Abstract
NGLY1 is a widely conserved eukaryotic cytosolic deglycosylating enzyme involved in the endoplasmic reticulum-associated degradation (ERAD) process, which eliminates misfolded proteins through retrograde translocation and proteasomal degradation. A human genetic disorder called NGLY1-deficiency has been reported, indicating the functional importance of NGLY1 in humans. Evidence suggests that Ngly1-KO is embryonic lethal in mice, while additional deletion of the Engase gene, encoding another cytosolic deglycosylating enzyme (endo-β-N-acetylglucosaminidase; ENGase), partially rescued lethality. Upon compromised Ngly1 activity, ENGase-mediated deglycosylation of misfolded glycoproteins may cause excess formation of N-GlcNAc proteins in the cytosol, leading to detrimental effects in the mice. Whether endogenous N-GlcNAc proteins are really formed in Ngly1-KO cells/animals or not remains unclarified. Here, comprehensive identification of O- and N-GlcNAc proteins was carried out using purified cytosol from wild type, Ngly1-KO, Engase-KO, and Ngly1/Engase double KO mouse embryonic fibroblasts. It was revealed that while there is no dramatic change in the level of O-GlcNAc proteins among cells examined, there was a vast increase of N-GlcNAc proteins in Ngly1-KO cells upon proteasome inhibition. Importantly, few N-GlcNAc proteins were observed in Engase-KO or Ngly1/Engase double-KO cells, clearly indicating that the cytosolic ENGase is responsible for the formation of N-GlcNAc proteins. The excess formation of N-GlcNAc proteins may at least in part account for the pathogenesis of NGLY1-deficiency.
Keywords: NGLY1, O-GlcNAc, N-GlcNAc, Glycoproteomics, ENGase, NGLY1-deficiency
Graphical Abstract
INTRODUCTION
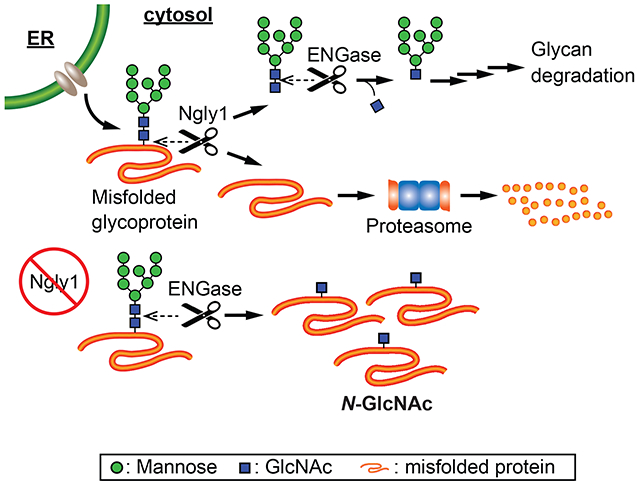
Endoplasmic reticulum (ER)1-associated degradation (ERAD) constitutes one of the protein homeostasis/quality control systems for newly synthesized proteins in the ER [1]. It has been proposed that N-glycosylated proteins have dedicated ERAD machinery [2]. Misfolded N-glycoproteins are recognized by the ERAD system and are retrotranslocated into the cytosol, where the proteasome plays a critical role in their degradation. During degradation, N-glycans are released by cytosolic peptide:N-glycanase (PNGase) (Fig. 1A) [3].
1.

A) Ngly1 and ENGase deglycosylating enzymes function in relation to endoplasmic reticulum associated degradation. Ngly1 removes N-linked glycans from retrotranslocated, misfolded glycoproteins before proteosomal degradation. ENGase cleaves between the two core GlcNAcs of previously released glycans in the cytosol. B) MEF (wild type, Ngly1-KO, Engase-KO, Ngly1/Engase double-KO) cells were permeabilized with 0.015% digitonin to selectively release cytosol. Tryptic glycopeptides were enriched using wheat germ agglutinin-based lectin weak affinity chromatography. Ubiquitinated peptides were enriched from LWAC flowthrough. Enriched peptides were analyzed by mass spectrometry.
Cytosolic PNGase was first identified and reported in mammalian cells [4,5]. The gene encoding the cytosolic PNGase (Ngly1 in mammals [6]) is well conserved in eukaryotes [7]. Recently, patients bearing mutations in the NGLY1 gene have been described and the clinical disorder termed NGLY1-deficiency [8–10]. Patients with NGLY1-deficiency display systemic symptoms, including developmental delay, movement disorders, peripheral neuropathy, hypo/alacrima, and hypotonia [9,10], clearly indicating the functional importance of NGLY1.
