Abstract
Background
Mushrooms are increasingly popular around the world as a nutritional food which is an excellent source of vitamin D2. Although natural mushrooms often contain very little vitamin D2 as many are grown in the dark, they are rich in ergosterol, a precursor to vitamin D2. Ergosterol can be converted to vitamin D2 under ultraviolet radiation. Due to the high water content of fresh mushroom, its quality deteriorates rapidly after harvest, and drying is the most commonly used technology to extend the shelf life. The vitamin D2 content of dried mushrooms depends on the drying conditions used.
Scope and approach
In this review, the chemistry of the photo-conversion process of ergosterol to vitamin D2 under ultraviolet radiation is introduced. The ergosterol and vitamin D contents in different mushroom varieties are discussed. The effects of several drying methods and the influence of different drying conditions are reviewed.
Key findings and conclusions: Thermal drying in the presence of UV has been proven to convert ergosterol into vitamin D and enhance the nutritional content of all types of edible mushrooms. Solar drying, hot air drying, freeze drying, microwave drying and infrared drying can be used for mushrooms drying under selected operating conditions. A critical evaluation of published literature demonstrates the importance of applying appropriate drying methodology to maximize the nutritional value of various types of edible mushrooms.
Keywords: Ergosterol, Vitamin D2, UV, Conversion, Mushrooms, Drying
1. Introduction
Vitamin D is valued as a micronutrient required by the human body. Structurally, vitamin D is a cyclopentane polyphenolic compound associated with sterols. Classified as a fat-soluble vitamin. Vitamin D was first recognized for its role in promoting calcium absorption and bone health (Kennel, Drake, & Hurley, 2010; Pittas, Laskowski, Kos, & Saltzman, 2010). A classic symptom of vitamin D deficiency is known as rickets, which is mostly found in children. Lack of vitamin D in adults can lead to problems such as osteoporosis (Holick, 2010). In addition, insufficient vitamin D intake often affects the endocrine pancreas and immune system, leading to a variety of diseases (Arabi, Bahrami, Ranjbar, Tabesh, & Norouzy, 2020; Leuven, 2020; Wu, Cai, Liu, Zhu, & Guan, 2020). More recently, many studies have linked vitamin D deficiency to non-skeletal diseases, such as cancer (Carlberg & Muñoz, 2020), heart disease (Mokhtar, Fawzy, Allam, Amer, & Hamed, 2019), arterial stiffness (Chen et al., 2019), neuropsychiatric disorders (Kim et al., 2020), diabetes (Mirzavandi et al., 2020; Zhang, Wu, Lu, & Fei, 2020) and other chronic illnesses. In addition, studies have shown that vitamin D can be used as an adjunct to the treatment of COVID-19, which ravaged the world in 2020 and left 612,054 people dead as of 6:18pm CEST, July 22, 2020 (Annweiler, Cao, & Sabatier, 2020; Martín Giménez et al., 2020; Razdan, Singh, & Singh, 2020; WHO, 2020). Thus, vitamin D is critical for human health, both skeletal and non-skeletal.
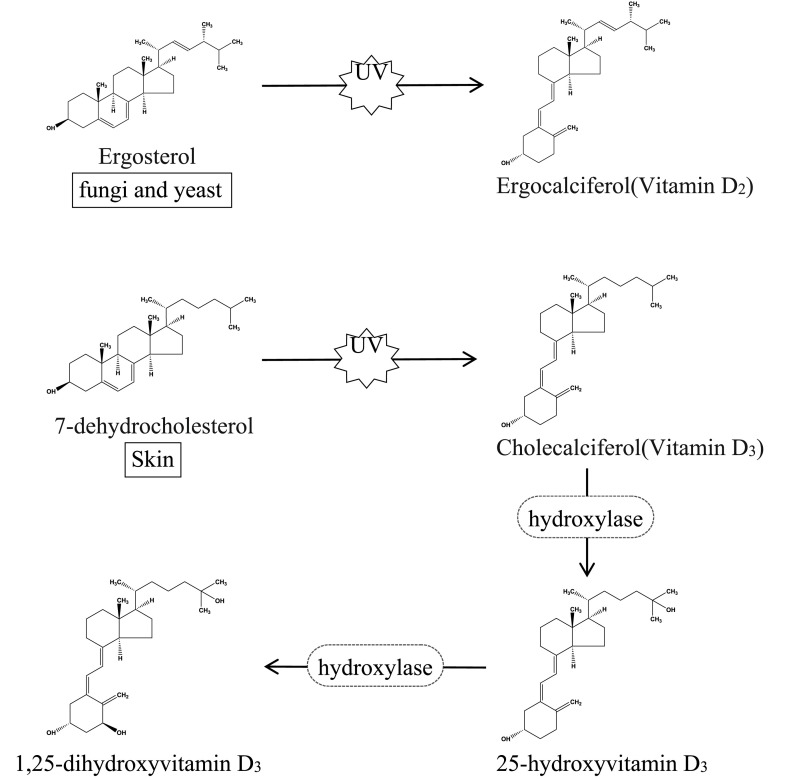
Vitamin D contains many forms, the most common of which are vitamin D2 and vitamin D3 (Taofiq, Fernandes, Barros, Barreiro, & Ferreira, 2017). Vitamin D3 (cholecalciferol) is produced mainly by ultraviolet B (UVB) radiation of skin exposed to sunlight. Unlike vitamin D3, vitamin D2 (ergocalciferol) is found mainly in fungi and yeast. Once in the blood circulation, both vitamins D2 and D3 are metabolized by enzymes in the liver to produce 25-hydroxyvitamin D (25(OH)D). Then, 25(OH)D is transported to the kidney, where it is metabolized to 1,25-dihydroxyvitamin D (1,25(OH)2D) by enzymes. Through this process of metabolism, vitamin D is eventually converted to 1,25(OH)2D, which is biologically active and thus beneficial to the body (Cardwell, Bornman, James, & Black, 2018; Charoenngam, Shirvani, & Holick, 2019; Kennel et al., 2010; Zerwekh, 2008). Fig. 1 shows the two types of vitamin D and their conversion to activity.
Fig. 1.
Two types of vitamin D and their conversion to activity.
It is now recognized that exposure to sunlight and dietary foods are the most important ways for humans to obtain vitamin D. Due to lifestyle changes, however, regular exposure to the sun has decreased resulting in vitamin D deficiency (Holick, 2010). Vitamin D in the diet is also a problem because only a few foods contain it naturally (McCourt, McNulty, Walton, & O'Sullivan, 2020). Studies have found that about 1 billion people around the world need vitamin D fortification, this has become a major global public health problem and a cause for concern (Charoenngam et al., 2019). Food fortification is an important option for getting adequate vitamin D (Moulas & Vaiou, 2018), however, it is limited by strict regulatory policies. Thus, the exploration of new foods rich in vitamin D and the promotion of their consumption has become a theme.
Mushrooms are an excellent source of vitamin D in addition to animal foods (Guan et al., 2016; Mau, Chen, & Yang, 1998; Simon, Phillips, Horst, & Munro, 2011). Although cultivated mushrooms lack vitamin D during the growth stage due to the absence of sunlight, they are rich in ergosterol, a precursor of vitamin D2 that can be converted into vitamin D2 by exposure to ultraviolet light and heat (Nölle, Argyropoulos, Ambacher, Müller, & Biesalski, 2017a). Ultraviolet light from sunlight or artificial light catalyzes this conversion process through a series of photochemical and thermal reactions. It's similar to how human skin produces vitamin D3 when exposed to ultraviolet light (Plum & DeLuca, 2009). Fresh mushrooms are perishable and have a limited shelf life due to their high water activity. Drying is a common method used in the post-harvest processing of fresh food. It can not only prolong their storage life, but also enhance their nutritional value and improve their functional properties (Yu, Huang, Zhang, Zhu, & Qin, 2020; Xu, Yuan, et al., 2020; Hnin, Zhang, Wang, & Devahastin, 2019; Li, Zhang, Chitrakar, & Jiang, 2020). In addition to achieving these traditional drying purposes, mushroom drying is particularly notable for the enrichment of vitamin D. A large number of studies on vitamin D enrichment in mushroom drying have been carried out (Sławińska et al., 2016; Argyropoulos, Heindl, & Müller, 2011b; Mutukwa, 2014; Nölle, Argyropoulos, Ambacher, Müller, & Biesalski, 2017b; Urbain & Jakobsen, 2015; Zhao et al., 2019). The results suggest that vitamin D in mushrooms can be fortified by appropriate drying methods, such as sun drying, hot air drying, and so on. Dried mushrooms also have a more intense flavor and are used as a valuable ingredient in a variety of recipes (Argyropoulos, Heindl, & Müller, 2011a).
This paper reviews the potential of different types of mushrooms as vitamin D supplements. Since degree of ultraviolet light exposure of mushrooms during drying leads to significant differences in vitamin D production, the paper will also introduce different drying methods for mushrooms and their effects on the production of vitamin D. The aim is to draw attention of producers and consumers to the most suitable mushroom drying method to maximize the nutritional as well as other key properties of dried mushrooms.
2. Vitamin D in mushroom
2.1. Nutritional content of mushrooms
Mushrooms are consumed widely in many countries in fresh and dried forms for their unique sensory quality (Kalač, 2009; Wang et al., 2014). They are rich in carbohydrates (glycogen, chitin, mannitol, trehalose and β-glucans), proteins, peptides, dietary fibers, vitamins, mineral elements, and unsaturated fatty acids. Mushrooms are a good source of protein with amino acids composition comparable to that of animal proteins. Glutamine, leucine, glutamic, valine and aspartic acids are abundant in mushrooms (Reis, Barros, Martins, & Ferreira, 2012). It is estimated that the dry matter of most mushrooms contains 50 to 65 percent carbohydrates, 19 to 35 percent protein and 2 to 6 percent fat, more of which are unsaturated fatty acids such as oleic acid and linoleic acid (Rathore, Prasad, & Sharma, 2017). Table 1 shows the proximate composition of some edible mushrooms. In addition, mushrooms have been found to contain specific bioactive compounds such as phenolic compounds and other antioxidants, making them of therapeutic value, including strengthening the immune system, treating and preventing heart disease, high blood pressure, and cancers. Mushrooms are also claimed to have anti-diabetes, anti-tumor, anti-fungal, anti-inflammatory, antiviral, antibacterial, and liver protection effects (Heleno et al., 2015).
Table 1.
