Abstract
Supplemental Digital Content is available in the text.
Keywords: abatacept, aging, heart failure, inflammation, mice
Meet the First Author, see p 952
Heart failure (HF) is the most frequent cause of hospitalization of elderly patients. Although it can be the end result of diverse risk factors, aging per se of cardiac-associated tissues can lead to cardiac function degeneration, as well as trigger cardiac inflammation. To study the effects of aging on cardiac function and inflammation, in the absence of confounding causes, we examined healthy wild-type C57BL6/J male mice, at 2 months of age (young), 12 months (adult), or 18 months (aging). Through echocardiography, we observed (Figure I in the Data Supplement) that aging led to reduced fractional shortening and ejection fraction, increased left ventricle internal dimension in systole and diastole, increased heart weight to tibia length ratio, and reduced relative wall thickness. This phenotype matches previously reported systolic dysfunction occurring in this age range.1 To analyze the effect of aging in cardiac inflammation, we examined the hearts via real-time quantitative polymerase chain reaction to identify the presence of key immune mediators and cell subsets, the latter by using lineage-specific markers (Figure II in the Data Supplement). Gene expression for cytokines, such as TNFα (tumor necrosis factor α) and IFNγ (interferon γ), chemokines CCL2 (CC motif Ligand 2) and CXCL10 (CXC motif Ligand 10), markers of proinflammatory type 1-polarized responses, increased significantly between 2 and 12 months of age, matching findings in pressure-overload HF.2 This was accompanied by innate immune cell presence (CD11b), which includes key cell subsets, such as macrophages.3 T cells (CD3e) were present, with increasing abundance of CD8+ T rather than CD4+ T with aging, as previously observed,1 whereas B cells (CD19) appeared more abundant in aging mouse hearts. Notably, a few of these markers displayed an early peak. To validate the mRNA expression data using a protein-based assay, we performed immunohistochemical staining on paraffin-embedded heart sections of mice at 2, 12, and 18 months of age (Figure III in the Data Supplement). Confirming the gene expression analysis, macrophages (Iba1 [ionized calcium- binding adapter molecule 1]) and T cells (CD3e, a marker of T cell presence and thus proxy of cardiac infiltration/proliferation of T cells) were significantly expanded in the hearts of mice at 12 and 18 months compared with 2 months of age, whereas mast cell abundance (toluidine blue) and collagen deposition, a sign of fibrosis and a hallmark of HF, also increased with age.
The cardiac inflammation induced by aging alone displayed great similarity to that occurring during pressure-overload HF.4 We recently demonstrated that pressure-overload HF can be treated by targeting this inflammation via T-cell costimulation inhibitors, such as Food and Drug Administration–approved drug CTLA4-Ig (cytotoxic T lymphocyte associated antigen 4) (abatacept), which blocks T cell activation, dampens inflammation, and averts further cardiac damage.4 The drug is also efficient in rescuing tumor therapy-induced myocarditis in humans.5 To follow a protocol with theoretical translational potential for age-related HF, we decided to intervene only once reduced cardiac function was evident. We treated adult-to-aging C57BL6/J male mice, starting at 15 months of age, with 200 µg of abatacept in 100 µL of PBS or PBS alone, twice a month (Figure [A]) for 7 months, that is, up to 22 months of age (old mice). Mice above 18 months develop far more substantial diastolic rather than systolic dysfunction,3 as indeed do old-age humans. We thus monitored both sets of parameters via echocardiography. Abatacept led to significantly higher systolic heart function (Figure [B], %fractional shortening, %ejection fraction). Most importantly, after 7 months of treatment with abatacept, 22-month-old mice exhibited significantly better diastolic function compared to control-treated mice (Figure [C]), evaluated as doppler mitral valve (MV) E wave, tissue Doppler e′ wave, and E/e′. As expected from studies on pressure-overload HF, abatacept-treated old mice displayed significantly reduced cardiac infiltration by macrophages, T cells, and, consequently, reduced fibrosis (Figure [D]). However, mast cells, whose activity lies upstream of the adaptive immunity targets of costimulation blockade, were unaffected by treatment (Figure [D]).
Figure.