Previously it was revealed that Ngly1-KO in C57BL/6 mice is embryonic/perinatal lethal [11]. Unexpectedly, the embryonic lethality of Ngly1-KO mice was partially rescued by the additional deletion of the Engase gene [11]. Engase encodes a cytosolic endo-β-N-acetylglucosaminidase (ENGase), another cytosolic N-glycan deglycosylating enzyme [12]. ENGase is involved in the catabolism of free N-glycans generated by multiple pathways (Fig. 1A) [13]. This observation led to the hypothesis that ENGase may be a drug target candidate for NGLY1-deficiency. We previously proposed the “N-GlcNAc hypothesis” as a potential mechanism for how ENGase can partially rescue the defects caused by Ngly1 deletion [11,14]. In this proposed process, ENGase cleaves glycans from cytosolic misfolded glycoproteins in a stochastic fashion, and N-GlcNAc modified proteins would be generated in the cytosol. In fact, degradation of a model ERAD substrate (RTAΔm derived from ricin toxin A chain) was delayed in Ngly1-KO mouse embryonic fibroblasts (MEF) cells, and the substrate was still deglycosylated by ENGase, forming N-GlcNAc RTAΔm [14]. Moreover, the N-GlcNAc-modified RTAΔm was found to form detergent-insoluble aggregates, implying that some N-GlcNAc proteins may be prone to form proteasome-resistant aggregates. These observations suggest that upon compromised NGLY1 activity, excess formation of N-GlcNAc proteins is expected, and the formation of excessive levels of N-GlcNAc proteins could lead to detrimental effects in part accounting for the pathophysiology of NGLY1-deficiency. The N-GlcNAc hypothesis also proposes that the presence of N-GlcNAc proteins may affect pre-existing O-GlcNAc mediated intracellular signaling.
While N-GlcNAc-containing peptides/proteins have been identified in nature from various sources [15–20], how NGLY1 and ENGase are involved in their formation remains unclear. In this study, a comprehensive analysis to detect and identify cytosolic N-GlcNAc and O-GlcNAc glycopeptides in wild type, Ngly1-KO, Engase-KO and Ngly1/Engase double KO MEF cells was performed to provide insight into the roles of Ngly1 and ENGase in the formation of N-GlcNAc proteins.
MATERIALS AND METHODS
Cell Culture Procedures
MEF cells from all genetic backgrounds were prepared and cultivated as described previously [14].
Cytosol Enrichment
Two million MEF cells were seeded on a 150 cm2 flask 48 h before extraction. For MG132 treatment, the MEF cells were cultured with medium containing 5 μM of MG132 for 3 h. Detergent extraction was used to separate cytosolic contents [21]. Briefly, MEF cells were washed 3 times with 10 mL of PBS. Cytosol was released with 1 mL of permeabilization buffer (25 mM KHEPES (pH 7.2), 110 mM potassium acetate, 2.5 mM magnesium acetate, 1 mM ethyleneglycoltetraacetic acid (EGTA), 1 mM dithiothreitol (DTT), 1 mM phenylmethylsulfonyl fluoride (PMSF), 100 μM O-(2-Acetamido-2-deoxy-D-glucopyranosylidenamino) N-phenylcarbamate (PUGNAc), 0.015% (w/v) digitonin) while slowly rocked at 4°C for 5 min. The collected buffer was then centrifuged at 7,500×g for 10 min at 4°C. The supernatant was used as the cytosolic fraction for glycoproteomic analysis.
Glycopeptide Enrichment
Glycopeptide enrichment by lectin weak affinity chromatography (LWAC) was described previously [16,19]. Briefly, 6-11 mg of protein from MEF cytosol were denatured in 8 M urea, reduced with 2.5 mM DTT for 1 hour at 25°C, and cysteines were alkylated with 5 mM iodoacetic acid at 25°C in the dark for 45 mins. Samples were diluted to 2 M urea with 25 mM KHEPES (pH 7.2) and digested overnight at 37°C with 5% (w/w) sequencing-grade trypsin (Promega). Digested samples were acidified with formic acid (Sigma-Aldrich) and subsequently desalted using a 360 mg C18 Sep-Pak SPE cartridge (Waters). Desalted samples were dried using a SpeedVac concentrator (Thermo). Desalted cytosolic peptides were resuspended in LWAC buffer (100 mM Tris-HCl (pH 7.5), 150 mM NaCl, 10 mm MgCl2, 10 mm CaCl2, and 5% acetonitrile). Glycopeptides were enriched with a POROS- wheat germ agglutinin (WGA) column, collected, and desalted inline using a Luna 10μ C18 column (Phenomenex). A total of three rounds of LWAC enrichment were performed. LWAC-enriched glycopeptides were subsequently fractionated by high-pH reversed-phase liquid chromatography.
Ubiquitin-Peptide Enrichment
Peptides from LWAC flowthrough were desalted as above. Ubiquitin modified peptides were enriched using the PTMScan ubiquitin remnant motif (K;-ε-GG) kit (Cell Signaling Technology) according to the protocol from Udeshi et al. [22].
