Abstract
Background:
Bone-graft substitutes are commonly used for the augmentation of traumatic bone defects in tibial plateau fractures. However, their clinical performance compared with that of autologous bone-grafting, the gold standard in bone defect reconstruction, still remains under debate. This study investigates the differences in quality of life, pain, and radiographic outcomes in the treatment of tibial plateau fracture-associated bone defects with either autologous bone grafts or a bioresorbable hydroxyapatite and calcium sulfate cement (CERAMENT BONE VOID FILLER [CBVF]; BONESUPPORT).
Methods:
In this study, 135 patients with acute depression and split-depression fractures of the proximal part of the tibia (OTA/AO types 41-B2 and 41-B3) were enrolled in a prospective, controlled, randomized, multicenter trial including 20 hospitals in Germany. Patients were randomized to receive either autologous iliac bone graft or CBVF for reconstruction of the bone defect. The primary outcome measures were the Short Form (SF)-12 version 2 Physical Component Summary (PCS) score at week 26 (the study was designed to show noninferiority of the CBVF with regard to the PCS with a prespecified margin of −5 points) and the pain level at 26 weeks postoperatively measured by a visual analog scale (VAS). The secondary outcomes were the SF-12 version 2 Mental Component Summary (MCS) and SF-12 PCS scores at weeks 1, 6, and 12 and bone-healing on radiographs.
Results:
Age, sex, fixation methods, and fracture pattern were comparable in both groups. There were no significant differences (p > 0.05) in the SF-12 PCS or VAS scores at postoperative week 26. There was a significant reduction of blood loss (p = 0.007) and pain levels (p = 0.008) at postoperative day 1 in the CBVF group. The rates of fracture-healing, defect remodeling, and articular subsidence were not significantly different (p > 0.05) in both groups.
Conclusions:
Bioresorbable CBVF was noninferior to autologous bone graft with regard to both patient-reported and radiographic outcomes in tibial plateau fractures of OTA/AO types 41-B2 and 41-B3.
Level of Evidence:
Therapeutic Level I. See Instructions for Authors for a complete description of levels of evidence.
Autologous iliac bone graft has been the most frequently recommended material to fill bone defects in tibial plateau fractures1,2. Despite the wide acceptance of autologous iliac bone graft as the gold standard3, some reports have shown that 0.76% to 39% of cases sustain complications at the harvest site that are capable of negatively influencing functional outcome; these include pain, hematoma, infection, and nerve injury4-7. Additionally, both operative duration and length of stay may be prolonged following bone-graft harvest6,8. To overcome such shortcomings, a large number of synthetic bone-graft substitutes have been developed. However, with an increasing number of synthetic bone-graft substitutes becoming available, the debate on their clinical performance in comparison with autologous bone-grafting is ongoing.
Surprisingly, given the large number of available synthetic bone-graft substitutes, to our knowledge, there have currently been a limited number of randomized controlled trials (RCTs) that have reported evidence outcomes following the use of these substitutes9. We know of only 3 RCTs that have been published on tibial plateau fractures comparing clinical outcomes and fracture union rates with autologous iliac bone graft and synthetic bone-graft substitutes9-11. The outcomes used in most studies on synthetic bone-graft substitutes relate to the quality of the surgical reconstruction rather than to the biological and biomechanical characteristics of synthetic bone-graft substitutes. Far more importantly, none of the published studies investigated patient-reported outcome measures such as pain and quality of life.
We hypothesized that the reconstruction of tibial plateau fracture-associated bone defects using synthetic bone-graft substitutes will be noninferior to that using autologous iliac bone graft with regard to both patient-reported outcome measures and radiographic outcomes. This RCT has been designed as a multicenter, prospective, randomized, controlled, open-label, clinical, noninferiority trial comparing these parameters in patients with well-defined, low-energy OTA/AO12 type 41-B2 and 41-B3 fractures.
Materials and Methods
The study design and implementation followed the Consolidated Standards of Reporting Trials (CONSORT) statement guidelines. The study protocol was prospectively designed and published previously13. Twenty orthopaedic trauma centers in Germany participated in the study. The study was approved by the ethical committee of Rhineland-Palatinate as well as by all local institutional review boards. This study was registered at ClinicalTrials.gov (NCT01828905). The trial was conducted in cooperation with the Interdisciplinary Center for Clinical Trials (IZKS) Mainz, which provided support in trial coordination, biometry, data management, and clinical monitoring.
Eligibility Criteria
Patients who were between 18 and 65 years of age and had sustained an isolated, acute, traumatic, closed, depression-type, proximal tibial fracture classified as OTA/AO type 41-B2 or 41-B3 (Schatzker type II or III) requiring reconstruction of the metaphyseal bone defect were prospectively enrolled in the study and were randomly allocated to 1 of the 2 study groups (Table I). Patients were excluded if they had any of the following: more than a single isolated injury, compartment syndrome, previous iliac crest bone-graft harvest, soft-tissue compromise or local infection, chronic pain, malignancy, rheumatoid arthritis, chronic cortisone therapy, questionable fracture classification, or unstable medical or surgical conditions that may have prevented safe and complete study participation. Device-related contraindications were strictly respected.
TABLE I.
Demographic Characteristics of the Intention-to-Treat Population
| Variable | Autologous Iliac Bone-Graft Group (N = 68) | CBVF Group (N = 65) | Total (N = 133) | P Value |
| Age (yr) | 0.7358* | |||
| Mean and std. dev. | 46.3 ± 11.2 | 47.0 ± 12.4 | 46.7 ± 12.0 | |
| Minimum | 18.0 | 18.0 | 18.0 | |
| Quartile 1 | 34.5 | 37.0 | 37.0 | |
| Median | 48.0 | 49.0 | 49.0 | |
| Quartile 3 | 54.0 | 58.0 | 56.0 | |
| Maximum | 65.0 | 66.0 | 66.0 | |
| Missing | 0 | 0 | 0 | |
| Sex | 0.8190† | |||
| Female‡ | 39 (57.4%) | 36 (55.4%) | 75 (56.4%) | |
| Male‡ | 29 (42.6%) | 29 (44.6%) | 58 (43.6%) | |
| Ethnicity | 0.2169† | |||
| Caucasian‡ | 67 (98.5%) | 63 (96.9%) | 130 (97.7%) | |
| African‡ | 1 (1.5%) | 0 (0.0%) | 1 (0.8%) | |
| Other‡ | 0 (0.0%) | 2 (3.1%) | 2 (1.5%) |
T test.
Chi-square test.
The values are given as the number of patients, with the percentage in parentheses.
Randomization
The randomization list was created with permuted blocks of length 4 or 6. After informed consent, patients were randomized with a 1:1 ratio using a web-based randomization tool. Randomization was stratified by age group (18 to 39 years and 40 to 65 years) and sex.
Surgical Technique
The study sites followed their preferred locally established protocols for pain management according to the World Health Organization standard. Open reduction and internal fixation was performed through a standard anterolateral approach using screws and a buttress plate after reduction of the depressed articular surface. The choice of fixation method (locking plates compared with non-locking plates) was left to the surgeon’s preference. The bone defect remaining after the reduction of the depressed articular fragments was reconstructed according to the result of randomization either with autologous iliac bone graft or with synthetic bone-graft substitute. In the first group, autologous bone grafts were harvested from the ipsilateral anterior part of the iliac crest following a well-established surgical technique currently presented by Shaw et al.14.
