Abstract
OBJECTIVES:
Analyses performed by the Sarcopenia Definitions and Outcomes Consortium (SDOC) identified cutpoints in several metrics of grip strength for consideration in a definition of sarcopenia. We describe the associations between the SDOC-identified metrics of low grip strength (absolute or standardized to body size/composition); low dual-energy x-ray absorptiometry (DXA) lean mass as previously defined in the literature (appendicular lean mass [ALM]/ht2); and slowness (walking speed <.8 m/s) with subsequent adverse outcomes (falls, hip fractures, mobility limitation, and mortality).
DESIGN:
Individual-level, sex-stratified pooled analysis. We calculated odds ratios (ORs) or hazard ratios (HRs) for incident falls, mobility limitation, hip fractures, and mortality. Follow-up time ranged from 1 year for falls to 8.8 ± 2.3 years for mortality.
SETTING:
Eight prospective observational cohort studies.
PARTICIPANTS:
A total of 13,421 community-dwelling men and 4,828 community-dwelling women.
MEASUREMENTS
Grip strength by hand dynamometry, gait speed, and lean mass by DXA.
RESULTS:
Low grip strength (absolute or standardized to body size/composition) was associated with incident outcomes, usually independently of slowness, in both men and women. ORs and HRs generally ranged from 1.2 to 3.0 for those below vs above the cut-point. DXA lean mass was not consistently associated with these outcomes. When considered together, those who had both muscle weakness by absolute grip strength (<35.5 kg in men and <20 kg in women) and slowness were consistently more likely to have a fall, hip fracture, mobility limitation, or die than those without either slowness or muscle weakness.
CONCLUSION:
Older men and women with both muscle weakness and slowness have a higher likelihood of adverse health outcomes. These results support the inclusion of grip strength and walking speed as components in a summary definition of sarcopenia.
Keywords: sarcopenia, grip strength, gait speed, hip fracture, mobility limitation
Many definitions of sarcopenia have been proposed.1-4 Early definitions relied on lean mass alone, whereas more recent definitions have incorporated strength and performance measures in addition to lean mass. Given the previous work in this field, why is a new definition of sarcopenia warranted? There are several important motivating factors for the work in this article and accompanying manuscripts of the Sarcopenia Definitions and Outcomes Consortium (SDOC).5-10
First, aside from the effort sponsored by the Foundation for the National Institutes of Health (FNIH), all other consensus definitions have largely relied on expert opinion rather than data-driven approaches.1-4 Second, all previous approaches to define sarcopenia presupposed that lean mass is an important metric required for defining sarcopenia despite the fact that lean mass has not been consistently related to poor outcomes in older adults. Assessment of lean mass is somewhat costly and burdensome, particularly in resource-poor settings, because it requires the use of expensive imaging machinery with exposure to ionizing radiation. Therefore, lean mass should only be included in sarcopenia definitions if it meaningfully predicts outcomes. Additionally, inclusion of a measure that is not associated with the risk of clinically meaningful outcomes in a composite definition will add noise to the data, potentially increasing measurement error. Finally, the development of previous sarcopenia definitions was done with little input from the outside scientific community. The SDOC approach addressed this shortcoming by holding a Position Development Conference to review the data and interpret the findings. The summary of the SDOC Position Development Conference is published elsewhere.5,11
Thus to address the need for a refined definition of sarcopenia, in 2016 the SDOC was formed to complete analyses and inform an updated operational definition of sarcopenia. The SDOC is a collaboration among content experts and many cohort studies and clinical populations.5 The main results of the efforts of the SDOC project are reported in a series of concurrent reports. In the first stage of the analyses for the SDOC, reported by Manini et al,8 we completed a pooled analysis of several cohort studies and primarily utilized classification and regression trees (CART) and receiver operator characteristics including area under the curve as tools to identify variables (and cut-points in these variables) that best discriminated older adults with slowness (objectively defined as usual walking speed <.8 m/s) from those without (Table 1). The primary discriminators of slowness identified in those analyses were all metrics of grip strength, either the absolute value or standardized to body size or composition. However, whether these variables (and the cut-points derived for them) in the CART models are predictive of subsequent adverse health outcomes such as falls, hip fractures, mobility limitation, and mortality (independent of slowness) in older adults is unknown.
Table 1.
Putative Sarcopenia Variables and Their Cut-Points
| Men | Women | |
|---|---|---|
| Primary variables: identified by SDOC CART analysis8 | ||
| Grip maximum value | <35.5 kg | <20 kga |
| Grip strength/BMI | <1.05 kg/kg/m2,b | <.79 kg/kg/m2 |
| Grip strength/TBF | <1.66 kg/kga | <.65 kg/kg |
| Grip/Arm lean mass | <6.08 kg/kga | <3.26 kg/kg |
| Grip/Weight | <.45 kg/kga | <.337 kg/kg |
| Secondary lean mass variables identified by previous efforts | ||
| ALM/ht2,12 | 7.26 kg/m2 | 5.45 kg/m2 |
| ALM13 | <19.75 kg | 15.02 kg |
Abbreviations: ALM, appendicular lean mass; BMI, body mass index; CART, classification and regression tree analysis; SDOC, Sarcopenia Definitions and Outcomes Consortium; TBF, total body fat.
