Abstract
In 1992, the Brugada brothers published a patient series of aborted sudden death, who were successfully resuscitated from ventricular fibrillation (VF). These patients had a characteristic coved ST-segment elevation in the right precordial leads on their 12-lead electrocardiogram with no apparent structural heart abnormality. This disease was referred to as “right bundle branch block, persistent ST-segment elevation, and sudden death syndrome.” The term Brugada syndrome (BrS) was first coined for this new arrhythmogenic entity in 1996. BrS is more prevalent in Southeast Asian ethnic groups and was considered a familial disease due to the presence of syncope and/or sudden deaths in several members of the same family, however, the genetic alteration was only noted in 1998. The genetic characterization of BrS has proven to be challenging. The most common and well-established BrS genotype involves loss-of-function mutations in the SCN5A gene, but only represents between 15% and 30% of the diagnosed patients. Patients with BrS can present with a range of symptoms which can include syncope, seizures, and nocturnal agonal breathing due to polymorphic ventricular tachycardia or VF. If these arrhythmias are sustained, sudden cardiac death may result. Despite the significant progress on the understanding of BrS over the last two decades, there remain a number of uncertainties and challenges; we present an update review on the subject.
Keywords: Brugada syndrome, electrical storm, epicardial ablation, polymorphic ventricular tachycardia, right ventricular outflow tract ablation, sudden cardiac death, ventricular fibrillation
HISTORICAL PERSPECTIVE
In 1917, Guazon et al.[1] described a sudden nocturnal death syndrome among apparently Philippine young healthy adults, and autopsy revealed no pathological evidence to explain the cause of the death. The condition had become known locally as “Bangungut,” the meaning of which is to “rise and moan in sleep” – representing the most common preterminal clinical feature. In 1992, the Brugada brothers published a series of eight patients of aborted sudden death, who were successfully resuscitated from ventricular fibrillation (VF).[2] These patients had a characteristic coved ST-segment elevation in the right precordial leads on their 12-lead electrocardiogram (ECG) with no apparent structural heart abnormality. This disease was referred to as “right bundle branch block, persistent ST-segment elevation, and sudden death syndrome.” The term Brugada syndrome (BrS) was first coined for this new arrhythmogenic entity in 1996[3] and in 1997,[4] it was recognized as the same entity of sudden nocturnal death syndrome described by Guazon et al. in 1917.
Sudden unexplained nocturnal death syndrome is an entity more prevalent in Southeast Asian ethnic groups and was considered a familial disease due to the presence of syncope and/or sudden deaths in several members of the same family, however, the genetic alteration was first noted in 1998 by Chen et al.[5] Despite the significant progress on the understanding of BrS over the last two decades, there remain a number of uncertainties and challenges. This review will focus primarily on the clinical manifestation, risk stratification, and management of patients with BrS.
PREVALENCE
The prevalence of BrS is believed to range from 1 in 5000 to 1 in 2000. However, the incidence of BrS pattern on ECG is higher, ranging from 0.12% to 0.8% in several studies. It is considered to be responsible for up to 20% of sudden deaths in patients with structurally normal hearts and has an 8–10 times male preponderance. The highest prevalence appears to be in South-East Asia.[6]
CLINICAL FEATURES
Patients with BrS can present with a range of symptoms which can include syncope, seizures, and nocturnal agonal breathing due to polymorphic ventricular tachycardia (PMVT) or VF. If these arrhythmias are sustained, sudden cardiac death (SCD) may result. Syncope or SCD is reported to range from 17% to 42%,[7] but this likely overestimates the true incidence, as most asymptomatic patients are never diagnosed. Recent data suggest a substantially lower rate of SCD as the first symptom, approximately 4.5%, and also a lower incidence of recurrent arrhythmia during follow-up, 5%.[8]
Life-threatening arrhythmias usually occur during resting or sleeping, suggesting a possible association with bradycardia or vagal events. However, there is no data discrimination between bradycardia-dependent or vagal events, but the majority of events occur during vagal predominance, which implies bradycardia at the same time. Febrile episodes have also been frequently associated with symptoms.[9] Symptoms typically first occur during adulthood, with a mean age of SCD presentation of 41 ± 15 years. There is an 8–10-fold male predominance and thus may be linked to hormonal influences, particularly as this gender difference is not seen in children under the age of 16 years.[10]
DIAGNOSTIC CRITERIA
Until 2013, BrS diagnosis required demonstration of the presence of a Type 1 ECG pattern and clinical manifestations such as resuscitated SCD, documented PMVT, a history of nonvasovagal syncope, and/or a family history of noncoronary SCD age <45 years. However, because many patients with a Type 1 ECG are asymptomatic, the 2013 expert consensus statement proposed the following definition:[11] “BrS is diagnosed when a type 1 ST elevation is observed either spontaneously or after intravenous administration of a sodium channel blocker in at least one right pre-cordial lead (V1 and V2), placed in a standard or superior position (up to the 2nd intercostal space),” without the need for evidence of malignant arrhythmias.
ELECTROCARDIOGRAPHIC FEATURES
Following the original description of BrS, there was much uncertainty about its precise ECG features. To clarify these ambiguities, an expert consensus document was published in 2012 establishing two descriptive ECG abnormalities for BrS,[12] when previously there had been three:
Type 1 (coved type) [Figure 1a]: This alteration is the only diagnostic pattern for BrS. It is characterized by an ST-segment elevation ≥2 mm in ≥1 right precordial lead (V1 to V3), followed by an r′-wave and a concave or straight ST segment, but not convex. The descending ST-segment crosses the isoelectric line and is accompanied by a negative and symmetric T-wave. These changes should be observed either spontaneously or following a provocative drug challenge following intravenous administration of a sodium-channel blocker, such as ajmaline, flecainide, or procainamide. All other known causes of ST-segment elevation in right precordial leads (known as phenocopies or mimics) should be excluded before making the diagnosis of BrS.[13]
Figure 1.
