Abstract
Background
In diabetes, cognitive impairment is linked with oxidative stress and neuroinflammation. As the only chimeric member of the galectin family, galectin-3 (Gal3) induces neuroinflammation and cognitive impairment in models of Alzheimer’s disease (AD); however, its role in diabetes-associated cognitive impairment is not established.
Methodology
Here, we investigated the effects of Gal3 inhibition on cognitive impairment and the possible underlying molecular events in diabetes. We investigated the effects of the Gal3 inhibitor modified citrus pectin (MCP; 100 mg/kg/day oral for 6 weeks) in vivo in high-fat diet (HFD)/streptozotocin (STZ)-induced diabetic rats. Additionally, the effects of MCP on high glucose (HG)-stimulated BV-2 microglial cells were investigated in vitro.
Results
We found that MCP attenuated memory impairment in diabetic rats in the Morris water maze test and reduced insulin resistance, oxidative stress, and neuroinflammation. In HG-stimulated BV-2 microglial cells, MCP increased cell viability and decreased oxidative stress and the production of proinflammatory cytokines.
Conclusion
The results of this study indicate that the inhibition of Gal3 by MCP ameliorates diabetes-associated cognitive impairment, oxidative stress, and neuroinflammation, suggesting that Gal3 could be a potential new target for therapeutic intervention to prevent cognitive impairment in diabetes.
Keywords: galectin-3, oxidative stress, neuroinflammation, cognitive impairment, diabetes
Introduction
Galectin-3 (Gal3), the only chimeric member of the galectin family, is expressed in different cell types and found in both the intracellular and extracellular spaces.1 It regulates several biological processes, such as cell adhesion, proliferation, apoptosis, tumor progression, oxidative stress, inflammation and innate and adaptative immune system modulation.2,3 Furthermore, Gal3 has been shown to cause cognitive impairment in rats.4,5 Thus, Gal3 may be a potential therapeutic target for brain disorders.
Cognitive impairment and dementia have been shown to be common diabetic complications.6 In recent years, a new term-“diabetes-associated cognitive impairment” was proposed to facilitate the researcher’s attention into this area and to increase recognition of the disorder.7 This term does not imply a particular pathogenesis, but merely describes a state of mild to moderate cognitive impairment, in particular, psychomotor slowing and reduced mental flexibility, not attributable to other causes.8 An increased risk of Alzheimer’s disease (AD) has been found in patients diagnosed with type 2 diabetes mellitus (T2DM), and this association is likely related to disease processes such as AD.9 It is hypothesized that insulin resistance, oxidative stress, and neuroinflammation are all the main mechanisms of diabetes-associated cognitive impairment and AD.6,9 Additionally, T2DM is one of the most important risk factors for ischemic stroke, which causes vascular cognitive impairment in patients with T2DM.7 T2DM aggravates brain injury induced by cerebral ischemia, it was shown that hyperglycemic condition in diabetes leads to the generation of reactive oxygen species (ROS), suppression of endothelial nitric oxide synthase (NOS) and causes vascular endothelial damage.10 Further research is needed to understand the potential underlying molecular mechanism of diabetes-associated impairment and AD.
Gal3 has been increasingly associated with the mechanisms underlying the pathogenesis of diabetes and has been identified as a potential serum marker of prediabetes and a diabetes risk factor in humans.11,12 Mechanistically, Gal3 directly binds to the insulin receptor, antagonizing its downstream metabolic signaling pathways and leading to insulin resistance and glucose intolerance.13,14 It is reported that Gal3 can bind to the triggering receptor expressed on myeloid cells 2 (TREM2), activating microglia and inducing neuroinflammation in an AD mouse model.4 Moreover, Gal3 can induce oxidative stress, resulting in neurodegeneration in diabetic retinopathy and diabetic optic neuropathy.2 In our recent study, T2DM patients with mild cognitive impairment (MCI) showed significantly higher levels of serum Gal3 than normal subjects. Gal3 is a potential risk factor for MCI development in patients with T2DM, suggesting that Gal3 is considered as a novel biomarker in clinical diagnosis and may be a new therapeutic target for combatting diabetes-associated cognitive impairment.15 We also found that Gal3 levels are significantly increased in the serum, cerebral cortex and hippocampus in high-fat diet (HFD)/streptozotocin (STZ)-induced T2DM rats.14 However, no experimental studies have revealed the functional significance of Gal3 in diabetes-associated cognitive impairment. Modified citrus pectin (MCP) is a compound polysaccharide extracted from the peel and pulp of citrus fruits.16 MCP binds to Gal3 carbohydrate recognition domains (CRDs), decreasing its expression and activity, and is known as a Gal3 inhibitor.16–18 Previous research has indicated that MCP exerts neuroprotective effects by inhibiting the upregulation of Gal3 and its downstream pathways following subarachnoid hemorrhage.19 Our previous study showed that MCP ameliorates cognitive impairment and decreases Gal3 levels in the serum and brain in HFD/STZ-induced T2DM rats.15 The aim of the current study was to further evaluate whether MCP prevents diabetes-associated cognitive impairment by inhibiting Gal3 and, if so, the possible mechanisms related to oxidative stress and neuroinflammation.
Materials and Methods
Animals and Experimental Design
Six-week-old male Wistar rats purchased from the Experimental Animal Center of Shandong University, Jinan, China, were used as the experimental animals. They were housed at 22±2°C under a 12-h light/dark cycle in a standard laboratory of Shandong Provincial Hospital and provided food and water ad libitum. The animal experiments in this study were approved by the Animal Care Committee of Shandong First Medical University Council and conducted in accordance with the Provisions and General Recommendation of Chinese Experimental Animals Administration Legislation.
After a week of adaptation, the rats were randomly divided into four groups. Group I was fed a normal diet and i.p. injected with 0.1 mL of 0.1 M citrate buffer solution. Group II rats were fed an HFD for 4 weeks and then intraperitoneally injected once with low-dose STZ (30 mg/kg; dissolved in 0.1 M citrate buffer) according to previous protocols.15,20 Rats with fasting blood glucose (FBG) levels ≥16.7 mmol/L were considered T2DM models, and they were continuously fed an HFD for an additional 6 weeks. MCP is Gal3 activity inhibitor, obtained from Centrax International Corporation, San Francisco, USA. Group III comprised T2DM rats that were fed an HFD and administered MCP (100 mg/kg/day), in drinking water for an additional 6 weeks.15 MCP has good water solubility and is readily accepted by rats, MCP solution was quantified daily to make sure the dosage of MCP. Group IV rats received MCP (100 mg/kg/day) only. Body weights were recorded, and fasting blood plasma glucose concentrations in abdominal aorta samples were measured using a glucose monitor before and after MCP treatment. At the end of dietary treatment, the learning ability and memory of the rats in all groups were tested by the Morris water maze test. Finally, the rats were decapitated under anesthesia after blood samples were collected from the abdominal aorta, and neurobehavioral experiments were carried out. We immediately removed the brains of each rat, then placed them on dry ice to isolate the cerebral cortex and hippocampus. Afterwards, the homogenates of brain tissues were prepared with 0.1 M phosphate buffer saline (PBS), centrifuged at 8000×g 4°C for 10 min, aliquoted and stored at −80°C.