From Youden’s index.
This cut-point is from the primary CART analyses; another cut-point of Grip/BMI <1.46 was found for men with secondary walking speed outcome.
Thus in this next stage of analysis using a subset of SDOC data from cohort studies of older adults, we had several objectives. First, we evaluated the association between the grip strength variables (with or without standardization for body size or composition) identified in the SDOC CART analyses with incident clinical outcomes of falls, self-reported mobility limitation, hip fracture, and mortality. The SDOC CART models did not identify dual-energy x-ray absorptiometry (DXA)-derived lean mass measures as important discriminators of slowness. Nonetheless, because many definitions of sarcopenia based on expert opinion include DXA lean mass and to allow full consideration of the association between DXA-derived measures of lean mass with these adverse outcomes in the SDOC analyses, we also included commonly used measures of DXA-derived lean mass in our analyses as potential risk factors for these adverse outcomes.
Second, for absolute grip strength measure, we determined whether the associations with these outcomes were independent of walking speed. Third, we quantified whether participants with low walking speed alone (without low grip strength), low grip strength alone (without low walking speed), or their combination (both low walking speed and low grip strength) had a greater likelihood of clinical outcomes than those who had neither slow walking speed nor low grip strength.
METHODS
Study Population
We used data from eight cohort studies participating in the SDOC (Supplementary Tables S1 and S2). These studies were described extensively elsewhere; each study includes community-dwelling older adults. Analyses were limited to older adults aged 65 years and older who had data on body composition measured by DXA, grip strength, and walking speed. Assessment of grip strength and walking speed are described in the Supplementary Methods and Supplementary Tables S3 and S4.
Defining Slowness and Muscle Weakness
In our main analyses, slowness was defined as walking speed less than .8 m/s and muscle weakness as absolute grip strength less than 20 kg in women or 35.5 kg in men.
Adverse Health Outcomes
In all cohorts, we harmonized data for falls, death, hip fracture, and self-reported incident mobility limitation. Falls were analyzed as one or more self-reported falls in the year after the sarcopenia assessment. Details regarding assessment of falls, deaths, self-reported mobility limitation, and hip fracture are reported in the Supplementary Methods and Supplementary Tables S5, S6, S7, and S8.
Statistical Analysis
Means plus or minus standard deviation and number (%) for selected characteristics are presented by category of grip strength for men and women separately. The current analysis evaluated the primary nodes of the SDOC CART analyses (that were all measures of grip strength with or without standardization to body size). Measures of lean mass as historically presented in literature were also evaluated despite not being selected by CART. Each predictor was defined as “low” (below cut-point) vs not. Given known sex differences in walking speed, strength, and body size, analyses were stratified by sex. We calculated odds ratios (ORs) for incident falls and incident self-reported mobility limitation for the sarcopenia predictors identified in Table 1 using logistic regression.
These metrics and cut-points were derived by Manini et al8 from both primary and secondary CART models (and logistic models using Youden’s index when CART did not identify a cut-point for a particular metric). We used proportional hazards models to estimate the hazard ratio for mortality and hip fractures. In sensitivity models for hip fracture, we accounted for the influence of the competing risk of mortality using the Fine-Gray extension to Cox models.14 We decided a priori to include covariates (age, self-rated health, pain, use of statins, cognitive function, cancer, congestive heart failure, stroke, chronic obstructive pulmonary disease, and diabetes, plus bone mineral density for hip fracture models) based on the factors that are most likely to confound the association between the putative sarcopenia variables.
Methods and description of the harmonization of these covariates across cohorts is reported in the Supplementary Methods and Supplementary Table S9. Separate models were run for each putative sarcopenia predictor variable. We also ran models that combined muscle weakness (defined by grip strength cut-points, not adjusted for body size) and walking speed as a four-category variable (muscle weakness and slowness; not weak but slow; weak but not slow; and not weak and not slow that served as the referent group). Further, for each outcome, we included both slowness (defined by walking speed <.8 m/s) and muscle weakness (defined by absolute grip strength cut-points) in a single model adjusted for the covariates listed earlier to test whether slowness and muscle weakness predict outcomes independent of each other. Finally, for each outcome, we tested the interaction between slowness (defined by walking speed <.8 m/s) and muscle weakness (defined by absolute grip strength cut-points) to determine if the effect of muscle weakness varied by the presence or absence of slowness (and vice versa). All hypotheses were tested using a two-sided α level of .05. Analyses were completed in SAS v.9.4 (Cary, NC).
