Abstract
Background:
Superficial femoral artery lesion is one of the main causes for intermittent claudication or critical limb ischemia. Percutaneous transluminal angioplasty is one of the approved therapies for this medical entity. Anatomical factors should be considered in choosing the right approach and puncture.
The purpose of this study is to discuss the anatomical, radiological, and technical factors which determine the preference of various approaches and to determine its safety, efficacy, and mid-term clinical and radiological outcome.
Methods:
Retrospectively, data were collected from patients who underwent angioplasty to superficial femoral arteries for total occlusion from January 2015 and June 2018 in our center, we performed angioplasty to 59 occluded superficial femoral artery patients at our center. The ipsilateral femoral artery, ipsilateral popliteal artery, contralateral femoral artery, or upper limb approaches were used depending on the various anatomical factors determined by radiological imaging before the procedure.
Results:
Acute success rate was 91.52%. There were no significant periprocedural complications. At the latest clinical follow-up of mean 25.8 months (10–51), a restenosis rate of 16.67% in infrainguinal arteries and 5.88% in suprainguinal arteries were noted.
Conclusions:
Percutaneous transluminal angioplasty of superficial femoral artery is a proven, viable, safer, and effective option, with good mid-term clinical results and patency rates. Different approaches to be chosen depends on the anatomical and technical factors to get the best possible outcome.
Keywords: Common femoral artery, infrainguinal arteries, left upper limb approach, percutaneous transarterial angioplasty, suprainguinal arteries
INTRODUCTION
Endovascular or surgical revascularization is the treatment of choice for superficial femoral artery (SFA) stenosis or occlusion. [1] Most common location of SFA involvement is at its origin or at the adductor canal.[2] Multiple approaches, ipsilateral common femoral artery (CFA),[3] crossover from the contralateral CFA,[4] popliteal artery,[5] and upper limb artery have been described for the access to and endovascular treatment of SFA.[6,7]
The ipsilateral femoral artery approach has the advantage of permitting the use of shorter tools which gives good support to manipulate the catheters and the guide wires.[6] Its main drawback, are the more demanding technical skills required for percutaneous puncture of the CFA and the potential difficulties of entering the SFA, avoiding its origin.
Ultrasound-guided or fluoroscopy-guided puncture may help to increase the success rate, especially in obese patients.[3,6]
When both iliac arteries and superficial femoral arteries are diseased, contralateral crossover or upper limb artery approach is preferred.[7] Narrow aortic bifurcation angle, concentric or eccentric calcific plaque at the bifurcation, significant stenosis at the bifurcation may discourage entry of hardware from contralateral femoral artery approach.[7] The use of longer devices within tortuous iliac arteries may prove a major inconvenience for correct navigation and deployment of larger devices.
Upper limb artery approach has its own disadvantages, radial artery would not accept bigger sheaths which are required for larger peripheral artery balloons or stents.[6] It may go into spasm. Brachial artery or axillary artery approach may have higher local access site complications such as bleeding, hematoma, nerve compression, and limb ischemia. If the lesions are below the popliteal artery, the length of the hardware’s may not be sufficient enough to reach the lesion site from the upper limb approach.[6] Retrograde transpopliteal[5] or pedal artery[8,9] approach has proved to be a reliable alternative vascular access in patients, not suitable for the transfemoral artery approach.
In this study, we describe various approaches to SFA occlusion, its advantages, disadvantages, technique, outcome, and its mid-to-long-term patency and follow-up.
METHODS
Patient population
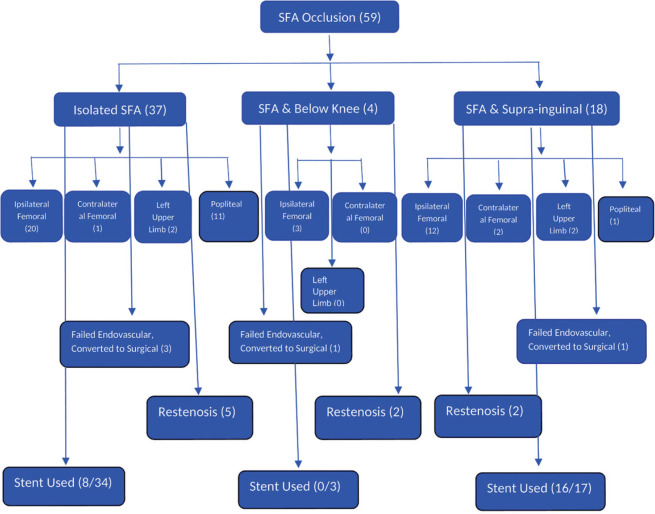
We at our center performed angioplasty to SFA occlusions in 59 patients from Jan 2015 to June 2018. The mean age of the patients was 63.58 years (46–76). Significant number of patients were diabetics (41, 69.49%), hypertensives (24, 40.68%), and tobacco smokers (34, 57.63%).