In the second group, CERAMENT BONE VOID FILLER (CBVF; BONESUPPORT) was used. CBVF is a bioresorbable synthetic bone-graft substitute, consisting of 60% calcium sulfate and 40% hydroxyapatite with an initial porosity of 40% to 50% and a mean pore size of <1 mm. It has been previously investigated in preclinical studies15,16 and clinical studies17-19. The preparation strictly followed the manufacturer’s instructions for use. Prior to implantation, bone defects were carefully cleaned. During application of CBVF, a tourniquet was applied to avoid mixture of the material with blood and to allow proper curing of the cement. Drains were routinely used at the donor site and the tibial plateau wound. The duration of the surgical procedure (incision to suture time) and blood loss were measured and were recorded in the surgical notes.
Postoperatively, all patients were mobilized with assistive devices and were allowed toe-touch weight-bearing for 6 weeks. Thereafter, progressive weight-bearing was permitted on the basis of the surgeon’s judgment. Clinical evaluation and trial documentation consisted of 7 visits: screening (visit 1), intervention (visit 2), and 5 follow-up examinations (visits 3 to 7) until week 26 (Table II)13.
TABLE II.
Time Points and Type of Assessment
| Visit 1 (Day −7 to 0): Screening | Visit 2 (Day 0): Day of Surgery | Visit 3 (Day 1 [± 1 day]) | Visit 4 (Day 7 [± 3 days]) | Visit 5 (Week 6 [± 1 week) | Visit 6 (Week 12 [± 2 weeks]) | Visit 7 (Week 26 [± 3 weeks]) | |
| First patient enrolled April 24, 2013 | |||||||
| Informed consent, demography, medical history, physical examination, radiographic assessment, randomization | X | ||||||
| Surgery (autologous iliac bone graft or CBVF) and procedure information | X | ||||||
| Adverse events | X | X | X | X | X | X | X |
| Device symptoms reported | X | X | X | X | X | X | |
| Clinical examination | X | X | X | X | X | X | |
| SF-12 | X | X | X | X | X | ||
| Pain (VAS) | X | X | X | X | X | X | |
| Assessment of other outcome measures | X | X | X | X | |||
| End of the trial | X | ||||||
| Last patient enrolled November 27, 2017 | |||||||
| Last follow-up completed June 8, 2018 |
Outcome Measures
The primary outcome measures evaluated were the Short Form (SF)-12 version 2 Physical Component Summary (PCS) score at week 26 (the PCS uses the scores of 12 questions and ranges from 0 to 100, where a 0-point score indicates the lowest level of physical health and a score of 100 points indicates the highest level of physical health) and the visual analog scale (VAS) for pain at week 26 (the VAS uses values from 0 [no pain] to 10 [worst pain ever]). We also assessed the VAS score at 1, 6, and 12 weeks in addition to the original study protocol registered at ClinicalTrials.gov to detect differences in pain levels over a whole period of 26 weeks.
The secondary outcome measures evaluated were the SF-12 version 2 Mental Component Summary (MCS) scores at 1, 6, and 12 weeks (the MCS score ranges from 0 to 100 points [from the lowest to the highest level of mental health]); SF-12 PCS scores at 1, 6, and 12 weeks; and bone-healing evaluated on radiographs. Utilization of costs of care-related resources was a secondary outcome of the original study protocol registered at ClinicalTrials.gov, but we did not report the results of this measure, because the precise calculation was not available for a sufficient number of patients.
All adverse events were evaluated and were recorded according to the principles of good clinical practice.
Radiographic Evaluation
Radiographs were made according to the standard procedure. No additional radiographs were made specifically for the purpose of the study. Available radiographs were pseudonymized by the participating centers and were sent for evaluation, which was performed by a single radiologist specialized in orthopaedic trauma, who was blinded to the kind of material used.
The radiographs were reviewed in chronological order to identify any subsidence of the articular surface according to the Rasmussen score20, which was assessed over a period of 26 weeks. Fracture-healing, bone-defect remodeling, and lack of resorption or premature resorption of the material were investigated using the Jerosch score21 over a period of 26 weeks.
Statistical Analysis
With regard to the sample size calculation, a previous cohort of patients with tibial plateau fractures treated in the Department of Orthopaedics and Traumatology of the University Medical Center Mainz was used for calculation of the sample size. In this cohort, the SF-12 PCS score showed a standard deviation of 10 points. The noninferiority margin of half of the standard deviation was chosen, corresponding to 5 points on the scale (range, 0 to 100). A shifted 2-sample t test with a 1-sided significance level of 2.5% and a power of 80% required 128 patients to show noninferiority. After assuming that 5% of randomized patients would be ineligible for per-protocol analysis, 136 patients were planned for randomization.
The confirmatory efficacy analysis was planned by employing a hierarchical testing procedure. The differences between the SF-12 PCS scores were tested by analysis of covariance (ANCOVA) with the SF-12 PCS score at week 26 as the independent variable; the age group (18 to 39 years and 40 to 65 years), sex, and treatment as fixed effects; and the SF-12 PCS score at screening as a covariate. Noninferiority of CBVF was concluded if the adjusted 2-sided 95% confidence interval (CI) of the treatment effect was entirely above the prespecified noninferiority margin of −5. The primary outcome of the SF-12 PCS score at week 26 was analyzed in the per-protocol population; it was repeated for the intention-to-treat population in a secondary analysis. Pain VAS underwent confirmatory testing as the other primary outcome using a Wilcoxon-Mann-Whitney U test with a 2-sided significance level of 5%. Analysis was performed primarily for the intention-to-treat population. All other testing was exploratory and utilized the t test, Wilcoxon-Mann-Whitney U test, and chi-square test, as required.
Results
Study Population
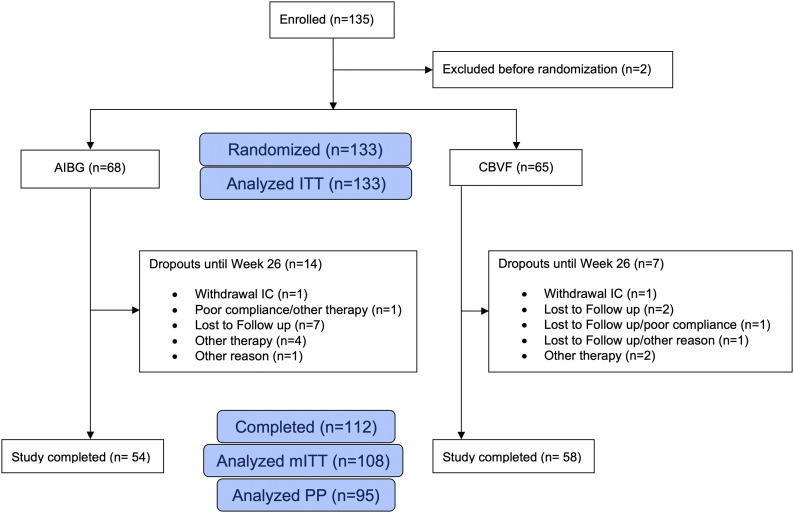
In this study, 135 patients were enrolled (Fig. 1). Demographic data of the study population are summarized in Table I. Two patients were excluded, resulting in an intention-to-treat population of 133 patients (Table III). One of the excluded patients was treated by a surgeon who had no good clinical practice certification. The second patient was excluded because an incorrect version of the SF-12 questionnaire was accidentally used. The modified intent-to-treat population was defined as patients with a valid SF-12 assessment at baseline and after 26 weeks, resulting in a population of 108 patients (52 in the autologous iliac bone-graft group and 56 patients in the CBVF group) (Fig. 1). The per-protocol population, defined as patients completing the study without major protocol deviations, included 95 patients (47 in the autologous iliac bone-graft group and 48 patients in the CBVF group). The reasons for exclusion from the per-protocol and intention-to-treat populations are listed in Figure 1.