RESULTS
The total number of participants and events by study are reported in Supplementary Table S2 (13,421 men and 4,828 women); follow-up time for each cohort and measure are noted in the supplementary materials. Characteristics of participants by grip strength cut-points (based on absolute maximum grip strength) are reported in Supplementary Table S10. For both men and women, those classified with low grip strength were older, weighed less, had lower DXA appendicular lean mass (ALM) and DXA ALM/ht2 than those with higher grip strength (P < .001 for all). Moreover, for both men and women, a greater percentage of participants with muscle weakness (low grip strength) had an incident adverse outcome (mortality, fall, hip fracture, and self-reported mobility limitation) than did those without muscle weakness (P < .05 for all).
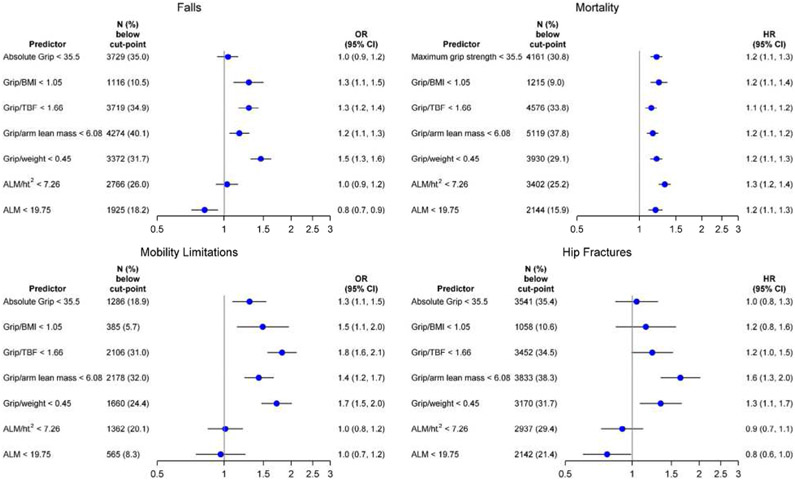
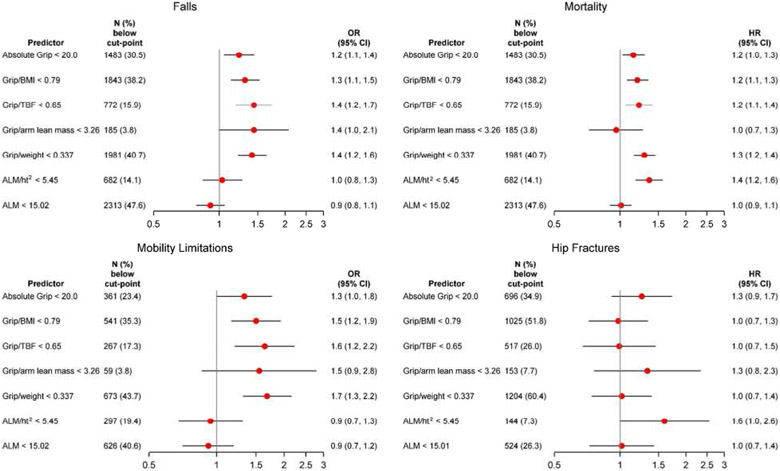
In multivariate models, we estimated the likelihood of falls and self-reported mobility limitation and the risk of mortality and hip fracture separately for men and women (Figures 1 and 2). In general, participants with low grip strength (absolute or with adjustment for body size) were more likely to experience an incident fall, self-reported mobility limitation, hip fracture, or death than those without. In contrast, DXA-derived measures of lean mass were not consistently associated with these outcomes. In women, only ALM/ht2 was significantly associated with mortality but no other outcomes. In men, ALM/ht2 and ALM were associated with mortality. In men, the significant association of low ALM and falls or hip fractures was in an opposite to what may be hypothesized (ie, low absolute ALM was protective against falls and hip fractures).There was no association with other outcomes in men.
Figure 1.
Association (odds ratio [OR] or hazards ratio [HR], 95% confidence interval [CI]) of putative sarcopenia variables and adverse outcomes in the Sarcopenia Definitions and Outcomes Consortium cohorts in men. Models are adjusted for age, self-rated health, pain, use of statins, cognitive function, cancer, congestive heart failure, stroke, chronic obstructive pulmonary disease. and diabetes. Hip fracture model is additionally adjusted for bone mineral density. Falls model includes 9,994 men (1,987 [19.9%] falls); mortality model includes 12,856 men (4,109 [32%] deaths); mobility limitation model includes 6,505 men (888 [13.7%] mobility limitation); and the hip fracture model includes 9,512 men (392 [4.1%] hip fractures). ALM, appendicular lean mass; BMI, body mass index; TBF, total body fat.
Figure 2.
Association (odds ratio [OR] or hazards ratio [HR], 95% confidence interval [CI]) of putative sarcopenia variables and adverse outcomes in the Sarcopenia Definitions and Outcomes Consortium cohorts in women. Models are adjusted for age, self-rated health, pain, use of statins, cognitive function, cancer, congestive heart failure, stroke, chronic obstructive pulmonary disease, and diabetes. Hip fracture model is additionally adjusted for bone mineral density. Falls model includes 4,551 women (913 [20.1%] falls]); mortality model includes 4,736 women (1,258 [26.6%] deaths); mobility limitation model includes 1,500 women (344 ]22.9%] mobility limitation); and the hip fracture model includes 1,745 women (166 ]9.5%] hip fractures). ALM, appendicular lean mass; BMI, body mass index; TBF, total body fat.