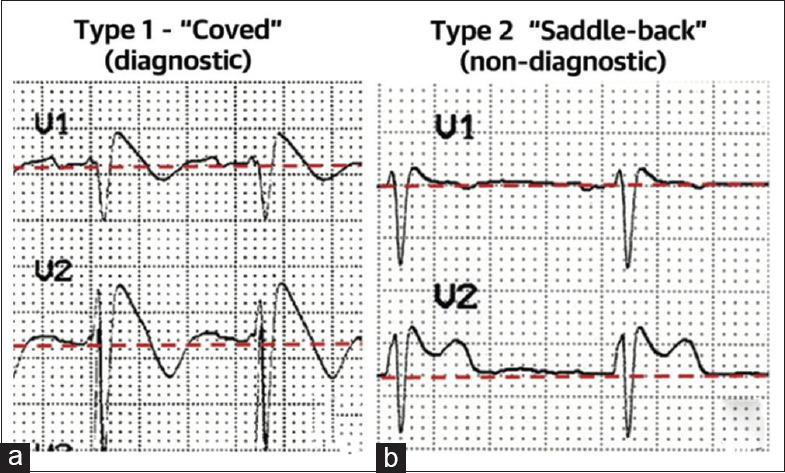
(a) Type 1 Brugada electrocardiogram pattern showing a concave ST-segment elevation ≥2 mm in ≥1 right precordial lead, followed by a negative T-wave. (b) Type 2 Brugada electrocardiogram pattern showing a convex ST-segment elevation ≥0.5 mm (generally >2 mm) in ≥1 right precordial lead followed by a positive T-wave[31]
Type 2 (saddle-back type) [Figure 1b]: This ECG anomaly is only suggestive of BrS. It is characterized by an ST-segment elevation ≥0.5 mm (often ≥2 mm in V2) in ≥1 right precordial lead (V1 to V3), followed by a convex ST. The rʹ-wave may or may not overlap the J point, but it has a slow downward slope. The ST segment is followed by a positive T-wave in V2 and is of variable morphology in V1.
The placement of the right precordial leads in more cranial positions (in the 2nd or 3rd intercostal spaces) increases sensitivity in some patients due to the variable anatomical correlation between the right ventricular outflow tract (RVOT) and V1 to V2 in the standard positions.[14] The identification of a spontaneous Type 1 pattern in the higher intercostal spaces conferred a similar prognosis to individuals with a Type 1 pattern in the standard positions of V1 to V2.[15]
GENETICS OF BRUGADA SYNDROME
The genetic characterization of BrS has proven to be challenging. The most common and well-established BrS genotype involves loss-of-function mutations in the SCN5A gene, representing between 15% and 30% of the diagnosed patients.[16] The SCN5A gene encodes for the cardiac voltage-gated sodium channel that is activated during the initial rapid depolarization of the cardiac action potential cycle. More than 250 mutations in the SCN5A gene have already been described, yet the role for genetic testing in the diagnosis of BrS is limited due to the presence of “benign” SCN5A variants in the general population.[4] It is clear that the genetic basis for BrS is heterogeneous and no clear pathogenic genotype can be currently identified in the majority of patients.[9] Furthermore, several large registry studies have failed to establish an independent association between genotype status and prognosis in BrS.[17] However, recent data suggest that mutations specifically involving the pore region of the SCN5A gene may carry a more severe phonotype and were found to be independently associated with the risk of adverse cardiac events in both asymptomatic and symptomatic BrS patients.[18]
Despite the ongoing progress in understanding the genetic causes of BrS, only 30%–35% of clinically diagnosed cases are genetically diagnosed, and most of these (25%–30%) result from pathogenic alterations in SCN5A.
PATHOPHYSIOLOGY – CHANNELOPATHY OR CARDIOMYOPATHY?
Two main pathophysiological mechanisms have been described for ventricular tachyarrhythmias (VTs) leading to SCD in BrS. Conventionally, BrS was thought to be a repolarization disorder, caused by the disparate expression of transient outward potassium current, mediated by a reduction in early sodium inflow, between the epicardium and inner myocardial layers. This results in curtailment of the epicardial action potential and a susceptibility to re-entry PMVT triggered by premature ventricular complexes, due to the epicardial-to-endocardial transmembrane ionic imbalance.[19,20]
The depolarization theory is modeled around action potential conduction delay in the RVOT relative to the surrounding myocardium. In such conditions, VT can be triggered by the unequal membrane potential around the RVOT border, similar to the formation of VT seen in THE circumstances of regional myocardial ischemia.[21] This theory is supported by a number of clinical studies demonstrating relative RVOT conduction delay.[22]
BrS was originally described as a disease of cardiac ion channel dysfunction without the presence of associated structural heart disease, leading to sudden death in otherwise healthy people.[17] However, evidence demonstrating normalization of the pathognomonic ECG pattern and apparent significant reduction of the tendency to arrhythmia in most patients following radiofrequency ablation (RFA) of the RVOT epicardium strengthens the theory that structural abnormalities have a critical role in arrhythmogenesis in BrS.[23]
Microanatomical changes such as increased collagen, fibrosis, and reduced gap junction expression, which may be mediated by the underlying myocardial inflammation, could be responsible for the characteristic ECG pattern and susceptibility to arrhythmia.[24] Based on these findings, it has been suggested that BrS and arrhythmogenic cardiomyopathy represent different parts of the same disease spectrum.[25] Although these two conditions have distinct macropathological appearances, there are many overlapping clinical features and genetic predispositions.[17] The BrS ECG pattern has been noted in patients with arrhythmogenic cardiomyopathy, and SCD may occur in the early phase of the arrhythmogenic cardiomyopathy, in the absence of the associated characteristic structural changes.[26]
There are a complex mix of underlying pathophysiological features which are variably expressed in individuals, which contribute toward BrS and risk of arrhythmia; these are summarized in Figure 2.[27]
Figure 2.