Fig. 1.
CONSORT flowchart of the study population. AIBG = autologous iliac bone graft, ITT = intention to treat, IC = informed consent, mITT= modified intention to treat, and PP = per protocol.
TABLE III.
Study Cohort: Regular End of Study and Dropouts Until Week 26*
| Autologous Iliac Bone-Graft Group† (N = 68) | CBVF Group† (N = 65) | Not Randomized† (N = 2) | Total† (N = 135) | |
| Written consent available | 68 (100.0%) | 65 (100.0%) | 2 (100.0%) | 135 (100.0%) |
| Safety population | 62 (91.2%) | 62 (95.4%) | 0 (0.0%) | 124 (91.9%) |
| Intention-to-treat population | 68 (100.0%) | 65 (100.0%) | 0 (0.0%) | 133 (98.5%) |
| Modified intention-to-treat population | 52 (76.5%) | 56 (86.2%) | 0 (0.0%) | 108 (80.0%) |
| Per-protocol population | 47 (69.1%) | 48 (73.8%) | 0 (0.0%) | 95 (70.4%) |
| No. of patients with major protocol violations | 5 (7.4%) | 8 (12.3%) | 0 (0.0%) | 13 (9.6%) |
| No. of patients with study termination | 14 (20.6%) | 7 (10.8%) | 2 (100.0%) | 23 (17.0%) |
| Total no. of major protocol violations‡ | 24 (100.0%) | 19 (100.0%) | 0 (0.0%) | 43 (100.0%) |
| Violation of inclusion criteria | 3 (12.5%) | 5 (26.3%) | 0 (0.0%) | 8 (18.6%) |
| Meeting any exclusion criteria | 3 (12.5%) | 3 (15.8%) | 0 (0.0%) | 6 (14.0%) |
| Time interval between visit 2 (day 0, surgery) and visit 7 (week 26) is not 23 to 29 weeks§ | 18 (75.0%) | 11 (57.9%) | 0 (0.0%) | 29 (67.4%) |
| Regular end of study | 54 (79.4%) | 58 (89.2%) | 0 (0.0%) | 112 (83.0%) |
| Reasons for study termination‡ | ||||
| Withdrawal of informed consent | 1 (6.7%) | 1 (11.1%) | 0 (0.0%) | 2 (7.7%) |
| Poor compliance | 1 (6.7%) | 1 (11.1%) | 0 (0.0%) | 2 (7.7%) |
| Lost to follow-up | 7 (50.0%) | 4 (44.4%) | 0 (0.0%) | 11 (42.3%) |
| Other interfering therapy of the study participant | 4 (28.6%) | 2 (22.2%) | 0 (0.0%) | 6 (26.9%) |
| Screening error | 0 (0.0%) | 0 (0.0%) | 1 (50.0%) | 1 (3.8%) |
| Administrative or regulatory reasons | 0 (0.0%) | 0 (0.0%) | 1 (50.0%) | 1 (3.8%) |
| Other | 1 (6.7%) | 1 (11.1%) | 0 (0.0%) | 2 (7.7%) |
The analysis set was the enrolled population (n = 135).
The values are given as the number of patients, with the percentage in parentheses.
There were multiple entries possible for this category.
This category included cases in which 1 or both visits did not take place.
Most of the fractures were OTA/AO type 41-B3.1 (n = 61) and OTA/AO type 41-B2.2 (n = 21) (Table IV). There were no significant differences in the fracture type distribution between the groups.
TABLE IV.
Fracture Types of the Intention-to-Treat Population* (N = 133)
| Classification | Autologous Iliac Bone-Graft Group† (N = 68) | CBVF Group† (N = 65) | Total† (N = 133) |
| OTA/AO 41-B2.1 | 8 (11.8%) | 7 (10.8%) | 15 (11.3%) |
| OTA/AO 41-B2.2 | 11 (16.2%) | 10 (15.4%) | 21 (15.8%) |
| OTA/AO 41-B2.3 | 2 (2.9%) | 2 (3.1%) | 4 (3.0%) |
| OTA/AO 41-B3.1 | 35 (51.5%) | 26 (40.0%) | 61 (45.9%) |
| OTA/AO 41-B3.2 | 4 (5.9%) | 9 (13.8%) | 13 (9.8%) |
| OTA/AO 41-B3.3 | 8 (11.8%) | 10 (15.4%) | 18 (13.5%) |
| Other | 0 (0.0%) | 1 (1.5%) | 1 (0.8%) |
P = 0.6064, chi-square test.
The values are given as the number of patients, with the percentage in parentheses.
Surgical Treatment
The mean duration (and standard deviation) of the surgical procedure was 112 ± 42 minutes in the autologous iliac bone graft group and 104 ± 36 minutes in the CBVF group (p = 0.27; t test) (Table V). The mean blood loss was 196 ± 160 mL in the autologous iliac bone-graft group and 109 ± 110 mL in the CBVF group (p = 0.0007; t test) (Table V). According to the volumes of CBVF needed to fill the bone voids, the mean defect size was 5.8 mL (range, 1 to 18 mL). In most cases, locking plates were used (93 locking plates [75%] compared with 31 non-locking plates), with a similar distribution in both groups (Table V). The mean length of hospital stay was 11 ± 6.2 days (median, 9 days [range, 3 to 37 days]) in the modified intention-to-treat population, with no significant difference between the autologous iliac bone-graft group (11.0 ± 5.7 days) and the CBVF group (11.1 ± 6.6 days) (p = 0.88; t test).
TABLE V.
Surgical Parameters of the Intention-to-Treat Population (N = 133)
| Variable | Autologous Iliac Bone-Graft Group (N = 68) | CBVF Group (N = 65) | Total (N = 133) | P Value |
| Could the planned surgery be performed? | 0.7131* | |||
| Yes† | 62 (93.9%) | 62 (95.4%) | 124 (94.7%) | |
| No† | 4 (6.1%) | 3 (4.6%) | 7 (5.3%) | |
| Missing† | 2 | 0 | 2 | |
| Delay from injury to open reduction and internal fixation | 0.2110‡ | |||
| No. of patients | 62 | 62 | 124 | |
| Mean and std. dev. (days) | 6.9 ± 4.2 | 6.0 ± 4.0 | 6.4 ± 4.1 | |
| Duration of surgery | 0.2689‡ | |||
| No. of patients | 62 | 62 | 124 | |
| Mean and std. dev. (min) | 112.1 ± 41.6 | 104.3 ± 36.5 | 108.2 ± 39.2 | |
| Minimum (min) | 46.0 | 50.0 | 46.0 | |
| Quartile 1 (min) | 80.0 | 75.0 | 78.5 | |
| Median (min) | 102.0 | 98.0 | 100.0 | |
| Quartile 3 (min) | 139.0 | 131.0 | 133.0 | |
| Maximum (min) | 210.0 | 215.0 | 215.0 | |
| Missing† | 6 | 3 | 9 | |
| Blood loss | 0.0007‡ | |||
| No. of patients | 60 | 59 | 119 | |
| Mean and std. dev. (mL) | 196 ± 160 | 109 ± 110 | 153 ± 144 | |
| Minimum (mL) | 0.0 | 0.0 | 0.0 | |
| Quartile 1 (mL) | 85.0 | 15.0 | 50.0 | |
| Median (mL) | 200.0 | 100.0 | 100.0 | |
| Quartile 3 (mL) | 250.0 | 150.0 | 200.0 | |
| Maximum (mL) | 850.0 | 400.0 | 850.0 | |
| Missing† | 8 | 6 | 14 | |
| Fracture reduction | 0.0420* | |||
| Closed reduction and fixation† | 0 (0%) | 4 (6.5%) | 4 (3.2%) | |
| Open reduction and fixation† | 62 (100%) | 58 (93.5%) | 120 (96.8%) | |
| Missing† | 6 | 3 | 9 | |
| Osteosynthesis material | ||||
| Lag screws | 0.0309* | |||
| Yes† | 38 (61.3%) | 49 (79.0%) | 87 (70.2%) | |
| No† | 24 (38.7%) | 13 (21.0%) | 37 (29.8%) | |
| Missing† | 6 | 3 | 9 | |
| Buttress plates | 0.5139* | |||
| Yes† | 15 (24.2%) | 12 (19.4%) | 27 (21.8%) | |
| No† | 47 (75.8%) | 50 (80.6%) | 97 (78.2%) | |
| Missing† | 6 | 3 | 9 | |
| Kirschner wires | 0.4545* | |||
| Yes† | 8 (12.9%) | 11 (17.7%) | 19 (15.3%) | |
| No† | 54 (87.1%) | 51 (82.3%) | 105 (84.7%) | |
| Missing† | 6 | 3 | 9 | |
| Angle stable plate | 0.2998* | |||
| Yes† | 49 (79.0%) | 44 (71.0%) | 93 (75.0%) | |
| No† | 13 (21.0%) | 18 (29.0%) | 31 (25.0%) | |
| Missing† | 6 | 3 | 9 | |
| Other | 0.5589* | |||
| Yes† | 2 (3.2%) | 1 (1.6%) | 3 (2.4%) | |
| No† | 60 (96.8%) | 61 (98.4%) | 121 (97.6%) | |
| Missing† | 6 | 3 | 9 |
Chi-square test.