The patient’s characteristics, clinical symptoms, cardiovascular risk factors, and comorbidities are summarized in Table 1. Based on the radiological and angiographic imaging, 37 patients had involvement of SFA alone sparing suprainguinal or popliteal or below-knee arteries. 18 patients had significant stenosis of suprainguinal segment in addition to occlusion at SFA. 4 patients had significant stenosis in below-knee vessel in addition to SFA occlusion. Only SFA occlusion (100% lesion) has been considered for analysis.
Table 1.
Patient, lesion, procedure characteristics, and outcome
| Parameters | Number of patients, n (%) |
|---|---|
| Total number of patients | |
| Men | 57 (96.61) |
| Women | 2 (3.39) |
| Symptoms | |
| Symptoms of lower limb ischemia | 59 (100) |
| Gangrene | 22 (37.29) |
| Cardiovascular risk factors and comorbidities | |
| Arterial hypertension | 24 (40.68) |
| Diabetes mellitus | 41 (69.49) |
| Smoking | 34 (57.63) |
| Hyperlipidemia | 11 (18.64) |
| Coronary heart disease or previous myocardial infarction | 4 (6.78) |
| Previous arterial bypass surgery | 0 |
| Previous ischemic stroke or transient ischemic attack | 0 |
| Other peripheral arterial disease | 5 (8.47) |
| Lesion severity (suprainguinal) (%) | |
| 100 | 11 (61.11) |
| 90-100 | 7 (38.89) |
| Lesion severity (SFA) 100% | 59 (100) |
| Lesion severity (infrainguinal) 100% | 4 (100) |
| Lesion length (suprainguinal) (mm) | |
| <30 | 2 (11.11) |
| 30-60 | 10 (55.56) |
| >60 | 6 (33.33) |
| Lesion length (SFA) (mm) | |
| <100 | 13 (22.03) |
| 100-200 | 20 (33.90) |
| >200 | 26 (44.07) |
| Lesion length (infrainguinal) (mm) | |
| <100 | 0 |
| >100 | 4 (100) |
| Lesion duration | |
| <1 month | 6 (10.17) |
| 1-3 months | 19 (32.20) |
| 3-12 months | 31 (52.54) |
| >1 year | 3 (5.08) |
| Atherosclerotic | 59 (100) |
| Stent used (suprainguinal) | 16/17 (94.12) |
| Stent used (SFA) | 10/59 (16.95) |
| Stent used (infrainguinal) | 0/4 (0) |
| Acute success of endovascular therapy | 54/59 (91.52) |
| Restenosis | 9/54 (16.67) |
SFA: Superficial femoral artery
Details of the approach have been mentioned in the Figures 1 and 2a-c. Patients were selected for endovascular therapy after discussion with patient and their relatives, after informed consent. All interventions were part of routine clinical management. Patient data, technical and clinical success, complications of the procedure, and results of follow-up controls were reviewed retrospectively from the patient’s charts and radiologic reports. All patients underwent preinterventional clinical examination and duplex sonography.
Figure 1.
Approach used at our center from January 2015 to June 2018 for superficial femoral artery occlusions
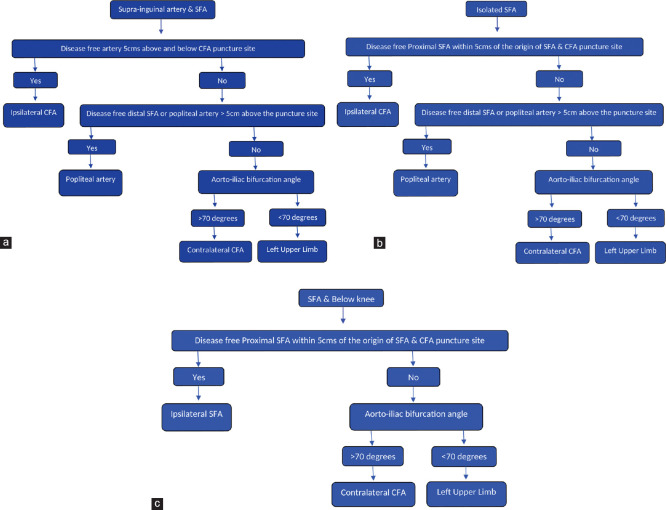
Figure 2.
Protocol to decide on the initial puncture site. (a) Protocol to decide the initial puncture site in combined suprainguinal and superficial femoral artery lesions. (b) Protocol to decide the initial puncture site in isolated superficial femoral artery lesion. (c) Protocol to decide the initial puncture site in combined superficial femoral artery and below-knee lesions
As part of the preoperative workup, patients underwent either computerized tomography angiogram or conventional angiogram to confirm the suspected lesion.
Technique
Percutaneous transarterial angioplasty (PTA) with or without stent placement was performed with the patient under local anesthesia, in the angiography suite on a high-resolution angiography system (Philips, FD 10). Factors which was considered to decide on the approach are, involvement of suprainguinal arteries, involvement of below-knee joint arteries, length of the disease-free SFA in its proximal segment, length of disease-free SFA above the knee joint, aortoiliac bifurcation angle, disease at the femoral artery or popliteal artery puncture site, and unilateral or bilateral disease.