Mass Spectrometry
Glycopeptide enriched samples were analyzed on an Orbitrap Velos (Thermo Fisher Scientific) equipped with a NanoAcquity UPLC (Waters). Peptides were fractionated on a 15 cm × 75 μm ID 3 μm C18 EASY-Spray column using a linear gradient from 2 to 30% solvent B over 60 min. Survey mass measurements were performed using the Orbitrap, scanning from m/z 350–2000. The three most abundant multiply charged ions were selected for higher energy collisional dissociation (HCD) and electron transfer dissociation (ETD) analysis. The trigger intensity was set to 2000. Supplemental activation was enabled. The ETD fragments were measured in the linear trap; HCD fragments were measured in the Orbitrap. Each sample was injected twice; the first analysis selected only 2+ precursor ions, and in the second analysis, 2+ precursor ions were excluded. Peaklists were extracted using Proteome Discoverer 1.4. The data were searched against the SwissProt Mus musculus and Bos taurus databases (22941 entries, downloaded January 1, 2017) (and concatenated with a randomized sequence for each entry) using Protein Prospector (version 5.23.0). Cleavage specificity was set as tryptic, allowing for two missed cleavages. Carboxymethylation of Cys was set as a constant modification. The required mass accuracy was 10 ppm for precursor ions and fragments ion mass accuracy was 0.6 Da for ETD files or 30 ppm for HCD files. Variable modifications are in Supplemental Table 8. Three modifications per peptide were permitted. Modified peptides were identified with a protein and peptide false discovery rate of 1%. HexNAc modified peptides were manually verified and O-GlcNAc and O-GalNAc modifications were differentiated based on protein localization and HexNAc oxonium ion fragment ratios [23]. Results can be viewed in MS Viewer (ETD search key: beypz6zf20, HCD search key: mriemlhilv) [24]. When comparing across cell conditions, peptide spectral matches (PSM) were normalized using total spectra.
Ubiquitin enriched samples were analyzed on a Q-Exactive Plus (Thermo Fisher Scientific) equipped with a NanoAcquity UPLC (Waters). Peptides were fractionated as above using a gradient from 2-25% B over 78 mins, then to 37% B over 4 mins, and to 40% B over 3 mins before washing and equilibrating the column. Survey mass measurements were scanned from m/z 350-1500. The ten most abundant multiply charged ions were selected with dynamic exclusion for HCD fragmentation with 25 normalized collision energy. Protein prospector search parameters were as above also allowing for GlyGly on uncleaved lysine as a variable modification. Modified peptides were identified with a protein and peptide false discovery rate of 1%. Results can be viewed in MS Viewer (search key: yxtxwkxdg0).
Raw mass spectrometry data has been deposited to MassIVE with the dataset identifier: MSV000084330.
O-GlcNAc Network Analysis
Network analysis was performed in Cytoscape v3.7.1 with stringApp v1.4.2 and Omics Visualizer v1.1.2. A confidence score cutoff of 0.9 was set for the network analysis and the functional enrichment analysis used an FDR value cutoff of 0.05.title.
RESULTS AND DISCUSSION
Formation of N-GlcNAc Proteins in Mouse Embryonic Fibroblasts
Cytosolic proteins were isolated from MEF cells [11] and digested with trypsin. Tryptic glycopeptides were enriched using WGA-LWAC and analyzed the by mass spectrometry (Fig. 1B). HCD and ETD fragmentation techniques were used for glycopeptide identification to ensure spectra identified as glycopeptides do in fact contain a glycan and to properly assign the type and site of the glycan modification.
The vast majority (90%) of assigned glycopeptide spectral matches (gPSMs) in wild type (WT) MEF cells were O-GlcNAc-modified (Supplemental Tables 1–4). However, glycopeptides from non-nucleocytoplasmic proteins were also identified and included O-GalNAc and N-linked glycans. The pool of N-linked glycans included N-GlcNAc modified peptides. The identification of N-GlcNAc modified peptides was used to assess ENGase activity in MEF cells.
The number of N-GlcNAc spectral matches in the cytosol of both WT and Ngly1-KO (NKO) MEF cells was similar, i.e. 1-2% of identified gPSMs (Fig. 2A, Supplemental Table 1)Over. Over 200 N-GlcNAc-containing peptides from almost 100 different proteins were identified in the NKO cells upon treatment with the proteosomal inhibitor. Ninety percent of these identified sites are annotated in Uniprot as known N-glycosylation sites. Figure 2B highlights an example of an N-GlcNAc peptide from thrombospondin-1, a secreted glycoprotein that mediates cell-cell and cell-matrix interactions and also plays a role in ER stress response [25]. The GlcNAc modification is present on Asn-1067, a known N-glycosylation site [26]. The c3 fragment ion confidently places the GlcNAc on Asn-1067, and the HCD fragmentation pattern (inset) is consistent with GlcNAc rather than GalNAc [23]. To determine if these peptides could originate from ERAD substrates, the LWAC flowthrough was analyzed for ubiquitin modifications. Tryptic digestion of ubiquitinated proteins results in Lys residues modified with two glycines (K-ε-GG). Peptides bearing this modification can be enriched with an antibody directed against the K-ε-GG motif [27]. More than 40 percent of the proteins with identified N-GlcNAc moieties in the MG132 treated NKO sample were found to also contain K-ε-GG moieties, establishing that these proteins represent in vivo ERAD substrates (Supplemental Table 5). Figure 2C illustrates an example of a thrombospondin-1 peptide containing a K-ε-GG modification. Three additional ubiquitination sites were identified on thrombospondin-1 from the MG132 treated NKO cytosol (Supplemental Table 6).