Proximate composition of some edible mushrooms (% of dry matter).
| Species | Photo | Carbohydrates | Crude protein | Lipids | Ash | Ref. |
|---|---|---|---|---|---|---|
| Agaricus bisporus (white) |  |
74.0 | 14.1 | 2.2 | 9.7 | Reis et al. (2012) |
| Agaricus bisporus (brown) |  |
71.5 | 15.4 | 1.7 | 11.4 | Reis et al. (2012) |
| Flammulina velutipes |  |
70.9 | 17.9 | 1.8 | 9.4 | Pereira, Barros, Martins, and Ferreira (2012) |
| Hericium erinaceus |  |
57.0 | 22.3 | 3.5 | 9.4 | Mau, Lin, Ma, and Song (2001) |
| Lactarius deliciosus |  |
51.5 | 26.6 | 7.4 | 6.6 | Akata, Ergonul, and Kalyoncu (2012) |
| Lentinula edodes |  |
64.4 | 22.8 | 2.1 | 6.0 | Bisen, Baghel, Sanodiya, Thakur, and Prasad (2010) |
| Pleurotus eryngii |  |
81.4 | 11.0 | 1.5 | 6.2 | Reis et al. (2012) |
| Pleurotus ostreatus |  |
35.4 | 28.4 | 4.7 | 8.6 | Ahmed, Abdullah, Ahmed, and Bhuyan (2013) |
| Tricholoma matsutake |  |
36.7 | 14.3 | 5.0 | 8.9 | Liu, Wang, Zhou, Guo, and Hu (2010) |
| Volvariella volvacea |  |
50.0 | 28.0 | 3.3 | 10.0 | Mshandete and Cuff (2008) |
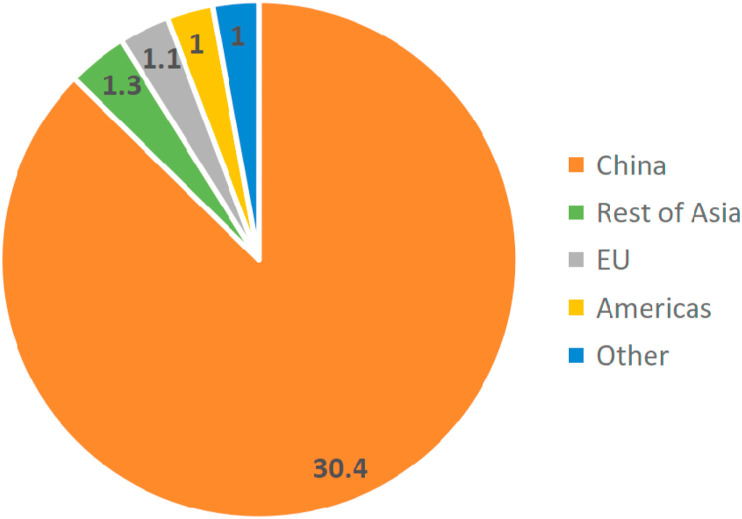
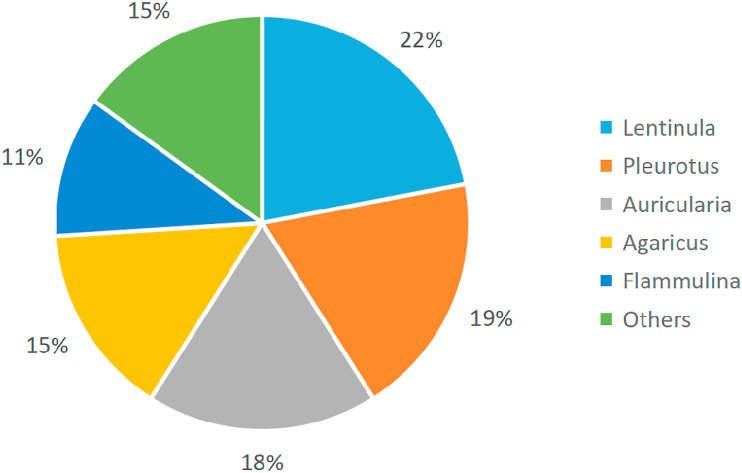
The global mushroom industry includes edible mushrooms, medicinal mushrooms and wild mushrooms. Since 1978, the yield of edible mushrooms grown around the world has increased significantly, with the yield in 2013 more than 30 times that of 1978. China is a major producer of cultivated edible mushrooms. Statistics show that China's mushroom production exceeded 30 billion kg in 2013, accounting for approximately 87% of the world's total output, as shown in Fig. 2 (Royse, Baars, & Tan, 2017). Although there are about 2000 edible mushrooms found in nature, only 25 are widely accepted worldwide (Haro et al., 2020; Kalač, 2013). Among them, Lentinula, Pleurotus, Auricularia, Agaricus and Flammulina are five main genera, accounting for approximately 85% of the world's total mushroom production. Lentinula (22%) is the most popular and widely planted, followed by Pleurotus (19%), Auricularia (17%), Agaricus (15%) and Flammulina (11%), as shown in Fig. 3 (Royse et al., 2017). The cultivation of these species is more simple and convenient, and requires less environmental controls and shorter growing period (Reis et al., 2012). Statistics show that the current per capita annual consumption of mushrooms is about 5 kg, and is still increasing year by year (Royse et al., 2017).
Fig. 2.
Mushroom production in major regions of the world, 2013 (billion kg).
Fig. 3.
World's main mushroom genera and their yields, 2013
2.2. Sterol and vitamin D content in mushrooms
Mushrooms belong to the class fungi that are biologically different from plants and animals, although they are often used as vegetables in cooking (Cardwell et al., 2018). Studies have found that fungi are rich in phytosterols, which have been shown to lower serum cholesterol levels and protect against colon cancer. Various sterols such as methylene cholesterol, ergosta-5,7-dienol, fungisterol and 24-methyl cholesterol have been identified in fungi (Mattila, Lampi, Ronkainen, Toivo, & Piironen, 2002). Of particular concern is ergosterol in the cell walls of mushrooms. Ergosterol can strengthen mushroom cell membrane, regulate membrane fluidity and assist membrane transport, which is similar to cholesterol in animals (Cardwell et al., 2018). When exposed to sunlight or ultraviolet light, ergosterol is converted to vitamin D2 precursors by photochemical reactions, and then isomerized to ergocalciferol, also known as vitamin D2, under thermal conditions. For this reason, mushrooms are the only vegetarian source of vitamin D, and provide far more vitamin D than the body needs every day, despite the fact that wild mushrooms naturally contain very little vitamin D (Mattila et al., 2002; Rathore et al., 2017). Although mushrooms contain other plant sterols, their total content also reduces the absorption of dietary cholesterol. However, it is far below the recommended amount of about 2 g of phytosterol per day to lower human serum cholesterol levels. Therefore, ergosterol is more interesting than plant sterols in transforming vitamin D perspective (Teichmann, Dutta, Staffas, & Jägerstad, 2007).
Fresh mushrooms contain low amounts of vitamin D2, and some were even identified to be vitamin D2 free. However, ergosterol is abundant in various mushrooms. In addition, some lesser amounts of sterols such as ergosta-7,22-dienol, ergosta-5,7-dienol and ergosta-7-enol are also found in fresh mushrooms. Ergosterol content was varied in different mushroom types, even in different tissues of the same type (Jasinghe & Perera, 2005). Jasinghe et al. studied the content of ergosterol in different cultivated edible mushrooms, including button, enoki, shiitake, abalone and oyster mushrooms. Results showed that the content of ergosterol was highest in button mushrooms (7.80 mg ergosterol/g DM (dry matter)) among them, followed by shiitake mushrooms (6.05 mg/g DM), oyster mushrooms (4.40 mg/g DM) and abalone mushrooms (4.35 mg/g DM), and enoki mushrooms (0.68 mg/g DM) was the lowest. Results also showed that ergosterol distribution was significantly different in different tissues of mushrooms, the highest content in gills and the lowest content in stems (Jasinghe & Perera, 2005). Phillips et al. studied the ergosterol and vitamin D content in 10 types of mushrooms, among which shiitake mushrooms had the highest value of 84.9 mg/100 g FW (fresh weight), followed by maitake mushrooms (79.2 mg/100 g FW). The lowest ergosterol content was found in morel mushrooms (26.3 mg/100 g FW) (Phillips et al., 2011). In these studies, natural vitamin D levels in mushrooms were also identified. Except for varieties that may be exposed to sunlight, mushroom varieties commonly consumed are relatively poor in vitamin D. The vitamin D content of white button, enoki, crimini, portabella and other mushrooms was <0.25 μg/100 g fresh weight. Jasinghe et al. showed that shiitake mushrooms did not contain any vitamin D2. Similar results were reported by Mattila et al. (Jasinghe & Perera, 2005; Mattila et al., 2002; Phillips et al., 2011). Table 2 shows ergosterol and vitamin D2 contents in some fresh mushroom samples.
Table 2.
Ergosterol and vitamin D2 contents in some fresh mushroom samples.
| Mushroom | Vitamin D2 (μg/100 g f. w.) | Ergosterol (mg/100 g f.w.) | Ergosta-7,22-dienol (mg/100 g f.w.) | Ergosta-5,7-dienol (mg/100 g f.w.) | Ergosta-7-enol (mg/100 g f.w.) | Total sterol (mg/100 g f.w.) | Ref. |
|---|---|---|---|---|---|---|---|
| Agaricus bisporus (white) | 0.6 | 44.6 | 1.9 | 1.9 | 0.9 | 47.2 | Teichmann et al. (2007) |
| Agaricus bisporus (white) | ___ | 50.3 | 1.2 | 7.2 | ___ | ___ | Mattila et al. (2002) |
| Agaricus bisporus (brown) | 0.3 | 39.5 | 2.6 | 3.3 | 6.5 | 68.4 | Teichmann et al. (2007) |
| Agaricus bisporus (brown) | ___ | 47.0 | 1.1 | 3.7 | ___ | ___ | Mattila et al. (2002) |
| Cantharellus | 5.3 | 46.3 | <1.7 | 4.5 | 2.0 | ___ | Phillips et al. (2011) |
| Flammulina velutipes | 0.1 | 35.5 | <1.5 | 16.5 | 2.3 | ___ | Phillips et al. (2011) |
| Lentinula edodes | 1.2 | 107.9 | 8.5 | 7.0 | 9.1 | 103.8 | Teichmann et al. (2007) |
| Lentinus edodes | 0.4 | 84.9 | 2.3 | 6.5 | 5.0 | ___ | Phillips et al. (2011) |
| Pleurotus ostreatus | 0.7 | 60.7 | 4.7 | 5.9 | 6.2 | 72.4 | Teichmann et al. (2007) |
| Pleurotus ostreatus | ___ | 53.9 | 1.2 | 6.7 | ___ | ___ | Mattila et al. (2002) |
It was found that although the natural vitamin D content in mushrooms is very low, high amount of sterols, especially ergosterol, may be a potential source of vitamin D. Jasinghe et al. showed a significant increase in vitamin D levels in mushrooms exposed to ultraviolet light, with a maximum vitamin D2 content of 184 μg/g DM. Even for Button mushrooms where the minimum vitamin D2 content was observed, the vitamin D2 content was 22.9 μg/g DM, far more than the original content. Five grams of fresh Shiitake exposed to ultraviolet light for 15 min can produce the daily amount of vitamin D required by adults (10 μg/day) (Jasinghe & Perera, 2006). Teichmann et al. found that vitamin D2 levels in Cantharellus tubaeformis and Agaricus bisporus (white) increased significantly after 2 h of UV-C exposure, from 1.6 ± 0.02 and 0.07 ± 0.004 μg/g DM to 14.0 ± 1.30 and 10.1 ± 0.31 μg/g DM, corresponding to 9-fold and 14-fold increase, respectively (Teichmann et al., 2007). Mau et al. found that after UV-B and UV-C irradiation on different type of mushrooms for 2 h, vitamin D2 content in all mushrooms increased significantly. Table 3 shows effects of UV irradiation on contents of vitamin D2 in mushrooms. In addition, it was also found that ergosterol decreased in mushrooms exposed to UV (Hu et al., 2020), which further indicated that UV promoted ergosterol conversion to vitamin D2.