Rat Blood Assays
After the neurobehavioral test, 5 rats from each group were fasted for 12 h, and 2 days before the rats were sacrificed, 3 mL blood samples were collected from the abdominal aorta, and then separated for serum and plasma and stored at −80°C. The FBG levels were measured by a glucose-oxidase biochemical analyzer, the fasting insulin (FINS) levels were determined by an enzyme linked immunosorbent assay (ELISA) kit (Cusabio, Wuhan, China). Serum Gal3 levels were measured by human-specific sensitive ELISA kits (R&D, Minneapolis, MN, USA). Homeostasis model assessment of insulin resistance (HOMA-IR) was calculated based on the formula FBG (mmol/L)×FINS (mU/L)/22.5.
Cognitive Function Test
The Morris water maze (MWM) test consisted of 5-day training (visible [days 1–2] and invisible [days 3–5] platform training sessions)21 was adopted to evaluate the spatial learning and retention of memory. Visible platform training was carried out for baseline differences in vision and motivation, and the escape latency of each rat during the 2-day visible platform test was recorded. Invisible platform training was carried out for spatial learning and retention memory to find the platform, the escape latency of each rat to reach the hidden platform during the 3-day invisible platform test was recorded. For details, please refer to our recent paper.15
Cell Culture
The mouse microglial BV-2 cell line was purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). The cells were cultured in Dulbecco’s Modified Eagle’s medium (DMEM, Invitrogen; Carlsbad, CA, USA) containing 10% fetal bovine serum (HyClone; Thermo, USA), 100 IU/mL penicillin (Sigma, St Louis, MO, USA) and 100 mg/mL streptomycin (Sigma) in a 37°C humidified 5% CO2 incubator. The cells were grown to 70–80% confluence in 60-mm dishes, and the medium was replaced every fourth day.
Cell Viability
The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was used to measure cell viability. BV-2 cells were seeded at a density of 1×105 cells/mL in 96-well plates. After culturing at 37°C for 24 h, the BV-2 cells were treated with different concentrations of glucose (50, 100, or 200 mmol/L) and the Gal3 activity inhibitor MCP (0.005%, 0.01%, or 0.02%). After the cells were cultured for another 48 h, MTT (20 μL, 5 mg/mL; Sigma) was added to each well and incubated at 37°C for 4 h. Then, the cultured medium was removed, and 150 μL dimethylsulfoxide (DMSO) was added to each well to dissolve the formazan generated by cells. The absorbance was read at 540 nm by a microplate reader (Bio-Rad, CA, USA). The cell viability of each group was analyzed by the ratio of OD value as a percentage of the control group.
Determination of Oxidative Stress and the Antioxidant Defense System
Hippocampal and cerebral cortex tissues isolated from Wistar rats and BV-2 cells were used for biochemical analysis. The ROS levels in the brain tissues and cells were quantified via the 20–70-dichlorofluorescein-diacetate (DCFHDA) assay as previously described in our studies.21,22 Protein carbonyl content (Jiancheng, Nanjing, China) and malondialdehyde (MDA) content, as well as activities of superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-Px), were assessed by spectrophotometry using commercially available kits (Beyotime, Shanghai, China).
Determination of Proinflammatory Cytokine Levels
The levels of interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α) in the supernatants of rat hippocampal and cerebral cortex homogenates as well as BV-2 cells were examined using commercially available ELISA kits. The experimental procedures were conducted in accordance with the manufacturer’s instructions (Elabscience Biotech, Wuhan, China).
Statistical Analysis
All results are expressed as the mean ± SEM. Except for the data from the acquisition phase of the MWM test, which was analyzed by repeated-measures analysis of variance (ANOVA), statistical significance was analyzed by one-way ANOVA followed by Tukey’s test for intergroup variation. P < 0.05 was considered to indicate statistical significance.
Results
Gal3 Inhibition Improves Insulin Sensitivity and Decreases Gal3 Levels in the Serum of HFD/STZ-Induced T2DM Rats
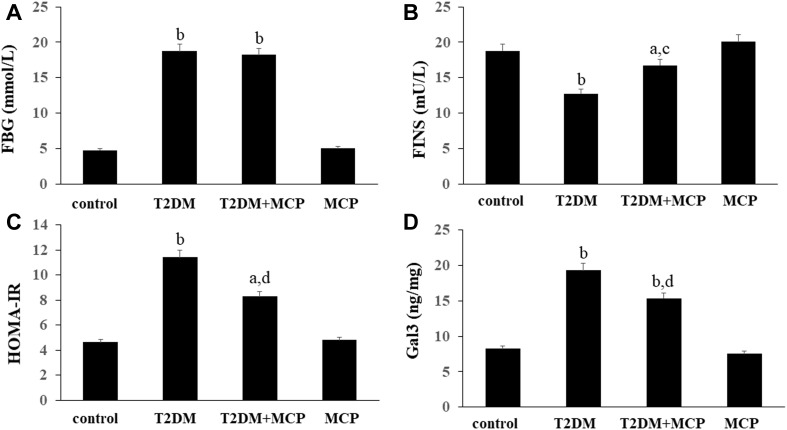
As seen from Figure 1, the levels of FBG, FINS, HOMA-IR, and serum Gal3 were significantly different between the T2DM group rats and the control rats. The rats in the T2DM+MCP group exhibited significantly lower levels of FBG, HOMA-IR, and Gal3 than those in the T2DM group. T2DM+MCP group rats showed increased levels of FBG, HOMA-IR, and Gal3 than that in the control group. The rats in the T2DM+MCP group exhibited significantly increased levels of FINS than those in the T2DM group. T2DM+MCP group rats showed decreased levels of FINS than that in the control group. No difference was found between control and MCP groups.
Figure 1.
Galectin-3 (Gal3) inhibitor modified citrus pectin (MCP) improves insulin sensitivity in T2DM rats.
Notes: Effects of MCP on the levels of FBG (A), FINS (B), HOMA-IR (C), and serum Gal3 (D) in T2DM rats. The data are from three independent experiments. aP<0.05, bP<0.01 compared to the control rats; cP<0.05, dP<0.01 compared to the T2DM rats.
Abbreviations: T2DM, type 2 diabetes mellitus; MCP, modified citrus pectin; FBG, fasting blood glucose; FINS, fasting insulin; HOMA-IR, homeostasis model assessment of insulin resistance.