The values are given as the number of patients, with or without the percentage in parentheses.
T test.
Outcomes
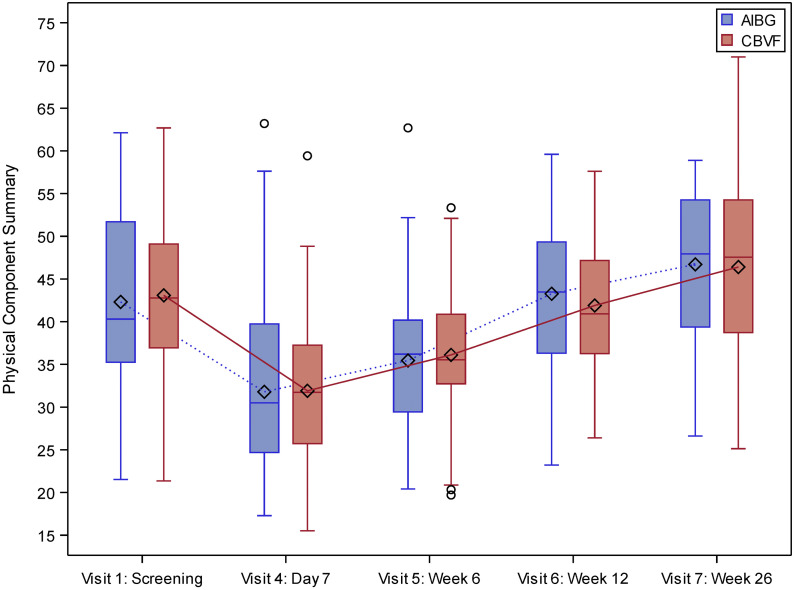
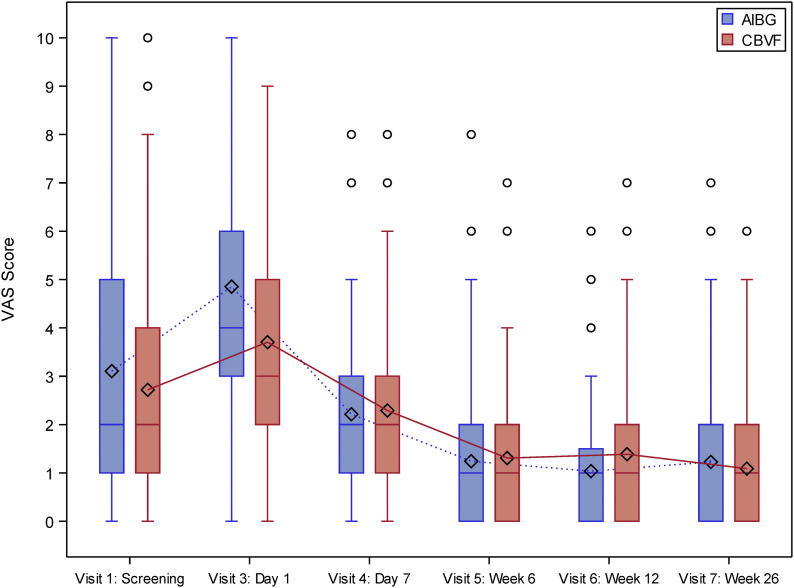
At 26 weeks, the noninferiority of the CBVF group compared with the autologous iliac bone-graft group could be demonstrated for the primary outcomes of the SF-12 PCS score (Fig. 2, Table VI) and the VAS for pain (Fig. 3).
Fig. 2.
SF-12 PCS scores in the intention-to-treat population (n = 133). AIBG = autologous iliac bone graft. The whiskers represent the minimum and the maximum, the circles represent outliers, and the diamonds represent the mean.
Fig. 3.
Pain score (VAS) in the intention-to-treat population (n = 133). AIBG = autologous iliac bone graft. The whiskers represent the minimum and the maximum, the circles represent outliers, and the diamonds represent the mean.
TABLE VI.
ANCOVA for SF-12 PCS in the Per-Protocol Population (N = 95) at Week 26
| Effect | SF-12 PCS* | P Value |
| Autologous iliac bone graft | 46.9 (44.0 to 49.9) | |
| CBVF | 47.1 (44.1 to 50.1) | |
| Difference between treatments | −0.1 (−3.9 to 3.7) | |
| Covariate | ||
| PCS score at screening | 0.2688 | |
| Age group | 0.2680 | |
| Sex | 0.4604 | |
| Treatment | 0.9579 |
The values are given as the mean, with the 95% CI in parentheses.
We also evaluated the secondary outcomes of this study: the SF-12 PCS and MCS scores at 1, 6, and 12 weeks and bone-healing on radiographs. The results of the SF-12 PCS score showed similar courses in both groups, beginning with 42 points in the autologous iliac bone-graft group and 43 points in the CBVF group on average, with a decrease on day 7 and a steady recovery during the later follow-up. The adjusted treatment difference was −0.1 (95% CI, −3.95 to 3.75), located entirely above the prespecified −5 noninferiority margin. The results were mirrored in the intention-to-treat population (treatment difference, 0.15 [95% CI, −3.44 to 3.74]).
