Abstract
Objectives
Patients with inflammatory rheumatic diseases (IRD) infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) may be at risk to develop a severe course of COVID-19. The influence of immunomodulating drugs on the course of COVID-19 is unknown. To gather knowledge about SARS-CoV-2 infections in patients with IRD, we established a registry shortly after the beginning of the pandemic in Germany.
Methods
Using an online questionnaire (www.COVID19-rheuma.de), a nationwide database was launched on 30 March 2020, with appropriate ethical and data protection approval to collect data of patients with IRD infected with SARS-CoV-2. In this registry, key clinical and epidemiological parameters—for example, diagnosis of IRD, antirheumatic therapies, comorbidities and course of the infection—are documented.
Results
Until 25 April 2020, data from 104 patients with IRD infected with SARS-CoV-2 were reported (40 males; 63 females; 1 diverse). Most of them (45%) were diagnosed with rheumatoid arthritis, 59% had one or more comorbidities and 42% were treated with biological disease-modifying antirheumatic drugs. Hospitalisation was reported in 32% of the patients. Two-thirds of the patients already recovered. Unfortunately, 6 patients had a fatal course.
Conclusions
In a short time, a national registry for SARS-CoV2-infected patients with IRD was established. Within 4 weeks, 104 cases were documented. The registry enables to generate data rapidly in this emerging situation and to gain a better understanding of the course of SARS-CoV2-infection in patients with IRD, with a distinct focus on their immunomodulatory therapies. This knowledge is valuable for timely information of physicians and patients with IRD, and shall also serve for the development of guidance for the management of patients with IRD during this pandemic.
Keywords: Antirheumatic Agents, Autoimmune Diseases, Epidemiology, Communicable Diseases, Imported, Health services research
INTRODUCTION
Since 12 March 2020, the WHO has declared the outbreak of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) disease 2019 (COVID-19) a pandemic. The main symptoms of respiratory infection include fever and cough.1 2 Eighty-one per cent of the patients show a mild course, 14% are seriously affected and 5% of the patients are critically ill.2 On 25 April 2020, the Johns Hopkins University reported 2 954 106 confirmed cases worldwide.3 The mortality rate at that time point was 6.95% (n=205 398).3 The presence and the number of comorbidities (eg, arterial hypertension (AHT), coronary heart disease), age (immunosenescence) and lifestyle factors (eg, smoking) appear to have deteriorating effects on the course of the infection.2 In this situation, patients with inflammatory rheumatic diseases (IRD) may face a particular risk as their disease, especially when clinically active, and their immunomodulatory treatment may impact the course of COVID-19 infection. However, firm knowledge of the course of SARS-CoV-2 infection in patients with IRD is missing, and therefore, evidence-based recommendations for the management of COVID-19 in patients with rheumatic disorders and antirheumatic treatments are lacking.
The German Society for Rheumatology (DGRh) developed at an early-stage first concise recommendations for the management of patients with IRD during the COVID-19 pandemic.4 Interruption or reduction of immunomodulators is not recommended as this might result in relapses, which would increase the risk of infections, and in addition may lead to the necessity of an even riskier treatment (ie, additional glucocorticoids (GC)).5 In a cross-sectional multicentric study, patients with IRD were asked anonymously in the first weeks of the pandemic on their opinion of their immunomodulating therapy. Over 90% of the patients followed the recommendation of the rheumatologists to continue the antirheumatic therapy, and only a small percentage of patients terminated the therapy on their own.6 Whether first recommendations based on responsible care and expert’s opinion is indeed the best option for the management of patients with IRD urgently needs to be validated.
Incidence and course of COVID-19, including lethal outcomes, vary considerably in different cohorts according to pre-existing conditions and healthcare systems. Investigation of special disease groups may contribute to a better understanding of the role of the immune system regarding the risk to get infected or to develop a more severe course of COVID-19. Therefore, patients with IRD, who are treated with different types, combinations and dosages of immunomodulatory therapies represent an interesting population to collect data regarding SARS-CoV-2 infection.
Registries with a large number of case reports are required to answer the questions, whether antirheumatic drugs increase or decrease the risk for a severe course of SARS-CoV-2 infection. As necessary data cannot be extracted from clinical charts or health insurance records, the DGRh and the Justus-Liebig University Giessen decided to establish a web-based registry, which allows a rapid and timely collection of IRD cases with confirmed SARS-CoV-2 infections in Germany to analyse the clinical course of SARS-CoV-2 infections in patients with IRD and to develop guidance for the management of patients with IRD during the COVID-19 pandemic.
PATIENTS AND METHODS
On 18 March 2020 the COVID-19 Registry Task Force was founded by the DGRh consisting of rheumatologists, epidemiologists and information technology specialists. In cooperation with biostatisticians and data-protection specialists to ensure mutual understanding of research objectives and scientifically and legally appropriate data collection, a database-driven online questionnaire was developed and has been launched on 30 March 2020.
Following are the key questions of the registry:
What is the course of COVID-19 in patients with IRD?
Does geographical location/different access to healthcare within Germany affect the course?
Does a specific immunomodulatory treatment ameliorate or deteriorate the course of COVID-19?
Which other factors are associated with a poor outcome of COVID-19 in patients with IRD?