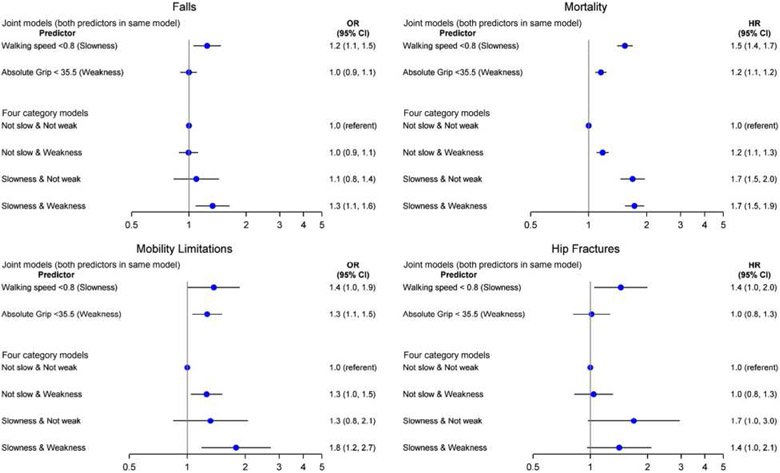
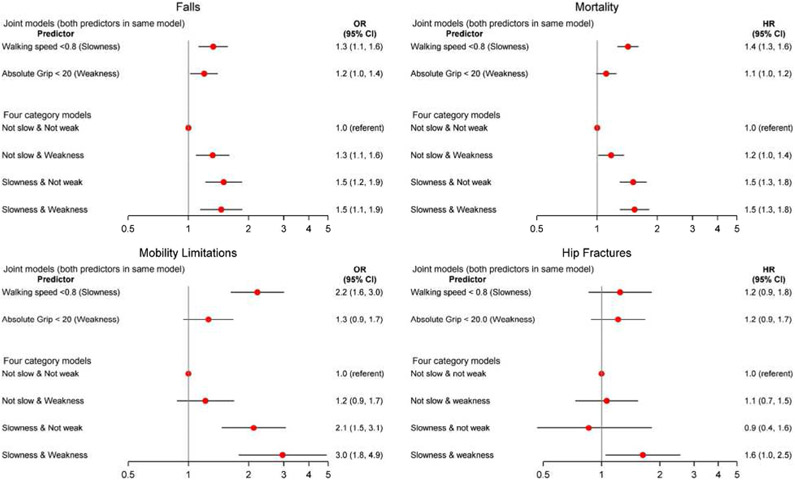
Figures 3 and 4 display the association between slowness (walking speed<.8 m/s) and muscle weakness (grip strength <20 kg for women and <35.5 kg for men), alone and in combination, for the outcomes of falls, mortality, hip fractures, and mobility limitation in men and women. The joint models are those that included both slowness and muscle weakness in the same model. In men, these models indicate that slowness was associated with all outcomes independently of grip strength. In women, slowness was associated with all outcomes (except hip fracture) independently of grip strength. These models also indicate that in men, muscle weakness was associated with mortality and mobility limitation (but not falls and hip fractures) independently of slowness. In women, muscle weakness was associated with falls (but not mortality, mobility imitation, and hip fractures) independently of slowness.
Figure 3.
Association (odds ratio [OR], 95% confidence interval [CI]) between grip strength and walking speed combinations and falls, mortality, mobility limitation, and hip fractures in men. Models are adjusted for age, self-rated health, pain, use of statins, cognitive function, cancer, congestive heart failure, stroke, chronic obstructive pulmonary disease, and diabetes. Hip fracture model additionally adjusted for bone mineral density. Falls model includes 9,994 men (1987 [19.9%] falls); mortality model includes 12,856 men (4,109 [32%] deaths); mobility limitation model includes 6,505 men (888 [13.7%] mobility limitation); and the hip fracture model includes 9,512 men (392 [4.1%] hip fractures). Interaction between slowness and muscle weakness was not significant for any model (P > .010).
Figure 4.
Association (odds ratio [OR], 95% confidence interval [CI]) between grip strength and walking speed combinations and falls, mortality, mobility limitation, and hip fractures in women. Models are adjusted for age, self-rated health, pain, use of statins, cognitive function, cancer, congestive heart failure, stroke, chronic obstructive pulmonary disease, and diabetes. Hip fracture model additionally adjusted for bone mineral density. Falls model includes 4,551 women ([913] 20.1% falls); Mortality model includes 4,736 women (1,258 [26.6%] deaths); mobility limitation model includes 1,500 women (344 [22.9%] mobility limitation); and the hip fracture model includes 1,745 women (166 [9.5%] hip fractures). Interaction between slowness and muscle weakness was significant for falls (P = .039), borderline significant for hip fracture (P = .076), and not significant for mobility limitation and mortality (P > .10).