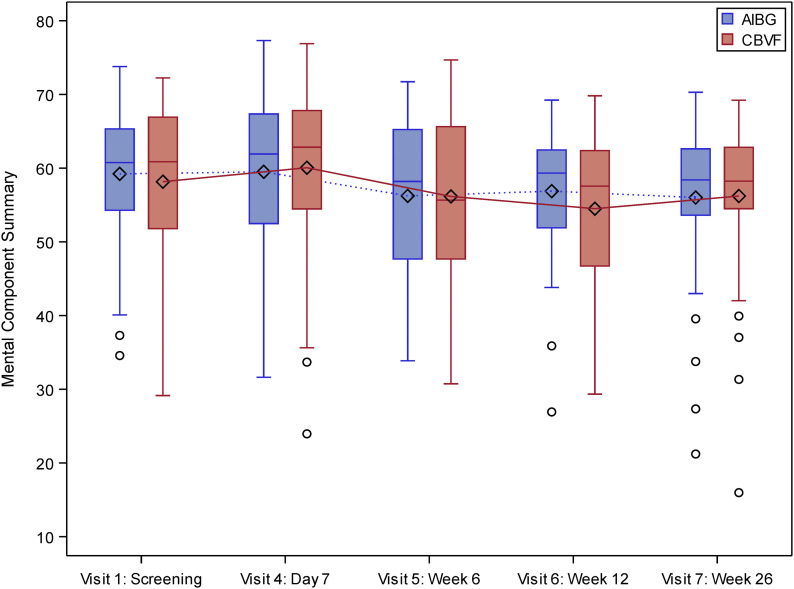
The SF-12 MCS score at week 26 showed similar courses in both groups; the mean values were around 60 points without any differences between the groups (Fig. 4). There were no differences in the rates of procedure-associated complications between the groups (Table VII22).
Fig. 4.
SF-12 MCS scores in the intention-to-treat population (n = 133). AIBG = autologous iliac bone graft. The whiskers indicate the minimum and the maximum, the circles indicate outliers, and the diamonds represent the mean.
TABLE VII.
Adverse Events in the Safety Population (N = 124) Coded According to MedDRA Terminology*
| System Organ Class or Preferred Term | Autologous Iliac Bone-Graft Group† | CBVF Group† | Total† | |||
| Patients (N = 62) | Patients with Adverse Events (N = 58) | Patients (N = 62) | Patients with Adverse Events (N = 52) | Patients (N = 124) | Patients with Adverse Events (N = 110) | |
| Subjects with any adverse event | 32 (51.6%) | 58 (100.0%) | 28 (45.2%) | 52 (100.0%) | 60 (48.4%) | 110 (100.0%) |
| Gastrointestinal disorders | 8 (12.9%) | 11 (19.0%) | 4 (6.5%) | 6 (11.5%) | 12 (9.7%) | 17 (15.5%) |
| Constipation | 4 (6.5%) | 4 (6.9%) | 1 (1.6%) | 1 (1.9%) | 5 (4.0%) | 5 (4.5%) |
| Flatulence | 1 (1.6%) | 1 (1.7%) | 2 (3.2%) | 2 (3.8%) | 3 (2.4%) | 3 (2.7%) |
| Lip blister | 1 (1.6%) | 1 (1.7%) | 0 (0.0%) | 0 (0.0%) | 1 (0.8%) | 1 (0.9%) |
| Nausea | 4 (6.4%) | 4 (6.9%) | 2 (3.2%) | 3 (5.8%) | 6 (4.8%) | 7 (6.4%) |
| Vomiting | 1 (1.6%) | 1 (1.7%) | 0 (0.0%) | 0 (0.0%) | 1 (0.8%) | 1 (0.9%) |
| Injury, poisoning, and procedural complications | 7 (11.3%) | 8 (13.8%) | 8 (12.9%) | 8 (15.4%) | 15 (12.1%) | 16 (14.5%) |
| Bone comminution | 0 (0.0%) | 0 (0.0%) | 1 (1.6%) | 1 (1.9%) | 1 (0.8%) | 1 (0.9%) |
| Fall | 2 (3.2%) | 2 (3.4%) | 1 (1.6%) | 1 (1.9%) | 3 (2.4%) | 3 (2.7%) |
| Fracture displacement | 1 (1.6%) | 1 (1.7%) | 0 (0.0%) | 0 (0.0%) | 1 (0.8%) | 1 (0.9%) |
| Fracture nonunion | 1 (1.6%) | 1 (1.7%) | 0 (0.0%) | 0 (0.0%) | 1 (0.8%) | 1 (0.9%) |
| Joint dislocation | 1 (1.6%) | 1 (1.7%) | 0 (0.0%) | 0 (0.0%) | 1 (0.8%) | 1 (0.9%) |
| Postprocedural hematoma | 1 (1.6%) | 1 (1.7%) | 1 (1.6%) | 1 (1.9%) | 2 (1.6%) | 2 (1.8%) |
| Procedural complication | 0 (0.0%) | 0 (0.0%) | 1 (1.6%) | 1 (1.9%) | 1 (0.8%) | 1 (0.9%) |
| Procedural nausea | 1 (1.6%) | 1 (1.7%) | 1 (1.6%) | 1 (1.9%) | 2 (1.6%) | 2 (1.8%) |
| Procedural vomiting | 1 (1.6%) | 1 (1.7%) | 1 (1.6%) | 1 (1.9%) | 2 (1.6%) | 2 (1.8%) |
| Seroma | 0 (0.0%) | 0 (0.0%) | 1 (1.6%) | 1 (1.9%) | 1 (0.8%) | 1 (0.9%) |
| Prolonged wound-healing | 0 (0.0%) | 0 (0.0%) | 1 (1.6%) | 1 (1.9%) | 1 (0.8%) | 1 (0.9%) |
| Musculoskeletal and connective tissue disorders | 7 (11.3%) | 7 (12.1%) | 6 (9.7%) | 9 (17.3%) | 13 (10.5%) | 16 (14.5%) |
| Arthralgia | 1 (1.6%) | 1 (1.7%) | 1 (1.6%) | 2 (3.8%) | 2 (1.6%) | 3 (2.7%) |
| Arthrofibrosis | 0 (0.0%) | 0 (0.0%) | 1 (1.6%) | 1 (1.9%) | 1 (0.8%) | 1 (0.9%) |
| Compartment syndrome | 1 (1.6%) | 1 (1.7%) | 0 (0.0%) | 0 (0.0%) | 1 (0.8%) | 1 (0.9%) |
| Joint swelling | 0 (0.0%) | 0 (0.0%) | 1 (1.6%) | 1 (1.9%) | 1 (0.8%) | 1 (0.9%) |
| Musculoskeletal pain | 0 (0.0%) | 0 (0.0%) | 1 (1.6%) | 1 (1.9%) | 1 (0.8%) | 1 (0.9%) |
| Musculoskeletal stiffness | 0 (0.0%) | 0 (0.0%) | 1 (1.6%) | 1 (1.9%) | 1 (0.8%) | 1 (0.9%) |
| Osteoarthritis | 0 (0.0%) | 0 (0.0%) | 1 (1.6%) | 1 (1.9%) | 1 (0.8%) | 1 (0.9%) |
| Osteopenia | 1 (1.6%) | 1 (1.7%) | 0 (0.0%) | 0 (0.0%) | 1 (0.8%) | 1 (0.9%) |
| Pain in extremity | 4 (6.4%) | 4 (6.9%) | 0 (0.0%) | 0 (0.0%) | 4 (3.2%) | 4 (3.6%) |
| Synovitis | 0 (0.0%) | 0 (0.0%) | 2 (3.2%) | 2 (3.8%) | 2 (1.6%) | 2 (1.8%) |
| General disorders and administration site conditions | 5 (8.1%) | 6 (10.3%) | 6 (9.7%) | 7 (13.5%) | 11 (8.9%) | 13 (11.8%) |
| Catheter site pain | 1 (1.6%) | 1 (1.7%) | 0 (0.0%) | 0 (0.0%) | 1 (0.8%) | 1 (0.9%) |
| Feeling abnormal | 0 (0.0%) | 0 (0.0%) | 1 (1.6%) | 1 (1.9%) | 1 (0.8%) | 1 (0.9%) |
| Impaired healing | 1 (1.6%) | 1 (1.7%) | 2 (3.2%) | 2 (3.8%) | 3 (2.4%) | 3 (2.7%) |
| Implant site inflammation | 0 (0.0%) | 0 (0.0%) | 1 (1.6%) | 1 (1.9%) | 1 (0.8%) | 1 (0.9%) |
| Edema | 0 (0.0%) | 0 (0.0%) | 1 (1.6%) | 1 (1.9%) | 1 (0.8%) | 1 (0.9%) |
| Pyrexia | 0 (0.0%) | 0 (0.0%) | 1 (1.6%) | 1 (1.9%) | 1 (0.8%) | 1 (0.9%) |
| Secretion discharge | 0 (0.0%) | 0 (0.0%) | 1 (1.6%) | 1 (1.9%) | 1 (0.8%) | 1 (0.9%) |
| Swelling | 3 (4.8%) | 3 (5.2%) | 0 (0.0%) | 0 (0.0%) | 3 (2.4%) | 3 (2.7%) |
| Tenderness | 1 (1.6%) | 1 (1.7%) | 0 (0.0%) | 0 (0.0%) | 1 (0.8%) | 1 (0.9%) |
| Infections and infestations | 5 (8.1%) | 6 (10.3%) | 3 (4.8%) | 3 (5.7%) | 8 (6.5%) | 9 (8.2%) |
| Diverticulitis | 1 (1.6%) | 1 (1.7%) | 0 (0.0%) | 0 (0.0%) | 1 (0.8%) | 1 (0.9%) |
| Gastrointestinal infection | 0 (0.0%) | 0 (0.0%) | 1 (1.6%) | 1 (1.9%) | 1 (0.8%) | 1 (0.9%) |