2.
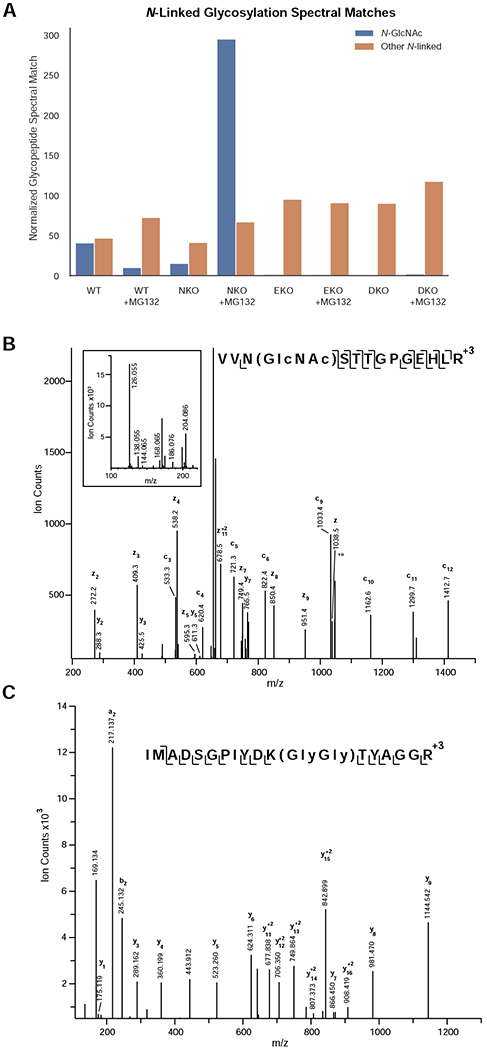
N-GlcNAc proteins are formed by the action of ENGase in the absence of Ngly1. A) Abundance of N-GlcNAc and extended N-glycopeptides in wild type, Ngly1-KO, Engase-KO, and Ngly1/Engase-KO treated with and without proteosomal inhibitor MG132. Peptide spectral matches were normalized to total spectra. Levels of extended N-linked glycopeptide-PSMs were relatively unchanged while the levels of N-GlcNAc modified PSMs were dramatically increased in Ngly1-KO cells upon proteosomal inhibition. B) Annotated ETD MS2 peaklist of N-GlcNAc modified peptide from Thrombospondin-1. C) Annotated HCD MS2 peaklist of K-GlyGly modified peptide from Thrombospondin-1.
The presence of N-GlcNAc moieties in the Ngly1-KO samples suggests that the cytoplasmic ENGase processes the protein-bound N-linked glycan structures of ERAD substrates in the cytosol. To verify this, cytosolic glycopeptides from Engase-KO (EKO) or Ngly1/Engase double KO (DKO) MEF cells were enriched and identified. Only a few N-GlcNAc peptides were identified in EKO or DKO cells irrespective of MG132 treatment (Fig. 2A, Supplemental Table 1). These data provide strong evidence that the cytosolic ENGase generates N-GlcNAc moieties.
It was interesting to note that, in the absence of proteasome inhibitor, there was little difference in the number of detected N-GlcNAc proteins between Ngly1-KO and WT MEF cells. This result indicates that N-GlcNAc formation by ENGase can occur under normal conditions. Upon treatment with proteasome inhibitor, however, there were fewer N-GlcNAc proteins identified in WT MEF, suggesting that the level of cytosolic N-GlcNAc proteins may be decreased in the presence of proteasome inhibitor. If N-GlcNAc modifications lead to protein aggregation, it is possible that cytosol enrichment with digitonin is not sensitive to the detection of those aggregated proteins. Cytosolic aggregates are reported to be segregated to mitochondria for degradation as a part of the cytosolic proteostasis machinery [28] and it is possible that mitochondria may be involved in the clearance of excess N-GlcNAc proteins. Mitochondrial function appears compromised in Ngly1-KO cells [29,30], and Ngly1-KO cells/animals are sensitive to proteasome inhibitors [31–34]. Those combination effects may be the reason for differential response towards proteasome inhibitor treatment between NKO and WT cells.
O-GlcNAcylation of ubiquitin-associated proteins
LWAC-based glycopeptide enrichment was originally developed to enrich O-GlcNAc modified peptides [15,16,35]. Unsurprisingly, nearly 90% of cytosolic-glycopeptide spectral matches across all cell conditions were O-GlcNAc-containing peptides (Fig. 3A). Across all samples, over 3000 unique O-GlcNAc modified peptides were identified from 466 proteins and nearly 700 unambiguous sites of O-GlcNAcylation were assigned. When normalized to total spectra, no large differences were apparent between cell conditions (Fig. 3B).
