Abstract
STUDY QUESTION
Are the European Association of Urology (EAU) guidelines for performing semen culture accurate enough for detecting a positive semen culture in Caucasian-European infertile men?
SUMMARY ANSWER
The majority (80%) of asymptomatic infertile men with a positive sperm culture may miss a proper diagnostic assessment when relying on EAU guidelines; no single parameter can assist in medical decision-making.
WHAT IS KNOWN ALREADY
The EAU guidelines suggest performing semen culture in case of increased leukocytes in semen (>106 peroxidase positive white blood cells/ml, i.e. leukocytospermia).
STUDY DESIGN, SIZE, DURATION
A cross-sectional validation study including 523 infertile men was carried out during 2010–2018.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Infertile men who were asymptomatic for genital infections were enrolled at a single academic center, and a semen culture was obtained in every case. A concentration of >103 cfu/ml urinary tract pathogens in the ejaculate was considered indicative of significant bacteriospermia. Semen analysis values were assessed on the basis of 2010 World Health Organization reference criteria. EAU guidelines for semen culture were used to predict positive semen culture in our cohort and thus validated. Moreover, we tested the predictive performance and accuracy of several clinical parameters and compared them to EAU guidelines.
MAIN RESULTS AND THE ROLE OF CHANCE
A positive semen culture was found in 54 men (10%). The application of EAU guidelines would have missed 43 out of 54 (80%) positive semen cultures with 120/131 (92%) useless examinations. EAU guidelines specificity, sensitivity and discrimination were 74%, 20% and 47%, respectively. When trying to improve positive semen culture prediction, we were unable to find any informative baseline parameter except for serum neutrophil-to-lymphocyte ratio (odds ratio 1.70 (95% CI 1.04–2.77)), although without any improvement in terms of discrimination (P = 0.10).
LIMITATIONS, REASONS FOR CAUTION
The study was limited by the lack of a control group of fertile men its retrospective nature. Moreover, monoclonal antibodies were not used for leukocyte assessment.
WIDER IMPLICATIONS OF THE FINDINGS
Since it is not possible to identify infertile men at risk of semen infection, further studies are needed to tailor the execution of semen culture.
STUDY FUNDING/COMPETING INTEREST(S)
No funding was received for this study. There are no competing interests.
Keywords: infertility, male infertility, leukocytospermia, infections, semen culture, guidelines
WHAT DOES THIS MEAN FOR PATIENTS?
The presence of bacteria in the semen of infertile men has been linked to poor sperm quality and quantity, and infections of the male urogenital tract may be curable causes of male infertility.
Current guidelines (which have not been fully validated by researchers) recommend measuring bacteria in a semen sample when there are more than a million leukocytes (white blood cells of the immune system that help to fight infection) per milliliter of ejaculate: a condition known as leukocytospermia. This approach is based on the idea that a bacterial infection would trigger an inflammatory response, easily identified by an increase in the number of leukocytes in semen.
We set out to validate these guidelines in infertile men by using semen culture, also seeking possible improvements in the ability to predict which infertile men have a bacterial infection.
This is important because more accurate tests will mean a reduced rate of misdiagnosis, lower anxiety for patients if unnecessary tests can be avoided, and it will help to identify those infertile patients who will benefit most from a semen culture assessment.
The results of the study, which included 523 White European men from infertile couples, showed that high levels of leukocytes in semen did not always predict a bacterial infection. In fact following the current guidelines in our study would have led to us miss 80% of the infected semen cultures and perform 120 examinations that would not have helped the patient.
We have therefore shown that the current criteria do not appear to be reliable, and there is a need for further research to improve the criteria for testing as well as the methods used.