Patients with a known IRD and positive testing for SARS-CoV-2 were included by their treating rheumatologists after registration. Patients had to have positive PCR-swabs for SARS-CoV-2 for inclusion in our registry. In contrast to other registries, presumptive diagnosis of COVID-19 based on suggestive symptoms, X-ray or CT findings or on other ‘unknown’ findings was not included.
The database includes, for example, federal state, age, weight, height, detailed rheumatological diagnosis, comorbidities, global disease activity antirheumatic medication at time of study and changes due to the infection. In addition, the course and outcome of the SARS-CoV-2 infection are also key parameters. Missing data on diagnosis, outcome and therapies can be queried by directly contacting the participating physicians. Periodic critical evaluation of the registry is carried out by the task force to ensure that the objectives are being met.
The disease activity from the last rheumatological visit was reported and divided into four groups: remission, low disease activity, moderate disease activity and high disease activity. This classification and approach are concordant to the EULAR/Global Rheumatology Alliance (GRA) database. Specific disease activity scores, for example, DAS-28, are not included in the survey.
Data entered in an electronic case report form with the URL https://www.COVID19-rheuma.de/ are directly stored into an SQL-database on a dedicated server located in Germany and certified according to DIN ISO/IEC 27001 using encryption and secure communication protocols (SSL/TLS and HTTPS). Data entered in these forms are checked for plausibility immediately. Web-forms use dynamic menus and subquestions. Data allowing for identification of individual patients are omitted, and reidentification is only possible via local files in the respective rheumatological unit.
In addition, patients with IRD affected by SARS-CoV-2 can contact a rheumatologist at the coordination site of the project, who collects the respective data sets via a telephone-interview (after informed consent of the patient) entering the pseudonymised data also directly into the same web-form/database. The lockdown appointments of in- and outpatients were reduced remarkably to spare capacity for the treatment of patients with severe COVID-19 infections and to minimise patients and physician’s infection risks during outpatients’ appointment. Especially, visits of patients which showed controlled disease activity were skipped or postponed. However, to give these patients the opportunity to report their case if they were tested positive for SARS-CoV-2, the physician telephone interview was set up. However, these data entries will be flagged and can be analysed separately from those directly entered by their treating physician.
After the study was approved by the ethics committee of the Justus-Liebig-University Giessen (#52-50) and registered (EuDRACT 2020-001958-21), the web-based questionnaire was announced to German rheumatologists on 30 March 2020. Later, the German database was, in part, adjusted to the EULAR COVID-19 database when the latter was launched (https://www.eular.org/eular_COVID-19_database.cfm) to facilitate the transfer of German anonymised data into the European database in the future.
Participating centres consist of academic and non-academic rheumatology clinics, and private practices in Germany. Most of them have been informed directly using established dissemination channels of the DGRh. Announcements of the project have been posted and are being regularly updated on the website of the DGRh, the homepage of the ‘www.COVID19-rheuma.de’ registry, via social media channels, and print media. The registry is also fully supported by the Professional Association of German Rheumatologists ‘Berufsverband Deutscher Rheumatologen’, and by the national patient organisation ‘Deutsche Rheuma-Liga’, its state representatives, the Association of Rheumatology Clinics ‘Verband der Rheumatologischen Akutkliniken e.V’ and several other disease-specific patient associations.
The completed data were reviewed and queried in case of uncertainties. Analysis was performed descriptively using SPSS Statistics. Median was calculated for age and body mass index (BMI) of the fatal courses. Data in figures are shown in percentages using GraphPad Prism 6 (GraphPad Software).
RESULTS
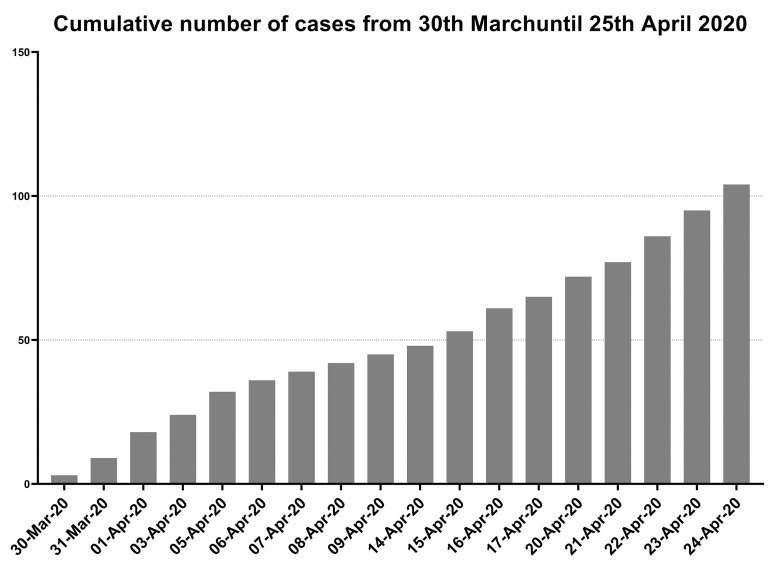
Between 30 March (first patient in) and 25 April, 104 patients with IRD and a SARS-CoV-2 infection were documented in the database by rheumatologists. The development of recruitment during the first 4 weeks is depicted in figure 1. To date, 138 rheumatologists have registered to take part in the project and 71 have already documented at least one patient. Entering a patient case took on average 5 min. Each data entry is logged by a time stamp allowing calculations of the time used for the documentation procedure up to the time required for single items in the survey.
Figure 1.