Gal3 Inhibition Ameliorates Memory Dysfunction in HFD/STZ-Induced T2DM Rats
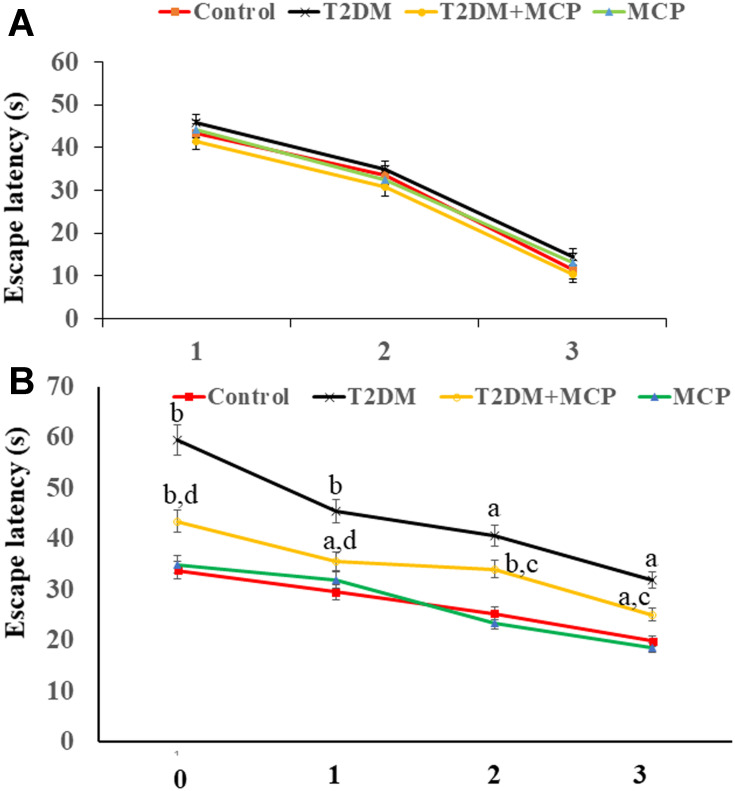
As seen from Figure 2A, consistent with our previous results,15 rats in each group exhibited a similar escape latency in a 2-day visible platform test, suggesting no differences in vision or basal motivation. In the 3-day invisible platform test, unlike the control group, rats in the T2DM group exhibited spatial learning and retention memory impairment, MCP administration improved spatial learning and retention memory function compared with the T2DM group (Figure 2B).
Figure 2.
Galectin-3 (Gal3) inhibitor modified citrus pectin (MCP) improves cognitive function in T2DM rats.
Notes: (A) No significant differences were found in escape latency during the 2-day visible platform test. (B) Changes in escape latency during the 3-day invisible platform test. aP<0.05, bP<0.01 compared to the control rats; cP<0.05, dP<0.01 compared to the T2DM rats.
Abbreviations: T2DM, type 2 diabetes mellitus; MCP, modified citrus pectin.
Gal3 Inhibition Improves Oxidant/Antioxidant Status in HFD/STZ-Induced T2DM Rats
As seen from Table 1, the T2DM group rats showed significantly higher levels of ROS, contents of MDA and protein carbonyl in both the hippocampus (P<0.05, P<0.01, and P<0.05, respectively) and cerebral cortex compared with the control rats (P<0.05, P<0.05, and P<0.05, respectively). The T2DM+MCP group rats showed lower levels of ROS, contents of MDA, and protein carbonyl in both the hippocampus (P<0.05, P<0.05, and P<0.05, respectively) and cerebral cortex (P<0.01, P<0.05, and P<0.05, respectively) compared with the T2DM group. Compared with the control group, the T2DM+MCP group showed significant increases in levels of ROS, contents of MDA, and protein carbonyl in the hippocampus (P<0.05, P<0.05, and P<0.01, respectively) and cerebral cortex (P<0.05, P<0.01, and P<0.05, respectively). The T2DM group rats showed lower activities of SOD, CAT and GSH-Px in both the hippocampus (P<0.01, P<0.01, and P<0.05, respectively) and cerebral cortex (P<0.01, P<0.05, and P<0.05, respectively) compared with control rats, and the T2DM+MCP group rats showed significant increases in activities of SOD, CAT and GSH-Px in both the hippocampus (P<0.05, P<0.05, and P<0.05, respectively) and cerebral cortex (P<0.01, P<0.05, and P<0.01, respectively) compared with T2DM group rats. Compared with the control group, the T2DM+MCP group showed significant decreases in activities of SOD, CAT and GSH-Px in the hippocampus (P<0.05, P<0.05, and P<0.05, respectively) and cerebral cortex (P<0.05, P<0.01, and P<0.05, respectively).
Table 1.
Effects of Galectin-3 (Gal3) Inhibitor MCP on ROS Level, MDA and Protein Carbonyl Contents, and Antioxidant Enzymes Catalase in Hippocampus and Cerebral Cortex of HFD/STZ-Induced T2DM Rats
| Treatment | Brain Regions and Cells |
ROS Level a | MDA Contentb | Protein Carbonyl Contentc |
Antioxidant Enzyme Activity | |||
|---|---|---|---|---|---|---|---|---|
| SODd | CATd | GSH-Pxd | ||||||
| Control group |
Hippocampus | 47.52 ± 2.45 | 1.53 ± 0.18 | 5.75 ± 0.45 | 3.25 ± 0.35 | 55.11 ± 2.75 | 25.11 ± 2.15 | |
| Cerebral cortex | 49.87 ± 3.13 | 1.57 ± 0.14 | 5.33 ± 0.69 | 5.19 ± 0.63 | 53.19 ± 2.63 | 23.19 ± 1.83 | ||
| T2DM group |
Hippocampus | 88.74 ± 6.27e | 3.33 ± 0.24f | 27.48 ± 1.79e | 1.49 ± 0.25f | 28.49 ± 1.75f | 12.49 ± 1.75e | |
| Cerebral cortex | 90.51 ± 5.25e | 3.21 ± 0.27e | 25.45 ± 0.19e | 1.54 ± 0.21f | 26.54 ± 1.11e | 10.54 ± 1.61e | ||
| T2DM+ MCP group |
Hippocampus | 74.65 ± 4.21eg | 2.27 ± 0.19eg | 17.56 ± 1.07fg | 2.74 ± 0.26eg | 33.11 ± 2.32eg | 17.23 ± 1.45eg | |
| Cerebral cortex | 70.43 ± 4.66eg | 2.65 ± 0.18fg | 20.34 ±1.75eg | 2.79 ± 0.35eh | 30.24 ± 1.95fg | 19.17 ± 0.95eh | ||
| MCP group |
Hippocampus | 48.54 ± 3.25 | 1.57 ± 0.15 | 5.65 ± 0.33 | 3.67 ± 0.29 | 53.74 ± 3.32 | 26.14 ± 1.55 | |
| Cerebral cortex | 48.54 ± 3.25 | 1.54 ± 0.09 | 5.49 ± 0.47 | 4.95 ± 0.56 | 56.32 ± 2.52 | 25.55 ± 1.79 | ||
Notes: aROS level, relative DCF fluorescence intensity; bMDA content, nmol/mg proteins; cProtein carbonyl content, nmol/mg proteins; dSOD, CAT and GSH-Px activities, U/mg proteins; eP<0.05, fP<0.01 versus control group; gP<0.05, hP<0.01versus T2DM model group.