| Infected bite | 1 (1.6%) | 1 (1.7%) | 0 (0.0%) | 0 (0.0%) | 1 (0.8%) | 1 (0.9%) |
| Nasopharyngitis | 1 (1.6%) | 1 (1.7%) | 0 (0.0%) | 0 (0.0%) | 1 (0.8%) | 1 (0.9%) |
| Upper respiratory tract infection | 0 (0.0%) | 0 (0.0%) | 1 (1.6%) | 1 (1.9%) | 1 (0.8%) | 1 (0.9%) |
| Urinary tract infection | 1 (1.6%) | 1 (1.7%) | 0 (0.0%) | 0 (0.0%) | 1 (0.8%) | 1 (0.9%) |
| Wound infection | 1 (1.6%) | 2 (3.4%) | 1 (1.6%) | 1 (1.9%) | 2 (1.6%) | 3 (2.7%) |
| Nervous system disorders | 3 (4.8%) | 3 (5.2%) | 6 (9.7%) | 6 (11.5%) | 9 (7.3%) | 9 (8.2%) |
| Dizziness | 0 (0.0%) | 0 (0.0%) | 1 (1.6%) | 1 (1.9%) | 1 (0.8%) | 1 (0.9%) |
| Dysesthesia | 1 (1.6%) | 1 (1.7%) | 1 (1.6%) | 1 (1.9%) | 2 (1.6%) | 2 (1.8%) |
| Ischemic cerebral infarction | 0 (0.0%) | 0 (0.0%) | 1 (1.6%) | 1 (1.9%) | 1 (0.8%) | 1 (0.9%) |
| Paresthesia | 2 (3.2%) | 2 (3.4%) | 1 (1.6%) | 1 (1.9%) | 3 (2.4%) | 3 (2.7%) |
| Sensory disturbance | 0 (0.0%) | 0 (0.0%) | 2 (3.2%) | 2 (3.8%) | 2 (1.6%) | 2 (1.8%) |
| Psychiatric disorders | 5 (8.1%) | 5 (8.6%) | 3 (4.8%) | 3 (5.7%) | 8 (6.5%) | 8 (7.3%) |
| Depression | 1 (1.6%) | 1 (1.7%) | 0 (0.0%) | 0 (0.0%) | 1 (0.8%) | 1 (0.9%) |
| Insomnia | 1 (1.6%) | 1 (1.7%) | 2 (3.2%) | 2 (3.8%) | 3 (2.4%) | 3 (2.7%) |
| Sleep disorder | 3 (4.8%) | 3 (5.2%) | 1 (1.6%) | 1 (1.9%) | 4 (3.2%) | 4 (3.6%) |
| Vascular disorders | 3 (4.8%) | 3 (5.2%) | 3 (4.8%) | 4 (7.7%) | 6 (4.8%) | 7 (6.4%) |
| Circulatory collapse | 2 (3.2%) | 2 (3.4%) | 0 (0.0%) | 0 (0.0%) | 2 (1.6%) | 2 (1.8%) |
| Deep vein thrombosis | 1 (1.6%) | 1 (1.7%) | 2 (3.2%) | 2 (3.8%) | 3 (2.4%) | 3 (2.7%) |
| Hypertension | 0 (0.0%) | 0 (0.0%) | 1 (1.6%) | 1 (1.9%) | 1 (0.8%) | 1 (0.9%) |
| Peripheral artery aneurysm | 0 (0.0%) | 0 (0.0%) | 1 (1.6%) | 1 (1.9%) | 1 (0.8%) | 1 (0.9%) |
| Skin and subcutaneous tissue disorders | 2 (3.2%) | 2 (3.4%) | 2 (3.2%) | 2 (3.8%) | 4 (3.2%) | 4 (3.6%) |
| Erythema | 1 (1.6%) | 1 (1.7%) | 1 (1.6%) | 1 (1.9%) | 2 (1.6%) | 2 (1.8%) |
| Rash | 0 (0.0%) | 0 (0.0%) | 1 (1.6%) | 1 (1.9%) | 1 (0.8%) | 1 (0.9%) |
| Skin warmth | 1 (1.6%) | 1 (1.7%) | 0 (0.0%) | 0 (0.0%) | 1 (0.8%) | 1 (0.9%) |
| Neoplasms: benign, malignant, and unspecified (including cysts and polyps) | 2 (3.2%) | 2 (3.4%) | 1 (1.6%) | 1 (1.9%) | 3 (2.4%) | 3 (2.7%) |
| Basal cell carcinoma | 1 (1.6%) | 1 (1.7%) | 0 (0.0%) | 0 (0.0%) | 1 (0.8%) | 1 (0.9%) |
| Invasive ductal breast carcinoma | 1 (1.6%) | 1 (1.7%) | 0 (0.0%) | 0 (0.0%) | 1 (0.8%) | 1 (0.9%) |
| Laryngeal cancer | 0 (0.0%) | 0 (0.0%) | 1 (1.6%) | 1 (1.9%) | 1 (0.8%) | 1 (0.9%) |
| Metabolism and nutrition disorders | 2 (3.2%) | 2 (3.4%) | 0 (0.0%) | 0 (0.0%) | 2 (1.6%) | 2 (1.8%) |
| Hypokalemia | 2 (3.2%) | 2 (3.4%) | 0 (0.0%) | 0 (0.0%) | 2 (1.6%) | 2 (1.8%) |
| Blood and lymphatic system disorders | 1 (1.6%) | 1 (1.7%) | 0 (0.0%) | 0 (0.0%) | 1 (0.8%) | 1 (0.9%) |
| Hemorrhagic anemia | 1 (1.6%) | 1 (1.7%) | 0 (0.0%) | 0 (0.0%) | 1 (0.8%) | 1 (0.9%) |
| Cardiac disorders | 1 (1.6%) | 1 (1.7%) | 0 (0.0%) | 0 (0.0%) | 1 (0.8%) | 1 (0.9%) |
| Cardiovascular disorder | 1 (1.6%) | 1 (1.7%) | 0 (0.0%) | 0 (0.0%) | 1 (0.8%) | 1 (0.9%) |
| Immune system disorders | 0 (0.0%) | 0 (0.0%) | 1 (1.6%) | 1 (1.9%) | 1 (0.8%) | 1 (0.9%) |
| Drug hypersensitivity | 0 (0.0%) | 0 (0.0%) | 1 (1.6%) | 1 (1.9%) | 1 (0.8%) | 1 (0.9%) |
| Investigations | 0 (0.0%) | 0 (0.0%) | 1 (1.6%) | 1 (1.9%) | 1 (0.8%) | 1 (0.9%) |
| Body temperature increased | 0 (0.0%) | 0 (0.0%) | 1 (1.6%) | 1 (1.9%) | 1 (0.8%) | 1 (0.9%) |
| Respiratory, thoracic, and mediastinal disorders | 0 (0.0%) | 0 (0.0%) | 1 (1.6%) | 1 (1.9%) | 1 (0.8%) | 1 (0.9%) |
| Oropharyngeal pain | 0 (0.0%) | 0 (0.0%) | 1 (1.6%) | 1 (1.9%) | 1 (0.8%) | 1 (0.9%) |
| Surgical and medical procedures | 1 (1.6%) | 1 (1.7%) | 0 (0.0%) | 0 (0.0%) | 1 (0.8%) | 1 (0.9%) |
| Open reduction of fracture | 1 (1.6%) | 1 (1.7%) | 0 (0.0%) | 0 (0.0%) | 1 (0.8%) | 1 (0.9%) |
MedDRA® the Medical Dictionary for Regulatory Activities terminology is the international medical terminology developed under the auspices of the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). MedDRA® trademark is registered by IFPMA (the International Federation of Pharmaceutical Manufacturers & Associations) on behalf of ICH. With use of MedDRA terminology, clinical signs and symptoms associated with possible complications of the surgical intervention were assessed at every study visit. All other adverse events were reported freely and were documented using a standard adverse event form together with intensity, relationship to study treatment, required actions, and outcome. Laboratory tests and vital signs were not included in the standard case report form. However, if they were considered abnormal by the treating surgeon, they had to be documented as an adverse event.