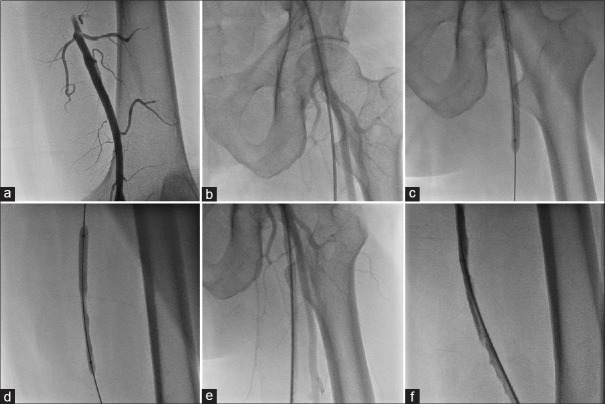
In ipsilateral suprainguinal and infrainguinal artery combined group [Figure 2a], if CFA puncture site and proximal 5 cm of the SFA is disease free, we used single common ipsilateral CFA [Figure 3] puncture.[10] Using retrograde femoral artery approach, 7F femoral artery sheath was inserted. Through retrograde CFA sheath, initially iliac artery lesions were addressed in all 12 patients with stent implantation [Figure 3a-c].
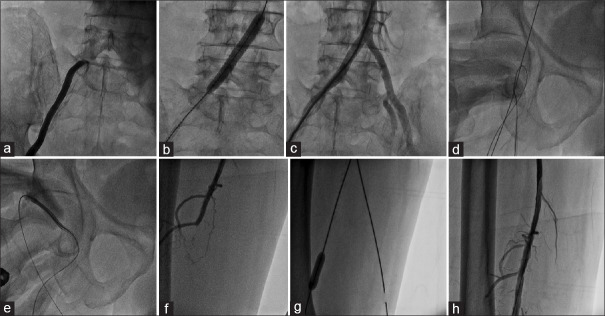
Figure 3.
PTA in combined right superficial femoral artery and right common iliac artery occlusion using ipsilateral common femoral artery puncture (First retrograde ipsilateral common femoral artery puncture is taken to address supringuinal artery lesion, then same puncture is converted into antegrade puncture to address superficial femoral artery occlusion). (a) Demonstration of right common iliac artery occlusion at its origin. (b) Stenting of right common iliac artery occlusion. (c) Final result after right common iliac artery stenting. (d) Entry into ipsilateral (right) superficial femoral artery after common iliac artery stenting with additional angled Terumo wire. (e) Sheath insertion antegradely into common femoral artery to address ipsilateral (right) superficial femoral artery lesion. (f) Demonstration of ipsilateral (right) superficial femoral artery occlusion. (g) Balloon dilatation of the superficial femoral artery lesion. (h) Final result of right superficial femoral artery after balloon dilatation
After obtaining a satisfactory result in iliac artery, angled 0.028-inch guide wire (Radio focus Guide Wire; Terumo, Tokyo, Japan) was parked retrogradely from the puncture site into the abdominal aorta. One more angled 0.025–0.028-inch guide wire (Radio focus Guide Wire; Terumo, Tokyo, Japan) was inserted into the same sheath. Gradually, the femoral artery sheath was withdrawn till the puncture site seeing on fluoroscopy, the angle of sheath, and location of initial puncture site, which was at the femoral head. Once sheath tip was at the level of the puncture site over the femoral head, second-angled guide wire was manipulated, antegradely into SFA under fluoroscopy guidance [Figure 3d and Video 1].
Entry into SFA was confirmed by cine angiography or fluoroscopic dye injection. Once confirmed, wire which was parked in the abdominal aorta was removed and sheath is inserted into SFA over dilator from the same CFA puncture, converting into antegrade technique for either superficial femoral, popliteal, or tibial arteries [Figure 3e and Video 2]. Using antegrade CFA puncture, infrainguinal artery angioplasty is done [Figure 3f-h].
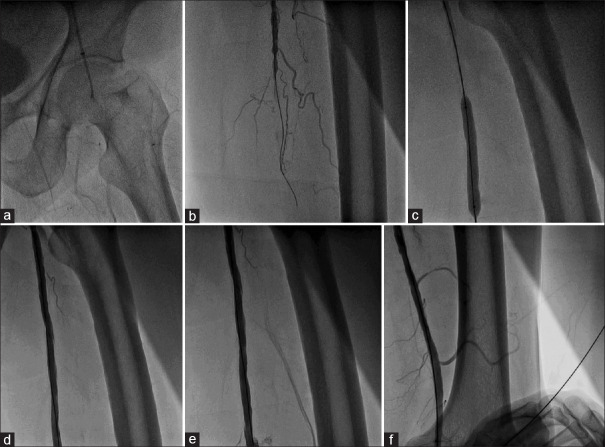
Rest of the 6 patients, in the combined group underwent alternative approaches (popliteal artery in 1, left upper limb in 2, or contralateral femoral artery in 2) because of the involvement of ipsilateral femoral artery by stenosis at the puncture site. Among the patients with isolated SFA occlusion [Figure 2b], if the proximal 5 cm of SFA is not diseased, CFA puncture site is free of atherosclerosis, ipsilateral CFA approach is used [Figure 4 and Video 3]. If either proximal 5 cm of SFA is diseased or CFA puncture site is diseased next, length of the disease-free distal SFA or popliteal artery above-knee joint is measured. If it is more than 5 cm and popliteal artery is free of disease at the level of malleoli, then popliteal artery puncture is preferred [Figure 5 and Video 4].