Table 3.
Effects of UV irradiation on contents of vitamin D2 in mushrooms.
| Mushroom | Radiation source | Vitamin D2 content (μg/g of dry weight) |
Ref. | |
|---|---|---|---|---|
| Control | UV irradiation | |||
| Agaricus bisporus | UV-C | 2.20 | 7.30 | Mau et al. (1998) |
| UV-C | 0.07 | 10.14 | Teichmann et al. (2007) | |
| UV-C | 7.90 | 13.40 | Guan et al. (2016) | |
| UV-C | ___ | 23.1 | Koyyalamudi, Jeong, Song, Cho, and Pang (2009) | |
| UV-B | 0.01 | 7.98 | Roberts, Teichert, and McHugh (2008) | |
| UV-B | 2.20 | 12.48 | Mau et al. (1998) | |
| UV-B | 0.05 | 4.10 | Simon et al. (2011) | |
| UV-B | <5 | 16.7 | Ko et al. (2008) | |
| UV-A | 0.07 | 0.15 | Teichmann et al. (2007) | |
| Agaricus bitorquis | UV-C | 4.01 | 5.32 | Mau et al. (1998) |
| Agaricus blazei | UV-B | ___ | 22.13 | Huang, Lin, and Tsai (2015) |
| Agrocybe cylindracea | UV-B | 0.95 | 42.36 | Huang et al. (2015) |
| Auricularia polytricha | UV-B | ___ | 60.29 | Huang et al. (2015) |
| Cantharellus tubaeformis | UV-A | 1.55 | 1.57 | Teichmann et al. (2007) |
| UV-C | 1.55 | 14.03 | Teichmann et al. (2007) | |
| Hypsizigus marmoreus | UV-B | 1.62 | 15.06 | Huang et al. (2015) |
| Lentinula edodes | UV-B | 2.16 | 6.58 | Mau et al. (1998) |
| UV-B | 0.35 | 15.10 | Huang et al. (2015) | |
| UV-B | 2.77 | 106.4 | Ko et al. (2008) | |
| Pholiota nameko | UV-B | ___ | 61.78 | Huang et al. (2015) |
| Pleurotus eryngii | UV-B | 1.56 | 28.71 | Huang et al. (2015) |
| Pleurotus citrinopileatus | UV-B | 3.93 | 208.65 | Huang et al. (2015) |
| Pleurotus ferulae | UV-B | 1.65 | 52.30 | Huang et al. (2015) |
| Pleurotus ostreatus | UV-B | 0.83 | 69.00 | Huang et al. (2015) |
| UV-B | 1.78 | 27.89 | Banlangsawan and Sanoamuang (2016) | |
| UV-C | ___ | 48.19 | Ruslan, Reza, and Damayanti (2011) | |
| Pleurotus salmoneostramineus | UV-B | 2.13 | 93.29 | Huang et al. (2015) |
| Volvariella volvacea | UV-B | 3.86 | 7.58 | Mau et al. (1998) |
3. Drying of mushrooms
Mushrooms are highly perishable as they have a high water content of about 87–95 percent (wet basis). Among the different preservation methods, drying is the most frequently used one (Huang & Zhang, 2012; Zhang et al., 2017). Mushroom drying is a standard postharvest technique to ensure long-term supply by reducing water activity to microbial safety levels. It also minimizes many moisture-mediated reactions and effectively inhibits enzymatic and nonenzymatic browning. In addition, drying also increases the variety of mushrooms for consumers. Dried mushrooms have unique texture, flavor and nutritional value and are used in a variety of food formulations (Argyropoulos et al., 2011b; Arora, Shivhare, Ahmed, & Raghavan, 2003; Mujumdar & Law, 2010). Suitable mushroom drying techniques can enhance the unique flavor of mushrooms, maintain color, produce better texture, and can be fully rehydrated in water (Argyropoulos, Khan, & Müller, 2011). To meet these demands, a number of studies have been published on mushroom drying techniques and better maintaining their quality. Different drying techniques such as solar drying (Jo et al., 2009), hot air drying (Arora et al., 2003), freeze drying (Sławińska et al., 2016), vacuum drying (Argyropoulos et al., 2011b), microwave or ultrasound assisted drying (Devi, Zhang, & Law, 2018) and infrared drying (Darvishi, Najafi, Hosainpour, Khodaei, & Aazdbakht, 2013) have been involved.
3.1. Solar drying of mushrooms
In many countries, natural sun drying is still often used because of its advantages of energy saving, low investment and simple operation. The most common operation is to lay the mushrooms thinly on a tray, and exposing them to sunlight and wind. However, due to vulnerability to pollution, incomplete drying, microbial growth and other reasons, the open sun drying process can cause considerable loss and quality deterioration. Moreover, natural sun drying is highly dependent on weather conditions and thus limited. In order to overcome these drawbacks, some solar dryers have been developed to improve the efficiency of drying, in which the mushrooms are kept inside a chamber (Nölle et al., 2017b). Solar drying is a good way to deal with the energy crisis and global greenhouse effect, since drying itself is an energy-intensive processing technology. Solar dryers used to be mainly used in high radiation areas, but are now being encouraged to be used in a wider range, including some areas of moderate radiation, through more advanced solar dryers. Reyes et al. used a hybrid solar dryer to dehydrate mushrooms, which included a 3 square meter solar panel and electrical resistances. Solar energy saved 3.5 to 12.5 percent of the total energy (Reyes, Mahn, Cubillos, & Huenulaf, 2013). A hybrid solar dryer used for mushroom dehydration is equipped with a solar panel with a total exposed surface of 10 square meter, electrical resistances and paraffin wax as phase change materials, which also achieve energy-saving effect in mushroom drying (Reyes, Mahn, & Vásquez, 2014). Jo et al. studied the effects of different drying methods on characteristics of postharvest Phellinus gilvus mushroom. The results showed that sun drying and far infrared drying were good for the appearance of mushrooms, while oven (60 °C) and hot air dryer (60 °C) were not. The solar drying method is superior to the hot air dryer (60 °C) in quality (appearance, chroma, anti-tumor activity) and economical (Jo et al., 2009).
3.2. Hot air drying
Hot air drying is the simplest dryer widely used worldwide (Argyropoulos et al., 2011b). This technique is commonly used for drying of wild mushroom species such as Craterellus cornucopioides, Boletus edulis, Morchella spp., Cantharellus cibarius (Argyropoulos et al., 2011), and Lentinula edodes (Zhao et al., 2019). A common method of hot air drying is to place mushrooms on a tray and push them into a cabinet or tunnel dryer. Heating to a temperature of 50–70 °C (Argyropoulos et al., 2011), along with air convection, the hot wind takes away the moisture inside the mushroom, thus achieving the purpose of drying. From the perspective of drying speed alone, high temperature and air convection speed are conducive to the drying of mushrooms. However, if the water on the mushroom surface moves too quickly, the internal water does not have time to move to the surface, the surface can become overheated. As this process progresses, the moisture content of the mushroom decreases, the drying rate requires more energy and the drying rate slows significantly, and drying becomes more and more difficult. Improper application of hot air drying on mushrooms can cause characteristic color changes and structural deformations (Kotwaliwale, Bakane, & Verma, 2007). In particular, if the drying temperature is higher than the optimal conditions, the mushroom will become hard, maillard and other browning reaction will occur through the sugar and protein in the mushroom, resulting in a significant browning effect. In addition, improper drying can also affect the flavor, resulting in smoky flavor (Argyropoulos et al., 2011). To overcome these problems, alternative methods such as freeze drying, microwave drying and infrared drying have emerged one after another. These drying methods have been implemented separately or combined with hot air drying.
3.3. Freeze drying
Freeze drying is a very effective drying method, which has been applied for dehydration of high value heat sensitive foods. Compared with other drying methods, the phase change of moisture in freeze-drying is special. In the freeze-drying process, frozen water sublimes directly from solid ice to water vapor. However, other dryer types, such as hot air drying, vacuum drying, water in the material is converted into vapor under liquid state. Due to low temperature and lack of liquid water, most microbial activity and enzyme reactions are stopped, resulting in high-quality products. Low temperature conditions can better protect the nutrients in mushrooms, especially the heat sensitive components. The loss of aromatic substances in mushrooms can be reduced to a minimum. In freeze drying, water molecules vaporize directly from the solid form, which protects the main structure and shape of the mushroom with minimum volume reduction. The surface of the material will not form hard thin skin, thus making the product rehydrating quickly after freeze drying. Currently, freeze drying is considered one of the best drying methods due to its excellent performance in maintaining the quality of dried products (Duan, Zhang, Li, & Mujumdar, 2008; Ratti, 2001; Wang, Zhang, Mujumdar, & Mothibe, 2013). Research on freeze drying has been carried out on some mushroom species such as button mushroom (Pei et al., 2014) and shiitake mushroom (Zhao et al., 2016). Nevertheless, despite its many advantages, freeze drying has long been known as the most expensive process for producing dehydrated products. Freeze drying is an energy-consuming operation because it requires freezing fresh products at very low temperatures. In addition, some processes involve reducing the pressure in the drying chamber to a certain degree of vacuum, sublimating water vapor by heating, and condensing water vapor, all of which require energy. Freeze drying also requires expensive equipment (Donsì, Ferrari, & Matteo, 2001; Irzyniec, Klimczak, & Michalowski, 1995). Therefore, the application of freeze drying technology is limited. To eliminate this problem, improve drying efficiency and reduce energy consumption, many attempts have focused on improving the entire freeze drying process. Some assistive technologies such as microwave, infrared and ultrasonic drying are increasingly used.
