Abstract
Objective:
We hypothesized the association of metabolic profile on cognition in postmenopausal women will be greater among ApoE4 carriers compared to non-carriers.
Methods:
Metabolic biomarkers and measures of global cognition, executive functions, and verbal memory, collected among postmenopausal females, were used in this analysis. Clustering analyses of metabolic biomarkers revealed three phenotypes: healthy, predominantly hypertensive, and poor metabolic with (borderline normal lab values). General linear models tested whether an association of metabolic cluster with cognition differed by ApoE4 genotype.
Results:
In the total sample of 497 women, verbal memory was lower in the poor metabolic cluster (p=0.04). Among ApoE4+ women, performance in all cognitive domains was lowest in the poor metabolic cluster. Differences in executive functions among metabolic clusters were detected only in ApoE4+ women (p-value for interaction=0.003).
Conclusions:
In a general population of postmenopausal women, association between poor metabolic profile with reduction in cognitive performance is more apparent in women who carry an ApoE4 allele. These data indicate a window of opportunity for interventions to reverse the trajectory of the preclinical phase of Alzheimer’s disease.
Keywords: Cognitive impairment, metabolic profile, biomarker, Alzheimer disease, menopause
INTRODUCTION
Postmenopausal women constitute over 60% of the affected AD population and carry the greatest burden of the disease1,2. While 11% of persons over the age of 65 are projected to develop AD, a challenge is to identify persons at greatest risk for AD when mitigation of risk to prevent AD is possible.
Metabolic and vascular factors have been consistently linked with cognitive decline and AD in both women and men3. Experimental and clinical research indicates that during the endocrine transition of perimenopause, the female brain undergoes a decline in glucose metabolism in brain leading to a bioenergetic crisis and activation of compensatory starvation pathways4,5. Emergence of decline in brain glucose metabolism and cognitive deficits can be early indicators of later life risk of AD4–8.
As an initial strategy to address the challenge of identifying persons at risk for AD, our previous work identified three metabolically distinct groups of postmenopausal women within a healthy cohort (specifically excluding individuals with cardiovascular disease or diabetes)9. Using a set of nine clinical metabolic biomarkers, we identified three metabolically distinct groups of postmenopausal women: metabolically healthy, high blood pressure (predominantly higher blood pressure levels compared to other two groups), and poor metabolic profile (borderline normal lab values)9. A cross-sectional analysis of these women indicated that postmenopausal women with a poor metabolic profile had significantly lower verbal memory compared to postmenopausal women with a healthy metabolic profile9. These findings in a cohort of healthy women are consistent with others that have shown that obese individuals with two or more metabolic abnormalities had faster cognitive decline compared to metabolically-normal participants who were not obese10.
The ApoE4 genotype is the most well-characterized genetic risk factor for cognitive impairment11–14. ApoE4 allele carriers have an earlier onset and faster progression of AD compared to non-carriers, suggesting a disproportionate acceleration of aging in ApoE4 allele carriers15. The accelerated aging process among ApoE4 carriers may be partially explained by the metabolic abnormalities associated with ApoE4 genotype. ApoE4 genotype has been consistently linked with metabolic risk factors, chronic metabolic diseases including atherosclerosis, and ischemic stroke16,17. Interestingly, ApoE4 genotype is reported to pose a greater risk for AD in women compared to men, such that ε4 heterozygote women have similar risk of AD as homozygote men2,11,18. While ApoE4 genotype is a known modifier of AD risk in women2, it is not known whether the association between poor metabolic profile and cognitive deficit in postmenopausal women is modified by ApoE4 genotype status.
To address the question of whether a poor metabolic profile and ApoE4 genotype are associated with cognitive decline, we conducted a clustering analysis using baseline data from the analyses of the women within the Early vs. Late Intervention Trial with Estradiol (ELITE) to identify metabolic phenotypes and the impact of these phenotypes on cognitive performance by ApoE4 genotype. We hypothesized that association of poor metabolic profile with cognition in postmenopausal women would be modified by ApoE4 genotype. Identification of at-risk populations during the preclinical phase, particularly among women, could contribute to effective prevention strategies for AD.
METHODS
ELITE trial design.
Methods and primary trial results from the ELITE trial have been detailed19,20. ELITE was a double-blind, placebo-controlled randomized clinical trial designed to test whether the effect of estrogen-containing hormone therapy on atherosclerosis progression and cognitive functions in postmenopausal women differed by time since menopause. Eligible women were postmenopausal, defined as absence of menses for at least 6 months or bilateral oophorectomy, with serum estradiol level below 25 pg/ml, and no clinical history of cardiovascular disease or diabetes. A total of 643 women were randomized into one of two strata: early menopause (within 6 years, n = 271) and late menopause (10 or more years post-menopause, n = 372). Randomized interventions were oral estradiol 1 mg daily, with (in women with a uterus) or without (in hysterectomized women) vaginal progesterone, or matching placebos.
The primary trial outcome was rate of change of distal common carotid artery far wall intima-media thickness20. A secondary outcome was change in cognitive function19. To measure cognitive function, a comprehensive battery of neuropsychological tests was administered at baseline prior to randomization, at about 2.5 years, and at each participant’s final study visit, approximately 5 years after randomization. The cognitive battery included 14 neuropsychological tests that emphasized standardized tests sensitive to age- and HT-associated change in middle-aged and older adults19. ELITE was approved by the Institutional Review Board of the University of Southern California. All participants provided written informed consent before trial-related procedures were conducted.
Clinical and laboratory measurements.