Figure 4.
PTA of isolated superficial femoral artery occlusion through ipsilateral common femoral artery puncture. (a and b) Demonstration of the superficial femoral artery occlusion by antegrade ipsilateral superficial femoral artery injection. (c) Balloon dilatation of superficial femoral artery lesion. (d-f) Final result after balloon dilatation
Figure 5.
PTA of isolated superficial femoral artery occlusion through ipsilateral popliteal artery puncture. (a) Demonstration of the superficial femoral artery occlusion by ipsilateral popliteal artery injection. (b) Demonstration of the superficial femoral artery occlusion at its ostium after crossing the lesion from the popliteal artery approach. (c and d) Balloon dilatation of the superficial femoral artery occlusion. (e and f) Final result after balloon dilatation
If both CFA and popliteal artery punctures are unsuitable, then depending on the aortoiliac bifurcation angle, if wide angle (>70°), contralateral femoral CFA approach [Figure 6 and Video 5] or if angle is narrow (<70°), then left upper limb approach is used [Figure 7 and Video 6]. If SFA occlusion is associated with below-knee joint arteries [Figure 2c], ipsilateral femoral artery approach [Figure 8] if it is suitable or else contralateral CFA [Figure 6] or left upper limb artery [Figure 7] approach depending on the aortoiliac angle is used. Angle or straight 0.025-, 0.028-, 0.032-, or 0.035-inch guide wire (Radio focus Guide Wire; Terumo, Tokyo, Japan) was used to cross the lesions in all the cases.
Figure 6.
PTA of isolated superficial femoral artery occlusion through contralateral common femoral artery puncture. (a) Demonstration of superficial femoral artery occlusion at its origin. (b) Crossing the superficial femoral artery lesion by Terumo wire. (c) Balloon dilatation of the superficial femoral artery lesion. (d.f) Final result after balloon dilatation
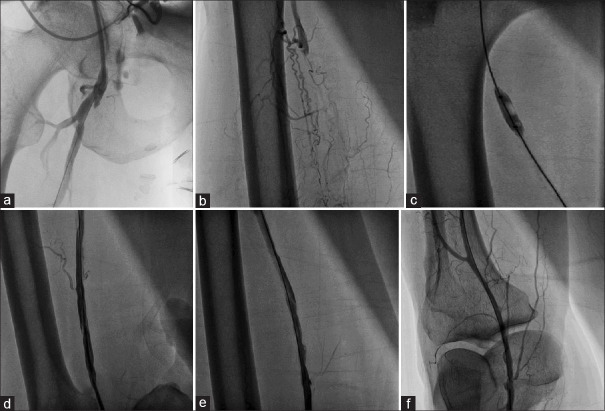
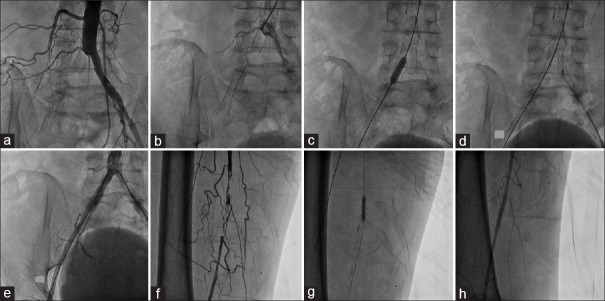
Figure 7.
PTA of combined right common iliac artery and right superficial femoral artery occlusion through the left upper limb artery (brachial) puncture. (a and b) Demonstration of right common iliac artery occlusion at its origin. (c) Balloon dilatation of right common iliac artery lesion. (d) Deployment of self-expandable stent at right common iliac artery lesion. (e) Angiogram of the right common iliac artery after stenting. (f) Demonstration of right superficial femoral artery occlusion. (g) Balloon dilatation of right superficial femoral artery occlusion. (h) Final result after right superficial femoral artery balloon dilatation
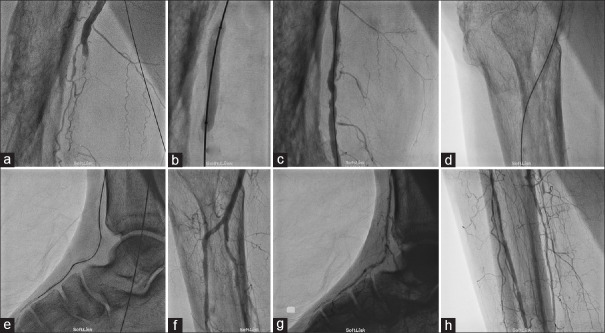
Figure 8.