When slowness and muscle weakness were combined into a four-level variable, for both men and women, those with slowness and muscle weakness were consistently more likely to experience falls, mobility limitation, and mortality than those who were not slow and not weak (although the association for those with slowness and muscle weakness for hip fracture did not reach statistical significance in men). The pattern of association between those who were slow (but not weak) and those who were weak (but not slow) varied by sex and outcome. The interaction between slowness and muscle weakness was not significant for any outcome in men, but it was significant for falls in women. Cohort-specific analyses and associations with alternative grip strength metrics/cut-points are described in Supplementary Table S11 and Supplementary Figures S1 and S2. Accounting for the competing risk of mortality did not materially alter results (data not shown).
DISCUSSION
We found that low grip strength (either the absolute value or the ratio of grip strength to body size or composition) was associated with incident outcomes of falls, mobility limitation, hip fracture, and mortality in both men and women. Slowness was also associated with these outcomes, and in general both muscle weakness and slowness were independent predictors of these outcomes. In contrast, DXA lean mass (analyzed as ALM or ALM/ht2) was not associated consistently with these outcomes. Our data served as the foundation for the development and consideration of the SDOC position statements described by Bhasin et al5 and as a basis for the SDOC’s recommended definition of sarcopenia.
Similar to other more recently proposed definitions of sarcopenia,3 the analyses here and the broader SDOC position statements5 support the inclusion of grip strength as a component of a definition of sarcopenia. Grip strength has long been considered an important measure of health15-20 and been included in most of the proposed definitions of sarcopenia.3,21,22 Grip strength is a measure of upper body strength, and previous studies suggest it is reasonably correlated with total body or lower extremity strength.23 Furthermore, grip strength can be measured easily and inexpensively in clinical and research settings, especially when compared with the complexity of measuring lower body strength and power.24 This is evidenced by the fact that although all of the cohort studies in this analysis collected measures of grip strength, there was not a single shared protocol for assessment of lower extremity strength or power across these studies. We acknowledge that lower extremity strength impairments may be a stronger predictor of slowness, particularly among those with less baseline impairment.23,25-28 However, assessment of lower extremity strength is expensive and time consuming, making it unlikely that currently available protocols could become widely available for research or clinical use.
Also, similar to previous reports,29,30 our results demonstrate a consistent association between slow walking speed and poor outcomes in older adults. Thus our data also support inclusion of walking speed as another component of the definition of sarcopenia. Similar to grip strength, walking speed is relatively simple to assess and has been used in both clinic- and home-based settings.30 Men and women with both slowness and muscle weakness together were consistently more likely to experience falls, mobility limitation, hip fracture, and mortality than those with neither slowness nor muscle weakness. However, our data showed some inconsistency between muscle weakness alone (without slowness) and slowness alone (without muscle weakness) in predicting all outcomes in both men and women.
Furthermore, although slow walking speed is a strong predictor of many outcomes, many causes of slowness are not due to muscle weakness; this fact was also noted in the SDOC position statements. Additional limitations of grip strength include equipment availability, potential variability among dynamometers, and difficulties of measurement in those with pain or arthritis. Nevertheless, incorporation of both slowness and muscle weakness into a single definition of sarcopenia may therefore identify those who are slow at least in part due to muscle weakness. Thus our results support the two position statements developed by the SDOC regarding the association of both slowness and muscle weakness with adverse outcomes, and the recommendation to include both of these factors in a summary definition of sarcopenia.5
Consistent with the SDOC CART analyses, our data do not show consistent associations between low lean mass and adverse outcomes in older adults.8 These results are largely comparable with the literature, with meta-analyses showing little to no effect of measures of DXA lean mass with adverse outcomes in older adults.31,32 Our data also closely align with the SDOC position statements that DXA-derived lean mass is not a good predictor of adverse outcomes in older adults, and they provide evidence for why the SDOC sarcopenia definition does not include measure of lean mass by DXA.5
At least two possible explanations are plausible for why DXA-derived measures of lean mass are not robustly associated with adverse outcomes. First, DXA does not measure muscle mass directly; rather it measures lean mass that is an approximation of muscle mass. DXA-derived lean mass includes not only muscle mass but also water and fibrotic tissue. Thus the measurement error inherent in DXA due to this inaccurate assessment of muscle may have biased the results we observed toward the null. Second, it is possible that muscle mass per se has no causal relation to these outcomes.
However, other methods to assess muscle mass that do not rely on the same assumptions as DXA, such as D3-creatine dilution,33 suggest that low muscle mass (when measured accurately) is associated with poor outcomes in older men. In addition, in older adults, measures of muscle cross-sectional area by computed tomography (CT) (centrally or in the periphery) are generally associated with poor outcomes (mortality, mobility limitation) while in the same population, measures of DXA-derived lean mass are not.34,35 In sum, these data suggest that muscle mass, when measured accurately, is likely related to poor outcomes in older people.
