Intravitreal injections are the standard of care in various retinal pathologies. Intravitreal anti-vascular endothelial growth factor (VEGF) therapy is the first-line treatment in diseases like neovascular age-related macular degeneration (nAMD), polypoidal choroidal vasculopathy (PCV), secondary choroidal neovascular membranes (CNVM), macular edema due to retinal vein occlusions (RVO), diabetic macular edema (DME) etc. in adults.[1] The recent COVID-19 (novel coronavirus disease-2019) pandemic has strained the overburdened health care system in India. There is a risk of human-to-human transmission of infection mainly by droplets and contact with contaminated surfaces or hands.[2] This has resulted in implementation of strict precautions to reduce its spread. There is also a tendency to delay elective procedures. Various guidelines have been issued by national and international organizations in ophthalmology to effectively treat patients while ensuring safety for patients and health care workers (HCW).[3,4]
There is limited literature available on the guidelines for the management of intravitreal injections in COVID times.[5,6] These pose unique challenges. Most of the patients who require intravitreal anti-VEGF injections are elderly or have systemic co-morbidities like diabetes mellitus, hypertension, obesity, chronic kidney disease, respiratory problems, etc., making them a 'high risk' group for contracting and transmitting COVID and also predispose them to serious complications associated with COVID.[2] These patients also require an increased number of routine follow-up visits to assess the response to therapy. These also require detailed ophthalmic evaluation and multiple ocular investigations like optical coherence tomography, fluorescein angiography (FFA), etc. for deciding the management. Therefore, there can be an increased risk of transmission of COVID to patients and HCWs. Delay in proper treatment can lead to serious blinding complications due to the risk of sub foveal haemorrhage, sub retinal fibrosis, damage to photoreceptors, or vitreous haemorrhage. Thus, proper patient selection, triaging, safe injection techniques, and proper sterilization are key to effectively manage injections in the COVID era.
We have implemented various modifications from routine intravitreal injection practices at our government tertiary eye care setup. These modifications have helped us in catering to patients during the countrywide COVID lockdown period and thereafter when the lockdown is lifted. The safety of patients and HCWs is foremost. Standard COVID precautions were practiced before taking up a patient for injection. The appointment of patients with respiratory symptoms suggestive of COVID was postponed until the resolution of symptoms, and the patient was directed to the physician/COVID treating facility. Patient triaging using the COVID questionnaire was done at the entrance of the hospital by HCW wearing full personal protective equipment (PPE). These include N95 mask, gloves, gown, cap, and face shield. COVID suspects were referred to the COVID treating facility. Patients were allowed after ensuring masks and hand hygiene/gloves. Ophthalmic examination was done by ophthalmologists wearing PPE (N95 mask, face shield, gloves) with adequate hand hygiene. Physical distancing was ensured. Open door ventilation was preferred with no internal recirculation of air. Home-based dilation of eyes was preferred for old follow-ups. Judicious use of imaging investigations was done. Invasive investigations like FFA were minimised. Non-invasive tests like OCT Angiography was favoured for follow-up visits. Patients were advised to actively practice home-based monitoring of vision using Amsler's grid and smartphone apps.
While planning for injections, patients were stratified based on the risk of vision loss, severity of the disease, and systemic stability. Non-ophthalmic life-threatening conditions were given priority over intravitreal injections. Conditions like neovascular AMD, PCV, active proliferative diabetic retinopathy with DME were given preference over DME, RVO patients. Those patients who had stable AMD/PCV or DME with vision better than 6/12 were deferred. Treat and extend or pro re nata regimen was preferred over fixed monthly dosing.[5,6] Longer-acting anti-VEGFs were recommended if possible. Patients were injected on the same day if required, to minimize the number of OPD visits. Consent for intravitreal injection was taken and COVID self-declaration form/consent was filled by the patient.[4]
Limited number of patients were called in each session and adequate physical distancing was ensured. Intravitreal injections were given in operation theatre (OT) maintaining utmost asepsis. Routine OPD based injections (like in western countries) are discouraged in Indian settings. Bilateral injections were also not performed due to the increased risk of post-injection endophthalmitis in our settings. Routine sterile precautions for intravitreal injections were observed. Importantly, the patient always wears a mask (cap, gown/OT dress also) and the ophthalmologist full PPE (N95 mask, gown, gloves, face shield or goggles) while injecting anti-VEGF [Fig. 1]. Any talking by the doctor or patient was discouraged. Additional drapes were applied to minimize contamination and exposure. Perioperative use of 10% betadine (Povidone Iodine) for skin and 5% betadine for conjunctival irrigation was ensured. Minimum number of personnel in OT were ensured with proper scrubbing and cough etiquettes. Adequate gap between two injections was maintained and overcrowding was discouraged. Next day follow-up visits were discouraged.[5,6] Patients were advised to contact doctors on phone for any query and eye casualty only in emergencies. Longer OPD follow-up (4-6 weeks) was advised.