Cumulative number of cases which have been reported until 25 April 2020.
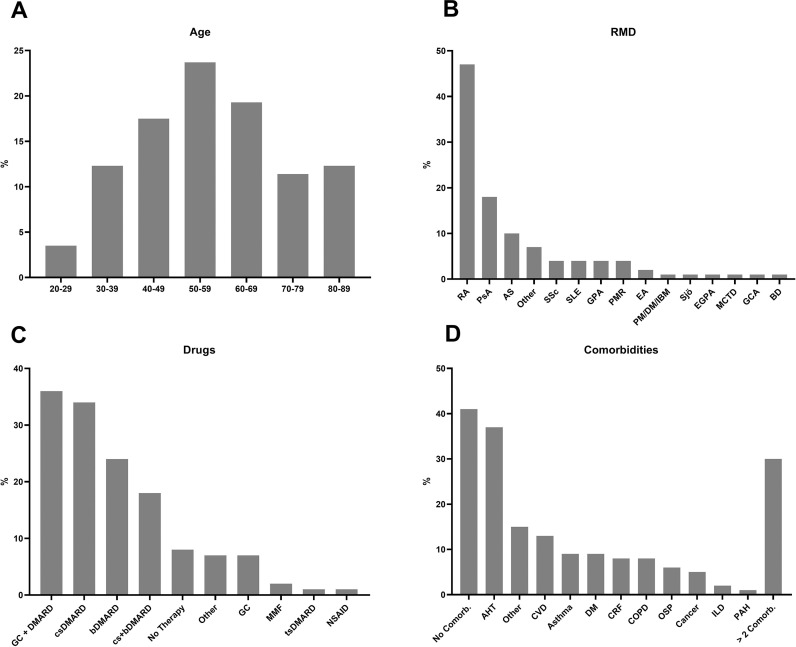
The 104 patients reported so far included 40 males, 63 females and 1 diverse, the age range was between 23 and 87 years (median age 56 years; figure 2A). Most patients with COVID-19 (24%) were between 50 and 59 years old. About two-thirds of the patients were reported from private practices, 32% from hospitals and 6% of the patients had a telephone interview by a rheumatologist at the coordination site. In relation to population numbers, most cases have been notified from Hamburg, Baden-Württemberg, Berlin, North Rhine-Westphalia and Rhineland Palatinate (table 1). The presence of antibodies was not included in our registry, which is comparable to the EULAR and GRA COVID-19 database.
Figure 2.
Overview of the reported data. (A) Age of the reported patients (in %): 4% of the patients were aged between 20 and 29 years, 12% between 30 and 39 years, 18% between 40 and 49 years, 24% between 50 and 59 years, 20% between 60 and 69 years, 11% between 70 and 79 and 12% between 80 and 89 years. (B) Distribution of inflammatory rheumatic diseases in the database (in %): 45% of the reported patients suffered from RA; 18% from PsA; 10% from AS; 7% from other inflammatory diseases (eg, gout, fever syndromes); 4% each from SSc, SLE, GPA and PMR; 2% from EA; 1% each from PM/DM/IBM, Sjö, MCTD, GCA and BD. (C) Distribution of antirheumatic drugs reported in the database (in %): 36% were with GC 6 DMARDs, 34% with csDMARDs, 24% with bDMARDs, 18% with csDMARDs and bDMARDs, 8% had no therapy, 7% received other medication, 7% were treated with glucocorticosteroids, 2% with MMF and 1% each with NSAIDs and tsDMARDs. (D) Distribution of comorbidities (in %): 41% had no comorbidities, 37% suffered from AHT, 15% from other relevant comorbidities, 13% from CVD, 9% each from bronchial asthma and DM, 8% each from COPD and CRF, 6% from OSP, 5% from cancer/history of cancer, 2% from ILD, 1% from PAH and 15% from other relevant comorbidities, 30% had more than two comorbidities. AHT, arterial hypertension; AS, ankylosing spondylitis; bDMARD, biological disease-modifying antirheumatic drugs; Comorb, comorbidities; COPD, chronic obstructive pulmonary disease; CRF, chronic renal failure; CVD, cardiovascular diseases; csDMARD, conventional synthetic disease-modifying antirheumatic drugs; DM, diabetes mellitus; DMARDs, disease-modifying antirheumatic drugs; EA, enteropathic arthritis; EGPA, eosinophilic granulomatosis with polyangiitis; GCA, giant cell arteritis; GPA, granulomatosis with polyangiitis; ILD, interstitial lung disease; MCTD, mixed connective tissue disease; MMF, mycophenolate-mofetil; NSAIDs, nonsteroidal anti-inflammatory drugs; OSP, osteoporosis; PAH, pulmonary arterial hypertension; PM/DM/IBM, polymyositis, dermatomyositis, inclusion body myositis; PMR, polymyalgia rheumatica; PsA, psoriatic arthritis; RA, rheumatoid arthritis; Sjö, Sjögren-syndrome; SSc, systemic sclerosis; SLE, systemic lupus erythematosus; tsDMARDs, targeted synthetic disease-modifying antirheumatic drugs.
Table 1.