Abbreviations: T2DM, type 2 diabetes mellitus; MCP, modified citrus pectin; HFD, high-fat diet; STZ, streptozotocin; ROS, reactive oxygen species; MDA, malondialdehyde; SOD, superoxide dismutase; CAT, catalase; GSH-Px, glutathione peroxidase.
Gal3 Inhibition Downregulates Proinflammatory Cytokine Levels in HFD/STZ-Induced T2DM Rats
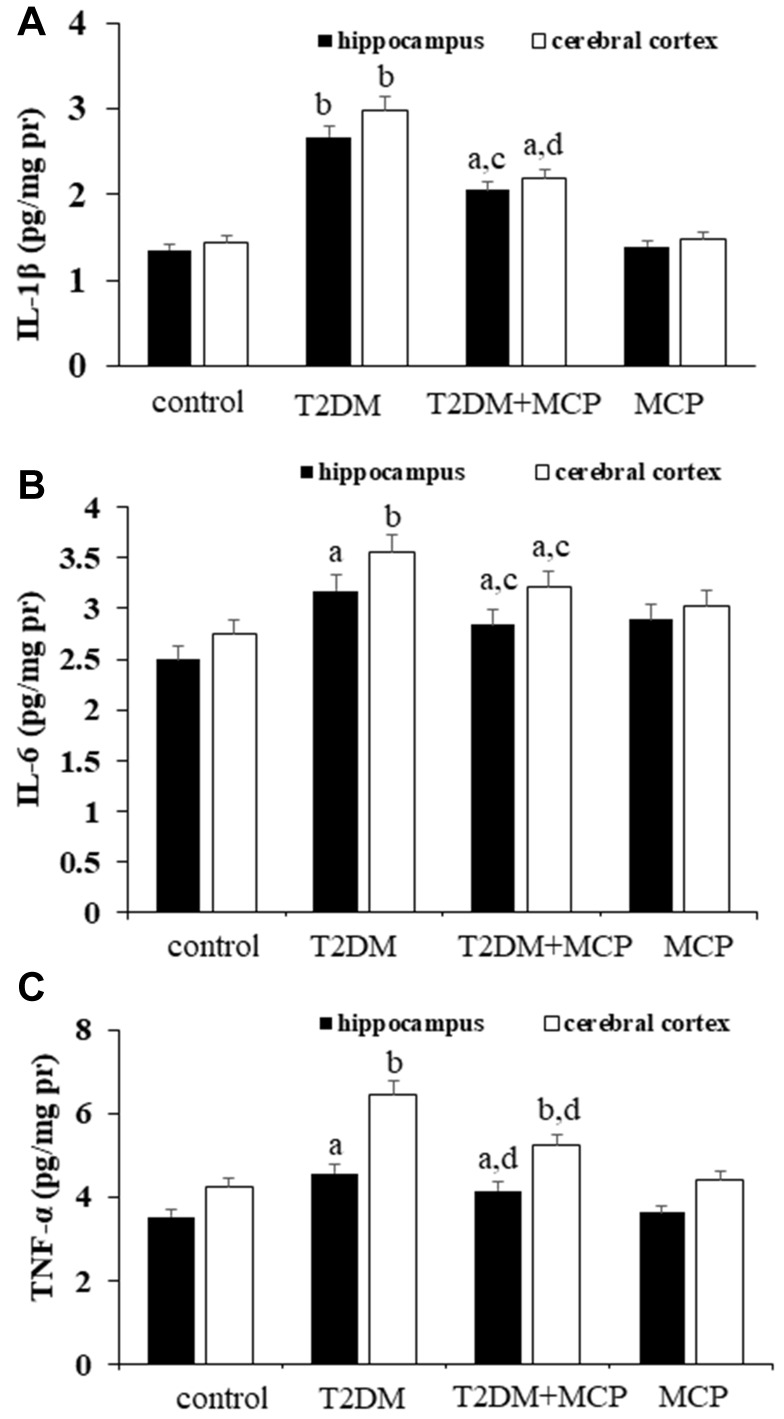
As seen from in Figure 3, significant increases in levels of IL-1β, IL-6 and TNF-α in both the hippocampus (P<0.01, P<0.05, and P<0.05, respectively) and cerebral cortex (P<0.01, P<0.01, and P<0.01, respectively) were observed in the T2DM group compared with the control group rats. The T2DM+MCP group rats showed decreases in levels of IL-1β, IL-6 and TNF-α in both hippocampus (P<0.05, P<0.05, P<0.01) and cerebral cortex (P<0.01, P<0.05, P<0.01) compared with the T2DM group rats. Compared with the control group, the T2DM+MCP rats were likely to show significant increases in IL-1β, IL-6 and TNF-α in hippocampus (P<0.05, P<0.05, and P<0.05, respectively) and cerebral cortex (P<0.05, P<0.05, and P<0.01, respectively).
Figure 3.
Galectin-3 (Gal3) inhibitor modified citrus pectin (MCP) attenuates neuroinflammation in T2DM rats.
Notes: MCP decreases the levels of IL-1β (A), IL-6 (B), and TNF-α (C) in the hippocampus and cerebral cortex in T2DM rats. aP<0.05, bP<0.01 compared to the control rats; cP<0.05, dP<0.01 compared to the T2DM rats.
Abbreviations: T2DM, type 2 diabetes mellitus; MCP, modified citrus pectin; IL-1β, interleukin-1β; IL-6, interleukin-6; TNF-α, tumor necrosis factor-alpha.