Introduction
The assessment of the infertile male is a complex diagnostic process requiring multidisciplinary competence, ranging from endocrinology and urology to genetics (Practice Committee of the American Society for Reproductive Medicine, 2015; Hwang et al., 2018; Jungwirtht et al., 2018). Though international guidelines (Practice Committee of the American Society for Reproductive Medicine, 2015; Hwang et al., 2018; Jungwirtht et al., 2018) assist this process, we are still far from having standardized and thoroughly evidence-based criteria for the evaluation of the infertile male. Recently, it has been shown that it is possible to achieve better precision during the diagnostic work-up in terms of both genetics and endocrine assessment (Ventimiglia et al., 2016a,b, 2017). This is not of limited importance, since more precision directly translates into a reduced rate of misdiagnosis, lower anxiety related to unnecessary diagnostics, more efficient result allocation and, ultimately, fewer idiopathic cases (Ventimiglia et al., 2016b; Verkuijlen et al., 2016).
In this regard, it is not yet clear which infertile patients benefit the most from a semen culture assessment. The European Association of Urology (EAU) guidelines recommend performing a semen culture when there is >106 peroxidase-positive white blood cells per milliliter of ejaculate (a condition known as leukocytospermia), an indicator of active inflammation (Jungwirtht et al., 2018). However, to the best of our current knowledge, this recommendation has never been validated. For these reasons, we sought to validate retrospectively the EAU guidelines in terms of semen culture in infertile men, seeking possible improvements of the predictive capability.
Materials and methods
Study population
The analyses included a homogeneous cohort of 523 White European men belonging to primary infertile couples assessed between 2010 and 2018 at a single academic center. Approval was given by the local Ethical Committee and written informed consent was obtained from each patient. Two semen samples were analyzed for every patient and evaluated according to the 2010 World Health Organization (WHO) guidelines. Male factor infertility (MFI) was defined and identified as at least one abnormal sperm parameter value in at least two consecutive semen analyses and after a comprehensive gynecological evaluation of the female partners.
Diagnostic work-up
A detailed patient history and physical examination were obtained for every patient. Comorbidities were scored with the Charlson Comorbidity Index (CCI), as previously described in infertile men (Ventimiglia et al., 2015). Further work-up included hormonal assessment (including, total testosterone and FSH), semen analyses (detailing sperm leukocyte concentration, peroxidase test (Ricci et al., 2000)) and semen culture. Semen analyses were performed in accordance with the WHO laboratory manual for the Examination and processing of human semen (Fifth edition) (World Health Organisation, 2010). The SEMinal QUAlity studies (SEMQUA) checklist was followed to improve accuracy and transparency of the study. Both an internal and external quality control program, according to the European Society of Human Reproduction and Embryology (ESHRE) guidelines, has been established in the laboratory in order to control for random and systematic errors and interlaboratory differences (Sánchez-Pozo et al., 2012). All the laboratory personnel were trained according to the SEMinal QUAlity studies Special Interest Group in Andrology Basic Semen Analysis Course (Sánchez-Pozo et al., 2012). According to our internal diagnostic work-up, semen culture was performed for every man after exclusion of clinically evident urethritis and bladder infection. Real-time PCR was used to identify Chlamydia trachomatis and Ureaplasma urealyticum in the semen (NIMBUS, Seegene, South Korea). A concentration of >103 cfu/ml urinary tract pathogens in the ejaculate was considered indicative of significant bacteriospermia.
Statistical analyses
Statistical analyses consisted of several steps. First, we assessed the prevalence of leukocytospermia and positive semen culture in our cohort. Second, we retrospectively validated the predictive performance of EAU guidelines in terms of positive semen culture, estimating their sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and discrimination. Third, we graphically explored the association between continuous values of semen leukocyte concentration and the multivariable probability (adjusted for age, BMI, CCI and sperm concentration) of positive semen cultures by the using a locally weighted scatterplot-smoothing approach in order to detect a possible non-linear relationship. Fourth, we developed a logistic regression-based model in order to predict positive semen culture according to age, BMI, CCI, patient sperm concentration and neutrophil-to-lymphocyte ratio (NLR). Eventually, decision curve analysis (DCA) (Van Calster et al., 2018) was used to determine the clinical net-benefit of the EAU guidelines, leukocyte concentration and the proposed predictive model. All statistical tests were two-sided with a significance value set a 0.05. The analyses were conducted using R: A language and environment for statistical computing (R Foundation for Statistical Computing, Vienna, Austria).