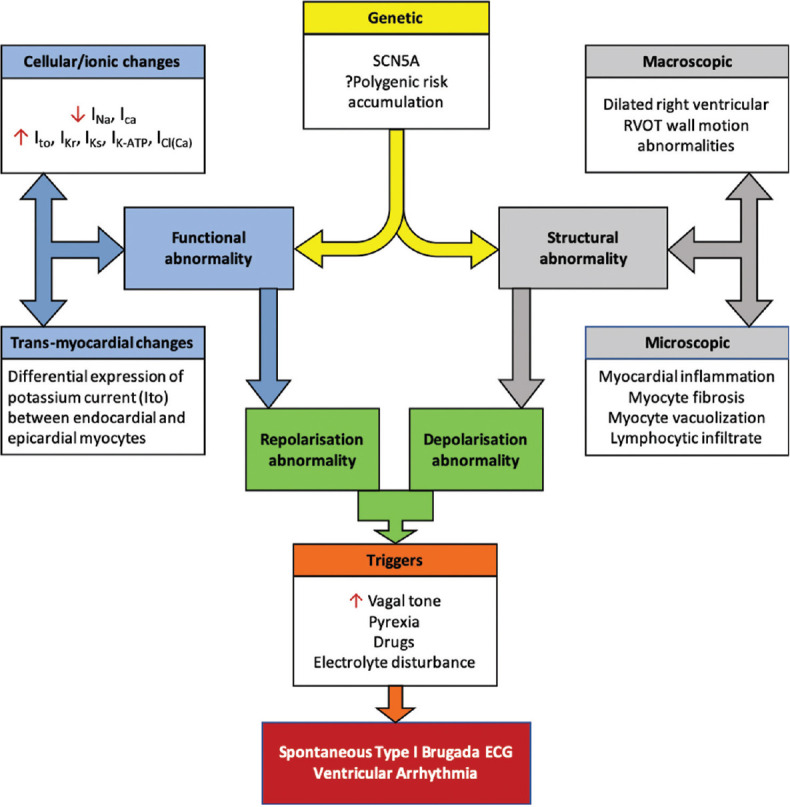
Pathophysiology of Brugada syndrome[27]
RISK STRATIFICATION
Mode of presentation – Cardiac arrest
Symptomatic presentation with aborted cardiac arrest or sustained VT confers the highest risk of future arrhythmic event in the form of PMVT/VF, with reported annual rates of approximately 8%, and secondary prevention with implantable cardioverter-defibrillator (ICD) implantation is recommended.[11,28]
Mode of presentation – Syncope
Syncope is a common presentation, occurring in about 30% of patients, and arrhythmia-related syncope is also a well-established risk factor, with an annual event rate of about 2%.[28,29] Therefore, it is recommended that a vasovagal cause of syncope is ruled out as these do not seem to clearly carry any additional risk.[30,31] However, there is evidence that vagal tone may play an important role in precipitating spontaneous VT/VF in some BrS patients.[32,33] Another challenge is that clinical symptoms suggestive of vagal syncope may be observed in syncope of cardiac origin.[34] Nevertheless, there are some pointers to help guide clinicians identified by Nordkamp et al.[35] in a study of 141 BrS patients, who, at the time of their diagnosis, had either experienced aborted sudden cardiac arrest (ACA) or syncope. Compared to suspected nonarrhythmic syncope patients, ACA patients tended to be older at the first event (45 vs. 20 years), were more likely to be male (relative risk 2.1) and to have urinary incontinence (relative risk 4.6), and were less likely to report prodromes. ACA was never triggered by hot/ crowded surroundings, pain or other emotional stress, seeing blood, or prolonged standing.
Although there is consensus that ICD therapy is indicated for patients with clear arrhythmic syncope and is not indicated for patients with neurocardiogenic syncope, management of patients whose cause of syncope is unclear remains challenging. Although not presently reflected in guidelines, there has been demonstration of the usefulness of implantable loop recorders for the evaluation of doubtful causes of syncope, which should be considered as an important diagnostic tool in select patients.[36,37,38]
Most patients diagnosed with BrS are asymptomatic and present a greater challenge for risk stratification and management decisions.[31,39] While the established risk of arrhythmic events is much lower for asymptomatic patients, about 1% annually overall, many cases of SCD still occur in this population.[28,31,40] Yet, regardless of several investigations into the predictors of arrhythmic events in asymptomatic patients, no consensus is available for a risk stratification strategy in this population.[31]
ELECTROCARDIOGRAM
A spontaneous Type 1 Brugada ECG pattern is an important and established risk factor for cardiac events, which has been reported to confer a three-to-fourfold higher risk in asymptomatic patients.[41,42] Despite this, only conservative surveillance and risk modification of lifestyle is universally recommended in asymptomatic patients.[43] In contrast, a family history of SCD at any age is not an independent prognostic marker for cardiac events in either symptomatic or asymptomatic patients.[39,44]
While a multitude of ECG parameters have been associated with increased risk of cardiac arrhythmias, two that have been consistently reported as independent risk factors are abnormal QRS fragmentation in leads V1, V2, and V3 and early repolarization pattern in inferior and/or lateral leads.[45,46,47] In addition, AF is more commonly seen in BrS than in the general population and has been reported as a risk factor for ventricular arrhythmias in several studies.[48]
A more recently reported[49] ECG finding which seems to confer greater risk for VT and VF was a significant S-wave (≥0.1 mV and/or ≥40 ms) in lead I, which showed a sensitivity of 90.6% and 96.9%, a specificity of 62.2% and 61.1%, a negative predictive value of 98.5% and 99.5%, and a positive predictive value of 19.6% and 20.5%, respectively. The usefulness of this simple ECG marker is in its high negative predictive value; however, it needs further corroboration in larger studies. Figure 3 summarizes the most commonly described high-risk features.
Figure 3.