Gal3 Inhibition Alleviates High Glucose (HG)-Induced Damage to BV-2 Cells
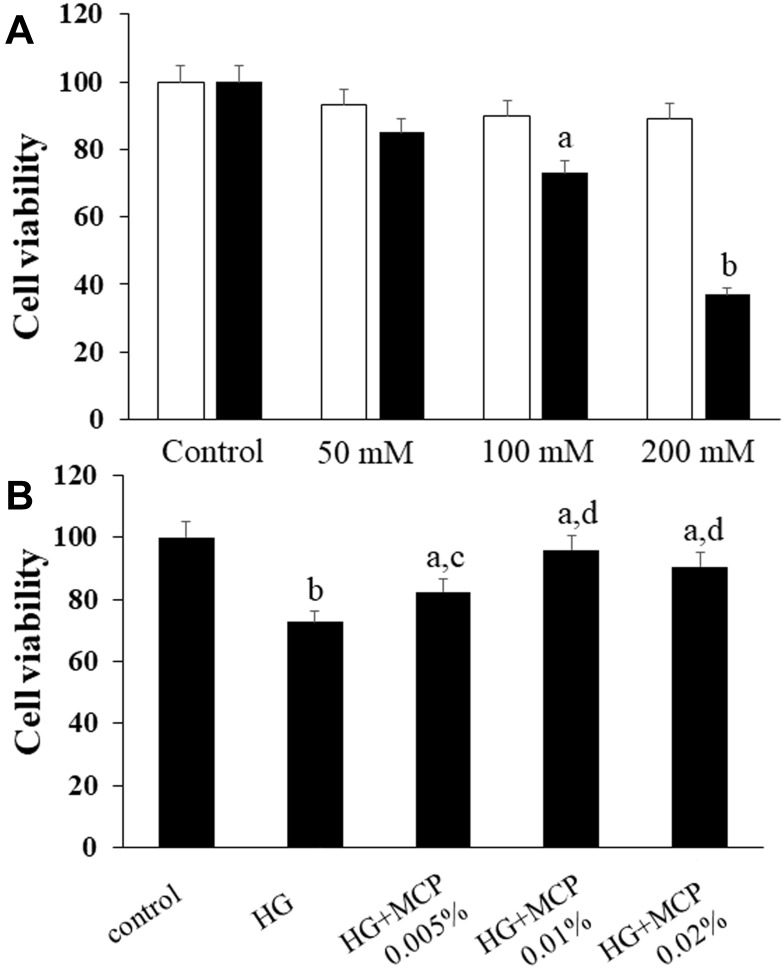
BV-2 cells were exposed to increasing concentrations of glucose (50, 100, or 200 mmol/L) for 48 h to establish the concentration of glucose that induced cell death, and mannitol was used as the isotonic control. A modified MTT assay was used to evaluate cell viability. As seen in Figure 4A, HG decreased the viability of BV-2 cells in a dose-dependent manner. We chose 100 mmol/L glucose treatment for subsequent experiments.
Figure 4.
Galectin-3 (Gal3) inhibitor modified citrus pectin (MCP) improves the viability of BV-2 microglial cells.
Notes: (A) The effects of different concentrations of glucose and mannitol on BV-2 cells. (B) Effects of the Gal3 inhibitor MCP on HG-stimulated BV-2 cells. The MTT assay was used to measure cell viability. The ratio of the OD value of each group to that of the control group was calculated as the cell viability. aP<0.05, bP<0.01 compared to the control cells; cP<0.05, dP<0.01 compared to the HG-stimulated cells.
Abbreviations: MCP, modified citrus pectin; HG, high glucose.
We administered gradient concentrations of MCP (0.005%, 0.01%, or 0.02%) to investigate the effects of MCP on HG-stimulated BV-2 cells. As seen in Figure 4B, MCP protected BV-2 cells from HG-induced cell death. However, the protective effects of MCP on BV-2 cells were not dose-dependent. It is possible that the drug concentration range of MCP needs to be further adjusted and further experiments should be conducted to exclude interfering factors. An MCP concentration of 0.01% was chosen for our subsequent study because it exerted the maximum effect.
Gal3 Inhibition Attenuates HG-Induced Cellular Oxidative Stress in BV-2 Cells
We found that 100 mmol/L glucose treatment induced cellular oxidative stress in BV-2 cells. As seen in Table 2, significant increases in the levels of ROS, contents of MDA and protein carbonyl (P<0.05, P<0.01, and P<0.05, respectively) were observed in the HG-treated group compared with the control group. Reduced levels of ROS, contents of MDA and protein carbonyl (P<0.01, P<0.05, and P<0.05, respectively) were observed in HG+MCP group cells compared with HG-stimulated cells. The HG+MCP group showed significant increases in levels of ROS, contents of MDA and protein carbonyl (P<0.01, P<0.05, and P<0.05, respectively) compared with the control group. The activities of SOD, CAT and GPx (P<0.05, P<0.01, and P<0.05, respectively) in HG-stimulated cells were significantly lower than those in control cells. The HG+MCP group cells showed significant increases in the activities of SOD, CAT and GPx (P<0.01, P<0.01, and P<0.05, respectively) compared with HG-stimulated cells. Compared to control cells, HG+MCP group cells showed significant decreases in the activities of SOD, CAT and GPx (P<0.01, P<0.05, and P<0.01, respectively).
Table 2.
Effects of Galectin-3 (Gal3) Inhibitor MCP on ROS Level, MDA and Protein Carbonyl Contents, and Antioxidant Enzymes Catalase in High Glucose Exposed BV-2 Microglial Cells
| Treatment | ROS Levela | MDA Contentb | Protein Carbonyl Contentc |
Antioxidant Enzyme Activity | ||
|---|---|---|---|---|---|---|
| SODd | CATd | GSH-Pxd | ||||
| Control group | 50.23 ± 2.48 | 0.94 ± 0.08 | 11.75 ± 0.75 | 22.56 ± 0.87 | 130.58 ± 3.78 | 48.23 ± 2.78 |
| HG group | 93.25 ± 6.11e | 1.33 ± 0.07f | 35.32 ± 0.39e | 14.41 ± 0.65e | 96.87 ± 4.13f | 31.41 ± 1.81e |
| HG+MCP group | 68.43 ± 3.11fh | 1.09 ± 0.08eg | 31.38 ± 0.74eg | 19.03 ± 1.05fg | 123.21 ± 5.32fg | 37.21 ± 0.75fh |
| MCP group | 51.78 ± 3.03 | 0.98 ± 0.09 | 12.98 ± 0.45 | 21.35 ± 0.87 | 132.32± 3.85 | 46.87 ± 0.98 |
Notes: aROS level, relative DCF fluorescence intensity; bMDA content, nmol/mg proteins; cProtein carbonyl content, pmol/mg proteins; dSOD, CAT and GSH-Px activities, U/mg proteins; eP<0.05, fP<0.01 versus control group; gP<0.05, hP<0.01 versus T2DM model group.
Abbreviations: HG, high glucose; MCP, modified citrus pectin; ROS, reactive oxygen species; MDA, malondialdehyde; SOD, superoxide dismutase; CAT, catalase; GSH-Px, glutathione peroxidase.