Results
Table I details descriptive statistics of the whole cohort of patients. Overall, isolated MFI and mixed infertility were observed in 354 (68%) and 169 (32%) men, respectively. A positive semen culture was found in 54 (10%) patients, whereas 131 (25%) men had leukocytospermia at semen analysis. The pathogen U. urealyticum was found more often (n = 16, 30%) than C. trachomatis (n = 4, 7%). No significant difference in the proportion of men with leukocytospermia was found between men with positive and negative semen cultures (11/54 (21%) vs 120/469 (26%), respectively, P = 0.55 χ2 test).
Table I.
Descriptive characteristics of the study population of infertile men.
| Positive semen culture | Negative semen culture | Overall | |
|---|---|---|---|
| n = 54 | n = 469 | n = 523 | |
| Age (years) | |||
| Median (IQR) | 38 (33–42) | 36 (34–40) | 37 (34–40) |
| BMI (kg/m2) | |||
| Median (IQR) | 25 (24–28) | 25 (23–27) | 25 (23–27) |
| BMI (kg/m2) | |||
| <25 | 27 (50) | 234 (50) | 261 (50) |
| 25–29.9 | 18 (33) | 195 (42) | 213 (41) |
| >30 | 9 (17) | 40 (9) | 49 (9) |
| CCI, n (%) | |||
| 0 | 52 (96) | 441 (94) | 493 (94) |
| 1 | 0 (0) | 18 (4) | 18 (3) |
| 2+ | 2 (4) | 10 (2) | 12 (2) |
| Mean testicular volume (Prader) | |||
| Median (IQR) | 15 (12–25) | 18 (14–20) | 18 (14–23) |
| Infertility type, n (%) | |||
| Primary | 48 (89) | 410 (87) | 458 (88) |
| Secondary | 6 (11) | 59 (13) | 65 (12) |
| Infertility factor, n (%) | |||
| MFI | 36 (67) | 318 (68) | 354 (68) |
| Mixed | 18 (33) | 151 (32) | 169 (32) |
| Total testosterone (ng/ml) | |||
| Median (IQR) | 4.2 (3.0–5.6) | 4.7 (3.6–5.9) | 4.7 (3.5–5.9) |
| FSH (mU/ml) | |||
| Median (IQR) | 5.5 (3.1–9.3) | 4.3 (2.9–7.8) | 4.4 (2.9–8.0) |
| NLR | |||
| Median (IQR) | 1.84 (1.51–2.35) | 1.58 (1.27–2.06) | 1.59 (1.28–2.08) |
| Sperm concentration (106/ml) | |||
| Median (IQR) | 19 (4–45) | 20 (4–48) | 19 (4–48) |
| Azoospermia, n (%) | |||
| No | 48 (89) | 432 (92) | 480 (92) |
| Yes | 6 (11) | 37 (8) | 43 (8) |
| Semen leukocytes (106/ml) | |||
| Median (IQR) | 299 (69–900) | 400 (120–1000) | 400 (112–997) |
| Leukocytospermia, n (%) | |||
| No | 43 (80) | 349 (74) | 392 (75) |
| Yes | 11 (20) | 120 (26) | 131 (25) |
CCI, Charlson Comorbidity Index; IQR, interquartile range; MFI, male factor infertility; mixed, infertility with a mixed male-female factor; NLR, neutrophil-to-lymphocyte ratio.