3.

O-GlcNAcylation in MEF cells. A) O-GlcNAc-modified peptides represent nearly 90% of the glycopeptide PSMs across all backgrounds and treatments from glycopeptide-enriched cytosol. B) O-GlcNAc PSM abundance remained relatively equal across the different samples. C) Network map of O-GlcNAcylated proteins with the Uniprot keyword “Ubl Conjugation Pathway.” The outer ring color represents MG132 treatment while the inner circle color represents genetic background of the sample.
Network and enrichment analysis of the O-GlcNAc dataset identified clustered networks involved in many cellular processes including transcriptional regulation, mRNA processing, nuclear and intracellular trafficking, and clathrinmediated endocytosis (Fig. S1, Supplemental Table 7). One such network contains proteins involved in the protein ubiquitination conjugation pathway (Fig. 3C). Nearly 40 percent of the identified O-GlcNAc modified proteins are related to ubiquitin conjugation (Uniprot keywords KW-0832 and KW-0833). Many were identified regardless of MG132 treatment or genetic background (Supplemental Table 7). Within this subset of proteins, at least one ERAD-associated protein, F-box only protein 6 (FBXO6), was shown to be O-GlcNAcylated. FBXO6 is part of an SCF complex (Skp, Cullin, F-box containing complex) which recognizes unfolded glycoproteins and targets them for degradation [36,37]. Although we only found O-GlcNAc-modified FBXO6 in EKO and DKO samples (Supplemental Table 4), further experimentation and analysis should be undertaken to determine the cellular mechanism of this modification and how it relates to NGLY1 expression.
General Discussion
Recent studies suggest NGLY1 is involved not only in the degradation of misfolded glycoproteins, but also activation of a transcription factor, NFE2L1 [31,32]. NFE2L1 is known to be involved in various cellular stress responses, including the proteasome recovery pathway upon inhibition of proteasome activity [38]. Accordingly, NGLY1-KO cells/animals exhibited sensitivity towards proteasome inhibitors [31–34]. It has been shown that NFE2L1-dependent NGLY1 function is independent from ENGase, as DKO MEF are as sensitive as NKO MEF to proteasome inhibitors [39]. While the contribution of N-GlcNAc protein accumulation to the pathophysiology of NGLY1-deficiency remains unclear, it is important to note that we can conclude that almost all the cytosolic N-GlcNAc proteins are formed by the action of ENGase.
Part of the N-GlcNAc hypothesis proposes that the presence of N-GlcNAc proteins may affect pre-existing O-GlcNAc mediated signaling. Our analysis showed little change in the number of O-GlcNAc proteins. It remains to be investigated whether the amount or localization of a subset of specific O-GlcNAc proteins may be altered. Our analysis also showed little difference in the level of ubiquitinated proteins between WT and NKO cells, with or without proteasome inhibitortreatment. This result is consistent with the recent observation that increase in the level of polyubiquitinated proteins are not observed in Ngly1-KO liver tissue [39] and ubiquitin-positive aggregates are only seen in limited tissue in Ngly1-KO rat [40]. We can therefore safely assume that accumulation of ubiquitinated proteins may be distinct among different tissues and cells. Further studies will help clarify how the accumulation of N-GlcNAc proteins impact the pathophysiology of NGLY1 deficiency.
Supplementary Material
1. String network and enrichment analysis of O-GlcNAcylated proteins across all MEF cell lines and treatments. Many cellular processes including transcriptional regulation, mRNA processing (pre-mRNA processing, mRNA decay, and splicing), nuclear and intracellular trafficking, and clathrin mediated endocytosis are clustered within these proteins.
Highlights.
In Ngly1-KO, ENGase is responsible for the formation of cytosolic N-GlcNAc proteins
Knockout of both Ngly1 and Engase decreases the level of cytosolic N-GlcNAc proteins
Identified 3000 unique O-GlcNAcylated peptides and 695 unambiguous O-GlcNAc sites
Acknowledgments
This work was partially supported by Mr. Hiroshi Mikitani (Rakuten Inc., Tokyo, Japan), Toray Science Foundation, Grace Science Foundation, RIKEN Pioneering Research Project (“Glycolipidologue Initiative”) (to TS), and Grant-in-Aids for Scientific Research (grant no. 26110725 to TS; 16K18520 to HF) from the MEXT, Japan. JCM, GD, and ALB were supported by the Dr. Miriam And Sheldon G. Adelson Medical Research Foundation (AMRF), the NIH (P41GM103481 and 1S10OD016229), and the Howard Hughes Medical Institute.
(1) All third-party financial support for the work in the submitted manuscript.
(2) All financial relationships with any entities that could be viewed as relevant to the general area of the submitted manuscript.
