Abstract
Objective
To compare characteristics, pregnancies and treatments during pregnancies of seronegative and seropositive antiphospholipid syndrome (APS), to analyse factors associated with obstetrical outcome.
Patients and methods
Inclusion criteria were: (1) thrombotic and/or obstetrical APS (Sydney criteria); (2) absence of conventional antiphospholipid antibodies (APL); (3) at least one persistent non-conventional APL among IgA anticardiolipin antibodies, IgA anti-B2GPI, anti-vimentin G/M, anti-annexin V G/M, anti-phosphatidylethanolamine G/M and anti-phosphatidylserine/prothrombin G/M antibodies. The exclusion criteria were: (1) systemic lupus erythematosus ( SLE) or SLE-like disease; and (2) other connective tissue disease.
Results
A total of 187 women (mean 33±5 years) with seronegative APS were included from 14 centres in Austria, Spain, Italy, Slovenia and France and compared with 285 patients with seropositive APS. Seronegative APS has more obstetrical rather than thrombotic phenotypes, with only 6% of venous thrombosis in comparison to seropositive APS. Cumulative incidence of adverse obstetrical events was similar in seronegative and seropositive APS patients, although higher rates of intrauterine deaths (15% vs 5%; p=0.03), of preeclampsia (7% vs 16%, p=0.048) and lower live birth term (36±3 vs 38±3 weeks of gestation; p=0.04) were noted in seropositive APS. The cumulative incidence of adverse obstetrical events was significantly improved in treated versus untreated seronegative APS (log rank<0.05), whereas there was no difference between patients who received aspirin or aspirin-low-molecular weighted heparin combination.
Conclusion
Several non-criteria APL can be detected in patients with clinical APS features without any conventional APL, with various rates. The detection of non-criteria APL and thus the diagnosis of seronegative APS could discuss the therapeutic management similar to seropositive APS, but well-designed controlled studies are necessary.
Keywords: Antibodies, Antiphospholipid, Antiphospholipid Syndrome, Outcome and Process Assessment, Health Care
INTRODUCTION
Antiphospholipid syndrome (APS) is an autoimmune disease characterised by vascular thrombosis, various obstetrical adverse events and persistent antiphospholipid antibodies (APL). The conventional APL includes lupus anticoagulant (LA), IgG/M anticardiolipin antibodies (aCL) and IgG/M antibodies against β2GPI (anti-β2GPI) antibodies.1 Seronegative APS has been recently defined in patients with obstetrical and thrombotic clinical APS features (Sydney criteria), but without detectable conventional APL.2 In seronegative APS, the possibility of persistent non-criteria APL has been raised. Several non-criteria antiphospholipid antibodies, such as IgA aCL and anti-β2GPI, anti-phosphatidylethanolamine (anti-PE), anti-phosphatidylserine/prothrombin (anti-PS/PT), anti-vimentin and anti-annexin V and II antibodies can actually be tested.3–6 However, there is a paucity of studies evaluating the link between these autoantibodies and clinical status, in particular for obstetrical outcomes.7 Studies mostly evaluated the prevalence of these persistent non-criteria APL in thrombotic clinical subsets, showing 10–15% of prevalent among patients with unexplained venous thrombosis. Accurate identification of patients with APS is essential, as treatment during pregnancy significantly improves the fetal and maternal outcomes.8 Studies reporting pregnancy outcomes in seronegative clinical APS with presence of non-criteria APL, treatments and comparison of this condition to seropositive APS are still lacking. From this retrospective European study we aimed to (1) describe the clinical and laboratory features of seronegative clinical APS with detectable persistent non-criteria APL; (2) describe the fetal, maternal outcomes and the treatments during pregnancies; (3) compare seronegative APS to seropositive APS women and (4) analyse factors associated with adverse obstetrical outcomes in seronegative and seropositive APS.
PATIENTS AND METHODS
Patients’ selection
A retrospective study was initiated with the European Forum on Antiphospholipid Antibodies from 2017 to 2019 and all practitioners were asked to fill a standardised excel form.