Epidemiological situation in Germany with distribution to the federal states on 25 April 2020
| Federal state | COVID-19-affected patients with IRD in national registry | COVID-19-affected patients in general population of the federal state7 |
|---|---|---|
| Baden-Wuerttemberg | 16 (15%) | 30.169 (20%) |
| Bavaria | 21 (20%) | 40.547 (27%) |
| Berlin | 6 (6%) | 5.525 (4%) |
| Brandenburg | 3 (3%) | 2.627 (2%) |
| Bremen | 0 (0%) | 719 (0,4%) |
| Hamburg | 11 (11%) | 4.400 (3%) |
| Hesse | 12 (12%) | 7.837 (5%) |
| Mecklenburg-West Pomerania | 0 (0%) | 667 (0,4%) |
| Lower Saxony | 5 (5%) | 9.691 (6%) |
| North Rhine-Westphalia | 14 (13%) | 31.465 (21%) |
| Rhineland-Palatinate | 8 (8%) | 5.767 (4%) |
| Saarland | 1 (1%) | 2.468 (2%) |
| Saxony | 3 (3%) | 4.406 (3%) |
| Saxony-Anhalt | 1 (1%) | 1.480 (1%) |
| Schleswig-Holstein | 2 (2%) | 2.612 (2%) |
| Thuringia | 1 (1%) | 2.058 (1%) |
| Deaths | 6 (6%) | 5.500 (4%) |
| Total number | 104 | 152.438 |
IRD, inflammatory rheumatic diseases.
Regarding diagnosis, 45% of the patients with IRD had rheumatoid arthritis, 18% were diagnosed with psoriatic arthritis, 10% with ankylosing spondylitis and 5% systemic sclerosis. The proportion of other IRD was below 5% (figure 2B).
Prior to the SARS-CoV-infection, 34% of the patients were receiving conventional synthetic disease-modifying antirheumatic drugs (csDMARDs), 42% were on bDMARDs, 24% in monotherapy and 18% in combination with csDMARDs. Forty-three per cent were treated with GC, 7% without any other immunomodulation and 8% of the patients did not receive DMARDs or GC (figure 2C).
In 59% of the cases, relevant comorbidities were reported. This included AHT in 37% of the patients, other relevant comorbidities in 15%, cardiovascular diseases (CVD) in 13%, 9% each suffered from bronchial asthma and diabetes, 8% each from chronic obstructive pulmonary disease and chronic renal failure, 6% from osteoporosis, 5% from cancer/history of cancer, 2% from interstitial lung disease, 1% from pulmonary arterial hypertension and 30% had more than two comorbidities (figure 2D). In 41% of the cases, no relevant comorbidities were reported.
Thirty-nine per cent of the patients had been vaccinated against seasonal influenza, 26% against pneumococci and 22% against both. Ten per cent of the documented patients were smokers, 1% were using e-cigarettes and 8% of the patients were drinking alcohol on a regular basis.
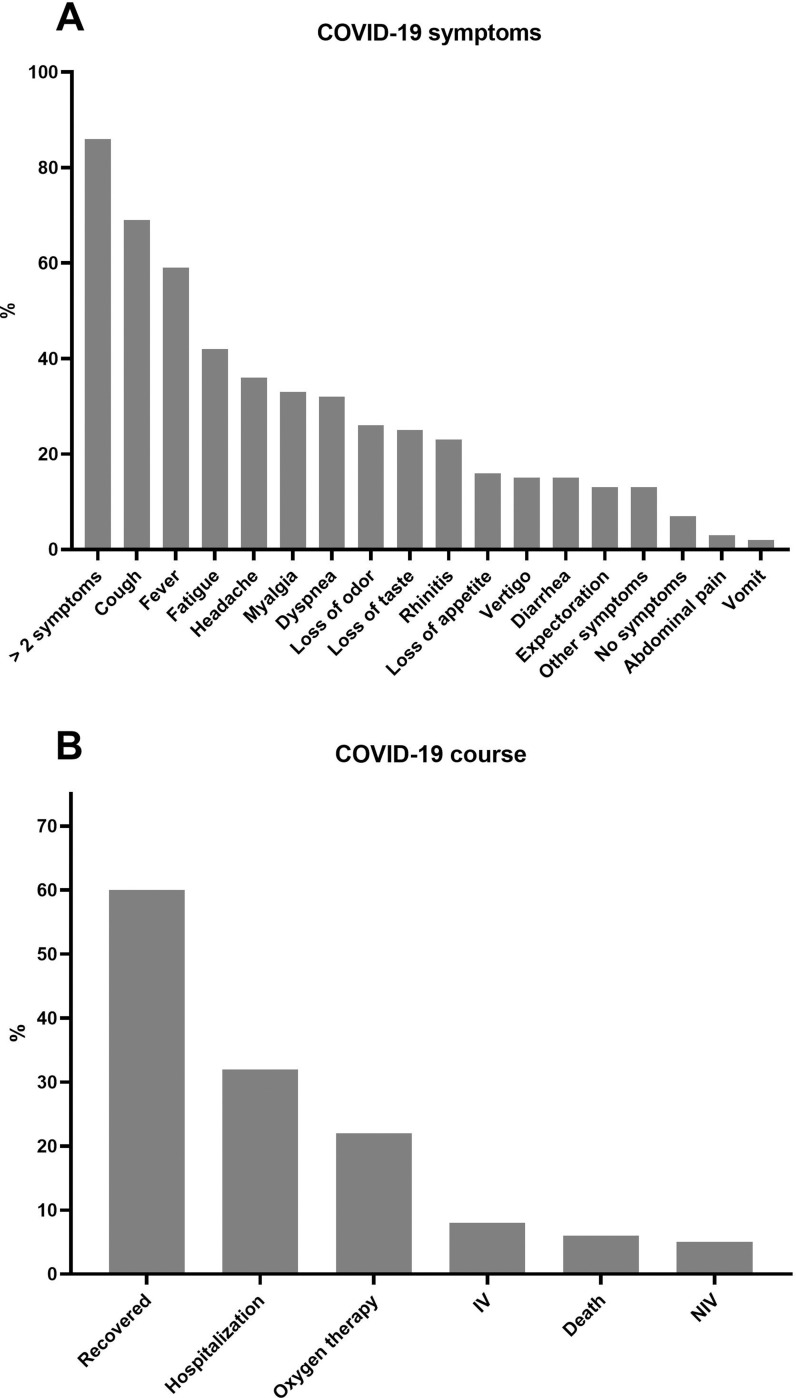
The most common reported symptoms of COVID-19 included cough (69%), fever (59%), fatigue (42%), headache (36%), myalgia (33%), dyspnea (32%), loss of odour (26%) or taste (25%), rhinitis (23%), loss of appetite (16%), vertigo (15%), diarrhoea (15%), expectoration (13%), other relevant symptoms (13%), abdominal pain (3%) and vomiting (2%) (figure 3A). Most of the patients (86%) had more than two symptoms, and 7% of the patients were reported as asymptomatic.