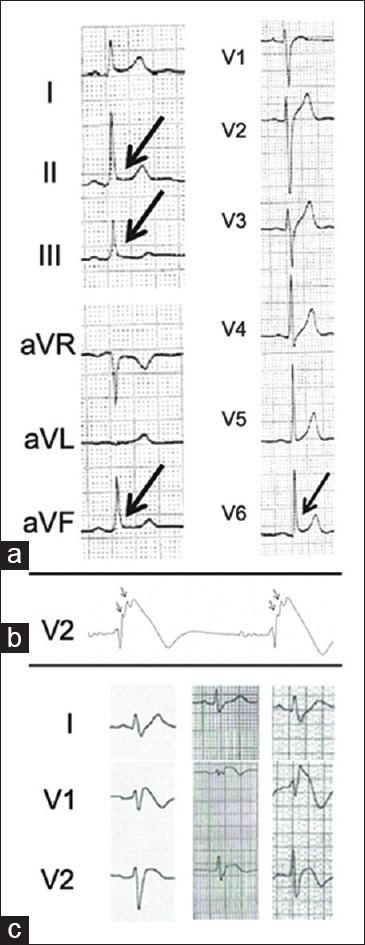
High-risk electrocardiogram features seen in some Brugada syndrome patients. (a) Early repolarization pattern in inferior and/or lateral leads.[45] (b) Fragmented QRS – arrows.[46] (c) Example of significant S wave in three patients with Brugada syndrome[49]
RISK STRATIFICATION WITH ELECTROPHYSIOLOGICAL STUDY
There is ongoing controversy regarding the prognostic value of electrophysiological study (EPS) with programmed electrical stimulation for risk stratification in patients without a history of cardiac arrest. Several studies have demonstrated a positive association between induced PMVT/VF during EPS and the future risk of ventricular arrhythmias, while many have not.[39,42,50,51]
An important limitation and bias of studies in this area is that patients with a positive EPS are more likely to proceed to ICD implantation. Therefore, this population is likely to have a higher frequency of recorded ventricular arrhythmias that may not result in cardiac arrest than in patients with negative EPS and no ICD.[28,30] Another limitation is the heterogeneity between different EPS protocols in the literature.[52] While aggressive stimulation protocols with ≥3 extrastimuli are more sensitive, they were found to be less specific than moderate protocols with up to two extrastimuli.[30,53]
In spite of previous reports indicating a strong negative predictive value of EPS in patients without cardiac arrest, a recent systematic review concluded that a negative EPS does not reliably indicate low risk in asymptomatic patients in the presence of other high-risk features, such as spontaneous Type 1 ECG.[30,52,54]
MANAGEMENT AND RISK MODIFICATION
The mainstay of treatment in high-risk patients remains ICD implantation.[31] Important risk-reducing strategies for all patients include avoidance of potentially exacerbating medications (a comprehensive list is available at http://www.brugadadrugs.org/), immediate treatment of fever with antipyretics as well as keeping away from excessive alcohol intake, and avoiding large meals.[55]
QUINIDINE THERAPY
Treatment with quinidine should be considered as an adjunct to ICD in patients experiencing electrical storms or frequent appropriate shocks, or as an alternative to ICD implantation where this is not possible. Quinidine seems to reduce the Ito current during epicardial repolarization and normalizes the action potential and prevent re-entry and PMVT.
The efficacy of quinidine monotherapy for long-term prevention of malignant ventricular arrhythmias following implantation of ICD, has been demonstrated in a number of studies.[56,57,58,59] A retrospective study showed total elimination of appropriate ICD shocks in 66% (19 of 29) of patients with previous arrhythmic storm or frequent shocks over a mean period of 60 ± 41 months, as well as a significant and clinically important reduction in the number of shocks experienced for the remaining patients.
The empirical use of high-dose quinidine (600–900 mg daily) for the prevention of arrhythmic events has so far been mainly evaluated by a randomized trial of quinidine versus placebo of fifty patients with previously implanted ICD.[56] While treatment appeared to be effective with no associated arrhythmic events observed, a significant result could not be obtained due to low event rate in the placebo group as well as high rates of treatment discontinuation. One substantial problem with quinidine therapy used as an alternative to ICD implantation is the issue of poor compliance and treatment discontinuation due to associated adverse effects.[60] While treatment with low-dose quinidine (<600 mg daily) is associated with greater tolerability, it has only been investigated in a small number of patients.[61] Another important practical limitation is the poor availability of quinidine in most geographic regions.[62]
ROLE OF RADIOFREQUENCY CATHETER ABLATION
RFA of arrhythmogenic zones in the RV epicardium has emerged over the past decade as a possible future curative treatment option for BrS. However, only a small number of studies with limited follow-up periods have reported successful results with RFA in symptomatic Brugada patients.
The first to describe a successful RFA procedure in BrS patients were Nademanee et al. using a selected cohort of nine high-risk patients with frequent ICD shocks for ventricular arrhythmias.[4] All patients were found to have a unique arrhythmogenic focus at the anterior RVOT on epicardial mapping as well as typical Type 1 ECG and inducible VT/VF at baseline. Following ablation, the ECG had normalized in 89% and VT/VF was no longer inducible in 78% of the cohort. Only one of the nine patients had a single subsequent arrhythmic event during the follow-up period (20 ± 6 months). More recently, Brugada et al.[63] and Pappone et al.[64] described an improved technique for successful elimination of the BrS phenotype with epicardial RFA. The mapping was performed before and after the administration of flecainide/ajmaline, which resulted in the identification of more extensive arrhythmogenic segments in the RV epicardium beyond the RVOT. In the larger and more recent study, the described RFA procedure showed normalization of ECG and nondeducibility of VT/VF in all the 135 patients with symptomatic BrS and previous ICD.[59] In addition, a Type 1 ECG could not be provoked with ajmaline following RFA in the vast majority. During a median follow-up period of 10 months, only two patients required a repeat procedure due to recurrent VF.
The only adverse effect reported for all the above studies was mild uncomplicated pericarditis after ablation. RFA treatment is, therefore, recommended for symptomatic patients with recurrent ICD shocks or as an alternative to ICD implantation when contraindicated.[43,55] Whether this is a suitable alternative to ICD for people with high risk, or even an option for low-risk people as a potential “cure,” remains to be determined.
Figure 4 summarizes the proposed management strategy for BrS patients.
Figure 4.