(3) All sources of revenue with relevance to the submitted work who made payments to you, or to your institution on your behalf, in the 36 months prior to submission.
(4) Any other interactions with the sponsor of outside of the submitted work should also be reported. (5) Any relevant patents or copyrights (planned, pending, or issued).
(6) Any other relationships or affiliations that may be perceived by readers to have influenced, or give the appearance of potentially influencing, what you wrote in the submitted work. As a general guideline, it is usually better to disclose a relationship than not.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DKO: Ngly1/Engase-knockout, EKO: Engase-knockout, ENGase: endo-β-N-acetylglucosaminidase, ERAD: Endoplasmic reticulum-associated degradation, ETD: Electron transfer dissociation, FBXO6: F-box only protein 6, GalNAc: N-Acetylgalactosamine, GlcNAc: N-Acetylglucosamine, gPSM: Glycopeptide spectral match, HCD: Higher energy collisional dissociation, HexNAc: N-Acetylhexosamine, LWAC: Lectin weak affinity chromatography, NFE2L1: Nuclear factor erythroid derived 2 like 1, NGLY1: Peptide-N(4)-(N-acetyl-beta-glucosaminyl)asparagine amidase, NKO: Ngly1-knockout, PNGase: cytosolic peptide:N-glycanase, PSM: Peptide spectral match, RTA: Ricin toxin A chain, SCF: Skp, Cullin, F-box containing, WGA: Wheat germ agglutinin
Declaration of competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Wu X; Rapoport TA Mechanistic insights into ER-associated protein degradation. Curr. Opin. Cell Biol 2018, 53, 22–28, doi: 10.1016/j.ceb.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roth J; Zuber C Quality control of glycoprotein folding and ERAD: the role of N-glycan handling, EDEM1 and OS-9. Histochem. Cell Biol 2017, 147, 269–284, doi: 10.1007/s00418-016-1513-9. [DOI] [PubMed] [Google Scholar]
- 3.Suzuki T; Huang C; Fujihira H The cytoplasmic peptide:N-glycanase (NGLY1) - Structure, expression and cellular functions. Gene 2016, 577, 1–7, doi: 10.1016/j.gene.2015.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suzuki T; Seko A; Kitajima K; Inoue Y; Inoue S Identification of peptide:N-glycanase activity in mammalianderived cultured cells. Biochem. Biophys. Res. Commun 1993, 194, 1124–1130, doi: 10.1006/bbrc.1993.1938. [DOI] [PubMed] [Google Scholar]
- 5.Suzuki T; Seko A; Kitajima K; Inoue Y; Inoue S Purification and enzymatic properties of peptide:N-glycanase from C3H mouse-derived L-929 fibroblast cells. Possible widespread occurrence of post-translational remodification of proteins by N-deglycosylation. J. Biol. Chem 1994, 269, 17611–17618. [PubMed] [Google Scholar]
- 6.Suzuki T; Kwofie MA; Lennarz WJ Ngly1, a mouse gene encoding a deglycosylating enzyme implicated in proteasomal degradation: expression, genomic organization, and chromosomal mapping. Biochem. Biophys. Res. Commun 2003, 304, 326–332, doi: 10.1016/s0006-291x(03)00600-4. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki T; Park H; Hollingsworth NM; Sternglanz R; Lennarz WJ PNG1, a yeast gene encoding a highly conserved peptide:N-glycanase. J. Cell Biol 2000, 149, 1039–1052, doi: 10.1083/jcb.149.5.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Need AC; Shashi V; Hitomi Y; Schoch K; Shianna KV; McDonald MT; Meisler MH; Goldstein DB Clinical application of exome sequencing in undiagnosed genetic conditions. J. Med. Genet 2012, 49, 353–361, doi: 10.1136/jmedgenet-2012-100819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Enns GM; Shashi V; Bainbridge M; Gambello MJ; Zahir FR; Bast T; Crimian R; Schoch K; Platt J; Cox R; et al. Mutations in NGLY1 cause an inherited disorder of the endoplasmic reticulum-associated degradation pathway. Genet. Med 2014, 16, 751–758, doi: 10.1038/gim.2014.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lam C; Ferreira C; Krasnewich D; Toro C; Latham L; Zein WM; Lehky T; Brewer C; Baker EH; Thurm A; et al. Prospective phenotyping of NGLY1-CDDG, the first congenital disorder of deglycosylation. Genet. Med 2017, 19, 160–168, doi: 10.1038/gim.2016.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujihira H; Masahara-Negishi Y; Tamura M; Huang C; Harada Y; Wakana S; Takakura D; Kawasaki N; Taniguchi N; Kondoh G; et al. Lethality of mice bearing a knockout of the Ngly1-gene is partially rescued by the additional deletion of the Engase gene. PLOS Genet. 