3.4. Microwave-related drying of mushrooms
Compared with conventional drying methods, microwave drying has the advantages of faster drying due to volumetric heating, less heat loss, smaller equipment footprint and easy automatic control. The correct microwave drying technology can greatly shorten the drying time and yield better product quality. Hence microwave technology has been paid increasing attention in various fields of drying (Funebo & Ohlsson, 1998; Mujumdar & Law, 2010; Soysal, Öztekin, & Eren, 2006; Zhang, Tang, Mujumdar, & Wang, 2006a, b). As a kind of heat-sensitive product, mushroom can be dried by microwave or microwave-assisted drying instead of conventional thermal drying. Nevertheless, improper use of microwave energy can also lead to irreversible changes in product quality, such as charred edge, excessive oxidation and so on. Continuous exposure to microwaves can also cause local overheating, especially if the product geometry is undefined (Das & Arora, 2018). Walde et al. studied effects of different drying methods on dehydration of button mushrooms and oyster mushrooms. The results showed that compared with vacuum drying, fluidized bed drying and cabinet moisture drying, microwave drying takes the shortest time and has the largest diffusion coefficient. However, the results also showed that prolonged exposure can cause the mushrooms to scorch, especially around the edges, and also cause the surfaces to harden. Microwaves alone are not ideal for mushroom drying, although the drying time is short (Walde, Velu, Jyothirmayi, & Math, 2006).
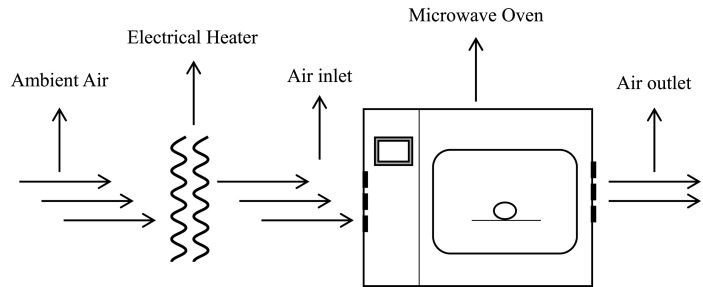
In order to overcome these limitations, some researches put forward strategies to improve the application effect of microwave. They are as follows: (1) the combination of microwave and conventional drying technology, such as microwave and vacuum drying to reduce the drying temperature; (2) pulse or intermittent application of microwave energy to avoid local overheating caused by continuous exposure (Gunasekaran, 1990, 1999; Kaensup, Chutima, & Wongwises, 2002). Das et al. developed a method to rapidly dry mushrooms by alternating microwave and convective hot air, which enabled the mushrooms to reach the commercial quality standard in a relatively short time of 72 min (Das & Arora, 2018). Fig. 4 shows the experimental principle of rapid drying by microwave-assisted convective hot air. Argyropoulos et al. found that compared with conventional hot air drying, the mushrooms combined with hot air and microwave vacuum drying were of excellent quality, with less overall color change, soft texture and high rehydration rate. Combined drying can make mushroom structure fluffy and crispy, which has the potential for developing novel snacks (Argyropoulos et al., 2011b).
Fig. 4.
Experimental principle of rapid drying by microwave-assisted convective hot air drying.
3.5. Far-infrared drying
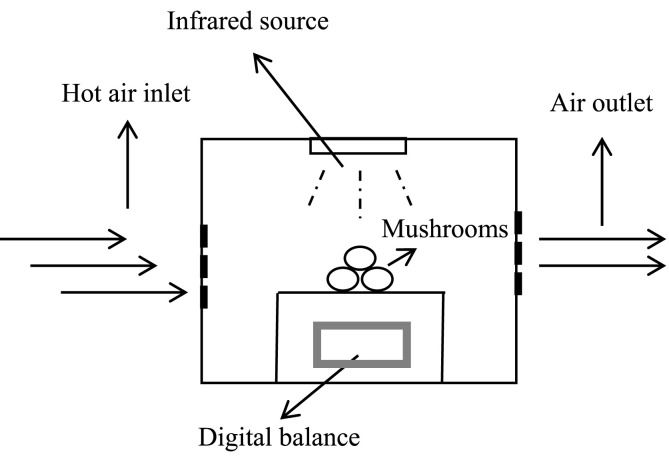
Infrared drying has the advantages of fast drying rate, high energy efficiency, uniform heating and high product quality. Among different infrared technologies, far infrared is more suitable for food drying, because most of the food ingredients can absorb far infrared radiation energy (Krishnamurthy, Khurana, Jun, Irudayaraj, & Demirci, 2008). This drying technique has been used successfully in some agricultural products, including onion, potato, apple, blueberry, and so on (Doymaz, 2012; Mongpraneet, Abe, & Tsurusaki, 2002; Sharma, Verma, & Pathare, 2005). There are also some studies focus on the application of infrared or infrared-assisted technology on mushrooms drying. Zhao et al. evaluated the nutritional properties, structural and sensory of lentinus edodes prepared by five drying methods, including hot air drying, hot air combined instant controlled pressure drop drying, freeze drying, heat pump drying and far-infrared radiation drying. The results showed that far infrared drying speed is fast, compared with freeze drying can save up to 66.25% time. The far infrared dried lentinus edodes showed better appearance quality, more flavor components, especially the content of certain sulfur compounds up to 24.59%. Among the five drying methods, far infrared dried lentinus edodes had better overall quality (Zhao et al., 2019). Jo et al. found that the exterior view of Phellinus gilvus mushroom after far infrared drying was better than that after 60 °C oven drying and 60 °C hot air drying (Jo et al., 2009). Kantrong et al. found that lentinus edodes dried by microwave vacuum combined with infrared drying had better quality in color, rehydration rate and texture (Kantrong, Tansakul, & Mittal, 2014). Wang et al. found that freeze-drying combined with infrared radiation saves 48% of the time compared with freeze-drying alone, and can better maintain the quality of lentinus edodes (Wang, Zhang, & Adhikari, 2015). Fig. 5 shows a typical experimental setup using the far infrared drying principle.
Fig. 5.
A typical experimental setup using the far infrared drying principle.
3.6. Ultrasound-assisted drying
Pretreatments prior on dehydration of mushroom involve the application of ultrasound, which can promote mass and heat transfer and water diffusion (Chen, Zhang, & Yang, 2020). Ultrasound has been widely used in the food industry, and its role in drying has attracted more and more attention in recent years (Cheng, Zhang, Xu, Adhikari, & Sun, 2015; Islam, Zhang, Adhikari, Xinfeng, & Xu, 2014; Wang, Xu, Wei, & Zeng, 2018; Xu, Yuan, et al., 2020). Some studies suggested that applying ultrasound to the drying or frying of green peppers (Szadzińska, Łechtańska, Kowalski, & Stasiak, 2017), potato chips (Su, Zhang, Zhang, Liu, & Adhikarie, 2018), strawberries (Szadzińska, Kowalski, & Stasiak, 2016) and mushrooms (Devi et al., 2018) improves the quality of products. Vallespir et al. studied the effects of ultrasound on low-temperature drying of white button mushrooms. The results showed that compared with drying without ultrasound, the drying time was reduced by 41%, 57% and 66% when ultrasound was applied at 5 °C, 10 °C and 15 °C, respectively (Vallespir, Crescenzo, Rodríguez, Marra, & Simal, 2019). Çakmak et al. found that the combination of electroplasmolysis and ultrasound pretreatments increased the drying rate of mushrooms by 37.10%. Compared with electroplasmolysis, the phenolic content and color value of mushrooms were better preserved by ultrasonic treatment (Çakmak, Tekeoğlu, Bozkır, Ergün, & Baysal, 2016). Jambrak et al. found that mushrooms subjected to ultrasonic pretreatment dried faster and had better rehydration performance than untreated samples (Jambrak, Mason, Paniwnyk, & Lelas, 2007). It can be seen that the application of ultrasonic technology has potential in the drying of mushrooms. In different drying methods for mushrooms, it can be used as a pretreatment or as an auxiliary technology to improve the drying of mushrooms.
3.7. Pretreatments before drying
In order to prevent color changes, inhibit enzyme activity, reduce the number of microorganisms, enhance flavor retention, and improve the overall stability of mushrooms during drying, some pretreatment methods are often applied before various drying. Traditional pretreatment methods include: blanching (Bernas, Jaworska, & Kmiecik, 2006), soaking in potassium metabisulphite (KMS) (Brennan, Le Port, Pulvirenti, & Gormley, 1999) and citric acid (Brennan, Le Port, & Gormley, 2000), and alternative methods such as curd fermentation, whey fermentation (Walde et al., 2006), etc., have been reported. Singh et al. showed that pretreatment with citric acid, KMS and EDTA affected the drying characteristics of button mushrooms, such as drying rate, diffusion rate and rehydration rate (Singh, Jain, Doshi, Jain, & Chahar, 2008). Walde et al. found that for oyster and button mushrooms, pretreatment with curds or fermented whey, the drying time was shorter than other treatments in all types of dryers (Walde et al., 2006). Hassan et al. studied the influence of sodium chloride, citric acid and sodium metabisulfite solution pretreatments on the quality of dried Pleurotus ostreatus and Pleurotus eryngii mushroom respectively. The results showed that both types of mushrooms could be prevented from browning by soaking them in the solutions for 10 min before hot air drying at 50 °C, especially by soaking in sodium metabisulfite. Also, soaking pretreatment resulted a satisfactory rehydration rate for the dried products (HASSAN & MEDANY, 2014). Nevertheless, there are also studies found that blanching can cause mushrooms to deteriorate in color and harden in texture, although it can inhibit enzyme activity and reduce microbial populations (Argyropoulos et al., 2011).