The values are given as the number of patients, with the percentage in parentheses.
The radiographic assessment revealed excellent and good results in >80% of all patients in both groups (Table VIII, Fig. 5). The results were fair only in 7 patients in the autologous iliac bone-graft group and 8 patients in the CBVF group, according to the Rasmussen score. The differences between the groups were not significant. Fracture union was observed in all patients. At week 26, remodeling of the bone defects with either nondirectional (R2) or directional (R3) trabecular structures was detected in >80% of all patients in each group (Table IX). The differences between the groups were not significant.
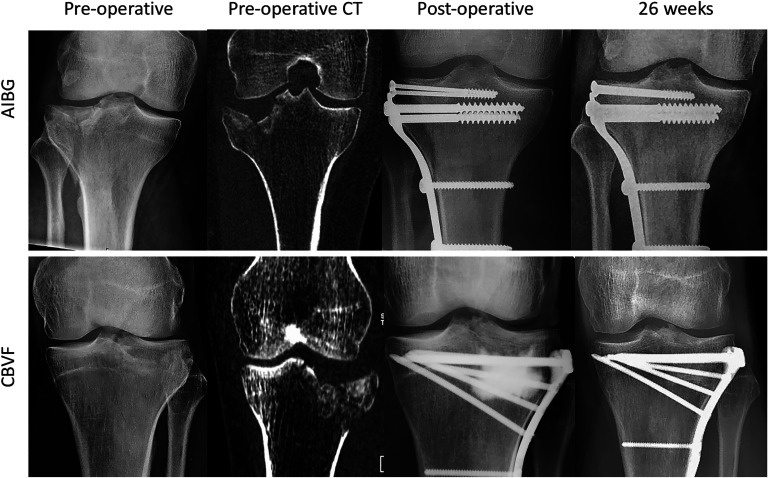
Fig. 5.
Representative radiographs showing the follow-up of a split-depression-type tibial plateau fracture (OTA/AO 41-B3, Schatzker type 2) in the autologous iliac bone-graft (AIBG) group and a depression-type tibial plateau fracture (OTA/AO 41-B2, Schatzker type 3) in the CBVF group. At the final follow-up visit, both fractures were healed without any complications (Rasmussen score, 18 points). The AIBG is visible in the postoperative radiograph directly underneath the lag screws. In the CBVF group, the radiopaque area in the lateral metaphysis corresponds to the Iohexol contrast agent in the applied bone cement. Iohexol diffuses away from the cement within 2 to 3 days and does not impair the assessment of the bone defect healing at later time points. At 26 weeks, bone defect healing with directional formation of bone trabecula was noticed in the standard anteroposterior radiograph in both groups (Jerosch score, 5 points).
TABLE VIII.
Radiographic Outcomes in the Intention-to-Treat Population (N = 133) According to the Rasmussen Score*
| Variable | Autologous Iliac Bone-Graft Group† (N = 68) | CBVF Group† (N = 65) | Total† (N = 133) | P Value‡ |
| Joint-line depression | 0.9144 | |||
| None | 21 (32.8%) | 21 (36.2%) | 42 (34.4%) | |
| <6 mm | 41 (64.1%) | 35 (60.3%) | 76 (62.3%) | |
| 6 to 10 mm | 2 (3.1%) | 2 (3.4%) | 4 (3.3%) | |
| Condylar widening | 0.3232 | |||
| None | 12 (18.7%) | 11 (19.0%) | 23 (18.8%) | |
| <6 mm | 52 (81.2%) | 45 (77.6%) | 97 (79.5%) | |
| 6 to 10 mm | 0 (0.0%) | 2 (3.4%) | 2 (1.6%) | |
| Varus and valgus angulation | 0.4371 | |||
| None | 22 (34.4%) | 26 (44.8%) | 48 (39.3%) | |
| <10° | 36 (56.2%) | 26 (44.8%) | 62 (50.8%) | |
| 10° to 20° | 6 (9.4%) | 6 (10.3%) | 12 (9.8%) | |
| Sum: score and grade | 0.1109 | |||
| 7 to 12 (fair) | 7 (11.0%) | 8 (13.7%) | 15 (13.3%) | |
| 13 to 17 (good)l | 21 (32.8%) | 8 (13.8%) | 29 (23.8%) | |
| 18 (excellent) | 36 (56.3%) | 42 (71.4%) | 78 (63.9%) | |
| Missing | 4 | 7 | 11 |
The Rasmussen score assesses articular subsidence (none, <6 mm, 6 to 10 mm), condylar widening (none, <6 mm, 6 to 10 mm), and varus or valgus deviation (none, <10°, 10° to 20°), which allows for grading the outcomes in excellent, good, fair, or poor.
†The values are given as the number of patients, with the percentage in parentheses.
Chi-square test.
TABLE IX.