Inclusion criteria were: (1) arterial and/or venous thrombotic; and/or obstetrical primary clinical APS (Sydney criteria) (not explained by usual causes, p.e. chromosomal, uterine, hormonal, etc., abnormalities for recurrent miscarriages); (2) absence of conventional antiphospholipid antibodies; (3) presence of at least one persistent non-conventional APL among IgA aCL, IgA anti-β2GPI, anti-vimentin G/M, anti-annexin V G/M, anti-PE G/M, anti-PS/PT G/M antibodies and (4) at least one pregnancy after the diagnosis of seronegative APS. The clinical APS criteria for thrombosis were otherwise unexplained thrombosis and for obstetrical events recurrent unexplained early miscarriages, unexplained intrauterine deaths and/or preeclampsia and prematurity from placental insufficiency otherwise unexplained.
The exclusion criteria were: (1) associated systemic lupus erythematosus (SLE) or SLE-like disease (SLE features and/or positive antinuclear autoantibodies) and (2) other systemic connective tissue disease (Sjogren’s syndrome, systemic sclerosis, myositis, etc.).
Maternal age, characteristics of previous thrombosis and obstetrical features, associated thrombotic and cardiovascular factors (obesity, hypercholesterolaemia, tobacco use, diabetes mellitus, arterial hypertension, constitutional thrombophilia), course and outcome of previous pregnancies and treatments during the pregnancies were recorded. Adverse pregnancy outcomes included early miscarriage (<10 weeks of gestation), fetal loss (≥10 weeks of gestation), intra-uterine growth restriction (IUGR), prematurity (<34 weeks of gestation), pre-eclampsia or eclampsia, hemolysis, elevated liver enzymes and low platelet count (HELLP) syndrome, placental abruption, gestational arterial hypertension and arterial and/or venous thrombosis during the pregnancy. All these data were included in standardised electronic files which were filled by the practitioner of each patient.
Non-criteria APL testing included anti-β2GPI IgA, anti-annexin V antibodies, anti-PE IgG and IgM antibodies, anti-PS/PT IgG and IgM antibodies, IgA aCL or anti-vimentin antibodies. For patients with at least one positive non-criteria APL, non-criteria APL positivity was confirmed to be persistent at 12 weeks. Because of non-standardisd tests for seronegative APL, APL were expressed as positives or negatives at 99° percentiles, without considering the titers.
The control group was selected among women with primary seropositive APS (Sydney criteria), included in the French APS and Lupus Registry. This registry retrospectively included APS patients, mainly in two referral centres (Cochin and Lille hospitals). The exclusion criteria were the same as for the seronegative APS: (1) associated SLE or SLE-like disease (SLE features and/or positive antinuclear autoantibodies) and (2) other systemic connective tissue disease (Sjogren’s syndrome, systemic sclerosis, myositis, etc.). For the first analysis comparing phenotypes among seropositive and seronegative APS patients, we selected all APS women included in the registry, including obstetrical and thrombotic APS phenotypes and excluding SLE-associated APS. For the comparison of subsequent pregnancies following APS diagnosis, we only selected women with seropositive APS (obstetrical and/or thrombotic phenotype), who had at least one registered pregnancy after the APS diagnosis similarly to the seronegative APS women.