4. Drying techniques to enhance vitamin D in dried mushrooms
Drying methods have been proven to have important effects on the quality of dried mushrooms. As described in Section 2.3 although the content of vitamin D in mushrooms is low, the abundant ergosterol content is a potential source of vitamin D. It is easy to convert ergosterol into vitamin D following ultraviolet radiation, which makes mushrooms become a rich source of vitamin D, as shown in Table 3. Therefore, in evaluating different drying methods for mushrooms, consideration should be given to optimization of the conditions to facilitate this conversion process as much as possible. Although the photo-conversion kinetics of ergosterol to vitamin D2 in mushrooms is not fully understood, UV exposure is an important condition. In various mushroom drying techniques, it is known that natural or artificial sun drying can meet this demand. While drying, mushrooms are exposed to ultraviolet light. Sun-dried mushrooms are rich in vitamin D (Cardwell et al., 2018; Huang, Cai, & Xu, 2016). However, as mentioned in 3.1 above, the sun-drying method has many drawbacks, such as slow drying speed, poor heat transfer, high environmental dependence, unhygienic, and so on. It is not suitable for the current industrial production mode and are rarely applied. Hot air drying, vacuum drying, microwave drying and infrared drying, often require additional ultraviolet irradiation conditions before or after drying to promote the production of vitamin D. In this process, UV radiation type (UV-A, UV-B or UV-C), irradiation dose, type of mushrooms, type of tissues exposed to UV, temperature and water content of irradiated mushrooms, etc. have been described as factors affecting vitamin D yield during mushroom drying (Jasinghe, Perera, & Sablani, 2007).
4.1. Effects of different types of UV radiation
UV-A, UV-B and UV-C are three ultraviolet wavelengths and can be used for mushroom irradiation. Their wavelengths are A (320–400), B (290–320) and C (190–290). Jasinghe et al. studied the effects of UV exposure on vitamin D2 production in edible mushrooms. Four types of fresh mushrooms were irradiated with different types of UV, respectively. The results showed that the content of vitamin D increased after exposure to different ultraviolet lights, and UV-B had the most significant effect (Jasinghe & Perera, 2006). Mau et al. found that UV-B exposure was associated with the most significant increase in vitamin D2 levels in common mushrooms compared to other types of UV exposure (Mau et al., 1998). Wu et al. found that the synthesis of vitamin D2 by UV-B light source was significantly more efficient than other light sources (Wu & Ahn, 2014). A similar conclusion was reported by Simon et al., that UV-B radiation (290–320 nm) was the most effective method to stimulate vitamin D2 production in button mushrooms. At the same time, no significant changes in contents of vitamin C, amino acids, fatty acids and other nutrients in mushrooms were observed after UV-B treatment. UV-B exposure is an appropriate and safe approach compared to natural light (Simon et al., 2011). It can be seen that UV-B is the most common type applied in the conversion of ergosterol to vitamin D in mushrooms, and can achieve a relatively satisfactory effect, although there are also studies involves the role of UV-A and UV-C on mushrooms.
4.2. Effects of different mushroom tissue and slice treatment
The content of vitamin D can also change if the mushrooms are exposed to ultraviolet light as a whole or sliced (Stamets, 2012). Nolle et al. found that sliced mushrooms treated with UV-B had higher levels of vitamin D than whole mushrooms treated with UV-B. Freeze-drying fresh whole mushrooms treated with UV-B produced 45 μg/g d.m. of vitamin D2. However, sliced mushrooms before being exposed to UV-B light and then dehydrated produce 406 μg/g d.m. of vitamin D2, about 10 times more than when the whole fruit is exposed to UV light (Nölle et al., 2017a). Kalaras et al. treated brown and white button mushroom slices and whole forms with pulsed UV light. The results showed that the vitamin D2 content of sliced mushrooms was significantly higher than that of the whole forms, and that of brown mushroom and white mushroom slices increased by 113% and 81%, respectively (Kalaras, Beelman, & Elias, 2011). Urbain et al. found that when Agaricus bisporus mushrooms were exposed to sunlight for a certain amount of time, the vitamin D2 content of 12 mm thick sliced mushrooms was significantly lower than that of 9 mm sliced mushrooms. The results also showed that after 120 min of sun exposure, the sliced mushroom samples which were flipped and exposed to sunlight on both sides had highest levels of vitamin D2, almost twice as high as when only one side was exposed to the same amount of UV (Urbain & Jakobsen, 2015). This is probably due to increased exposure of gill tissue in mushroom slices, since there are numerous studies have shown that the gill tissue of different mushrooms contains more ergosterol than other tissues (Jasinghe & Perera, 2005; Kalaras et al., 2011; Ko, Lee, Lee, & Park, 2008). Whether sliced or exposed to the sun on both sides, the tissue area of mushrooms exposed to ultraviolet light increases, resulting in an increase in vitamin D production. Similar results were obtained by Wu et al., that compared with intact fresh mushrooms, homogeneous mushroom powder significantly increased the exposure surface area, thus greatly increasing the ergosterol conversion efficiency. In addition, the opening of mushroom cap also affects the formation of vitamin D2 when the whole mushroom is irradiated by ultraviolet light. This is of practical importance for some mushrooms that are harvested according to their maturity, such as button mushrooms, which has different stages of maturity as button, cup and open flat. As the mushroom cap opens, more gills are exposed, which helps to receive ultraviolet light (Nölle et al., 2017a). Sławinska et al. also found that white button mushrooms, despite their higher ergosterol content compared to oyster and shiitake mushrooms, had the lowest vitamin D content after UV-B exposure because the white button mushrooms used in the study had closed caps and therefore limited UV-B access to the gill area (Sławińska et al., 2016). It can be seen that a certain amount of exposure area is necessary in the drying of mushrooms to achieve the required vitamin D content. In addition, from the perspective of drying demand, in order to improve the drying rate, accelerate the evaporation of water and shorten the distance of water transfer, it is often necessary to slice mushrooms.
4.3. Effects of moisture content and UV irradiation on dried mushrooms
Due to the different growth environments used to produce mushrooms, the water content of mature mushrooms will be varied, even in different tissues of the same mushroom, the water content is different. Studies have found that the water content in mushrooms can affect the efficiency of vitamin D conversion. Perera et al. showed that the optimum moisture content for vitamin D conversion of shiitake mushrooms was 70%. Therefore, moisture drying is also important in optimizing the conversion of ergosterol into vitamin D. During a continuous drying operation, mushrooms should be exposed to ultraviolet light before their moisture content drops to 70% (Perera, Jasinghe, Ng, & Mujumdar, 2003). Jasinghe et al. studied the vitamin content produced by ultraviolet irradiation on the gills of lentinus edodes with different water content, showing that the best conversion occurred on the basis of moisture content of about 78%. This may be due to the dilution effect of ergosterol at high water content, which may result in a lower conversion rate (Jasinghe & Perera, 2005). Considering the influence of water content, some studies have conducted ultraviolet irradiation after mushroom drying to evaluate the effect on vitamin D conversion. Sławinska et al. found that UV-B exposure to dried mushrooms had a significant effect on vitamin D production, and vitamin D content gradually increased with the extension of UV-B treatment time. Besides exposure time, the increased vitamin D content is influenced by drying methods and mushroom species. The results showed that lyophilized fruit bodies exposed to UV-B contained more vitamin D2 than those which were hot-air dried. White button mushroom has the highest ergocalciferol content in dried fruit bodies (Sławińska et al., 2016). Teichmann et al. showed that the vitamin D content of freeze-dried chanterelles mushrooms increased significantly under ultraviolet radiation (Teichmann et al., 2007).
4.4. Effects of irradiation temperature and irradiation dose
In the process of ergosterol conversion to vitamin D, there are usually thermal isomerism steps. Therefore, irradiation temperature also has an effect on the conversion rate. Jasinghe et al. showed that the optimal temperature of UV irradiation for lentinus edodes was about 35 °C, at which the vitamin D yield was highest. The decrease in the conversion rate beyond 35 °C may be due to a number of concurrent factors, including further conversion of vitamin D, photodegradation under UV radiation, cell death, oxidation, and formation of browning pigments (Jasinghe & Perera, 2005). A similar conclusion was reached by Wu et al. that the content of vitamin D2 enhanced with an increasing of temperature up to 35 °C (Wu & Ahn, 2014). Won et al. identified the optimal process conditions for strengthening vitamin D2 of Lentinus edodes by UV irradiation through response surface method. They found that 40.56 °C was the optimum temperature at UV dose of 36.27 kJ/m2 and moisture content of 80.46% (Won et al., 2017). It is worth noting that different conditions will have mutual influence, so the optimization of conditions needs to coordinate with each other. Lee et al. determined the optimal conditions for ergosterol conversion to vitamin D in Agaricus bisporus powder by response surface method (RSM). The results showed that the optimal condition was that the UV-B light source with irradiation intensity of 1.36 W/m2 irradiated the mushroom powder for 10.4 min at the ambient temperature of 26.33 °C. Under this condition, the final vitamin D content could reach 741.50 μg/g (Lee & Aan, 2016). Pyo et al. found that UV-B irradiation for 15 min was an optimal condition for vitamin D enhancement in Lentinus edodes, with a vitamin D content of 35.54 μg/g and a conversion rate of 5.56% (Pyo et al., 2020).
It can be seen that the conversion rate of ergosterol to vitamin D2 in mushrooms depends on many factors. Suitable drying conditions should be choose to increase the production of vitamin D2. In addition, the effect of drying on the quality of mushrooms should be considered at the same time, as some operations will cause damage to mushrooms. For example, studies have shown that the content of vitamin D2 increases significantly with the increase of irradiation intensity, and the effect of ergosterol on the conversion of vitamin D2 is positively correlated with irradiation intensity. However, the adverse effects of high doses of radiation are also significant, resulting in surface discoloration and reduced moisture content of mushrooms, thereby affecting the quality of mushrooms and the market value of products (Ko et al., 2008). Hu et al. found that although vitamin D content in mushrooms was increased under UV irradiation, only part of ergosterol was converted to vitamin D, and most of ergosterol may be degraded by UV. More suitable conversion conditions should be explored to avoid the photo-degradation of ergosterol and enhance the conversion rate (Hu et al., 2020). These limitations all need to be taken into account in the selection of drying conditions.
5. Conclusions
With the increasing consumption of mushrooms, the vitamin D value of mushrooms has been paid attention to. Thermal drying in the presence of UV has been proven to convert ergosterol into vitamin D and enhance the nutritional content of all types of edible mushrooms. Solar drying, hot air drying, freeze drying, microwave drying and infrared drying can be used for mushrooms drying under selected operating conditions. It can be seen that in the drying process of mushroom with different methods, the drying conditions need to be optimized to enhance the content of vitamin D2, because the photo-conversion process of ergosterol into vitamin D2 needs certain conditions. Ultraviolet radiation is the basic requirement, and other factors affecting ergosterol conversion to vitamin D2, such as irradiation light source, irradiation intensity, irradiation time, and moisture content of mushrooms, etc. should be considered. The aim is to enrich the vitamin D2 content in mushrooms under the precondition of meeting the drying parameters. In addition, numerous studies have shown that dried mushrooms can also boost vitamin D2 levels after exposure to ultraviolet light, which could also be used as an alternative. Therefore, with the demand for vitamin D2 production, drying can be expected to become a commercial standard for mushroom post-harvest processing. Even under appropriate conditions, the conversion efficiency of vitamin D2 in mushrooms remains to be further verified in industrial production. Furthermore, the adverse effects of irradiation on the texture, appearance and color of mushrooms cannot be ignored, because it will affect consumer acceptance. Considering that there are many factors affecting the treatment of mushrooms by ultraviolet radiation. Further research can focus on the isolation of ergosterol from fresh mushrooms and vitamin D2 from irradiated mushrooms. The conversion of ergosterol isolated to vitamin D2 by UV irradiation in vitro can be another research topic.