PTA of combined right superficial femoral artery and right infrapopliteal artery occlusion through ipsilateral common femoral artery puncture. (a) Demonstration of superficial femoral artery occlusion. (b) Balloon dilatation of superficial femoral artery lesion. (c) Final result of superficial femoral artery lesion after balloon angioplasty. (d and e) Crossing the infrapopliteal artery lesion by Terumo wire. (f-h) Final result of infrapopliteal artery lesion after balloon dilatation
All the lesions were balloon dilated to nominal to higher pressures for 30–180 s. 16 out of the 17 suprainguinalstenoses were stented, as stenting is considered better in suprainguinal arteries. Among SFA occlusions, only 8 patients out of 34 were stented because of either flow-limiting dissection, or residual stenosis of >30%, or vessel recoil or not establishment of good flow with balloon angioplasty alone. Vascular closure device was not used because of the financial reasons.
Stent placement
Stent selection was based on vessel reference diameter and lesion length. The stent diameter was chosen not to exceed the vessel reference diameter. We stented 16 out of 17 suprainguinal lesions. For SFA and popliteal arteries, angioplasty with balloon with provisional stenting was the strategy. Only 10 patients out of 54 required stents to SFA (8 out of 34 in isolated SFA occlusion and 2 out of 17 in SFA with suprainguinal combined lesion) because of flow limiting dissection.
We used both self-expandable (complete SE vascular from Medtronic, Absolute pro LL from Abbott vascular) and balloon expandable stents (Omnilink Elite from Abbott vascular, Genesis peripheral stent from Cordis Endovascular, and Invatecscuba from Medtronic) depending on the lesion location. We preferred balloon expandable in short -segment and ostial lesions, self-expandable in long-segment and nonostial locations, which is a standard practice in majority of the centers.
After the interventional procedure, a final angiography was always performed to confirm the technical success and patency of the vessel. Technical success was defined as a stenosis grade reduction of >80% of the target vessel. No vascular closure device was used in any patient because of financial reasons.
Pharmacologic treatment
All patients were given aspirin 325 mg, clopidogrel 300 mg as a loading dose, 40 mg of atorvastatin 2–12 h before the procedure and continued with aspirin 150 mg once a day, clopidogrel 75 mg once a day, atorvastatin 10–40 mg for 1 year followed by single antiplatelet, and atorvastatin 10–40 mg. During the procedure, 5000–8000 IU of heparin was administered.
Follow-up
Clinical follow-up consisted of a history of recurrent or new onset of symptoms, physical examination including peripheral pulses, and duplex sonography postinterventionally and at 1, 3, and 12 months and 6 monthly thereafter.
CT angiogram or conventional angiogram was done if significant change in the duplex examination or relevant clinical symptoms occurred and if duplex sonography was not conclusive or if additional new or progressive occlusive lesions were suspected.
RESULTS
Technical success
Stent deployment was done in 16 out of 17 patients for suprainguinal lesions in combined suprainguinal and SFA lesion, 10 patients out of 54 required stents to SFA (8 out of 34 in isolated SFA occlusion, and 2 out of 17 in suprainguinal and SFA combined lesion. No stents were used for combined SFA and below-knee group.
In combined suprainguinal and SFA group, one patient with initial contralateral femoral artery approach was switched over to left upper limb approach. In isolated SFA group, 2 patients with initial contralateral femoral artery puncture group were switched over to the left upper limb approach because of the technical difficulties, to negotiate into lesion. Three patients from initial ipsilateral femoral artery puncture group were switched over to popliteal artery approach as the lesion could not be crossed antegradely. Technical success rate was 91.52%. Hospital stay was mean of 53 h. Patients were mobilized at an average of 8 h. They were back to their routine activities at a mean of 8 days.
Endovascular interventions was not successful in 5 patients in spite of trying multiple approaches, they underwent surgical therapy (3 from isolated SFA lesion underwent femoropopliteal bypass grafting, 1 from combined SFA and suprainguinal lesion underwent iliofemoral and femoropopliteal bypass grafting, and 1 from SFA and below-knee group underwent above-knee amputation, as there were no revascularization options available surgically, which was decided by vascular surgeon).
Complications
No access-related or procedure-related complications were seen in any of the study patients.
Follow-up and restenosis
Follow-up varied between 10 and 51 months with a mean of 25.8 months. At least 1 clinical and radiologic follow-up could be performed in all patients. Immediate complete resolution or improvement of the symptoms could be achieved in 54 out of 59 patients. 22 out of 59 patients presented to us with lower limb gangrene. In 14 patients, gangrene healed completely. In 7 patients, there was limitation of amputation to either toes or to metatarsal joints. One patient had to undergo above - knee amputation, wherein endovascular treatment was a failure.
On follow-up, 6 patients (10.17%) needed repeat procedure (balloon angioplasty without stent) for symptomatic restenosis of the SFA lesion at a mean of 7 months (3–18 months) with good result. One patient (5.88%), needed repeat procedure for symptomatic in-stent restenosis (balloon angioplasty without stent) of the common iliac artery (suprainguinal) at 18 months, after the index procedure with good result. Two patients (66.67%) needed repeat procedure due to a symptomatic restenosis (balloon angioplasty without stent) of the infrainguinal arteries at a mean of 3 months after the index procedure. One restenosis patient of the infrainguinal group had to undergo above-knee amputation because of unsuccessful endovascular and surgical revascularization.