Statistical analysis
Descriptive analyses were expressed as proportions (%) for categorical variables and means (SD) for continuous variables. First, we compared phenotypes from all seronegative and seropositive APS patients, using t-tests for continuous variables and Chi-squared tests for categorical variables. Then, we compared the pregnancy outcomes occurring after APS diagnosis. To study obstetrical outcomes, we chose the following outcomes: fetal loss <10 weeks, fetal loss ≥10 weeks, premature birth <34 weeks of gestation, maternal complications (HELLP and/or preeclampsia, thrombosis) and the presence of any of these events. To assess the association between APL status and obstetrical outcomes, univariate analyses were performed using t-tests for continuous variables, and chi-squared tests for categorical variables. We subsequently used multivariate models using logistic regressions, with all variables associated with the outcome (p<0.2) in univariate analyses. In those analyses, missing variables were imputed using multiple imputations. Kaplan–Meier curves and log-rank tests were used to compare the cumulative incidence of adverse obstetrical events first according to the treatment among seronegative APS, then comparing seronegative and seropositive APS. All analyses were performed using R software, version 3.4.2 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
A total of 187 women (mean age 33±5 years) with seronegative APS were included from 14 centres in Austria, Spain, Italy, Slovenia and France and compared with 285 patients with seropositive APS (table 1). Seronegative APS had mostly obstetrical phenotypes rather than venous thrombosis, with only 6% having experienced a venous thrombosis. Non-criteria APL in seronegative APS were anti-β2GPI IgA antibodies (n=3/127), anti-annexin V antibodies (n=43/185), anti-PE IgG (n=19/124) and IgM antibodies (n=43/124), anti-PS/PT IgG (n=66/186) and IgM antibodies (n=69/186), without any IgA aCL (n=0/53) or anti-vimentin antibodies (n=0/65) (online supplemental table 1). A single non-criteria APL was noted in 131 women (70%), with double and triple positivity’s in 50 (27%) and 6 cases (3%), respectively. Single-triple and double-triple positive seronegative APS have similar age and frequencies of obstetrical ad thrombotic APS features (data not shown). Comparing pregnancy outcomes of seronegative APS, women with double-positive non-criteria APL had significantly more frequent fetal loss than single-positive APS (13/28 (46%) vs 16/70 (23%); p=0.03) despite the use of similar frequencies of aspirin, low-molecular weighted heparin (LMWH) and additional therapies during the pregnancies.
Table 1.
Characteristics of seronegative and seropositive APS women
|
Seronegative APS (n=187) |
Seropositive APS (n=285) |
|
|---|---|---|
| General characteristics | ||
| Caucasian (n; %) | 150 (82) | 188 (66) |
| Age (years) | 33±5 | 36±5† |
| Obesity (n; %) | 15 (9) | 32 (11) |
| Arterial hypertension (n; %) | 3 (2) | 54 (19)† |
| Diabetes mellitus (n; %) | 1 (1) | 18 (6)† |
| Tobacco use (n; %) | 12 (7) | 33 (12)† |
| Hypercholesterolaemia (n; %) | 0 | 69 (24)† |
| Protein S deficiency/V Leiden (n; %) | 0/1 (1) | 8 (3)/10 (4)† |
| APS features | ||
| Thrombotic APS | ||
| Arterial APS (n; %) | 0 | 105 (37)† |
| Venous APS (n; %) | 9 (6) | 154 (54)† |
| Obstetrical APS (n; %) | 168 (89) | 89 (31)† |
| Mix APS (n; %) | 8 (4) | 16 (6) |
| Non-criteria features (n; %) | 16 (9) | 141 (49)† |
| Obstetrical history | ||
| Miscarriages (n; %) | 66 (35) | 18 (6)† |
| Intrauterine deaths (n; %) | 60 (32) | 46 (16)† |
| Prematurity <34 weeks of gestation (n; %) | 43 (23) | 31 (11)† |
†p<0.05.
Values are numbers with frequencies and means with SD.
APS, antiphospholipid syndrome.
rmdopen-2020-001340s001.pdf (53KB, pdf)
The cumulative incidence of adverse obstetrical events was significantly improved in treated seronegative APS versus untreated ones (log rank <0.05), whereas there was no difference between patients who received aspirin or aspirin-LMWH combination (figure 1). The rates of live-birth pregnancies treated with aspirin-LMWH combination were significantly higher than pregnancies resulting in fetal loss (46/75 (61%) vs 16/43 (37%), p=0.002). Women with isolated recurrent miscarriages treated by aspirin and LMWH combination (n=48 pregnancies; 89%) have live birth pregnancies in 31 (57%), whereas those with prematurity related to placental insufficiency under aspirin and LMWH in 9 (45%) have live birth pregnancies in 18 (82%).
Figure 1.