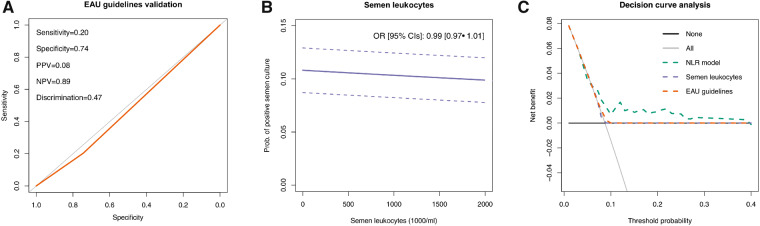
The strict application of EAU guidelines to our population would have missed 43 out of 54 (80%) positive semen cultures, representing 120/131 (92%) useless examinations. Overall, EAU guideline specificity, sensitivity, PPV and NPV were 74%, 20%, 8% and 89%, respectively (Fig. 1A). EAU guidelines discrimination was 47%.
Figure 1.
Predictive values and net benefits of using the European Association of Urology guidelines, semen leukocytes and the proposed logistic regression based model. (A) Receiver operating characteristic curve for European Association of Urology (EAU) guidelines application in our population (red line). The gray line represents the 50% line. (B) The locally weighted scatterplot-smoothing curve depicting the association between continuous values of semen leukocyte concentration and the multivariable probability (adjusted for age, BMI, Charlson Comorbidity Index and sperm concentration) of positive semen culture. (C) Decision curve analysis showing the net benefit in diagnosing positive semen culture through the application of EAU guidelines, using continuous values of semen leukocytes, and with the proposed logistic regression model. NLR, neutrophil-to-lymphocyte ratio; NPV, negative predictive value; OR, odds ratio; PPV, positive predictive value.
Semen leukocyte concentration was not associated with positive semen culture (adjusted odds ratio (OR) (95% CI): 0.99 (0.97 − 1.01)); this lack of association was observed for all the leukocyte thresholds studied (Fig. 1B). When we tried to predict the presence of a positive semen culture by the application of the aforementioned logistic regression model, we were not able to find any informative baseline parameter except for serum NLR (OR 1.70 (1.04–2.77)). The discrimination of this logistic regression model was 59%, not significantly different from EAU guidelines (P = 0.10 at DeLong’s test). DCA showed that EAU guidelines and continuously coded semen leukocyte concentration had a non-superior net benefit in terms of diagnosing positive semen cultures compared with either ‘screen-all’ or ‘screen-none’ strategies (Fig. 1C); moreover, the logistic regression model including NLR was only marginally superior to EAU guidelines in terms of net-benefit (Fig. 1C).
Discussion
In this study, we have assessed and reported the prevalence of both leukocytospermia (25%) and positive semen culture (10%) in a homogeneous large cohort of White European men presenting for primary couple’s infertility at a single academic institution. Leukocytospermia did not emerge as an informative predictive factor of positive semen culture. Therefore, the hypothetical application of EAU guidelines in our cohort would have led both to missing 43/54 (80%) of positive semen cultures and performing 120/131 (92%) useless examinations. Moreover, none of the evaluated parameters (namely age, BMI, CCI and sperm concentration) could properly identify men at risk of having a positive semen culture.