Figure 3.
Symptoms and courses of COVID-19 infection (in %). (A) Distribution of the symptoms of COVID-19 infection (in%): 86% of the affected patients had more than two symptoms, 69% reported cough, 59% fever, 42% fatigue, 36% headache, 33% myalgia, 32% dyspnea, 26% loss of odour, 25% loss of taste, 23% rhinitis, 16% loss of appetite, 15% vertigo, 15% diarrhoea, 13% expectoration, 13% other symptoms, 7% had no symptoms, 3% abdominal pain and 2% vomit. (B) Distribution of the course of COVID-19 infection (in%): 60% of the patients already recovered, 32% of the patients needed to be hospitalised, 22% of the patients were treated with oxygen (5% non-invasive ventilation (NIV), 8% invasive ventilation (IV)). Six deadly courses were already reported.
One-third (32%) of the patients was hospitalised, in 70% (23/33) of these cases, oxygen supply was necessary, 15% (5/33) received non-invasive and 24% (8/33) invasive ventilation. Unfortunately, six patients (6%) died in the context of COVID-19 (figure 3B), three male and three female patients (table 2). The median age in this group was 71 years (range 59–80 years), the median BMI 27.8 kg/m2 (range 23.9–40.6 kg/m2 ). All patients had at least AHT and/or other CVD (table 2). All deceased patients needed to be ventilated invasively. Five of the six patients were treated with low-dose GC (≤7.5 mg/day), which was not interrupted due to the infection in four cases. Only two of eight patients recovered after invasive ventilation.
Table 2.
Characteristics of fatalities
| IRD | PsA | RA | RA | RA | PsA | RA |
|---|---|---|---|---|---|---|
| Age (years) | 64 | 70 | 80 | 80 | 59 | 72 |
| Gender | M | M | F | F | M | F |
| BMI (kg/m2) | 40.6 | 27.8 | 28.7 | 23.9 | 27.8 | 26.6 |
| Antirheumatic therapy | GC | SSZ | GC, MTX | GC, MTX, RTX | GC, SSZ | GC, MTX, ABC |
| Comorbidities | CVD COPD |
AHT | CVD AHT COPD |
AHT Osteoporosis |
CVD AHT Cancer Other |
AHT COPD |
| Disease duration (COVID-19) |
6 days | 8 days | 14 days | 21 days | 18 days | 20 days |
| Symptoms (COVID-19) |
Fever Dyspnea |
Fever Cough |
Fever Dyspnea Chest pain |
Fever Cough Dyspnea Vertigo Fatigue |
Fever Dyspnea Cough |
Dyspnea |
| Invasive ventilation | Yes | Yes | Yes | Yes | Yes | Yes |
| Interruption DMARD | Yes | Yes | Yes, MTX | Yes, MTX and RTX | No | Yes, ABC and MTX |
ABC, abatacept; AHT, arterial hypertension; COPD, chronic obstructive pulmonary disease; CVD, cardiovascular diseases; DMARD, disease-modifying antirheumatic drugs; F, female; GC, glucocorticosteroids; IRD, inflammatory rheumatic diseases; M, male; MTX, methotrexate; PsA, psoriatic arthritis; RA, rheumatoid arthritis; RTX, rituximab; SSZ, sulphasalazine.