At the baseline clinic visit, 8-hour fasting blood was drawn and blood pressure was measured. Concomitant medications were recorded. Fasting glucose, β-hydroxybutyrate, insulin, total cholesterol, triglycerides, HDL- and LDL-cholesterol, and hemoglobin A1c (HbA1c) levels were measured as previously described9. The homeostatic model assessment (HOMA); a measure of insulin resistance was measured as: [glucose mmol/L*insulin]/22.5. Three isoforms (ε2, ε3, and ε4) of the apolipoprotein E gene were determined according to two nonsynonymous SNPs (rs429358 and rs7412)21 encoding arginine for cysteine amino acid variants at codon positions 112 and 158, respectively (TaqMan Assay-on-Demand Genotyping Service; Applied Biosystems)22. Thirty one percent of the participants were ApoE4 carriers, of which 13% were homozygous for the E4 allele.
Identification of metabolic risk phenotypes.
Nine biomarkers were used to determine metabolic risk phenotypes within the ELITE population: glucose, the HOMA score, ketones, triglycerides, HDL-cholesterol, LDL-cholesterol, HbA1c, and systolic and diastolic blood pressure9. As the clustering analysis was part of a larger longitudinal study, the full sample of 643 ELITE women was restricted to those completing cognitive testing at baseline and having complete biomarker data available for clustering (n = 502); the present analysis was further restricted to women with ApoE genotype data (n=497). A K-means clustering algorithm was used to identify three clusters that were descriptively identified based on their biomarker profile: Healthy Metabolic (n=208, 41.9%), High Blood Pressure (n=190, 38.2%), and Poor Metabolic (n=99, 19.9%) (Table 1). The majority of the mean metabolic biomarkers were within a normal range, consistent with recruitment of a healthy population of postmenopausal women in the study. Metabolic indicator means in the poor metabolic group were at the margins of clinically healthy values.
Table 1.
Baseline Characteristics of Participants by Cluster
| Cluster | |||||||
|---|---|---|---|---|---|---|---|
| Characteristic, N (%) | Healthy metabolic | High Blood Pressure | Poor Metabolic | P-valuea | |||
| Number of participants | 208 (41.9%) | 190 (38.2%) | 99 (19.9%) | - | |||
| Age, years (mean±SD) | 60.1 ± 7.3 | 60.9 ± 6.8 | 61.2 ± 6.6 | 0.30 | |||
| Time Since Menopause, years (mean±SD) | 9.9 ± 7.4 | 10.5 ± 7.7 | 11.5 ± 8.6 | 0.27 | |||
| Education, years (mean±SD) | 16.3 ± 2.2 | 16.2 ± 2.1 | 15.7 ± 2.2 | 0.06 | |||
| Genotype | |||||||
| ApoE4+ | 61 (29.3%) | 61 (32.1%) | 32 (32.3%) | 0.79 | |||
| ApoE4− | 147 (70.7%) | 129 (67.9%) | 67 (67.7%) | ||||
| Menopause Cohort | |||||||
| Early menopause | 94 (45.2%) | 81 (42.6%) | 39 (39.4%) | 0.62 | |||
| Late menopause | 114 (54.8%) | 109 (57.4%) | 60 (60.6%) | ||||
| Race or Ethnicity | % within cluster | % of racial group | % within cluster | % of racial group | % within cluster | % of racial group | |
| White, non-Hispanic | 157 (75.5%) | (44.4%) | 140 (73.7%) | (39.6%) | 57 (57.6%) | (16.1%) | |
| Black | 13 (6.3%) | (33.3%) | 17 (9.0%) | (43.6%) | 9 (9.1%) | (23.1%) | 0.002 |
| Hispanic | 17 (8.2%) | (27.4%) | 21 (11.1%) | (33.9%) | 24 (24.2%) | (38.7%) | |
| Asian | 21 (10.1%) | (50.5%) | 12 (6.3%) | (28.6%) | 9 (9.1%) | (21.4%) | |
| Biomarkers, (mean±SD) | |||||||
| Glucose (mg/dL) | 80.65 ± 7.56 | 80.25 ± 7.46 | 91.45 ± 9.67bc | <0.001 | |||
| Insulin Resistance (HOMA Score) | 0.98 ± 0.48 | 1.16 ± 0.46b | 2.63 ± 1.12bc | <0.001 | |||
| Ketones (mM) | 0.12 ± 0.06 | 0.10 ± 0.03b | 0.10 ± 0.04b | <0.001 | |||
| HDL cholesterol (mg/dL) | 75.05 ± 17.88 | 65.38 ± 15.71b | 52.16 ± 10.89bc | <0.001 | |||
| LDL cholesterol (mg/dL) | 130.08 ± 29.65 | 136.83 ± 28.92 | 144.10 ± 33.49b | <0.001 | |||
| Triglycerides (mg/dL) | 80.48 ± 27.04 | 97.47 ± 33.38b | 166.16 ± 66.07bc | <0.001 | |||
| HbA1c (%) | 5.59 ± 0.37 | 5.51 ± 0.40 | 5.79 ± 0.45bc | <0.001 | |||
| Systolic blood pressure (mmHg) | 105.89 ± 8.93 | 125.28 ± 10.29b | 121.37 ± 10.68bc | <0.001 | |||
| Diastolic blood pressure (mmHg) | 67.98 ± 5.53 | 80.81 ± 5.80b | 76.26 ± 7.78bc | <0.001 | |||
Abbreviations: SD = standard deviation
P-values comparing the three cluster phenotypes were obtained using ANOVA for continuous variables and χ2 test for discrete variables. The Tukey- Kramer method was used to adjust for multiple comparisons.
Average value is significantly different from the Healthy phenotype (p < 0.05).
Average value is significantly different from the High Blood Pressure phenotype (p < 0.05).
Cognitive composite scores.
Three cognitive composite scores (global cognition, executive functions, and verbal memory) were generated from the 14-item test battery. Composite scores were a linear sum of the standardized test scores within each domain, with each standard test score inversely weighted by its correlation with other contributing cognitive tests23. The verbal memory composite score was defined a priori by Word List Free Recall (a short version of the California Verbal Learning Test II) immediate and delayed recall, and Paragraph Recall (East Boston Memory Test) immediate and delayed recall23. Tests included in the executive functions composite score were Symbol Digit Modalities Test, Trail Making Test part B, Shipley Abstraction Scale, Letter-Number Sequencing, and category fluency (Animal Naming). These tests were determined by a principal components analysis of baseline scores23. The composite score for global cognition was similarly calculated as a weighted average of all tests in the battery, including Judgment of Line Orientation, Block Design, Visual Memory (Faces I, immediate recall and Faces II, delayed recall), and Boston Naming Test, in addition to the aforementioned tests.