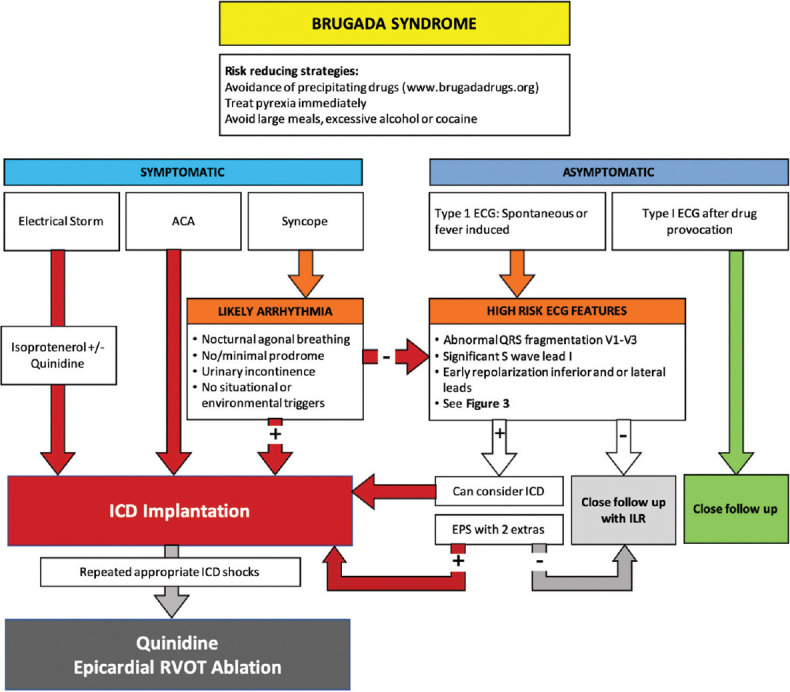
Summary flowchart for the management of Brugada syndrome patients[11]
CONCLUSIONS
BrS is a rare genetic entity characterized by a typical ECG with an associated risk of VF and SCD. Despite recent advances, pathophysiological mechanisms responsible for incomplete penetrance and variable expressivity remain incompletely understood. Almost two-thirds of patients with clinically diagnosed BrS patients have no clear associated genetic basis. The only gene convincingly implicated is SCN5A. The recent reclassification of pathogenic variants associated with BrS is likely to change the percentage of patients identified with a pathological gene.
The pathophysiology of disease generation and progression is more complex than initially described with some overlap with arrhythmogenic cardiomyopathy; a greater understanding of the underlying processes will hopefully lead to further improvements in the diagnosis and management of BrS.
Currently, the ICD is the most accepted therapy to protect patients at risk, but there remain significant shortcomings in the accurate risk assessment in BrS, particularly asymptomatic patients.
Epicardial ablation in selected patients has been used as a new therapy with promise, and our personal experience, albeit limited, mirrors the literature, but as of yet is largely indicated for the treatment of BrS patients with a high burden of ICD therapy despite optimization of other risk modifiers.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Guazon M. Algunas Notas Sobre Bangungut. Filipina Med Farm. 1917;8:437–42. [Google Scholar]
- 2.Brugada P, Brugada J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: A distinct clinical and electrocardiographic syndrome. A multicenter report. J Am Coll Cardiol. 1992;20:1391–6. doi: 10.1016/0735-1097(92)90253-j. [DOI] [PubMed] [Google Scholar]
- 3.Miyazaki T, Mitamura H, Miyoshi S, Soejima K, Aizawa Y, Ogawa S. Autonomic and antiarrhythmic drug modulation of ST segment elevation in patients with Brugada syndrome. J Am Coll Cardiol. 1996;27:1061–70. doi: 10.1016/0735-1097(95)00613-3. [DOI] [PubMed] [Google Scholar]
- 4.Nademanee K, Veerakul G, Nimmannit S, Chaowakul V, Bhuripanyo K, Likittanasombat K, et al. Arrhythmogenic marker for the sudden unexplained death syndrome in Thai men. Circulation. 1997;96:2595–600. doi: 10.1161/01.cir.96.8.2595. [DOI] [PubMed] [Google Scholar]
- 5.Chen Q, Kirsch GE, Zhang D, Brugada R, Brugada J, Brugada P, et al. Genetic basis and molecular mechanism for idiopathic ventricular fibrillation. Nature. 1998;392:293–6. doi: 10.1038/32675. [DOI] [PubMed] [Google Scholar]
- 6.Quan XQ, Li S, Liu R, Zheng K, Wu XF, Tang Q. A meta-analytic review of prevalence for Brugada ECG patterns and the risk for death. Medicine (Baltimore) 2016;95:e5643. doi: 10.1097/MD.0000000000005643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Milman A, Andorin A, Gourraud JB, Postema PG, Sacher F, Mabo P, et al. Profile of patients with Brugada syndrome presenting with their first documented arrhythmic event: Data from the Survey on Arrhythmic Events in BRUgada Syndrome (SABRUS) Heart Rhythm. 2018;15:716–24. doi: 10.1016/j.hrthm.2018.01.014. [DOI] [PubMed] [Google Scholar]
- 8.Casado-Arroyo R, Berne P, Rao JY, Rodriguez-Mañero M, Levinstein M, Conte G, et al. Long-term trends in newly diagnosed Brugada syndrome: Implications for risk stratification. J Am Coll Cardiol. 2016;68:614–23. doi: 10.1016/j.jacc.2016.05.073. [DOI] [PubMed] [Google Scholar]
- 9.Michowitz Y, Milman A, Sarquella-Brugada G, Andorin A, Champagne J, Postema PG, et al. Fever-related arrhythmic events in the multicenter survey on arrhythmic events in Brugada syndrome. Heart Rhythm. 2018;15:1394–401. doi: 10.1016/j.hrthm.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 10.Shimizu W, Matsuo K, Kokubo Y, Satomi K, Kurita T, Noda T, et al. Sex hormone and gender difference—role of testosterone on male predominance in Brugada syndrome. J Cardiovasc Electrophysiol. 2007;18:415–21. doi: 10.1111/j.1540-8167.2006.00743.x. [DOI] [PubMed] [Google Scholar]
- 11.Priori SG, Wilde AA, Horie M, Cho Y, Behr ER, Berul C, et al. HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes: Document endorsed by HRS, EHRA, and APHRS in May 2013 and by ACCF, AHA, PACES, and AEPC in June 2013. Heart Rhythm. 2013;10:1932–63. doi: 10.1016/j.hrthm.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 12.Bayés de Luna A, Brugada J, Baranchuk A, Borggrefe M, Breithardt G, Goldwasser D, et al. Current electrocardiographic criteria for diagnosis of Brugada pattern: A consensus report. J Electrocardiol. 2012;45:433–42. doi: 10.1016/j.jelectrocard.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 13.Baranchuk A, Nguyen T, Ryu MH, Femenía F, Zareba W, Wilde AA, et al. Brugada phenocopy: New terminology and proposed classification. Ann Noninvasive Electrocardiol. 2012;17:299–314. doi: 10.1111/j.1542-474X.2012.00525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miyamoto K, Yokokawa M, Tanaka K, Nagai T, Okamura H, Noda T, et al. Diagnostic and prognostic value of a type 1 Brugada electrocardiogram at higher (third or second) V1 to V2 recording in men with Brugada syndrome. Am J Cardiol. 2007;99:53–7. doi: 10.1016/j.amjcard.2006.07.062. [DOI] [PubMed] [Google Scholar]
