Abstract
At the end of November 2019, a novel coronavirus responsible for respiratory tract infections emerged in China. Despite drastic containment measures, this virus, known as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), spread in Asia and Europe. The pandemic is ongoing with a particular hotspot in southern Europe and America in spring 2020. Many studies predicted an epidemic in Africa similar to that currently seen in Europe and the USA. However, reported data do not confirm these predictions. Several hypotheses that could explain the later emergence and spread of the coronavirus disease 2019 (COVID-19) pandemic in African countries are being discussed, including the lack of health-care infrastructure capable of clinically detecting and confirming COVID-19 cases, the implementation of social distancing and hygiene, international air traffic flows, the climate, the relatively young and rural population, the genetic polymorphism of the angiotensin-converting enzyme 2 receptor, cross-immunity and the use of antimalarial drugs.
Keywords: Africa, antimalarial drugs, coronavirus disease 2019, malaria, severe acute respiratory syndrome coronavirus 2
Introduction
In December 2019, the Chinese health authorities reported the emergence of a novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), in Wuhan (China), after clinicians had published that this new Sarbecovirus was causing a disease subsequently named COVID-19 (coronavirus disease 2019) [1,2]. In fact, retrospective studies show that SARS-CoV-2 has already been circulating for several weeks, probably since October 2019, with a case of COVID-19 formally identified on 17 November 2019 [3]. Among 10 237 infected individuals with SARS-CoV-2 included in a nationwide cohort in South Korea (24 January to 9 April 2020), 6350 individuals (62%) were asymptomatic [4]. In another study on 1096 individuals in Kuwait (24 February to 20 April 2020), 46.3% of the infected people were asymptomatic on the sample day [5]. Thirty-five individuals (6.9%) who were initially asymptomatic developed symptoms later [5]. Asymptomatic infection can occur at any age, but had a higher prevalence in patients under 45 years old compared with symptomatic people [6]. In a meta-analysis, the most frequent clinical signs and symptoms reported in symptomatic patients were fever (78.5%), cough (53.8%) and fatigue (25.0%), and 6.8% of the patients were admitted to the intensive care unit and 7.7% died during hospitalization due to complications related to COVID-19 (pneumonia, secondary bacterial infection and respiratory failure) [7].
Because of its high transmission efficiency [8], SARS-CoV-2 spread worldwide, prompting the WHO to declare an international public health emergency on 30 January 2020 [9], and a pandemic on 11 March 2020 [10]. Following the early outbreak in China, COVID-19 spread to other Asian countries and western European countries and then to the USA in Spring 2020 (Fig. 1). COVID-19 later spread to Central and South America, Africa, India and Southeastern Asia. Cities most affected by COVID-19 are either those with intensive international economic exchanges, such as Hong Kong, Milan, London, Paris, Madrid, and more recently New York, or cities having recently hosted events bringing together many people. It is worth noting that in the ranking of countries that faced the most severe outbreaks, Italy was the first to be very strongly impacted in Europe.
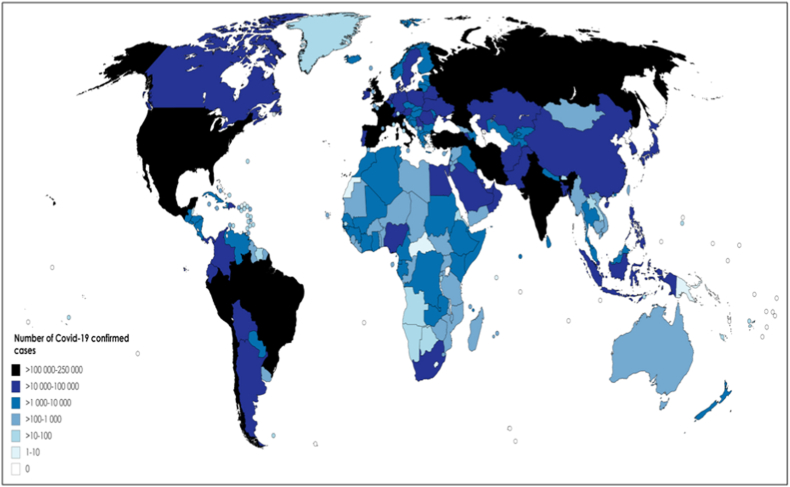
Fig. 1.
Confirmed cases of COVID-19 by country (4 June 2020). The colour gradient (dark to light) represents the graduated number of confirmed cases (highest to lowest). The figure was generated by compiling the data on COVID-19 cases on 4 June 2020, in each country worldwide (from Worldometer [124]).
Interestingly, it was reported that the Lombardia region, accounting for the highest number of COVID-19 cases in Italy, is the region with the highest percentage of Chinese residents (23% of its population being composed of Chinese residents) [11]. Although the pandemic has evolved rapidly at the global level (on 23 March 2020, 174 countries and territories had been affected by COVID-19, whereas on 4 June, almost all countries worldwide were involved (Fig. 1)), the African continent has so far reported only a very small number of cases.
As of 4 June 2020, 54 countries are affected in Africa with 150 610 cumulative confirmed cases and 4281 reported deaths [12]. The five most affected African countries are South Africa with 37 525 cases and 792 deaths, Nigeria with 11 166 cases and 315 deaths, Algeria with 9733 cases and 673 deaths, Ghana with 8548 cases and 38 deaths, and Cameroon with 6789 cases and 203 deaths, on June 4, 2020. The first confirmed African cases of SARS-CoV-2 were reported on 14 February 2020 in Egypt for north Africa [13] and on 27 February 2020 in Nigeria for Sub-Saharan Africa [14]. Gilbert et al. estimated the risk of importation of cases of COVID-19 to Africa according to air travel flows from infected provinces of China to Africa and taking into account the African countries' capacity to detect COVID-19 [15]. The countries at highest importation risk from China were Egypt, Algeria, South Africa, Nigeria and Ethiopia. Three of these countries (South Africa, Algeria and Nigeria) are the three most affected countries and Egypt has recorded 28 615 cases and 1088 deaths. Another study predicted a high risk of exposure in countries of small size like Mauritius (1 097 013 estimated cases for 1 265 303 inhabitants; 335 confirmed cases on 4 June 2020) or Equatorial Guinea (819 289 estimated cases for 1 308 974 inhabitants; 1306 confirmed cases on 4 June 2020) [16]. Using a branching process model, Pearson et al. estimated the number of cases of COVID-19 in each African country, and more especially the timing of reporting 10 000 cases for all African countries [17]. For instance, the timing of reporting 10 000 cases was between 11 and 18 April (3836 cases on 4 June 2020) in Senegal, 17 and 23 April (6585 cases on 4 June 2020) in Cameroon, 7 to 21 May (86 cases on 4 June 2020) in Angola and 23 April and 3 May (1486 cases on 4 June 2020) in Ethiopia. The data declared to WHO indicated the spread of COVID-19 in Africa but with lower confirmed cases than expected. We discuss several hypotheses that could explain the later emergence and spread of the COVID-19 pandemic in African countries.
Information gathering regarding COVID-19 cases and deaths
The gathering of available information regarding the number of infected people varies between countries depending on several factors. First, screening strategies vary considerably between countries according to the availability of virus-screening assays. For instance, in western countries, some countries (like France) have recommended testing only people with mild to severe symptoms, whereas other countries (like Germany) have adopted an open strategy to detect asymptomatic persons and then isolate positive persons. At the beginning of February 2020, only two countries in Africa (Senegal and South Africa) had laboratories able to carry out RT-PCR tests, the reference standard in COVID-19 testing, in the absence of available and reliable rapid diagnostic tests [18]. Between 2 February and 1 March 2020, the laboratory capacity for testing for SARS-CoV-2 had increased from two to 34 countries [19]. However, on 1 April 2020, South Africa had tested more than 47 000 people, but Zimbabwe had tested only 316 people and Namibia only 306 [20]. The lack of health-care infrastructure capable of clinically detecting and subsequently confirming COVID-19 cases is obviously the most important reason for the relatively low number of confirmed cases in Africa [21]. The most affected country is South Africa with 37 525 cases and 792 deaths (4 June 2020), one of the countries with the best diagnostic capacities with more than 3 million tests performed on 13 August 2020 [22]. However, between 2 February and 15 April 2020, laboratory testing capacity has increased from two countries (South Africa and Senegal) to 44 countries in the WHO African Region [23]. The situation is even more precarious in war zones and the many landlocked zones.