Statistical analysis.
The present analysis was confined to baseline cognitive performance assessed prior to randomization. Baseline characteristics were compared between clusters using analysis of variance for continuous variables and chi-square tests for categorical variables. ApoE genotype was classified as either ApoE4+ (E2/E4, E3/E4, E4/E4) or ApoE4- (E2/E2, E2/E3, E3/E3). General linear models were used to test whether an association of metabolic cluster with baseline cognitive performance differed by ApoE4 genotype (i.e., testing a cluster by ApoE4 genotype interaction); dependent variables were the three cognitive composite scores. ApoE4 interactions were also evaluated for individual cognitive tests; interaction p-values were adjusted for false discovery rate (FDR). All models were adjusted for menopausal cohort (within 6 years vs more than 10 years since menopause) and years of formal education. Additional models tested the confounding effects of chronological age and BMI by comparing the results between models adjusted and not adjusted for age and BMI. A sensitivity analysis was performed additionally adjusting all models for LDL- cholesterol (in light of a significant ApoE4-cluster interaction on LDL-cholesterol). P-values for pairwise comparisons among metabolic clusters were adjusted for multiple comparisons using the Tukey-Kramer method and significance was set at 0.05. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Inc., Cary, NC).
RESULTS
Study Sample.
Baseline demographic and clinical characteristics of the study population are presented by metabolic clusters in Table 1. A total of 497 postmenopausal women from the ELITE trial with available phenotype and genotype data contributed to the analysis. Women were on average (±SD) 60.6±7.0 years old, had 16.2±2.2 years of education, and were postmenopausal for an average duration of 10.4±7.7 years. Fifty-seven percent of the women were in late (more than 10 years) menopause. ApoE4 genotype distributions were comparable among the three clusters (p=0.79). The distribution of race/ethnicity significantly differed (p=0.002); a larger proportion of Hispanic participants were evident in the poor metabolic cluster (24.2%). Reflecting the clustering algorithm, the three clusters highly significantly differed on all of the nine metabolic biomarkers (p<0.001). Comparing the metabolic markers by ApoE4 genotype and cluster (see Table, Supplement Digital Content 1, which describes the metabolic biomarkers in by cluster and ApoE4 genotype), cluster differences in mean biomarker levels were in general equally evident in ApoE4+ and ApoE4- women (p-values for ApoE4-cluster interaction >0.05). Two exceptions were LDL-cholesterol and diastolic blood pressure. LDL-cholesterol significantly differed between clusters in ApoE4+ women only (p-value between clusters <0.001). ApoE4+ women in the poor metabolic phenotype exhibited a higher level of LDL-compared to both the high blood pressure (p=0.011) and healthy phenotype (p<0.001); differences in LDL-cholesterol among clusters were not observed for ApoE4- women (p-value for ApoE4-cluster interaction = 0.006). While the between cluster differences in DBP were similar for ApoE4+ and ApoE4- women, the ApoE4-cluster interaction remained statistically significant (p-value for interaction=0.03; Supplemental Digital Content 1).
Cognitive composite scores: main effects of metabolic clusters.
Cognitive composite score comparisons between clusters at baseline were adjusted for postmenopause cohort and years of education (Figure 1). As previously reported9, mean performance on all cognitive domains was lowest in the poor metabolic cluster. Metabolic groups did not significantly differ on global cognition (p=0.09; Figure 1a) or executive functions (p=0.11; Figure 1c). However, the verbal memory composite score significantly differed between clusters (p=0.04; Figure 1b), with pairwise comparisons indicating a significant difference in verbal memory between women in the healthy and poor metabolic clusters (p=0.03).
Figure 1. (Panels A, B, & C): Baseline Cognitive Composite Scores by Metabolic Phenotypes.