Acknowledgments
Financial supports from the China Key Research Program (Contract No. 2018YFD0700303), the 111 Project(BP0719028), Jiangsu Province (China) “Collaborative Innovation Center for Food Safety and Quality Control” Industry Development Program, Jiangsu Province Key Laboratory Project of Advanced Food Manufacturing Equipment and Technology (No. FMZ202003) are gratefully acknowledged.
References
- Ahmed M., Abdullah N., Ahmed K.U., Bhuyan M.H.M.B. Yield and nutritional composition of oyster mushroom strains newly introduced in Bangladesh. Pesquisa Agropecuaria Brasileira. 2013;48:197–202. [Google Scholar]
- Akata I., Ergonul B., Kalyoncu F. Chemical compositions and antioxidant activities of 16 wild edible mushroom species grown in Anatolia. International Journal of Pharmacology. 2012;8:134–138. [Google Scholar]
- Annweiler C., Cao Z., Sabatier J. Point of view: Should COVID-19 patients be supplemented with vitamin D? Maturitas. 2020;140:24–26. doi: 10.1016/j.maturitas.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arabi S.M., Bahrami L.S., Ranjbar G., Tabesh H., Norouzy A. The effect of vitamin D supplementation on inflammation in critically ill patients: A systematic review. PharmaNutrition. 2020;13:100196. [Google Scholar]
- Argyropoulos D., Heindl A., Müller J. Assessment of convection, hot-air combined with microwave-vacuum and freeze-drying methods for mushrooms with regard to product quality. International Journal of Food Science & Technology. 2011;46:333–342. [Google Scholar]
- Argyropoulos D., Heindl A., Müller J. Assessment of convection, hot-air combined with microwave-vacuum and freeze-drying methods for mushrooms with regard to product quality. International Journal of Food Science & Technology. 2011;46:333–342. [Google Scholar]
- Argyropoulos D., Khan M.T., Müller J. Effect of air temperature and pre-treatment on color changes and texture of dried Boletus edulis mushroom. Drying technology: International Journal. 2011;29:1890–1900. [Google Scholar]
- Arora S., Shivhare U.S., Ahmed J., Raghavan G.S.V. Drying kinetics OF agaricus bisporus and pleurotus Florida mushrooms. Transactions of the Asae American Society of Agricultural Engineers. 2003;46:721–724. [Google Scholar]
- Banlangsawan N., Sanoamuang N. Effect of UV-B irradiation on contents of ergosterol, vitamin D2, vitamin B1 and vitamin B2 in Thai edible mushrooms. Chiang Mai Journal of Science. 2016;43:45–53. [Google Scholar]
- Bernas E., Jaworska G., Kmiecik W. Storage and processing of edible mushrooms. Acta Scientarum Polonorum - Technologia Alimentaria. 2006;5:5–23. [Google Scholar]
- Bisen P.S., Baghel R.K., Sanodiya B.S., Thakur G.S., Prasad G.B.K.S. Lentinus edodes: A macrofungus with pharmacological activities. Current Medicinal Chemistry. 2010;17:2419–2430. doi: 10.2174/092986710791698495. [DOI] [PubMed] [Google Scholar]
- Brennan M., Le Port G., Gormley R. Post-harvest treatment with citric acid or hydrogen peroxide to extend the shelf life of fresh sliced mushrooms. Lebensmittel-Wissenschaft und -Technologie- Food Science & Technology. 2000;33:285–289. [Google Scholar]
- Brennan M., Le Port G., Pulvirenti A., Gormley R. The effect of sodium metabisulphite on the whiteness and keeping quality of sliced mushrooms. Lebensmittel-Wissenschaft und -Technologie- Food Science &Technology. 1999;32:460–463. [Google Scholar]
- Çakmak R.Ş., Tekeoğlu O., Bozkır H., Ergün A.R., Baysal T. Effects of electrical and sonication pretreatments on the drying rate and quality of mushrooms. Lebensmittel-Wissenschaft und -Technologie- Food Science & Technology. 2016;69:197–202. [Google Scholar]
- Cardwell G., Bornman J.F., James A.P., Black L.J. A review of mushrooms as a potential source of dietary vitamin D. Nutrients. 2018;10:1498. doi: 10.3390/nu10101498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlberg C., Muñoz A. An update on vitamin D signaling and cancer. Seminars in Cancer Biology. 2020 doi: 10.1016/j.semcancer.2020.05.018. [DOI] [PubMed] [Google Scholar]
- Charoenngam N., Shirvani A., Holick M.F. Vitamin D for skeletal and non-skeletal health: What we should know. Journal of Clinical Orthopaedics & Trauma. 2019;10:1082–1093. doi: 10.1016/j.jcot.2019.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X., Zhang M., Xu B., Adhikari B., Sun J. The principles of ultrasound and its application in freezing related processes of food materials: A review. Ultrasonics Sonochemistry. 2015;27:576–585. doi: 10.1016/j.ultsonch.2015.04.015. [DOI] [PubMed] [Google Scholar]
- Chen N., Hsu C., Mao P.C., Dreyer G., Wu F., Chen C. The effects of correction of vitamin D deficiency on arterial stiffness: A systematic review and updated meta-analysis of randomized controlled trials. The Journal of Steroid Biochemistry & Molecular Biology. 2019;198:105561. doi: 10.1016/j.jsbmb.2019.105561. [DOI] [PubMed] [Google Scholar]
- Chen F., Zhang M., Yang C. Application of ultrasound technology in processing of ready-to-eat fresh food: A review. Ultrasonics Sonochemistry. 2020;63:104953. doi: 10.1016/j.ultsonch.2019.104953. [DOI] [PubMed] [Google Scholar]
- Darvishi H., Najafi G., Hosainpour A., Khodaei J., Aazdbakht M. Far-infrared drying characteristics of mushroom slices. Chemical Product & Process Modeling. 2013;8:107–117. [Google Scholar]
- Das I., Arora A. Alternate microwave and convective hot air application for rapid mushroom drying. Journal of Food Engineering. 2018;223:208–219. [Google Scholar]
- Devi S., Zhang M., Law C.L. Effect of ultrasound and microwave assisted vacuum frying on mushroom (Agaricus bisporus) chips quality. Food Bioscience. 2018;25:111–117. [Google Scholar]
- Donsì G., Ferrari G., Matteo D.I. Utilization of combined processes in freeze-drying of shrimps. Food & Bioproducts Processing. 2001;79:152–159. [Google Scholar]
- Doymaz İ. Infrared drying of sweet potato (Ipomoea batatas L.) slices. Journal of Food Science & Technology. 2012;49:760–766. doi: 10.1007/s13197-010-0217-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan X., Zhang M., Li X., Mujumdar A.S. Microwave freeze drying of sea cucumber coated with nanoscale silver. Drying Technology. 2008;26:413–419. [Google Scholar]
- Funebo T., Ohlsson T. Microwave-assisted air dehydration of apple and mushroom. Journal of Food Engineering. 1998;38:353–367. [Google Scholar]
- Guan W., Lei J., Jie Z., Wang Z., Yan R., Suqin S., et al. Effects of UV-C treatment and cold storage on ergosterol and vitamin D2 contents in different parts of white and brown mushroom (Agaricus bisporus) Food Chemistry. 2016;210:129–134. doi: 10.1016/j.foodchem.2016.04.023. [DOI] [PubMed] [Google Scholar]
- Gunasekaran S. Grain drying using continuous and pulsed microwave energy. Drying Technology. 1990;8:1039–1047. [Google Scholar]
- Gunasekaran S. Pulsed microwave-vacuum drying OF food materials. Drying Technology. 1999;17:395–412. [Google Scholar]
- Haro A., Trescastro A., Lara L., Fernández-Fígares I., Nieto R., Isabel Mineral elements contents of wild growing edible mushrooms from the southeast of Spain. Journal of Food Composition & Analysis. 2020;91:103504. [Google Scholar]
- Hassan F.R.H., Medany G.M. Effect OF pretreatments and drying temperatures ON the quality OF dried pleurotus mushroom SPP. Egyptian Journal of Agricultural Research. 2014;92:1009–1023. [Google Scholar]
- Heleno S.A., Barros L., Martins A., Morales P., Fernández-Ruiz V., Glamoclija J., et al. Nutritional value, bioactive compounds, antimicrobial activity and bioaccessibility studies with wild edible mushrooms. Lebensmittel-Wissenschaft und -Technologie- Food Science & Technology. 2015;63:799–806. [Google Scholar]
- Hnin K.K., Zhang M., Wang B., Devahastin S. Different drying methods effect on quality attributes of restructured rose powder-yam snack chips. Food Bioscience. 2019;32:100486. [Google Scholar]
- Holick M.F. The vitamin D deficiency pandemic: A forgotten hormone important for health. Public Health Reviews. 2010;32:267–283. [Google Scholar]
- Huang G., Cai W., Xu B. Vitamin D2, ergosterol, and vitamin B2 content in commercially dried mushrooms marketed in China and increased vitamin D2 content following UV-C irradiation. International Journal for Vitamin & Nutrition Research. 2016;87:1–10. doi: 10.1024/0300-9831/a000294. [DOI] [PubMed] [Google Scholar]
- Huang S., Lin C., Tsai S. Vitamin D2 content and antioxidant properties of fruit body and mycelia of edible mushrooms by UV-B irradiation. Journal of Food Composition & Analysis. 2015;42:38–45. [Google Scholar]
- Huang L., Zhang M. Trends in development of dried vegetable products as snacks. Drying Technology. 2012;30:448–461. [Google Scholar]
- Hu D., Chen W., Li X., Yue T., Zhang Z., Feng Z., et al. Ultraviolet irradiation increased the concentration of vitamin D2 and decreased the concentration of ergosterol in shiitake mushroom (lentinus edodes) and oyster mushroom (Pleurotus ostreatus) powder in ethanol suspension. ACS Omega. 2020;5:7361–7368. doi: 10.1021/acsomega.9b04321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irzyniec Z., Klimczak J., Michalowski S. Freeze-drying of the Black currant juice. Drying Technology. 1995;13:417–424. [Google Scholar]