Gal3 Inhibition Attenuates Inflammation Induced by HG in BV-2 Cells
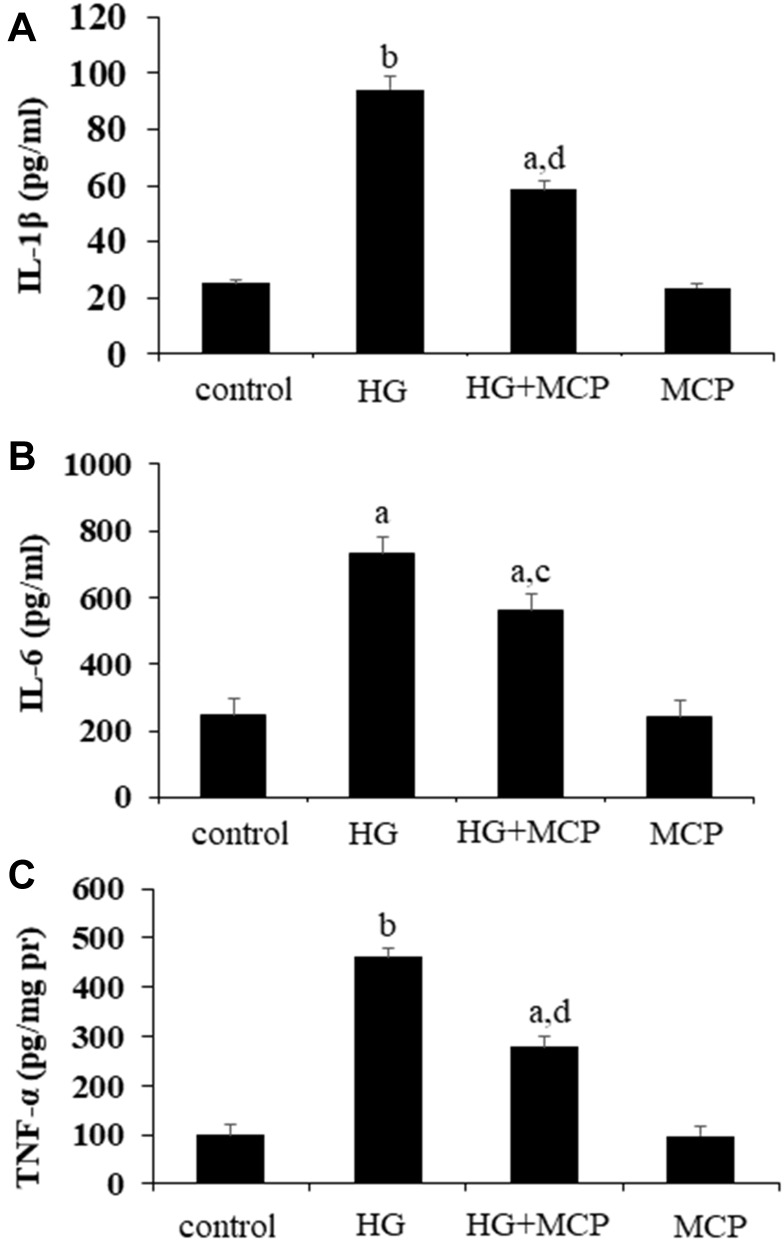
As seen in Figure 5, compared with the control group, the levels of inflammatory cytokines including IL-1β, IL-6 and TNF-α (P<0.01, P<0.05, and P<0.0, respectively) in cell supernatant were significantly increased in the HG-treated group. Reduced levels of IL-1β, IL-6 and TNF-α (P<0.01, P<0.05, and P<0.01, respectively) were observed in the cell supernatant of T2DM+MCP group compared with the T2DM group.
Figure 5.
Galectin-3 (Gal3) inhibitor modified citrus pectin (MCP) attenuates inflammation in high glucose (HG)-stimulated BV-2 cells.
Notes: The BV-2 cells were pretreated with 0.01% MCP for 24 h before being treated with 100 mM glucose for 24 h. The levels of IL-1β (A), IL-6 (B), and TNF-α (C) in the cell supernatant were measured. aP<0.05, bP<0.01 compared to the control cells; cP<0.05, dP<0.01 compared to the HG-stimulated cells.
Abbreviations: MCP, modified citrus pectin; IL-1β, interleukin-1β; IL-6, interleukin-6; TNF-α, tumor necrosis factor-alpha; HG, high glucose.
Discussion
Studies have recently demonstrated that MCP, a Gal3 inhibitor, improves renal remodeling in hyperaldosteronism,23 is beneficial in acute kidney injury24 and protects against hypertensive nephropathy.25 Moreover, MCP inhibits Gal3 function to reduce atherosclerotic lesions, which are common in T2DM patients.26 Several recent studies have shown a similar positive correlation between Gal3 and cognitive impairment,5,27,28 while the underlying mechanisms have yet to be clarified. In addition, no studies have shown an association between Gal3 and diabetes-associated cognitive impairment. Here, we report for the first time that Gal3 can modulate cognitive function in diabetes, as an inhibitor of Gal3 activity, MCP attenuated oxidative stress, neuroinflammation, and diabetes-associated cognitive impairment in vivo and in vitro.
Consistent with our previous study,15 our results also revealed that the Gal3 inhibitor MCP inhibited Gal3 upregulation and attenuated hyperglycemia, insulin resistance, and cognitive deficits in diabetic rats. Our present results showed that in vitro, MCP increased the viability of HG-stimulated BV-2 cells and participated in oxidative stress and neuroinflammation in both HFD/STZ-induced diabetic rats and HG-stimulated BV-2 cells. The hippocampus and temporal cortex are well known to play important roles in cognitive function.29 Our present study showed that MCP administration indeed rescued memory impairment in diabetic rats, and this rescuing effect of MCP on cognitive impairment was well correlated with its effect on oxidative stress and neuroinflammation.
Oxidative stress has been reported to be one of the key factors in the pathogenesis of diabetes-associated cognitive impairment. SOD is a major antioxidant enzyme that converts superoxide radicals to hydrogen peroxide, playing an essential role in maintaining redox equilibrium.30 CAT and GSH-Px can convert hydrogen peroxide to water, and both are primary antioxidant enzymes.31 When the antioxidant defense system is damaged by HFD/STZ, ROS are not sufficiently scavenged to regulate oxidative stress. Thus, lipid peroxidation is induced by oxidative stress, and the activities of SOD, CAT and GSH-Px are reduced. MDA, a marker of lipid peroxidation production, indirectly indicates the intensity of neuronal apoptosis induced by ROS, resulting in neurological behavior deficits.32 Gal3 has been reported as a new regulator of oxidative stress, and the Gal3 inhibitor MCP has been used to suppress oxidative stress.33 MCP can decrease ROS levels and increase antioxidant capacity in cardiac fibroblasts and cardiac lipotoxicity.16,33 Our in vivo studies showed that the activities of SOD, GSH-Px, and CAT were significantly decreased and that the levels of ROS, MDA, and carbonyl protein were significantly increased in the brains of HFD/STZ-induced diabetic rats compared to control rats. However, treatment with the Gal3 inhibitor MCP increased antioxidant enzyme activities and decreased the levels of oxidative stress markers. Our in vitro studies also indicated that MCP attenuated oxidative stress induced by HG in BV-2 cells. These results suggested that Gal-3 may be a new regulator of antioxidant defense and that MCP exerts antioxidant effects against diabetes-associated cognitive impairment.