However, alternative approaches to measure muscle mass have some drawbacks: magnetic resonance imaging and CT are expensive and not widely available. CT additionally includes radiation exposure, and many older adults cannot have MR scans due to implanted metal or claustrophobia. The D3-creatine method holds promise,33 but data using this approach are limited, and it is not yet approved for routine clinical use.
The SDOC cut-points for muscle weakness derived from CART analyses are similar to approaches to define muscle weakness from US nationally representative populations. The Health and Retirement Survey found cut-points in grip strength of 39 kg in men (compared with 35.5 kg in the present analyses) and 22 kg in women (compared with 20 kg in the present analyses).20 The FNIH Sarcopenia Project found a much lower cut-point for muscle weakness (<26 kg) in men. For women, the FNIH Sarcopenia Project was a little lower than ours (<16 kg) but found an intermediate cutpoint for muscle weakness that was identical to our definition of muscle weakness.36
The newly revised European Working Group for Sarcopenia in Older Persons (EWGSOP2)3 uses grip strength cut-points of 16 kg for women and 27 kg for men, but it is based on distributional analyses of grip strength across the life course18 not on an evaluation of what level of grip strength is associated with performance (as was done in the present analyses). The EWGSOP2 definition also includes DXA lean mass. With our higher cut-points for both men and women, more adults would be classified as having muscle weakness by the SDOC definition than by the previous FNIH definition or the EWGSOP2 recommendations.
Given the results of these and parallel SDOC analyses, and the input of the International Expert Panel, the SDOC has ultimately defined sarcopenia as the presence of both muscle weakness (absolute grip strength <35.5 kg in men and <20 kg women) and slowness (walking speed <.8 m/s).5 This leads to the question: How exactly should our cutpoints be used? The SDOC definition for muscle weakness identifies a relatively large proportion of adults (particularly men) as weak. This relatively high prevalence is likely reasonable should the cut-points be used to identify older adults for relatively low cost and safe treatment such as resistance training (although the effect of resistance training on all of the outcomes described in this article have not been established conclusively).
However, the prevalence of muscle weakness by the current SDOC definition may be too high for a future hypothetical situation in which patients would be recommended for treatment with an (not yet developed) expensive medication with significant side effects to prevent an outcome such as fracture or disability. Further, it is unknown how the SDOC definition performs as eligibility criteria or as an outcome in randomized trials. Finally, whether the SDOC’s sarcopenia definition or the individual components of slowness or weakness improve prediction of events such as fractures, above and beyond existing risk factors (such as the Fracture Risk Assessment Tool model for fractures) is not formally evaluated in this article. This is the topic of separate ongoing investigations.37,38
As noted, the EWGSOP recently updated their recommended definition (referred to as EWGSOP2).3 In addition to issues previously described with inclusion of DXA lean mass in a sarcopenia definition, another limitation of the EWGSOP2 is that the definition is not exact. The EWGSOP2 algorithm allows many different measures to be substituted for each other, for each component of sarcopenia. For example, muscle strength may be assessed by either grip strength or chair stands, and physical performance may be assessed by gait speed, the Short Physical Performance Battery, Timed Up and Go, or the 400-m walk. This means the same person, at the same point in time, may be classified as completely normal or with severe sarcopenia, solely depending on the measures used in the algorithm for case finding rather than their true physical condition. Although the flexibility of this method is appealing from an ease-of-implementation perspective, the lack of precision in defining sarcopenia by the EWGSOP2 algorithm significantly limits its utility in clinical and research settings given these significant concerns about misclassification.
Strengths of our project include the large data set with similar assessments of grip strength and walking speed and end points across cohorts. As part of the larger SDOC project, we also evaluated the harmonization of DXA measures8 that did not materially change our results, demonstrating that our results cannot be explained by lack of DXA harmonization. However, a few limitations must be noted.
First, the participants in our studies were generally healthy community-dwelling older adults not selected for poor function or disease status. Further, although our analyses included a diverse population in terms of race and geographic location, the population was still largely whites residing in the United States. We did not run CART models to identify race-specific or geography-specific cut-points. Our results suggest a variation in the prevalence of muscle weakness by race, and it is unclear if separate cut-points should be developed for nonwhite populations or for different geographic locations. For the mobility limitation outcome, time between the sarcopenia assessment and report of subsequent mobility limitation varied across the cohorts. This could have introduced informative censoring, thereby biasing the effect estimates.
Further, the multivariate models in our report may not have fully accounted for confounding factors. This includes factors that were not measured at all in the studies or factors measured so differently across all studies as to preclude their inclusion in our models. For example, the studies varied so substantially in how physical activity was assessed that these differences made it impossible to include a similar term for physical activity for all studies in the models. Finally, further work is needed to facilitate the implementation of these measures in clinical settings including standardization of equipment and procedures, staff training for assessing grip strength and walking speed in clinical practice, and identifying and removing barriers to use with patients.