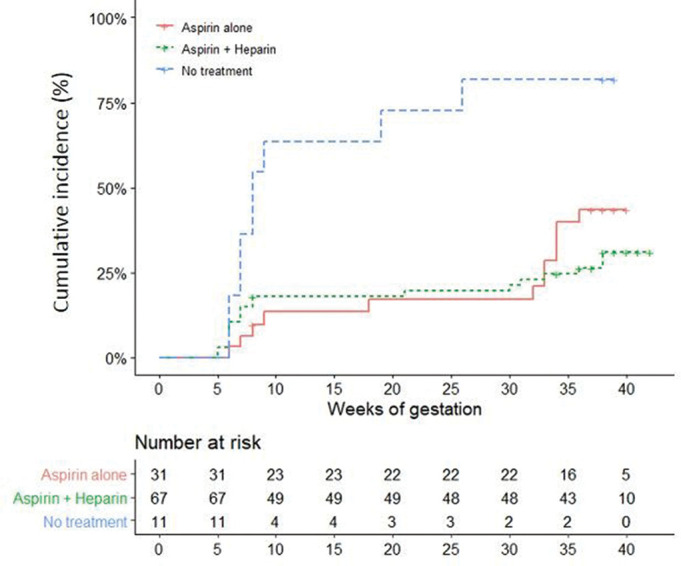
Adverse obstetrical events (fetal loss and/or premature birth before the 34 weeks of gestation because of eclampsia or severe preeclampsia or placental insufficiency) in 109 pregnancies of seronegative APS depending on treatment during the pregnancy and the type (aspirin or aspirin/LMWH combination). APS, antiphospholipid syndrome; LMWH, low-molecular weighted heparin.
The control group of seropositive APS included 285 women with a mean age of 36±5 years. The APL were the LA (n=211; 74%), aCL (n=228; 80%) and anti-β2GPI antibodies (183 cases; 64%), with triple positivity observed in 134 women (47%). Seropositive APS women had significantly more venous and/or arterial thrombosis than seronegative APS women (table 1). Cardiovascular risk factors including arterial hypertension, dyslipidaemia, and/or diabetes mellitus were significantly more frequent in seropositive APS patients, and the latter have more non-criteria APS manifestations (livedo racemosa, thrombocytopenia and migraines). Seronegative APS women were more prone to have obstetrical phenotypes, with more frequent intrauterine deaths, miscarriages and early prematurity (table 1).
For the comparisons of subsequent pregnancies following APS diagnoses, we included 108 seronegative APS and 75 seropositive APS women, who had at least one pregnancy after APS diagnosis (table 2). The frequencies of treated pregnancies and rates of aspirin-LMWH combination, prednisone and hydroxychloroquine used during the pregnancy were not significantly different in seronegative and seropositive APS, except for higher rates of curative LMWH use in seropositive APS and of aspirin alone in seronegative APS. Cumulative incidence of adverse obstetrical events was similar in seronegative and seropositive APS patients (figure 2), although higher rates of intrauterine deaths (15% vs 5%; p=0.03), preeclampsia (7% vs 16%, p=0.048) and lower live birth term (36±3 vs 38±3 weeks of gestation; p=0.04) were noted in seropositive treated APS.
Table 2.