Another possible explanation of the weakness of epidemiological signals regarding COVID-19 in Africa is the hypothesis that epidemiological surveillance cannot be carried out appropriately and that syndromic surveillance is difficult due to confounding factors linked to other respiratory infections. For instance, COVID-19 has been reported in the same areas in Brazil where cases of dengue fever, with similar clinical and laboratory characteristics, have been identified [24].
Physical distancing and enhanced hygiene
WHO anticipates a rapid increase in the number of individuals with COVID-19 on the African continent and Africa has responded quickly. African health ministers quickly implemented the Africa Taskforce for Coronavirus Preparedness and Response on 3 February 2020 [25]. Physical distancing and enhanced hygiene are two of the response measures. Lockdown measures, like closing schools, churches, mosques or markets to promote social distancing or shutting down airports, have been rapidly implemented in some African countries [[26], [27], [28]]. However, lockdown and working from home are unlikely to be implementable in many areas of Africa because of a cash economy, daily work requiring physical presence, and electricity or internet inaccessibility [29]. Sanitary measures including access to soap, water and hydroalcoholic gel, are also difficult to set up [30,31]. More than 50% of the population in sub-Saharan Africa was without access to handwashing in 2019 [31]. In three African countries (Nigeria, Ethiopia and Democratic Republic of the Congo), more than 50 million people were estimated to be without handwashing access [31]. Additionally, physical distancing and enhanced hygiene are unlikely to be implementable in refugee camps, shantytowns or overcrowded settings [[32], [33], [34]]. However, several African countries have benefited from previous initiatives to address Ebola [35,36]. For instance, the surveillance structure and screening measures in airports developed for Ebola virus disease became pillars in the COVID-19 response in Uganda [35] and Ebola standard operating procedures were updated for COVID-19 in the Democratic Republic of Congo [36].
Delay in the dynamic of the COVID-19 epidemic
The hypothesis of a marked delay of the onset of the epidemic in Africa could be linked to less frequent exchanges between China and Africa (a mono-directional exchanges model where it is especially the Chinese who travel to Africa) than between China and Europe or China and the USA (a bi-directional exchange model). According to air travel flows from infected provinces in China to Africa and the African country's capacity to detect COVID-19, Gilbert et al. estimated that Egypt, Algeria, South Africa, Nigeria and Ethiopia presented the highest risk of importation of cases of COVID-19 from China [15]. Another study, based on the analysis of 388 327 passengers travelling to 1297 airports, estimated that the risk of transmission to Africa was very low [37]. The only African countries identified as at high risk were Ethiopia (28th) and South Africa (40th). Egypt, Algeria and Nigeria were estimated as at low risk (59th, 88th and 100th, respectively). However, this hypothesis is questionable: the African continent attracted 121 million Chinese visitors in 2017 (a tremendous growth compared with 2005 (31 million)) [38], while according to the China Tourism Academy, about six million Chinese visitors were recorded as travelling to Europe in 2018 and with an 8% increase in the first 6 months of 2019 [39]. But the status of Chinese visitors is different between Europe and Africa. Most Chinese visitors in Europe are either students, tourists or on business trips. In contrast, most Chinese visitors to Africa are construction workers, engineers, translators, or company executives who stay in Africa for a long time [40] and the number of Chinese ranged between 1 and 2 million in 2020 [41]. In 2017, Chinese tourists accounted for only 11 million flights for a total of 121 million visitors [42]. The Chinese workers typically fly back to China once or twice a year, and especially for Chinese New Year. In contrast, many Chinese tourists leave China for Europe on vacation days before the Chinese New Year Day. Most of the first COVID-19 cases in Europe have been linked to Chinese tourists or people returning from China [43,44]: on 29 January 2020, two Chinese tourists from Hubei province coming to Italy [45]; on 27 January 2020, a Chinese individual working for a company in Munich who had been in contact with their parents from Wuhan before visiting Germany [46]; on 4 February 2020, a Belgian individual returning from Wuhan [47]; on 27 January 2020, two Chinese tourists coming to England [48]; or on 24 January 2020, a French individual returning from Wuhan and two Chinese tourists from Wuhan [49].
What is interesting is that the majority of the initial cases of COVID-19 in Africa have been linked to European travel rather than China. The first case of SARS-CoV-2 in Nigeria was identified in an Italian citizen on 27 February 2020 [14], in Italian people on 1 March 2020 in South Africa [50], in an Italian citizen on 17 February 2020 in Algeria [51], in a French citizen on 24 February 2020 in Cameroon [52], in a French resident in Dakar returning from France on 26 February 2020 in Senegal [53], in two citizens arrived in Ghana from Norway and Turkey on 12 March 2020 [54], in a Kenyan citizen returning to Kenya from the USA via London on 5 March 2020 [55] and in a Japanese man who travelled from Japan to Burkina Faso before coming to Ethiopia on 13 March 2020 [56]. Among the countries most affected by COVID-19, only the first case in Egypt came from Wuhan [57].
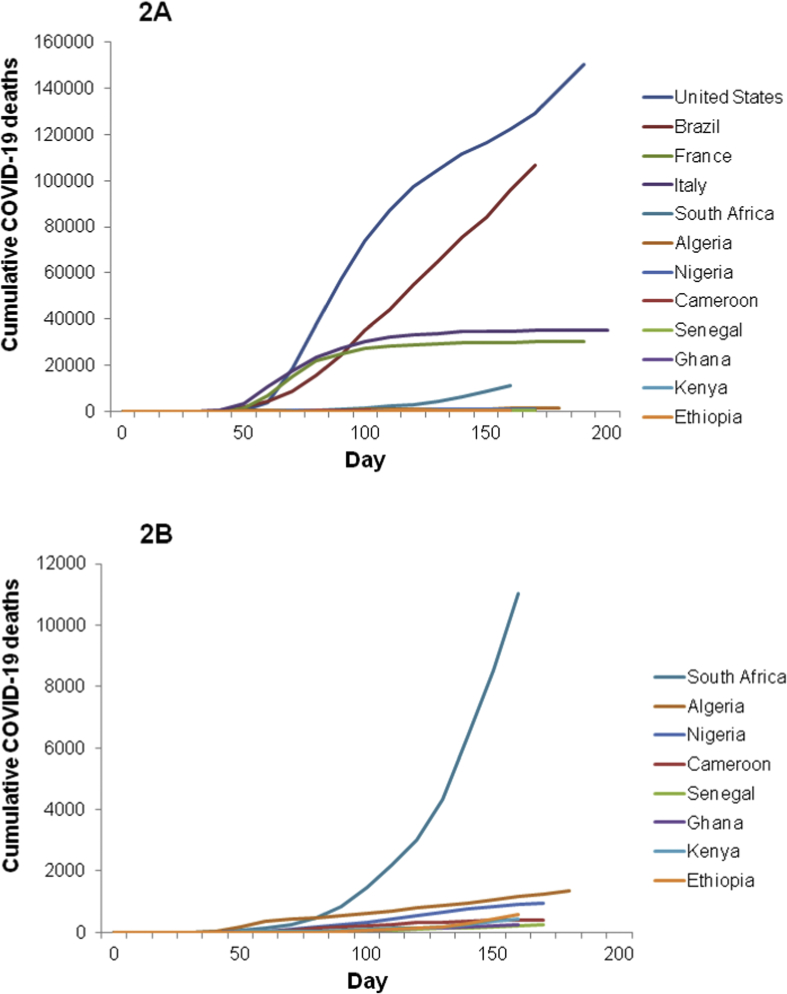
First cases of SARS-CoV-2 were identified preferably in January–February 2020 in Europe and the USA, and in February–March in Africa and Brazil. The dynamic of the expansion of COVID-19-related deaths was different between the countries (Fig. 2). Deaths emerged rapidly in African countries but stayed low after the first 150 days, Logarithmic analysis of the cumulative deaths (Fig. 2c, d) showed that the number of deaths evolved in a similar way in South Africa and Algeria during the first 30 days to what was observed in the USA, Brazil or Europe. The dynamic was slower in Nigeria, Cameroon, Senegal, Ghana, Kenya and Ethiopia than in South Africa or Algeria (Fig. 2d).