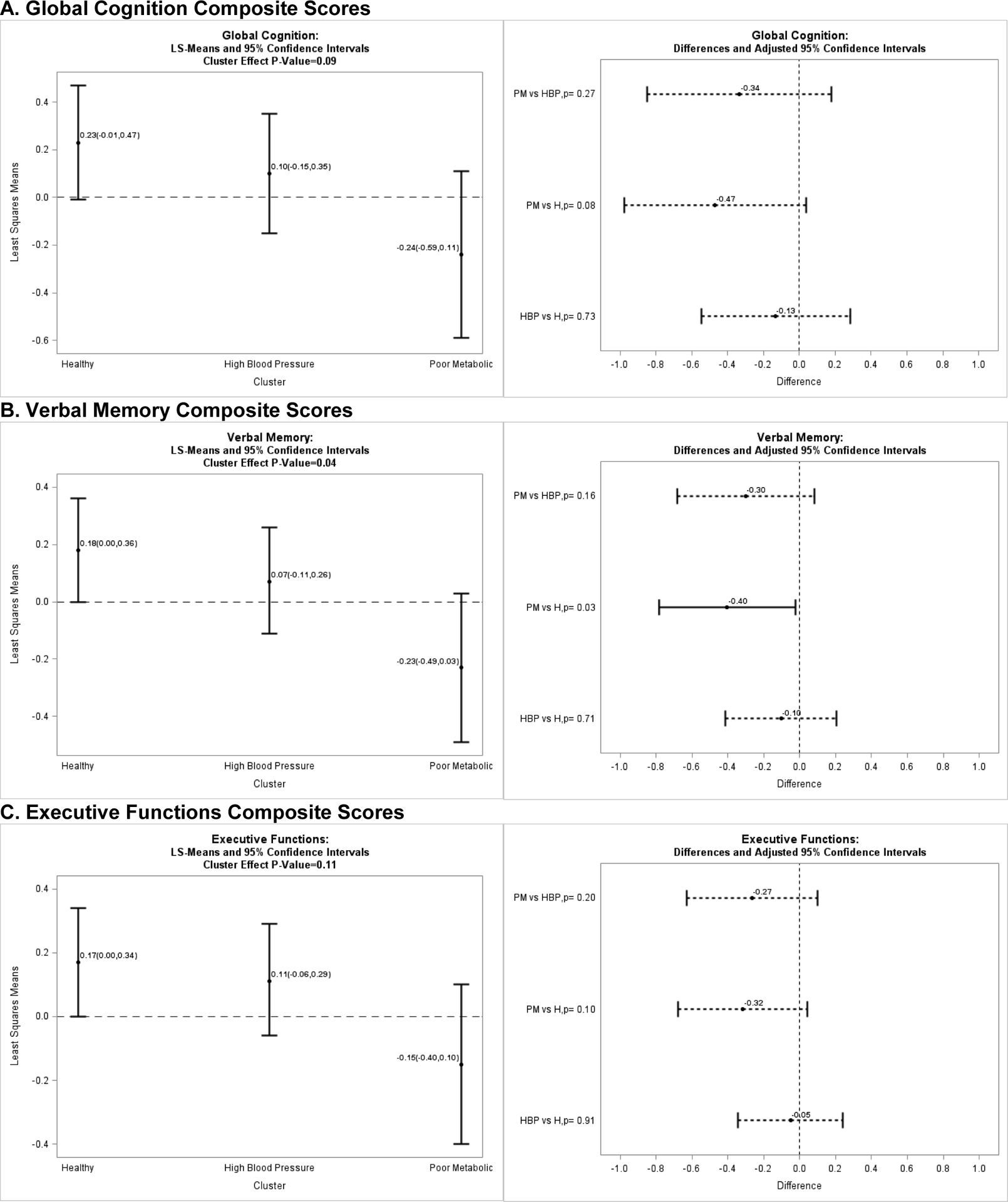
A. Global Cognition Composite Scores B. Verbal Memory Composite Scores C. Executive Functions Composite Scores
Cognitive composite scores: main effects of ApoE genotype.
Comparisons of cognitive composite score between genotypes at baseline were adjusted for postmenopause cohort and years of education (Figure 2). Mean performance for all three composite scores was lower for women who were ApoE4+ (global cognition composite p=0.03, Figure 2a; executive functions composite p=0.05, Figure 2c; verbal memory composite p=0.12, Figure 2b).
Figure 2. (Panels A, B, & C): Cognitive Composite Scores by ApoE4 Genotype.
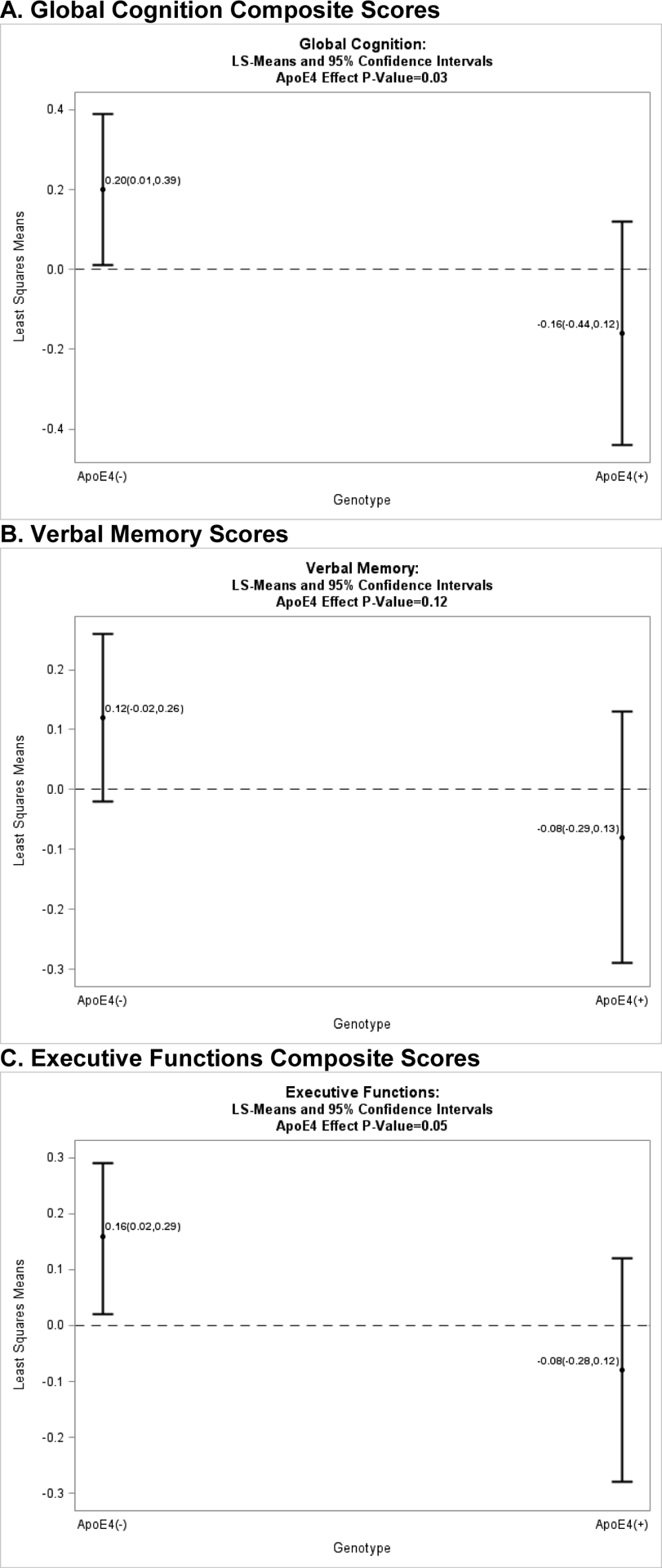
A. Global Cognition Composite Scores B. Verbal Memory Scores C. Executive Functions Composite Scores
Interaction between APOE4 genotype and metabolic clusters: cognitive composite scores.