2017, 13, e1006696, doi: 10.1371/journal.pgen.1006696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suzuki T; Yano K; Sugimoto S; Kitajima K; Lennarz WJ; Inoue S; Inoue Y; Emori Y Endo-β-N-acetylglucosaminidase, an enzyme involved in processing of free oligosaccharides in the cytosol. Proc. Natl. Acad. Sci 2002, 99, 9691–9696, doi: 10.1073/pnas.152333599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suzuki T Catabolism of N-glycoproteins in mammalian cells: Molecular mechanisms and genetic disorders related to the processes. Mol. Aspects Med 2016, 51, 89–103, doi: 10.1016/j.mam.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 14.Huang C; Harada Y; Hosomi A; Masahara-Negishi Y; Seino J; Fujihira H; Funakoshi Y; Suzuki T; Dohmae N; Suzuki T Endo-β-N-acetylglucosaminidase forms N-GlcNAc protein aggregates during ER-associated degradation in Ngly1-defective cells. Proc. Natl. Acad. Sci. U. S. A 2015, 112, 1398–1403, doi: 10.1073/pnas.1414593112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chalkley RJ; Thalhammer A; Schoepfer R; Burlingame AL Identification of protein O-GlcNAcylation sites using electron transfer dissociation mass spectrometry on native peptides. Proc. Natl. Acad. Sci. U. S. A 2009, 106, 8894–8899, doi: 10.1073/pnas.0900288106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trinidad JC; Barkan DT; Gulledge BF; Thalhammer A; Sali A; Schoepfer R; Burlingame AL Global identification and characterization of both O-GlcNAcylation and phosphorylation at the murine synapse. Mol. Cell. Proteomics 2012, 11, 215–229, doi: 10.1074/mcp.O112.018366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trinidad JC; Schoepfer R; Burlingame AL; Medzihradszky KF N- and O-Glycosylation in the Murine Synaptosome. Mol. Cell. Proteomics 2013, 12, 3474–3488, doi: 10.1074/mcp.M113.030007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim Y-C; Jahren N; Stone MD; Udeshi ND; Markowski TW; Witthuhn BA; Shabanowitz J; Hunt DF; Olszewski NE Identification and Origin of N-Linked β-d-N-Acetylglucosamine Monosaccharide Modifications on Arabidopsis Proteins. Plant Physiol. 2013, 161, 455–464, doi: 10.1104/pp.112.208900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim S; Maynard JC; Sasaki Y; Strickland A; Sherman DL; Brophy PJ; Burlingame AL; Milbrandt J Schwann Cell O-GlcNAc Glycosylation Is Required for Myelin Maintenance and Axon Integrity. J. Neurosci 2016, 36, 9633–9646, doi: 10.1523/JNEUROSCI.1235-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malaker SA; Penny SA; Steadman LG; Myers PT; Loke JC; Raghavan M; Bai DL; Shabanowitz J; Hunt DF; Cobbold M Identification of Glycopeptides as Posttranslationally Modified Neoantigens in Leukemia. Cancer Immunol. Res 2017, 5, 376–384, doi: 10.1158/2326-6066.CIR-16-0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stephens SB; Dodd RD; Lerner RS; Pyhtila BM; Nicchitta CV Analysis of mRNA partitioning between the cytosol and endoplasmic reticulum compartments of mammalian cells. Methods Mol. Biol. Clifton NJ 2008, 419, 197–214, doi: 10.1007/978-1-59745-033-1_14. [DOI] [PubMed] [Google Scholar]
- 22.Udeshi ND; Mertins P; Svinkina T; Carr SA Large-scale identification of ubiquitination sites by mass spectrometry. Nat. Protoc 2013, 8, 1950–1960, doi: 10.1038/nprot.2013.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Halim A; Westerlind U; Pett C; Schorlemer M; Rüetschi U; Brinkmalm G; Sihlbom C; Lengqvist J; Larson G; Nilsson J Assignment of Saccharide Identities through Analysis of Oxonium Ion Fragmentation Profiles in LC-MS/MS of Glycopeptides. J. Proteome Res 2014, 13, 6024–6032, doi: 10.1021/pr500898r. [DOI] [PubMed] [Google Scholar]
- 24.Baker PR; Chalkley RJ MS-viewer: a web-based spectral viewer for proteomics results. Mol. Cell. Proteomics 2014, 13, 1392–1396, doi: 10.1074/mcp.O113.037200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lynch JM; Maillet M; Vanhoutte D; Schloemer A; Sargent MA; Blair NS; Lynch KA; Okada T; Aronow BJ; Osinska H; et al. A thrombospondin-dependent pathway for a protective ER stress response. Cell 2012, 149, 1257–1268, doi: 10.1016/j.cell.2012.