Fig. 2.
Comparison of the cumulative total COVID-19 deaths reported in the first 200 days of the pandemic in the USA, Brazil, France, Italy and the most affected countries in Africa (South Africa, Algeria, Nigeria, Cameroon, Senegal, Ghana, Kenya and Ethiopia). (a) Cumulative deaths for the 12 selected countries, (b) cumulative deaths for the eight African countries, (c) cumulative deaths for the 12 selected countries in log. The number of deaths was evaluated every 10 days from the first SARS-CoV-2 detected case to 18 August 2020, in each country. The graph was generated with daily WHO data [12]. (d) cumulative deaths for the eight selected countries in log.
Demographic hypothesis
One hypothesis that has been rarely mentioned to explain the weakness of the epidemiological signal in Africa regarding the COVID-19 epidemic (and potentially a delay in its dynamic) is the fact that its population is extremely young. In 2015, 40% of its population was aged 0–14 years and 19% was aged 15–24 years, whereas those aged over 65 years accounted for only 3.5% The proportion of people over 65 years of age in 2019 was higher in Southeastern Asia (7%), in Central America (7%) and South America (9%) but remained much lower than in Europe (21%) [58]. The relatively young and rural population has certainly limited the spread and severity of COVID-19 in Africa [59]. Yet, there is a consensus to consider that COVID-19 is far less severe in children than in adults, and that in this group, most of them are asymptomatic. For example, in China, in a study conducted on 7736 individuals who had been hospitalized with COVID-19, only 0.9% were younger than 15 years [60]. Disease severity in patients under 15 years of age represented only 0.6% of the total number of cases. Individuals over 65 years of age accounted for 15.1% of the patients and 27.0% of the total severity. In an investigation of 72 314 cases, Wu et al. reported that children under 9 and from 10 to 19 years old represented 1% of the total number of cases, respectively [61]. In a clinical and epidemiological study conducted on 36 children, 28% were asymptomatic versus <5% in adults, 36% showed fever versus 89% in adults, 0% showed severe disease versus 23% in adults [62].
Role of climate
Environmental factors, especially temperature and relative humidity, may influence SARS-CoV-2 transmission [63]. High temperature and high humidity may reduce coronavirus transmission [64], which could explain the relatively low number of confirmed cases in Africa. Around 60% of the world cases occurred in areas where temperature ranged from 5 to 15°C [65]. The rate of confirmed cases significantly decreased with maximum temperature above 22.5°C [66]. Mean daily cases and deaths were significantly lower in the hottest countries with highest temperatures reported between 29 December 2019 and 12 May 2020 (26.3°C; 407.1 daily cases and 17.8 daily deaths) than in the coldest countries with lowest temperatures (6.2°C; 1876.7 daily cases and 100.4 daily deaths) [67]. The viral transmissibility is estimated by the reproduction number (R0), which represents the number of people that will be infected by an individual who has an infection. The SARS-CoV-2 R0 was >2 for temperatures below 0°C, decreased to R0 = 1 for temperatures increasing from 0°C to 11°C, increased to R0 = 1.6 for temperatures increasing from 11°C to 20°C, and gradually decreased to R0 = 1 for temperatures above 20°C [68]. However, a study carried out in Australia suggested that even with high temperature, COVID-19 could persist [69]. Another study showed that in Brazil, high temperatures and relative humidity favoured COVID-19 transmission [70].
Genetic polymorphism
Genetic polymorphism of angiotensin-converting enzyme 2 (ACE2), the cell entry receptor for SARS-CoV-2, could play a role in SARS-CoV-2 transmission [71,72]. Analyses of different geographical populations demonstrated the existence of at least 32 variants of ACE2 (single mutation, deletion, truncation) [73]. Expression of a specific variant allele in African subpopulations could alter the process of interaction between the viral S protein and the ACE2 receptor on the alveolar cells of the lung. In addition, expression quantitative trait loci variants were reported to regulate the expression of the ACE2 gene. ACE2 gene expression was down-regulated by variants rs11271234 and rs75979613 and up-regulated by the other variants [[74], [75], [76]].
Moreover, the ACE1 gene, an analogue of ACE2, can have insertion (I) or deletion (D) of a 287-bp Alu repeat sequence in intron 16 [77]. Three different genotypes exist: II, ID and DD. The number of patients infected with SARS-CoV-2 and the number of deaths from COVID-19 were negatively associated with the ACE1 II genotype frequency [78]. The European population has a lower ACE1 II genotype frequency and a higher prevalence of infected patients and mortality due to COVID-19 than the Asian population [78]. These data are consistent with an epidemiological study that reported a significant positive correlation of the D allele with SARS-CoV-2 infection prevalence and mortality rate in Asian population [79]. The DD genotype was also increased in acute respiratory distress syndrome [80]. Increase of ID genotype frequency was found to increase recovery rate after COVID-19 [81]. The frequencies of D allele were different between ethnic groups: it ranged from 72% to 77% in North African individuals (Moroccans, Egyptians, Algerians, Tunisians), 59% in West African individuals (Nigerians), 64% to 73% in East African individuals (Sudanese, Somalis), only 14% in South African individuals (Zambians), 29% in Chinese individuals, 9% in Samoan individuals, and 46% in Caucasian individuals [82]. For instance, the DD frequency was 43% for Sudanese individuals [83], 51% for Somali individuals [83], 36% for Burkinabe individuals [84], 34% for South African individuals [85] and 30% for European individuals [86]. Prospective studies are needed to investigate ACE genotype frequencies in different world areas and a potential role in delayed transmission. Moreover, other SARS-CoV-2 receptors could be involved in COVID-19 cases and mortality rate, like the transmembrane protease serine 2 (encoded by TMPRSS2) [87]. TMPRSS2 up-regulating variants were found at higher frequencies in European and American populations than in the Asian and African populations, which implies that these populations might be relatively susceptible to SARS-CoV-2 infection [87].
Cross-immunity to SARS-CoV-2
Protective immune responses to viral infection occur following the combined actions of B cells (humoral immunity) and T cells (cellular immunity). In COVID-19, B cells produce IgM, IgG and IgA antibodies that neutralize SARS-CoV-2 entry by competition with ACE2 for binding the receptor-binding domain of the viral spike protein [88,89].
T helper cells (CD4) are responsible for cellular immunity and for helping B cells to produce neutralizing antibodies. Importantly, all B and T cells have immunological memory after a first contact with a pathogen. Memory CD4+ T cells mediate protective immunity against respiratory coronaviruses as well as neutralizing antibodies that bind the receptor binding domain in the spike of SARS-CoV were reported [90,91], suggesting that a similar immune response could be observed in individuals with COVID-19. The SARS-CoV-2 Spike glycoprotein is closely related to that of SARS-CoV S and some antibodies identified in SARS-CoV-infected patients could also neutralize SARS-CoV-2 [92]. But although cross-reactivity in antibodies binding to the Spike protein is relatively common, cross-neutralization responses between SARS-CoV and SARS-CoV-2 remain rare [93]. Convalescent sera from SARS-CoV and SARS-CoV-2 patients showed only limited cross-neutralization [94,95]. Pre-existing immunity could explain the relatively low confirmed cases in Africa. Pre-existing SARS-CoV-2-cross-reactive CD4+ and CD8+ T cells responses were observed in healthy people [[96], [97], [98]]. SARS-CoV-2 Spike glycoprotein-reactive CD4+ cells were found in 20%–60% of healthy individuals [[96], [97], [98]]. Very recently, the analysis of immune responses to SARS-CoV-2 from patients who recovered from COVID-19 identified targets of T-cell responses to SARS-CoV-2 and revealed cross-reaction with circulating ‘common cold’ betacoronaviruses due to past infections [[97], [98], [99], [100], [101], [102]]. Children appear to be protected and develop mild COVID-19 or no disease [[103], [104], [105]]. Common human coronaviruses (HCoV-229E, HCoV-NL63, HCoV-OC43, HCoV-HKU1) were isolated from 7% to 11% in children hospitalized for acute respiratory tract infections [[106], [107], [108]]. Cross-reactivity between SARS-CoV-2 and human coronavirus is a plausible explanation for why children appear to be protected.