Hyperglycemia is thought to induce neuronal apoptosis by activating microglial-mediated inflammatory responses and is thus involved in the pathogenesis of diabetes-associated cognitive impairment.34,35 IL-1β, IL-6 and TNF-α are major proinflammatory cytokines that regulate the inflammatory response. A recent study showed that HG induces the production of proinflammatory cytokines, including IL-1β and TNF-α, in BV-2 microglial cells.36 Experimental studies in models of multiple sclerosis, traumatic brain injury, stroke, and AD have revealed that Gal3 plays a crucial role in promoting inflammatory activation of microglia in the injured brain.4,37–39 It has been shown that the Gal3 inhibitor MCP can protect against hypertensive renal damage by inhibiting inflammation.40 Our in vivo study showed significant elevations of the levels of IL-1β, IL-6 and TNF-α in the hippocampus and cerebral cortex in HFD/STZ-induced diabetic rats and revealed that MCP treatment reversed these effects. Our in vitro studies also indicated that MCP attenuated the inflammatory response induced by HG in BV-2 cells, suggesting that the Gal3 inhibitor MCP blocks hyperglycemia-induced neuroinflammation.
Our in vivo study showed that the Gal3 inhibitor MCP attenuated cognitive impairment, improved insulin resistance, blocked Gal3, and reduced oxidative stress and neuroinflammation. In vitro, Gal3 inhibition by MCP increased the viability of HG-stimulated BV-2 cells and alleviated oxidative stress and the inflammatory response. We conclude that inhibition of Gal3 by MCP ameliorates diabetes-associated cognitive impairment via inhibition of oxidative stress and neuroinflammation, suggesting that Gal3 may be a novel key regulator of oxidative stress and neuroinflammation in diabetes-associated cognitive impairment. Our findings suggest that inhibition of Gal3 represents a new valuable approach for the management of diabetes-associated cognitive impairment.
In the present study, we provide the first evidence that Gal3 inhibitor MCP could improve insulin sensitivity, attenuate memory impairment, and inhibit oxidative stress and neuroinflammation in T2DM rats. However, the mechanisms by which Gal3 modulates diabetes-associated cognitive impairment are far from completely elucidated. We are continuing to investigate the downstream signaling pathways of Gal3 and the alterations that occur in the brains of Gal3−/- transgenic animals and BV-2 cells transfected with shRNA-Gal3. Further studies are warranted before Gal3 can be applied clinically.
Acknowledgments
This work was supported by Shandong Provincial Natural Science Foundation (grant no. ZR2019PH017), Jinan Municipal Science and Technology Project (grant no. 201805073), Project funded by China Postdoctoral Science Foundation (grant no. 2017M622217), and National Natural Science Foundation of China (grant no.81601018).
Data Sharing Statement
All the figures and tables adopted to support the findings of the present study are included in the article.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that there is no conflict of interest.
References
- 1.Rahimian R, Béland LC, Kriz J. Galectin-3: mediator of microglia responses in injured brain. Drug Discov Today. 2017;23:375–381. doi: 10.1016/j.drudis.2017.11.004 [DOI] [PubMed] [Google Scholar]
- 2.Mendonca HR, Carpi-Santos R, da Costa Calaza K, et al. Neuroinflammation and oxidative stress act in concert to promote neurodegeneration in the diabetic retina and optic nerve: galectin-3 participation. Neural Regen Res. 2020;15(4):625–635. doi: 10.4103/1673-5374.266910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dong R, Zhang M, Hu Q, et al. Galectin-3 as a novel biomarker for disease diagnosis and a target for therapy (Review). Int J Mol Med. 2018;41(2):599–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boza-Serrano A, Ruiz R, Sanchez-Varo R, et al. Galectin-3, a novel endogenous TREM2 ligand, detrimentally regulates inflammatory response in Alzheimer’s disease. Acta Neuropathol. 2019;138:251–273. doi: 10.1007/s00401-019-02013-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tao C, Cheng K, Ma Y, et al. Galectin-3 promotes Aβ oligomerization and Aβ toxicity in a mouse model of Alzheimer’s disease. Cell Death Differ. 2020;27(1):192–209. doi: 10.1038/s41418-019-0348-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simó R, Ciudin A, Simó-Servat O, Hernández C. Cognitive impairment and dementia: a new emerging complication of type 2 diabetes-The diabetologist’s perspective. Acta Diabetol. 2017;54:417–424. doi: 10.1007/s00592-017-0970-5 [DOI] [PubMed] [Google Scholar]
- 7.Biessels GJ, Despa F. Cognitive decline and dementia in diabetes mellitus: mechanisms and clinical implications. Nat Rev Endocrinol. 2018;14(10):591–604. doi: 10.1038/s41574-018-0048-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang LY, Tang SS, Wang XY, et al. PPARγ agonist pioglitazone reverses memory impairment and biochemical changes in a mouse model of type 2 diabetes mellitus. CNS Neurosci Ther. 2012;18(8):659–666. doi: 10.1111/j.1755-5949.2012.00341.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arnold SE, Arvanitakis Z, Macauley-Rambach SL. Brain insulin resistance in type 2 diabetes and Alzheimer disease: concepts and conundrums. Nat Rev Neurol. 2018;14(3):168–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Versari D, Daghini E, Virdis A, et al. Endothelial dysfunction as a target for prevention of cardiovascular disease. Diabetes Care. 2009;32(2):S314–S321. doi: 10.2337/dc09-S330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pugliese G, Iacobini C, Ricci C, et al. Galectin-3 in diabetic patients. Clin Chem Lab Med. 2014;52(10):1413–1423. doi: 10.1515/cclm-2014-0187 [DOI] [PubMed] [Google Scholar]
- 12.Atalar MN, Abuşoğlu S, Ünlü A, et al. Assessment of serum galectin-3, methylated arginine and Hs-CRP levels in type 2 diabetes and prediabetes. Life Sci. 2019;231:116577. doi: 10.1016/j.lfs.2019.116577 [DOI] [PubMed] [Google Scholar]
- 13.Li PP, Liu SN, Lu M, et al. Hematopoietic-derived Galectin-3 causes cellular and systemic insulin resistance. Cell. 2016;167:973–984. doi: 10.1016/j.cell.2016.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson AMF, Hou S, Li P. Inflammation and insulin resistance: new targets encourage new thinking: galectin-3 and LTB are pro-inflammatory molecules that can be targeted to restore insulin sensitivity. Bioessays. 2017;39(9):1700036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma S, Li S, Lv R, et al. Prevalence of mild cognitive impairment in type 2 diabetes mellitus is associated with serum galectin-3 level. J Diabetes Investig. 2020. doi: 10.1111/jdi.13256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao X, Zhi Y, Zhang T, et al. Analysis of the neutral polysaccharide fraction of MCP and its inhibitory activity on galectin‑3. Glycoconj J. 2012;29:159‑165. doi: 10.1007/s10719-012-9382-5 [DOI] [PubMed] [Google Scholar]
