigating a new paradigm of treatment toxicities. Asia Pac J Clin Oncol 2017;13:277–88. doi: 10.1111/ajco.12698. [DOI] [PubMed] [Google Scholar]
- 5. Zibelman M, Pollak N, Olszanski AJ. Autoimmune inner ear disease in a melanoma patient treated with pembrolizumab. J Immunother Cancer 2016;4:8. doi: 10.1186/s40425-016-0114-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thompson JA. New NCCN guidelines: recognition and management of immunotherapy-related toxicity. J Natl Compr Canc Netw 2018;16:594–6. doi: 10.6004/jnccn.2018.0047. [DOI] [PubMed] [Google Scholar]