Another explanation is protection against SARS-CoV-2 induced by vaccines used against other pathogens. It seems that bacillus Calmette–Guérin (BCG) vaccination could protect from COVID-19. A strong association was observed between BCG vaccination deployment and COVID-19 mortality [109,110]. Countries where BCG vaccination is given at birth showed lower numbers of COVID-19 cases and deaths [111]. In fact, a SARS-CoV-2 envelope protein is closely related to a Mycobacterium bovis protein and BCG vaccine could induce a specific immunity against SARS-CoV-2 by targeting its essential viral envelope protein [112]. Moreover, the fatality rate due to COVID-19 was lower in Asian countries where Japanese encephalitis immunization is recommended compared with those were the population is not vaccinated [113].
Protection by repeated antimalarial treatments?
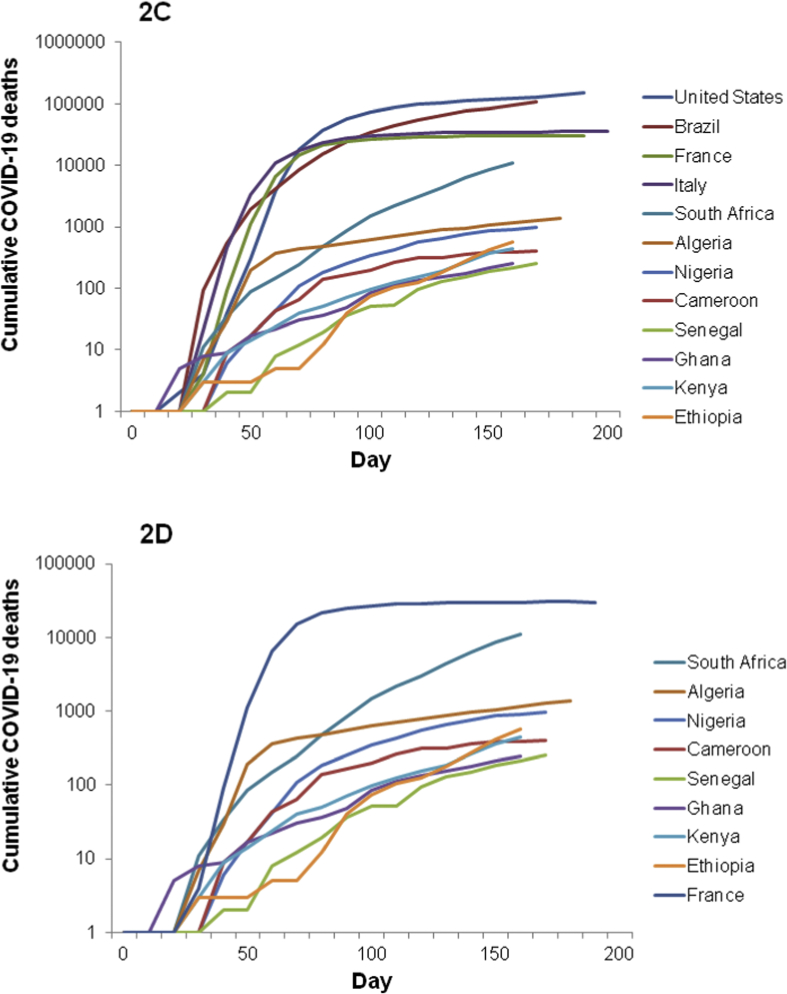
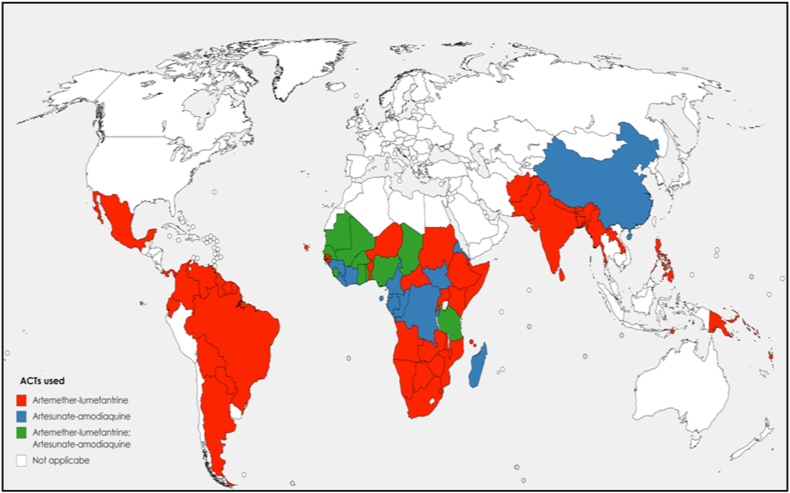
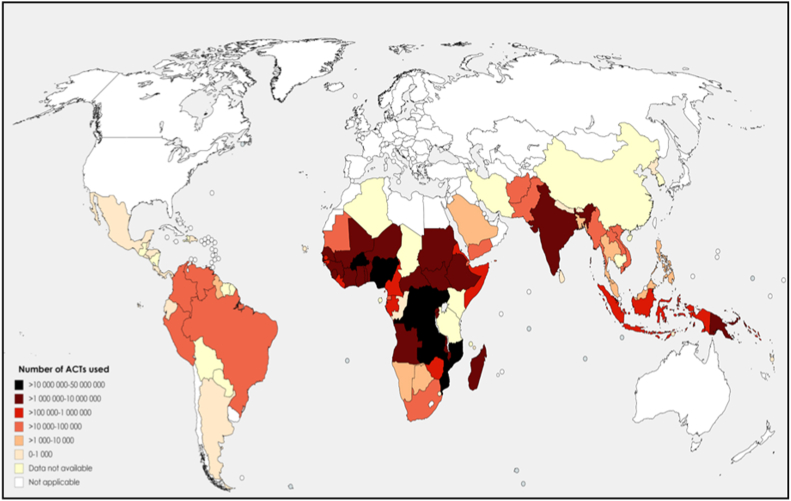
There seems to be an inverse relationship in the overall number of COVID-19 cases and malaria cases [110,[114], [115], [116]]. For instance, three of the African countries most affected by COVID-19 on 4 June 2020 are South Africa (37 525 cases and 792 deaths), Algeria (9733 cases and 673 deaths) and Egypt (28 615 cases and 1088 deaths), which belong to the countries less affected by malaria [117]. The use of antimalarial drugs to treat uncomplicated malaria in Africa could be another hypothesis explaining the relatively low number of confirmed cases in Africa [118]. Since 2002, the WHO has recommended the use of artemisinin-based combination therapy (ACT) in the treatment of uncomplicated falciparum malaria (artemether-lumefantrine, artesunate-amodiaquine, dihydroartemisinin-piperaquine or artesunate-mefloquine) (Fig. 3). In 2018, these ACTs were widely used (Fig. 4). These ACTs, evaluated at plasma concentrations expected after oral uptake at the recommended doses used in uncomplicated malaria treatment, showed an in vitro inhibition of SARS-CoV-2 replication that ranged from 30% to 70% [119]. The combination mefloquine-artesunate was found to be the most effective in vitro against SARS-CoV-2. A patient treated with ACT for uncomplicated malaria could be protected from SARS-CoV-2 during treatment. Another study reported that some artemisinin derivatives used alone (dihydroartemisinin and artesunate) showed in vitro activity with median effective concentration (EC50) around 10 μM [120]. Moreover, amodiaquine, a quinoleine antimalarial and one of the partners of artemisinin derivative, was found to be active in vitro at a micromolar concentration against SARS-CoV-1 (2.5 μM) [121]. Another ACT partner, mefloquine, exerts in vitro cytopathic effects on Vero cells infected by SARS-CoV-2 at 10 μM [122]. A molecular docking study showed that pyronaridine, another ACT partner (artesunate-pyronaridine), can interact with the non-structural protein 5 (Nsp5), which is an essential protein for the transcription and replication of SARS-CoV-2 [123]. Pyronaridine showed effective antiviral activity with EC50 of 0.72 μM and pyronaridine is 165 times more concentrated in lungs than in plasma (Pradines B, unpublished data). The use of antimalarial drugs and the dynamics of COVID-19 should now be compared on a country-by-country basis to confirm the potential effects of antimalarial drugs on COVID-19 transmission. It could be necessary now to evaluate clinically the efficacy of ACT to treat COVID-19, and more particularly that of mefloquine-artesunate, and the potential prevention of ACT against SARS-CoV-2 during malaria season, which currently begins in some African countries, taking into account the antimalarial drug, its dose, the number of treated patients, the number of cases of COVID-19 and deaths.