The addition of a cluster-by-ApoE4 genotype interaction term to the regression model showed that cluster differences in executive functions significantly varied by ApoE genotype (interaction p=0.003; Table 2). The interaction was of borderline significance for global cognition (interaction p=0.055). Cluster differences on verbal memory did not vary by ApoE genotype (interaction p=0.21).
Table 2.
Composite cognitive scores by metabolic phenotypes and ApoE4 genotype
| Cluster | Pairwise p-value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Outcome | Genotype | (1) Healthy | (2) High Blood Pressure | (3) Poor Metabolic | P-value | (1) vs. (2) | (1) vs. (3) | (2) vs. (3) | ApoE4 Main Effect P-value | Interaction P-value |
| Executive Functions |
ApoE4− ApoE4+ |
0.29(0.10) −0.13(0.16) |
0.034 (0.11) 0.28(0.16) |
0.11(0.15) −0.68(0.22) |
0.22 0.002 |
0.53 0.44 |
0.92 0.31 |
1.00 0.005 |
0.012 | 0.003 |
| Global Cognition |
ApoE4− ApoE4+ |
0.37(0.14) −0.098 (0.23) |
0.068(0.15) 0.17(0.22) |
0.086(0.21) −0.91(0.31) |
0.30 0.019 |
0.71 0.96 |
0.88 0.28 |
1.00 0.058 |
0.011 | 0.055 |
| Verbal Memory |
ApoE4− ApoE4+ |
0.20(0.11) 0.13(0.17) |
0.098(0.12) 0.019(0.17) |
−0.022(0.16) −0.65(0.23) |
0.51 0.019 |
0.99 1.00 |
0.87 0.074 |
0.99 0.17 |
0.051 | 0.21 |
Data represent the least-square mean (SE) for each composite cognitive outcome. All models were adjusted for postmenopause cohort and years of education. The Tukey-Kramer method was used to adjust for multiple comparisons.
Adjusting for education and menopause cohort and correcting for multiple comparisons, differences by cluster were evident for executive function in ApoE4+ women (p=0.002), but not for ApoE4- women (p=0.22).
Among ApoE4+ women, pairwise comparisons revealed significantly lower performance on executive functions in poor metabolic compared to the high blood pressure cluster; mean (SE) executive function 0.28 (0.16) vs −0.68 (0.22) in high blood pressure vs poor metabolic cluster (p-value = 0.005, Table 2). Similarly lower performances were observed in ApoE4+ women in the high blood pressure and poor metabolic clusters for global cognition (mean (SE) 0.17 (0.22) vs −0.91 (0.31) in high blood pressure vs poor metabolic cluster (p-value = 0.058), and between women in the healthy phenotypes and poor metabolic for verbal memory (mean (SE) 0.13 (0.17) vs −0.65 (0.23) in healthy vs poor metabolic cluster (p-value = 0.074).
Similar results emerged when composite cognitive scores in the combined healthy and high blood pressure phenotypes were contrasted with the poor metabolic phenotype within each ApoE4 genotype. Among E4 carriers, women characterized by a poor metabolic phenotype performed significantly lower on all 3 composite scores compared to women in the combined healthy and high blood pressure clusters (p=0.007, p=0.006, and p=0.002 for global cognition, verbal memory, and executive functions, respectively). Among ApoE4- women, no such differences were observed (p=0.58, p=0.34, and p=0.74 for global cognition, verbal memory, and executive functions, respectively; results not shown). Further adjusting the analysis for age and BMI showed very similar results (results not shown).
Interaction between APOE4 genotype and metabolic clusters: individual cognitive test scores.
Interactions between the metabolic clusters and ApoE4 genotype were tested for each of the individual cognitive tests of the cognitive composite scores (significant interaction results shown in a table of Supplemental Digital Content 2, that describes individual cognitive test scores by metabolic phenotype and ApoE4 genotype ). After adjusting for false discovery rate (FDR) over all individual tests, two of the five interaction tests within the executive functions composite score showed a statistically significant interaction of the ApoE4 gene with the metabolic phenotype: the Shipley Institute of Living Abstraction scale (FDR-adjusted p-value for interaction=0.04) and category fluency (FDR-adjusted p-value for interaction=0.04), while the Trail Making test showed a marginally significant interaction (FDR-adjusted p=0.09). Of note, among ApoE4+ women, adjusted means differed by cluster on Trail Making (p=0.018), Shipley Abstraction scale (p=0.002), and Category Fluency (p=0.015). In each of these tests, the average performance of ApoE4+ women in the poor metabolic cluster was lowest among the three clusters. Adjusted means for these cognitive tests did not differ among ApoE4- women.
Within the verbal memory cognitive composite score, a statistically significant interaction between ApoE4 genotype and the delayed paragraph recall test was evident (FDR-adjusted p=0.04) while a trend for significance was noted for the immediate recall test (FDR-adjusted p=0.09). In pairwise comparisons among ApoE4+ women, women in the poor metabolic cluster performed more poorly on immediate paragraph recall than women in the healthy cluster (p=0.003); women in the poor metabolic cluster performed more poorly on the delayed paragraph recall compared to both the high blood pressure cluster (p=0.043) and the healthy cluster (p=0.01). In ApoE4- women, paragraph recall performance did not differ across clusters.