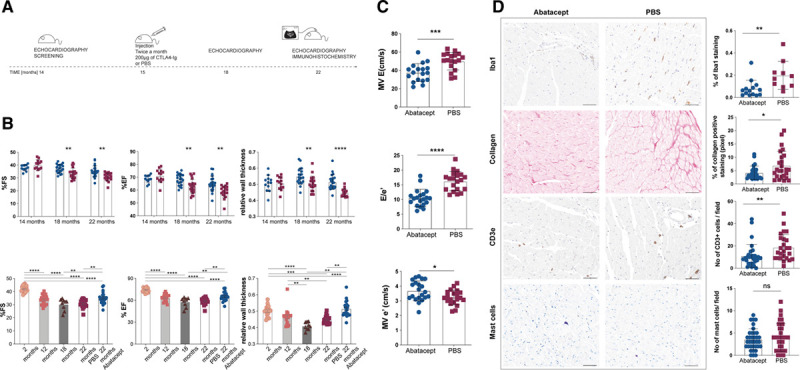
Abatacept treatment significantly improves systolic and diastolic function in old mice. A. Schematic representation of the therapeutic protocol applied to aging mice. C57BL6/J male mice were screened via echocardiography at 14 mo of age, and starting at 15 mo of age received 2×/mo 200 µg abatacept in 100 µL of PBS or 100 µL of PBS via intraperitoneal injection, for further 7 mo. Systolic heart functionality at 18 and 22 mo of age, and diastolic heart functionality at 22 mo of age via echocardiography was recorded and analyzed by a blinded operator. B, Abatacept treatment improves systolic function. %fractional shortening (FS), %ejection fraction (EF), and relative wall thickness (RWT) of 14-, 18- and 22-mo-old C57BL6/J mice treated with abatacept (white column/blue circles) or with PBS (white column/red squares) are plotted as mean±SEM (abatacept-treated n=24, PBS-treated n=20). %FS, %EF and RWT of 2- (white column/orange circles), 12-(light gray column/pink squares), 18-mo-old (dark gray column/burgundy triangles), 22 mo-old treated with PBS (white column/red squares) and 22-mo-old abatacept-treated (white column/blue circles) C57BL6/J male mice are plotted as Scatter plot with bar; columns represent the mean and each dot represents one mouse (2 mo n=18, 12 mo n=19, 18 mo n=9, abatacept-treated n=24, PBS-treated n=20). Full legend and statistical details in the Data Supplement. C, Abatacept treatment improves diastolic function. Echocardiographic analysis of transmitral early peak velocity (E), early diastolic mitral annulus velocity (e′) and E/e′ estimated by transmitral Doppler and tissue Doppler of 22 mo-old C57BL6/J mice treated with abatacept (white column/blue circles) or with PBS (white column/red squares) are plotted as mean±SEM (n=20). Shapiro-Wilk test was performed to confirm normal distribution. Unpaired t test. *P=2.04×10-2; ****P=1.12×10-6. D, Macrophage infiltration, collagen deposition, and T celll infiltration but not mast cell presence is reduced by abatacept treatment. Full legend is given in the Data Supplement.
The cardioprotective effect of abatacept in pressure-overload HF is lost in the absence of anti-inflammatory cytokine IL (interleukin)-10, although the levels of the cytokine are unaffected by treatment.4 Similarly, IL-10–deficient mice, treated with abatacept starting at 12 months of age for 3 months (an accelerated protocol to avoid IL-10-deficiency–driven autoimmunity) had similar systolic and diastolic heart function as IL-10–deficient mice treated with control (Figure IV in the Data Supplement).
Our results show that T cell costimulation blockade inhibits age-related systolic and diastolic cardiac dysfunction. As with the application of the same drug in pressure-overload–induced HF, this dampens cardiac infiltration by immune cells, leading to reduced fibrosis formation in the heart, and may involve an IL-10–dependent loop.4 Given the recently demonstrated efficacy of costimulation blockade in clinical treatment of tumor immunotherapy-induced myocarditis,5 our findings expand the range of settings in which T cell function has been shown to affect cardiac pathology.
Data and materials are available upon request.
Experiments were performed after institutional and national authorization.
Sources of Funding
We acknowledge European Research Council (ERC) Advanced Grant (CardioEpigen, No. 294609), European Research Area Network-Cardiovascular Diseases (ERANET-CVD) (Exploring New Pathways in Age-Related Heart Diseases [EXPERT] project; G. Condorelli), and Umberto Veronesi Foundation (M. Kallikourdis).
Disclosures
None.
Supplementary Material
Footnotes
Nonstandard Abbreviation and Acronyms
- HF
- heart failure
- IFNγ
- interferon γ
- IL
- interleukin
- TNFα
- tumor necrosis factor α
G.C. and M.K. contributed equally to this article and MC and CP contributed equally to this article.
The Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/CIRCRESAHA.119.316530.
For Sources of Funding and Disclosures, see page 1116.
References
- 1.Ramos GC, van den Berg A, Nunes-Silva V, Weirather J, Peters L, Burkard M, Friedrich M, Pinnecker J, Abeßer M, Heinze KG, et al. Myocardial aging as a T-cell-mediated phenomenon. Proc Natl Acad Sci USA. 2017;114:E2420–E2429. doi: 10.1073/pnas.1621047114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nevers T, Salvador AM, Velazquez F, Ngwenyama N, Carrillo-Salinas FJ, Aronovitz M, Blanton RM, Alcaide P. Th1 effector T cells selectively orchestrate cardiac fibrosis in nonischemic heart failure. J Exp Med. 2017;214:3311–3329. doi: 10.1084/jem.20161791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hulsmans M, Sager HB, Roh JD, Valero-Muñoz M, Houstis NE, Iwamoto Y, Sun Y, Wilson RM, Wojtkiewicz G, Tricot B, et al. Cardiac macrophages promote diastolic dysfunction. J Exp Med. 2018;215:423–440. doi: 10.1084/jem.20171274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kallikourdis M, Martini E, Carullo P, Sardi C, Roselli G, Greco CM, Vignali D, Riva F, Ormbostad Berre AM, Stølen TO, et al. T cell costimulation blockade blunts pressure overload-induced heart failure. Nat Commun. 2017;8:14680 doi: 10.1038/ncomms14680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salem JE, Allenbach Y, Vozy A, Brechot N, Johnson DB, Moslehi JJ, Kerneis M. Abatacept for severe immune checkpoint inhibitor-associated myocarditis. N Engl J Med. 2019;380:2377–2379. doi: 10.1056/NEJMc1901677 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