Fig. 3.
Global distribution of the two most used artemisinin-based combination therapies (ACTs) for the treatment of uncomplicated falciparum malaria in 2018, especially artemether-lumefantine and artesunate-amodiaquine, the most deployed ACT in malaria endemic countries. The figure was generated by compiling the data obtained by WHO in 2018 [117].
Fig. 4.
Number of artemisinin-based combination therapy (ACTs) used in 2018, by malaria-affected country. The colour gradient (dark to light) depends on the graduated number of ACTs used (highest to lowest). The figure was generated by compiling the data obtained by WHO in 2018 [117].
Conclusion
The comparison of European and African models of SARS-CoV-2 prevalence could provide important clues to the spread of the virus. However, it is essential to increase the capacity to clinically detect COVID-19 cases and then to confirm them by RT-PCR or reliable rapid diagnostic tests in African countries. Epidemiological, clinical and pharmacological data should be carefully recorded to follow the evolution of the spread of COVID-19 over the coming months in malaria endemic areas in Africa.
Acknowledgements
The authors thank CookieTrad for proofreading the text.
Conflict of Interest statement
CD declares a link of interest with the Sanofi company, which sells hydroxychloroquine. The other authors have no competing interests to declare.
Funding source
This study was supported by the Institut Hospitalo-Universitaire (IHU) Méditerranée Infection, the National Research Agency under the program Investissements d'avenir, reference (ANR-10-IAHU-03).
References
- 1.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frutos R., Lopez Roig M., Serra-Cobo J., Devaux C.A. COVID-19: the conjunction of events leading to the coronavirus pandemic and lessons to learn for future threats. Front Med. 2020;7:223. doi: 10.3389/fmed.2020.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jung C.Y., Park H., Kim D.W., Choi Y.J., Kim S.W., Chang T.I. Clinical characteristics of asymptomatic patients with COVID-19: a nationwide cohort study in South Korea. Int J Infect Dis. 2020 doi: 10.1016/j.ijid.2020.08.001. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Almazeedi S., Al-Youba S., Jamal M.H., Al-Haddad M., Al-Muhaini A., Al-Ghimlas F. Characteristics. risk factors and outcomes among the first consecutive 1096 patients diagnosed with COVID-19 in Kuwait. EClinicalMedicine. 2020;24:100448. doi: 10.1016/j.eclinm.2020.100448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu C., Zhou M., Liu Y., Guo T., Ou C., Yang L. Characteristics of asymptomatics COVID-19 infection and progression: a multicenter, retrospective study. Virulence. 2020 doi: 10.1080/21505594.2020. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jutzeler C.R., Bourguignon L., Weis C.V., Tong B., Wrong C., Rieck B. Comorbidities, clinical signs and symptoms, laboratory findings, imaging features, treatment strategies, and outcomes in adults and pediatric patients with COVID-19: a systematic review and meta-analysis. Travel Med Infect Dis. 2020;4:101825. doi: 10.1016/j.tmaid.2020.101825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lai C.C., Shih T.P., Ko W.C., Tang H.J., Hsueh P.R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents. 2020;55:105924. doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.https://www.euro.who.int/en/health-topics/health-emergencies/international-health-regulations/news/news/2020/2/2019-ncov-outbreak-is-an-emergency-of-international-concern Accessed.
- 10.https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 Accessed.
- 11.Chirumbolo S. Might the many positive COVID19 subjects in Italy have been caused by resident bat-derived zoonotic β-coronaviruses instead of the Wuhan (China) outbreak? J Med Virol. 2020 doi: 10.1002/jmv.25777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.https://www.afro.who.int/health-topics/coronavirus-covid-19 Accessed.
- 13.https://www.africanews.com/2020/02/14/covid-19-egypt-confirms-first-coronavirus-case-in-africa/ Accessed.
- 14.Kalu B. COVID-19 in Nigeria: a disease of hunger. Lancet Resp Med. 2020;8:556–557. doi: 10.1016/S2213-2600(20)30220-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilbert M., Pullano G., Pinotti F., Valdano E., Poletto C., Boëlle P.Y. Preparedness and vulnerability of African countries against importations of COVID-19: a modelling study. Lancet. 2020;395:871–877. doi: 10.1016/S0140-6736(20)30411-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cabore J.W., Karamagi H.C., Kipruto H., Asamani J.A., Droti B., Seydi A.B.W. The potential effects of widespread community transmission of SARS-CoV-2 infection in the World Health Organization African Region: a predictive model. BMJ Glob Heal. 2020;5 doi: 10.1136/bmjgh-2020-002647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pearson C.A., Van Schalkwyk C., Foss A.M., O’Reilly K.M. SACEMA Modelling and Analysis Response Team, CMMID Covid-Working Group, et al. Projected early spread of COVID-19 in Africa through 1 June 2020. Euro Surveill. 2020;25:2000543. doi: 10.2807/1560-7917.ES.2020.25.18.2000543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nkengasong J.N., Mankoula W. Looming threat of COVID-19 infection in Africa: act collectively, and fast. Lancet. 2020;395:841–842. doi: 10.1016/S0140-6736(20)30464-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organization . 4 March 2020. COVID-19. Situation update for the WHO african region.https://apps.who.int/iris/bitstream/handle/10665/331330/SITREP_COVID-19_WHOAFRO_20200304-eng.pdf External Situation Report 1. Available from: [Google Scholar]
- 20.Adebisi Y.A., Oke G.I., Ademola P.S., Chinemelum I.G., Ogun-Kola I.O., Lucera-Prisno D.E. SARS-CoV-2 diagnostic testing in Africa: needs and chalenges. Pan Afr Med J. 2020;35:4. doi: 10.11604/pamj.2020.35.4.22703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kobia F., Gitaka J. COVID-19: are Africa’s diagnostic challenges blunting response effectiveness? AAS Open Res. 2020;3:4. doi: 10.12688/aasopenres.13061.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.https://www.statista.com/statistics/1028731/covid19-tests-select-countries-worldwide/ Accessed.
- 23.World Health Organization . 15 April 2020. COVID-19. Situation update for the WHO african region.https://apps.who.int/iris/bitstream/handle/10665/331763/SITREP_COVID-19_WHOAFRO_20200415-eng.pdf External Situation Report 7. [Google Scholar]
- 24.Ribeiro V.S.T., Telles J.P., Tuon F.F. Arboviral diseases and COVID-19 in Brazil: concerns regarding climatic, sanitation and endemic scenario. J Med Virol. 2020 doi: 10.1002/jmv.26079. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.https://africacdc.org/news-item/africa-cdc-establishes-continent-wide-task-force-to-respond-to-global-coronavirus-epidemic/ Accessed.