At the latest clinical follow-up, all patients were clinically asymptomatic, radiologically having normal flow on color Doppler.
DISCUSSION
Peripheral arterial disease of the SFA is the most common cause of intermittent claudication. Occlusion or stenosis of the SFA often results in decreased perfusion of the leg, resulting in demand related, reversible, ischemic pain localized to the calf, or ischemic rest pain and tissue loss, also known as critical limb ischemia (CLI).
Endovascular treatment is one of the common therapeutic strategies for these lesions. Endovascular treatment of the SFA was first described by Dotter and Judkins in 1964.[11] In Dotters original description, he used Teflon-coated dilators to sequentially dilate the SFA in an 82-year-old woman to treat CLI that was considered nonoperable.[11] Subsequently, Gruntzig popularized the concept of catheter-directed balloon angioplasty.[12]
Tran-Atlantic Inter-Society Consensus Document II recommends percutaneous transluminal angioplasty (PTA) with provisional stenting or primary stenting as one of the treatment options for SFA lesion or occlusion.[13] Whether provisional stenting or a primary stenting is better is always debatable. Neither newer nitinol stents[14,15,16,17] nor older stainless stents[13,18,19] proved any advantage of primary stenting strategy compared to provisional stenting in SFA, popliteal, or below-knee arteries.
We employed provisional stenting strategy for SFA, popliteal and below-knee arteries, and primary stenting strategy for suprainguinal arteries in our study. When compared to bypass grafting, BASIL trial demonstrated that similar amputation-free survival and overall survival, with less morbidity and a reduced overall health-care cost for up to 2 years.[20] After 2 years, however, bypass surgery offers a 5.9-month amputation-free survival advantage and a 7.3-month overall survival advantage.[21]
The BASIL survival prediction model introduced by the BASIL investigators suggests that for patients with a 2 years predicted survival of ≥90%, bypass surgery is preferable. On the other hand, if the 2-year predicted survival is ≤10%, PTA is favorable.[22] For patients with a predicted survival between 10% and 90%, the choice of treatment is based on the disease pattern, patient characteristics, and surgeon comfort and preference.[23]
Role of drug-eluting balloons is coming up as the primary strategy with provisional stenting, which is showing decent success rate and good short- to mid-term results.[24] However, with the availability of newer techniques such as cryoplasty [25] and laser technique, newer hardwares such as cutting balloon and scoring balloons, reentry devices (Frontrunner system from Cordis, Outback from Cordis, Pioneer from Medtronic) more and more centers are preferring endovascular treatment over open surgical bypass with good success rate, good short-term and mid-term results. The most common approach used for PTA of the SFA is the contralateral retrograde femoral arterial approach[4] [Figure 6].
The primary advantages of this technique are ease of arterial access and vascular access management; however, tracking across aortic bifurcation may be difficult because of the narrow aortoiliac angle, plaque, and calcium at the aortic bifurcation of proximal common iliac arteries.
SFA occlusions can also be alternatively approached with antegrade ipsilateral CFA access,[3] [Figures 3, 4 and 8] which is the most common approach employed in our center. Hardware support, maneuverability is better, length to be transgressed is lesser, suitable irrespective of the aortoiliac bifurcation anatomy. We, in fact, used this approach even for combined suprainguinal and infrainguinal artery involvement if CFA puncture site is spared[10] [Figure 3].
Ultrasound-guided puncture is more safer and easy. However, it has some disadvantages, antegrade CFA puncture needs some expertise and learning curve. It is difficult in obese patients, not recommended if CFA puncture site is diseased, in high SFA takeoff.[3,4,5] SFA angioplasty can also be performed with popliteal artery access,[5] needs at least 5-cm disease-free popliteal artery above the popliteal artery puncture site [Figure 5]. Distal cap is thought to be softer than proximal cap, hence success rate is better.[26,27]
However, difficulty puncturing the popliteal artery, difficulty for the patient being in prone position throughout the procedure, theoretical possibility of pseudoaneurysm formation, difficulty taking one more antegrade puncture if required in the prone position, and involvement of the popliteal artery puncture site by atherosclerosis are few of the technical difficulties, one may encounter.
In our study, neither operators nor patients did not find any difficulty during the procedure. Immediate or long-term complications were not seen in our study with the popliteal artery approach. Left upper limb approach gives coaxial ness to work, helps to address suprainguinal disease as well [Figure 7]. However, upper limb artery approach may have its own disadvantages, radial artery would not accept bigger sheaths which are required for larger peripheral artery balloons or stents. It may go into spasm.
Brachial artery or axillary artery approach may have higher local access site complications such as bleeding, hematoma, nerve compression, and limb ischemia. If the lesions are below popliteal artery, the length of the hardware’s may not be sufficient enough to reach the lesion site.