- 17.Marín-Royo G, Gallardo I, Martínez-Martínez E, et al. Inhibition of galectin-3 ameliorates the consequences of cardiac lipotoxicity in a rat model of diet-induced obesity. Dis Model Mech. 2018;11:2. doi: 10.1242/dmm.032086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma Z, Han Q, Wang X, et al. Galectin-3 inhibition is associated with neuropathic pain attenuation after peripheral nerve injury. PLoS One. 2016;11:e0148792. doi: 10.1371/journal.pone.0148792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nishikawa H, Liu L, Nakano F, et al. Modified citrus pectin prevents Blood-Brain Barrier disruption in mouse subarachnoid hemorrhage by inhibiting galectin-3. Stroke. 2018;49(11):2743–2751. doi: 10.1161/STROKEAHA.118.021757 [DOI] [PubMed] [Google Scholar]
- 20.Li H, Luo Y, Xu Y, et al. Meloxicam improves cognitive impairment of diabetic rats through COX2-PGE2-EPs-cAMP/pPKA Pathway. Mol Pharm. 2018;15(9):4121–4131. doi: 10.1021/acs.molpharmaceut.8b00532 [DOI] [PubMed] [Google Scholar]
- 21.Yin Q, Ma Y, Hong Y, et al. Lycopene attenuates insulin signaling deficits, oxidative stress, neuroinflammation, and cognitive impairment in fructose-drinking insulin resistant rats. Neuropharmacology. 2014;86:389–396. doi: 10.1016/j.neuropharm.2014.07.020 [DOI] [PubMed] [Google Scholar]
- 22.Yin Q, Dong C, Dong S, et al. AGEs induce cell death via oxidative and endoplasmic reticulum stresses in both human SH-SY5Y neuroblastoma cells and rat cortical neurons. Cell Mol Neurobiol. 2012;32:1299–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calvier L, Martinez-Martinez E, Miana M, et al. The impact of galectin-3 inhibition on aldosterone-induced cardiac and renal injuries. JACC Heart Fail. 2015;3(1):59–67. doi: 10.1016/j.jchf.2014.08.002 [DOI] [PubMed] [Google Scholar]
- 24.Kolatsi-Joannou M, Price KL, Winyard PJ, et al. Modified citrus pectin reduces galectin-3 expression and disease severity in experimental acute kidney injury. PLoS One. 2011;6(4):e18683. doi: 10.1371/journal.pone.0018683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frenay AR, Yu L, van der Velde AR, et al. Pharmacological inhibition of galectin-3 protects against hypertensive nephropathy. Am J Physiol Renal Physiol. 2015;308(5):F500–9. doi: 10.1152/ajprenal.00461.2014 [DOI] [PubMed] [Google Scholar]
- 26.Lu Y, Zhang M, Zhao P, et al. Modified citrus pectin inhibits galectin-3 function to reduce atherosclerotic lesions in apoE-deficient mice. Mol Med Rep. 2017;16(1):647–653. doi: 10.3892/mmr.2017.6646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trompet S, Jukema W, Mooijaart SP, et al. Genetic variation in galectin-3 gene associates with cognitive function at old age. Neurobiol Aging. 2012;33:2232. [DOI] [PubMed] [Google Scholar]
- 28.Boziki M, Polyzos SA, Deretzi G, et al. A potential impact of Helicobacter pylori-related galectin-3 in neurodegeneration. Neurochem Int. 2018;113:137–151. doi: 10.1016/j.neuint.2017.12.003 [DOI] [PubMed] [Google Scholar]
- 29.Yin Q, Pei -J-J, Xu S, et al. Pioglitazone improves cognitive function via increasing insulin sensitivity and strengthening antioxidant defense system in fructose-drinking insulin resistance rats. PLoS One. 2013;8(3):e59313. doi: 10.1371/journal.pone.0059313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nurdiana S, Goh YM, Hafandi A, et al. Improvement of spatial learning and memory, cortical gyrification patterns and brain oxidative stress markers in diabetic rats treated with Ficus deltoidea leaf extract and vitexin. J Tradit Complement Med. 2017;8(1):190–202. doi: 10.1016/j.jtcme.2017.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumar P, Kumar A. Prolonged pretreatment with carvedilol prevents 3-nitropropionic acid-induced behavioral alterations and oxidative stress in rats. Pharmacol Rep. 2008;60:706–715. [PubMed] [Google Scholar]
- 32.Jiao C, Gao F, Qu L, et al. Tetrahydroxy stilbene glycoside (TSG) antagonizes abeta-induced hippocampal neuron injury by suppressing mitochondrial dysfunction via Nrf2-dependent HO-1 pathway, Biomed. Pharmacother. 2017;96:222–228. doi: 10.1016/j.biopha.2017.09.134 [DOI] [PubMed] [Google Scholar]
- 33.Ibarrola J, Arrieta V, Sádaba R, et al. Galectin-3 down-regulates antioxidant peroxiredoxin-4 in human cardiac fibroblasts: a new pathway to induce cardiac damage. Clin Sci (Lond). 2018;132(13):1471–1485. doi: 10.1042/CS20171389 [DOI] [PubMed] [Google Scholar]
- 34.Sadeghi A, Hami J, Razavi S, et al. The effect of diabetes mellitus on apoptosis in hippocampus: cellular and molecular aspects. Int J Prev Med. 2016;7:57. doi: 10.4103/2008-7802.178531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pouvreau C, Dayre A, Butkowski EG, et al. Inflammation and oxidative stress markers in diabetes and hypertension. J Inflamm Res. 2018;11:61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang L, Tong Y, Chen PF, et al. Neuroprotection of dihydrotestosterone via suppression of the toll-like receptor 4/nuclear factor-kappa B signaling pathway in high glucose-induced BV-2 microglia inflammatory responses. Neuroreport. 2020;31(2):139–147. doi: 10.1097/WNR.0000000000001385 [DOI] [PubMed] [Google Scholar]
- 37.James RE, Hillis J, Adorjan I, et al. Loss of galectin-3 decreases the number of immune cells in the subventricular zone and restores proliferation in a viral model of multiple sclerosis. Glia. 2016;64:105–121. doi: 10.1002/glia.22906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burguillos MA, Svensson M, Schulte T, et al. Microglia-secreted galectin-3 acts as a toll-like receptor 4 ligand and contributes to microglial activation. Cell Rep. 2015;10(9):1626–1638. doi: 10.1016/j.celrep.2015.02.012 [DOI] [PubMed] [Google Scholar]
- 39.Yip PK, Carrillo-Jimenez A, King P, et al. Galectin-3 released in response to traumatic brain injury acts as an alarmin orchestrating brain immune response and promoting neurodegeneration. Sci Rep. 2017;7:41689. doi: 10.1038/srep41689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martínez-Martínez E, Ibarrola J, Fernández-Celis A, et al. Galectin-3 pharmacological inhibition attenuates early renal damage in spontaneously hypertensive rats. J Hypertens. 2018;36(2):368–376. doi: 10.1097/HJH.0000000000001545 [DOI] [PubMed] [Google Scholar]