- 15.Cerrato N, Giustetto C, Gribaudo E, Richiardi E, Barbonaglia L, Scrocco C, et al. Prevalence of type 1 Brugada electrocardiographic pattern evaluated by twelve-lead twenty-four-hour Holter monitoring. Am J Cardiol. 2015;115:52–6. doi: 10.1016/j.amjcard.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 16.Veerman CC, Mengarelli I, Guan K, Stauske M, Barc J, Tan HL, et al. hiPSC-derived cardiomyocytes from Brugada Syndrome patients without identified mutations do not exhibit clear cellular electrophysiological abnormalities. Sci Rep. 2016;6:309671–10. doi: 10.1038/srep30967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Antzelevitch C, Brugada P, Borggrefe M, Brugada J, Brugada R, Corrado D, et al. Brugada syndrome: Report of the second consensus conference: Endorsed by the Heart Rhythm Society and the European Heart Rhythm Association. Circulation. 2005;111:659–70. doi: 10.1161/01.CIR.0000152479.54298.51. [DOI] [PubMed] [Google Scholar]
- 18.Kosmidis G, Veerman CC, Casini S, Verkerk AO, van de Pas S, Bellin M, et al. Readthrough-promoting drugs gentamicin and PTC124 Fail to Rescue Nav1.5 Function of human-induced pluripotent stem cell-derived cardiomyocytes carrying nonsense mutations in the sodium channel gene SCN5A. Circ Arrhythm Electrophysiol. 2016;9:e004227. doi: 10.1161/CIRCEP.116.004227. [DOI] [PubMed] [Google Scholar]
- 19.Yan GX, Antzelevitch C. Cellular basis for the Brugada syndrome and other mechanisms of arrhythmogenesis associated with ST-segment elevation. Circulation. 1999;100:1660–6. doi: 10.1161/01.cir.100.15.1660. [DOI] [PubMed] [Google Scholar]
- 20.Antzelevitch C. The Brugada syndrome: Ionic basis and arrhythmia mechanisms. J Cardiovasc Electrophysiol. 2001;12:268–72. doi: 10.1046/j.1540-8167.2001.00268.x. [DOI] [PubMed] [Google Scholar]
- 21.Meregalli PG, Wilde AA, Tan HL. Pathophysiological mechanisms of Brugada syndrome: Depolarization disorder, repolarization disorder, or more? Cardiovasc Res. 2005;67:367–78. doi: 10.1016/j.cardiores.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 22.Nagase S, Kusano KF, Morita H, Fujimoto Y, Kakishita M, Nakamura K, et al. Epicardial electrogram of the right ventricular outflow tract in patients with the Brugada syndrome: Using the epicardial lead. J Am Coll Cardiol. 2002;39:1992–5. doi: 10.1016/s0735-1097(02)01888-0. [DOI] [PubMed] [Google Scholar]
- 23.Pieroni M, Notarstefano P, Oliva A, Campuzano O, Santangeli P, Coll M, et al. Electroanatomic and pathologic right ventricular outflow tract abnormalities in patients with Brugada syndrome. J Am Coll Cardiol. 2018;72:2747–57. doi: 10.1016/j.jacc.2018.09.037. [DOI] [PubMed] [Google Scholar]
- 24.Nademanee K, Raju H, de Noronha SV, Papadakis M, Robinson L, Rothery S, et al. Fibrosis, Connexin-43, and conduction abnormalities in the Brugada syndrome. J Am Coll Cardiol. 2015;66:1976–86. doi: 10.1016/j.jacc.2015.08.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moncayo-Arlandi J, Brugada R. Unmasking the molecular link between arrhythmogenic cardiomyopathy and Brugada syndrome. Nat Rev Cardiol. 2017;14:744–56. doi: 10.1038/nrcardio.2017.103. [DOI] [PubMed] [Google Scholar]
- 26.Agullo-Pascual E, Cerrone M, Delmar M. Arrhythmogenic cardiomyopathy and Brugada syndrome: Diseases of the connexome. FEBS Lett. 2014;588:1322–30. doi: 10.1016/j.febslet.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Isbister JC, Krahn AD, Semsarian C, Sy RW. Brugada syndrome: Clinical care amidst pathophysiological uncertainty. Heart Lung Circ. 2020;29:538–46. doi: 10.1016/j.hlc.2019.11.016. [DOI] [PubMed] [Google Scholar]
- 28.Letsas KP, Asvestas D, Baranchuk A, Liu T, Georgopoulos S, Efremidis M, et al. Prognosis, risk stratification, and management of asymptomatic individuals with Brugada syndrome: A systematic review. Pacing Clin Electrophysiol. 2017;40:1332–45. doi: 10.1111/pace.13214. [DOI] [PubMed] [Google Scholar]
- 29.Polovina MM, Vukicevic M, Banko B, Lip GYH, Potpara TS. Brugada syndrome: A general cardiologist’s perspective. Eur J Intern Med. 2017;44:19–27. doi: 10.1016/j.ejim.2017.06.019. [DOI] [PubMed] [Google Scholar]