In our center, based on the anatomical and technical factors, we choose an approach based on the flow diagram discussed in the methodology, we do not hesitate to take one more puncture or approach depending on the technical issues we encounter. We, authors and operators from our experience feel, when we employ systematic approach understanding the anatomy and technicalities, success rate, and long-term patency rate improve in endovascular therapy of SFA occlusion.
Limitations
This was a retrospective, single-center study. Modern reentry devices or hardwares, vascular closure devices, and ultrasound guidance to puncture could not be used because of financial reasons.
CONCLUSION
Peripheral artery disease involving SFA is one of the most common causes of intermittent claudication or CLI. Systematic approach to different access site based on the anatomical and technical factors increase the technical success and long-term patency rates. Prospective, large, randomized control study is needed to validate the technicalities in various approaches more effectively and to compare it with surgical revascularization.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Video available on: www.heartviews.org
REFERENCES
- 1.Aboyans V, Ricco JB, Bartelink ME, Björck M, Brodmann M, Cohnert T, et al. 2017 ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteriesEndorsed by: The European Stroke Organization (ESO) The task force for the diagnosis and treatment of peripheral arterial diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS) Eur Heart J. 2018;39:763–816. doi: 10.1093/eurheartj/ehx095. [DOI] [PubMed] [Google Scholar]
- 2.Wiesinger B, Heller S, Schmehl J, Claussen CD, Wiskirchen J, Tepe G, et al. Percutaneous vascular interventions in the superficial femoral artery. A review. Minerva Cardioangiol. 2006;54:83–93. [PubMed] [Google Scholar]
- 3.Marcus AJ, Lotzof K, Howard A. Access to the superficial femoral artery in the presence of a “hostile groin”: A prospective study. Cardiovasc Intervent Radiol. 2007;30:351–4. doi: 10.1007/s00270-005-0347-y. [DOI] [PubMed] [Google Scholar]
- 4.Nice C, Timmons G, Bartholemew P, Uberoi R. Retrograde vs. antegrade puncture for infra-inguinal angioplasty. Cardiovasc Intervent Radiol. 2003;26:370–4. doi: 10.1007/s00270-003-2721-y. [DOI] [PubMed] [Google Scholar]
- 5.Saha S, Gibson M, Magee TR, Galland RB, Torrie EP. Early results of retrograde transpopliteal angioplasty of iliofemoral lesions. Cardiovasc Intervent Radiol. 2001;24:378–82. doi: 10.1007/s00270-001-0043-5. [DOI] [PubMed] [Google Scholar]
- 6.Tadros RO, Vouyouka AG, Ting W, Teodorescu V, Kim SY, Marin ML, et al. A review of superficial femoral artery angioplasty and stenting. J Vasc Med Surg. 2015;3:1. [Google Scholar]
- 7.Grenon SM, Reilly LM, Ramaiah VG. Technical endovascular highlights for crossing the difficult aortic bifurcation. J Vasc Surg. 2011;54:893–6. doi: 10.1016/j.jvs.2011.03.268. [DOI] [PubMed] [Google Scholar]
- 8.Iyer SS, Dorros G, Zaitoun R, Lewin RF. Retrograde recanalization of an occluded posterior tibial artery by using a posterior tibial cutdown: Two case reports. Cathet Cardiovasc Diagn. 1990;20:251–3. doi: 10.1002/ccd.1810200408. [DOI] [PubMed] [Google Scholar]
- 9.Botti CF, Jr, Ansel GM, Silver MJ, Barker BJ, South S. Percutaneous retrograde tibial access in limb salvage. J Endovasc Ther. 2003;10:614–8. doi: 10.1177/152660280301000330. [DOI] [PubMed] [Google Scholar]
- 10.Krishnappa S, Rachaiah JM, Hegde SS, Sadananda KS, Ramasanjeevaiah G, Nanjappa MC. Endovascular Approach to Combined Ipsilateral Supra-inguinal and Infra-inguinal Artery Stenosis through a Single Common Puncture in a Single Sitting: Technique and Follow-Up. J Cardiovasc Disease Res. 2018;9:123–6. [Google Scholar]
- 11.Dotter CT, Judkins MP. Transluminal treatment of arteriosclerotic obstruction. Description of a new technic and a preliminary report of its application. Circulation. 1964;30:654–70. doi: 10.1161/01.cir.30.5.654. [DOI] [PubMed] [Google Scholar]
- 12.Grüntzig AR, Senning A, Siegenthaler WE. Nonoperative dilatation of coronary-artery stenosis: Percutaneous transluminal coronary angioplasty. N Engl J Med. 1979;301:61–8. doi: 10.1056/NEJM197907123010201. [DOI] [PubMed] [Google Scholar]