Figure 1.
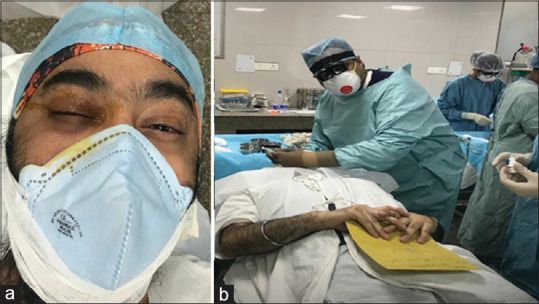
(a) Patient is wearing mask with surgical cap during intravitreal injection. (b) Photograph of the injection OT showing surgeon wearing full PPE and patient draped after changing to OT dress. Other HCWs are also wearing PPE
Some modifications have also been done in the OT sterilization protocol. After cleaning floors and walls with detergent, they were cleaned with Ecoshield solution (11% hydrogen peroxide). The cleaning of floors can also be done by 1% hypochlorite. OT surfaces were sprayed and cleaned with Bacillocid rasant (rapid disinfectant containing glutaraldehyde). Routine use of 1% hypochlorite or 70% isopropyl alcohol-based disinfectant was done in-between cases. At the end of the day, fogging was done by using Ecoshield or Bacillocid special disinfectant. To maintain strict asepsis, Ultraviolet (UV) light was switched on for overnight. UV is also effective against coronaviruses.
Following these precautions and practices, more than 100 patients received intravitreal injections during the lockdown period for various indications mentioned above. There was no case of serious ophthalmic/systemic adverse event in any patient. None of the patient or HCW contracted COVID or sent for quarantine. Thus, proper patient selection, proper personal protection measures (for patient and HCW) and strict OT sterilization protocols can help in assuring safe intravitreal injection practices during COVID times.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Tah V, Orlans HO, Hyer J, Casswell E, Din N, Sri Shanmuganathan V, et al. Anti-VEGF therapy and the retina: An Update. J Ophthalmol. 2015;2015:627674. doi: 10.1155/2015/627674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. People who are at higher risk for severe illness. 2020. [Last accessed on 2020 May 05]. Available from https://wwwcdcgov/coronavirus/2019-ncov/need-extra-precautions/people-at-higher-riskhtml .
- 3.American Academy of Ophthalmology. Important coronavirus updates for ophthalmologists. 2020. [Last accessed on 2020 May 05]. Available from https://wwwaaoorg/headline/alert-important-coronavirus-context .
- 4.Sengupta S, Honavar SG, Sachdev MS, Sharma N, Kumar A, Ram J, et al. Writing Committee on behalf of the All India Ophthalmological Society - Indian Journal of Ophthalmology Expert Group for COVID Practice Guidelines. All India Ophthalmological Society – Indian Journal of Ophthalmology consensus statement on preferred practices during the COVID-19 pandemic. Indian J Ophthalmol. 2020;68:711–24. doi: 10.4103/ijo.IJO_871_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Korobelnik JF, Loewenstein A, Eldem B, Joussen AM, Koh A, Lambrou GN, et al. Guidance for anti-VEGF intravitreal injections during the COVID-19 pandemic. Graefes Arch Clin Exp Ophthalmol. 2020:1–8. doi: 10.1007/s00417-020-04703-x. doi: 101007/s00417-020-04703-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shmueli O, Chowers I, Levy J. Current safety preferences for intravitreal injection during COVID-19 pandemic. Eye. 2020:1–3. doi: 10.1038/s41433-020-0925-x. doi: 101038/s41433-020-0925-x. [DOI] [PMC free article] [PubMed] [Google Scholar]