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gundry RL; Raginski K; Tarasova Y; Tchernyshyov I; Bausch-Fluck D; Elliott ST; Boheler KR; Van Eyk JE; Wollscheid B The mouse C2C12 myoblast cell surface N-linked glycoproteome: identification, glycosite occupancy, and membrane orientation. Mol. Cell. Proteomics 2009, 8, 2555–2569, doi: 10.1074/mcp.M900195-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim W; Bennett EJ; Huttlin EL; Guo A; Li J; Possemato A; Sowa ME; Rad R; Rush J; Comb MJ; et al. Systematic and quantitative assessment of the ubiquitin modified proteome. Mol. Cell 2011, 44, 325–340, doi: 10.1016/j.molcel.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruan L; Zhou C; Jin E; Kucharavy A; Zhang Y; Wen Z; Florens L; Li R Cytosolic proteostasis through importing of misfolded proteins into mitochondria. Nature 2017, 543, 443–446, doi: 10.1038/nature21695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kong J; Peng M; Ostrovsky J; Kwon YJ; Oretsky O; McCormick EM; He M; Argon Y; Falk MJ Mitochondrial function requires NGLY1. Mitochondrion 2018, 38, 6–16, doi: 10.1016/j.mito.2017.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang K; Huang R; Fujihira H; Suzuki T; Yan N N-glycanase NGLY1 regulates mitochondrial homeostasis and inflammation through NRF1. J. Exp. Med 2018, 215, 2600–2616, doi: 10.1084/jem.20180783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lehrbach NJ; Ruvkun G Proteasome dysfunction triggers activation of SKN-1A/Nrf1 by the aspartic protease DDI-1. eLife 2016, 5, doi: 10.7554/eLife.17721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tomlin FM; Gerling-Driessen UIM; Liu Y-C; Flynn RA; Vangala JR; Lentz CS; Clauder-Muenster S; Jakob P; Mueller WF; Ordoñez-Rueda D; et al. Inhibition of NGLY1 Inactivates the Transcription Factor Nrf1 and Potentiates Proteasome Inhibitor Cytotoxicity. ACS Cent. Sci 2017, 3, 1143–1155, doi: 10.1021/acscentsci.7b00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodriguez TP; Mast JD; Hartl T; Lee T; Sand P; Perlstein EO Defects in the Neuroendocrine Axis Contribute to Global Development Delay in a Drosophila Model of NGLY1 Deficiency. G3 Bethesda Md 2018, 8, 2193–2204, doi: 10.1534/g3.118.300578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lehrbach NJ; Breen PC; Ruvkun G Protein Sequence Editing of SKN-1A/Nrf1 by Peptide:N-Glycanase Controls Proteasome Gene Expression. Cell 2019, 177, 737–750.e15, doi: 10.1016/j.cell.2019.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vosseller K; Trinidad JC; Chalkley RJ; Specht CG; Thalhammer A; Lynn AJ; Snedecor JO; Guan S; Medzihradszky KF; Maltby DA; et al. O-Linked N-Acetylglucosamine Proteomics of Postsynaptic Density Preparations Using Lectin Weak Affinity Chromatography and Mass Spectrometry. Mol. Cell. Proteomics 2006, 5, 923–934, doi: 10.1074/mcp.T500040-MCP200. [DOI] [PubMed] [Google Scholar]
- 36.Yoshida Y; Tokunaga F; Chiba T; Iwai K; Tanaka K; Tai T Fbs2 Is a New Member of the E3 Ubiquitin Ligase Family That Recognizes Sugar Chains. J. Biol. Chem 2003, 278, 43877–43884, doi: 10.1074/jbc.M304157200. [DOI] [PubMed] [Google Scholar]
- 37.Yoshida Y; Adachi E; Fukiya K; Iwai K; Tanaka K Glycoprotein-specific ubiquitin ligases recognize N-glycans in unfolded substrates. EMBO Rep. 2005, 6, 239–244, doi: 10.1038/sj.embor.7400351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Radhakrishnan SK; Lee CS; Young P; Beskow A; Chan JY; Deshaies RJ Transcription factor Nrf1 mediates the proteasome recovery pathway after proteasome inhibition in mammalian cells. Mol. Cell 2010, 38, 17–28, doi: 10.1016/j.molcel.2010.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fujihira H; Masahara-Negishi Y; Akimoto Y; Hirayama H; Lee H-C; Story BA; Mueller WF; Jakob P; Clauder-Münster S; Steinmetz LM; et al. Liver-specific deletion of Ngly1 causes abnormal nuclear morphology and lipid metabolism under food stress. Biochim. Biophys. Acta Mol. Basis Dis 2020, 1866, 165588, doi: 10.1016/j.bbadis.2019.165588. [DOI] [PubMed] [Google Scholar]
- 40.Asahina M; Fujinawa R; Nakamura S; Yokoyama K; Tozawa R; Suzuki T Ngly1−/− rats develop neurodegenerative phenotypes and pathological abnormalities in their peripheral and central nervous systems. Hum. Mol. Genet 2020, doi: 10.1093/hmg/ddaa059. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1. String network and enrichment analysis of O-GlcNAcylated proteins across all MEF cell lines and treatments. Many cellular processes including transcriptional regulation, mRNA processing (pre-mRNA processing, mRNA decay, and splicing), nuclear and intracellular trafficking, and clathrin mediated endocytosis are clustered within these proteins.