Pregnancy outcome and treatment in seronegative and seropositive APS
|
Seronegative APS pregnancies (n=108) |
Seropositive APS pregnancies (n=75) |
|
|---|---|---|
| APS features | ||
| Thrombotic APS | ||
| Arterial APS (n; %) | 0 | 20 (27)† |
| Venous APS (n; %) | 8 (7) | 42 (56)† |
| Obstetrical APS (n; %) | 93 (86) | 36 (48)† |
| Mix APS (n; %) | 6 (6) | 18 (24)† |
| Non-criteria features (n; %) | 18 (17) | 34 (45)† |
| Previous obstetrical history | ||
| Miscarriages (n; %) | 49 (45) | 8 (11)† |
| Intrauterine deaths (n; %) | 29 (27) | 16 (21) |
| Prematurity <34 weeks of gestation (n; %) | 24 (22) | 14 (19) |
| Subsequent pregnancy treatments | ||
| Aspirin (n; %)/aspirin alone | 95 (88)/32 (30) | 57 (76)†/2 (3)† |
| LMWH isocoagulant amounts (n; %) | 63 (58) | 39 (52) |
| LMWH curative amounts (n; %) | 2 (2) | 33 (44)† |
| Aspirin-LMWH (n; %) | 65 (60) | 55 (73) |
| Prednisone (n; %) | 10 (9) | 4 (5) |
| Hydroxychloroquine (n; %) | 10 (9) | 8 (11) |
| Venous thrombosis (n; %) | 0 | 1 (1) |
| Pregnancy outcome | ||
| Preeclampsia/HELLP syndrome (n; %) | 7 (7) | 12 (16)† |
| Intrauterin growth restriction (IUGR) (n; %) | 5 (5) | 7 (10) |
| Oligoamnios (n; %) | 2 (2) | 1 (1) |
| Fetal loss (n; %) miscarriage/intrauterine deaths |
33 (31) 23 (21)/5 (5) |
22 (29) 11 (15)/11 (15)† |
| Prematurity <34 weeks of gestation (n; %) | 6 (6) | 9 (12) |
| Term of fetal loss (weeks of gestation) | 10±8 | 13±7 |
| Live births (n; %) | 75 (69) | 53 (70) |
| Term of live birth (weeks of gestation) | 38±3 | 36±3† |
†p<0.05.
Values are numbers with frequencies and means with SD.
APS, antiphospholipid syndrome; HELLP, hemolysis, Elevated Liver enzymes and Low platelet count; LMWH, low-molecular weighted heparin.
Figure 2.
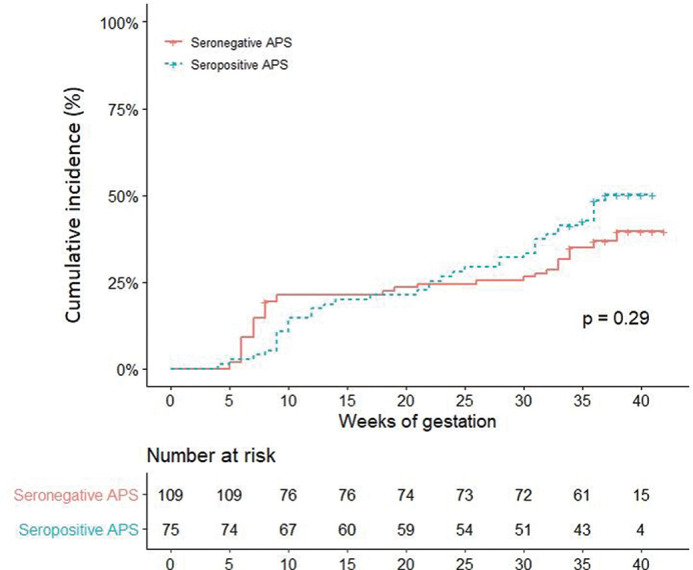
Adverse obstetrical events (fetal loss and/or premature birth before the 34 weeks of gestation because of eclampsia or severe preeclampsia or placental insufficiency) among 183 pregnancies depending on APS seropositive and seronegative status. APS, antiphospholipid syndrome.
Then, we analysed factors associated with fetal loss (<10 weeks of gestation), intrauterine deaths (>10 weeks of gestation), premature birth (<34 weeks of gestation) and preeclampsia/HELLP syndrome considering all pregnancies of both seropositive and seronegative APS women. The factors analysed in univariate analysis for these different obstetrical outcomes included: age, seropositive or seronegative APS status, cardiovascular risk factors (obesity, arterial hypertension, diabetes, tobacco use, hereditary thrombophilia), clinical APS phenotype (thrombotic, obstetrical or mix), presence of non-criteria APS features (livedo, thrombocytopenia, headaches, Libman–Sachs endocarditis, etc.), type of therapies during the pregnancy (aspirin, LMWH and isocoagulant or curative amounts, prednisone, hydroxychloroquine), maternal adverse obstetrical events (preeclampsia/HELLP, thrombosis), fetal complication (IUGR, oligoamnios) and term of delivery. Considering the risk of fetal loss (<10 weeks of gestation), in multivariate analyses, no differences have been shown with regard to seropositive/seronegative APL status and the history of previous early miscarriage (OR 3.4 [1.34, 9.08] (p=0.01)) and smoking (OR 4.70 [1.07, 21.01] (p=0.038)) were associated with an increased risk of early fetal loss, while aspirin was associated with a lower risk (OR 0.25 [0.1, 0.61] (p=0.003)). Considering intrauterine deaths (>10 weeks of gestation), in multivariate analysis, only previous history of intra-uterine death was significant with OR 4.8 [1.6; 15.6] (p=0.006).