The diagnostic work-up in the infertile male may be a challenging process. Though several improvements have been reported in recent years (Tournaye et al., 2016), the way we assess and treat infertile couples still remains unsatisfactory in a relevant proportion of cases (Olesen et al., 2017). During this process, it is not clear when a semen culture should be performed and who should be the most appropriate candidate for this examination, especially among asymptomatic men. EAU guidelines recommend the performing semen culture in men with leukocytospermia (Jungwirtht et al., 2018), whereas the American Society for Reproductive Medicine recommendations do not provide specific statements in this regard (Practice Committee of the American Society for Reproductive Medicine, 2015; Hwang et al., 2018). In this context, the EAU findings are based on the pathophysiological concept that a bacterial infection would trigger an inflammatory response, easily documented by an increase in semen leukocyte concentration (Jue and Ramasamy, 2017). However, this concept has never been systematically validated. The rationale for the identification of semen pathogens in asymptomatic infertile men is not fully clear. There are claims stating that infections of the male urogenital tract are potentially curable causes of male infertility (Liu et al., 2002; Sanocka et al., 2004). Semen pathogens have been associated with decreased sperm concentration and progressive motility, lower chromatin condensation and a higher rate of protamine1/protamine2 ratio abnormalities (Zeyad et al., 2018). In the clinical setting, asymptomatic C. trachomatis infection is found to be more common in infertile men than in fertile controls, and subsequent antibiotic treatment is associated with improved semen parameters and reduced levels of reactive oxygen species in the infertile cohort (Ahmadi et al., 2018b). Similar results are observed in asymptomatic infertile men with evidence of seminal Mycoplasma genitalium (Ahmadi et al., 2018a). Such results would suggest the systematic screening for, and treating, asymptomatic semen pathogens; however, it should be noted that these studies have not been replicated, and provide rather poor evidence. Moreover, the possible detrimental impact on reproduction owing to extensive antibiotic use in asymptomatic infertile men should be taken into account, both in terms of negative effects on semen parameters values and the increased risk of inoculating the female partner’s reproductive tract with antibiotic-resistant bacteria (Schlegel et al., 1991; Liversedge et al., 1996).
The setting where a semen culture is obtained might influence its results, mainly because of possible contamination. The WHO guidelines recommend washing the hands and penis with soap in order to reduce the risk of contamination of the specimen with commensal organisms from the skin (World Health Organisation, 2010); this user-friendly recommendation was always used in our study for obtaining semen samples. This procedure has been questioned by Kim and Goldstein (Kim and Goldstein, 1999), who showed that an antibacterial skin preparation consisting of a shower followed by perineal, penile and hand washing with 4% chlorhexidine and 10% povidone-iodine was less frequently associated with semen cultures harboring enteric organisms. Unfortunately, this promising approach was never adopted by other groups and therefore we lack further corroborating data. We also tried to restrict our analyses to non-enteric organisms, and our results remained substantially unchanged (data not shown).
Our study is not devoid of limitations. First, the aforementioned lack of an antibacterial skin preparation might have inflated the proportion of men with positive semen culture due to contamination; however, this kind of preparation is not currently recommended by international guidelines, and therefore is not easily implementable outside the research setting. Second, several lifestyle factors (Saleh et al., 2002; Maneesh et al., 2006; Cocuzza et al., 2008), could be implicated in increasing the semen leukocyte concentration, thus contributing to possible unmeasured confounding. Third, more accurate methodology for sperm leukocyte assessment has been reported combining flow cytometry and monoclonal antibodies (anti-CD45, anti-CD53), possibly providing more informative insights (Ricci et al., 2000). The lack of certified and unquestionable modifiers of semen leukocyte concentration prevents not only us, but every researcher working in the field, from disentangling this issue. Unfortunately, if this study suggests that the way international guidelines currently recommend us to perform a semen culture during MFI work-up is not satisfying, we have not been able to suggest a better diagnostic algorithm owing to the lack of informative predictors. However, the introduction of sperm DNA fragmentation analysis as well as other proxy markers in the clinic might provide useful insights in this regard (Pratap et al., 2019). More specifically, analysis of the direct detrimental effects produced by pathogens, rather than its epiphenomenon (i.e. leukocytospermia), might turn out to be more clinically manifest. Until sound and consistent studies finally solve this intricate issue, we will lack the evidence-based common ground to rely on when selecting the best candidate for semen culture in our daily clinical practice.
Conclusion
The vast majority (80%) of asymptomatic infertile men with a positive sperm culture may not have a proper diagnostic assessment when relying on EAU guidelines. No single baseline parameter was found to assist with medical decision-making to improve diagnostic accuracy. We believe that further studies are needed to identify the most appropriate patients for sperm culture.