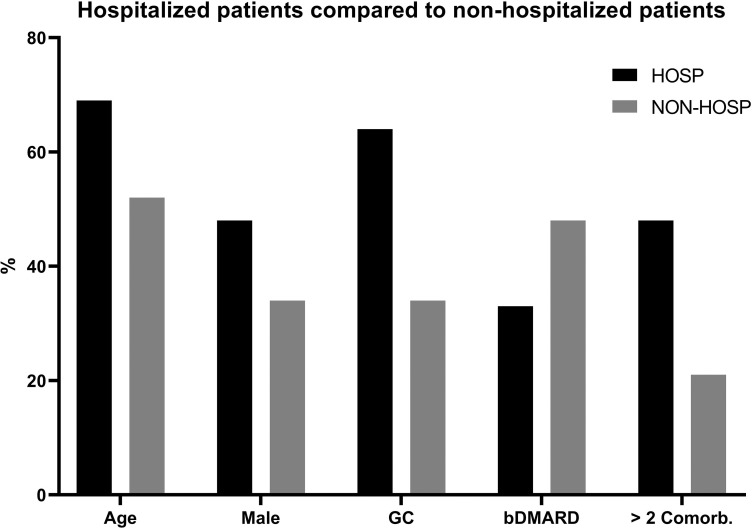
Hospitalised patients were older than non-hospitalised patients (median age 69 vs 52 years). Even though women represented 62% of all registered patients, the hospitalisation rate was equal in both genders (48% male vs 52% female). More hospitalised patients were on GC (64% vs 34%), but less were treated with bDMARD (33% vs 48%). In addition, more comorbidities (>2) were documented in hospitalised patients (48% vs 21%, figure 4).
Figure 4.
Distribution between hospitalised and non-hospitalised patients.
Median age in group of hospitalised patients (HOSP) was 69 years and in non-hospitalised (NON-HOSP) was 52 years. More male patients were hospitalised (48%) compared to NON-HOSP (34%). HOSP were treated in 64% of the cases with GC, NON-HOSP in 34%. Thirty-three per cent of HOSP received bDMARDs compared to 48% of NON-HOSP. In HOSP, more comorbidities were observed (48%) compared to NON-HOSP (21%). bDMARD, biological disease-modifying antirheumatic drugs; Comorb., comorbidities; GC, glucocorticosteroids.
At the last rheumatological visit, 37% (38/104) of the patients were in remission, 17% (18/104) patients were reported to have low disease activity, 13% (13/104) moderate and 2% (2/104) high disease activity. In 29% (30/104), disease activity was not reported. Interestingly, the two cases with reported high disease activity developed a severe course of COVID-19 leading to hospitalisation. Comparing remission (33% vs 38%) and moderate (9% vs 14%) disease activity, the proportion was similar in the hospitalised versus non-hospitalised groups, and different in patients with low disease activity (27% vs 13%).
DISCUSSION
Patients affected by IRD are at an increased overall risk of infection compared to the general population.8–10 However, the infection risk varies and is highly dependent on the type and activity of the autoimmune disease, on co-morbidities and on the intensity of the immunosuppressive/immunomodulatory treatment.8 11 Most patients with IRD are treated with GC, csDMARDs, bDMARDs and tsDMARDs on a regular basis. Especially, GC increase the risk of serious infection in a dose-dependent manner. Moreover, treatment with DMARDs can be associated with infectious complications. Most frequently, these are of bacterial origin,12 but also certain viral infections like Herpes zoster may complicate the course of many antirheumatic therapies.13 Treatment with tumour necrosis factor (TNF) inhibitors is associated with an increased risk of serious infections at the beginning of treatment, but when effective, the risk decreases due to better functional capacity and decreased use of GC.14
Currently, there is no evidence, whether and to what extent patients with IRD are at an increased risk for COVID-19 and if antirheumatic treatment, especially GC and DMARDs, are harmful to patients in the context of COVID-19. Interestingly, some of the antirheumatic drugs such as hydroxychloroquine,15 16 anakinra and interleukin 6 inhibitors17 18 have been discussed to have a beneficial role in the course of SARS-CoV-2 infection, whereas others may exert deleterious effects similar to that recently observed in rituximab-treated patients.19 20 Data from the global rheumatology alliance physician-reported registry indicate that GC exposure of ≥10 mg/day is associated with higher ORs of hospitalisation.21
This is the first report of a cross-sectional study of patients with IRD and COVID-19 in Germany. The distribution of cases within the 16 federal states of Germany is consistent with the validated infection rates in the general German population that have been electronically reported to the Robert Koch Institute (table 1). Of all patients in our database, more women were affected by COVID-19 (62% female/38% male, what had to be expected, since most IRD show a considerable preponderance of females (~78%).22 In our cohort, there was a slight relative preponderance of male patients with IRD suffering from severe COVID-19, which is consistent with data from the Chinese general population.2 The proportion of male patients with IRD was even considerably higher in our register among those who needed to be hospitalised, in which both genders were represented nearly equally (52% female/48% male, figure 4). This was not as clear in the global register, in which the distribution for hospitalised patients was 67% females vs 33% males and 74% vs 36% for non-hospitalised patients, respectively.21 These findings argue that—as in the general population—also in IRD, male patients tend to develop COVID-19 more than female patients23 and might be at risk for a more severe course of COVID-19.22
With respect to infections of the airways, TNF-α is discussed to mediate pulmonary inflammation in viral pneumonia.24 Of note, TNF-α inhibition might inherit positive effects on symptoms and severity of virus-specific lung immunopathology, especially the inflammatory burst that finally damages the lungs.25 In the RABBIT registry (German register for the long-term observation of therapy with biologics in adult patients with rheumatoid arthritis), TNF-inhibitors seemed to be beneficial for the course of severe infections by lowering the risk of sepsis and fatal outcome.14 In the global registry, anti-TNF treatment was associated with a decreased hospitalisation rate.21
In our case series, 24 patients (23%) were treated with TNF-α-inhibitors, and none of these needed oxygen treatments. Conversely, hospitalised patients tended to be treated predominantly with GC and/or csDMARDs, and 33% of them have been treated with bDMARDs compared to 48% in the non-hospitalised group. However, at present, the use of bDMARDs other than TNF-inhibition is too scarce to draw conclusions regarding risks or benefits of individual biologics, but with growing numbers of patients entered, we are confident in being able to do so in the near future.
