Abstract
Purpose:
To study the correlation between thyroid eye disease (TED) with type-2 diabetes mellitus.
Methods:
A cross-sectional cohort study was conducted from Jan 2018 to Dec 2018, in patients presenting with thyroid eye disease to orbit and oculoplasty clinic of a tertiary eye care hospital. A total of 105 patients were included in the study. All patients underwent detailed ophthalmic evaluation and thyroid eye disease workup. Patients were categorized into mild, moderate, and severe/sight-threatening TED based on EUGOGO classification. Systemic history of diabetes was noted. RBS was done in all patients.
Results:
Mild disease was noted 61 patients of which 11 were diabetics, moderate in 26 patients (8 diabetics), and severe disease in 18 patients (14 diabetics). All patients were treated accordingly. Among the TED patients, the percentage of diabetic patients was noted to be in increasing order toward the severity spectrum of TED. The prevalence of severe TED was found to be much higher in diabetic patients accounting upto 77.77% of 18 patients. A statistically significant correlation was noted (P = 0.014) between severe TED and type-2 diabetes mellitus. In addition, early onset of thyroid eye disease was noted in type-2 diabetes patients. Even though female preponderance was noted, severe TED was more in men (66.6%).
Conclusion:
An autoimmune etiology for the association of thyroid and type-1diabetes has been well established. This study shows that type-2 diabetic patients can have more severity in the clinical presentation of TED. Therefore, the presence of type-2 DM in patients with TED can be a predictive factor for onset, progression, and severity of disease. Hence, a high concern of interest among treating ophthalmologists and endocrinologists regarding this entity would help in early prediction and decreased morbidity among such patients.
Keywords: Diabetes mellitus, thyroid eye disease, thyroid-related orbitopathy, type-2 DM
Thyroid-related eye disease (TED) is a most commonly encountered orbital disorder in adults which has a significant impact on the quality of life and may be sight-threatening. TED is characterized by inflammation and expansion of retroocular soft tissues. Proptosis, malfunctioning of the extraocular muscles, corneal exposure, and optic nerve damage are the major clinical consequences noted. Thyroid-related eye disease (TED) occurs only in a subset of patients with thyroid disorders and the factors and its underlying mechanisms remain unclear. Various risk factors have been described for the development or worsening of the condition, which include gender and ancestral group, genetic, environmental, and mechanical factors, and also factors related to thyroid dysfunction.[1] Environmental factors involve smoking (either active or passive) while mechanical factor involves orbital anatomy and its venous or lymphatic return.[1] Diabetes mellitus is emerging to be an additional risk factor.
Diabetes mellitus (DM) is the most common endocrine disorder, with significant morbidity and mortality.[2] The underlying pathogenesis in explaining diabetes and thyroid-related orbitopathy is unclear. The common factor of autoimmunity in type-1 diabetes and Graves' disease can be attributed to their association.[3] However, severe TED cases are seen in type-2 diabetes which is more prevalent and has no associated autoimmunity. In this study, we have analyzed the correlation between the severity of TED and type-2 diabetes mellitus.
Methods
The aim of our research was to study the correlation between severity of thyroid eye disease and type-2 diabetes mellitus with an objective to know the epidemiological aspects of TED with type-2 diabetes mellitus in terms of gender distribution, its prevalence in diabetics, and its predictability and as a contributing factor for the development or progression of TED.
We conducted a cross-sectional cohort study in norms with ethical committee guidelines of the hospital over a duration of 12 months from Jan 2018 to Dec 2018, including a total of 105 TED patients. The study adhered to the guidelines of the declaration of Helsinki.
Patients
All patients attending orbit and oculoplasty clinic diagnosed as TED, based on the clinical picture, and supported by immunological and biochemical findings were included. Systemic thyroid status was noted. Age-group ranging from 18 to 85 was included. A special note was made on preexisting diabetic status of the patient, type of diabetes, duration, and treatment aspect. Mild alteration in blood sugar levels after oral or intravenous steroids for TED was not considered as diabetics. However, these patients were referred to a physician for a detailed evaluation to rule out prediabetes. In female patients with TED, a history of gestational diabetic status (GDM) was noted.
A general evaluation was done in terms of pulse, blood pressure, tremors, thyroid swelling, and body weight measurements. The routine ophthalmological evaluation included visual acuity, routine anterior segment evaluation, fundus examination, and intraocular pressure measurements. All patients were tested for color vision, central fields by confrontation. Humphrey's 30-2 visual field testing was done in patients who had defective central field testing.
TED evaluation
TED severity and clinical activity stage were evaluated at the patient presentation. TED severity was evaluated according to the EUGOGO guidelines.[4] Palpebral fissure width was evaluated in millimeters by a ruler and proptosis with a Hertel exophthalmometer [Fig. 1]. Diplopia was classified according to the Gorman score.[5] TED was defined a) mild when features of TED have only a minor impact on daily life and patients had one or more of the following: minor lid retraction (< 2 mm), mild soft tissue involvement, exophthalmos <3 mm above 21, transient or no diplopia, and corneal exposure responsive to lubricants; b) moderate to severe when eye disease had sufficient impact on daily life with one or more of the following: lid retraction 2 mm or more, moderate or severe soft tissue involvement, exophthalmos 3 mm or more above 21, and inconstant or constant diplopia; [Figs. 1 and 2] and c) severe and sight-threatening [Fig. 3] in the case of dysthyroid optic neuropathy (DON) and/or corneal breakdown.[6] In patients with DON, findings included sluggishly reacting pupil with altered color vision, disc edema, visual field defects on HFA, and enlarged extraocular muscles at orbital apex (apical crowding) or optic nerve stretching on imaging. Clinical activity scoring was done.
Figure 1.
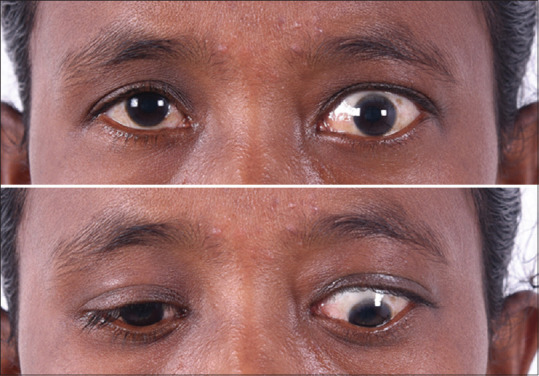
Unilateral TED in a female showing lid retraction and lid lag
Figure 2.
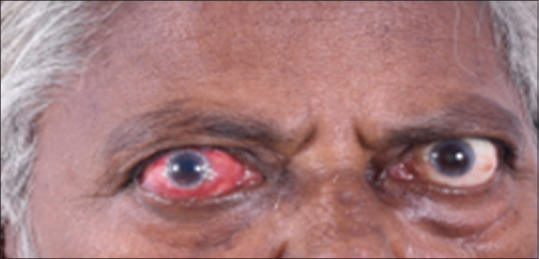
Female patient presenting with unilateral thyroid eye disease
Figure 3.
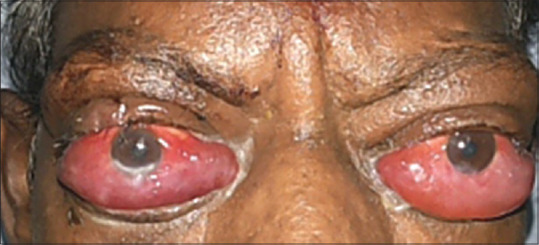
A 48-year-old male patient with features of bilateral severe TED and right eye showing features of corneal decompensation
Laboratory measurements
Free T3, free T4, and TSH were measured by microparticle enzyme immunoassays. Anti TPO antibodies and antimicrosomal antibodies were measured. An assessment was made regarding diabetic status. Random blood sugar was done in all patients. Those who had abnormal RBS and not a prior diagnosed case of diabetes were evaluated further by a physician and were reviewed back. Based on physician note, patients were later grouped either newly diagnosed diabetic or nondiabetic in the respective category of TED. Among diabetics, fasting and postprandial sugar levels were noted and physician referral was sought if patient had uncontrolled sugars in spite of medications. Imaging: magnetic resonance imaging (MRI) orbits [Fig. 4] was performed to look for extraocular muscle crowding at apex and optic nerve streching. B-scan in orbital mode was done to assess extraocular muscle thickness.
Figure 4.
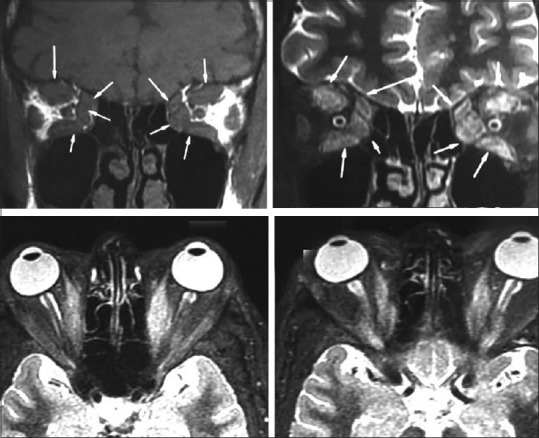
Magnetic resonance imaging of TED showing bulky extraocular muscles at apex in coronal view and tendon sparing of the same in axial view
Treatment
Patients were treated according to the stage of disease. The mild disease given lubricants in cases of lid retraction. Moderate cases were treated with either oral steroids and prism glasses for diplopia or lubricants. Aesthetically concerned patients were given injection botulinum toxin into levator muscle for unsightly lid retraction. Severe TED cases were given pulse IV steroid therapy (500 mg methylprednisolone IV weekly doses for 6 weeks and 250 mg doses for next 6 weeks). Sight threatening cases were treated with intravenous methylprednisolone 1 g over 3 days, followed by pulse IV steroid therapy. Surgical interventions like blepharotomy, tarsorrhaphy, therapeutic keratoplasty, and orbital decompression were done in sight-threatening cases not responding to IVMP therapy within 72 h.
Statistical analysis
Student's t-test was used to evaluate continuous variables with the normal distribution. The Chi-square test was used to perform categorical data.
Results
A total of 105 patients were included. The mild disease was seen in 61 patients accounting to 58.1%. Among these mild cases, there were 11 diabetics. The moderate disease was seen in 26 patients accounting for 24.8% out of which 8 were diabetics. Severe/sight-threatening TED was seen in 18 patients accounting for 17.1% out of which 14 were diabetics [Table 1 and Graph 1]. Percentage of diabetics in mild, moderate, and severe TED is 18%, 30.7%, and 77%, respectively. [Table 1 and Graph 2] Mean age of the cohort included was 48.4 years.
Table 1.
Distribution of TED in the cohort along with mean age values at presentation
| TED | No of patients | Diabetes | Mean Age | No diabetes | Mean Age |
|---|---|---|---|---|---|
| Mild | 61 | 11 | 38.3 | 50 | 40.1 |
| Moderate | 26 | 8 | 41.7 | 18 | 43.2 |
| Severe/sight threatening | 18 | 14 | 49.9 | 4 | 55.5 |
| Total | 105 | 33 | 72 |
Graph 1.
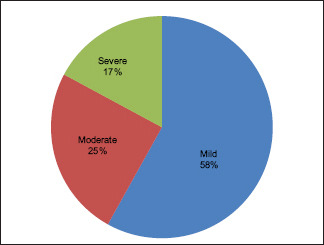
Showing the distribution of patients among spectrum of TED severity
Graph 2.
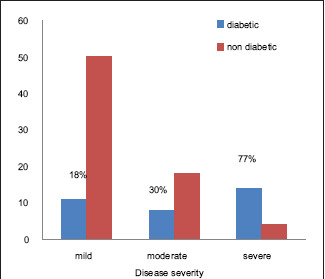
Showing association of TED severity with diabetes mellitus and percentage of diabetics in each group
Out of 105 patients, there were 58 females and 47 males accounting for 55.3% and 44.6%, respectively [Table 2]. Female preponderance was noted. Out of 58 females, three patients had a history of gestational diabetes mellitus. Among females, the mild-to-moderate disease was seen in 88.7% and severe TED in 10.3%. Though the overall incidence of TED was more in females, severe TED was noted more often in males. Out of 47 males, the mild-to-moderate disease was seen in 74.5% and severe TED was seen in 25.5% (12 patients). There was a male predilection accounting for 66.6% of the entire cohort of severe TED.
Table 2.
Gender distribution
| TED | Males | Percentage | Females | Percentage |
|---|---|---|---|---|
| Mild | 21 | 45% | 33 | 57% |
| Moderate | 14 | 30% | 19 | 33% |
| Severe/sight threatening | 12 | 25% | 6 | 10% |
| Total | 47 | 100 | 58 | 100 |
Out of 105 patients, 33 were diabetics, accounting to 31.42%. All patients were type-2 diabetics and three females had gestational diabetic mellitus. Among the TED patients, the severity spectrum of disease had a greater percentage of diabetic patients, as observed in Graph 2. In patients with a thyroid disorder and concurrent diabetes, the onset of TED was earlier in the course of disease compared to those without diabetes. Out of 18 severe TED, 14 were diabetics out of which 12 were on insulin therapy [Table 3]. Ten of the 14 diabetics in severe TED group also had uncontrolled sugars. Three patients with a history of GDM, presently with controlled sugar levels had moderate TED and were on oral hypoglycaemics. Out of 33 diabetics, 7 (3 = moderate, 4 = severe GO) patients were newly diagnosed at ophthalmic presentation. Endocrinologist opinion regarding the confirmation and treatment of this newly diagnosed diabetes was taken. Diabetic retinopathy was looked for in all patients except the ones with exposure keratopathy. However, no correlation of retinopathy severity with TED was noted.
Table 3.
Details of diabetic status in TED
| TED | Diabetics | New onset DM | Oral hypoglycemics | On Insulin |
|---|---|---|---|---|
| Mild | 11 | 0 | 11 | 0 |
| Moderate | 8 | 3 | 8 | 0 |
| Severe/sight threatening | 14 | 4 | 2 | 12 |
| Total | 33 | 7 | 21 | 12 |
The prevalence of severe TED was found to be much higher in diabetic patients accounting upto 77.77% of 18 severe TED patients. Among them, 10 patients had dysthyroid optic neuropathy (9 were diabetics) and 8 patients had corneal decompensation (5 were diabetics) [Table 4]. All features of TED severity like proptosis and extraocular muscle involvement were more frequently seen in diabetic patients than in nondiabetics. On statistical analysis as reflected in Table 2, the association between diabetes and severe TED was found to be statistically significant (P < 0.0001) based on Chi-square test.
Table 4.
Distribution of diabetics in severe TED
| Severe/sight threatening TED | Number of patients | Diabetics |
|---|---|---|
| DON | 10 | 9 |
| Corneal decompensation | 8 | 5 |
| Total | 18 | 14 |
Discussion
Thyroid-related eye disease may develop in euthyroid, hypothyroid, or hyperthyroid status with various contributing factors. Wiersinga et al. recently published a composition of 4 aspects as a predictive score for the development and progression of Graves' orbitopathy in newly diagnosed Graves' disease. They included clinical activity score, TSH-binding inhibitory immunoglobulins, duration of hyperthyroid symptoms, and smoking.[6] We wanted to evaluate whether the coexisting diabetes mellitus has an impact on the development and progression of TED and whether DM can be used as a predictive factor for the prognosis. Among the total recruited patients with TED, 31.4% were diabetics. A study done by Moli et al. states that 53.2% of their patients had TED with diabetes.[7] This difference in the prevalence in our study could be attributed to both type-1 and type-2 diabetes included in their study. A similar observation was made by Kalman and Mouritz 18 years ago in a series of 462 TED patients in the Netherlands, with the prevalence of diabetes then being 3.1%.[8] This could be attributed to an increased overall incidence of type-2 DM in recent times.
Gender distribution in our study shows a similar trend in accordance with the previous data, and the usual predominance of autoimmunity in women, with a female predilection in the ratio of 1.23:1, as with study by Burch and Wartofsky being 2:1.[9] Male gender was shown to confer higher risk of TED and males with Graves' disease have either the same chances or even higher chances of developing TED. TED in males has a more severe form of ocular involvement and later age of onset.[1,10] Our study also shows a higher number of males in severe TED group accounting to 67.7% and mean age among males was 49.8 years, in accordance with above-mentioned studies. However, the increased severity of TED in men can also be confounded by smoking.
Severe TED was seen in 18 (17.1%) patients. Out of these, 77.7% were diabetics. Moli et al. also state that TED is most severe and more frequent in type-2 DM patients.[7] Among the severe TED patients, 55.5% had DON, out of which 90% were diabetics. Neigel et al. described a group of 58 patients with DON, with 15.5 of them being diabetics.[11] Following this, the study by Kalman and Mouritz in 1999 showed 26.3% diabetics in the DON group, compared with 3.1% diabetics in the whole GO population.[8] The increased incidence of DON in diabetics could be explained by marginal oxygenation of the optic nerve in the diabetics due to its vasculopathy, which makes the optic nerve more susceptible to pressure due to apical crowding by enlarged extraocular muscles.[8] Further, recent radiological studies mention that either apical crowding or optic nerve stretching is seen in all cases of DON.[12] Studies have also shown that patients with DON and diabetics have poor or no response to orbital decompression.[8,13] This higher incidence of DON in diabetics alerts the possible coexisting nature of this entity and considering it as a predictive factor for severe TED. Moreover, 7 patients in our study as described earlier presented to us with TED and then diagnosed to be type-2 diabetics. This supports our objective that DM is more often associated with TED, which is overlooked in routine clinical practice. Being an inexpensive prognosticating tool, it also should prompt clinicians to check blood sugar levels of all patients presenting with features of TED.
A more recent study by Khurana et al.[14] found a high prevalence (16%) of thyroid disorders in type-2 DM and recommends screening of thyroid dysfunction in all diabetic patients, especially with poor diabetic control.[14] In our study also, diabetics with uncontrolled sugars on insulin were found in severe TED group. American Thyroid association in its recent archives, mentions diabetes as a high-risk factor and recommends frequent TSH assays.[15,16] This further strengthens the correlation between thyroid disorder and type-2 diabetes.
Upon considering all the above aspects, our study combines the following data regarding the higher incidence of severe TED in diabetics, newly diagnosed diabetics among TED patients, and raising number of diabetics at the severity end of TED spectrum and also supports the prior studies regarding the increased incidence of DON in these patients.
Recent endocrinological data explains multiple mechanisms for thyroid dysfunction in type-2 diabetics and stresses upon detection of thyroid dysfunction among type-2 diabetic patients.[16] From ophthalmological point of view, we would like to stress the need to look for type-2 DM in all TED patients. Thus, type-2 diabetes mellitus can be considered a risk factor for disease progression and also a predictive factor for prognosis of thyroid eye disease. A better analysis of correlation regarding the status of controlled or uncontrolled diabetics on TED could have been assessed by measuring HbA1C levels. This remains the drawback of our study.
Conclusion
Severe TED is more often associated with diabetes. The presence of type-2 DM in patients with TED can be a predictive factor for onset, progression, and severity of TED. Hence, every TED patient has to be evaluated for diabetes. Further studies for the possible underlying mechanisms would help in proving this clinical association. This calls for special attention among ophthalmologists and endocrinologists to be extra vigilant in considering this correlation, especially in Asian countries with the high prevalence of type-2 diabetes mellitus.
The part of this research work was presented as a paper at oculoplasty session in south-regional ophthalmic conference [SROC-KOS2018] held at Mangalore in December-2018.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Stan M, Bahn R. Risk factors for development or deterioration of Graves' ophthalmopathy. Thyroid. 2010;20:777e83. doi: 10.1089/thy.2010.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Niffenegger JH, Fong D, Cavallerano J. Diabetes mellitus. In: Jakobiec FA, Albert DM, editors. Principles and practice of ophthalmology: Basic sciences. Vol. 5. Philadelphia: WB Saunders; 1994. pp. 2925–36. [Google Scholar]
- 3.Greco D, Pisciotta M, Gambina F, Maggio F. Graves' disease in subjects with type 1 diabetes mellitus: A prevalence study in Western Sicily (Italy) Prim Care Diabetes. 2011;5:241e4. doi: 10.1016/j.pcd.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 4.Bartalena L, Baldeschi L, Dickinson A, Eckstein A, Kendall-Taylor P, Marcocci C, et al. Consensus statement of the European Group on Graves' orbitopathy (EUGOGO) on management of GO. Eur J Endocrinol. 2008;158:273–85. doi: 10.1530/EJE-07-0666. [DOI] [PubMed] [Google Scholar]
- 5.Bahn RS, Gorman CA. Choice of therapy and criteria for assessing treatment outcome in thyroid e associated ophthalmopathy. Endocrinol Metab Clin North Am. 1987;16:391e407. [PubMed] [Google Scholar]
- 6.Wiersinga W, Žarković M, Bartalena L, Donati S, Perros P, Okosieme O, et al. Predictive score for the development or progression of Graves' orbitopathy in patients with newly diagnosed Graves' hyperthyroidism. Eur J Endocrinol. 2018;178:635–43. doi: 10.1530/EJE-18-0039. [DOI] [PubMed] [Google Scholar]
- 7.Le Moli R, Muscia V, Tumminia A, Frittitta L, Buscema M, Palermo F, et al. Type 2 diabetic patients with Graves' disease have more frequent and severe Graves'orbitopathy? Nutr Metab Cardiovasc Dis. 2015;25:452–7. doi: 10.1016/j.numecd.2015.01.003. doi: 10.1016/j.numecd. 2015.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Kalman R, Mouritz MP. Diabetes mellitus: A risk factor in patients with Graves' orbitopathy. Br J Opthalmol. 1999;83:463e5. doi: 10.1136/bjo.83.4.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burch HB, Wartofsky L. Graves' ophthalmopathy current concepts regarding pathogenesis and management. Endocr Rev. 1993;14:747–93. doi: 10.1210/edrv-14-6-747. [DOI] [PubMed] [Google Scholar]
- 10.Lim SL, Lim AK, Mumtaz M, Hussein E, Wan Bebakar WM, Khir AS. Prevalence, risk factors, and clinical features of thyroid-associated ophthalmopathy in multiethnic Malaysian patients with Graves' disease. Thyroid. 2008;18:1297–301. doi: 10.1089/thy.2008.0044. [DOI] [PubMed] [Google Scholar]
- 11.Neigel JM, Rootman J, Belkin RI, Nugent RA, Drance SM, Beattie CW, et al. Dysthyroid optic neuropathy. The crowded orbital apex syndrome. Ophthalmology. 1988;95:1515–21. doi: 10.1016/s0161-6420(88)32978-7. [DOI] [PubMed] [Google Scholar]
- 12.Rutkowska-Hinc B, Maj E, Jabłońska A, Milczarek-Banach J, Bednarczuk T, Miśkiewicz P. Prevalence of radiological signs of dysthyroid optic neuropathy in magnetic resonance imaging in patients with active, moderate-to-severe, and very severe graves orbitopathy. Eur Thyroid J. 2018;7:88–94. doi: 10.1159/000486828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mourits M Ph, Koornneef L, Wiersinga WM, Prummel MF, Berghout A, van der Gaag R. Orbital decompression for Graves' ophthalmopathy by inferomedial, inferomedial plus lateral, and by coronal approach. Ophthalmology. 1990;97:636–41. doi: 10.1016/s0161-6420(90)32532-0. [DOI] [PubMed] [Google Scholar]
- 14.Khurana A, Dhoat P, Jain G. Prevalence of thyroid disorders in patients of type 2 diabetes mellitus. J Indian Acad Clin Med. 2016;17:13. [Google Scholar]
- 15.Ladenson PW, Singer PA, Ain KB, Bagchi N, Bigos ST, Levy EG, et al. American thyroid association guidelines for detection of thyroid dysfunction. Arch Intern Med. 2000;160:1573–5. doi: 10.1001/archinte.160.11.1573. [DOI] [PubMed] [Google Scholar]
- 16.Wang C. The relationship between type 2 diabetes mellitus and related thyroid diseases. J Diabetes Res. 2013;2013:390534. doi: 10.1155/2013/390534. [DOI] [PMC free article] [PubMed] [Google Scholar]