- 13.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG, et al. Inter-society consensus for the management of peripheral arterial disease (TASC II) J Vasc Surg. 2007;45(S):S5–67. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 14.Krankenberg H, Schlüter M, Steinkamp HJ, Bürgelin K, Scheinert D, Schulte KL, et al. Nitinol stent implantation versus percutaneous transluminal angioplasty in superficial femoral artery lesions up to 10 cm in length: The femoral artery stenting trial (FAST) Circulation. 2007;116:285–92. doi: 10.1161/CIRCULATIONAHA.107.689141. [DOI] [PubMed] [Google Scholar]
- 15.Schillinger M, Sabeti S, Loewe C, Dick P, Amighi J, Mlekusch W, et al. Balloon angioplasty versus implantation of nitinol stents in the superficial femoral artery. N Engl J Med. 2006;354:1879–88. doi: 10.1056/NEJMoa051303. [DOI] [PubMed] [Google Scholar]
- 16.Laird JR, Katzen BT, Scheinert D, Lammer J, Carpenter J, Buchbinder M, et al. Nitinol stent implantation versus balloon angioplasty for lesions in the superficial femoral artery and proximal popliteal artery: Twelve-month results from the RESILIENT randomized trial. Circ Cardiovasc Interv. 2010;3:267–76. doi: 10.1161/CIRCINTERVENTIONS.109.903468. [DOI] [PubMed] [Google Scholar]
- 17.Duda SH, Bosiers M, Lammer J, Scheinert D, Zeller T, Tielbeek A, et al. Sirolimus-eluting versus bare nitinol stent for obstructive superficial femoral artery disease: The SIROCCO II trial. J Vasc Interv Radiol. 2005;16:331–8. doi: 10.1097/01.RVI.0000151260.74519.CA. [DOI] [PubMed] [Google Scholar]
- 18.Grimm J, Müller-Hülsbeck S, Jahnke T, Hilbert C, Brossmann J, Heller M, et al. Randomized study to compare PTA alone versus PTA with palmaz stent placement for femoropopliteal lesions. J Vasc Interv Radiol. 2001;12:935–42. doi: 10.1016/s1051-0443(07)61572-3. [DOI] [PubMed] [Google Scholar]
- 19.Muradin GS, Bosch JL, Stijnen T, Hunink MG. Balloon dilation and stent implantation for treatment of femoropopliteal arterial disease: Meta-analysis. Radiology. 2001;221:137–45. doi: 10.1148/radiol.2211010039. [DOI] [PubMed] [Google Scholar]
- 20.Adam DJ, Beard JD, Cleveland T, Bell J, Bradbury AW, Forbes JF, et al. Bypass versus angioplasty in severe ischaemia of the leg (BASIL): Multicentre, randomised controlled trial. Lancet. 2005;366:1925–34. doi: 10.1016/S0140-6736(05)67704-5. [DOI] [PubMed] [Google Scholar]
- 21.Bradbury AW, Adam DJ, Bell J, Forbes JF, Fowkes FG, Gillespie I, et al. Bypass versus angioplasty in severe ischaemia of the leg (BASIL) trial: An intention-to-treat analysis of amputation-free and overall survival in patients randomized to a bypass surgery-first or a balloon angioplasty-first revascularization strategy. J Vasc Surg. 2010;51:5S–17S. doi: 10.1016/j.jvs.2010.01.073. [DOI] [PubMed] [Google Scholar]
- 22.Bradbury AW, Adam DJ, Bell J, Forbes JF, Fowkes FG, Gillespie I, et al. Bypass versus angioplasty in severe ischaemia of the leg (BASIL) trial: A survival prediction model to facilitate clinical decision making. J Vasc Surg. 2010;51:52S–68S. doi: 10.1016/j.jvs.2010.01.077. [DOI] [PubMed] [Google Scholar]
- 23.Forbes JF, Adam DJ, Bell J, Fowkes FG, Gillespie I, Raab GM, et al. Bypass versus angioplasty in severe ischaemia of the leg (BASIL) trial: Health-related quality of life outcomes, resource utilization, and cost-effectiveness analysis. J Vasc Surg. 2010;51:43S–51S. doi: 10.1016/j.jvs.2010.01.076. [DOI] [PubMed] [Google Scholar]
- 24.Tepe G, Zeller T, Albrecht T, Heller S, Schwarzwälder U, Beregi JP, et al. Local delivery of paclitaxel to inhibit restenosis during angioplasty of the leg. N Engl J Med. 2008;358:689–99. doi: 10.1056/NEJMoa0706356. [DOI] [PubMed] [Google Scholar]
- 25.Laird J, Jaff MR, Biamino G, McNamara T, Scheinert D, Zetterlund P, et al. Cryoplasty for the treatment of femoropopliteal arterial disease: Results of a prospective, multicenter registry. J Vasc Interv Radiol. 2005;16:1067–73. doi: 10.1097/01.RVI.0000167866.86201.4E. [DOI] [PubMed] [Google Scholar]
- 26.Ochiai M. Retrograde approach for chronic total occlusion: Present status and prospects. EuroIntervention. 2007;3:169–73. doi: 10.4244/eijv3i2a30. [DOI] [PubMed] [Google Scholar]
- 27.Villas PA, Cohen G, Goyal A, Putnam SG, 3rd, Ball D. The merits of percutaneous transluminal angioplasty of a superficial femoral artery stenosis via a retrograde popliteal artery approach. J Vasc Interv Radiol. 1999;10:325–8. doi: 10.1016/s1051-0443(99)70038-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.