Taken together, as a first result of our project, it could be shown that establishing an online-registry of patients with IRD and COVID-19 is feasible in short time and allows for rapid collection of possibly relevant data in the context of the SARS-CoV-2 pandemic. As in the general population, also in our study, male gender might be a potential risk factor for COVID-19 in patients with IRD, and as in the global register, treatment with GC seemed to be a disease-related risk factor in patients with IRD. With recruitment of more patients in the near future the trend of lower hospitalisation rates in patients with IRD treated with bDMARDs compared to those treated with csDMARDs will be further investigated. It is also worthwhile to compare data from Germany with those from other countries in and outside the European Union.
Key messages.
What is already known about this subject?
Management of patients with inflammatory rheumatic diseases (IRD) in the current pandemic is a major challenge for rheumatologists and they may be at an increased risk due to the IRD itself as well as due to immunomodulating drugs.
What does this study add?
In this group of 104 patients with IRD, hospitalised patients were more often treated with glucocorticoids while bDMARDs were used less often.
As in the general population, patients with IRD having comorbidities are at higher risk to develop a more severe course of COVID-19.
How might this impact on clinical practice?
Also, in view of the actual COVID-19 pandemic, GC should be kept as low as possible in the therapy of patients with IRD as they seem to enhance the risk of hospitalisation. This was not the case for bDMARD.
Whether, and if so which single biological DMARD enhances or alleviates the risk of hospitalisation in COVID-19 may become clear with recruitment of more patients in the future.
Acknowledgments
The authors would like to thank all physicians (Boeddecker, Stephanie; Kurthen, Reiner; Mattar, Johannes; Anita Viardot; Brandt-Juergens, Jan; Rihl, Markus; Menne, Hans-Jürgen; Bremer, Jan-Phillip; Bauhammer, Jutta; Maerz, Vanessa; Dörfler, Rainer; Andriopoulos, Nikolaos; Merwald-Fraenk, Helga; Henes, Jörg; Noethe, Matthias; Wiesent, Franziska; Weigelt, Martin; Hauf, Maura-Maria; Riemekasten, Gabriela; Aries, Peer; Bloching, Hans; Reindl, Christiane; Hein, Liane; Herzer, Peter; Decker, Elvira; Gilly, Jasmin; De Groot, Kirsten; Korsten, Peter; Krusche, Martin; Kovacs, Magdolna; Endokrinologikum Frankfurt; Raub, Wolfgang; Kittel, Birgit; Röser, Markus; Sievert, Iris; Weiß, Angela; Ospina; Purschke, Michael; Scholz, Michaela; Anders, Nils; Rossmanith, Christopf; Krüger, Klaus; Bruckner, Andreas; Hartmann, Urs; Nerenheim, Anabell; Gniezinski- Schwister, Agnes; Alexander, Tobias; Rockwitz, Karin; Haibel, Hildrun; Schieweck-Güsmer, Andreas; Steinchen, Nicolai; Bender, Nico; Boche, Konrad; Winau, Lea; Bäuerle, Michael; Saech, Jaesmine; Klink, Claudia; Feuchtenberger, Martin; Avemarg, Sarah; Drexler, Elke; Weiner, Stefan; Sonn, Sigrid; Blendea, Daniel; Becker, Klaus; Witt, Matthias; Melzer, Adelheid; Eder, Roman; Fleck, Martin; Löffler, Christian; Hoese, Guido; Schönherr, Jutta; Vallbracht-Ackermann, Inka; Baerwald, Christopf; Sekura, Matthias; Kreutzberger, Rene; Schiebel, Magnus; Nottarp, Dirk; Zaus, Monika; Wysocki, Nina; Birkner, Gerhard; Krause, Dietmar; Amberger, Christopher; Sensse, Jörg; Grünke, Mathias; Röther, Ekkehard) and personnel involved in the documentation of the cases in our registry.
Footnotes
Contributors: RH, UM-L, TS, HS-K and CS performed the study design. RH performed the research, analysed, and interpreted the data. RH, UM-L, TS, HS-K and CS wrote the manuscript. RH, UM-L, TS, BFH, AK, H-ML, ACR, JGR, AS, REV, HS-K and CS performed physician recruitment. All authors contributed to preparation of the project, and read and approved the final manuscript.
Funding: RH was supported by the Justus-Liebig University Giessen Clinician Scientist Program in Biomedical Research (JLU-CAREER) to work on this registry.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: The study has been approved by the ethics committee of the Justus-Liebig-University Giessen (#52-50), Germany.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article.