A sensitivity analysis additionally adjusting for LDL-cholesterol did not alter the results for cluster differences by composite outcomes (see table, Supplemental Digital Content 3, that describes composite cognitive scores by metabolic phenotypes and ApoE4 genotype: Adjustment for LDL cholesterol). LDL-adjustment did however push significant cluster by individual cognitive test score interactions to a marginal level of significance (see table, Supplemental Digital Content 4, that describes individual cognitive test scores by metabolic phenotype and ApoE4 genotype: Adjustment for LDL cholesterol).
DISCUSSION
In the current analysis, we incorporated ApoE genotype status to further define a metabolically at-risk group of postmenopausal women with relatively lower cognitive performance. Our analyses using baseline data from the ELITE trial indicated that women with a poor metabolic phenotype were characterized by metabolic biomarkers at the borderline of normal had significantly lower performance on executive functions compared to the healthy and high blood pressure phenotypes if they carried at least one ApoE4 allele (metabolic phenotype x ApoE4 genotype interaction p-value = 0.003; Table 2). Global weighted cognition and verbal memory were also significantly lower among ApoE4 allele carriers with poor metabolic phenotype compared to the healthy and high blood pressure phenotypes carrying the ApoE4 allele. It is noteworthy that ApoE4+ women within the poor metabolic phenotype had the worst global cognition and verbal memory compared to ApoE4+ women in the high blood pressure and healthy metabolic phenotypes (p-value for both composite scores = 0.019; Table 2). Collectively, these data provide evidence for a specific interaction between the ApoE4 genotype and metabolic function that drives adverse impact on specific domains of cognition. Further, these data suggest that the adverse impact of the ApoE4 genotype is dependent upon metabolic dysregulation, as ApoE4+ women within the metabolically healthy cluster performed significantly better on cognitive functions compared to ApoE4+ within the poor metabolic cluster.
Vascular and metabolic risk factors have been extensively investigated in relation to dementia, cognitive decline, and AD. High blood pressure, total cholesterol and other lipid parameters, diabetes and insulin resistance, body mass index and obesity have been linked to dementia and cognition24–26. Metabolic syndrome, a constellation of metabolic factors, has also been linked to age-related cognitive decline, mild cognitive impairment, and vascular dementia27,28. We used a metabolic clustering approach in this sample of health postmenopausal women using a set of nine clinically accessible biomarkers to identify metabolically-distinct groups of women9, with one cluster characterized with a relatively worse metabolic profile9. Women in this poor metabolic group had significantly lower verbal memory performance compared to the other (healthy and high blood pressure) profiles9. Our current analyses extend these findings to demonstrate an association of poor metabolic profile with lower cognitive functions that is specific to women carrying at least one ApoE4 allele.
Detecting at-risk individuals within a healthy population is critical for preventing or delaying Alzheimer’s disease. Postmenopausal women constitute more than 60% of the affected Alzheimer population, and thus it is of immense public health importance to establish successful screening tools in order to identify healthy postmenopausal women at risk for cognitive impairment and AD.
ApoE4 genotype is one of the strongest risk factors for cognitive impairment11,12, and Alzheimer’s disease (AD)13,14, in addition to increasing age29, female sex29, and metabolic abnormalities27. ApoE4 allele carriers have an earlier onset of AD compared to non-carriers, suggesting a disproportionate acceleration of aging in ApoE4 allele carriers15. The accelerated aging process among ApoE4 carriers may be partially explained by the metabolic abnormalities associated with the ApoE4 genotype30. In the majority of studies that have evaluated the predictive value of metabolic risk factors for cognitive outcomes, ApoE4 carrier status has, in general, only been considered as a covariate, and not as a modifier of metabolic associations. Using these baseline data from the ELITE trial, we show that associations of poor metabolic profiles with reduced cognitive performance are evident only in healthy postmenopausal women who are ApoE4+.
A strong body of evidence indicate an interaction between female sex and ApoE genotype for AD risk11,31, such that ApoE4 confers greater risk for AD in women compared to men11,31–33. The effect of ApoE4 genotype on AD biomarkers including tau levels are also reported to be higher among women compared to men11. Female ApoE4 carriers had significantly greater brain hypometabolism and cortical thinning34, and increased vulnerability of connection between hippocampus and precuneus/posterior cingulate cortex compared to male carriers35.
Only a limited number of studies have investigated the interaction between ApoE4 genotype and metabolic risk factors for their joint effect on cognitive function. Our findings are consistent with a recent study on a total of 1800 participants from two independent cohorts reporting a significant interaction between ApoE4 genotype and hypercholesterolemia on cognitive function36. Perna et al. showed that the negative effects of ApoE4 genotype on cognitive function was significantly amplified by the presence of hypercholesterolemia and CVD; consequently, hypercholesterolemia was negatively associated with cognitive function only among ApoE4 carriers36. In another study cholesterol homeostasis was significantly negatively associated with cognitive decline only among the ApoE4 carriers37. Contrasting our study with Perna et al. study, we considered a total of 9 metabolic markers to define a comprehensive metabolic profile since perturbation in multiple metabolic factors tend to coexist; instead of total cholesterol we evaluated LDL- and HDL-cholesterol levels specifically. Our data extend the results reported by Perna et al. in addition to validating and confirming their results. It is also noteworthy that when we evaluated the individual metabolic factors, high LDL-cholesterol emerged as the defining characteristic differentiating the ApoE4 carriers and non-carriers among women with a poor metabolic profile (Supplemental Digital Content 1). Therefore, it is possible that LDL-cholesterol is a key metabolic factor contributing to reduced cognition among ApoE4 carriers. Interestingly, in a sensitivity analysis adjusting for LDL-cholesterol, our primary findings of ApoE4-metabolic cluster interactions on executive functions composite scores remained (Supplemental Digital Content 3), suggesting that some ApoE4 effect other than LDL-cholesterol was responsible for our findings. Further research is warranted to investigate the mechanisms for the differential associations reported here.