Considering preeclampsia and HELLP syndrome, in multivariate analysis, the age at the APS diagnosis and the preventive use of LMWH were associated with decreased risk (OR at 0.89 [0.81, 0.98] (p=0.007) and 0.20 [0.03, 0.96] (p=0.02), respectively).
Considering the occurrence of at least one adverse obstetrical events among early fetal loss, intrauterine deaths, premature birth and preeclampsia/HELLP syndrome, the multivariate analysis showed that aspirin use (OR at 0.29 [0.11, 0.69]; p=0.003) was associated with obstetrical outcomes, independently of seropositive or seronegative APL status.
DISCUSSION
In this large European case-series of seronegative APS with at least one detectable non-criteria APL, we described clinical APS features and compared them to seropositive APS. Seronegative APShave less frequent thrombotic and non-criteria features than seropositive APS. Pregnancy outcomes of seronegative APS were managed similarly to seropositive APS especially with a similar use of isocoagulant LMWH and combined aspirin-LMWH treatment during the pregnancy and associated with better obstetrical outcomes in seronegative APS women than no treatment.
Patients with clinical features of APS considering Sydney criteria but with negative APL tests are quite usual, and the diagnosis of seronegative APS has been introduced for these patients by Hughes and Khamashta in 2003.9 10 In this study, 67 patients with major clinical APS criteria and at least two additional non-criteria features without conventional APL were compared with seropositive APS (n=87).10 Thrombotic and obstetrical features were similar in both seronegative and seropositive APS patients, seronegative APS tended to have more pregnancy morbidity, and non-conventional APL have not been tested in this study. Other studies described seronegative APS considering only major clinical APS criteria, without adding APS non-criteria features, with mainly clinical APS features and not all having at least one detectable non-criteria APL. Seronegative APS have been found to present more frequent obstetrical features, in particular recurrent miscarriages in another case-series, but only with 37% of patients that have detectable non-criteria APL.11 A major limitation of seronegative APS studies is actually the heterogeneity of tested non-criteria APL, the clinical inclusion criteria, the absence of diagnostic criteria; studies of seronegative APS with at least on detectable non-criteria APL are lacking. Non-criteria APL have also been evaluated in seropositive APS patients, considering the potential use to define high-risk subgroups. Thus, anti-PS/PT antibodies have been shown to be associated to adverse pregnancy outcome,12 and included in the GAPSS scale, defining the relapse risk for thrombotic APS.13 Among APS women seeking conception, anti-PS/PT antibodies have been found to be an interesting marker for APL-related complications, in particular for IUGR.14 The prevalence of different non-criteria APL in our study varied from 0% for anti-vimentin and IgA aCL antibodies to 37% for anti-PS/PT autoantibodies and could be useful to consider in routine screening of the seronegative APS.