In summary, we found that muscle weakness defined by low absolute grip strength (<35.5 kg in men and <20 kg in women) and slowness defined by low walking speed (<.8 m/s) were generally associated with a higher likelihood of adverse health outcomes (falls, mobility limitation, hip fractures, and mortality) in older men and women. Likelihood of these outcomes was also consistently elevated for men and women who had both muscle weakness and slowness when compared with those who had neither condition. No consistent relationship was found between low DXA-derived lean mass and these outcomes in either men or women. These data support the summary SDOC definition of sarcopenia as the presence of both slowness and muscle weakness regardless of the level of DXA-derived lean mass.
Supplementary Material
Supplementary Table S1: Characteristics of included cohorts.
Supplementary Table S2: Studies included in analysis and frequency of outcomes by cohort.
Supplementary Table S3: Grip strength protocols.
Supplementary Table S4: Walking speed protocols.
Supplementary Table S5: Falls outcome protocol.
Supplementary Table S6: Mortality outcome protocol.
Supplementary Table S7: Hip fracture outcome protocol.
Supplementary Table S8: Self-reported incident mobility limitation protocol.
Supplementary Table S9: Covariate protocols.
Supplementary Table S10: Characteristics [N (%) or mean ± SD] of participants by categories of grip strength.
Supplementary Table S11: Association* of Grip/BMI <1.46 in men and clinical outcomes in the SDOC project.
Supplementary Figure S1: Association (odds ratio*, 95% CI) of grip strength and incident falls for each cohort individually.
Supplementary Figure S2: Association (hazard ratio, 95% CI) of grip strength and mortality for each cohort individually.
Supplemental Text S1: Financial Statement
ACKNOWLEDGMENTS
Financial Disclosure: The Sarcopenia Definitions and Outcomes Consortium (SDOC) is supported by the National Institute on Aging (NIA; grant number AG51421), the Foundation for the National Institutes of Health (FNIH; grant numbers CAWT16SARC2 and BHAS16SARC2), and the California Pacific Medical Center Foundation. This research was supported in part by the intramural research program at the NIA. Conflict of interest statements and funding information for the participating cohort studies can be found in Supplementary Material.
Footnotes
Sponsor’s Role: The sponsors of this work had no role in the analysis or interpretation of results and no role in the decision to publish these data.
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article.
REFERENCES
- 1.Fielding RA, Vellas B, Evans WJ, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International Working Group on Sarcopenia. J Am Med Dir Assoc. 2011;12(4):249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis. Age Ageing. 2010;39(4):412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen LK, Liu LK, Woo J, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc. 2014;15(2):95–101. [DOI] [PubMed] [Google Scholar]
- 5.Bhasin S, Travison TG, Manini TM, et al. Sarcopenia definition: the position statements of the sarcopenia definition and outcomes consortium. J Am Geriatr Soc. 2019. 10.1111/jgs.16372. [DOI] [PubMed] [Google Scholar]
- 6.Erlandson KM, Travison TG, Zhu H, et al. Application of selected muscle strength and body mass cut-points for the diagnosis of sarcopenia in men and women with or at risk for HIV infection. J Gerontol Ser A. 2020. 10.1093/gerona/glaa083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grosicki GJ, Travison TG, Zhu H, et al. Application of selected cut-points for low muscle strength and lean mass in mobility-limited older adults. J Am Geriatr Soc. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manini TM, Patel SM, Newman AB, et al. Identification of sarcopenia components that discriminate slow walking speed: a pooled data analysis from the Sarcopenia Definitions and Outcomes Consortium. J Am Geriatr Soc. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Orwig D, Travison TG, Zhu H, et al. Application of selected cut-points for low muscle strength and lean mass for recovery of gait speed after hip fracture. J Gerontol Med Sci. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel SM, Duchowny KA, Kiel DP, et al. Performance of weakness using SDOC cut points in two nationally representative population-based cohorts. J Am Geriatr Soc. In press. [Google Scholar]
- 11.Cawthon PM, Travison TG, Manini TM, et al. Establishing the link between lean mass and grip strength cut-points with mobility disability and other health outcomes: proceedings of the Sarcopenia Definition and Outcomes Consortium conference. J Gerontol A Biol Sci Med Sci. 2019. 10.1093/gerona/glz081.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baumgartner RN, Koehler KM, Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147(8):755–763. [DOI] [PubMed] [Google Scholar]
- 13.Cawthon PM, Peters KW, Shardell MD, et al. Cutpoints for low appendicular lean mass that identify older adults with clinically significant weakness. J Gerontol A Biol Sci Med Sci. 2014;69(5):567–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496W–509W. [Google Scholar]
- 15.Rantanen T, Avlund K, Suominen H, Schroll M, Frandin K, Pertti E. Muscle strength as a predictor of onset of ADL dependence in people aged 75 years. Aging Clin Exp Res. 2002;14(3 Suppl):10–15. [PubMed] [Google Scholar]
- 16.Rantanen T, Guralnik JM, Foley D, et al. Midlife hand grip strength as a predictor of old age disability. JAMA. 1999;281(6):558–560. [DOI] [PubMed] [Google Scholar]