- 30.Sroubek J, Probst V, Mazzanti A, Delise P, Hevia JC, Ohkubo K, et al. Programmed ventricular stimulation for risk stratification in the Brugada syndrome: A pooled analysis. Circulation. 2016;133:622–30. doi: 10.1161/CIRCULATIONAHA.115.017885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brugada J, Campuzano O, Arbelo E, Sarquella-Brugada G, Brugada R. Present status of Brugada syndrome: JACC state-of-the-art review. J Am Coll Cardiol. 2018;72:1046–59. doi: 10.1016/j.jacc.2018.06.037. [DOI] [PubMed] [Google Scholar]
- 32.Teodorovich N, Kogan Y, Paz O, Swissa M. Vagally mediated ventricular arrhythmia in Brugada syndrome. HeartRhythm Case Rep. 2016;2:530–5. doi: 10.1016/j.hrcr.2016.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mizumaki K, Fujiki A, Tsuneda T, Sakabe M, Nishida K, Sugao M, et al. Vagal activity modulates spontaneous augmentation of ST elevation in the daily life of patients with Brugada syndrome. J Cardiovasc Electrophysiol. 2004;15:667–73. doi: 10.1046/j.1540-8167.2004.03601.x. [DOI] [PubMed] [Google Scholar]
- 34.Alboni P, Brignole M, Menozzi C, Raviele A, Del Rosso A, Dinelli M, et al. Diagnostic value of history in patients with syncope with or without heart disease. J Am Coll Cardiol. 2001;37:1921–8. doi: 10.1016/s0735-1097(01)01241-4. [DOI] [PubMed] [Google Scholar]
- 35.Olde Nordkamp LR, Vink AS, Wilde AA, de Lange FJ, de Jong JS, Wieling W, et al. Syncope in Brugada syndrome: Prevalence, clinical significance, and clues from history taking to distinguish arrhythmic from nonarrhythmic causes. Heart Rhythm. 2015;12:367–75. doi: 10.1016/j.hrthm.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 36.Sacher F, Arsac F, Wilton SB, Derval N, Denis A, de Guillebon M, et al. Syncope in Brugada syndrome patients: Prevalence, characteristics, and outcome. Heart Rhythm. 2012;9:1272–9. doi: 10.1016/j.hrthm.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 37.Kubala M, Aïssou L, Traullé S, Gugenheim AL, Hermida JS. Use of implantable loop recorders in patients with Brugada syndrome and suspected risk of ventricular arrhythmia. Europace. 2012;14:898–902. doi: 10.1093/europace/eur319. [DOI] [PubMed] [Google Scholar]
- 38.Hamilton G, O’Donnell D, Han HC. Brugada syndrome and undifferentiated syncope: Use of an implantable loop recorder to document causation. Med J Aust. 2018;209:113–4. doi: 10.5694/mja17.01117. [DOI] [PubMed] [Google Scholar]
- 39.Probst V, Veltmann C, Eckardt L, Meregalli PG, Gaita F, Tan HL, et al. Long-term prognosis of patients diagnosed with Brugada syndrome: Results from the FINGER Brugada Syndrome Registry. Circulation. 2010;121:635–43. doi: 10.1161/CIRCULATIONAHA.109.887026. [DOI] [PubMed] [Google Scholar]
- 40.Raju H, Papadakis M, Govindan M, Bastiaenen R, Chandra N, O’Sullivan A, et al. Low prevalence of risk markers in cases of sudden death due to Brugada syndrome relevance to risk stratification in Brugada syndrome. J Am Coll Cardiol. 2011;57:2340–5. doi: 10.1016/j.jacc.2010.11.067. [DOI] [PubMed] [Google Scholar]
- 41.Gehi AK, Duong TD, Metz LD, Gomes JA, Mehta D. Risk stratification of individuals with the Brugada electrocardiogram: A meta-analysis. J Cardiovasc Electrophysiol. 2006;17:577–83. doi: 10.1111/j.1540-8167.2006.00455.x. [DOI] [PubMed] [Google Scholar]
- 42.Letsas KP, Liu T, Shao Q, Korantzopoulos P, Giannopoulos G, Vlachos K, et al. Meta-Analysis on Risk Stratification of Asymptomatic Individuals With the Brugada Phenotype. Am J Cardiol. 2015;116:98–103. doi: 10.1016/j.amjcard.2015.03.044. [DOI] [PubMed] [Google Scholar]
- 43.Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, et al. 2017 AHA/ACC/HRS Guideline for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2018;72:1677–749. doi: 10.1016/j.jacc.2017.10.053. [DOI] [PubMed] [Google Scholar]
- 44.Sarkozy A, Sorgente A, Boussy T, Casado R, Paparella G, Capulzini L, et al. The value of a family history of sudden death in patients with diagnostic type I Brugada ECG pattern. Eur Heart J. 2011;32:2153–60. doi: 10.1093/eurheartj/ehr129. [DOI] [PubMed] [Google Scholar]
- 45.Kawata H, Morita H, Yamada Y, Noda T, Satomi K, Aiba T, et al. Prognostic significance of early repolarization in inferolateral leads in Brugada patients with documented ventricular fibrillation: A novel risk factor for Brugada syndrome with ventricular fibrillation. Heart Rhythm. 2013;10:1161–8. doi: 10.1016/j.hrthm.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 46.Tokioka K, Kusano KF, Morita H, Miura D, Nishii N, Nagase S, et al. Electrocardiographic parameters and fatal arrhythmic events in patients with Brugada syndrome: Combination of depolarization and repolarization abnormalities. J Am Coll Cardiol. 2014;63:2131–8. doi: 10.1016/j.jacc.2014.01.072. [DOI] [PubMed] [Google Scholar]