In our case series, more than 80% of seronegative APS women have received aspirin and/or LMWH combination during the pregnancy, with a similar rate of combined aspirin-LMWH to seropositive APS. Seropositive APS seems to have poorer obstetrical outcome, despite more prevalent curative APS amounts. Moreover, whether combined aspirin-LMWH treatment significantly reduces placenta-mediated complications in women receiving low-dose aspirin for previous severe preeclampsia diagnosed before 34 weeks of gestation and no previously recognised APS has been challenged.15 16 Data about the management of seronegative APS are quite scarce. We previously reported 65 patients with seronegative APS with at least one non-criteria APL, compared with 83 seropositive APS and to the control group without any APL.17 The conventional aspirin/heparin combination was significantly associated with live births in both seropositive and seronegative APS groups, at the difference of women without any detectable APL. Our study provides data about the management of seronegative APS during the pregnancy and shows the benefit of aspirin alone or combined with LMWH to prevent adverse obstetrical outcomes. Prospective studies are lacking to determine the value and the types of therapies for seronegative APS, in particular the need for prophylactic vitamin K antagonists therapies in thrombotic seronegative APS.
Some important limitations should be discussed such as the retrospective design, the possible inclusion of mainly obstetrical subtypes and the absence of centralised screening of both conventional and non-criteria APL in our cases. Seronegative and seropositive APS were not matched which can account for the differences which have been noted between these groups. Even thrombotic and obstetrical APS should be included, the need for at least one pregnancy, more frequent non-criteria APL screening after adverse obstetrical event probably could biased the prevalence of thrombotic subtypes. APS is still a diagnostic challenge, as no international standardised laboratory tests are available, and it is also conceivable that positive APL becomes negative over the time. Despite these important limitations, one has to consider that, previously, anti-B2GPI have been added to the laboratory criteria of APS and that new autoantigens target antibodies could be an additional tool to help to the APS diagnosis.11 According to the different prevalent of these non-criteria APL, in particular, for IgA and anti-vimentin antibodies, the screening of non-criteria APL could be organised in ‘step by step’ approach.
CONCLUSION
Several non-criteria APL can be detected in patients with clinical features consistent with APS without any conventional APL, with various prevalent rates. The detection of non-criteria APL and thus the diagnosis of seronegative APS could discuss the therapeutic management similar to seropositive APS, but well-designed controlled studies are necessary.
Key messages.
What is already known about this subject?
Several studies reported about seronegative APS mainly defined by clinical criteria and among them the series with clinical criteria and at least one positive non-criteria APL are lacking.
What does this study add?
Several non-criteria APL can be detected in patients with clinical features consistent with APS without any conventional APL, with various prevalent rates.
How might this impact on clinical practice?
The detection of non-criteria APL and thus the diagnosis of seronegative APS could discuss the therapeutic management similar to seropositive APS, but well-designed controlled studies are necessary.
Acknowledgments
We thank the European Forum on Antiphospholipid Antibodies for his help in this study.
Footnotes
Contributors: All authors were involved in drafting the article. NAand AM have full access to all data in the study and takes responsibility for the integrity of the data and the accuracy of data analysis. Study conception and design: NA, YN, LM, EEV, CDM, PB, DEP, LC, GK, MB, CJ, PNR, GU, PZ, HB, KP, YB, JAR, OF, AM. Acquisition of data: NA, YN, LM, EV, CDM, PB, DEP, LC, GK, MB, CJ, PNR, GU, PZ, HB, KP, YB, JAR, OF, AM. Analysis and interpretation of data: NA, YN, MA, OF, AM.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: Ethical committee not required for this observational study according to Helsinki law.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available in a public, open access repository.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