- 26.Quaresima V., Naldini M.M., Cirillo D.M. The prospects for the SARS-CoV-2 pandemic in Africa. EMBO Mol Med. 2020;12 doi: 10.15252/emmm.202012488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tolu L.B., Ezeh A., Feyissa G.T. How prepared is Africa for the COVID-19 pandemic response? The case of Ethiopia. Risk Manag Healthc Policy. 2020;13:771–776. doi: 10.2147/RMHP.S258273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mbow M., Lell B., Jochems S.P., Cisse B., Mboup S., Dewals B.G. COVID-19 in Africa: dampening the storm? Science. 2020;369:624–626. doi: 10.1126/science.abd3902. [DOI] [PubMed] [Google Scholar]
- 29.Coetzee B.J., Kagee A. Structural barriers to adhering to health behaviours in the context of the COVID-19 crisis: considerations for low- and middle-income countries. Glob Public Health. 2020;15:1093–1102. doi: 10.1080/17441692.2020.1779331. [DOI] [PubMed] [Google Scholar]
- 30.Jiwani S.S., Antiporta D.A. Inequalities in access to water and soap matter for the COVID-19 response in sub-Saharan Africa. Int J Equity Health. 2020;19:82. doi: 10.1186/s12939-020-01199-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brauer M., Zhao J.T., Bennitt F.B., Stanaway J.D. Global access to handwashing: implications for COVID-19 control in low-income countries. Environ Health Perspect. 2020;128:57005. doi: 10.1289/EHP7200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vonen H.D., Olsen M.L., Eriksen S.S., Jervelund S.S., Eikemo T.A. Refugee camp and COVID-19: can we prevent a humanitarian crisis. Scand J Public Health. 2020 doi: 10.1177/1403494820934952. 1403494820934952. [DOI] [PubMed] [Google Scholar]
- 33.Subbaraman N. Distancing is impossible': refugee camp race to avert coronavirus catastrophe. Nature. 2020;581:18. doi: 10.1038/d41586-020-01219-6. [DOI] [PubMed] [Google Scholar]
- 34.Kassem Refugees besieged: the lurking threat of COVID-19 in Syrian war refugee camps. Travel Med Infect Dis. 2020:101736. doi: 10.1016/j.tmaid.2020.101736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lumu I. COVID-19 response in sub-Saharan Africa: lessons from Uganda. Disaster Med Public Health Prep. 2020;15:1–5. doi: 10.1017/dmp.2020.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nachega J.B., Mbala-Kingebeni P., Otshudiema J., Mobula L.M., Preiser W., Kallay O Responding to the challenge of the dual COVID-19 and Ebola epidemics in the Democratic Republic of Congo—priorities for achieving control. Am J Trop Med Hyg. 2020;103:597–602. doi: 10.4269/ajtmh.20-0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haider N., Yavlinsky A., Simons D., Osman A.Y., Ntoumi F., Zumla A. Passengers’ destinations from China: low risk of novel coronavirus (2019-nCoV) transmission into Africa and South America. Epidemiol Infect. 2020;148:e41. doi: 10.1017/S0950268820000424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Diallo A. Jeune Afrique. Face à l’épidémie de coronavirus, les pays africains adoptent des mesures de prévention. https://www.jeuneafrique.com/886692/societe/face-a-lepidemie-de-coronavirus-les-pays-africains-adoptent-des-mesures-de-prevention/ n.d. Available from:
- 39.Citrinot L. Coronavirus : le trafic aérien entre la Chine et l’Europe en danger. https://www.voyages-d-affaires.com/coronavirus-trafic-aerien-chine-europe-20200128.html n.d. Available from:
- 40.Zhou Y. Why Chinese are traveling to Africa. and why Africans are traveling to China. https://qz.com/africa/1680094/why-chinese-are-traveling-to-africa-and-why-africans-are-traveling-to-china/ Accessed.
- 41.https://qz.com/africa/1865111/chinese-migrant-workers-in-africa-and-myths-of-self-segregation/ Accessed.
- 42.https://www.agenceecofin.com/economie/0512-52633-l-afrique-est-desormais-la-destination-touristique-de-10-des-chinois-voyageant-a-l-exterieur-50-par-an Accessed.
- 43.Spiteri G., Fielding J., Diercke M., Campase C., Enouf V., Gaymard A. First cases of coronavirus disease 2019 (VOVID-19) in the WHO European region, 24 january to 21 february 2020. Euro Surveill. 2020;25:2000178. doi: 10.2807/1560-7917.ES.2020.25.9.2000178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Olsen S.J., Chen M.Y., Liu Y.L., Witschi M., Ardoin A., Calba C. Early introduction of severe acute respiratory syndrome coronavirus 2 into Europe. Emerg Infect Dis. 2020;26:1567–1570. doi: 10.3201/eid2607.200359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Capobianchi M.R., Rueca M., Messina F., Giombini E., Carletti F., Colavita F. Molecular characterization of SARS-CoV-2 from the first case of COVID-19 in Italy. Clin Microbiol Infect. 2020;26:954–956. doi: 10.1016/j.cmi.2020.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bohmer M.M., Buchholz U., Corman V.M., Hoch M., Katz K., Marosevic D.V. Investigation of a COVID-19 outbreak in Germany resulting from a single travel-asspciated primary case: a case series. Lancet Infect Dis. 2020;20:920–928. doi: 10.1016/S1473-3099(20)30314-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.http://www.xinhuanet.com/english/2020-03/01/c_138832869.htm Accessed.
- 48.https://www.thetimes.co.uk/article/hunt-for-contacts-of-coronavirus-stricken-pair-in-york-dh363qf8k Accessed.
- 49.Stoecklin S.B., Rolland P., Silue Y., Mailles A., Campese C., Simondon A. First cases of coronavirus disease 2019 (COVID-19) in France: surveillance. investigations and control measures. Ero Surveill. January 2020;25:2000094. doi: 10.2807/1560-7917.ES.2020.25.6.2000094. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.https://www.nicd.ac.za/first-case-of-covid-19-coronavirus-reported-in-sa/ Accessed.
- 51.https://africatimes.com/2020/02/25/algerian-health-minister-confirms-first-covid-19-case/ Accessed.
- 52.https://www.aa.com.tr/en/africa/cameroon-confirms-first-coronavirus-case/1756866 Accessed.
- 53.https://www.afro.who.int/news/senegal-reports-first-covid-19-case Accessed.
- 54.https://www.ghanabusinessnews.com/2020/03/12/ghana-confirms-first-covid-19-cases/ Accessed.
- 55.https://www.health.go.ke/first-case-of-coronavirus-disease-confirmed-in-kenya/ Accessed.
- 56.https://www.afro.who.int/news/first-case-covid-19-confirmed-ethiopia Accessed.