Authors’ roles
Conception and design: E.V. and A.S. Acquisition of data: E.V., P.C., L.B., E.P., F.C., W.C., R.M. and C.A. Analysis and interpretation of data: E.V. and A.S. Drafting of the manuscript: E.V. Critical revision: A.S., P.V. and F.M. Statistical analysis: E.V. and A.S. Administrative, technical, or material support: A.S. and F.M. Supervision: A.S. and F.M.
Funding
No funding was received for this study.
Conflict of interest
None
References
- Ahmadi MH, Mirsalehian A, Gilani MAS, Bahador A, Talebi M. Improvement of semen parameters after antibiotic therapy in asymptomatic infertile men infected with Mycoplasma genitalium. Infection 2018. a;46:31–38. [DOI] [PubMed] [Google Scholar]
- Ahmadi MH, Mirsalehian A, Sadighi Gilani MA, Bahador A, Afraz K. Association of asymptomatic Chlamydia trachomatis infection with male infertility and the effect of antibiotic therapy in improvement of semen quality in infected infertile men. Andrologia 2018. b;doi:10.1111/and.12944. [DOI] [PubMed] [Google Scholar]
- Cocuzza M, Athayde KS, Agarwal A, Sharma R, Pagani R, Lucon AM, Srougi M, Hallak J. Age-related increase of reactive oxygen species in neat semen in healthy fertile men. Urology 2008;71:490–494. [DOI] [PubMed] [Google Scholar]
- Hwang K, Smith JF, Coward RM, Penzias A, Bendikson K, Butts S, Coutifaris C, Falcone T, Fossum G, Gitlin S. et al. Evaluation of the azoospermic male: a committee opinion. Fertil Steril 2018;109:777–782. [DOI] [PubMed] [Google Scholar]
- Jue JS, Ramasamy R. Significance of positive semen culture in relation to male infertility and the assisted reproductive technology process. Transl Androl Urol 2017;6:916–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungwirtht A, Diemer T, Kopa Z, Krausz C, Minhas S, Tournaye H. European Association of Urology Guidelines on Male Infertility. European Association of Urology, 2018 Edition. 2018. [DOI] [PubMed]
- Kim FY, Goldstein M. Antibacterial skin preparation decreases the incidence of false-positive semen culture results. J Urol 1999;161:819–821. [PubMed] [Google Scholar]
- Liu J-H, Li H-Y, Cao Z-G, Duan Y-F, Li Y, Ye Z-Q. Influence of several uropathogenic microorganisms on human sperm motility parameters in vitro. Asian J Androl 2002;4:179–182. [PubMed] [Google Scholar]
- Liversedge NH, Jenkins JM, Keay SD, McLaughlin EA, Al-Sufyan H, Maile LA, Joels LA, Hull MG. Antibiotic treatment based on seminal cultures from asymptomatic male partners in in-vitro fertilization is unnecessary and may be detrimental. Hum Reprod 1996;11:1227–1231. [DOI] [PubMed] [Google Scholar]
- Maneesh M, Dutta S, Chakrabarti A, Vasudevan DM. Alcohol abuse-duration dependent decrease in plasma testosterone and antioxidants in males. Indian J Physiol Pharmacol 2006;50:291–296. [PubMed] [Google Scholar]
- Olesen IA, Andersson AM, Aksglaede L, Skakkebaek NE, Rajpert-de Meyts E, Joergensen N, Juul A. Clinical, genetic, biochemical, and testicular biopsy findings among 1,213 men evaluated for infertility. Fertil Steril 2017;107:74–82.e7. [DOI] [PubMed] [Google Scholar]
- Practice Committee of the American Society for Reproductive Medicine. Diagnostic evaluation of the infertile male: a committee opinion. Fertil Steril 2015;103:e18–e25. [DOI] [PubMed] [Google Scholar]