- 17.Rantanen T, Masaki K, Foley D, Izmirlian G, White L, Guralnik JM. Grip strength changes over 27 yr in Japanese-American men. J Appl Physiol (1985). 1998;85(6):2047–2053. [DOI] [PubMed] [Google Scholar]
- 18.Dodds RM, Syddall HE, Cooper R, et al. Grip strength across the life course: normative data from twelve British studies. PLOS One. 2014;9(12):e113637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dodds RM, Syddall HE, Cooper R, Kuh D, Cooper C, Sayer AA. Global variation in grip strength: a systematic review and meta-analysis of normative data. Age Ageing. 2016;45(2):209–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duchowny KA, Peterson MD, Clarke PJ. Cut points for clinical muscle weakness among older Americans. Am J Prev Med. 2017;53(1):63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cruz-Jentoft AJ, Landi F, Schneider SM, et al. Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the international sarcopenia initiative (EWGSOP and IWGS). Age Ageing. 2014;43(6):748–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Studenski SA, Peters KW, Alley DE, et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci. 2014;69(5):547–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fragala MS, Alley DE, Shardell MD, et al. Comparison of handgrip and leg extension strength in predicting slow gait speed in older adults. J Am Geriatr Soc. 2016;64(1):144–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blum MR, Bauer DC, Collet TH, et al. Subclinical thyroid dysfunction and fracture risk: a meta-analysis. JAMA. 2015;313(20):2055–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohta J, Seki M, Ao M, et al. Comparison of lower leg muscle strength and grip strength for diagnosing slower gait speed in the elderly. Osteoporos Sarcopenia. 2017;3(3):128–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pinter D, Ritchie SJ, Gattringer T, et al. Predictors of gait speed and its change over three years in community-dwelling older people. Aging (Albany NY). 2018;10(1):144–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glenn JM, Gray M, Binns A. Relationship of sit-to-stand lower-body power with functional fitness measures among older adults with and without sarcopenia. J Geriatr Phys Ther. 2017;40(1):42–50. [DOI] [PubMed] [Google Scholar]
- 28.Buchner DM, Larson EB, Wagner EH, Koepsell TD, de Lateur BJ. Evidence for a non-linear relationship between leg strength and gait speed. Age Ageing. 1996;25(5):386–391. [DOI] [PubMed] [Google Scholar]
- 29.Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305(1):50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332(9):556–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manini TM, Clark BC. Dynapenia and aging: an update. J Gerontol A Biol Sci Med Sci. 2012;67(1):28–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schaap LA, Koster A, Visser M. Adiposity, muscle mass, and muscle strength in relation to functional decline in older persons. Epidemiol Rev. 2013;35:51–65. [DOI] [PubMed] [Google Scholar]
- 33.Cawthon PM, Orwoll ES, Peters KE, et al. Strong relation between muscle mass determined by D3-creatine dilution, physical performance and incidence of falls and mobility limitations in a prospective cohort of older men. J Gerontol A Biol Sci Med Sci. 2019; 74(6):844–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Santanasto AJ, Miljkovic I, Cvejkus RC, et al. Body composition remodeling and incident mobility limitations in African ancestry men. J Gerontol A Biol Sci Med Sci. 2019;74(3):400–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Santanasto AJ, Goodpaster BH, Kritchevsky SB, et al. Body composition remodeling and mortality: the health aging and body composition study. J Gerontol A Biol Sci Med Sci. 2017;72(4):513–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alley DE, Shardell MD, Peters KW, et al. Grip strength cutpoints for the identification of clinically relevant weakness. J Gerontol A Biol Sci Med Sci. 2014;69(5):559–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harvey NC, Kanis JA, Liu E, Johansson H, Lorentzon M, McCloskey E. Appendicular lean mass and fracture risk assessment: implications for FRAX(R) and sarcopenia. Osteoporos Int. 2019;30:537–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harvey NC, Oden A, Orwoll E, et al. Measures of physical performance and muscle strength as predictors of fracture risk independent of FRAX, falls, and aBMD: a meta-analysis of the osteoporotic fractures in men (MrOS) study. J Bone Miner Res. 2018;33(12):2150–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1: Characteristics of included cohorts.
Supplementary Table S2: Studies included in analysis and frequency of outcomes by cohort.
Supplementary Table S3: Grip strength protocols.
Supplementary Table S4: Walking speed protocols.
Supplementary Table S5: Falls outcome protocol.
Supplementary Table S6: Mortality outcome protocol.
Supplementary Table S7: Hip fracture outcome protocol.
Supplementary Table S8: Self-reported incident mobility limitation protocol.
Supplementary Table S9: Covariate protocols.
Supplementary Table S10: Characteristics [N (%) or mean ± SD] of participants by categories of grip strength.
Supplementary Table S11: Association* of Grip/BMI <1.46 in men and clinical outcomes in the SDOC project.
Supplementary Figure S1: Association (odds ratio*, 95% CI) of grip strength and incident falls for each cohort individually.
Supplementary Figure S2: Association (hazard ratio, 95% CI) of grip strength and mortality for each cohort individually.
Supplemental Text S1: Financial Statement