- 57.Kattab N.M., Vermund S.H., Hu Y. How coronavirus disease enterd Africa and the Middle East: a case study from Egypt. Trans R Soc Trop Med Hyg. 2020:traa065. doi: 10.1093/trstmh/traa065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gilles Pison. 2019. Tous les pays du monde; p. 2019. [Google Scholar]
- 59.Diop B.Z., Ngom M., Pougué Biyong C., Pougué Biyong J.N. The relatively young and rural population may limit the spread and severity of COVID-19 in Africa: a modelling study. BMJ Glob Heal. 2020;5 doi: 10.1136/bmjgh-2020-002699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guan W.-J., Ni Z.-Y., Hu Y., Liang W.-H., Ou C.-Q., He J.-X. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. J Am Med Assoc. 2020 doi: 10.1001/jama.2020.2648. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 62.Qiu H., Wu J., Hong L., Luo Y., Song Q., Chen D. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: an observational cohort study. Lancet Infect Dis. 2020;20:689–696. doi: 10.1016/S1473-3099(20)30198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Adedokun K.A., Olarinmoye A.O., Mustapha J.O., Kamorudeen R.T. A close look at the biology of SARS-CoV-2, and the potential influence of weather conditions and seasons on COVID-19 case spread. Infect Dis Poverty. 2020;9:77. doi: 10.1186/s40249-020-00688-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang J., Tang K., Feng K., Lv W. High temperature and high humidity reduce the transmission of COVID-19. SSRN Electron J. 2020 epub ahead of print. [Google Scholar]
- 65.Huang Z., Huang J., Gu Q., Du P., Liang H., Dong Q. Optimal temperature zone for the dispersal of COVID-19. Sci Total Environ. 2020;736:139487. doi: 10.1016/j.scitotenv.2020.139487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Triplett M. Evidence that higher temperatures are associated with lower incidence of COVID-19 in pandemic state, cumulative cases reported up to March 27, 2020. MedRxiv. 2020 epub ahead of print. [Google Scholar]
- 67.Meo S.A., Abukhalaf A.A., Alomar A.A., Al-Beeshi I.Z., Alhowikan A., Shafi K.M. Climate and COVID-19 pandemic: effect of heat and humidity on the incidence and mortality in world's top ten hottest and top ten coldest countries. Eur Rev Med Pharmacol Sci. 2020;24:8232–8238. doi: 10.26355/eurrev_202008_22513. [DOI] [PubMed] [Google Scholar]
- 68.Rubin D., Huang J., Fisher B.T., Gasparrini A., Tam V., Song L. Association of social distancing, population density, and temerature with the instantaneous reproduction number of SARS-CoV-2 in countries across the Unites States. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.16099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ward M.P., Xiao S., Zhang Z. The role of climate during the COVID-19 epidemic in New South Wales, Australia. Transbound Emerg Dis. 2020 doi: 10.1111/tbed.13631. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Auler A.C., Cássaro F.A.M., da Silva V.O., Pires L.F. Evidence that high temperatures and intermediate relative humidity might favor the spread of COVID-19 in tropical climate: a case study for the most affected Brazilian cities. Sci Total Environ. 2020;729:139090. doi: 10.1016/j.scitotenv.2020.139090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Qiu Y., Zhao Y.B., Wang Q., Li J.Y., Zhou Z.J., Liao C.H. Predicting the angiotensin converting enzyme 2 (ACE2) utilizing capability as the receptor of SARS-CoV-2. Microbe. Infect. 2020;22:221–225. doi: 10.1016/j.micinf.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Devaux C.A., Rolain J.M., Raoult D. ACE2 receptor polymorphism: susceptibility to SARS-CoV-2, hypertension, multi-organ failure, and COVID-19 disease outcome. J Microbiol Immunol Infect. 2020;53:425–435. doi: 10.1016/j.jmii.2020.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cao Y., Li L., Feng Z., Wan S., Huang P., Sun X. Comparative genetic analysis of the novel coronavirus (2019-nCoV/SARS-CoV-2) receptor ACE2 in different populations. Cell Discov. 2020;6:11. doi: 10.1038/s41421-020-0147-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen Y.Y., Zhang P., Zhou X.M., Liu D., Zhong J.C., Zhang C.J. Relationship between genetic variants of ACE2 gene and circulating levels of ACE2 and its metabolites. J Clin Pharm Ther. 2018;43:189–195. doi: 10.1111/jcpt.12625. [DOI] [PubMed] [Google Scholar]
- 75.Benjafield A.V., Wang W.Y.S., Morris B.J. No association of angiotensin-converting enzyme 2 gene (ACE2) polymorphisms with essential hypertension. Am J Hypertens. 2004;17:624–628. doi: 10.1016/j.amjhyper.2004.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Palmer B.R., Jarvis M.D., Pilbrow A.P., Ellis K.L., Frampton C.M., Skelton L. Angiotensin-converting enzyme 2 A1075G polymorphism is associated with survival in an acute coronary syndromes cohort. Am Heart J. 2008;156:752–758. doi: 10.1016/j.ahj.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 77.Rieder M.J., Taylor S.L., Clark A.G., Nickerson D.A. Sequence variation in the human angiotensin converting enzyme. Nat Genet. 1999;22:59–62. doi: 10.1038/8760. [DOI] [PubMed] [Google Scholar]
- 78.Yamamoto N., Ariumi Y., Nishida N., Yamamoto R., Bauer G., Gojobori T. SARS-CoV-2 infections and COVID-19 mortalities strongly corretated with ACE1 I/D genotype. Gene. 2020;758:144944. doi: 10.1016/j.gene.2020.144944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pati A., Mahto H., Padhi S., Panda A.K. ACE deletion allele is associated with susceptibility to SARS-CoV-2 infection and mortality rate: an epidemiological study in the Asian population. Clin Chim Acta. 2020;510:455–458. doi: 10.1016/j.cca.2020.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Marshall R.P., Webb S., Bellingan G.J., Montgomery H.E., Chaudhari B., McAnulty R.J. Angiotensin converting enzyme insertion/deletion polymorphism is associated with susceptibility and outcome in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2002;166:646–650. doi: 10.1164/rccm.2108086. [DOI] [PubMed] [Google Scholar]
- 81.Hatami N., Ahi S., Sadeghinikoo A., Foroughian M., Javdani F., Kalani N. Worldwide ACE (I/D) polymorphism may affect COVID-19 recovery rate: an ecological meta-regression. Endocrine. 2020;68:479–484. doi: 10.1007/s12020-020-02381-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Atadzhanov M., Mwaba M.H., Mukomena P.N., Lakhi S., Mwaba P., Rayaprolu S. Frequency of APOE, MTHFR and ACE polymorphisms in the Zambian population. BMC Res Notes. 2014;7:194. doi: 10.1186/1756-0500-7-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bayoumi R.A., Simsek M., Yahya T.M., Bendict S., Al-Hinai A., Al-Barwani H. Insertion-deletion polymorphisms in the angiotensin-converting enzyme (ACE) gene among Sudanese, Somalis, Emiratis, and Omanis. Hum Biol. 2006;78:103–108. doi: 10.1353/hub.2006.0022. [DOI] [PubMed] [Google Scholar]
- 84.Tchelougou D., Kologo J.K., Karou S.D., Yaméogo V.N., Bisseye C., Djigma F.W. Renin-angiotensin system genes polymorphisms and essential hypertension in Burkina-Faso, West Africa. Int J Hypertens. 2015;2015:979631. doi: 10.1155/2015/979631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mohilisa R.R., Rayner B.R., Owen E.P., Schwager S.L.U., Stark J.S., Badri M. Association of B2 receptor polymorphisms and ACE activity with ACE inhibitor-induced angioedema in Black and mixed-race South Africans. J Clin Hypertens. 2013;15:413–419. doi: 10.1111/jch.12104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bonnet F., Patel S., Laville M., Balkau B., Favuzzi A., Monti L.D., Lalic N. Influence of the ACE gene insertion/deletion polymorphism on insulin sensitivity and impaired glucose tolerance in healthy subjects. Diabetes Care. 2008;31:789–794. doi: 10.2337/dc07-1788. [DOI] [PubMed] [Google Scholar]
- 87.Irham L.M., Chou W.H., Calkins M.J., Adikusama W., Hsieh S.L., Chang W.C. Genetic variants that influence SARS-CoV-2 receptor TMPRSS2 expression among population cohorts from multiple continents. Biochem Biophys Res Commun. 2020;529:263–269. doi: 10.1016/j.bbrc.2020.05.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ju B., Zhang Q., Ge J., Wang R., Sun J., Ge X. Human neutralizing antibodies elicited by SARS-CoV-2 infection. Nature. 2020;584:115–119. doi: 10.1038/s41586-020-2380-z. [DOI] [PubMed] [Google Scholar]
- 89.Seydoux E., Homad L.J., MacCamy A.J., Parks R., Hurlburt N.K., Jennewein M.F. Analysing of a SARS-CoV-2 infected individual reveals development of potent neutralizing antibodies with limites somatic mutation. Immunity. 2020;53:98–105. doi: 10.1016/j.immuni.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhao J., Zhao J., Mangalam A.K., Channappanavar R., Fett C., Meyerholz D.K. Airway memory CD4+ T cells mediate protective immunity against emerging respiratory coronaviruses. Immunity. 2016;44:1379–1391. doi: 10.1016/j.immuni.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhu Z., Chakraborti S., He Y., Roberts A., Sheahan T., Xiao D. Potent cross-reactive neutralization of SARS coronavirus isolates by human monoclonal antibodies. Proc Natl Acad Sci U S A. 2007;104:12123–12128. doi: 10.1073/pnas.0701000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pinto D., Park Y.J., Beltramello M., Walls A.C., Tortorici M.A., Bianchi S. Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody. Nature. 2020;583:290–295. doi: 10.1038/s41586-020-2349-y. [DOI] [PubMed] [Google Scholar]