- 47.Takagi M, Aonuma K, Sekiguchi Y, Yokoyama Y, Aihara N, Hiraoka M, et al. The prognostic value of early repolarization (J wave) and ST-segment morphology after J wave in Brugada syndrome: Multicenter study in Japan. Heart Rhythm. 2013;10:533–9. doi: 10.1016/j.hrthm.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 48.Kewcharoen J, Rattanawong P, Kanitsoraphan C, Mekritthikrai R, Prasitlumkum N, Putthapiban P, et al. Atrial fibrillation and risk of major arrhythmic events in Brugada syndrome: A meta-analysis. Ann Noninvasive Electrocardiol. 2019;24:e12676. doi: 10.1111/anec.12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Calò L, Giustetto C, Martino A, Sciarra L, Cerrato N, Marziali M, et al. A new electrocardiographic marker of sudden death in Brugada syndrome: The S-Wave in Lead I. J Am Coll Cardiol. 2016;67:1427–40. doi: 10.1016/j.jacc.2016.01.024. [DOI] [PubMed] [Google Scholar]
- 50.Priori SG, Gasparini M, Napolitano C, Della Bella P, Ottonelli AG, Sassone B, et al. Risk stratification in Brugada syndrome: Results of the PRELUDE (PRogrammed ELectrical stimUlation preDictive valuE) registry. J Am Coll Cardiol. 2012;59:37–45. doi: 10.1016/j.jacc.2011.08.064. [DOI] [PubMed] [Google Scholar]
- 51.Fauchier L, Isorni MA, Clementy N, Pierre B, Simeon E, Babuty D. Prognostic value of programmed ventricular stimulation in Brugada syndrome according to clinical presentation: An updated meta-analysis of worldwide published data. Int J Cardiol. 2013;168:3027–9. doi: 10.1016/j.ijcard.2013.04.146. [DOI] [PubMed] [Google Scholar]
- 52.Delise P, Allocca G, Marras E, Giustetto C, Gaita F, Sciarra L, et al. Risk stratification in individuals with the Brugada type 1 ECG pattern without previous cardiac arrest: Usefulness of a combined clinical and electrophysiologic approach. Eur Heart J. 2011;32:169–76. doi: 10.1093/eurheartj/ehq381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Makimoto H, Kamakura S, Aihara N, Noda T, Nakajima I, Yokoyama T, et al. Clinical impact of the number of extrastimuli in programmed electrical stimulation in patients with Brugada type 1 electrocardiogram. Heart Rhythm. 2012;9:242–8. doi: 10.1016/j.hrthm.2011.09.053. [DOI] [PubMed] [Google Scholar]
- 54.Sieira J, Conte G, Ciconte G, de Asmundis C, Chierchia GB, Baltogiannis G, et al. Prognostic value of programmed electrical stimulation in Brugada syndrome: 20 years experience. Circ Arrhythm Electrophysiol. 2015;8:777–84. doi: 10.1161/CIRCEP.114.002647. [DOI] [PubMed] [Google Scholar]
- 55.Priori SG, Blomström-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac Death. The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology. G Ital Cardiol (Rome) 2016;17:108–70. doi: 10.1714/2174.23496. [DOI] [PubMed] [Google Scholar]
- 56.Andorin A, Gourraud JB, Mansourati J, Fouchard S, le Marec H, Maury P, et al. The QUIDAM study: Hydroquinidine therapy for the management of Brugada syndrome patients at high arrhythmic risk. Heart Rhythm. 2017;14:1147–54. doi: 10.1016/j.hrthm.2017.04.019. [DOI] [PubMed] [Google Scholar]
- 57.Hermida JS, Denjoy I, Clerc J, Extramiana F, Jarry G, Milliez P, et al. Hydroquinidine therapy in Brugada syndrome. J Am Coll Cardiol. 2004;43:1853–60. doi: 10.1016/j.jacc.2003.12.046. [DOI] [PubMed] [Google Scholar]
- 58.Belhassen B. Assessing the clinical efficacy of quinidine in Brugada syndrome: “Mission: Impossible”? Heart Rhythm. 2017;14:1155–6. doi: 10.1016/j.hrthm.2017.04.034. [DOI] [PubMed] [Google Scholar]
- 59.Márquez MF, Rivera J, Hermosillo AG, Iturralde P, Colín L, Moragrega JL, et al. Arrhythmic storm responsive to quinidine in a patient with Brugada syndrome and vasovagal syncope. Pacing Clin Electrophysiol. 2005;28:870–3. doi: 10.1111/j.1540-8159.2005.00183.x. [DOI] [PubMed] [Google Scholar]
- 60.Brodie OT, Michowitz Y, Belhassen B. Pharmacological therapy in Brugada syndrome. Arrhythm Electrophysiol Rev. 2018;7:135–42. doi: 10.15420/aer.2018.21.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Márquez MF, Bonny A, Hernández-Castillo E, De Sisti A, Gómez-Flores J, Nava S, et al. Long-term efficacy of low doses of quinidine on malignant arrhythmias in Brugada syndrome with an implantable cardioverter-defibrillator: A case series and literature review. Heart Rhythm. 2012;9:1995–2000. doi: 10.1016/j.hrthm.2012.08.027. [DOI] [PubMed] [Google Scholar]
- 62.Viskin S, Wilde AA, Guevara-Valdivia ME, Daoulah A, Krahn AD, Zipes DP, et al. Quinidine, a life-saving medication for Brugada syndrome, is inaccessible in many countries. J Am Coll Cardiol. 2013;61:2383–7. doi: 10.1016/j.jacc.2013.02.077. [DOI] [PubMed] [Google Scholar]
- 63.Brugada J, Pappone C, Berruezo A, Vicedomini G, Manguso F, Ciconte G, et al. Brugada syndrome phenotype elimination by epicardial substrate ablation. Circ Arrhythm Electrophysiol. 2015;8:1373–81. doi: 10.1161/CIRCEP.115.003220. [DOI] [PubMed] [Google Scholar]
- 64.Pappone C, Brugada J, Vicedomini G, Ciconte G, Manguso F, Saviano M, et al. Electrical substrate elimination in 135 consecutive patients with Brugada syndrome. Circ Arrhythm Electrophysiol. 2017;10:e005053. doi: 10.1161/CIRCEP.117.005053. [DOI] [PubMed] [Google Scholar]