REFERENCES
- 1. Dai H, Zhang X, Xia J, et al. High-resolution chest CT features and clinical characteristics of patients infected with COVID-19 in Jiangsu, China. Int J Infect Dis 2020. 10.1016/j.ijid.2020.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese cEnter for Disease Control And Prevention. JAMA 2020;323:1239 10.1001/jama.2020.2648 [DOI] [PubMed] [Google Scholar]
- 3. John Hopkins University Medicine Coronavirus resource center. Available https://coronavirus.jhu.edu/map.html
- 4. Schulze-Koops H, Specker C, Iking-Konert C, et al. Preliminary recommendations of the German Society of Rheumatology (DGRh eV) for the management of patients with inflammatory rheumatic diseases during the SARS-CoV-2/COVID-19 pandemic. Ann Rheum Dis 2020. 10.1136/annrheumdis-2020-217628 [DOI] [PubMed] [Google Scholar]
- 5. Mikuls TR, Johnson SR, Fraenkel L, et al. American College of Rheumatology guidance for the management of adult patients with rheumatic disease during the COVID-19 pandemic. Arthritis Rheumatol (Hoboken, NJ) 2020;72:1241–51. 10.1002/art.41301 [DOI] [PubMed] [Google Scholar]
- 6. Schmeiser T, Broll M, Dormann A, et al. A cross sectional study on patients with inflammatory rheumatic diseases in terms of their compliance to their immunosuppressive medication during COVID-19 pandemic. Z Rheumatol 2020. 10.1007/s00393-020-00800-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Robert-Koch-Institute Daily situation report of the robert koch institute - 25-Apr-2020. Available https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Situationsberichte/2020-04-25-de.pdf?__blob=publicationFile
- 8. Doran MF, Crowson CS, Pond GR, et al. Frequency of infection in patients with rheumatoid arthritis compared with controls: a population-based study. Arthritis Rheum 2002;46:2287–93. 10.1002/art.10524 [DOI] [PubMed] [Google Scholar]
- 9. Lahaye C, Tatar Z, Dubost J-J, et al. Management of inflammatory rheumatic conditions in the elderly. Rheumatology (Oxford) 2019;58:748–64. 10.1093/rheumatology/key165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hsu C-Y, Ko C-H, Wang J-L, et al. Comparing the burdens of opportunistic infections among patients with systemic rheumatic diseases: a nationally representative cohort study. Arthritis Res Ther 2019;21:211 10.1186/s13075-019-1997-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bello SL, Serafino L, Bonali C, et al. Incidence of influenza-like illness into a cohort of patients affected by chronic inflammatory rheumatism and treated with biological agents. Reumatismo 2012;64:299–306. 10.4081/reumatismo.2012.299 [DOI] [PubMed] [Google Scholar]
- 12. Ozen G, Pedro S, England BR, et al. Risk of serious infection in patients with rheumatoid arthritis treated with biologic versus nonbiologic disease-modifying antirheumatic drugs. ACR Open Rheumatol 2019;1:424–32. 10.1002/acr2.11064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Veetil BMA, Myasoedova E, Matteson EL, et al. Incidence and time trends of herpes zoster in rheumatoid arthritis: a population-based cohort study. Arthritis Care Res (Hoboken) 2013;65:854–61. 10.1002/acr.21928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Richter A, Listing J, Schneider M, et al. Impact of treatment with biologic DMARDs on the risk of sepsis or mortality after serious infection in patients with rheumatoid arthritis. Ann Rheum Dis 2016;75:1667–73. 10.1136/annrheumdis-2015-207838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Choudhary R, Sharma AK. Potential use of hydroxychloroquine, ivermectin and azithromycin drugs in fighting COVID-19: trends, scope and relevance. New Microbes New Infect 2020;100684 10.1016/j.nmni.2020.100684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sinha N, Balayla G. Hydroxychloroquine and COVID-19. Postgrad Med J 2020. 10.1136/postgradmedj-2020-137785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang C, Wu Z, Li J-W, et al. The cytokine release syndrome (CRS) of severe COVID-19 and interleukin-6 receptor (IL-6R) antagonist tocilizumab may be the key to reduce the mortality. Int J Antimicrob Agents 2020;55:105954 10.1016/j.ijantimicag.2020.105954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhao M. Cytokine storm and immunomodulatory therapy in COVID-19: role of chloroquine and anti-IL-6 monoclonal antibodies. Int J Antimicrob Agents 2020;55:105982 10.1016/j.ijantimicag.2020.105982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jordan RE, Adab P, Cheng KK. COVID-19: risk factors for severe disease and death. BMJ. 2020;368:m1198 10.1136/bmj.m1198 [DOI] [PubMed] [Google Scholar]
- 20. Schulze-Koops H, Krueger K, Vallbracht I, et al. Increased risk for severe COVID-19 in patients with inflammatory rheumatic diseases treated with rituximab. Ann Rheum Dis 2020. 10.1136/annrheumdis-2020-218075 [DOI] [PubMed] [Google Scholar]
- 21. Gianfrancesco M, Hyrich KL, Al-Adely S, et al. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 global rheumatology alliance physician-reported registry. Ann Rheum Dis 2020;79:859–66. 10.1136/annrheumdis-2020-217871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fairweather D, Frisancho-Kiss S, Rose NR. Sex differences in autoimmune disease from a pathological perspective. Am J Pathol 2008;173:600–9. 10.2353/ajpath.2008.071008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020;323:1061–9. 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Peper RL, van Campen H. Tumor necrosis factor as a mediator of inflammation in influenza A viral pneumonia. Microb Pathog 1995;19:175–83. 10.1006/mpat.1995.0056 [DOI] [PubMed] [Google Scholar]
- 25. Hussell T, Pennycook A, Openshaw PJ. Inhibition of tumor necrosis factor reduces the severity of virus-specific lung immunopathology. Eur J Immunol 2001;31:2566–73. [DOI] [PubMed] [Google Scholar]