- 93.Lv H., Wu N.C., Tsang O.T.Y., Yuan M., Perera R.A.P.M., Leung W.S. Cross-reactive antibody response between SARS-CoV-2 and SARS-CoV infections. Cell Rep. 2020;31:107725. doi: 10.1016/j.celrep.2020.107725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yang R., Lan J., Huang B., Mingqing Lu R.A., Wang W., Wang W. Lack of antibody-mediated cross-protection between SARS-CoV-2 and SARS-CoV infections. EBioMed. 2020;58:102890. doi: 10.1016/j.ebiom.2020.102890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Anderson D.E., Tan C.W., Cia W.N., Young B.E., Linster M., Low J.H. Lack of cross-neutralization by SARS patient sera towards SARS-CoV-2. Emerg Microbe. Infect. 2020;9:900–902. doi: 10.1080/22221751.2020.1761267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Grifoni A., Weiskopf D., Ramirez S.I., Mateus J., Dan J.M., Moderbacher C.R. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020;181:1489–1501. doi: 10.1016/j.cell.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mateus J., Grifoni, Tarke A., Sidney J., Ramirez S.I., Dan J.M. Selective and cross-reactive SARS-CoV-2 T cell epitopes in unexposed humans. Science. 2020 doi: 10.1126/science.abd3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Braun J., Loyal L., Frentsch M., Wendisch D., Georg P., Kurth F. SARS-CoV-2-reactive T cells in healthy donors and patients with COVID-19. Nature. 2020 doi: 10.1038/s41586-020-2598-9. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 99.Le Bert N., Tan A.T., Kunasegaran K., Tham C.Y.L., Hafezi M., Chia A. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS. and uninfected controls. Nature. 2020 doi: 10.1038/s41586-020-2550-z. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 100.Sekine T., Perez-Potti A., Rivera-Ballesteros O., Stralin K., Gorin J.B., Olsson A. Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID-19. BioRxiv. 2020 doi: 10.1016/j.cell.2020.08.017. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Weiskopf D., Schmitz K.S., Raadsen M.P., Grifoni A., Okba N.M.A., Endeman H. Phenotype and kinetic of SARS-CoV-2-specific T cells in COVID-19 patients with acute respiratory distress syndrome. Sci Immunol. 2020;5 doi: 10.1126/sciimmunol.abd2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hicks J., Klumpp-Thomas C., Kalish H., Shunmugavel A., Mehalko J., Denson J.P. Serologic cross-reactivity of SARS-CoV-2 with endemic and seasonal Betacoronaviruses. MedRxiv. 2020 doi: 10.1007/s10875-021-00997-6. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kim L., Whitaker M., O'Halloran A., Kambhampati A., Chai S.J., Reingold A. Hospitalization rates and characteristics of children aged <18 years hospitalized with laboratory-confirmed COVID-19 - COVID-NET, 14 states, March 1 – july 25, 2020. MMWR Morb Mortal Wkly Rep. 2020;39:1081–1088. doi: 10.15585/mmwr.mm6932e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Maltezou H.C., Vorou R., Papadima K., Kossyvakis A., Spanakis N., Gioula G. Transmission dynamis of SARS-CoV-2 within families with children in Greece: a study of 23 clusters. J Med Virol. 2020 doi: 10.1002/jmv.26394. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Morand A., Fabre A., Minodier P., Boutin A., Vanel N., Bosdure E. COVID-19 virus and children: what do we know? Arch Pediatr. 2020;27:117–118. doi: 10.1016/j.arcped.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Raymond F., Carbonneau J., Boucher N., Robitaille L., Boisvert S., Wu W.K. Comparison of automated microarray detection with real-time PCR assays for detection of respiratory viruses in specimens obtained from children. J Clin Microbiol. 2009;47:743–750. doi: 10.1128/JCM.01297-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhao Y., Lu R., Shen J., Xie Z., Liu G., Tan W. Comparison of viral and epidemiological profiles of hospitalized children with severe acute respiratory infection in Beijing and Shanghai, China. BMC Infect Dis. 2019;19:729. doi: 10.1186/s12879-019-4385-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gilca R., Carazo S., Amini R., Charest H., De Serres G. Common human coronavirus seem at least as severe as influenza in patients hospitalized with acute respiratory infection: results from 8-year hospital-based surveillance in Quebec, Canada. J Infect Dis. 2020:jiaa477. doi: 10.1093/infdis/jiaa477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Escobar L.E., Molina-cruz A., Barillas-Mury C. BCG caccine protection from severe coronavirus disease 2019 (COVID-19) Proc Natl Acad Sci USA. 2020;117:17720–17726. doi: 10.1073/pnas.2008410117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.El-Gendy A.O., Saeed H., Ali A.M.A., Zawbaa H.M., Gomaa D., Harb H.S. Bacillus Calmette–Guérin vaccine, antimalarial, age and gender relation to COVID-19 spread and mortality. Vaccine. 2020;38:5564–5568. doi: 10.1016/j.vaccine.2020.06.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Covian C., Retamal-Diaz A., Bueno S.M., Kalergis A.M. Could BCG vaccination induce protective trained immunity for SARS-CoV-2? Front Immunol. 2020;11:970. doi: 10.3389/fimmu.2020.00970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nuovo G., Tili E., Suster D., Matys E., Hupp L., Magro C. Strong homology between SARS-CoV-2 envelope protein and a Mycobacterium sp. antigen allows rapid diagnosis of mycobacterial infections and may provide specific anti-SARS-CoV-2 immunity via BCG vaccine. Ann Diagn Pathol. 2020;48:151600. doi: 10.1016/j.anndiagpath.2020.151600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Katoh S., Obayashi T., Ganesh J.S., Iwasaki M., Preethy S., Abraham S.J. Cross-protection induced by encephalitis vaccines against COVID-19 might be a reason for relatively lower mortality rate in some countries. Arch Acad Emerg Med. 2020;8:e54. [PMC free article] [PubMed] [Google Scholar]
- 114.Napoli P.E., Nioi M. Global Spread of coronavirus disease 2019 and malaria: an epidemiological paradox in the early stage of a pandemic. J Clin Med. 2020;9:1138. doi: 10.3390/jcm9041138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hajizadeh R., Behnemoon M. Is the new coronavirus (COVID-19) pandemic halted by malaria epidemics? Arch Bone Jt Surg. 2020;8:319–320. doi: 10.22038/abjs.2020.47662.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Panda A.K., Tripathy R., Das B.K. Plasmodium falciparum infection may protect a population from SARS-CoV-2 infection. J Infect Dis. 2020:jiaa455. doi: 10.1093/infdis/jiaa455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.WHO . 2019. World malaria report 2019.https://www.who.int/malaria/publications/world-malaria-report-2019/en/ Availbale from: [Google Scholar]
- 118.Izoulet M. Countries which primarily use antimalarial drugs as COVID-19 treatment see slower dynamic of daily deaths. SSRN Electron J. 2020 epub ahead of print. [Google Scholar]
- 119.Gendrot M., Duflot I., Boxberger M., Delandre O., Jardot P., Le Bideau M. Antimalarial artemisinin-based combination therapies (ACT) and COVID-19 in Africa: in vitro inhibition of SARS-CoV-2 replication by mefloquine-artesunate. Int J Infect Dis. 2020 doi: 10.1016/j.ijid.2020.08.032. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cao R., Hu H., Li Y., Wang X., Xu M., Liu J. Anti-SARS-CoV-2 potentials of artemisinins in vitro. ACS Infect Dis. 2020 doi: 10.1021/acsinfecdis.0c00522. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 121.Barnard D.L., Day C.W., Bailey K., Heiner M., Montgomery R., Lauridsen L. Evaluation of immunomodulators, interferons and known in vitro SARS-CoV inhibitors for inhibition of SARS-coV replication in BALB/c mice. Antivir Chem Chemother. 2006;17:275–284. doi: 10.1177/095632020601700505. [DOI] [PubMed] [Google Scholar]
- 122.Fan H.H., Wang L.Q., Liu W.L., An X.P., Liu Z.D., He X.Q. Repurposing of clinically approved drugs for treatment of coronavirus disease 2019 in a 2019-novel coronavirus (2019-nCoV) related coronavirus model. Chin Med J (Engl) 2020;133:1051–1056. doi: 10.1097/CM9.0000000000000797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hosseini F.S., Amanlou M. Anti-HCV and anti-malaria agent, potential candidates to repurpose for coronavirus infection: virtual screening, molecular docking, and molecular dynamics simulation study. Life Sci. 2020;258:118205. doi: 10.1016/j.lfs.2020.118205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Coronavirus update (live): 6,607,848 cases and 388,575 deaths from COVID-19 virus pandemic—worldometer. https://www.worldometers.info/coronavirus/ n.d. Available from: