Abstract
Objective
To conduct a systematic review of case reports documenting the development of antiphospholipid syndrome (APS) or APS-related features after an infection.
Methods
We searched Medline, EMBASE, Web of Science, PubMed ePubs, and The Cochrane Library – CENTRAL through March 2015 without restrictions. Studies reporting cases of APS or APS-related features following an infection were included.
Results
259 publications met inclusion criteria, reporting on 293 cases. Three different groups of patients were identified; group 1 included patients who fulfilled the criteria for definitive APS (24.6%), group 2 included patients who developed transient antiphospholipid (aPL) antibodies with thromboembolic phenomena (43.7%), and group 3 included patients who developed transient aPL antibodies without thromboembolic events (31.7%). The most common preceding infection was viral (55.6%). In cases that developed thromboembolic events Human immunodeficiency (HIV) and Hepatitis C (HCV) viruses were the most frequently reported. Parvovirus B19 was the most common in cases that developed antibodies without thromboembolic events. Hematological manifestations and peripheral thrombosis were the most common clinical manifestations. Positive anticardiolipin antibodies were the most frequent antibodies reported, primarily coexisting IgG and IgM isotypes. Few patients in groups 1 and 2 had persistent aPL antibodies for more than 6 months. Outcome was variable with some cases reporting persistent APS features and others achieving complete resolution of clinical events.
Conclusions
Development of aPL antibodies with all traditional manifestations of APS were observed after variety of infections, most frequently after chronic viral infections with HIV and HCV. The causal relationship between infection and APS cannot be established, but the possible contribution of various infections in the pathogenesis of APS need further longitudinal and controlled studies to establish the incidence, and better quantify the risk and the outcomes of aPL-related events after infection.
Keywords: anticardiolipin antibodies, antiphospholipid antibodies, lupus anticoagulant, infection, systematic review
Antiphospholipid syndrome (APS) is a systemic autoimmune disease with persistent elevation of antiphospholipid (aPL) antibodies that can result in recurrent thromboembolic events, and pregnancy-related morbidity with recurrent fetal losses.(1) The disease may be life-threatening with multiple organ failure in about 1% of cases, who develop catastrophic antiphospholipid syndrome (CAPS).(2)
The reported prevalence of elevated aPL antibodies, mainly anticardiolipin (aCL) and lupus anticoagulant (LA), among healthy individuals is 1–5%; higher among elderly individuals with chronic diseases. It is not clear how many people with elevated aPL antibodies develop APS.(3–7) APS often occurs in association with other autoimmune diseases, most commonly systemic lupus erythematosus (SLE).(2)
The molecular pathogenesis of APS is complex, and environmental triggers may play a crucial role in genetically predisposed individuals.(8) APS may occur in association with an infection or malignancy, or may be induced by certain drugs (e.g., interferon-alpha).(9) The pathogenesis of these associations is unclear.(10) Molecular mimicry with shared genetic epitopes with infectious agents has been proposed as a possible mechanism.(11, 12) Previous studies suggested that infection may lead to the development of transiently elevated non-thrombogenic aPL antibodies lacking anti-β2 glycoprotein-I (anti-β2 GPI) activity.(13, 14) However, there are increasing case reports of patients with various types of infections who develop aPL antibodies and thromboembolic events.
We conducted a systematic review of all such reported cases in the literature to summarize existing evidence. Although a systematic review of case reports cannot support causality between infection and APS, it can identify unrecognized or rare associations, and can generate hypotheses for subsequent studies. Our objective was to identify potentially putative infections identified in the literature in association with APS, and to describe related clinical and immunologic features.
METHODS
Data sources and searches
We searched electronic databases (Medline, EMBASE, Web of Science, PubMed ePubs, and The Cochrane Library - CENTRAL) with no language restrictions, from inception through March 2015 to identify case reports of patients with elevated aPL antibodies after an infection. References of included articles were also searched manually. Search terms are provided in Appendix 1.
Study selection
The screening of eligible publications was carried out independently by two raters. First, the titles and abstracts of all citations were reviewed. Next, the full text of potentially relevant citations was reviewed. Discrepancies were resolved by consensus. Cases were only included if they reported patients with a history of infection that was diagnosed before elevated aPL antibodies were identified in those patients, whether or not they had APS-related clinical features. We considered any type of infection as long as the infectious agent was identified. To meet the definition of aPL antibodies elevation, one positive laboratory test either LA, aCL, or anti-β2 GPI antibodies after a prior diagnosis of infection was required. For the diagnosis of APS, infection must be followed by thromboembolic manifestations (arterial or venous), or pregnancy-related complications, with persistent elevation of aPL antibodies that remained positive for at least 12 weeks.(1) Diagnosis of CAPS was considered when the authors of the reported cases considered the diagnosis of CAPS, or when thromboembolic events developed in three or more organs simultaneously with persistent aPL antibodies positivity and small vessels occlusion confirmed by biopsy.(15) A time frame was not chosen between the earlier diagnosis of infection and the subsequent recognition of aPL antibodies positivity as there are no published validated criteria to define this time window. Nevertheless, studies were excluded if they reported patients with APS diagnosed before the infection was acquired, or coexisted with the diagnosis of infection. Studies were also excluded if they reported patients with a definite history of SLE diagnosed prior to APS or infection.
Data extraction and quality assessment
Data was extracted by one reviewer and crosschecked by another. Data from articles published in languages other than English were extracted by physician collaborators proficient in the original language (Chinese, Japanese, Spanish, French, and Germany). We extracted data on the potentially putative infections (whether viral, bacterial, fungal, or parasitic), clinical presentation following infection and prior comorbidities, laboratory abnormalities, aPL antibodies elevation (whether LA, aCL, or anti-β2 GPI antibodies), aPL antibodies positivity (whether persistent or transient), treatment required and patient outcomes.
We used a modified version of a tool for quality appraisal of case reports.(16) The assessment was carried out by one investigator and a random sample was crosschecked by another. For articles published in languages other than English only one reviewer performed the assessment. We used four items: i) patient was described adequately (i.e., chief complaint, history, clinical and laboratory evaluations, treatments), ii) an accurate diagnosis was provided (i.e., valid and reliable outcome measures were utilized), iii) convincing evidence in support of the diagnosis was presented (i.e., according to the criteria for diagnosis of APS/CAPS, or describing the evidence for diagnosis), and iv) alternate explanations were considered and refuted (differential diagnosis was illustrated and scientifically excluded, or underlying possible mechanisms that could explain the finding were addressed). Possible item ratings were yes, partially, or no.
Data synthesis and analysis
Data were summarized using descriptive statistics, with means and standard deviations for continuous variables and frequencies and percentages for dichotomous variables.
RESULTS
Publication characteristics
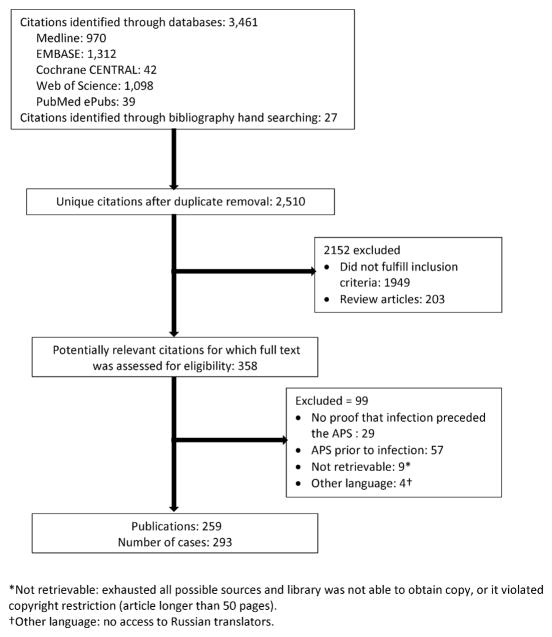
A total of 2,510 unique citations were initially retrieved (Figure 1). We identified 358 citations as potentially relevant and reviewed the full publication. We excluded 29 publications reporting cases in which no proof that infection preceded the development of APS, 57 publications reporting cases in which diagnosis of APS preceded the infection, 9 publications were not retrievable, and 4 Russian language publications as we were unable to translate them. We included 259 publications (reporting on 293 cases where clinical description of each reported case was provided separately). Bibliographic references for the case reports are included in Appendix 2. Cases from the United States were most common (20.5%), followed by Spain (14.7%), and France (12.6%).
Figure 1.
Study selection flow chart.
Quality appraisal
The overall quality of the cases was good to moderate. Most cases reported an adequate description of the chief complaint, patient past medical history, laboratory and image investigations, and treatments (87.0%). Accurate diagnosis with valid and reliable outcomes measures were reported for two thirds (65.9%). Convincing evidence of diagnosis was provided in 81.6% and an alternate explanation was reported in 73.0% (Appendix 2).
Patient characteristics
The mean age of the cases was 34.0 years (standard deviation, 19.4 years). One hundred and fifty-three patients (52.2%) were male. Patients were categorized into 1 of 3 groups according to the clinical presentation reported. Group 1 included 72 patients (24.6%) whose infection was followed by symptoms that fulfilled the classification criteria for definitive APS, including 17 patients (5.8%) who fulfilled the most up-to-date CAPS criteria.(1, 13) Group 2 included 128 patients (43.7%) who developed thromboembolic phenomena associated with elevated aPL antibodies during the course of infection, but did not fulfill APS/CAPS criteria (either transient antibodies or not enough follow-up duration). Group 3 included 93 patients (31.7%) who developed transient elevated aPL antibodies after an infection but did not develop thromboembolic manifestations or pregnancy-related complications.
Types of infections
The most common type of infection across all groups was viral (55.6%) (Table 1). In general, Human immunodeficiency (HIV) and Hepatitis C (HCV) viruses were the most frequent infections reported primarily in cases that developed thromboembolic events in group 1 (17.0%) and group 2 (9.9%). Parvovirus B19 (PVB19) was the most frequently reported viral infection in group 3 (antibodies with no thromboembolic or pregnancy events) (16.1%).
Table 1.
Types of infections reported in the 3 patient groups
| Infection | N (%)a | |||
|---|---|---|---|---|
| Total, N=293 | Group 1 APS/CAPS criteria N=72 | Group 2 Incomplete criteria N=128 | Group 3 No clinical events N=93 | |
| Viral | 163 (55.6) | 38 (52.8) | 78 (60.9) | 47 (50.5) |
| Human immunodeficiency virus | 47 (16.0) | 13 (18.1) | 26 (20.3) | 8 (8.6) |
| Human immunodeficiency virus + Hepatitis C virus | 3 (1.0) | 1 (1.4) | 1 (0.8) | 1 (1.1) |
| Hepatitis C virus | 29 (9.9) | 11 (15.3) | 14 (10.9) | 4 (4.3) |
| Hepatitis A virus | 3 (1.0) | 0 | 2 (1.6) | 1 (1.1) |
| Hepatitis B virus | 2 (0.7) | 0 | 2 (1.6) | 0 |
| Parvovirus B19 | 19 (6.5) | 2 (2.8) | 2 (1.6) | 15 (16.1) |
| Cytomegalovirus | 17 (5.8) | 4 (5.6) | 9 (7.0) | 4 (4.3) |
| Varicella-zoster virus | 15 (5.1) | 3 (4.2) | 12 (9.4) | 0 |
| Epstein-Barr virus | 9 (3.1) | 1 (1.4) | 6 (4.7) | 2 (2.2) |
| Herpes simplex virus | 2 (0.7) | 1 (1.4) | 0 | 1 (1.1) |
| Cytomegalovirus + Epstein-Barr virus | 2 (0.7) | 0 | 0 | 2 (2.2) |
| Human herpes virus 6 | 1 (0.3) | 0 | 0 | 1 (1.1) |
| Adenovirus | 6 (2) | 0 | 0 | 6 (6.5) |
| Dengue virus | 2 (0.7) | 0 | 2 (1.6) | 0 |
| Influenza | 3 (1.0) | 2 (2.8) | 1 (0.8) | 0 |
| Rubella | 1 (0.3) | 0 | 0 | 1 (1.1) |
| Measles | 1 (0.3) | 0 | 0 | 1 (1.1) |
| Japanese encephalitis | 1 (0.3) | 0 | 1 (0.8) | 0 |
| Bacterial | 108 (36.9) | 22 (30.6) | 48 (37.5) | 38 (40.9) |
| Coxiella burnetii | 21 (7.2) | 0 | 5 (3.9) | 16 (17.2) |
| Coxiella burnetii + Rickettsia typhi | 1 (0.3) | 0 | 0 | 1 (1.1) |
| Coxiella burnetii + Helicobacter pylori | 1 (0.3) | 0 | 1 (0.8) | 0 |
| Coxiella burnetii + Mycoplasma pneumonia | 1 (0.3) | 0 | 0 | 1 (1.1) |
| Mycoplasma pneumonia | 14 (4.8) | 1 (1.4) | 11 (8.6) | 2 (2.2) |
| Streptococci | 9 (3.1) | 5 (6.9) | 2 (1.6) | 2 (2.2) |
| Staphylococci | 5 (1.7) | 3 (4.2) | 2 (1.6) | 0 |
| Mycobacterium tuberculosis | 8 (2.7) | 1 (1.4) | 5 (3.9) | 2 (2.2) |
| Mycobacterium tuberculosis + Staphylococci | 1 (0.3) | 0 | 1 (0.8) | 0 |
| Mycobacterium lepra | 6 (2.0) | 3 (4.2) | 2 (1.6) | 1 (1.1) |
| Escherichia coli | 4 (1.4) | 1 (1.4) | 2 (1.6) | 1 (1.1) |
| Escherichia coli + Bacteroides fragilis | 1 (0.3) | 0 | 1 (0.8) | 0 |
| Escherichia coli + Bacteroides ovatus + Fusobacterium necrophorum | 1 (0.3) | 0 | 1 (0.8) | 0 |
| Salmonella | 3 (1.0) | 1 (1.4) | 2 (1.6) | 0 |
| Klebsiella | 2 (0.7) | 0 | 1 (0.8) | 1 (1.1) |
| Bartonella henselae | 2 (0.7) | 0 | 1 (0.8) | 1 (1.1) |
| Rickettsia africae | 2 (0.7) | 0 | 0 | 2 (2.2) |
| Pseudomonas aeruginosa | 2 (0.7) | 1 (1.4) | 1 (0.8) | 0 |
| Mycoplasma penetrans | 1 (0.3) | 1 (1.4) | 0 | 0 |
| Proteus mirabilis | 1 (0.3) | 1 (1.4) | 0 | 0 |
| Helicobacter pylori | 1 (0.3) | 0 | 0 | 1 (1.1) |
| Chlamydia | 1 (0.3) | 0 | 0 | 1 (1.1) |
| Listeria monocytogenes | 1 (0.3) | 0 | 0 | 1 (1.1) |
| Neisseria meningitides | 1 (0.3) | 0 | 1 (0.8) | 0 |
| Bacteroides fragilis | 1 (0.3) | 0 | 1 (0.8) | 0 |
| Fusobacterium necrophorum | 1 (0.3) | 0 | 1 (0.8) | 0 |
| Campylobacter jejuni | 1 (0.3) | 0 | 1 (0.8) | 0 |
| Eubacterium Limosum | 1 (0.3) | 1 (1.4) | 0 | 0 |
| Spirochetal | 13 (4.4) | 3 (4.2) | 6 (4.7) | 4 (4.3) |
| Borellia burgdorferi | 6 (2.0) | 1 (1.4) | 3 (2.3) | 2 (2.2) |
| Syphilis | 5 (1.7) | 2 (2.8) | 1 (0.8) | 2 (2.2) |
| Leptospirosis | 2 (0.7) | 0 | 2 (1.6) | 0 |
| Parasitic | 12 (4.1) | 3 (4.2) | 4 (3.1) | 5 (5.4) |
| Malaria | 5 (1.7) | 0 | 2 (1.6) | 3 (3.2) |
| Fasciola hepatica | 2 (0.7) | 0 | 2 (1.6) | 0 |
| Toxoplasmosis | 1 (0.3) | 1 (1.4) | 0 | 0 |
| Entamoeba histolytica | 1 (0.3) | 1 (1.4) | 0 | 0 |
| Enterobius vermicularis | 1 (0.3) | 1 (1.4) | 0 | 0 |
| Sarcoptes scabies | 1 (0.3) | 0 | 0 | 1 (1.1) |
| Trypanosoma brucei | 1 (0.3) | 0 | 0 | 1 (1.1) |
| Fungal | 5 (1.7) | 1 (1.4) | 2 (1.6) | 2 (2.2) |
| Candida | 2 (0.7) | 0 | 1 (0.8) | 1 (1.1) |
| Aspergillus fumigatus | 1 (0.3) | 1 (1.4) | 0 | 0 |
| Bipolaris spicifera | 1 (0.3) | 0 | 0 | 1 (1.1) |
| Cryptococcus | 1 (0.3) | 0 | 1 (0.8) | 0 |
| Unidentified organism | 22 (7.5) | 10 (3.9) | 5 (3.9) | 7 (7.5) |
Fifteen patients (2 in group 1, 8 in group 2, and 5 in group 3) reported more than 1 type of infection (viral, bacterial, parasitic, and fungal).
Bacterial infections were reported in 108 patients (36.9%), most commonly secondary to Coxiella burnetii, Mycoplasma pneumonia, streptococci, and Mycobacterium tuberculosis. Most Coxiella cases resulted in development of antibodies without clinical manifestations, while for the other infections the majority of the cases reported had APS.
Parasitic and fungal infections were less common across all groups; only 12 patients (4.1%) had a parasitic infection and 5 patients (1.7%) had a fungal infection. Fifteen patients (5.1%) were reported to have more than one type of infection (viral, bacterial, spirochetal, parasitic, and fungal). In 22 cases (7.5%) the infectious agent was not clearly identified. These cases were reported to have gastrointestinal, urinary, upper respiratory tract infections, or other unspecified infections.
In cases that developed CAPS, HCV was the most common infection reported, although another 9 different viral and bacterial infections were also observed (Appendix 3).
Most commonly, infection alone was reported as the precipitating factor for APS or elevated aPL antibodies with no other comorbidities were identified (83.6%) (Table 2). A history of other concomitant diseases was reported in the remainder of the cases, most frequently an autoimmune or inflammatory disease (7.5%) (cases with SLE were excluded), or a previous diagnosis of cancer (2.4%). In addition, a prior history of congenital, cardiovascular, blood, or allergic diseases was reported in a few cases. For these cases, there was no evidence of the presence of aPL antibodies before the onset of infection. Viral infection was predominant in cases with a prior history of autoimmune diseases (81.8%), with PVB19 occurring in approximately one third of the cases (31.8%) (Appendix 4).
Table 2.
Possible factors precipitating APS or elevated aPL antibodies in each group
| Precipitating factor | N (%) | |||
|---|---|---|---|---|
| Total, N=293 | Group 1 APS/CAPS criteria N=72 | Group 2 Incomplete criteria N=128 | Group 3 No clinical events N=93 | |
| Infection only | 245 (83.6) | 61 (84.7) | 113 (88.3) | 71 (76.3) |
| Infection and concomitant disease | 48 (16.4) | 11 (15.3) | 15 (11.7) | 22 (23.7) |
| Autoimmune diseasesa | 22 (7.5) | 6 (8.3) | 3 (2.3) | 13 (14.0) |
| Acute rheumatic fever | 2 (2.8) | 0 | 0 | |
| Cutaneous sarcoidosis and leukocytoclastic vasculitis | 1 (1.4) | 0 | 0 | |
| Discoid lupus | 1 (1.4) | 0 | 0 | |
| Drug-induced SLE | 0 | 0 | 1 (1.1) | |
| Kikuchi-Fujimoto disease | 1 (1.4) | 0 | 0 | |
| Seronegative spondyloarthropathies | 1 (1.4) | 1 (0.8) | 4 (4.3) | |
| Polyarticular JIA | 0 | 0 | 4 (4.3) | |
| Vasculitisb | 0 | 0 | 4 (4.3) | |
| Sjögren syndrome | 0 | 1 (0.8) | 0 | |
| Multiple sclerosis | 0 | 1 (0.8) | 0 | |
| Tumora | 7 (2.4) | 0 | 5 (3.9) | 2 (2.2) |
| Hairy cell leukemia | 0 | 0 | 1 (1.1) | |
| Lymphoma in complete remission | 0 | 1 (0.8) | 0 | |
| Acute myeloid leukemia | 0 | 0 | 1 (1.1) | |
| Epidermoid carcinoma of the mouth in complete remission | 0 | 1 (0.8) | 0 | |
| Benign tumor near optic chiasma | 0 | 1 (0.8) | 0 | |
| Idiopathic inflammatory pseudotumor of the orbits + tolosa Hunt syndrome | 0 | 1 (0.8) | 0 | |
| Squamous cell carcinoma of the cervix incomplete remission | 0 | 1 (0.8) | 0 | |
| Congenital diseases | 6 (2.0) | 3 (4.2) | 2 (1.6) | 1 (1.1) |
| Cardiovascular diseases | 5 (1.7) | 1 (1.4) | 3 (2.3) | 1 (1.1) |
| Blood diseases | 4 (1.4) | 0 | 1 (0.8) | 3 (3.2) |
| Congenital afibrinogenemia | 0 | 1 (0.8) | 0 | |
| Chronic hemolytic anemia | 0 | 0 | 1 (1.1) | |
| Mild hemophilia A | 0 | 0 | 1 (1.1) | |
| Factor VIII deficiency | 0 | 0 | 1 (1.1) | |
| Allergic and hypersensitivity diseases | 4 (1.4) | 1 (1.4) | 1 (0.8) | 2 (2.2) |
SLE: systemic lupus erythematosus; JIA: juvenile idiopathic arthritis.
Serum level of antiphospholid antibodies was not determined in patients with autoimmune diseases or patients with malignancy before the onset of infection.
Four case reports with vasculitis including Wegener granulomatosis, central nervous system vasculitis secondary to neurosyphilis, and 2 cases with leukocytoclastic vasculitis secondary to infection.
Clinical features
Table 3 shows the most common features in patients who presented with thromboembolic or pregnancy related events (with or without fulfilling APS criteria). Hematologic manifestations, were reported in 33.5% of the cases, with 5.0% developing disseminated intravascular coagulopathy (DIC). Thrombocytopenia was reported in 33.3% of cases fulfilling the diagnosis of APS or CAPS, in 21.9% of those who developed thromboembolic events with elevated aPL antibodies and in 6 out of 10 cases complicated by DIC. Peripheral thrombosis was the most commonly reported thromboembolic complication occurring in 30.0% of the cases, followed by stroke or transient ischemic attacks (23.5%) and pulmonary thromboembolism (16.5%). Obstetric complications (with up to 5 recurrent abortions) were reported among 7 patients in the group fulfilling APS criteria (9.7%). Other less frequent manifestations are shown in Table 3.
Table 3.
Clinical presentations of antiphospholipid syndrome in group 1 and thromboembolic phenomena associated with elevated aPL antibodies in group 2a
| Clinical presentation | N (%) | ||
|---|---|---|---|
| Total, N = 200 | Group 1 APS/CAPS criteria N=72 | Group 2 Incomplete criteria N=128 | |
| Hematologic manifestations | 65 (33.5) | 31 (43.1) | 34 (26.6) |
| Thrombocytopenia and/or hemolytic anemia | 52 (26.0) | 24 (33.3) | 28 (21.9) |
| Pancytopenia | 3 (1.0) | 2 (2.8) | 1 (0.8) |
| Disseminated intravascular coagulopathy | 10 (5.0) | 5 (6.9) | 5 (3.9) |
| Peripheral thrombosis | 60 (30.0) | 28 (38.9) | 32 (25.0) |
| Vascular thrombosis in UL/LL | 53 (26.5) | 23 (31.9) | 30 (23.4) |
| Jugular and/or subclavian vein thrombosis | 4 (2.0) | 2 (2.8) | 2 (1.6) |
| Jugular and subclavian veins thrombosis + vascular thrombosis in LL | 1 (0.5) | 1 (1.4) | 0 |
| Testicular thrombosis | 1 (0.5) | 1 (1.4) | 0 |
| Penile infarction + vascular thrombosis in UL/LL | 1 (0.5) | 1 (1.4) | 0 |
| Neurologic manifestations | 54 (27.0) | 21 (29.2) | 33 (25.8) |
| Stroke and/or transient ischemic attack | 47 (23.5) | 17 (23.6) | 30 (23.4) |
| Chorea | 1 (0.3) | 1 (1.4) | 0 |
| Seizures | 3 (1.0) | 1 (1.4) | 2 (1.6) |
| Multi-infarct dementia | 1 (0.3) | 1 (1.4) | 0 |
| Transverse myelopathy | 1 (0.3) | 1 (1.4) | 0 |
| Encephalopathy | 1 (0.3) | 0 | 1 (0.8) |
| Cutaneous manifestations | 39 (19.5) | 15 (20.8) | 24 (18.7) |
| Cutaneous necrosis and/or capillary thrombosis (livedo reticularis/pseudovasculitis/purpura) | 22 (11.0) | 10 (13.9) | 12 (9.4) |
| Digital gangrene | 14 (7.0) | 5 (6.9) | 9 (7.0) |
| Penile leukocytoclastic vasculitis | 3 (1.5) | 0 | 3 (2.3) |
| Respiratory manifestations | 38 (19.0) | 14 (19.4) | 24 (18.8) |
| Pulmonary thromboembolism | 33 (16.5) | 12 (16.7) | 21 (16.4) |
| Pulmonary hypertension | 1 (0.5) | 0 | 1 (0.8) |
| Pulmonary and diffuse alveolar hemorrhage | 1 (0.5) | 0 | 1 (0.8) |
| Pulmonary thromboembolism + pulmonary and diffuse alveolar hemorrhage | 2 (1.0) | 1 (1.4) | 1 (0.8) |
| Acute respiratory distress syndrome | 1 (0.5) | 1 (1.4) | 0 |
| Cardiac manifestations | 34 (17.0) | 17 (23.6) | 17 (13.3) |
| Intra-cardiac thrombus | 7 (3.5) | 5 (6.9) | 2 (1.6) |
| Superior and/or inferior vena cava thrombosis | 10 (5.0) | 4 (5.6) | 6 (4.7) |
| Internal carotid artery thrombosis | 3 (1.5) | 0 | 3 (2.3) |
| Aortic occlusion | 2 (1.0) | 0 | 2 (1.6) |
| Intra-cardiac thrombus + aortic occlusion | 1 (0.5) | 1 (1.4) | 0 |
| Myocardial infarction | 8 (4.0) | 6 (8.3) | 2 (1.6) |
| Valve thickening and/or vegetation | 3 (15) | 1 (1.4) | 2 (1.6) |
| Renal manifestations | 23 (11.5) | 13 (18.1) | 10 (7.8) |
| Renal vessels occlusion | 9 (4.5) | 5 (6.9) | 4 (3.1) |
| Acute renal failure | 11 (5.5) | 6 (8.3) | 5 (3.9) |
| End stage renal disease | 2 (1.0) | 2 (2.8) | 0 |
| Membranous/focal proliferative glomerulonephritis | 1 (0.5) | 0 | 1 (0.8) |
| Splenic infarction | 19 (9.5) | 8 (11.1) | 11 (8.6) |
| Gastrointestinal manifestations | 13 (6.5) | 5 (6.9) | 8 (6.2) |
| Abdominal vessels (mesenteric/iliac/abdominal aorta) occlusion | 11 (5.5) | 5 (6.9) | 6 (4.7) |
| Gastric ulcer | 2 (1.0) | 0 | 2 (1.6) |
| Osteo-articular manifestations | 12 (6.0) | 3 (4.2) | 9 (7.0) |
| Arthralgia/arthritis | 2 (1.0) | 2 (2.8) | 0 |
| Avascular necrosis | 10 (5.0) | 1 (1.4) | 9 (7.0) |
| Hepatic manifestations | |||
| Portal and/or hepatic vessels thrombosis | 11 (5.5) | 4 (5.6) | 7 (5.5) |
| Ophthalmologic manifestations | |||
| Retinal thrombosis and/or optic neuropathy | 8 (4.0) | 2 (2.8) | 6 (4.7) |
| Obstetric manifestations | 7 (3.5) | 7 (9.7) | 0 |
| Adrenal crisis | 2 (1.0) | 2 (2.8) | 0 |
LL: lower limb; UL: upper limb.
Patients in group 3 did not show postinfectious thromboembolic complications related to antiphospholipid syndrome.
In patients with HIV, avascular necrosis was the main presentation followed by peripheral thrombosis, stroke, and cutaneous necrosis as well. Whereas in patients with HCV infection, thrombocytopenia, peripheral thrombosis, and stroke were the main clinical features similarly observed (Appendix 5).
By definition, patients in group 3 did not develop thromboembolic manifestations or pregnancy complications related to APS or CAPS. Transient thrombocytopenia after the infection was detected in 9 patients (9.7%); 4 (4.3%) of whom had platelet counts of less than 100,000/mm3, but was not associated with any clinical consequences. No other laboratory abnormalities were reported.
aPL antibody profiles
All cases were tested for at least one positive aPL antibody as per our inclusion criteria, but not all cases were tested for the same antibodies (Table 4). Positive aCL antibodies were the most frequently reported in groups 1 (89.2%) and 2 (93.3%), mainly as coexisting IgG and IgM antibodies. Positive LA was the most common in group 3 (92.3%) and anti-β2 GPI antibodies were reported in 60.6% of all cases among the three groups. Additionally, positive aPL antibodies with unspecified isotype were reported in 8.5%.
Table 4.
Antiphospholipid antibody isotypes in each patient group among reported cases
| Antiphospholipid antibodies | N (%) | |||
|---|---|---|---|---|
| Total, N=293 | Group 1 APS/CAPS criteria N=72 | Group 2 Incomplete criteria N=128 | Group 3 No clinical events N=93 | |
| Anticardiolipin antibodies (reported data) | n = 243 | n = 65 | n = 105 | n = 73 |
| IgG alone | 54 (22.2) | 19 (29.2) | 22 (21.0) | 13 (17.8) |
| IgM alone | 40 (16.5) | 4 (6.2) | 23 (21.9) | 13 (17.8) |
| IgA alone | 5 (2.1) | 0 | 1 (1.0) | 4 (5.5) |
| IgG + IgM | 87 (35.8) | 28 (43.1) | 36 (34.3) | 23 (31.5) |
| IgG + IgM + IgA | 2 (0.8) | 0 | 2 (1.9) | 0 |
| Unspecified | 25 (10.3) | 7 (10.8) | 14 (13.3) | 4 (5.5) |
| Positive for any isotype | 213 (87.7) | 58 (89.2) | 98 (93.3) | 57 (78.1) |
| Negative for all isotypes | 30 (12.3) | 7 (10.8) | 7 (6.7) | 16 (21.9) |
| Lupus anticoagulant antibodies (reported data) | n = 170 | n = 48 | n = 70 | n = 52 |
| Positive | 120 (70.6) | 30 (62.5) | 42 (60.0) | 48 (92.3) |
| Negative | 50 (29.4) | 18 (37.5) | 28 (40.0) | 4 (7.7) |
| Anti-β2 glycoprotein-I antibodies (reported data) | n = 99 | n = 20 | n = 44 | n = 35 |
| Positive | 60 (60.6) | 15 (75.0) | 25 (56.8) | 20 (57.1) |
| Negative | 39 (39.4) | 5 (25.0) | 19 (43.2) | 15 (42.9) |
| Unspecified isotypea | 25 (8.5) | 5 (6.9) | 15 (11.7) | 5 (5.4) |
IgG: immunoglobulin G; IgM: immunoglobulin M; IGA: immunoglobulin A.
Unspecified isotype: antiphospholipid antibodies without defining the isotype, antiphosphatidylserine, antiphosphatidylcholine, and antiphosphatidylserine-prothrombin complex.
In cases that developed anti-β2 GPI antibodies, viral infections were predominant (65.0%); HCV in group 1 (40.0%), CMV in group 2 (20.0%) followed by HIV and varicella (16.0% each), and PVB19 in group 3 (45.0%).
Follow-up was reported for 168 cases, and among them 120 (71.4%) had transient aPL antibodies (in general considered for most case as less than 6 months). Persistent positive antibodies were observed in 28 patients in group 1 (84.8%) in contrast to only 11 patients in group 2 (14.3%) and 9 patients in group 3 (15.5%). Nine patients in group 1 (27.3%) and 7 patients in group 2 (9.2%) showed persistent positive aPL antibodies for more than 6 months. Follow-up data was reported in 41 cases that developed anti-β2 GPI antibodies, and revealed transient antibodies not associated with any clinical consequences in 70.7%.
Treatment
Details of treatment were available for 266 cases. All patients received antimicrobial therapy. Anticoagulation was given to most patients who develop thromboembolic events; anticoagulants and/or antiplatelet therapy (aspirin or clopidogrel) were administered to 54 patients in group 1 (88.5%) and 72 patients in group 2 (63.2%). In group 3, only 1 HIV infected case (1.1%) received anticoagulation for stroke thought to be secondary to neurosyphilis with brain vasculitis.
Outcomes
Data on patient outcomes was available in 236 cases. In group 1, 7 patients (15.2%) died (5 from CAPS, 1 from active acquired immunodeficiency, and the cause of death was not defined in 1 patient with HIV infection); 17 (37.0%) continued to have recurrent APS manifestations, and 22 (47.8%) became asymptomatic on antithrombotic therapy.
HIV and HCV were the most common infections reported in patients who died; no specific aPL antibody was identified. Two thirds of cases that developed CAPS, had persistent APS or died.
In group 2, 9 (8.1%) died from thrombotic complications and the remainder (91.9%) had complete resolution of thrombotic events with no recurrences. Four patients in group 3 (5.1%) died during the course of their infection, the rest recovered completely with no complications of APS or CAPS.
Patients with anti-β2 GPI antibodies and HCV had worse outcomes (persistent APS and death) than those with PVB19, where antibodies were transient and not associated with thromboembolic events.
Sixty-five cases were 18 years old or younger. Of these, 38 (58.5%) had viral, and 22 (33.9%) had bacterial infections (Appendix 6). Infection was the sole precipitating factor in 52 cases (80.0%), and 37 cases (56.9%) developed clinical manifestations (groups 1 and 2) mainly hematologic and cutaneous, followed by peripheral thrombosis and stroke. Non-typical presentations such as cardiac, vena cava, carotid artery, pulmonary thrombosis, and splenic infarction were also reported in the pediatric group. Three cases diagnosed were diagnosed with CAPS where the identified agents were Escherichia coli, Pseudomonas aeruginosa, and PVB19. Persistent APS and death were reported in 6 cases; 88.2% had complete recovery.
DISCUSSION
Since their discovery, aPL antibodies have been a subject of great interest. Cardiolipin was the major tissue extract for reactive non-treponemal tests for syphilis since 1906.(17, 18) Although cardiolipin antibodies (IgG, IgM or IgA) were considered a serological marker for syphilis, many other infections, such as hepatitis, varicella, measles, scarlet fever, or viral pneumonia, were associated with transient positive tests considered to be false positive tests for syphilis.(19–21) It was subsequently observed that patients with autoimmune diseases, primarily SLE, could develop persistent false positive tests for syphilis.(22–24) In the early 1980s, aCL antibodies cross-reacting with negatively charged phospholipids were discovered using an enzyme-linked immunosorbent assay (ELISA) and its association with APS syndrome was described.(23, 25–28) Further studies identified the role of β2 GPI, a cofactor with anticoagulant properties required to enhance aCL binding to target phospholipids.(13, 29, 30) Generally, it had been thought that aCL antibodies in patients with infection were not associated with β2 GPI.(14, 31) However, increasingly, case reports of patients have shown that aCL, LA and β2 GPI can occur after infection with clinical consequences, not just as a transient non-pathogenic process.
To our knowledge, we are reporting the largest and most comprehensive systematic review of case reports on the association of infection with subsequent APS or APS-related features. Our review identified 293 case reports with more than 50 different infections associated with subsequent development of aPL antibodies. We classified cases according to the clinical presentation reported: APS or CAPS, as per diagnostic criteria (group 1), APS events not fulfilling criteria (group 2), and elevated aPL antibodies alone with no associated thromboembolic or pregnancy events (group 3). The most common putative infections in all three groups were viral, with HIV and HCV as the most frequent infections in patients with clinical manifestations (group 1 and 2), and PVB19 in group 3. Bacterial infections were the second most common infections in all 3 groups with Mycoplasma pneumonia, streptococci, and Mycobacterium tuberculosis being the most frequently reported in group 1 and 2, while Coxiella cases were more frequent in group 3. Infection alone was the sole precipitating factor in the majority of the reported cases (83.6%), with the remainder reporting primarily pre-existing autoimmune or inflammatory disorders (SLE was excluded) or cancer. In cases with pre-existing autoimmune disease, PVB19 infection was the predominant infection.
Thrombocytopenia and peripheral vascular thrombosis were the most common presenting features among patients with clinical manifestations (groups 1 and 2). Outcomes were variable and ranged from complete resolution of clinical manifestations and antibodies to persistent APS with recurrent events, and CAPS. In general, patients who fulfilled criteria for APS or CAPS were more likely to develop chronic persistent disease. HIV and HCV were the most common infections reported in those cases where persistent APS or death occurred. Non-typical presentations such as retinal, aortic, and abdominal vessel occlusion, splenic infarction, and/or adrenal crisis were also reported.
Among the included cases, 65 were children with 23 different infectious agents identified. Similar to adults, viral infection was the most common putative infection followed by bacterial. More than half of the cases developed clinical manifestations (groups 1 and 2) with occasional atypical presentations. Persistent APS and death were reported in few cases, but the majority had complete recovery.
The coexistence of infection and thromboembolic events has been previously reported in two other reviews, and in two case reports with a literature search included.(32–35) The first review of 100 patients with APS thrombotic manifestations and infection reported skin infections, HIV, pneumonia, HCV, and urinary infection as the most common infections, with pulmonary, skin, and renal thromboembolic events as the main clinical presentations.(32) In their review, cases with aPL antibodies and infection were not included unless thromboembolic events occurred. They reported a lower proportion of HIV cases compared to our findings, while the prevalence of HCV was relatively similar. Sixty eight percent of their cases had primary APS, with the reminder reporting other autoimmune diseases (27 cases had SLE). The timing of infection in relation to the diagnosis of primary APS was not clearly specified. The other comprehensive review described 82 patients with chronic HCV or HIV and reported non-typical presentations compared to patients from other case series of APS without infection.(35) Their findings, as ours, showed that avascular necrosis, followed by cutaneous necrosis and peripheral thrombosis, and neurological manifestations were most common in patients with HIV. Intra-abdominal thrombosis and myocardial infarction were more frequent in patients with HCV. Both reviews had a more limited search (only one database), and did not follow the specific steps required for systematic reviews, such as specific inclusion criteria, and quality appraisal.(32, 35, 36) Another previous review of 80 patients with CAPS pointed to infection as a possible triggering factor in 35% of the cases, but the majority of them had previous diagnosis of SLE or primary APS. Respiratory tract infection was the most common precipitating factor.(36) Analysis of 280 patients from a CAPS Registry also showed that infection was the most common precipitating factor in 22%, but the infectious agents were not identified.(37)
With respect to the frequency of aPL antibodies, aCL antibodies were the most commonly reported in groups 1 and 2, primarily coexisting IgG and IgM isotypes, and LA was the most frequently reported antibody in group 3. Many reports however, were old and did not test for the presence of anti-β2 GPI. Genetic polymorphism of β2 GPI may be an important risk factor in susceptibility to APS. (38–42) Molecular mimicry between infectious agents and β2 GPI has been proposed as a possible etiology of APS (11, 12) but the relationship between mimicry and genetic variants is unknown. In our review, positive anti-β2 GPI antibodies were identified across all three groups. Overall, more than two thirds of anti-β2 GPI antibodies were transient and not associated with any clinical consequences. However, in HCV infections, anti-β2 GPI appeared to be associated with persistent APS and/or death. None of the previous reviews had information on anti-β2 GPI.(32, 35, 36)
Our systematic review included a comprehensive literature search without any language restrictions, with specific criteria for inclusion and quality appraisal. Our findings are limited nevertheless by the quality and breadth of the data in the reports, which was not uniform or consistent (e.g. all reported cases were not tested for all aPL antibodies). Publication bias could account for increased number of cases with HIV and HCV. Most importantly, case series and reports are uncontrolled, and while they can suggest hypotheses they cannot establish robust associations. Nevertheless, clinicians should be aware of the large number of cases reported in the literature suggesting that infection may be implicated in the pathogenesis of APS, perhaps in genetically predisposed individuals. While case reports can identify signals, they are not robust enough for statistical inference. Therefore, the evidence provided is not sufficient to recommend systematic screening in patients with infections, but should alert physician of the possible putative association in patients with both signs and symptoms of infection and clinical features of APS.
In conclusion, development of aPL antibodies with all traditional manifestations of APS was observed after a variety of infections including viruses, bacteria, fungi and parasites. Our findings warrant the need for controlled longitudinal studies to establish the incidence and outcomes of aPL-related events after infection, and to help identify if specific infections may warrant systematic screening for aPL antibodies.
Acknowledgments
We are grateful to Harish R. Siddhanamatha, Saurabh P. Talathi, Huifang Lu, Xin Pan, for assisting in study selection or translation of the studies, and to Erica Goodoff, Scientific Editor in the Department of Scientific Publications at The University of Texas MD Anderson Cancer Center for their valuable contributions.
FUNDING SOURCE: Dr. Suarez-Almazor has a K24 career award from the National Institute for Arthritis, Musculoskeletal and Skin Disorders (NIAMS: grant # AR053593). The funding agency had no role in the study’s design, conduct, and reporting.
Appendix 1. MEDLINE search strategy
Database(s): Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations and Ovid MEDLINE(R) 1946 to 03/2015
| 1 | exp “BACTERIAL INFECTIONS AND MYCOSES”/ |
| 2 | exp VIRUS DISEASES/ |
| 3 | exp PARASITIC DISEASES/ |
| 4 | exp BACTERIA/ |
| 5 | exp VIRUSES/ |
| 6 | PARASITES/ |
| 7 | or/1–6 [pathogen or pathogen dis MeSH terms] |
| 8 | ANTIPHOSPHOLIPID SYNDROME/ |
| 9 | 7 and 8 |
| 10 | exp ANTIBODIES, ANTIPHOSPHOLIPID/ |
| 11 | BETA 2-GLYCOPROTEIN I/ |
| 12 | or/10–11 |
| 13 | (exp *“BACTERIAL INFECTIONS AND MYCOSES”/ or exp *VIRUS DISEASES/ or exp *PARASITIC DISEASES/) and (exp *ANTIBODIES, ANTIPHOSPHOLIPID/ or *BETA 2-GLYCOPROTEIN I/) [pathogen dis major MeSH AND antibody terms major MeSH] |
| 14 | ((antiphospholipid* adj3 (syndrom* or antibod*)) or (anti phospholipid* adj3 (syndrom* or antibod*)) or “lupus anticoagula*” or “lupus anti coagula*” or “lupus coagulation inhibitor*” or anticardiolipin* or anti-cardiolipin* or “beta2 glycoprotein i” or “beta 2 glycoprotein i” or “beta2 gpi” or beta2gpi or “beta 2 gpi” or “beta 2gpi” or “apolipoprotein h” or “apo h” or apoh).ti. [antibody terms in titles] |
| 15 | (infect* or coinfect* or co-infect* or bacter* or fungus* or fungem* or fungi* or fungal* or mycoses* or mycotic* or communicable* or virus* or viral* or viremi* or viridae or parasit* or microorganism* or micro-organism* or pathogen*1 or microbe*1 or microbial* or parvovir* or ebv or “epstein barr vir*” or mononucleos* or (human adj2 herpesvirus 4) or (burkitt* adj2 herpesvirus*) or (burkitt* adj2 lymphoma adj2 virus*) or “hhv 4”).ti. [infectious disease or pathogen terms in titles] |
| 16 | 14 and 15 |
| 17 | (((antiphospholipid* adj3 (syndrom* or antibod*)) or (anti phospholipid* adj3 (syndrom* or antibod*)) or “lupus anticoagula*” or “lupus anti coagula*” or “lupus coagulation inhibitor*” or anticardiolipin* or anti-cardiolipin* or “beta2 glycoprotein i” or “beta 2 glycoprotein i” or “beta2 gpi” or beta2gpi or “beta 2 gpi” or “beta 2gpi” or “apolipoprotein h” or “apo h” or apoh) adj10 (infect* or coinfect* or co-infect* or bacter* or fungus* or fungem* or fungi* or fungal* or mycoses* or mycotic* or communicable* or virus* or viral* or viremi* or viridae or parasit* or microorganism* or micro-organism* or pathogen*1 or microbe*1 or microbial* or parvovir* or ebv or “epstein barr vir*” or mononucleos* or (human adj2 herpesvirus 4) or (burkitt* adj2 herpesvirus*) or (burkitt* adj2 lymphoma adj2 virus*) or “hhv 4”)).ab. [keyword phrases within 10 words of each other in an abstract] |
| 18 | 7 and 14 [MeSH pathogen or dis and antibody keyword term] |
| 19 | (8 or 12) and 15 [APS MeSH term AND infect term in titles] |
| 20 | 9 or 13 or 16 or 17 or 18 or 19 [all facets merged] |
| 21 | (animals not (humans and animals)).sh. |
| 22 | 20 not 21 |
Appendix 2. Reported cases and their quality appraisal
| Author | Year | Country | Adequate description | Reliable outcome | Convincing evidence | Alternate explanation |
|---|---|---|---|---|---|---|
| Abernethy (1) | 1995 | USA | Yes | Partially | Partially | Yes |
| Abulafia (2, 3)a | 2004 | Brazil | Partially | Partially | No | No |
| Aguilar (4) | 2005 | Spain | Yes | Yes | Yes | Yes |
| Akerkar (5) | 2005 | India | Yes | Yes | Yes | Yes |
| Alcock (6) | 2011 | Australia | Yes | Yes | Yes | Yes |
| Aldamiz-Echebarria (7) | 1991 | Spain | Yes | Partially | No | Yes |
| Alric (8) | 1998 | France | Yes | Yes | Yes | Yes |
| Amiral (9) | 1997 | Greek | Yes | Yes | Yes | Yes |
| Amit (10) | 2012 | Israel | Yes | Yes | Yes | Yes |
| Anton-Martinez (11) | 2011 | Spain | Yes | Yes | Yes | Partially |
| Appert-Flory (12) | 2010 | France | Yes | Yes | Yes | Yes |
| Yes | Yes | Yes | Yes | |||
| Arnason (13) | 1995 | USA | Yes | Yes | Partially | Yes |
| Arruda (14) | 1993 | Brazil | Yes | Partially | Yes | Yes |
| Asano (15) | 2006 | Japan | Yes | Yes | Yes | Yes |
| Ascer (16) | 2011 | Brazil | Yes | Partially | Partially | Partially |
| Asherson (17) | 2001 | South Africa | Yes | Partially | Partially | No |
| Yes | Partially | Partially | No | |||
| Ashrani (18) | 2003 | USA | Yes | Yes | Partially | Yes |
| Yes | Yes | Partially | Yes | |||
| Aydin (19) | 2006 | Turkey | Yes | Partially | Yes | Yes |
| Baid (20) | 1999 | USA | Yes | Partially | Yes | Yes |
| Yes | Partially | Yes | Yes | |||
| Bakos (21) | 1996 | Brazil | Yes | Partially | Yes | Yes |
| Bakshi (22) | 2006 | India | Yes | Yes | Yes | Yes |
| Balderramo (23) | 2009 | Spain | Yes | Yes | Yes | Yes |
| Barfield (24) | 1997 | USA | Yes | Yes | Yes | Yes |
| Belmonte (25) | 1993 | Spain | Yes | Partially | Yes | Yes |
| Yes | Partially | Yes | Yes | |||
| Yes | Partially | Yes | Yes | |||
| Ben-Chetrit (26) | 2013 | Israel | Partially | Partially | Partially | No |
| Bibler (27) | 1986 | USA | Yes | Partially | Yes | Yes |
| Bloom (28) | 1986 | USA | Partially | Partially | Partially | Partially |
| Bouchard (29) | 1998 | France | Partially | Partially | Yes | No |
| Brackett (30) | 2011 | USA | Yes | Yes | Yes | No |
| Brown (31) | 2001 | USA | Yes | Partially | Yes | Yes |
| Yes | Partially | Yes | Yes | |||
| Yes | Partially | Yes | Yes | |||
| Brown (32) | 2008 | UK | Yes | Yes | Yes | Yes |
| Bulucu (33) | 2002 | Turkey | Yes | Yes | Partially | Yes |
| Cagatay (34) | 2004 | Turkey | Yes | Partially | Yes | Partially |
| Cailleux (35, 36)a | 1999 | France | Yes | Yes | Yes | Yes |
| Calvo (37) | 1998 | Spain | Partially | Yes | Yes | Partially |
| Campanelli (38) | 2004 | Switzerland | Yes | Yes | Yes | Yes |
| Campos-Alvarez (39) | 1992 | Spain | Yes | Partially | Yes | No |
| Canpolat (40) | 2008 | Turkey | Yes | Yes | Yes | Yes |
| Cappell (41) | 1993 | USA | Yes | Partially | Yes | Yes |
| Carli (42) | 1993 | France | Partially | Yes | Yes | No |
| Partially | Yes | Yes | No | |||
| Catteau (43) | 1995 | France | Yes | Yes | Yes | Yes |
| Yes | Yes | Yes | Yes | |||
| Charloux (44) | 1993 | France | Yes | Partially | Partially | Yes |
| Chen (45) | 2005 | Taiwan | Yes | Yes | Yes | Yes |
| Chen (46) | 2006 | Taiwan | Yes | Yes | Yes | Yes |
| Chevalier (47) | 1993 | France | Yes | Yes | Yes | Yes |
| Cho (48) | 2006 | Korea | Yes | Partially | Partially | Yes |
| Chou (49) | 2000 | Taiwan | Yes | Yes | Yes | Yes |
| Yes | Yes | Yes | Yes | |||
| Yes | Yes | Yes | Yes | |||
| Clark (50) | 2003 | UK | Yes | Yes | Yes | Yes |
| Collazos (51) | 1994 | Spain | Yes | Partially | Yes | Yes |
| Cooray (52) | 2013 | Canada | Yes | Yes | Yes | Yes |
| Corti (53) | 2001 | Spain | Yes | Yes | Yes | Yes |
| Cross (54) | 1999 | USA | Yes | Yes | Yes | Partially |
| Cull (55) | 2012 | USA | Yes | Yes | Yes | Yes |
| Damian (56) | 2004 | Romania | Yes | Partially | Yes | No |
| Daniels (57) | 2008 | USA | Yes | Partially | Yes | Yes |
| De Argila Fernandez-Auran (58) | 1996 | Spain | Yes | Partially | Partially | Partially |
| de, Corla-Souza André (59) | 2003 | USA | Yes | Partially | Yes | Yes |
| de, Lucas (60) | 1998 | Spain | Partially | Yes | Yes | No |
| De, Larranaga (61) | 2005 | Argentina | Yes | Yes | Yes | Yes |
| del, Arco (62) | 2001 | Spain | Partially | Yes | Yes | No |
| Del, Castillo (63) | 1997 | Spain | Yes | Yes | Partially | Yes |
| Delbos (64) | 2007 | France | Yes | Yes | Yes | Yes |
| Demey (65) | 1997 | Belgium | Yes | Yes | Partially | No |
| Yes | Yes | Partially | No | |||
| Devars (66) | 1997 | France | Partially | Yes | Yes | Partially |
| Diaz (67) | 2010 | Spain | Yes | Yes | Yes | Yes |
| Doyle (68) | 1998 | USA | Yes | Yes | Yes | Yes |
| Drulovic (69) | 2000 | Yugoslavia | Yes | Partially | Yes | Yes |
| Durkin (70) | 2013 | USA | Yes | Yes | Yes | Yes |
| Economou (71) | 2003 | Greece | Yes | Yes | Yes | Yes |
| Enomoto (72) | 2010 | Japan | Partially | Partially | Yes | Yes |
| Ergas (73) | 2008 | Israel | Yes | Partially | Yes | Yes |
| Ertem (74, 75)a | 2001 | Turkey | Yes | Yes | Yes | Yes |
| Yes | Yes | Yes | Yes | |||
| Fain (76) | 2009 | France | Partially | Partially | Partially | No |
| Faller (77) | 1999 | France | Yes | Partially | Partially | Yes |
| Fanlo (78) | 2010 | Spain | Yes | Yes | Yes | Yes |
| Yes | Yes | Yes | Yes | |||
| Faria (79) | 2011 | Portugal | Partially | Yes | Yes | No |
| Fernandez (80) | 2007 | Spain | Yes | Yes | Yes | Yes |
| Yes | Yes | Yes | Yes | |||
| Yes | Yes | Yes | Yes | |||
| Flateau (81) | 2013 | France | Yes | Yes | Yes | Yes |
| Freeman (82) | 2014 | UK | Yes | Yes | Yes | Yes |
| Frontino (83) | 2009 | Italy | Yes | Yes | Yes | Yes |
| Galvez (84) | 1997 | Spain | Partially | Yes | Partially | Yes |
| Garcia Rincon (85) | 2014 | Colombia | Yes | Yes | Yes | Yes |
| Germano (86) | 2005 | Portugal | Yes | Yes | Yes | Yes |
| Ghosh (87) | 2008 | India | Yes | Yes | Yes | Yes |
| Giordano (88) | 2005 | Italy | Yes | Yes | Yes | Yes |
| Girard (89) | 2005 | France | Yes | Partially | Yes | Yes |
| Gologorsky (90) | 2011 | USA | Yes | Partially | Yes | Yes |
| Gorczyca (91) | 2005 | Poland | Yes | Partially | Partially | Partially |
| Graffin (92) | 2007 | France | Yes | Yes | Yes | Yes |
| Granel (93) | 1998 | France | Yes | Yes | Yes | Partially |
| Grau (94) | 1991 | Spain | Partially | Yes | Yes | No |
| Partially | Yes | Yes | No | |||
| Partially | Yes | Yes | No | |||
| Graw-Panzer (95) | 2009 | USA | Yes | Yes | Yes | Yes |
| Greco (96) | 2011 | USA | Yes | Yes | Yes | Yes |
| Yes | Partially | Partially | Partially | |||
| Yes | Partially | Partially | No | |||
| Gru (97) | 2010 | USA | Yes | Yes | Yes | Yes |
| Guedes-Barbosa (98) | 2008 | Brazil | Yes | Yes | Yes | Yes |
| Haire (99) | 1986 | USA | Yes | Partially | Yes | Yes |
| Hal Sebastiaan (100) | 2005 | Australia | Yes | Yes | Yes | Yes |
| Hamidou (101) | 1993 | France | Yes | Partially | No | No |
| Hansen (102) | 1998 | USA | Yes | Yes | Yes | Yes |
| Harada (103) | 2003 | Japan | Yes | Partially | Yes | Yes |
| Hassoun (104) | 2004 | USA | Yes | Partially | Partially | Yes |
| Hernandez (105) | 2000 | Spain | Yes | Partially | Yes | Yes |
| Herscovici (106) | 2012 | Israel | Yes | Partially | Partially | Yes |
| Hoxha (107, 108)a | 2008 | Italy | Yes | Yes | Partially | Partially |
| Humphries (109) | 1994 | USA | Yes | Yes | Partially | Yes |
| Ignatov (110) | 2004 | Bulgaria | Yes | Partially | Yes | Yes |
| Ihle (111) | 2002 | Australia | Yes | Yes | Partially | Yes |
| Inglot (112) | 2013 | Poland | Yes | Yes | Yes | Yes |
| Inomata (113) | 2008 | Japan | Yes | Partially | Yes | No |
| Iqbal Belkys (114) | 2012 | UK | Partially | Partially | Yes | Yes |
| Izhevsky (115) | 2004 | USA | Yes | Yes | Yes | Yes |
| Jacq (116) | 1997 | France | Yes | Yes | Yes | Yes |
| Jani (117) | 1997 | India | Yes | Partially | Yes | Partially |
| Jarrett (118) | 1998 | New Zealand | Yes | Partially | Yes | Yes |
| Jin (119) | 2011 | Korea | Yes | Yes | Yes | Yes |
| Johnston (120) | 2000 | UK | Yes | Yes | Yes | Yes |
| Kalt (121) | 2001 | USA | Yes | Yes | Yes | Yes |
| Kang (122) | 2013 | Korea | Yes | Yes | Yes | Yes |
| Karunatilaka (123) | 2007 | UK | Yes | Yes | Yes | Yes |
| Keeling (124) | 1990 | UK | Partially | Partially | Yes | Yes |
| Kida (125) | 2009 | Japan | Yes | Yes | Yes | Partially |
| Kirrstetter (126) | 2004 | Cameroon | Yes | Yes | Yes | Yes |
| Kobayashi (127) | 2008 | Japan | Yes | Partially | Yes | Yes |
| Korkmaz (128) | 2001 | Turkey | Yes | Yes | Yes | Yes |
| Ku (129) | 2003 | USA | Yes | Yes | Yes | No |
| Kurugol (130) | 2001 | Turkey | Yes | Yes | Yes | Yes |
| Labarca (131) | 1997 | USA | Yes | Yes | Yes | Yes |
| Lamaury (132) | 1996 | France | Partially | Yes | Yes | Yes |
| Le Goff (133) | 2004 | France | Yes | Partially | Yes | Yes |
| Leder (134) | 2001 | South Africa | Yes | Partially | Yes | Partially |
| Lee (135) | 2011 | Taiwan | Yes | Yes | Yes | Yes |
| Lefebvre (136) | 2010 | France | Yes | Yes | Yes | Yes |
| Yes | Yes | Yes | Yes | |||
| Lehmann (137) | 2004 | Germany | Yes | Partially | Yes | Yes |
| Yes | Partially | Yes | Yes | |||
| Yes | Partially | Yes | Yes | |||
| Lehmann (138) | 2008 | Germany | Yes | Partially | Yes | Yes |
| Yes | Partially | Yes | Yes | |||
| Yes | Partially | Yes | Yes | |||
| Yes | Partially | Yes | Yes | |||
| Liappis (139) | 2003 | USA | Yes | Yes | Yes | Yes |
| Lijfering (140) | 2007 | Netherlands | Yes | Yes | Yes | Yes |
| Linares (141) | 2006 | Spain | Yes | Yes | Yes | Yes |
| Lioger (142) | 2013 | France | Yes | Yes | Yes | Yes |
| Lobrano (143) | 2006 | USA | Yes | Yes | Yes | Yes |
| Lydakis (144) | 2005 | Greece | Yes | Yes | Partially | Yes |
| Magdalena (145) | 2006 | Poland | Yes | Yes | Yes | Partially |
| Maldonado (146) | 2004 | Spain | Yes | Yes | Yes | Yes |
| Maldonado (147) | 2014 | Mexico | Yes | Yes | Yes | Yes |
| Malnick (148) | 1997 | Israel | Yes | Yes | Yes | Yes |
| Manas (149) | 2006 | Spain | Yes | Yes | Yes | Partially |
| Manco-Johnson (150) | 1992 | USA | Partially | Yes | Yes | Partially |
| Marruchella (151) | 2010 | Italy | Yes | Yes | Yes | Yes |
| Martin (152) | 2011 | USA | Yes | Yes | Yes | Yes |
| Martin-Aspas (153) | 2006 | Spain | Yes | Yes | Yes | No |
| Massano (154) | 2008 | Portugal | Yes | Yes | Yes | Yes |
| McKinley (155) | 2010 | USA | Yes | Partially | Partially | No |
| Medina (156) | 2009 | Mexico | Yes | Yes | Partially | Partially |
| Meissner (157) | 2013 | Germany | Yes | Partially | Yes | No |
| Merino (158) | 1996 | Spain | Partially | Yes | Yes | No |
| Partially | Yes | Yes | No | |||
| Mizumoto (159) | 2006 | Japan | Yes | Yes | Yes | Yes |
| Yes | Yes | Yes | Yes | |||
| Yes | Yes | Yes | Yes | |||
| Molina-Ruiz (160) | 2012 | Spain | Yes | Yes | Yes | Yes |
| Moreira (161) | 2000 | Spain | Yes | Partially | No | Partially |
| Morino (162) | 2009 | Japan | Yes | Yes | Yes | Yes |
| Muntean (163) | 1980 | Austria | Yes | Yes | Yes | Yes |
| Muwakkit (164) | 2002 | Lebanon | Yes | Yes | Yes | Yes |
| Nadir (165) | 2000 | USA | Partially | Partially | Partially | Yes |
| Partially | Partially | Partially | Yes | |||
| Partially | Partially | Partially | Yes | |||
| Nagashima (166) | 2010 | Japan | Yes | Yes | Yes | Yes |
| Nakayama (167) | 2014 | Japan | Yes | Yes | Yes | Yes |
| Naranjo (168) | 1992 | Spain | Yes | Yes | Yes | Partially |
| Nasilowska-Adamska (169) | 2014 | Poland | Yes | Yes | Yes | Yes |
| Ndimbie (170) | 1989 | Germany | Yes | Partially | Yes | Yes |
| Newcombe (171) | 2013 | Australia | Yes | Yes | Yes | Yes |
| Niitsuma (172) | 2003 | Japan | Yes | Partially | Yes | No |
| Nishio (173) | 2013 | Japan | Yes | Partially | No | Yes |
| Noureddine (174) | 2003 | Moraco | Yes | Partially | Yes | Yes |
| Noval (175) | 1999 | Spain | Yes | Partially | Yes | Yes |
| Novelli (176) | 2011 | Italy | Partially | Yes | Yes | Yes |
| Nunzie (177) | 2014 | Ecuador | Yes | Yes | Yes | Yes |
| Orbea (178) | 1999 | Spain | Yes | Partially | Yes | No |
| Padmakumar (179) | 2004 | UK | Yes | Yes | Yes | Yes |
| Padovan (180) | 2001 | Munich | Yes | Yes | Yes | Yes |
| Pamuk (181) | 2003 | Turkey | Yes | Yes | Yes | Yes |
| Parola (182) | 1998 | France | Yes | Yes | Yes | Yes |
| Pelletier (183) | 1995 | France | Partially | Yes | Yes | Yes |
| Pers (184) | 2008 | France | Yes | Yes | Yes | Yes |
| Peter (185) | 2013 | USA | Partially | Partially | Partially | Partially |
| Peyton (186) | 1998 | USA | Yes | Yes | Yes | No |
| Yes | Yes | Yes | Yes | |||
| Pittschieler (187) | 2011 | Austria | Partially | Yes | Yes | No |
| Poon Michelle (188) | 2012 | Singapore | Yes | Yes | Yes | Yes |
| Pourrat (189) | 2003 | France | Yes | Yes | Yes | Yes |
| Poux (190) | 1995 | France | Partially | Yes | Yes | No |
| Puri (191) | 1999 | Canada | Yes | Partially | Yes | No |
| Reitblat (192) | 2000 | Israel | Yes | Partially | Partially | No |
| Rennke (193) | 1999 | USA | Yes | Yes | Partially | Yes |
| Rivoisy (194) | 2014 | France | Yes | Partially | Yes | Yes |
| Rizzi (195) | 1994 | Italy | Yes | Yes | Partially | Partially |
| Rodriguez-Hernandez (196) | 1996 | Spain | Partially | Yes | Yes | Yes |
| Rodriguez-Quinonez (197) | 2004 | USA | Yes | Yes | Yes | Yes |
| Ronayne (198) | 2013 | New Zealand | Yes | Yes | Yes | Yes |
| Rosca (199) | 2010 | Romania | Yes | Partially | Yes | Yes |
| Rose (200) | 1998 | France | Yes | Yes | Yes | Partially |
| Saberi (201, 202)a | 2009 | USA | Yes | Partially | Yes | No |
| Sanchez (203) | 2004 | Spain | Yes | Yes | Yes | Yes |
| Sanli (204) | 2002 | Turkey | Yes | Yes | Yes | Yes |
| Santos (205) | 2004 | Spain | Yes | Partially | Yes | Yes |
| Schattner (206) | 1994 | Israel | Yes | Yes | Yes | Yes |
| Schmidt (207) | 1990 | USA | Yes | Yes | Yes | Yes |
| Schmugge (208) | 2001 | Switzerland | Yes | Yes | Partially | Yes |
| Scimeca (209) | 1987 | USA | Yes | Partially | Yes | Yes |
| Sedlak (210) | 2008 | Slovakia | Yes | Partially | Partially | Yes |
| Selman (211) | 2011 | UK | Yes | Partially | Partially | No |
| Senda (212) | 2010 | Japan | Yes | Yes | Yes | Yes |
| Shah (213) | 2006 | India | Yes | Yes | Yes | Yes |
| Shahnaz (214) | 2004 | USA | Yes | Partially | Yes | Partially |
| Shimizu (215) | 2009 | Japan | Yes | Partially | Yes | Partially |
| Shimizu (216) | 2014 | Japan | Yes | Yes | Yes | Yes |
| Shimura (217) | 2013 | Japan | Yes | Yes | Yes | Yes |
| Shinohara (218) | 2009 | USA | Yes | Partially | Yes | Partially |
| Shiomou (219) | 2002 | Greece | Yes | Yes | Yes | Yes |
| Shroff (220) | 2011 | Canada | Yes | Yes | Yes | Yes |
| Sinnreich (221) | 2003 | Switzerland | Yes | Yes | Yes | Yes |
| Sonoda (222) | 2005 | Japan | Yes | Partially | No | Yes |
| Soper (223) | 2000 | Egypt | Yes | Yes | Yes | Partially |
| Soweid (224) | 1995 | USA | Partially | Partially | Partially | Partially |
| Steuerwald (225) | 1995 | Pakistan | Partially | Yes | Yes | Yes |
| Suero (226) | 2005 | Spain | Yes | Yes | Yes | Yes |
| Sztajzel (227) | 2000 | Switzerland | Yes | Yes | Yes | Yes |
| Tanir (228) | 2006 | Turkey | Yes | Yes | Yes | Yes |
| Tanizawa (229) | 2009 | Japan | Yes | Yes | Yes | Yes |
| Tattevin (230) | 2003 | France | Yes | Yes | Yes | Yes |
| Tavakoli (231) | 2011 | Iran | Yes | Yes | Yes | Yes |
| Thirumalai (232) | 1994 | USA | Yes | Partially | Yes | Yes |
| Tolosa-Vilella (233) | 1995 | Spain | Yes | Yes | Yes | Yes |
| Toyoshima (234) | 2007 | Japan | Yes | Yes | Yes | Partially |
| Tullett (235) | 1989 | UK | Yes | Partially | Yes | Yes |
| Tung (236) | 2011 | Spain | Yes | Yes | Yes | Yes |
| Turhal (237) | 2001 | Turkey | Yes | Partially | Yes | Partially |
| Yes | Partially | Yes | Partially | |||
| Yes | Partially | Yes | Partially | |||
| Yes | Partially | Yes | Yes | |||
| Turtle (238) | 1999 | Australia | Yes | Yes | Yes | Yes |
| Ulvestad (239) | 2000 | Norway | Yes | Yes | Yes | Yes |
| Uthman (240) | 1999 | Lebanon | Yes | Yes | Partially | No |
| Uthman (241) | 2001 | Lebanon | Yes | Yes | Yes | No |
| Uthman (242) | 2002 | Lebanon | Yes | Yes | Partially | No |
| Vassalluzzo (243) | 1995 | USA | Partially | Yes | Yes | No |
| Venugopalan (244) | 2001 | Oman | Yes | Partially | Partially | Yes |
| Vidal (245) | 2005 | France | Yes | Yes | Yes | Yes |
| Yes | Yes | Yes | Yes | |||
| Viseux (246) | 2000 | France | Yes | Yes | Yes | Yes |
| Waller Elizabeth (247) | 2008 | USA | Yes | Partially | Yes | Yes |
| Wallin (248) | 2009 | Brazil | Yes | Yes | Yes | Yes |
| Wiegering (249) | 2010 | Germany | Yes | Yes | Yes | Yes |
| Witmer (250) | 2007 | USA | Yes | Yes | Yes | Yes |
| Yes | Yes | Yes | Yes | |||
| Witz (251) | 2000 | Isreal | Yes | Yes | Yes | Yes |
| Wong (252) | 2001 | Australia | Yes | Yes | Yes | Yes |
| Yes | Yes | Yes | Yes | |||
| Wong (253) | 2004 | USA | Partially | Partially | Partially | Yes |
| Yamazaki (254) | 1991 | Japan | Yes | Yes | Yes | Partially |
| Yanez (255) | 1999 | USA | Yes | Yes | Yes | No |
| Yilmaz (256) | 2002 | Turkey | Yes | Yes | Yes | Partially |
| Yoo (257) | 2004 | Korea | Yes | Yes | Yes | Partially |
| Younes (258) | 2002 | Tunisie | Partially | Yes | Partially | Yes |
| Zhang (259) | 2012 | China | Yes | Yes | Partially | Yes |
Two publications for the same case reports.
Appendix 3. Infections reported in patients with catastrophic antiphospholipid syndrome
| Infection | N (%) |
|---|---|
| Catastrophic antiphospholipid syndrome, N = 17a | |
| Viral | 7 (41.2) |
| Hepatitis C virus | 3 (17.7) |
| Cytomegalovirus | 2 (11.8) |
| Parvovirus B19 | 1 (5.9) |
| Influenza A virus (subtype H1N1) | 1 (5.9) |
| Bacterial | 7 (41.2) |
| Staphylococci | 2 (11.8) |
| Streptococci | 1 (5.9) |
| Escherichia coli | 1 (5.9) |
| Mycobacterium tuberculosis | 1 (5.9) |
| Pseudomonas aeruginosa | 1 (5.9) |
| Spirochetal | |
| Treponema pallidum | 1 (5.9) |
| Unidentified organisms | 4 (23.5) |
One case had both viral and bacterial infections.
Appendix 4. Infections reported in patients with a history of autoimmune or inflammatory diseases
| Infection | N (%) |
|---|---|
| Autoimmune diseases N = 22 | |
| Viral | 18 (81.8) |
| Parvovirus B19 | 7 (31.8) |
| Cytomegalovirus | 3 (13.6) |
| Human immunodeficiency virus | 2 (9.1) |
| Hepatitis C virus | 2 (9.1) |
| Hepatitis B virus | 1 (4.6) |
| Herpes simplex virus | 1 (4.6) |
| Influenza A virus (subtype H1N1) | 1 (4.6) |
| Varicella-zoster virus | 1 (4.6) |
| Bacterial | 4 (18.2) |
| Streptococci | 2 (9.1) |
| Listeria monocytogenes | 1 (4.6) |
| Spirochetal | |
| Treponema pallidum | 1 (4.6) |
Appendix 5. Thromboembolic events in patients with human immunodeficiency virus and hepatitis C virus
| Clinical presentation | N (%) | |
|---|---|---|
| Human immunodeficiency N = 47a |
Hepatitis C N = 29 |
|
| Hematologic manifestations | 6 (12.8) | 6 (20.7)b |
| Thrombocytopenia and/or hemolytic anemia | 4 (8.5) | 6 (20.7) |
| Pancytopenia | 2 (4.3) | 0 |
| Disseminated intravascular coagulopathy | 0 | 1 (3.5) |
| Peripheral thrombosis | 8 (17.0) | 6 (20.7) |
| Vascular thrombosis in UL/LL | 7 (14.9) | 5 (17.2) |
| Jugular and/or subclavian vein thrombosis | 0 | 1 (3.5) |
| Testicular thrombosis | 1 (2.1) | 0 |
| Neurologic manifestations | ||
| Stroke and/or transient ischemic attack | 8 (17.0) | 6 (20.7) |
| Cutaneous manifestations | 8 (17.0) | 5 (17.2) |
| Cutaneous necrosis and/or capillary thrombosis (livedo reticularis/ pseudovasculitis/purpura) | 6 (12.8) | 2 (6.9) |
| Digital gangrene | 2 (4.3) | 2 (6.9) |
| Penile leukocytoclastic vasculitis | 0 | 1 (3.5) |
| Respiratory manifestations | ||
| Pulmonary thromboembolism | 6 (12.8) | 1 (3.5) |
| Cardiac manifestations | 3 (6.4) | 5 (17.2) |
| Superior and/or inferior vena cava thrombosis | 1 (2.1) | 1 (3.5) |
| Intra-cardiac thrombus + aortic occlusion | 0 | 1 (3.5) |
| Myocardial infarction | 1 (2.1) | 3 (10.3) |
| Valve thickening and/or vegetation | 1 (2.1) | 0 |
| Renal manifestations | 0 | 5 (17.2) |
| Renal vessels occlusion | 0 | 3 (10.3) |
| Acute renal failure | 0 | 1 (3.5) |
| End stage renal disease | 0 | 1 (3.5) |
| Splenic infarction | 2 (4.3) | 2 (6.9) |
| Gastrointestinal manifestations | ||
| Abdominal vessels (mesenteric/iliac/abdominal aorta) occlusion | 0 | 2 (6.9) |
| Osteo-articular manifestations | ||
| Avascular necrosis | 9 (19.2) | 1 (3.5) |
| Hepatic manifestations | ||
| Portal and/or hepatic vessels thrombosis | 2 (4.3) | 3 (10.3) |
| Ophthalmologic manifestations | ||
| Retinal thrombosis and/or optic neuropathy | 1 (2.1) | 3 (10.3) |
| Obstetric manifestations | 0 | 2 (6.9) |
| Adrenal crisis | 0 | 1 (3.5) |
HIV: human immunodeficiency syndrome; HCV: hepatitis c virus.
Twenty four cases have been diagnosed with acquired immune deficiency syndrome.
Only 1 case was complicated by disseminated intravascular coagulopathy among the 6 cases of HCV infection who develop thrombocytopenia and/or hemolytic anemia.
Appendix 6. Infections reported in patients 18 years old or younger
| Infection | N (%) |
|---|---|
| Pediatric Cases N = 65a | |
| Viral | 38 (58.5) |
| Varicella-zoster virus | 9 (13.9) |
| Parvovirus B19 | 7 (10.8) |
| Adenovirus | 5 (7.7) |
| Epstein-Barr virus | 5 (7.7) |
| Human immunodeficiency virus | 3 (4.6) |
| Hepatitis A virus | 3 (4.6) |
| Hepatitis B virus | 1 (1.5) |
| Hepatitis C virus | 1 (1.5) |
| Herpes simplex virus | 1 (1.5) |
| Cytomegalovirus | 1 (1.5) |
| Dengue virus | 1 (1.5) |
| Measles | 1 (1.5) |
| Bacterial | 22 (33.9) |
| Mycoplasma pneumonia | 8 (12.3) |
| Streptococci | 4 (6.2) |
| Pseudomonas aeruginosa | 2 (3.1) |
| Bartonella henselae | 1 (1.5) |
| Coxiella burnetii | 1 (1.5) |
| Escherichia coli | 1 (1.5) |
| Mycoplasma penetrans | 1 (1.5) |
| Mycobacterium tuberculosis | 1 (1.5) |
| Rickettsia africae | 1 (1.5) |
| Spirochetal | |
| Borellia burgdorferi | 2 (3.1) |
| Parasitic | 1 (1.5) |
| Malaria | |
| Unidentified organisms | 6 (9.2) |
Two patients reported more than 1 type of infection (viral, and bacterial).
References
- 1.Abernethy ML, McGuinn JL, Callen JP. Widespread cutaneous necrosis as the initial manifestation of the antiphospholipid antibody syndrome. J Rheumatol. 1995;22(7):1380–3. [PubMed] [Google Scholar]
- 2.Abulafia LA, Spinelli LP, Kac BK. Lucio’s leprosy and phenomenon: A case in Brazil. [Spanish] Lepra de Lucio y fenomeno de Lucio: Un caso en Brasil. Dermatologia Revista Mexicana. 2004;48:279–83. [Google Scholar]
- 3.Azulay-Abulafia L, Pereira S, Hardmann D, Kawa K, Levy RA, Talhari C, et al. Lucio phenomenon: Vasculitis or occlusive vasculopathy? [German] Lucio-phanomen: Vaskulitis oder okklusive vaskulopathie? Hautarzt. 2006;57:1101–5. doi: 10.1007/s00105-005-1086-3. [DOI] [PubMed] [Google Scholar]
- 4.Aguilar C, Ortega JL, Caro N. Autoimmune type antiphospholipid antibodies in a patient with Q fever. Haematologica. 2005;90:ECR12. [PubMed] [Google Scholar]
- 5.Akerkar SM, Bichile LS. Leprosy & gangrene: a rare association; role of anti phospholipid antibodies. BMC Infect Dis. 2005;5:74. doi: 10.1186/1471-2334-5-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alcock R, Elsik M, Yiannikas C, Yiannikas J. Antiphospholipid syndrome and rheumatic fever: a case spanning three decades of changing concepts and common immunological mechanisms. Lupus. 2011;20:1316–20. doi: 10.1177/0961203311403023. [DOI] [PubMed] [Google Scholar]
- 7.Aldamiz-Echebarria San S, Agud A, Ayensa D, Zubizarreta G. Primary pulmonary hypertension, anticardiolipin antibodies and human immunodeficiency virus infection. Medicina Clinica. 1991;97:199. [PubMed] [Google Scholar]
- 8.Alric L, Oskman F, Sanmarco M, Izopet J, Bonnet E, Garcia-Ricart F, et al. Association of antiphospholipid syndrome and chronic hepatitis C. Br J Rheumatol. 1998;37:589–90. doi: 10.1093/rheumatology/37.5.589. [DOI] [PubMed] [Google Scholar]
- 9.Amiral J, Aronis S, Adamtziki E, Garoufi A, Karpathios T. Association of lupus anticoagulant with transient antibodies to prothrombin in a patient with hypoprothrombinemia. Thrombosis Research. 1997;86:73–8. doi: 10.1016/s0049-3848(97)00047-9. [DOI] [PubMed] [Google Scholar]
- 10.Amit S, Gadoth A, Giladi M, Justo D. Transient ischemic attack associated with acute cytomegalovirus infection. Journal of Medical Virology. 2012;84:487–9. doi: 10.1002/jmv.23225. [DOI] [PubMed] [Google Scholar]
- 11.Anton-Martinez D, Polo-Romero FJ, Atienza-Morales MP, Esteso-Perona M. Acute Q fever with secondary autoimmune hepatitis and antiphospholipid antibodies. [Spanish] Fiebre Q aguda con hepatitis autoinmune y anticuerpos antifosfoli pido secundarios. Inmunologia. 2011;30:90–3. [Google Scholar]
- 12.Appert-Flory A, Fischer F, Amiral J, Monpoux F. Lupus Anticoagulant- Hypoprothrombinemia syndrome (HLAS): report of one case in a familial infectious context. Thrombosis Research. 2010;126:e139–40. doi: 10.1016/j.thromres.2009.11.025. [DOI] [PubMed] [Google Scholar]
- 13.Arnason JA, Graziano FM. Adrenal insufficiency in the antiphospholipid antibody syndrome. Seminars in Arthritis and Rheumatism. 1995;25:109–16. doi: 10.1016/s0049-0172(95)80024-7. [DOI] [PubMed] [Google Scholar]
- 14.Arruda VR, Bizzacchi JM, Metze IL. Hairy cell leukemia and multiple autoimmune manifestations in a human immunodeficiency virus-infected patient. Ann Hematol. 1993;66:325–7. doi: 10.1007/BF01695977. [DOI] [PubMed] [Google Scholar]
- 15.Asano Y, Sarukawa M, Idezuki T, Harada S, Kaji K, Nakasu I, et al. Multiple small pulmonary emboli associated with transient antiphospholipid syndrome in human Parvovirus B19 infection. Clinical Rheumatology. 2006;25:585–7. doi: 10.1007/s10067-005-0032-1. [DOI] [PubMed] [Google Scholar]
- 16.Ascer E, Marques M, Gidlund M. M pneumoniae infection, pulmonary thromboembolism and antiphospholipid antibodies. BMJ Case Reports. 2011;2011 doi: 10.1136/bcr.12.2010.3561. bcr1220103561-bcr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asherson RA, Cervera R, Piette JC, Shoenfeld Y, Espinosa G, Petri MA, et al. Catastrophic antiphospholipid syndrome - Clues to the pathogenesis from a series of 80 patients. Medicine. 2001;80:355–77. doi: 10.1097/00005792-200111000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Ashrani AA, Aysola A, Al-Khatib H, Nichols WL, Key NS. Lupus anticoagulant associated with transient severe factor X deficiency: a report of two patients presenting with major bleeding complications. Br J Haematol. 2003;121:639–42. doi: 10.1046/j.1365-2141.2003.04325.x. [DOI] [PubMed] [Google Scholar]
- 19.Aydin K, Sert A, Ati G, Kiresi DA. Acute childhood hemiplegia associated with chickenpox and elevated anticardiolipin antibody. J Child Neurol. 2006;21:890–3. doi: 10.1177/08830738060210101101. [DOI] [PubMed] [Google Scholar]
- 20.Baid S, Pascual M, Cosimi AB, Chung RT, Colvin RB, Tolkoff-Rubin N. Viruses and thrombotic microangiopathy. Transplantation. 1999;68:710–1. doi: 10.1097/00007890-199909150-00022. [DOI] [PubMed] [Google Scholar]
- 21.Bakos L, Correa CC, Bergmann L, Bonamigo RR, Muller LF. Antiphospholipid antibodies thrombotic syndrome misdiagnosed as Lucio’s phenomenon. International Journal of Leprosy & Other Mycobacterial Diseases. 1996;64:320–3. [PubMed] [Google Scholar]
- 22.Bakshi M, Khemani C, Vishwanathan V, Anand RK, Khubchandani RP. Mycoplasma pneumonia with antiphospholipid antibodies and a cardiac thrombus. Lupus. 2006;15:105–6. doi: 10.1191/0961203306lu2258cr. [DOI] [PubMed] [Google Scholar]
- 23.Balderramo DC, García O, Colmenero J, Espinosa G, Forns X, Ginès P. Antiphospholipid syndrome during pegylated interferon alpha-2a therapy for chronic hepatitis C. Digestive and Liver Disease. 2009;41:e4–e7. doi: 10.1016/j.dld.2007.11.029. [DOI] [PubMed] [Google Scholar]
- 24.Barfield W, Gardner R, Lett S, Johnsen C. Congenital rubella reinfection in a mother with anti-cardiolipin and anti-platelet antibodies. Pediatric Infectious Disease Journal. 1997;16:249–51. doi: 10.1097/00006454-199702000-00017. [DOI] [PubMed] [Google Scholar]
- 25.Belmonte MA, Garcia-Portales R, Domenech I, Fernandez-Nebro A, Camps MT, De R. Avascular necrosis of bone in human immunodeficiency virus infection and antiphospholipid antibodies. J Rheumatol. 1993;20:1425–8. [PubMed] [Google Scholar]
- 26.Ben-Chetrit E, Wiener-Well Y, Fadeela A, Wolf DG. Antiphospholipid antibodies during infectious mononucleosis and their long term clinical significance. Journal of Clinical Virology. 2013;56:312–5. doi: 10.1016/j.jcv.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 27.Bibler MR, Luber HJ, Glueck HI, Estes SA. Disseminated sporotrichosis in a patient with HIV infection after treatment for acquired factor VIII inhibitor. JAMA. 1986;256:3125–6. [PubMed] [Google Scholar]
- 28.Bloom EJ, Abrams DI, Rodgers G. Lupus anticoagulant in the acquired immunodeficiency syndrome. JAMA. 1986;256:491–3. [PubMed] [Google Scholar]
- 29.Bouchard O, Bosseray A, Leclercq P, Micoud M. Portal thrombosis and anticardiolipin antibodies association in an HIV-2 infected patient. Presse Medicale. 1998;27:965. [PubMed] [Google Scholar]
- 30.Brackett J, King K, Lin PH, Yee D. Phlegmasia cerulea dolens in a child due to group a streptococcal infection. Pediatric Blood and Cancer. 2011;56(6):918. [Google Scholar]
- 31.Brown P, Crane L. Avascular necrosis of bone in patients with human immunodeficiency virus infection: report of 6 cases and review of the literature. Clinical Infectious Diseases. 2001;32:1221–6. doi: 10.1086/319745. [DOI] [PubMed] [Google Scholar]
- 32.Brown Sarah MN, Padley S, Bush A, Cummins D, Davidson S, Buchdahl R. Mycoplasma pneumonia and pulmonary embolism in a child due to acquired prothrombotic factors. Pediatric Pulmonology. 2008;43:200–2. doi: 10.1002/ppul.20739. [DOI] [PubMed] [Google Scholar]
- 33.Bulucu F, Can C, Oktenli C, Koc B, Polat Z. Membranous glomerulonephritis, antiphospholipid syndrome, and persistent low C3 levels associated with meningococcal disease. Nephron. 2002;91:336–8. doi: 10.1159/000058415. [DOI] [PubMed] [Google Scholar]
- 34.Cagatay AA, Kucukkaya R, Akyildiz M, Berk H, Cagatay Y, Yildirmak T, et al. Human immunodeficiency virus and avascular necrosis of the femoral head: a case report. Chinese Medical Journal. 2004;117:1437–40. [PubMed] [Google Scholar]
- 35.Cailleux N, Marie I, Jeanton M, Lecomte F, Levesque H, Courtois H. Are antiphospholipid antibodies thrombogenic in the course of human immunodeficiency virus infection? Journal des Maladies Vasculaires. 1999;24:53–6. [PubMed] [Google Scholar]
- 36.Cailleux N, Marie I, Lecomte F, Levesque H, Courtois H. Are antiphospholipid antibodies thrombogenic in human immunodeficiency virus infection? Journal of Vascular Research. 1998;35:94. [PubMed] [Google Scholar]
- 37.Calvo R, JM, Diaz R. Chronic hepatitis C virus positive hepatitis and antiphospholipid syndrome. Gastroenterologia y Hepatologia. 1998;21:437–8. [PubMed] [Google Scholar]
- 38.Campanelli A, Kaya G, Ozsahin AH, La S, Jacquier C, Stauffer M, et al. Purpura fulminans in a child as a complication of chickenpox infection. Dermatology. 2004;208:262–4. doi: 10.1159/000077315. [DOI] [PubMed] [Google Scholar]
- 39.Campos-Alvarez RM, Jimenez-Mejias ME, Moreno M, Cuello C, JA Q fever and anticardiolipin antibodies. Revista Clinica Espanola. 1992;191:454–5. [PubMed] [Google Scholar]
- 40.Canpolat N, Topal N, Civilibal M, Caliskan S, Sever L, Kasapcopur O, et al. A case of catastrophic antiphospholipid syndrome in an adolescent girl with parvovirus B19 infection. Clin Pediatr (Phila) 2008;47:593–7. doi: 10.1177/0009922808315216. [DOI] [PubMed] [Google Scholar]
- 41.Cappell MS, Simon T, Tiku M. Splenic infarction associated with anticardiolipin antibodies in a patient with acquired immunodeficiency syndrome. Dig Dis Sci. 1993;38:1152–5. doi: 10.1007/BF01295735. [DOI] [PubMed] [Google Scholar]
- 42.Carli P, Carpentier JP, Chagnon A, Yao N’Dri A, Chauveau E. Antiphospholipid antibodies during malaria: Two case reports. Revue de Medecine Interne. 1993 [Google Scholar]
- 43.Catteau B, Delaporte E, Hachulla E, Piette F, Bergoend H. Mycoplasma infection with Stevens-Johnson syndrome and antiphospholipid antibodies: apropos of 2 cases. Revue de Medecine Interne. 1995;16:10–4. doi: 10.1016/0248-8663(96)80659-x. [DOI] [PubMed] [Google Scholar]
- 44.Charloux A, Espinassouze F, Cribier B, Quoix E, Pauli G. Multiple complications of a mycoplasma pneumoniae infection. Revue des Maladies Respiratoires. 1993;10:259–61. [PubMed] [Google Scholar]
- 45.Chen WH, Kao YF, Liu JS. An increase of blood anti-beta2-glycoprotein I antibody in Japanese encephalitis associated with cerebral ischemia. Blood Coagul Fibrinolysis. 2005;16:55–9. doi: 10.1097/00001721-200501000-00009. [DOI] [PubMed] [Google Scholar]
- 46.Chen W. An unusual transitory increase of lupus anticoagulant in dengue virus infection complicated with cerebral ischaemia. Journal of Infection. 2006;52:e87–e91. doi: 10.1016/j.jinf.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 47.Chevalier X, Larget-Piet B, Hernigou P, Gherardi R. Avascular necrosis of the femoral head in HIV-infected patients. Journal of Bone & Joint Surgery - British Volume. 1993;75:160. doi: 10.1302/0301-620X.75B1.8421018. [DOI] [PubMed] [Google Scholar]
- 48.Cho YP, Choi SJ, Jung BH, Hwang JW, Han MS, Kim YH, et al. Lemierre’s syndrome in a patient with antiphospholipid syndrome. Ann Vasc Surg. 2006;20:274–7. doi: 10.1007/s10016-006-9004-4. [DOI] [PubMed] [Google Scholar]
- 49.Chou TN, Hsu TC, Chen RM, Lin LI, Tsay GJ. Parvovirus B19 infection associated with the production of anti-neutrophil cytoplasmic antibody (ANCA) and anticardiolipin antibody (aCL) Lupus. 2000;9:551–4. doi: 10.1177/096120330000900714. [DOI] [PubMed] [Google Scholar]
- 50.Clark BM, Zenios M, Wilkins EGL, Sochart DH. Avascular necrosis in five patients with human immunodeficiency virus (HIV) infection. HIP International. 2003;13:229–34. [Google Scholar]
- 51.Collazos J, Diaz F, Ayarza R, de Miguel J. Actinobacillus actinomycetemcomitans: a cause of pulmonary-valve endocarditis of 18 months’ duration with unusual manifestations. Clin Infect Dis. 1994;18(1):115–6. doi: 10.1093/clinids/18.1.115. [DOI] [PubMed] [Google Scholar]
- 52.Cooray M, Manolakos JJ, Wright DS, Haider S, Patel A. Parvovirus infection mimicking systemic lupus erythematosus. Cmaj. 2013;185(15):1342–4. doi: 10.1503/cmaj.121565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Corti M, Trione N, Corbera K. Spleen infarction associated with the presence of lupic inhibitor in a patient with AIDS. Enfermedades Infecciosas y Microbiologia Clinica. 2001;19:408–9. doi: 10.1016/s0213-005x(01)72677-8. [DOI] [PubMed] [Google Scholar]
- 54.Cross KJ, Patmas MA. Antiphospholipid antibody syndrome and lyme disease: A possible association. Journal of Spirochetal and Tick borne Diseases. 1999 [Google Scholar]
- 55.Cull E, Stein Brady L. Splenic infarction, warm autoimmune hemolytic anemia and antiphospholipid antibodies in a patient with infectious mononucleosis. International Journal of Hematology. 2012;95:573–6. doi: 10.1007/s12185-012-1047-4. [DOI] [PubMed] [Google Scholar]
- 56.Damian L, Rednic S, Cristea A, Felea I, Nicola M, Nicoara I, et al. Viral-induced catastrophic antiphospholipid syndrome: Two cases with favourable outcome. Ann Rheum Dis. 2004;63:330. [Google Scholar]
- 57.Daniels AH, Wilson CL, Harrison RA. Hepatitis C-associated leukocytoclastic vasculitis with anticardiolipin antibodies causing penile necrosis and deep venous thrombosis in the absence of cryoglobulinemia. Journal of Hospital Medicine (Online) 2008;3:170–2. doi: 10.1002/jhm.261. [DOI] [PubMed] [Google Scholar]
- 58.de Argila F-A, Revenga A, Iglesias D. Perniosis and lupus anticoagulant. Revista Clinica Espanola. 1996;196:24–7. [PubMed] [Google Scholar]
- 59.de C-S, André, Cunha Burke A. Streptococcal viridans subacute bacterial endocarditis associated with antineutrophil cytoplasmic autoantibodies (ANCA) Heart & Lung: The Journal of Acute and Critical Care. 2003;32:140–3. doi: 10.1067/mhl.2003.2. [DOI] [PubMed] [Google Scholar]
- 60.de L, EM, Valladares MP, Banuls SR, Regadera MPM. Antiphospholipid antibodies in acute hepatitis C infection. Medicina Clinica. 1998;110:238–9. [PubMed] [Google Scholar]
- 61.De L, GF, Remondino GI, Forastiero RR, Cunto ER, Narbaitz M, et al. Catastrophic antiphospholipid syndrome and Kikuchi-Fujimoto disease: The first case reported. Lupus. 2005;14:967–9. doi: 10.1191/0961203305lu2232cr. [DOI] [PubMed] [Google Scholar]
- 62.del A, de La T, Luis P, Garcia J. Pulmonary embolism in a patient with Q fever. Are the anticardiolipin antibodies the origin? Enfermedades Infecciosas y Microbiologia Clinica. 2001;19:137–8. doi: 10.1016/s0213-005x(01)72588-8. [DOI] [PubMed] [Google Scholar]
- 63.Del C, LF, Soria C, Schoendorff C, Garcia G, Diez-Caballero N, et al. Widespread cutaneous necrosis and antiphospholipid antibodies: two episodes related to surgical manipulation and urinary tract infection. J Am Acad Dermatol. 1997;36:872–5. doi: 10.1016/s0190-9622(97)70045-8. [DOI] [PubMed] [Google Scholar]
- 64.Delbos V, Abgueguen P, Chennebault JM, Fanello S, Pichard E. Acute cytomegalovirus infection and venous thrombosis: Role of antiphospholipid antibodies. Journal of Infection. 2007;54:e47–e50. doi: 10.1016/j.jinf.2006.03.031. [DOI] [PubMed] [Google Scholar]
- 65.Demey HE, Lambrecht G, Moorkens G, Michielsen P, VandenEnde J, Bossaert LL. Thrombolysis in central splanchnic thrombosis. Journal of Intensive Care Medicine. 1997;12:269–75. [Google Scholar]
- 66.Devars du M, JF, Molinie V, Pradalier A. Hepatic granulomatosis associated with a circulating anticoagulant, disclosing Q fever. Presse Medicale. 1997;26:666. [PubMed] [Google Scholar]
- 67.Diaz JS, Octavio JG, Fernandez-Guerrero ML. Antiphospholipid syndrome and acute HIV infection. Emerging Infectious Diseases. 2010;16:360–1. doi: 10.3201/eid1602.090728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Doyle G, Simmons M, Granke K. Axillary vein thrombosis during pregnancy in association with a lupus anticoagulant. The West Virginia medical journal. 1998;94(2):87–9. [PubMed] [Google Scholar]
- 69.Drulovic J, Dujmovic I, Stojsavlevic N, Tripkovic I, Apostolski S, Levic Z, et al. Transverse myelopathy in the antiphospholipid antibody syndrome: pinworm infestation as a trigger? Journal of Neurology, Neurosurgery & Psychiatry. 2000;68:249. doi: 10.1136/jnnp.68.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Durkin ML, Marchese D, Robinson MD, Ramgopal M. Catastrophic antiphospholipid syndrome (CAPS) induced by influenza A virus subtype H1N1. BMJ Case Reports. 2013 doi: 10.1136/bcr-2013-200474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Economou M, Lithoxopoulou M, Aivazis V, Tsakalidis C, Athanassiou-Metaxa M. Bartonella henselae: association with the development of transient lupus anticoagulant and asymptomatic prolongation of activated partial thromboplastin time. Scandinavian Journal of Infectious Diseases. 2003;35:149. doi: 10.1080/0036554021000027004. [DOI] [PubMed] [Google Scholar]
- 72.Enomoto M, Negoro N, Fujii H, Kobayashi S, Iwai S, Morikawa H, et al. Hepatitis B-associated cryoglobulinemia and antiphospholipid antibodies. [Japanese. Acta Hepatologica Japonica. 2010;51:454–6. [Google Scholar]
- 73.Ergas D, Herskovitz P, Skurnik Y, Mavor E, Sthoeger ZM. Superior mesenteric vein thrombosis with pulmonary embolism: A rare presentation of acute cytomegalovirus infection. Israel Medical Association Journal. 2008;10:235–6. [PubMed] [Google Scholar]
- 74.Ertem D, Acar Y, Arat C, Pehlivanoglu E. Thrombotic and thrombocytopenic complications secondary to hepatitis A infection in children [2. American Journal of Gastroenterology. 1999;94:3653–5. doi: 10.1111/j.1572-0241.1999.01627.x. [DOI] [PubMed] [Google Scholar]
- 75.Ertem D, Acar Y, Pehlivanoglu E. Autoimmune complications associated with hepatitis A virus infection. The Pediatric Infectious Disease Journal. 2001 doi: 10.1097/00006454-200108000-00020. [DOI] [PubMed] [Google Scholar]
- 76.Fain O, Landon C, Stirnemann J. Your images. Parvovirus B19 infection in adult. [French] Vos images. Infection a parvovirus B19. Revue du Praticien. 2009;59:1344. [PubMed] [Google Scholar]
- 77.Faller JP, Mangin I, Couqueberg L, Ruyer O, Feissel M. Latent adrenal insufficiency in an HIV-infected subject with antiphopholipids: A case report [1]. [French] Insuffisance surrenalienne lente au cours d’une infection VIH avec presence d’anticorps antiphospholipides. Presse Medicale. 1999;28:1419. [PubMed] [Google Scholar]
- 78.Fanlo P, Ibanez J, Arnaez R, Cia M, Artola V, Perez C. Coxiella burnetii (Q fever) induced antiphospholipid antibodies. Lupus. 2010;19:1–185. [Google Scholar]
- 79.Faria A, Carvalho AF, Silva RP, Mascarenhas A. Antiphospholipid Syndrome and Cronic Hepatitis C – a Case Report. European Journal of Internal Medicine. 2011;22:S37. [Google Scholar]
- 80.Fernandez M, Salvador O, Godoy A, Padron N, Soria B, Sevil F, et al. Lupus anticoagulant (LA) in infectious disease. A report of three cases. Haematologica-the Hematology Journal. 2007;92:515. [Google Scholar]
- 81.Flateau C, Asfalou I, Deman AL, Ficko C, Andriamanantena D, Fontan E, et al. Aortic thrombus and multiple embolisms during a Mycoplasma pneumoniae infection. Infection. 2013;41:867–73. doi: 10.1007/s15010-013-0475-2. [DOI] [PubMed] [Google Scholar]
- 82.Freeman H, Patel J, Fernandez D, Sharples P, Ramanan AV. Fitting and flailing: Recognition of paediatric antiphospholipid syndrome. Archives of Disease in Childhood: Education and Practice Edition. 2014;99(1):28–36. doi: 10.1136/archdischild-2012-302404. [DOI] [PubMed] [Google Scholar]
- 83.Frontino G, Passoni A, Piscopo Maria A, Grechi E, Cammarata B, Pozzobon G. Bilateral cavo-ilio-femoral thrombosis in an adolescent with transient anti-phospholipid antibodies and Factor V heterozygous mutation: a case report. Cases Journal. 2009;2:6830. doi: 10.4076/1757-1626-2-6830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Galvez J, Martin I, Merino D, Pujol E. Thrombophlebitis in a patient with acute Q fever and anticardiolipin antibodies. Medicina Clinica. 1997;108:396–7. [PubMed] [Google Scholar]
- 85.Garcia Rincon CI, Restrepo NM, Munoz-Grajales C, Cardozo Avendano SL, Calle J, Velasquez Franco CJ, et al. Extensive skin necrosis secondary to antiphospholipid syndrome in an HIV infected patient: A case report. [Spanish. Revista Colombiana de Reumatologia. 2014;21(3):155–9. [Google Scholar]
- 86.Germano N, Mendonca P, Murinello A. Pulmonary embolism associated to HIV infection. Revista Portuguesa de Pneumologia. 2005;11:407–12. doi: 10.1016/s0873-2159(15)30517-1. [DOI] [PubMed] [Google Scholar]
- 87.Ghosh K, Shetty S. Deep venous thrombosis associated with antiphospholipid antibodies following tuberculosis lymphadenitis in a predisposed patient. Blood Coagulation & Fibrinolysis. 2008;19:464–5. doi: 10.1097/MBC.0b013e328304e056. [DOI] [PubMed] [Google Scholar]
- 88.Giordano N, Amendola A, Papakostas P, Cipolli F, Agate VM, Martini G, et al. Possible pathogenetic role of antiphospholipid antibodies in a clinical case of human immunodeficiency virus infection with peripheral polyneuropathy and arterial thrombosis. New Microbiol. 2005;28:261–3. [PubMed] [Google Scholar]
- 89.Girard C, Guillot B, Biron C, Lavabre-Bertrand T, Navarro R, Bessis D. Digital skin necrosis in congenital afibrinogenaemia associated with hepatitis C virus infection, mixed cryoglobulinaemia and anticardiolipin antibodies. Acta Dermato-Venereologica. 2005;85:56–9. doi: 10.1080/00015550410001053. [DOI] [PubMed] [Google Scholar]
- 90.Gologorsky E, Andrews DM, Gologorsky A, Sampathi V, Sundararaman L, Govindaswamy R, et al. Devastating intracardiac and aortic thrombosis: A case report of apparent catastrophic antiphospholipid syndrome during liver transplantation. Journal of Clinical Anesthesia. 2011;23:398–402. doi: 10.1016/j.jclinane.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 91.Gorczyca I, Stanek M, Podlasin B, Furmanek M, Pniewski J. Recurrent cerebral infarcts as the first manifestation of infection with the HIV virus. Folia Neuropathol. 2005;43:45–9. [PubMed] [Google Scholar]
- 92.Graffin B, Goutorbe P, Poyet R, Raymond A, Paris JF, Carli P. Multi-organ failures during septic shock from Escherichia coli urinary tract infection: catastrophic antiphospholipid syndrome? Revue de Medecine Interne. 2007;28:52–5. doi: 10.1016/j.revmed.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 93.Granel F, Amaudo JP, Reichert S, Barbaud A, Schmutz JL. chronic atrophic acrodermatitis and circulating anticoagulants. Revue de Medecine Interne. 1998;19:583–4. doi: 10.1016/s0248-8663(99)80032-0. [DOI] [PubMed] [Google Scholar]
- 94.Grau M, Vicens V, Franco C, Masabeu U, Garcia C, Oller V. Antiphospholipid antibodies in Q fever. Anales de Medicina Interna. 1991;8:256–7. [PubMed] [Google Scholar]
- 95.Graw-Panzer KD, Verma S, Rao S, Miller ST, Lee H. Venous thrombosis and pulmonary embolism in a child with pneumonia due to Mycoplasma pneumoniae. J Natl Med Assoc. 2009;101:956–8. doi: 10.1016/s0027-9684(15)31045-2. [DOI] [PubMed] [Google Scholar]
- 96.Greco TP, Jr, Conti-Kelly AM, Greco TP. Antiphospholipid antibodies in patients with purported ‘chronic Lyme disease’. Lupus. 2011;20:1372–7. doi: 10.1177/0961203311414098. [DOI] [PubMed] [Google Scholar]
- 97.Gru A, Dehner LP. Catastrophic antiphospholipid syndrome in a child with trisomy 21. An acquired thrombopathy with a discussion of thrombopathies in childhood. Pediatric and Developmental Pathology. 2010;13:178–83. doi: 10.2350/09-06-0667-OA.1. [DOI] [PubMed] [Google Scholar]
- 98.Guedes-Barbosa LS, Batista EV, Martins DC, Neder L, Crepaldi N, Martins EV. Necrotizing cutaneous vasculitis in multibacillary leprosy disease (lucio’s phenomenon) Journal of Clinical Rheumatology. 2008;14:57–9. doi: 10.1097/RHU.0b013e318163bd9a. [DOI] [PubMed] [Google Scholar]
- 99.Haire WD. The acquired immunodeficiency syndrome and lupus anticoagulant. Ann Intern Med. 1986;105:301–2. doi: 10.7326/0003-4819-105-2-301_2. [DOI] [PubMed] [Google Scholar]
- 100.Hal Sebastiaan V, Senanayake S, Hardiman R. Splenic infarction due to transient antiphospholipid antibodies induced by acute Epstein-Barr virus infection. Journal of Clinical Virology. 2005;32:245–7. doi: 10.1016/j.jcv.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 101.Hamidou M, Tiab M, Jego P, Canfrere I, Merrien D, Grolleau JY. Q fever with anticardiolipin antibodies simulating juvenile rheumatoid arthritis in adults. Revue de Medecine Interne. 1993;14:560. [Google Scholar]
- 102.Hansen KE, Arnason J, Bridges AJ. Autoantibodies and common viral illnesses. Seminars in Arthritis and Rheumatism. 1998;27:263–71. doi: 10.1016/s0049-0172(98)80047-4. [DOI] [PubMed] [Google Scholar]
- 103.Harada M, Nishi Y, Tamura S, Iba Y, Abe K, Yanbe Y, et al. Infective endocarditis with a huge mitral vegetation related to atopic dermatitis and high serum level of infection-related antiphospholipid antibody: a case report. Journal of Cardiology. 2003;42:135–40. [PubMed] [Google Scholar]
- 104.Hassoun A, Al-Kadhimi Z, Cervia J. HIV infection and antiphospholipid antibody: literature review and link to the antiphospholipid syndrome. AIDS Patient Care STDS. 2004;18:333–40. doi: 10.1089/1087291041444032. [DOI] [PubMed] [Google Scholar]
- 105.Hernandez JL, Zarrabeitia R, Fernandez-Llaca H, Hortal L, Gonzalez-Macias J. Multifactorial thrombotic-type microangiopathy with skin ulcers and hepatitis C infection. European journal of internal medicine. 2000;11(3):165–7. doi: 10.1016/s0953-6205(00)00073-x. [DOI] [PubMed] [Google Scholar]
- 106.Herscovici R, Szyper-Kravitz M, Altman A, Eshet Y, Nevo M, Agmon-Levin N, et al. Superior vena cava syndrome - changing etiology in the third millennium. Lupus. 2012;21:93–6. doi: 10.1177/0961203311412412. [DOI] [PubMed] [Google Scholar]
- 107.Hoxha A, Calligaro A, Bortolati M, Tonello M, Guariso G, Ruffatti A. A close relationship between infections and anti-phospholipid syndrome in a child with trisomy 21. Clinical and Experimental Rheumatology. 2008;26:S91-S. [Google Scholar]
- 108.Hoxha A, Calligaro A, Bortolati M, Tonello M, Guariso G, Ruffatti A. The antiphospholipid syndrome and infections in a child with trisomy 21. Autoimmunity Reviews. 2008;8:121–3. doi: 10.1016/j.autrev.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 109.Humphries JE, Acker MN, Pinkston JE, Ruddy S. Transient lupus anticoagulant associated with prothrombin deficiency: unusual cause of bleeding in a 5-year-old girl. The American journal of pediatric hematology/oncology. 1994;16(4):372–6. [PubMed] [Google Scholar]
- 110.Ignatov K, Georgiev G, Lukanov T, Petrova P. A case of bleeding peptic ulcer associated with antiphospholipid syndrome. Clinical Application of Immunology. 2004;3:416–9. [Google Scholar]
- 111.Ihle BU, Oziemski P. Multi-organ failure secondary to catastrophic anti-phospholipid syndrome. Anaesth Intensive Care. 2002;30:82–5. doi: 10.1177/0310057X0203000116. [DOI] [PubMed] [Google Scholar]
- 112.Inglot M, Szymanek A, Szymczak A, Rymer W, Pawlowski T, Pacan P, et al. Three episodes of brain stroke as a manifestation of neurosyphilis in an HIV-infected man. Acta Dermato-Venereologica. 2013;93:234–5. doi: 10.2340/00015555-1482. [DOI] [PubMed] [Google Scholar]
- 113.Inomata S, Takeyama Y, Tanaka T, Ueda S, Morihara D, Nishizawa S, et al. Budd-Chiari syndrome: two cases with different courses. Case Reports Gastroenterology. 2008;2:256–61. doi: 10.1159/000146063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Iqbal Belkys H, Javaid Epstein-Barr Virus and Cerebral Vein Thrombosis. Infectious Diseases in Clinical Practice. 2012 [Google Scholar]
- 115.Izhevsky D, Maple JT, Ommen SR. Acute myocardial infarction: an unusual culmination of rheumatic pancarditis and antiphospholipid antibody syndrome. J Intern Med. 2004;255:296–8. doi: 10.1046/j.0954-6820.2003.01254.x. [DOI] [PubMed] [Google Scholar]
- 116.Jacq F, Emmerich J, Heron E, Lortholary O, Bruneval P, Fiessinger JN. Distal gangrene and cryoglobulinemia related to hepatitis C virus infection with presence of anticardiolipin antibodies. Revue de Medecine Interne. 1997;18:324–7. doi: 10.1016/s0248-8663(97)84019-2. [DOI] [PubMed] [Google Scholar]
- 117.Jani C, Bichile SK, Sampat N, Thacker H. Lupus anticoagulant presenting as a stroke in young. Journal of the Association of Physicians of India. 1997;45:329–30. [PubMed] [Google Scholar]
- 118.Jarrett P, Snow J. Scabies presenting as a necrotizing vasculitis in the presence of lupus anticoagulant. Br J Dermatol. 1998;139:701–3. doi: 10.1046/j.1365-2133.1998.02472.x. [DOI] [PubMed] [Google Scholar]
- 119.Jin SJ, Kim HW, Kim H, Yoon JH, Rim SJ, Song YG. A case of Libman-Sacks endocarditis that developed after infective endocarditis. Infection and Chemotherapy. 2011;43:416–20. [Google Scholar]
- 120.Johnston AM, Hill K, Woodcock BE. Lupus anticoagulant in a patient with parvovirus B19 infection. Clin Lab Haematol. 2000;22:109–10. doi: 10.1046/j.1365-2257.2000.00298.x. [DOI] [PubMed] [Google Scholar]
- 121.Kalt M, Gertner E. Antibodies to beta 2-glycoprotein I and cardiolipin with symptoms suggestive of systemic lupus erythematosus in parvovirus B19 infection. J Rheumatol. 2001;28:2335–6. [PubMed] [Google Scholar]
- 122.Kang HJ, Jung HK, Kim MY, Ryu MS, Ahn SY, Cho HW, et al. Antiphospholipid syndrome presenting variceal bleeding in patient with systemic anaerobic bacterial infection. EWHA Medical Journal. 2013;36(2):149–52. [Google Scholar]
- 123.Karunatilaka DH, De S JR, Ranatunga PK, Gunasekara TM, Faizal MA, et al. Idiopathic purpura fulminans in dengue hemorrhagic fever. Indian Journal of Medical Sciences. 2007;61:471–3. [PubMed] [Google Scholar]
- 124.Keeling DM, Birley H, Machin SJ. Multiple transient ischaemic attacks and a mild thrombotic stroke in a HIV-positive patient with anticardiolipin antibodies. Blood Coagul Fibrinolysis. 1990;1:333–5. doi: 10.1097/00001721-199008000-00012. [DOI] [PubMed] [Google Scholar]
- 125.Kida Y, Maeshima E, Yamada Y. Portal vein thrombosis in a patient with hepatitis C virus-related cirrhosis complicated with antiphospholipid syndrome. Rheumatol Int. 2009;29:1495–8. doi: 10.1007/s00296-009-0856-0. [DOI] [PubMed] [Google Scholar]
- 126.Kirrstetter M, Lerin-Lozano C, Heintz H, Manegold C, Gross WL, Lamprecht P. Trypanosomiasis in a woman from Cameroon mimicking systemic lupus erythematosus. Deutsche medizinische Wochenschrift. 2004;129(23):1315–7. doi: 10.1055/s-2004-826866. [DOI] [PubMed] [Google Scholar]
- 127.Kobayashi H, Sano A, Aragane N, Fukuoka M, Tanaka M, Kawaura F, et al. Disseminated infection by Bipolaris spiciferain an immunocompetent subject. Medical Mycology. 2008;46:361–5. doi: 10.1080/13693780701883490. [DOI] [PubMed] [Google Scholar]
- 128.Korkmaz C, Harmanci E, Metintas I, Gulbas Z. Antiphospholipid syndrome associated with intestinal amoebiasis. Scand J Infect Dis. 2001;33:938–40. doi: 10.1080/00365540110076804. [DOI] [PubMed] [Google Scholar]
- 129.Ku EW, Mizrachi A, Cohn J. Catastrophic Antiphospholipid Syndrome secondary to mycobacterium tuberculosis infection: A case report. Blood. 2003;102:107B-B. [Google Scholar]
- 130.Kurugol Z, Vardar F, Ozkinay F, Kavakli K, Cetinkaya B, Ozkinay C. Lupus anticoagulant and protein S deficiency in a child who developed disseminated intravascular coagulation in association with varicella. Turk J Pediatr. 2001;43:139–42. [PubMed] [Google Scholar]
- 131.Labarca JA, Rabaggliati RM, Radrigan FJ, Rojas PP, Perez CM, Ferres MV, et al. Antiphospholipid syndrome associated with cytomegalovirus infection: case report and review. Clin Infect Dis. 1997;24:197–200. doi: 10.1093/clinids/24.2.197. [DOI] [PubMed] [Google Scholar]
- 132.Lamaury I, Brouzes F, Sow MT, Pelczar S, Roul S, Strobel M. Portal thrombosis, mesenteric infarction and anticardiolipin antibodies in a patient with AIDS. Annales de Medecine Interne. 1996;147:344–5. [PubMed] [Google Scholar]
- 133.Le G, Agard C, Hamidou M, Tessier M, Boutoille D, Bonnel C, et al. Endocarditis due to Bacteroides fragilis revealed by portal thrombosis: A case report. [French] Une observation d’endocardite a Bacteroides fragilis revelee par une thrombose portale. Revue de Medecine Interne. 2004;25:473–5. doi: 10.1016/j.revmed.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 134.Leder AN, Flansbaum B, Zandman-Goddard G, Asherson R, Shoenfeld Y. Antiphospholipid syndrome induced by HIV. Lupus. 2001;10:370–4. doi: 10.1191/096120301669209574. [DOI] [PubMed] [Google Scholar]
- 135.Lee CH, Chuah SK, Pei SN, Liu JW. Acute Q fever presenting as antiphospholipid syndrome, pneumonia, and acalculous cholecystitis and masquerading as Mycoplasma pneumoniae and hepatitis C viral infections. Jpn J Infect Dis. 2011;64:525–7. [PubMed] [Google Scholar]
- 136.Lefebvre M, Grossi O, Agard C, Perret C, Le P, Patrice, et al. Systemic Immune Presentations of Coxiella burnetii Infection (Q Fever) Seminars in Arthritis and Rheumatism. 2010;39:405–9. doi: 10.1016/j.semarthrit.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 137.Lehmann HW, Plentz A, von L, Muller-Godeffroy E, Modrow S. Intravenous immunoglobulin treatment of four patients with juvenile polyarticular arthritis associated with persistent parvovirus B19 infection and antiphospholipid antibodies. Arthritis Research & Therapy. 2004;6:R1–R6. doi: 10.1186/ar1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lehmann Hartwig W, Plentz A, Landenberg P, Küster Rolf M, Modrow S. Different patterns of disease manifestations of parvovirus B19-associated reactive juvenile arthritis and the induction of antiphospholipid-antibodies. Clinical Rheumatology. 2008;27:333–8. doi: 10.1007/s10067-007-0718-7. [DOI] [PubMed] [Google Scholar]
- 139.Liappis AP, Roberts AD, Schwartz AM, Simon GL. Thrombosis and infection: a case of transient anti-cardiolipin antibody associated with pylephlebitis. Am J Med Sci. 2003;325:365–8. doi: 10.1097/00000441-200306000-00008. [DOI] [PubMed] [Google Scholar]
- 140.Lijfering WM, Sprenger HG, van S, WJ, van der M. Mesenteric vein thrombosis associated with primary cytomegalovirus infection: a case report. Blood Coagul Fibrinolysis. 2007;18:509–11. doi: 10.1097/MBC.0b013e3281a3bef9. [DOI] [PubMed] [Google Scholar]
- 141.Linares P, Fernandez-Gundin MJ, Vivas S, Suarez P, Olcoz JL. Unusual thrombotic manifestations secondary to antiphospholipid syndrome and hepatic fascioliasis. Journal of Infection. 2006;52:75–6. doi: 10.1016/j.jinf.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 142.Lioger B, Debiais S, Lauvin MA, Bonnaud I, Maillot F, Ferreira-Maldent N. Anticardiolipin antibodies-associated stroke in primary CMV infection. European Journal of Neurology. 2013;20:e105–6. doi: 10.1111/ene.12179. [DOI] [PubMed] [Google Scholar]
- 143.lobrano a, blanchard k, abell tl, minocha a, boone w, wyatt-ashmead j, et al. Postinfectious gastroparesis related to autonomic failure: a case report. Neurogastroenterology and Motility. 2006;18:162–7. doi: 10.1111/j.1365-2982.2005.00728.x. [DOI] [PubMed] [Google Scholar]
- 144.Lydakis C. Stroke-Complicated Endocarditis with Positive Lupus Anticoagulant: A Case Report. Angiology. 2005;56:503–6. doi: 10.1177/000331970505600421. [DOI] [PubMed] [Google Scholar]
- 145.Magdalena K, Anna K, Eugeniusz JK, Robert P. Secondary antiphospholipid syndrome due to unrecognized acquired immunodeficiency syndrome. A case report. Reumatologia. 2006 [Google Scholar]
- 146.Maldonado JA, Belinda M, Rodriguez V, FF Deep venous thrombosis associated with varicella pneumonia and anticardiolipin antibody. Anales de Medicina Interna. 2004;21:100–1. doi: 10.4321/s0212-71992004000200014. [DOI] [PubMed] [Google Scholar]
- 147.Maldonado R, Faugier E, Flores A, Lara PB. Tuberculosis the great simulator, really is lupus? A clinical case report. Pediatric Rheumatology. 2014;12 [Google Scholar]
- 148.Malnick SD, Abend Y, Evron E, Sthoeger ZM. HCV hepatitis associated with anticardiolipin antibody and a cerebrovascular accident. Response to interferon therapy. J Clin Gastroenterol. 1997;24:40–2. doi: 10.1097/00004836-199701000-00009. [DOI] [PubMed] [Google Scholar]
- 149.Manas MD, Calderon P, Yanes J, Ballester B, Gijon J. Antiphospholipid syndrome in a patient with Salmonella enteritidis bacteremia. Anales de Medicina Interna. 2006;23:97–8. doi: 10.4321/s0212-71992006000200014. [DOI] [PubMed] [Google Scholar]
- 150.Manco-Johnson MJLJ, Nuss R, Hays T. Blood, 1992;80:510a. Purpura fulminans (PF) in a child with varicella, a lupus anticoagulant (LA) and severe protein S (PS) deficiency [Abstract. Blood. 1992;80:510a. [Google Scholar]
- 151.Marruchella A, Corpolongo A, Tommasi C, Lauria Francesco N, Narciso P. A case of pulmonary tuberculosis presenting as diffuse alveolar haemorrhage: is there a role for anticardiolipin antibodies? BMC Infectious Diseases. 2010;10:33. doi: 10.1186/1471-2334-10-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Martin E, Winn R, Nugent K. Catastrophic antiphospholipid syndrome in a community-acquired methicillin-resistant Staphylococcus aureus infection: A review of pathogenesis with a case for molecular mimicry. Autoimmunity Reviews. 2011;10:181–8. doi: 10.1016/j.autrev.2010.09.023. [DOI] [PubMed] [Google Scholar]
- 153.Martin-Aspas A, Marin-Iglesia R, Gamiz-Sanchez R, Garcia-Lara E. Acute Q-fever with an uncommon presentation. Enfermedades Infecciosas y Microbiologia Clinica. 2006;24:288–9. doi: 10.1016/s0213-005x(06)73781-8. [DOI] [PubMed] [Google Scholar]
- 154.Massano J, Ferreira D, Toledo T, Mansilha A, Azevedo E, Carvalho M. Stroke and multiple peripheral thrombotic events in an adult with varicella. European Journal of Neurology. 2008;15:e90–1. doi: 10.1111/j.1468-1331.2008.02267.x. [DOI] [PubMed] [Google Scholar]
- 155.McKinley L. A case of blue toe syndrome in a patient with lupus anticoagulant and;proteus mirabilis septicemia. Journal of the American Academy of Dermatology. 2010 [Google Scholar]
- 156.Medina G, Calleja C, Moran M, Vera-Lastra O, Jara LJ. Catastrophic antiphospholipid syndrome in a patient with Down syndrome. Lupus. 2009;18:1104–7. doi: 10.1177/0961203309105878. [DOI] [PubMed] [Google Scholar]
- 157.Meissner MS, Schmitt DV, Penov K, Ender J, Mohr FW. Report of a case history of an acquired Fulminates, infection-associated Antiphospholipid Syndrome (APS) after CABG Surgery with Thrombi and Emboli in Six Locations. Medizinische Klinik-Intensivmedizin Und Notfallmedizin. 2013;108:358–9. [Google Scholar]
- 158.Merino R, Garcia-Consuegra J, Cuesta MV, Pascual-Salcedo D. Antiphospholipid antibodies in childhood: Case reports of 9 patients. [Spanish] Anticuerpos antifosfolipido en la infancia: Descripcion de 9 pacientes. Revista Espanola de Reumatologia. 1996;23:307–10. [Google Scholar]
- 159.Mizumoto H, Maihara T, Hiejima E, Shiota M, Hata A, Seto S, et al. Transient antiphospholipid antibodies associated with acute infections in children: a report of three cases and a review of the literature. European Journal of Pediatrics. 2006;165:484–8. doi: 10.1007/s00431-006-0117-0. [DOI] [PubMed] [Google Scholar]
- 160.Molina-Ruiz AM, Luque R, Zulueta T, Bernabeu J, Requena L. Cytomegalovirus-induced cutaneous microangiopathy manifesting as lower limb ischemia in a human immunodeficiency virus-infected patient. Journal of Cutaneous Pathology. 2012;39:945–9. doi: 10.1111/j.1600-0560.2012.01974.x. [DOI] [PubMed] [Google Scholar]
- 161.Moreira MC, Garcia VV, Garcia BDH, Mola EM. Anticardiolipin antibodies in a patient with polyarthritis due to Parvovirus B19 infection. Medicina Clinica. 2000;115:198–9. doi: 10.1016/s0025-7753(00)71506-5. [DOI] [PubMed] [Google Scholar]
- 162.Morino M, Yamano H, Sasaki N. Role of varicella virus and anticardiolipin antibodies in the development of stroke in a patient with Down syndrome associated with Moyamoya syndrome. Pediatrics International. 2009;51:300–2. doi: 10.1111/j.1442-200X.2009.02805.x. [DOI] [PubMed] [Google Scholar]
- 163.Muntean W, Petek W. Lupus anticoagulant after measles. Eur J Pediatr. 1980;134:135–8. doi: 10.1007/BF01846032. [DOI] [PubMed] [Google Scholar]
- 164.Muwakkit S, Al-Ajam M, Arayssi T, Hasbini D, Masri AF, Mikati M. Isolated digital gangrene complicating hepatitis A infection in a child. J Clin Rheumatol. 2002;8:223–7. doi: 10.1097/00124743-200208000-00013. [DOI] [PubMed] [Google Scholar]
- 165.Nadir A, Amin A, Chalisa N, van T, DH Retinal vein thrombosis associated with chronic hepatitis C: a case series and review of the literature. Journal of Viral Hepatitis. 2000;7:466–70. doi: 10.1046/j.1365-2893.2000.00245.x. [DOI] [PubMed] [Google Scholar]
- 166.Nagashima M, Higaki T, Satoh H, Nakano T. Cardiac thrombus associated with Mycoplasma pneumoniae infection. Interactive CardioVascular and Thoracic Surgery. 2010;11:849–51. doi: 10.1510/icvts.2010.242115. [DOI] [PubMed] [Google Scholar]
- 167.Nakayama T, Akahoshi M, Irino K, Kimoto Y, Arinobu Y, Niiro H, et al. Transient antiphospholipid syndrome associated with primary cytomegalovirus infection: a case report and literature review. case report. 2014;2014(3):271548. doi: 10.1155/2014/271548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Naranjo IC, Santos JAT, Rodriguez MAA. Ischemic stroke as the sole manifestation of human immunodeficiency virus infection [2. Stroke. 1992;23:117–8. [PubMed] [Google Scholar]
- 169.Nasilowska-Adamska B, Grabarczyk P, Dzieciatkowski T, Windyga J, Ejduk A, Tomaszewska A, et al. Early post-transplantation lymphoproliferative disorder with central nervous system involvement and EBV infection following allogeneic hematopoietic stem cells transplantation. [Polish. Hematologia. 2014;5(1):81–8. [Google Scholar]
- 170.Ndimbie OK, Raman BK, Saeed SM. Lupus anticoagulant associated with specific inhibition of factor VII in a patient with AIDS. Am J Clin Pathol. 1989;91:491–3. doi: 10.1093/ajcp/91.4.491. [DOI] [PubMed] [Google Scholar]
- 171.Newcombe JP, Gray PE, Palasanthiran P, Snelling TL. Q Fever with transient antiphospholipid antibodies associated with cholecystitis and splenic infarction. Pediatric Infectious Disease Journal. 2013;32:415–6. doi: 10.1097/INF.0b013e3182843d7e. [DOI] [PubMed] [Google Scholar]
- 172.Niitsuma T, Nukaga M, Izawa A, Tsuyuguchi M, Tsuboi N, Hayashi T. Antiphospholipid syndrome during allergic bronchopulmonary aspergillosis. Allergy. 2003;58:454–5. doi: 10.1034/j.1398-9995.2003.00114.x. [DOI] [PubMed] [Google Scholar]
- 173.Nishio S, Nakano Y, Sato H, Masuyama A, Tsuruta S, Haraoka H. Case report: a case of severe Lemierre’s syndrome with transient elevation of antiphospholipid antibody. Nippon Naika Gakkai Zasshi - Journal of Japanese Society of Internal Medicine. 2013;102:966–8. doi: 10.2169/naika.102.966. [DOI] [PubMed] [Google Scholar]
- 174.Noureddine M, Bennis A, Raquim S, Tahiri A, Chraibi N. Cardiovascular complications of the antiphospholipid antibody syndrome. [French] Anomalies cardiovasculaires revelatrices du syndrome des anticorps anti-phospholipides. Archives des Maladies du Coeur et des Vaisseaux. 2003;96:324–31. [PubMed] [Google Scholar]
- 175.Noval J, Nuno FJ, Bustamante JF, Baro U. Splenic infarction in a patient with anticardiolipin antibodies and leptospirosis. Enfermedades Infecciosas y Microbiologia Clinica. 1999;17:98–9. [PubMed] [Google Scholar]
- 176.Novelli M, Pilato A, Bertello P. Acute cytomegalovirus infection and venous thromboembolism. Recenti Progressi in Medicina. 2011;102:294–5. doi: 10.1701/913.10048. [DOI] [PubMed] [Google Scholar]
- 177.Nunzie E, Ortega Cabrera LV, Macanchi Moncayo FM, Ortega Espinosa PF, Clapasson A, Massone C. Lucio Leprosy with Lucio’s phenomenon, digital gangrene and anticardiolipin antibodies. Lepr Rev. 2014;85(3):194–200. [PubMed] [Google Scholar]
- 178.Orbea R, Venero G, Justo A, Colmenero C, Rodriguez P. Deep venous thrombosis of inferior extremity in a patient with AIDS and anticardiolipin antibodies. Anales de Medicina Interna. 1999;16:268. [PubMed] [Google Scholar]
- 179.Padmakumar B, Sun J, Satchithananthan G, Sills JA, Alwaidh MA. Deep venous thrombosis and pulmonary embolism following chickenpox. Ann Trop Paediatr. 2004;24:271–4. doi: 10.1179/027249304225019028. [DOI] [PubMed] [Google Scholar]
- 180.Padovan CS, Pfister HW, Bense S, Fingerle V, Abele-Horn M. Detection of Mycoplasma pneumoniae DNA in cerebrospinal fluid of a patient with M. pneumoniae infection-“associated” stroke. Clin Infect Dis. 2001;33(10):E119–21. doi: 10.1086/323461. [DOI] [PubMed] [Google Scholar]
- 181.Pamuk ON, Cakir N, Soy M, Aktoz M, Celik Y, Akdemir O. Mitral valve vegetation and cerebral emboli in a primary antiphospholipid syndrome patient who had hepatitis C virus infection: report of a case and review of the literature. Clinical Rheumatology. 2003;22:136–9. doi: 10.1007/s10067-002-0662-5. [DOI] [PubMed] [Google Scholar]
- 182.Parola P, Jourdan J, Raoult D. Tick-borne infection caused by Rickettsia africae in the West Indies [7. New England Journal of Medicine. 1998;338:1391. doi: 10.1056/NEJM199805073381918. [DOI] [PubMed] [Google Scholar]
- 183.Pelletier S, Chosidow O, Rogeaux O, Lenoir S, Piette JC, Frances C, et al. Probable antiphospholipid syndrome, secondary to fascioliasis. Annales de Medecine Interne. 1995;146:276–8. [PubMed] [Google Scholar]
- 184.Pers YM, Puygrenier M, Borlot F, Simorre B, Barazer I, Oziol E, et al. Acute Q fever, antiphopholipid antibodies and renal artery thrombosis: case report and literature review. Revue de Medecine Interne. 2009;30:250–4. doi: 10.1016/j.revmed.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 185.Peter R, Sadigh K, Bloom M. Is there an association between hepatitis C and anti-phospholipid syndrome? The heart says yes! American Journal of Gastroenterology. 2013;108:S345–S6. [Google Scholar]
- 186.Peyton BD, Cutler BS, Stewart FM. Spontaneous tibial artery thrombosis associated with varicella pneumonia and free protein S deficiency. J Vasc Surg. 1998;27:563–7. doi: 10.1016/s0741-5214(98)70335-0. [DOI] [PubMed] [Google Scholar]
- 187.Pittschieler S, Wiedermann FJ. Catastrophic antiphospholipid syndrome associated with methicillin-resistant Staphylococcus aureus infection. Autoimmunity Reviews. 2011;10:238. doi: 10.1016/j.autrev.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 188.Poon Michelle LM, Tang Julian W, Chee Yen L. Case report: Cytomegalovirus-induced thrombosis in an immunocompetent patient. Journal of Medical Virology. 2012;84:116–8. doi: 10.1002/jmv.22253. [DOI] [PubMed] [Google Scholar]
- 189.Pourrat O, Roblot F, Gombert JM, Pierre F. Clinical manifestations and abnormal laboratory findings in pregnant women with primary cytomegalovirus infection [4. BJOG: An International Journal of Obstetrics and Gynaecology. 2003;110:1139–40. doi: 10.1111/j.1471-0528.2003.03028.x. [DOI] [PubMed] [Google Scholar]
- 190.Poux JM, Jauberteau MO, Boudet R, Leroux-Robert C, Liozon F. Anti-cardiolipin antibodies in acute Coxiella burnetii infection. Value of the search and course of different isotypes. Presse Medicale. 1995;24:416. [PubMed] [Google Scholar]
- 191.Puri V, Bookman A, Yeo E, Cameron R, Heathcote EJ. Antiphospholipid antibody syndrome associated with hepatitis C infection. J Rheumatol. 1999;26:509–10. [PubMed] [Google Scholar]
- 192.Reitblat T, Drogenikov T, Sigalov I, Oren S, London D. Transient anticardiolipin antibody syndrome in a patient with parvovirus B19 infection. American Journal of Medicine. 2000;109:512–3. doi: 10.1016/s0002-9343(00)00561-1. [DOI] [PubMed] [Google Scholar]
- 193.Rennke HG, Laposata M. A 54-year-old woman with acute renal failure and thrombocytopenia - Antiphospholipid-antibody syndrome. New England Journal of Medicine. 1999;340:1900–8. doi: 10.1056/NEJM199906173402408. [DOI] [PubMed] [Google Scholar]
- 194.Rivoisy C, D’Oiron R, Cherin M, Segeral O, Meynard JL, Lambert T, et al. Acquired haemophilia A associated with HIV infection: A rare disease. Aids. 2014;28(6):931. doi: 10.1097/QAD.0000000000000172. [DOI] [PubMed] [Google Scholar]
- 195.Rizzi M, Avogadri M, Minola E, Quinzan P. Transient amaurosis and high level of anticardiolipin antibodies in AIDS patient. [Italian] Amaurosi Transitoria Ed Elevato Titolo Di Anticorpi Anticardiolipina in Un Paziente Con Aids. Giornale di Malattie Infettive e Parassitarie. 1994;46:327–9. [Google Scholar]
- 196.Rodriguez H, MJ, Cisneros H, JM, Digon P, Canas O, et al. Paludism and antiphospholipid antibodies. Anales de Medicina Interna. 1996;13:614–5. [PubMed] [Google Scholar]
- 197.Rodriguez-Quinonez A, Schneck MJ, Biller J, Brown HG. AIDS, stroke, and cryptococcus infection. Seminars in Cerebrovascular Diseases and Stroke. 2004;4:234–7. [Google Scholar]
- 198.Ronayne C. Malaria and lupus anticoagulant: A case study and review. New Zealand Journal of Medical Laboratory Science. 2013;67(3):98–103. [Google Scholar]
- 199.Rosca T, Tanaseanu C, Sarafoleanu C, Serban AT. Orbital pseudotumour with Wegener’s granulomatosis developing antiphospholipid syndrome. Neuro-Ophthalmology. 2010 [Google Scholar]
- 200.Rose C, Daneshpouy M, Leleu X, Maes P, Jeanjean ME, Mahieu M. Original course of autoimmune manifestations in cytomegalovirus infection inducing late disclosure of marginal zone lymphoma [3]. [French] Evolution inhabituelle de manifestations auto-immunes au decours d’une primo-infection a cytomegalovirus revelant tardivement un lymphome de la zone marginale. Revue de Medecine Interne. 1998;19:939–40. doi: 10.1016/s0248-8663(99)80071-x. [DOI] [PubMed] [Google Scholar]
- 201.Saberi A, Morrison RE. Catastrophic antiphospholipid syndrome in secondary syphilis. Infections in Medicine. 2009;26:18–22. [Google Scholar]
- 202.Saberi Robert EM, Ali Catastrophic antiphospholipid syndrome in secondary syphilis. Health Reference Center Academic - Clinical Report Document (not identified) 2009 [Google Scholar]
- 203.Sanchez AN, Gavela E. HIV and renal failure. Nefrologia. 2004;24:97–100. [PubMed] [Google Scholar]
- 204.Sanli H, Ozdemir E. Ig M class anticardiolipin antibody and anti-Ro/SS-A positivity in urticarial vasculitis associated with hepatitis C virus infection. International Journal of Dermatology. 2002;41:930–2. doi: 10.1046/j.1365-4362.2002.01681_2.x. [DOI] [PubMed] [Google Scholar]
- 205.Santos JL, Cruz I, Martin H, Albarran C, Gonzalez M, JM, et al. Recurrent coronary thrombosis, factor V Leiden, primary antiphospholipid syndrome and HIV. Revista Espanola de Cardiologia. 2004;57:997–9. [PubMed] [Google Scholar]
- 206.Schattner A, Sthoeger Z, Geltner D. Effect of acute cytomegalovirus infection on drug-induced SLE. Postgraduate Medical Journal. 1994;70:738–40. doi: 10.1136/pgmj.70.828.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Schmidt JL, Yaremchuk KL, Mickelson SA. Abnormal coagulation profiles in tonsillectomy and adenoidectomy patients. Henry Ford Hospital medical journal. 1990;38(1):33–5. [PubMed] [Google Scholar]
- 208.Schmugge M, Tolle S, Marbet GA, Laroche P, Meili EO. Gingival bleeding, epistaxis and haematoma three days after gastroenteritis: the haemorrhagic lupus anticoagulant syndrome. Eur J Pediatr. 2001;160(1):43–6. doi: 10.1007/pl00008415. [DOI] [PubMed] [Google Scholar]
- 209.Scimeca PG, Weinblatt ME, Kochen JA. Acquired coagulation inhibitor in association with Rocky Mountain spotted fever. With a review of other acquired coagulation inhibitors. Clin Pediatr (Phila) 1987;26(9):459–63. doi: 10.1177/000992288702600905. [DOI] [PubMed] [Google Scholar]
- 210.Sedlak T, Payer J, Horvathova D, Killinger Z, Hatalova A, Rovensky J, et al. Severe hemolytic uremia syndrome and antiphospholipid antibodies following bowel infection in the absence of major vascular occlusions: an example of MAPS? Israel Medical Association Journal: Imaj. 2008;10:896–8. [PubMed] [Google Scholar]
- 211.Selman A, Lumsden DE, Lim M, Martinez-Alier N, Lin JP. Retropharyngeal abscess with arterial thrombosis and stroke associated with Pseudomonas aeruginosa infection – Expanding the description of Lemierre’s like illness? European Journal of Paediatric Neurology. 2011 [Google Scholar]
- 212.Senda J, Ito M, Atsuta N, Watanabe H, Hattori N, Kawai H, et al. Paradoxical Brain Embolism Induced by Mycoplasma pneumoniae Infection with Deep Venous Thrombus. Internal Medicine. 2010;49:2003–5. doi: 10.2169/internalmedicine.49.3570. [DOI] [PubMed] [Google Scholar]
- 213.Shah I, Chudgar P. Antiphospholipid syndrome in a human immunodeficiency virus 1-infected child. The Pediatric Infectious Disease Journal. 2006;25:185–6. doi: 10.1097/01.inf.0000200140.04406.d0. [DOI] [PubMed] [Google Scholar]
- 214.Shahnaz S, Parikh G, Opran A. Antiphospholipid antibody syndrome manifesting as a deep venous thrombosis and pulmonary embolism in a patient with HIV. Am J Med Sci. 2004;327:231–2. doi: 10.1097/00000441-200404000-00010. [DOI] [PubMed] [Google Scholar]
- 215.Shimizu M, Yamazaki M, Horisawa T, Seno A, Ohta K, Furuichi K, et al. Catastrophic anti-phospholipid syndrome associated with Escherichia coli O157 infection. Rheumatology. 2009;48:1168–9. doi: 10.1093/rheumatology/kep159. [DOI] [PubMed] [Google Scholar]
- 216.Shimizu T, Ishiguro A, Takayanagi T, Matsui T, Tonegawa N, Maekawa T, et al. A case of lupus anticoagulant hypoprothrombinemia syndrome following adenovirus gastroenteritis and mycoplasma pneumonia. Nihon Rinsho Meneki Gakkai Kaishi. 2014;37(1):55–60. doi: 10.2177/jsci.37.55. [DOI] [PubMed] [Google Scholar]
- 217.Shimura H, Imai Y, Ieko M, Shiseki M, Mori N, Teramura M, et al. Transient lupus anticoagulant with a prolonged activated partial thromboplastin time secondary to cytomegalovirus-related infectious mononucleosis. Ann Hematol. 2013;92:143–4. doi: 10.1007/s00277-012-1532-0. [DOI] [PubMed] [Google Scholar]
- 218.Shinohara MM, Davis C, Olerud J. Concurrent antiphospholipid syndrome and cutaneous [corrected] sarcoidosis due to interferon alfa and ribavirin treatment for hepatitis C. Journal of Drugs in Dermatology: JDD. 2009;8:870–2. [PubMed] [Google Scholar]
- 219.Shiomou K, Galanakis E, Tzoufi M, Tsaousi C, Papadopoulou ZL. Transient lupus anticoagulant and prolonged activated partial thromboplastin time secondary to Epstein-Barr virus infection. Scand J Infect Dis. 2002;34:67–9. doi: 10.1080/003655402753395229. [DOI] [PubMed] [Google Scholar]
- 220.Shroff A, Chung HO, Khalidi NA, Spyropoulos A. Antiphospholipid syndrome and the aorta: A rare presentation. J Rheumatol. 2011;38:1808–9. doi: 10.3899/jrheum.101231. [DOI] [PubMed] [Google Scholar]
- 221.Sinnreich M, Rossillion B, Landis T, Burkhard PR, Sztajzel R. Bilateral optic ischemic neuropathy related to chronic hepatitis C-associated anticardiolipin antibodies. European Neurology. 2003;49:243–5. doi: 10.1159/000070195. [DOI] [PubMed] [Google Scholar]
- 222.Sonoda S, Ikegami R, Ashino N, Maki I, Atsumi T. A case of transient lupus anticoagulant associated with hypoprothrombinemia following adenovirus infection. [Japanese. Skin Research. 2005;4:361–5. [Google Scholar]
- 223.Soper CP, Sampson SA, Velasco N. Renal thrombotic microangiopathy, campylobacter gastroenteritis and anti-cardiolipin antibody. Nephrology Dialysis Transplantation. 2000;15:1261–2. doi: 10.1093/ndt/15.8.1261-a. [DOI] [PubMed] [Google Scholar]
- 224.Soweid AM, Hajjar RR, Hewan-Lowe KO, Gonzalez EB. Skin necrosis indicating antiphospholipid syndrome in patient with AIDS. South Med J. 1995;88:786–8. doi: 10.1097/00007611-199507000-00022. [DOI] [PubMed] [Google Scholar]
- 225.Steuerwald M, Gilli L. A case from practice (330). 1. HIV infection stage CDC A2. 2. Anticardiolipin syndrome. Praxis. 1995;84:944–6. [PubMed] [Google Scholar]
- 226.Suero JA, Franco MJM, Carrillo JLS, Gomez JV. Antiphospholipidic antibodies induced by an infection by Chlamydia pneumoniae. Medicina Clinica. 2005;124:519. doi: 10.1157/13073572. [DOI] [PubMed] [Google Scholar]
- 227.Sztajzel R, Heft S, Negro F. Transitory ischemic attack and acute hepatitis C. American Journal of Gastroenterology. 2000;95:2405–6. doi: 10.1111/j.1572-0241.2000.02358.x. [DOI] [PubMed] [Google Scholar]
- 228.Tanir G, Aydemir C, Yilmaz D, Tuygun N. Internal carotid artery occlusion associated with Mycoplasma pneumoniae infection in a child. Turk J Pediatr. 2006;48:166–71. [PubMed] [Google Scholar]
- 229.Tanizawa K, Nakatsuka D, Tanaka E, Inoue T, Sakuramoto M, Minakuchi M, et al. Pulmonary thrombosis with transient antiphospholipid syndrome after mononucleosis-like illness. Internal Medicine. 2009;48:1231–4. doi: 10.2169/internalmedicine.48.1949. [DOI] [PubMed] [Google Scholar]
- 230.Tattevin P, Dupeux S, Hoff J. Leptospirosis and the antiphospholipid syndrome. American Journal of Medicine. 2003;114:164. doi: 10.1016/s0002-9343(02)01365-7. [DOI] [PubMed] [Google Scholar]
- 231.Tavakoli M, Roghaee S, Soheilian R, Soheilian M. Antiphospholipid syndrome following toxoplasma retinochoroiditis. Ocul Immunol Inflamm. 2011;19:311–3. doi: 10.3109/09273948.2011.596302. [DOI] [PubMed] [Google Scholar]
- 232.Thirumalai S, Kirshner HS. Anticardiolipin antibody and stroke in an HIV-positive patient. AIDS. 1994;8:1019–20. doi: 10.1097/00002030-199407000-00027. [DOI] [PubMed] [Google Scholar]
- 233.Tolosa-Vilella C, Rodriguez-Jornet A, Font-Rocabanyera J, Andreu-Navarro X. Mesangioproliferative glomerulonephritis and antibodies to phospholipids in a patient with acute Q fever: case report. Clin Infect Dis. 1995;21:196–8. doi: 10.1093/clinids/21.1.196. [DOI] [PubMed] [Google Scholar]
- 234.Toyoshima M, Maegaki Y, Yotsumata K, Takei S, Kawano Y. Antiphospholipid Syndrome Associated With Human Herpesvirus-6 Infection. Pediatric Neurology. 2007;37:449–51. doi: 10.1016/j.pediatrneurol.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 235.Tullett JP, Bowman CA, Greaves M. Lupus anticoagulant in psoriatic-type arthropathy. Journal of the Royal Society of Medicine. 1989;82:505–6. doi: 10.1177/014107688908200826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Tung Y, Escutia B, Blanes M, Navarrro M, Pujol C. Sulfasalazine-induced hypersensitivity syndrome associated with human herpesvirus 6 reactivation and induction of antiphospholipid syndrome. Actas Dermo-Sifiliograficas. 2011;102:537–40. doi: 10.1016/j.ad.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 237.Turhal NS, Peters VB, Rand JH. Antiphospolipid syndrome in HIV infection - Report on four cases and review of literature. Allergy and Clinical Immunology International. 2001:268–71. [Google Scholar]
- 238.Turtle CJ, Coyle LA, Kotsiou G. Q-fever associated with splenic infarction and an anti-cardiolipin antibody. Australian & New Zealand Journal of Medicine. 1999;29:755–6. doi: 10.1111/j.1445-5994.1999.tb01635.x. [DOI] [PubMed] [Google Scholar]
- 239.Ulvestad E, Kanestrom A, Tengner P, Gjerde S, Sundal J, Haga HJ. Anti-cardiolipin autoantibodies and pulmonary embolism. A case for a common cause. Scand J Rheumatol. 2000;29:330–3. doi: 10.1080/030097400447750. [DOI] [PubMed] [Google Scholar]
- 240.Uthman I, Tabbarah Z, Gharavi AE. Hughes syndrome associated with cytomegalovirus infection. Lupus. 1999;8:775–7. doi: 10.1191/096120399678841034. [DOI] [PubMed] [Google Scholar]
- 241.Uthman I, Taher A, Khalil I. Hughes syndrome associated with varicella infection. Rheumatol Int. 2001;20:167–8. doi: 10.1007/s002960000086. [DOI] [PubMed] [Google Scholar]
- 242.Uthman I, Taher A, Khalil I, Bizri AR, Gharavi AE. Catastrophic antiphospholipid syndrome associated with typhoid fever: comment on the article by Hayem et al. Arthritis & Rheumatism. 2002;46:850. doi: 10.1002/art.10086. [DOI] [PubMed] [Google Scholar]
- 243.Vassalluzzo CJ, McGee DL, Glynn MJ. Antiphospholipid antibody syndrome: An unusual cause of stroke in a 22- year-old female. Journal of Emergency Medicine. 1995;13:485–8. doi: 10.1016/0736-4679(95)80005-0. [DOI] [PubMed] [Google Scholar]
- 244.Venugopalan P, Bushra R, Gravell D. Accidental detection of lupus anticoagulants in children. Ann Trop Paediatr. 2001;21:277–9. doi: 10.1080/02724930120077880. [DOI] [PubMed] [Google Scholar]
- 245.Vidal M, Corbin V, Chanet V, Ruivard M, Gourdon F, Laurichesse H, et al. Infections associated to severe thrombotic events and antiphospholipid antibodies. Medecine et Maladies Infectieuses. 2005;35:552–5. doi: 10.1016/j.medmal.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 246.Viseux V, Darnige L, Carmi E, Chaby G, Poulain JF, Cevallos R, et al. Pulmonary embolism and transitory anti-beta2-GPI antibodies in an adult with chicken pox. Lupus. 2000;9:558–60. doi: 10.1177/096120330000900716. [DOI] [PubMed] [Google Scholar]
- 247.Waller Elizabeth R, Siatkowski RM, Pardo G. Abnormal Eye Movements Due to Radiographically Silent Ischaemia as a Presenting Sign of Antiphospholipid Syndrome. Neuro- Ophthalmology. 2008;32:87–92. [Google Scholar]
- 248.Wallin L, Beckhauser AP, Haider O, Araujo F, Silva MB, Skare TL. Leprosy, antiphospholipid antibodies and bilateral fibular arteries obstruction. Revista Brasileira de Reumatologia. 2009:181–7. [Google Scholar]
- 249.Wiegering V, Balling G, Wirbelauer J, Sturm A, Girschick HJ. Post varicella disseminated intravascular coagulation and transient protein S deficiency in an otherwise healthy 6-year-old boy: a case report. Infection. 2010;38:505–8. doi: 10.1007/s15010-010-0053-9. [DOI] [PubMed] [Google Scholar]
- 250.Witmer CM, Steenhoff AP, Shah SS, Raffini LJ. Mycoplasma pneumoniae, Splenic Infarct, and Transient Antiphospholipid Antibodies: A New Association? Pediatrics. 2007;119:e292–e5. doi: 10.1542/peds.2006-1340. [DOI] [PubMed] [Google Scholar]
- 251.Witz M, Lehmann J, Korzets Z. Acute brachial artery thrombosis as the initial manifestation of human immunodeficiency virus infection. Am J Hematol. 2000;64:137–9. doi: 10.1002/(sici)1096-8652(200006)64:2<137::aid-ajh13>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 252.Wong RCW, Wilson R, Silcock R, Kratzing LM, Looke D. Unusual combination of positive IgG autoantibodies in acute Q-fever infection. Internal Medicine Journal. 2001;31:432–5. doi: 10.1046/j.1445-5994.2001.00099.x. [DOI] [PubMed] [Google Scholar]
- 253.Wong W, Denton M, Rennke HG, Lin J. Hepatitis C, proteinuria, and renal insufficiency. American Journal of Kidney Diseases. 2004;44:924–9. [PubMed] [Google Scholar]
- 254.Yamazaki M, Asakura H, Kawamura Y, Ohka T, Endo M, Matsuda T. Transient lupus anticoagulant induced by Epstein-Barr virus infection. Blood Coagul Fibrinolysis. 1991;2:771–4. doi: 10.1097/00001721-199112000-00012. [DOI] [PubMed] [Google Scholar]
- 255.Yanez A, Cedillo L, Neyrolles O, Alonso E, Prevost MC, Rojas J, et al. Mycoplasma penetrans bacteremia and primary antiphospholipid syndrome. Emerg Infect Dis. 1999;5:164–7. doi: 10.3201/eid0501.990122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Yilmaz I, Koc B, Kantarcioglu M, Akinci S, Ayta H, Bulucu F, et al. Pulmonary alveolar microlithiasis after Varicella zoster infection in a patient presenting with antiphospholipid syndrome and discoid lupus. Rheumatol Int. 2002;22:213–5. doi: 10.1007/s00296-002-0209-8. [DOI] [PubMed] [Google Scholar]
- 257.Yoo JH, Min JK, Kwon SS, Jeong CH, Shin WS. Symmetrical peripheral gangrene complicating Klebsiella pneumoniae sepsis associated with antiphospholipid antibodies. Ann Rheum Dis. 2004;63:459–60. doi: 10.1136/ard.2003.012542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Younes S, Chebel S, Boukhris S, Frih-Ayed M. Central nervous system involvement in patients with hepatitis C infection. [French] Virus de l’hepatite C et atteinte neurologique centrale: A propos d’une observation. Revue Neurologique. 2002;158:1202–4. [PubMed] [Google Scholar]
- 259.Zhang J, Jiang JJ, Zhang X, Bai CX. A 67-year-old man with persistent fever and high titers of serum anticardiolipin antibody. Internal Medicine Journal. 2012;42:344–5. doi: 10.1111/j.1445-5994.2012.02718.x. [DOI] [PubMed] [Google Scholar]
Footnotes
AUTHOR CONTRIBUTIONS. Dr. Suarez-Almazor had full access to all of the data in the study and takes responsibility for the integrity and the accuracy of the data analysis.
Study concept and design: Dr. Suarez-Almazor, Dr. Lopez-Olivo, Dr. Abdel-Wahab, Dr. Pinto-Patarroyo.
Acquisition of data: Dr. Suarez-Almazor, Dr. Lopez-Olivo.
Analysis and interpretation of data: Dr. Abdel-Wahab, Dr. Pinto-Patarroyo, Dr. Lopez-Olivo.
Quality appraisal: Dr. Abdel-Wahab, Dr. Lopez-Olivo.
Drafting of the manuscript: Dr. Abdel-Wahab, Dr. Lopez-Olivo, Dr. Suarez-Almazor.
Critical revision of the manuscript for important intellectual content: Dr. Suarez-Almazor, Dr. Lopez-Olivo.
Statistical analysis: Dr. Abdel-Wahab, Dr. Lopez-Olivo.
Administrative, technical, or material support: Dr. Suarez-Almazor.
Study supervision: Dr. Suarez-Almazor.
Disclosures: All authors report no conflicts of interest.
References
- 1.Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS) Journal of thrombosis and haemostasis : JTH. 2006;4(2):295–306. doi: 10.1111/j.1538-7836.2006.01753.x. [DOI] [PubMed] [Google Scholar]
- 2.Cervera R, Piette JC, Font J, Khamashta MA, Shoenfeld Y, Camps MT, et al. Antiphospholipid syndrome: clinical and immunologic manifestations and patterns of disease expression in a cohort of 1,000 patients. Arthritis and rheumatism. 2002;46(4):1019–27. doi: 10.1002/art.10187. [DOI] [PubMed] [Google Scholar]
- 3.Juby AG, Davis P. Prevalence and disease associations of certain autoantibodies in elderly patients. Clinical and investigative medicine Medecine clinique et experimentale. 1998;21(1):4–11. [PubMed] [Google Scholar]
- 4.Levine JS, Branch DW, Rauch J. The antiphospholipid syndrome. The New England journal of medicine. 2002;346(10):752–63. doi: 10.1056/NEJMra002974. [DOI] [PubMed] [Google Scholar]
- 5.Mchrani TPM. Epidemiology of the antiphospholipd syndrome. In: Asherson RA, editor. Handbook of systemic autoimmune diseases. Vol. 10. Amsterdam: Elsevier; 2009. pp. 13–34. [Google Scholar]
- 6.Petri M. Epidemiology of the antiphospholipid antibody syndrome. Journal of autoimmunity. 2000;15(2):145–51. doi: 10.1006/jaut.2000.0409. [DOI] [PubMed] [Google Scholar]
- 7.Shi W, Krilis SA, Chong BH, Gordon S, Chesterman CN. Prevalence of lupus anticoagulant and anticardiolipin antibodies in a healthy population. Australian and New Zealand journal of medicine. 1990;20(3):231–6. doi: 10.1111/j.1445-5994.1990.tb01025.x. [DOI] [PubMed] [Google Scholar]
- 8.Harel M, Aron-Maor A, Sherer Y, Blank M, Shoenfeld Y. The infectious etiology of the antiphospholipid syndrome: links between infection and autoimmunity. Immunobiology. 2005;210:743–7. doi: 10.1016/j.imbio.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Asherson RA, Cervera R. ‘Primary’, ‘secondary’ and other variants of the antiphospholipid syndrome. Lupus. 1994;3(4):293–8. doi: 10.1177/096120339400300417. [DOI] [PubMed] [Google Scholar]
- 10.Amin NM. Antiphospholipid syndromes in infectious diseases. Hematology - Oncology Clinics of North America. 2008;22:131–43. vii. doi: 10.1016/j.hoc.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 11.Blank M, Krause I, Fridkin M, Keller N, Kopolovic J, Goldberg I, et al. Bacterial induction of autoantibodies to beta2-glycoprotein-I accounts for the infectious etiology of antiphospholipid syndrome. The Journal of clinical investigation. 2002;109(6):797–804. doi: 10.1172/JCI12337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shoenfeld Y, Blank M, Cervera R, Font J, Raschi E, Meroni PL. Infectious origin of the antiphospholipid syndrome. Ann Rheum Dis. 2006;65(1):2–6. doi: 10.1136/ard.2005.045443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galli M, Comfurius P, Maassen C, Hemker HC, de Baets MH, van Breda-Vriesman PJ, et al. Anticardiolipin antibodies (ACA) directed not to cardiolipin but to a plasma protein cofactor. Lancet. 1990;335(8705):1544–7. doi: 10.1016/0140-6736(90)91374-j. [DOI] [PubMed] [Google Scholar]
- 14.Hunt JE, McNeil HP, Morgan GJ, Crameri RM, Krilis SA. A phospholipid-beta 2-glycoprotein I complex is an antigen for anticardiolipin antibodies occurring in autoimmune disease but not with infection. Lupus. 1992;1(2):75–81. doi: 10.1177/096120339200100204. [DOI] [PubMed] [Google Scholar]
- 15.Asherson RA, Cervera R, de Groot PG, Erkan D, Boffa MC, Piette JC, et al. Catastrophic antiphospholipid syndrome: international consensus statement on classification criteria and treatment guidelines. Lupus. 2003;12(7):530–4. doi: 10.1191/0961203303lu394oa. [DOI] [PubMed] [Google Scholar]
- 16.Haneline MT. Book: Evidence-Based Chiropractic Practice. 2006. [Google Scholar]
- 17.Michaelis L. Precipitin reaction bei syphilis. Berl KlinWochenschr. 1907;44 [Google Scholar]
- 18.Wasserman ANA, Bruck C. Eine serodiagnostiche Reaction bei Syphilis. Deutsche medizinische Wochenschrift. 1906;32:745. [Google Scholar]
- 19.Landsteiner KMR, Potzl O. Zur Frage der Komplementbindungsreaktion bei Syphilis. Wien Klin Wochenschr. 1907;20:1565. [Google Scholar]
- 20.Lynch FWBR, Kimball AC. False positive serologic reactions for syphilis due to smallpox vaccination (vaccinia) JAMA. 1941;115:591. [Google Scholar]
- 21.Putkonen T. Biologic false-positive seroreactions for syphilis: Type, incidence, and cause. JAMA. 1952;150:467. [PubMed] [Google Scholar]
- 22.Harvey AMSL. Systemic lupus erythematosus and chronic biological false positive test for syphilis. In: Dubois E, editor. Lupus Erythematosus. 4. Los Angeles: University of California Press; 1974. pp. 196–209. [Google Scholar]
- 23.Koike T, Sueishi M, Funaki H, Tomioka H, Yoshida S. Anti-phospholipid antibodies and biological false positive serological test for syphilis in patients with systemic lupus erythematosus. Clinical and experimental immunology. 1984;56(1):193–9. [PMC free article] [PubMed] [Google Scholar]
- 24.Moore JELW. Natural history of SLE: Approach to its study through chronic biologic false positive reactions. J Chron Dis. 1949;1:297. doi: 10.1016/0021-9681(55)90039-4. [DOI] [PubMed] [Google Scholar]
- 25.Harris EN, Gharavi AE, Boey ML, Patel BM, Mackworth-Young CG, Loizou S, et al. Anticardiolipin antibodies: detection by radioimmunoassay and association with thrombosis in systemic lupus erythematosus. Lancet. 1983;2(8361):1211–4. doi: 10.1016/s0140-6736(83)91267-9. [DOI] [PubMed] [Google Scholar]
- 26.Harris EN, Gharavi AE, Loizou S, Derue G, Chan JK, Patel BM, et al. Crossreactivity of antiphospholipid antibodies. Journal of clinical & laboratory immunology. 1985;16(1):1–6. [PubMed] [Google Scholar]
- 27.Harris ENBE, Asherson RA, Hughes GRVH. Clinical and serological features of the “antiphospholipid syndrome” [abstract] Br J Rheumatol. 1987;26(19) [Google Scholar]
- 28.Hughes GR, Harris NN, Gharavi AE. The anticardiolipin syndrome. J Rheumatol. 1986;13(3):486–9. [PubMed] [Google Scholar]
- 29.Matsuura E, Igarashi Y, Fujimoto M, Ichikawa K, Koike T. Anticardiolipin cofactor(s) and differential diagnosis of autoimmune disease. Lancet. 1990;336(8708):177–8. doi: 10.1016/0140-6736(90)91697-9. [DOI] [PubMed] [Google Scholar]
- 30.McNeil HP, Simpson RJ, Chesterman CN, Krilis SA. Anti-phospholipid antibodies are directed against a complex antigen that includes a lipid-binding inhibitor of coagulation: beta 2-glycoprotein I (apolipoprotein H) Proceedings of the National Academy of Sciences of the United States of America. 1990;87(11):4120–4. doi: 10.1073/pnas.87.11.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Asherson RA, Cervera R. Antiphospholipid antibodies and infections. Ann Rheum Dis. 2003;62:388–93. doi: 10.1136/ard.62.5.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cervera R, Asherson RA, Acevedo ML, Gomez-Puerta JA, Espinosa G, De La Red G, et al. Antiphospholipid syndrome associated with infections: clinical and microbiological characteristics of 100 patients. Ann Rheum Dis. 2004;63(10):1312–7. doi: 10.1136/ard.2003.014175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Flateau C, Asfalou I, Deman AL, Ficko C, Andriamanantena D, Fontan E, et al. Aortic thrombus and multiple embolisms during a Mycoplasma pneumoniae infection. Infection. 2013;41:867–73. doi: 10.1007/s15010-013-0475-2. [DOI] [PubMed] [Google Scholar]
- 34.Nakayama T, Akahoshi M, Irino K, Kimoto Y, Arinobu Y, Niiro H, et al. Transient antiphospholipid syndrome associated with primary cytomegalovirus infection: a case report and literature review. case report. 2014;2014(3):271548. doi: 10.1155/2014/271548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramos-Casals M, Cervera R, Lagrutta M, Medina F, Garcia-Carrasco M, de la Red G, et al. Clinical features related to antiphospholipid syndrome in patients with chronic viral infections (hepatitis C virus/HIV infection): description of 82 cases. Clin Infect Dis. 2004;38(7):1009–16. doi: 10.1086/382537. [DOI] [PubMed] [Google Scholar]
- 36.Asherson RA, Cervera R, Piette JC, Shoenfeld Y, Espinosa G, Petri MA, et al. Catastrophic antiphospholipid syndrome - Clues to the pathogenesis from a series of 80 patients. Medicine. 2001;80:355–77. doi: 10.1097/00005792-200111000-00002. [DOI] [PubMed] [Google Scholar]
- 37.Cervera R, Bucciarelli S, Plasin MA, Gomez-Puerta JA, Plaza J, Pons-Estel G, et al. Catastrophic antiphospholipid syndrome (CAPS): descriptive analysis of a series of 280 patients from the “CAPS Registry”. Journal of autoimmunity. 2009;32(3–4):240–5. doi: 10.1016/j.jaut.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 38.Gushiken FC, Arnett FC, Ahn C, Thiagarajan P. Polymorphism of beta2-glycoprotein I at codons 306 and 316 in patients with systemic lupus erythematosus and antiphospholipid syndrome. Arthritis and rheumatism. 1999;42(6):1189–93. doi: 10.1002/1529-0131(199906)42:6<1189::AID-ANR15>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 39.Koike T. Antiphospholipid syndrome: 30 years and our contribution. International journal of rheumatic diseases. 2014 doi: 10.1111/1756-185X.12438. [DOI] [PubMed] [Google Scholar]
- 40.Sanghera DK, Kristensen T, Hamman RF, Kamboh MI. Molecular basis of the apolipoprotein H (beta 2-glycoprotein I) protein polymorphism. Human genetics. 1997;100(1):57–62. doi: 10.1007/s004390050465. [DOI] [PubMed] [Google Scholar]
- 41.Steinkasserer A, Dorner C, Wurzner R, Sim RB. Human beta 2-glycoprotein I: molecular analysis of DNA and amino acid polymorphism. Human genetics. 1993;91(4):401–2. doi: 10.1007/BF00217367. [DOI] [PubMed] [Google Scholar]
- 42.Yasuda S, Atsumi T, Matsuura E, Kaihara K, Yamamoto D, Ichikawa K, et al. Significance of valine/leucine247 polymorphism of beta2-glycoprotein I in antiphospholipid syndrome: increased reactivity of anti-beta2-glycoprotein I autoantibodies to the valine247 beta2-glycoprotein I variant. Arthritis and rheumatism. 2005;52(1):212–8. doi: 10.1002/art.20741. [DOI] [PubMed] [Google Scholar]