REFERENCES
- 1. Mekinian A, Costedoat-Chalumeau N, Masseau A, et al. Obstetrical APS: is there a place for hydroxychloroquine to improve the pregnancy outcome? Autoimmun Rev 2015;14:23–9. 10.1016/j.autrev.2014.08.040 [DOI] [PubMed] [Google Scholar]
- 2. Nayfe R, Uthman I, Aoun J, et al. Seronegative antiphospholipid syndrome. Rheumatology (Oxford) 2013;52:1358–67. 10.1093/rheumatology/ket126 [DOI] [PubMed] [Google Scholar]
- 3. Alessandri C, Conti F, Pendolino M, et al. New autoantigens in the antiphospholipid syndrome. Autoimmun Rev 2011;10:609–16. 10.1016/j.autrev.2011.04.011 [DOI] [PubMed] [Google Scholar]
- 4. Litvinova E, Darnige L, Kirilovsky A, et al. Prevalence and significance of non-conventional antiphospholipid antibodies in patients with clinical APS criteria. Front Immunol 2018;9:2971 10.3389/fimmu.2018.02971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhu H, Wang M, Dong Y, et al. Detection of non-criteria autoantibodies in women without apparent causes for pregnancy loss. 2019:e22994 10.1002/jcla.22994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Truglia S, Capozzi A, Mancuso S, et al. A monocentric cohort of obstetric seronegative anti-phospholipid syndrome. Front Immunol 2018;9:1678 10.3389/fimmu.2018.01678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Conti F, Andreoli L, Crisafulli F, et al. Does seronegative obstetric APS exist? “pro” and “cons”. Autoimmun Rev 2019;18:102407 10.1016/j.autrev.2019.102407 [DOI] [PubMed] [Google Scholar]
- 8. Alijotas-Reig J, Ferrer-Oliveras R, Ruffatti A, et al. The European Registry on Obstetric Antiphospholipid Syndrome (EUROAPS): a survey of 247 consecutive cases. Autoimmun Rev 2015;14:387–95. 10.1016/j.autrev.2014.12.010 [DOI] [PubMed] [Google Scholar]
- 9. Hughes GR, Khamashta MA. Seronegative antiphospholipid syndrome. Ann Rheum Dis 2003;62:1127 10.1136/ard.2003.006163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rodriguez-Garcia JL, Bertolaccini ML, Cuadrado MJ, et al. Clinical manifestations of antiphospholipid syndrome (APS) with and without antiphospholipid antibodies (the so-called ‘seronegative APS’). Ann Rheum Dis 2012;71:242–4. 10.1136/annrheumdis-2011-200614 [DOI] [PubMed] [Google Scholar]
- 11. Zohoury N, Bertolaccini ML, Rodriguez-Garcia JL, et al. Closing the serological gap in the antiphospholipid syndrome: the value of “non-criteria” antiphospholipid antibodies. J Rheumatol 2017;44:1597–602. 10.3899/jrheum.170044 [DOI] [PubMed] [Google Scholar]
- 12. Zigon P, Perdan Pirkmajer K, Tomsic M, et al. Anti-phosphatidylserine/prothrombin antibodies are associated with adverse pregnancy outcomes. J Immunol Res 2015;2015:975704 10.1155/2015/975704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sciascia S, Sanna G, Murru V, et al. GAPSS: the global anti-phospholipid syndrome score. Rheumatology (Oxford) 2013;52:1397–403. 10.1093/rheumatology/kes388 [DOI] [PubMed] [Google Scholar]
- 14. Canti V, Del Rosso S, Tonello M, et al. Antiphosphatidylserine/prothrombin antibodies in antiphospholipid syndrome with intrauterine growth restriction and preeclampsia. J Rheumatol 2018;45:1263–72. 10.3899/jrheum.170751 [DOI] [PubMed] [Google Scholar]
- 15. Haddad B, Winer N, Chitrit Y, et al. Enoxaparin and aspirin compared with aspirin alone to prevent placenta-mediated pregnancy complications: a randomized controlled trial. Obstet Gynecol 2016;128:1053–63. 10.1097/AOG.0000000000001673 [DOI] [PubMed] [Google Scholar]
- 16. Rodger MA, Hague WM, Kingdom J, et al. Antepartum dalteparin versus no antepartum dalteparin for the prevention of pregnancy complications in pregnant women with thrombophilia (TIPPS): a multinational open-label randomised trial. Lancet (London, England) 2014;384:1673–83. 10.1016/S0140-6736(14)60793-5 [DOI] [PubMed] [Google Scholar]
- 17. Mekinian A, Bourrienne MC, Carbillon L, et al. Non-conventional antiphospholipid antibodies in patients with clinical obstetrical APS: prevalence and treatment efficacy in pregnancies. Semin Arthritis Rheum 2016;46:232–7. 10.1016/j.semarthrit.2016.05.006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2020-001340s001.pdf (53KB, pdf)