- Islam M.N., Zhang M., Adhikari B., Xinfeng C., Xu B. The effect of ultrasound-assisted immersion freezing on selected physicochemical properties of mushrooms. International Journal of Refrigeration. 2014;42:121–133. [Google Scholar]
- Jambrak A.R., Mason T.J., Paniwnyk L., Lelas V. Accelerated drying of button mushrooms, Brussels sprouts and cauliflower by applying power ultrasound and its rehydration properties. Journal of Food Engineering. 2007;81:88–97. [Google Scholar]
- Jasinghe V.J., Perera C.O. Distribution of ergosterol in different tissues of mushrooms and its effect on the conversion of ergosterol to vitamin D2 by UV irradiation. Food Chemistry. 2005;92:541–546. [Google Scholar]
- Jasinghe V.J., Perera C.O. Ultraviolet irradiation: The generator of Vitamin D2 in edible mushrooms. Food Chemistry. 2006;95:638–643. [Google Scholar]
- Jasinghe V.J., Perera C.O., Sablani S.S. Kinetics of the conversion of ergosterol in edible mushrooms. Journal of Food Engineering. 2007;79:864–869. [Google Scholar]
- Jo W., Park S., Park S., Chang Z., Seo G., Uhm J., et al. Changes in quality of Phellinus gilvus mushroom by different drying methods. Mycoscience. 2009;50:70–73. [Google Scholar]
- Kaensup W., Chutima S., Wongwises S. Experimental study ON drying OF chilli IN a combined microwave-vacuum-rotary drum dryer. Drying Technology. 2002;20:2067–2079. [Google Scholar]
- Kalač P. Chemical composition and nutritional value of European species of wild growing mushrooms: A review. Food Chemistry. 2009;113:9–16. [Google Scholar]
- Kalač P. A review of chemical composition and nutritional value of wild-growing and cultivated mushrooms. Journal of the Science of Food & Agriculture. 2013;93:209–218. doi: 10.1002/jsfa.5960. [DOI] [PubMed] [Google Scholar]
- Kalaras M.D., Beelman R.B., Elias R.J. Effects of postharvest pulsed UV light treatment of white button mushrooms (Agaricus bisporus) on vitamin D2 content and quality attributes. Journal of Agricultural and Food Chemistry. 2011;60:220–225. doi: 10.1021/jf203825e. [DOI] [PubMed] [Google Scholar]
- Kantrong H., Tansakul A., Mittal G.S. Drying characteristics and quality of shiitake mushroom undergoing microwave-vacuum drying and microwave-vacuum combined with infrared drying. Journal of Food Science & Technology. 2014;51:3594–3608. doi: 10.1007/s13197-012-0888-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennel K.A.M., Drake M.T.M.P., Hurley D.L.M. Vitamin D deficiency in adults: When to test and how to treat. Mayo Clinic Proceedings. 2010;85:752–758. doi: 10.4065/mcp.2010.0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Jeon S., Lim W., Oh K., Shin D., Cho S.J., et al. Vitamin D deficiency and suicidal ideation: A cross-sectional study of 157,211 healthy adults. Journal of Psychosomatic Research. 2020;134:110125. doi: 10.1016/j.jpsychores.2020.110125. [DOI] [PubMed] [Google Scholar]
- Ko J.A., Lee B.H., Lee J.S., Park H.J. Effect of UV-B exposure on the concentration of vitamin D2 in sliced shiitake mushroom (lentinus edodes) and white button mushroom (Agaricus bisporus) Journal of Agricultural & Food Chemistry. 2008;56:3671–3674. doi: 10.1021/jf073398s. [DOI] [PubMed] [Google Scholar]
- Kotwaliwale N., Bakane P., Verma A. Changes in textural and optical properties of oyster mushroom during hot air drying. Journal of Food Engineering. 2007;78:1207–1211. [Google Scholar]
- Koyyalamudi S.R., Jeong S., Song C., Cho K.Y., Pang G. Vitamin D2 formation and bioavailability from Agaricus bisporus button mushrooms treated with ultraviolet irradiation. Journal of Agricultural & Food Chemistry. 2009;57:3351–3355. doi: 10.1021/jf803908q. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy K., Khurana H.K., Jun S., Irudayaraj J., Demirci A. Infrared heating in food processing: An overview. Comprehensive Reviews in Food Science & Food Safety. 2008;7:2–13. [Google Scholar]
- Lee N.K., Aan B. Optimization of ergosterol to vitamin D2 synthesis in Agaricus bisporus powder using ultraviolet-B radiation. Food Science & Biotechnology. 2016;25:1627–1631. doi: 10.1007/s10068-016-0250-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuven R.K. In memoriam AW Norman, the founding father of the Vitamin D workshops. The Journal of Steroid Biochemistry & Molecular Biology. 2020;200:105610. [Google Scholar]
- Liu G., Wang H., Zhou B., Guo X., Hu X. Compositional analysis and nutritional studies of Tricholoma matsutake collected from Southwest China. Journal of Medicinal Plants Research. 2010;4:1222–1227. [Google Scholar]
- Li L., Zhang M., Chitrakar B., Jiang H. Effect of combined drying method on phytochemical components, antioxidant capacity and hygroscopicity of Huyou (Citrus changshanensis) fruit. Lwt-Food Science & Technology. 2020;123:109102. [Google Scholar]
- Martín Giménez V.M., Inserra F., Tajer C.D., Mariani J., Ferder L., Reiter R.J., et al. Lungs as target of COVID-19 infection: Protective common molecular mechanisms of vitamin D and melatonin as a new potential synergistic treatment. Life Sciences. 2020;254:117808. doi: 10.1016/j.lfs.2020.117808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattila P., Lampi A., Ronkainen R., Toivo J., Piironen V. Sterol and vitamin D2 contents in some wild and cultivated mushrooms. Food Chemistry. 2002;76:293–298. [Google Scholar]
- Mau J., Chen P., Yang J. Ultraviolet irradiation increased vitamin D2 content in edible mushrooms. Journal of Agricultural & Food Chemistry. 1998;46:5269–5272. [Google Scholar]
- Mau J., Lin H., Ma J., Song S. Non-volatile taste components of several speciality mushrooms. Food Chemistry. 2001;73:461–466. [Google Scholar]
- McCourt A., McNulty B.A., Walton J., O'Sullivan A. Efficacy and safety of food fortification to improve Vitamin D intakes of older adults. Nutrition. 2020;75–76:110767. doi: 10.1016/j.nut.2020.110767. [DOI] [PubMed] [Google Scholar]
- Mirzavandi F., Babaie S., Rahimpour S., Razmpoosh E., Talenezhad N., Zarch S.M.A., et al. The effect of high dose of intramuscular vitamin D supplement injections on depression in patients with type 2 diabetes and vitamin D deficiency: A randomized controlled clinical trial. Obesity Medicine. 2020;17:100192. [Google Scholar]
- Mokhtar W.A., Fawzy A., Allam R.M., Amer R.M., Hamed M.S. Maternal vitamin D level and vitamin D receptor gene polymorphism as a risk factor for congenital heart diseases in offspring; an Egyptian case-control study. Genes & Diseases. 2019;6:193–200. doi: 10.1016/j.gendis.2018.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongpraneet S., Abe T., Tsurusaki T. Accelerated drying of Welsh onion by far infrared radiation under vacuum conditions. Journal of Food Engineering. 2002;55:147–156. [Google Scholar]
- Moulas A.N., Vaiou M. Vitamin D fortification of foods and prospective health outcomes. Journal of Biotechnology. 2018;285:91–101. doi: 10.1016/j.jbiotec.2018.08.010. [DOI] [PubMed] [Google Scholar]
- Mshandete A.M., Cuff J. Proximate and nutrient composition of three types of indigenous edible wild mushroom grown in Tanzania and their utilization prospects. African Journal of Food, Agriculture, Nutrition & Development. 2008;219:1235–1243. [Google Scholar]
- Mujumdar A.S., Law C.L. Drying technology: Trends and applications in postharvest processing. Food & Bioprocess Technology. 2010;3:843–852. [Google Scholar]
- Mutukwa I. 2014. Drying and pretreatments affect the nutritional and sensory quality of oyster mushrooms. [Google Scholar]
- Nölle N., Argyropoulos D., Ambacher S., Müller J., Biesalski H.K. Vitamin D2 enrichment in mushrooms by natural or artificial UV-light during drying. Lebensmittel-Wissenschaft und -Technologie- Food Science & Technology. 2017;85:400–404. [Google Scholar]
- Nölle N., Argyropoulos D., Ambacher S., Müller J., Biesalski H.K. Vitamin D2 enrichment in mushrooms by natural or artificial UV-light during drying. Lebensmittel-Wissenschaft und -Technologie- Food Science & Technology. 2017;85:400–404. [Google Scholar]
- Pei F., Shi Y., Mariga A.M., Yang W., Tang X., Zhao L., et al. Comparison of freeze-drying and freeze-drying combined with microwave vacuum drying methods on drying kinetics and rehydration characteristics of button mushroom (Agaricus bisporus) slices. Food & Bioprocess Technology. 2014;7:1629–1639. [Google Scholar]
- Pereira E., Barros L., Martins A., Ferreira I.C.F.R. Towards chemical and nutritional inventory of Portuguese wild edible mushrooms in different habitats. Food Chemistry. 2012;130:394–403. [Google Scholar]
- Perera C.O., Jasinghe V.J., Ng F.L., Mujumdar A.S. The effect of moisture content on the conversion of ergosterol to vitamin D in shiitake mushrooms. Drying Technology. 2003;21:1091–1099. [Google Scholar]
- Phillips K.M., Ruggio D.M., Horst R.L., Minor B., Simon R.R., Feeney M.J., et al. Vitamin D and sterol composition of 10 types of mushrooms from retail suppliers in the United States. Journal of Agricultural & Food Chemistry. 2011;59:7841–7853. doi: 10.1021/jf104246z. [DOI] [PubMed] [Google Scholar]
- Pittas A.G., Laskowski U., Kos L., Saltzman E. Role of vitamin D in adults requiring nutrition support. Journal of Parenteral & Enteral Nutrition. 2010;34:70–78. doi: 10.1177/0148607109349061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plum L.A., DeLuca H.F. The functional metabolism and molecular biology of vitamin D action. Clinical Reviews in Bone & Mineral Metabolism. 2009;7:20–41. [Google Scholar]