In addition to the composite cognitive measures used in our primary analyses, we also evaluated the interaction between ApoE4 genotype and metabolic cluster for individual cognitive tests. Notably, our results demonstrating poorer composite executive functions among women with a poor metabolic profile who carried the ApoE4 allele was validated by two of five individual tests, namely Shipley Abstraction Scale and Category Fluency (Animal Naming) (Supplemental Digital Content 2). It is also interesting to note that, although the interaction between ApoE4 genotype and the composite verbal memory summary score was not statistically significant, the interaction was significant for the delayed paragraph recall test.
While the accelerated cognitive aging process among ApoE4 carriers can be presumably explained by the metabolic abnormalities inflicted by the ApoE4 genotype, the distribution of ApoE4 genotype did not differ among metabolic clusters in this sample of healthy postmenopausal women (p = 0.79; Table 1). The impact of ApoE4 genotype is not consistent across metabolic factors; the ApoE gene regulates the clearance of lipoproteins and consequently lipid profile levels. ApoE was not associated with other metabolic factors including glucose, insulin, HbA1c, and systolic blood pressure in a pooled analysis among 60,883 individuals16.
The primary strength of the study is the considerably large sample size of 497 participants that reduces the probability of chance findings. Our analytic approach also allowed us to use metabolic profiles incorporating multiple key metabolic factors as an indicator instead of considering them individually. Finally, inclusion of only clinically healthy mid- to late-age postmenopausal women adds to the strength of this study. Demonstrating an association of relatively poorer metabolic profile with reduced cognitive performance in healthy ApoE4+ postmenopausal women, these results have significant prevention potential and public health implications. Using ApoE4 genotype and metabolic profile, as our data suggest, may define a population at potentially greater risk for cognitive decline and thus emphasis for development of intervention and prevention strategies. Identification and implementation of effective prevention strategies in such at-risk populations can reduce the burden of cognitive impairment and AD in women.
A limitation of the study is that our population was predominantly white (71%), therefore, these results may not be generalizable to other racial/ethnic groups. Despite the fact that a larger proportion of Hispanic women belonged to the poor metabolic cluster in our data, we were not able to stratify our analysis by racial/ethnic groups due to limited number of Hispanic women. Of note, as verbal memory tests are dependent upon English language proficiency, Hispanic ethnicity might confound the contribution of metabolic factors on cognitive performance. These results should be repeated in larger sample along with sufficient representation of all ethnic groups as interactions that were not significant in our study might be due to lack of enough power. Finally, the longitudinal effect of the interaction between ApoE4 genotype and metabolic phenotype on changes in cognitive skills should also be evaluated in future studies.
CONCLUSIONS
In this sample of healthy postmenopausal women, associations of poor metabolic profiles with reduced cognitive performance were particularly apparent in women who carried an ApoE4 allele. Further research should corroborate and extend these findings, and evaluate possible mechanisms. Such research may ultimately suggest targeted preventive measures based on a combination of ApoE4 genotyping and poor metabolic profiles.
Supplementary Material
Supplemental Digital Content 1: Metabolic biomarkers in by cluster and ApoE4 genotype
Supplemental Digital Content 2: Individual cognitive test scores by metabolic phenotype and ApoE4 genotype
Supplemental Digital Content 3: Composite cognitive scores by metabolic phenotypes and ApoE4 genotype: Adjustment for LDL cholesterol
Supplemental Digital Content 4: Individual cognitive test scores by metabolic phenotype and ApoE4 genotype: Adjustment for LDL cholesterol
Funding Source:
This work was supported by the National Institute of Health (grant numbers: P01AG026572, R01AG024154).
Footnotes
Financial disclosures/conflicts of interest: None reported.
REFERENCES:
- 1.Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM. Forecasting the global burden of Alzheimer’s disease. Alzheimers Dement. 2007;3(3):186–191. [DOI] [PubMed] [Google Scholar]
- 2.Riedel BC, Thompson PM, Brinton RD. Age, APOE and sex: Triad of risk of Alzheimer’s disease. J Steroid Biochem Mol Biol. 2016;160:134–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Panza F, Solfrizzi V, Logroscino G, et al. Current epidemiological approaches to the metabolic-cognitive syndrome. J Alzheimers Dis. 2012;30 Suppl 2:S31–75. [DOI] [PubMed] [Google Scholar]
- 4.Brinton RD, Yao J, Yin F, Mack WJ, Cadenas E. Perimenopause as a neurological transition state. Nat Rev Endocrinol. 2015;11(7):393–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yin F, Yao J, Sancheti H, et al. The perimenopausal aging transition in the female rat brain: decline in bioenergetic systems and synaptic plasticity. Neurobiol Aging. 2015;36(7):2282–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao L, Mao Z, Woody SK, Brinton RD. Sex differences in metabolic aging of the brain: insights into female susceptibility to Alzheimer’s disease. Neurobiol Aging. 2016;42:69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rettberg JR, Yao J, Brinton RD. Estrogen: a master regulator of bioenergetic systems in the brain and body. Front Neuroendocrinol. 2014;35(1):8–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henderson VW, Brinton RD. Menopause and mitochondria: windows into estrogen effects on Alzheimer’s disease risk and therapy. Prog Brain Res. 2010;182:77–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rettberg JR, Dang H, Hodis HN, et al. Identifying postmenopausal women at risk for cognitive decline within a healthy cohort using a panel of clinical metabolic indicators: potential for detecting an at-Alzheimer’s risk metabolic phenotype. Neurobiol Aging. 2016;40:155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh-Manoux A, Kivimaki M, Glymour MM, et al. Timing of onset of cognitive decline: results from Whitehall II prospective cohort study. BMJ. 2012;344:d7622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Altmann A, Tian L, Henderson VW, Greicius MD, Alzheimer’s Disease Neuroimaging Initiative I. Sex modifies the APOE-related risk of developing Alzheimer disease. Ann Neurol. 2014;75(4):563–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caselli RJ, Reiman EM, Osborne D, et al. Longitudinal changes in cognition and behavior in asymptomatic carriers of the APOE e4 allele. Neurology. 2004;62(11):1990–1995. [DOI] [PubMed] [Google Scholar]