- Pratap H, Hottigoudar SY, Nichanahalli KS, Rajendran S, Bheemanathi HS. Sperm DNA integrity in leukocytospermia and its association with seminal adenosine deaminase. J Hum Reprod Sci 2019;12:182–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci G, Presani G, Guaschino S, Simeone R, Perticarari S. Leukocyte detection in human semen using flow cytometry. Hum Reprod 2000;15:1329–1337. [DOI] [PubMed] [Google Scholar]
- Saleh RA, Agarwal A, Sharma RK, Nelson DR, Thomas AJJ. Effect of cigarette smoking on levels of seminal oxidative stress in infertile men: a prospective study. Fertil Steril 2002;78:491–499. [DOI] [PubMed] [Google Scholar]
- Sánchez-Pozo MC, Mendiola J, Serrano M, Mozas J, Björndahl L, Menkveld R, Lewis SEM, Mortimer D, Jørgensen N, Barratt CLR. et al. Proposal of guidelines for the appraisal of SEMen QUAlity studies (SEMQUA). Hum Reprod 2012;28:10–21. [DOI] [PubMed] [Google Scholar]
- Sanocka D, Fraczek M, Jedrzejczak P, Szumala-Kakol A, Kurpisz M. Male genital tract infection: an influence of leukocytes and bacteria on semen. J Reprod Immunol 2004;62:111–124. [DOI] [PubMed] [Google Scholar]
- Schlegel PN, Chang TS, Marshall FF. Antibiotics: potential hazards to male fertility. Fertil Steril 1991;55:235–242. [DOI] [PubMed] [Google Scholar]
- Tournaye H, Krausz C, Oates RD. Novel concepts in the aetiology of male reproductive impairment. Lancet Diabetes Endocrinol 2016;8587:1–10. [DOI] [PubMed] [Google Scholar]
- Van Calster B, Wynants L, Verbeek JFM, Verbakel JY, Christodoulou E, Vickers AJ, Roobol MJ, Steyerberg EW. Reporting and interpreting decision curve analysis: a guide for investigators. Eur Urol 2018;74:796–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventimiglia E, Capogrosso P, Boeri L, Ippolito S, Scano R, Moschini M, Gandaglia G, Papaleo E, Montorsi F, Salonia A. Validation of the American Society for Reproductive Medicine guidelines/recommendations in White European men presenting for couple’s infertility. Fertil Steril 2016. a;106:1076–1082.e1. [DOI] [PubMed] [Google Scholar]
- Ventimiglia E, Capogrosso P, Boeri L, Pederzoli F, Montorsi F, Salonia A, Catto J, Cazzaniga W, Scano R, Ippolito S. et al. When to perform karyotype analysis in infertile men? Validation of the European Association of Urology Guidelines with the proposal of a new predictive model. Eur Urol 2016. b;70:920–923. [DOI] [PubMed] [Google Scholar]
- Ventimiglia E, Capogrosso P, Boeri L, Serino A, Colicchia M, Ippolito S, Scano R, Papaleo E, Damiano R, Montorsi F. et al. Infertility as a proxy of general male health: results of a cross-sectional survey. Fertil Steril 2015;104:48–55. [DOI] [PubMed] [Google Scholar]
- Ventimiglia E, Ippolito S, Capogrosso P, Pederzoli F, Cazzaniga W, Boeri L, Cavarretta I, Alfano M, Vigano P, Montorsi F. et al. Primary, secondary and compensated hypogonadism: a novel risk stratification for infertile men. Andrology 2017;5:505–510. [DOI] [PubMed] [Google Scholar]
- Verkuijlen J, Verhaak C, Nelen WLDM, Wilkinson J, Farquhar C. Psychological and educational interventions for subfertile men and women. Cochrane database Syst Rev 2016;3:CD011034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organisation. WHO laboratory manual for the Examination and processing of human semen. 2010. http://www.who.int/iris/handle/10665/44261 (1 June 2020, date last accessed).
- Zeyad A, Hamad MF, Hammadeh ME. The effects of bacterial infection on human sperm nuclear protamine P1/P2 ratio and DNA integrity. Andrologia 2018;50. doi:10.1111/and.12841. [DOI] [PubMed] [Google Scholar]