- Pyo J., Gu J., Kim T.H., Lee J.J., Hwang M.S., Kang J.S., et al. A study on increased content of vitamin D in different types of mushrooms. Journal of the Korean Society of Food Science & Nutrition. 2020;49:311–315. [Google Scholar]
- Rathore H., Prasad S., Sharma S. Mushroom nutraceuticals for improved nutrition and better human health: A review. PharmaNutrition. 2017;5:35–46. [Google Scholar]
- Ratti C. Hot air and freeze-drying of high-value foods: A review. Journal of Food Engineering. 2001;49:311–319. [Google Scholar]
- Razdan K., Singh K., Singh D. Vitamin D levels and COVID-19 susceptibility: Is there any correlation? Medicine in Drug Discovery. 2020;7:100051. doi: 10.1016/j.medidd.2020.100051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis F.S., Barros L., Martins A., Ferreira I.C.F.R. Chemical composition and nutritional value of the most widely appreciated cultivated mushrooms: An inter-species comparative study. Food and Chemical Toxicology. 2012;50:191–197. doi: 10.1016/j.fct.2011.10.056. [DOI] [PubMed] [Google Scholar]
- Reyes A., Mahn A., Cubillos F., Huenulaf P. Mushroom dehydration in a hybrid-solar dryer. Energy Conversion & Management. 2013;70:31–39. [Google Scholar]
- Reyes A., Mahn A., Vásquez F. Mushrooms dehydration in a hybrid-solar dryer, using a phase change material. Energy Conversion and Management. 2014;83:241–248. [Google Scholar]
- Roberts J.S., Teichert A., McHugh T.H. Vitamin D2 formation from post-harvest UV-B treatment of mushrooms (Agaricus bisporus) and retention during storage. Journal of Agricultural & Food Chemistry. 2008;56:4541–4544. doi: 10.1021/jf0732511. [DOI] [PubMed] [Google Scholar]
- Royse D.J., Baars J., Tan Q. In: Edible and medicinal mushrooms: Technology and applications. Diego C.Z., Giménez A.P., editors. John Wiley & Sons Ltd.; 2017. Current overview of mushroom production in the world; pp. 5–13. [Google Scholar]
- Ruslan K., Reza R.A., Damayanti S. Effect of ultraviolet radiation on the formation of ergocalciferol (vitamin D2) in Pleurotus ostreatus. Bionatura-Jurnal Ilmu-ilmu Hayati dan Fisik. 2011;13:255–261. [Google Scholar]
- Sharma G.P., Verma R.C., Pathare P.B. Thin-layer infrared radiation drying of onion slices. Journal of Food Engineering. 2005;67:361–366. [Google Scholar]
- Simon R.R., Phillips K.M., Horst R.L., Munro I.C. Vitamin D mushrooms: Comparison of the composition of button mushrooms (Agaricus bisporus) treated postharvest with UVB light or sunlight. Journal of Agricultural & Food Chemistry. 2011;59:8724–8732. doi: 10.1021/jf201255b. [DOI] [PubMed] [Google Scholar]
- Singh U., Jain S.K., Doshi A., Jain H.K., Chahar V.K. Effects of pretreatments on drying characteristics of button mushroom. International Journal of Food Engineering. 2008;4 [Google Scholar]
- Soysal Y., Öztekin S., Eren Ö. Microwave drying of parsley: Modelling, kinetics, and energy aspects. Biosystems Engineering. 2006;93:403–413. [Google Scholar]
- Stamets P. Place mushrooms in sunlight to get your vitamin D. 2012. https://fungi.com/blogs/articles/place-mushrooms-in-sunlight-to-get-your-vitamin-d
- Su Y., Zhang M., Zhang W., Liu C., Adhikarie B. Ultrasonic microwave-assisted vacuum frying technique as a novel frying method for potato chips at low frying temperature. Food & Bioproducts Processing. 2018;108:95–104. [Google Scholar]
- Szadzińska J., Kowalski S.J., Stasiak M. Microwave and ultrasound enhancement of convective drying of strawberries: Experimental and modeling efficiency. International Journal of Heat & Mass Transfer. 2016;103:1065–1074. [Google Scholar]
- Szadzińska J., Łechtańska J., Kowalski S.J., Stasiak M. The effect of high power airborne ultrasound and microwaves on convective drying effectiveness and quality of green pepper. Ultrasonics Sonochemistry. 2017;34:531–539. doi: 10.1016/j.ultsonch.2016.06.030. [DOI] [PubMed] [Google Scholar]
- Sławińska A., Fornal E., Radzki W., Skrzypczak K., Zalewska-Korona M., Michalak-Majewska M., et al. Study on vitamin D2 stability in dried mushrooms during drying and storage. Food Chemistry. 2016;199:203–209. doi: 10.1016/j.foodchem.2015.11.131. [DOI] [PubMed] [Google Scholar]
- Taofiq O., Fernandes Â., Barros L., Barreiro M.F., Ferreira I.C.F.R. UV-irradiated mushrooms as a source of vitamin D2: A review. Trends in Food Science & Technology. 2017;70:82–94. [Google Scholar]
- Teichmann A., Dutta P.C., Staffas A., Jägerstad M. Sterol and vitamin D2 concentrations in cultivated and wild grown mushrooms: Effects of UV irradiation. Lebensmittel-Wissenschaft und -Technologie- Food Science & Technology. 2007;40:815–822. [Google Scholar]
- Urbain P., Jakobsen J. Dose–response effect of sunlight on vitamin D2 production in Agaricus bisporus mushrooms. Journal of Agricultural & Food Chemistry. 2015;63:8156–8161. doi: 10.1021/acs.jafc.5b02945. [DOI] [PubMed] [Google Scholar]
- Vallespir F., Crescenzo L., Rodríguez Ó., Marra F., Simal S. Intensification of low-temperature drying of mushroom by means of power ultrasound: Effects on drying kinetics and quality parameters. Food & Bioprocess Technology. 2019;12:839–851. [Google Scholar]
- Walde S.G., Velu V., Jyothirmayi T., Math R.G. Effects of pretreatments and drying methods on dehydration of mushroom. Journal of Food Engineering. 2006;74:108–115. [Google Scholar]
- Wang L., Xu B., Wei B., Zeng R. Low frequency ultrasound pretreatment of carrot slices: Effect on the moisture migration and quality attributes by intermediate-wave infrared radiation drying. Ultrasonics Sonochemistry. 2018;40:619–628. doi: 10.1016/j.ultsonch.2017.08.005. [DOI] [PubMed] [Google Scholar]
- Wang H., Zhang M., Adhikari B. Drying of shiitake mushroom by combining freeze-drying and mid-infrared radiation. Food & Bioproducts Processing. 2015;94:507–517. [Google Scholar]
- Wang Y., Zhang M., Mujumdar A.S., Mothibe K.J. Microwave-assisted pulse-spouted bed freeze-drying of stem lettuce slices—effect on product quality. Food & Bioprocess Technology. 2013;6:3530–3543. [Google Scholar]
- Wang X., Zhang J., Wu L., Zhao Y., Li T., Li J., et al. A mini-review of chemical composition and nutritional value of edible wild-grown mushroom from China. Food Chemistry. 2014;151:279–285. doi: 10.1016/j.foodchem.2013.11.062. [DOI] [PubMed] [Google Scholar]
- WHO WHO coronavirus disease (COVID-19) dashboard. 2020. https://covid19.who.int/
- Won D.J., Kim S.Y., Jang C.H., Lee J.S., Ko J.A., Park H.J. Optimization of UV irradiation conditions for the vitamin D2-fortified shiitake mushroom (Lentinula edodes) using response surface methodology. Food Science & Biotechnology. 2017 doi: 10.1007/s10068-017-0266-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W., Ahn B. Statistical optimization of ultraviolet irradiate conditions for vitamin D2 synthesis in oyster mushrooms (Pleurotus ostreatus) using response surface methodology. PloS One. 2014;9 doi: 10.1371/journal.pone.0095359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Cai Y., Liu M., Zhu D., Guan Y. The potential immunoregulatory roles of vitamin D in neuromyelitis optica spectrum disorder. Multiple Sclerosis & Related Disorders. 2020;43:102156. doi: 10.1016/j.msard.2020.102156. [DOI] [PubMed] [Google Scholar]
- Xu B., Yuan J., Wang L., Lu F., Wei B., Azam R.S.M., et al. Effect of multi-frequency power ultrasound (MFPU) treatment on enzyme hydrolysis of casein. Ultrasonics Sonochemistry. 2020;63:104930. doi: 10.1016/j.ultsonch.2019.104930. [DOI] [PubMed] [Google Scholar]
- Yu P., Huang M., Zhang M., Zhu Q., Qin J. Rapid detection of moisture content and shrinkage ratio of dried carrot slices by using a multispectral imaging system. Infrared Physics & Technology. 2020;108:103361. [Google Scholar]
- Zerwekh J.E. Blood biomarkers of vitamin D status. American Journal of Clinical Nutrition. 2008;87:1087S–1091S. doi: 10.1093/ajcn/87.4.1087S. [DOI] [PubMed] [Google Scholar]
- Zhang M., Chen H., Mujumdar A.S., Tang J., Miao S., Wang Y. Recent developments in high-quality drying of vegetables, fruits, and aquatic products. Critical Reviews in Food Science & Nutrition. 2017;57:1239–1255. doi: 10.1080/10408398.2014.979280. [DOI] [PubMed] [Google Scholar]
- Zhang M., Tang J., Mujumdar A.S., Wang S. Trends in microwave-related drying of fruits and vegetables. Trends in Food Science & Technology. 2006;17:524–534. [Google Scholar]
- Zhang M., Tang J., Mujumdar A.S., Wang S. Trends in microwave-related drying of fruits and vegetables. Trends in Food Science & Technology. 2006;17:524–534. [Google Scholar]
- Zhang Q., Wu Y., Lu Y., Fei X. Role of vitamin D in risk factors of patients with type 2 diabetes mellitus. Medicina Clínica. 2020;154:151–156. doi: 10.1016/j.medcli.2019.04.019. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Bi J., Yi J., Jin X., Wu X., Zhou M. Evaluation of sensory, textural, and nutritional attributes of shiitake mushrooms (Lentinula edodes) as prepared by five types of drying methods. Journal of Food Process Engineering. 2019;42 [Google Scholar]
- Zhao J., Ding Y., Nie Y., Xiao H., Zhang Y., Zhu Z., et al. Glass transition and state diagram for freeze-dried Lentinus edodes mushroom. Thermochimica Acta. 2016;637:82–89. [Google Scholar]