- 13.Okuizumi K, Onodera O, Tanaka H, et al. ApoE-epsilon 4 and early-onset Alzheimer’s. Nat Genet. 1994;7(1):10–11. [DOI] [PubMed] [Google Scholar]
- 14.Strittmatter WJ, Saunders AM, Schmechel D, et al. Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci U S A. 1993;90(5):1977–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261(5123):921–923. [DOI] [PubMed] [Google Scholar]
- 16.Khan TA, Shah T, Prieto D, et al. Apolipoprotein E genotype, cardiovascular biomarkers and risk of stroke: systematic review and meta-analysis of 14,015 stroke cases and pooled analysis of primary biomarker data from up to 60,883 individuals. Int J Epidemiol. 2013;42(2):475–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bennet AM, Di Angelantonio E, Ye Z, et al. Association of apolipoprotein E genotypes with lipid levels and coronary risk. JAMA. 2007;298(11):1300–1311. [DOI] [PubMed] [Google Scholar]
- 18.Neu SC, Pa J, Kukull W, et al. Apolipoprotein E Genotype and Sex Risk Factors for Alzheimer Disease: A Meta-analysis. JAMA Neurol. 2017;74(10):1178–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Henderson VW, St John JA, Hodis HN, et al. Cognitive effects of estradiol after menopause: A randomized trial of the timing hypothesis. Neurology. 2016;87(7):699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hodis HN, Mack WJ, Henderson VW, et al. Vascular Effects of Early versus Late Postmenopausal Treatment with Estradiol. N Engl J Med. 2016;374(13):1221–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henderson VW, Guthrie JR, Dudley EC, Burger HG, Dennerstein L. Estrogen exposures and memory at midlife: a population-based study of women. Neurology. 2003;60(8):1369–1371. [DOI] [PubMed] [Google Scholar]
- 22.den Heijer T, Geerlings MI, Hofman A, et al. Higher estrogen levels are not associated with larger hippocampi and better memory performance. Arch Neurol. 2003;60(2):213–220. [DOI] [PubMed] [Google Scholar]
- 23.Henderson VW, St John JA, Hodis HN, et al. Cognition, mood, and physiological concentrations of sex hormones in the early and late postmenopause. Proc Natl Acad Sci U S A. 2013;110(50):20290–20295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peters R The prevention of dementia. Int J Geriatr Psychiatry. 2009;24(5):452–458. [DOI] [PubMed] [Google Scholar]
- 25.Panza F, Capurso C, D’Introno A, et al. Vascular risk factors, alcohol intake, and cognitive decline. J Nutr Health Aging. 2008;12(6):376–381. [DOI] [PubMed] [Google Scholar]
- 26.Panza F, D’Introno A, Colacicco AM, et al. Cognitive frailty: Predementia syndrome and vascular risk factors. Neurobiol Aging. 2006;27(7):933–940. [DOI] [PubMed] [Google Scholar]
- 27.Panza F, Frisardi V, Capurso C, et al. Metabolic syndrome and cognitive impairment: current epidemiology and possible underlying mechanisms. J Alzheimers Dis. 2010;21(3):691–724. [DOI] [PubMed] [Google Scholar]
- 28.Panza F, Frisardi V, Seripa D, et al. Metabolic syndrome, mild cognitive impairment, and dementia. Curr Alzheimer Res. 2011;8(5):492–509. [DOI] [PubMed] [Google Scholar]
- 29.Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology. 2013;80(19):1778–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perkins M, Wolf AB, Chavira B, et al. Altered Energy Metabolism Pathways in the Posterior Cingulate in Young Adult Apolipoprotein E varepsilon4 Carriers. J Alzheimers Dis. 2016;53(1):95–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Payami H, Montee KR, Kaye JA, et al. Alzheimer’s disease, apolipoprotein E4, and gender. JAMA. 1994;271(17):1316–1317. [PubMed] [Google Scholar]
- 32.Damoiseaux JS, Seeley WW, Zhou J, et al. Gender modulates the APOE epsilon4 effect in healthy older adults: convergent evidence from functional brain connectivity and spinal fluid tau levels. J Neurosci. 2012;32(24):8254–8262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Farrer LA, Cupples LA, Haines JL, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA. 1997;278(16):1349–1356. [PubMed] [Google Scholar]
- 34.Sampedro F, Vilaplana E, de Leon MJ, et al. APOE-by-sex interactions on brain structure and metabolism in healthy elderly controls. Oncotarget. 2015;6(29):26663–26674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heise V, Filippini N, Trachtenberg AJ, Suri S, Ebmeier KP, Mackay CE. Apolipoprotein E genotype, gender and age modulate connectivity of the hippocampus in healthy adults. Neuroimage. 2014;98:23–30. [DOI] [PubMed] [Google Scholar]
- 36.Perna L, Mons U, Rujescu D, Kliegel M, Brenner H. Apolipoprotein E e4 and Cognitive Function: A Modifiable Association Results from Two Independent Cohort Studies. Dement Geriatr Cogn Disord. 2016;41(1–2):35–45. [DOI] [PubMed] [Google Scholar]
- 37.van den Kommer TN, Dik MG, Comijs HC, et al. The role of extracerebral cholesterol homeostasis and ApoE e4 in cognitive decline. Neurobiol Aging. 2012;33(3):622 e617–628. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content 1: Metabolic biomarkers in by cluster and ApoE4 genotype
Supplemental Digital Content 2: Individual cognitive test scores by metabolic phenotype and ApoE4 genotype
Supplemental Digital Content 3: Composite cognitive scores by metabolic phenotypes and ApoE4 genotype: Adjustment for LDL cholesterol
Supplemental Digital Content 4: Individual cognitive test scores by metabolic phenotype and ApoE4 genotype: Adjustment for LDL cholesterol