Bone-Defect Healing in the Intention-to-Treat Population (N = 133) According to the Jerosch Score*
| Visit and Stage of Remodeling | Autologous Iliac Bone-Graft Group† (N = 68) | CBVF Group† (N = 65) | Total† (N = 133) | P Value‡ |
| Visit 5: week 6 | 0.6612 | |||
| R0 (no bone remodeling visible) | 1 (1.8%) | 3 (5.9%) | 4 (3.8%) | |
| R1 (beginning bone remodeling with periosteal bridging) | 27 (50.0%) | 24 (47.1%) | 51 (48.6%) | |
| R2 (bone remodeling with nondirectional trabecular structure) | 21 (38.9%) | 21 (41.2%) | 42 (40.0%) | |
| R3 (complete bone remodeling with directional trabecular structure) | 5 (9.3%) | 3 (5.9%) | 8 (7.6%) | |
| Missing | 14 | 14 | 28 | |
| Visit 7: week 26 | 0.2306 | |||
| R1 (beginning bone remodeling with periosteal bridging) | 9 (18.0%) | 5 (10.4%) | 14 (14.3%) | |
| R2 (bone remodeling with nondirectional trabecular structure) | 26 (52.0%) | 33 (68.7%) | 59 (60.2%) | |
| R3 (complete bone remodeling with directional trabecular structure) | 15 (30.0%) | 10 (20.8%) | 25 (25.5%) | |
| Missing | 18 | 17 | 35 |
The Jerosch score assesses osteolysis, premature resorption of the graft (1 point), unchanged size of the void, no resorption of the bone graft (2 points), beginning marginal bone-defect remodeling (3 points), bone-defect remodeling with nondirectional formation of trabeculae (4 points), and bone-defect remodeling with directional formation of trabeculae (5 points).
The values are given as the number of patients, with or without the percentage in parentheses.
Chi-square test.
We also assessed the VAS scores at 1, 6, and 12 weeks in addition to the original study protocol. The mean VAS score was 2 points in both groups at visit 1, increased on the first postoperative day, and decreased until week 6. At 6 months, there was no significant difference between the groups in the VAS for pain (Fig. 3). The only significant difference was found on the first postoperative day, when the mean VAS was 4.8 points in the autologous iliac bone-graft group and 3.7 points in the CBVF group (p = 0.0079).
Discussion
Patient-reported outcome measures have become the standard measure for treatment effectiveness following surgical procedures23-26, because they reflect the patient’s perception of an abnormal physical or emotional state and are not reported by an observer. Perception of health and well-being (quality of life), although the most subjective of all patient-reported outcome measure elements, reflects patient recovery at the highest hierarchical level24. The most commonly used patient-reported outcome measures include pain scales (VAS)26 and quality-of-life questionnaires such as the SF-12, SF-36, and EQ-5D (EuroQol 5-Dimensions). In this study, we showed that the use of bioresorbable hydroxyapatite and calcium sulfate biomaterial for augmentation of bone defects in OTA/AO 41-B2 and OTA/AO 41-B3 fractures is noninferior to autologous iliac crest bone in terms of patient-reported functional outcomes and pain levels at 26 weeks. There were also no significant differences in the SF-12 MCS score, fracture-healing and bone-defect healing, complication rates, and numbers of adverse events between the 2 groups. The use of CBVF was associated with lower pain levels and reduced blood loss on the first postoperative day.
In 2008, Russell et al. published an RCT on 120 patients with tibial plateau fractures randomized to receive either autologous iliac bone graft or a calcium phosphate cement10. Both study groups showed similar union rates and time to union. Interestingly, there was a significantly higher rate of articular subsidence in the bone-graft group. In an RCT including 20 patients with tibial plateau fractures, Jónsson and Mjöberg used either porous titanium granules or autologous iliac bone graft27. Articular subsidence was lower and operative time was shorter when titanium granules were used. There were no significant differences between the 2 groups in terms of knee pain or functional outcome at 12 months. However, patients with autologous iliac bone graft experienced pain at the donor site. In these studies, the indication for bone void augmentation was to prevent articular subsidence and to promote bone-healing. These studies provided evidence for the effectiveness of the material to justify its use. Our results indicate that CBVF is noninferior to autologous iliac bone graft for bone void augmentation in fractures of the tibial plateau.
The primary strengths of this trial are the strict inclusion and exclusion criteria for the selection of patients with a well-defined fracture type, the large number of patients recruited, and the high rate of follow-up. By focusing on patients with an isolated tibial plateau fracture, we believe that the possible bias associated with multiple injuries has been reduced and the effect of the bone-harvesting procedure afforded greater weight in the study design.
One main limitation of this study was that our results reflected the early patient-reported outcome measures at 26 weeks. With a longer follow-up, patients might have exhibited a deterioration of the assessed parameters. The second important limitation was the unavoidable lack of blinding of both surgeon and patient. Despite our best efforts, 11 patients were lost to follow-up. Bone-grafting may not always be necessary in the treatment of fractures of the tibial plateau. The indication for bone-defect augmentation was confirmed by the most experienced surgeon in the respective study centers prior to randomization. However, we cannot entirely exclude all possibility of selection bias influencing the results. The assessment of bone-defect healing was performed using conventional radiographs, which do not allow a precise assessment of articular subsidence or bone remodeling. Postoperative computed tomography (CT) scans were not obtained, as they were not considered the standard of care, but might have allowed more accurate measurements of both the amount of subsidence and bone defect remodeling. However, we believe that the requirement that an experienced radiologist reliably identified the different grades of the Rasmussen score obviated this concern. Although the radiologist was blinded as to treatment group, we cannot fully exclude his ability to detect the type of treatment on the radiograph, resulting in an element of detection bias. Tibial plateau fractures may be associated with concomitant ligamentous injury, which was not assessed in magnetic resonance imaging (MRI) preoperatively because it was not considered to be the standard of care. Therefore, we cannot exclude possible functional deficits due to a concomitant ligamentous injury resulting in some degree of assessment bias. The intake of pain medication was not measured. However, it might have been helpful for the validation of pain assessment.
In conclusion, this prospective, multicenter, randomized trial showed noninferiority of CBVF compared with autologous iliac bone graft in tibial plateau fractures, with noninferior patient-reported outcomes.
Acknowledgments
Note: *The CERTiFy (CERament BVF-Treatment in Tibial Fractures) Study Group includes: Onays Al Sadi, MD, University Centre for Orthopaedics and Trauma Surgery, Dresden, Germany; Hagen Andruszkow, MD, PhD, Department of Trauma and Reconstructive Surgery, University Hospital RWTH Aachen, Aachen, Germany; Charlotte Arand, MD, Department of Orthopedics and Traumatology, University Medical Center Mainz, Mainz, Germany; Peter Biberthaler, MD, PhD, Department of Trauma Surgery, University of Munich, Munich, Germany; Tim Danko, MD, Department of Traumatology and Orthopaedics, Westpfalz-Clinics Kaiserslautern, Kaiserslautern, Germany; Michael Diefenbeck, MD, PhD, BONESUPPORT AB, Lund, Sweden; Sven-Oliver Dietz, MD, PhD, Department of Orthopedics and Traumatology, University Medical Center Mainz, Mainz, Germany; Jochen Franke, MD, BG Traumacenter Ludwigshafen, Ludwigshafen, Germany; Holger Freischmidt, MD, BG Traumacenter Ludwigshafen, Ludwigshafen, Germany; Stephan Frosch, MD, Department of Trauma Surgery, Plastic and Reconstructive Surgery, University Medical Centre Göttingen, Göttingen, Germany; Erol Gercek, MD, PhD, Center for Trauma and Orthopedic Surgery, Gemeinschaftsklinikum Mittelrhein, Evangelischer Stift Koblenz, Germany; Matthias Geyer, MD, Department of Trauma Surgery, GPR-Klinikum, Rüsselsheim, Germany; Martin Glombitza, MD, Department of Septic Surgery, BG Traumacenter Duisburg, Duisburg, Germany; Marc Hanschen, MD, PhD, Department of Trauma Surgery, University of Munich, Munich, Germany; Matthias Hansen, MD, PhD, Department of Trauma Surgery, Hochtaunus-Kliniken Bad Homburg, Bad Homburg, Germany; Martin Henri Hessmann, MD, PhD, Department of Orthopedics and Trauma Surgery, Academic Teaching Hospital, Fulda, Germany; Martijn Hofman, MD, Department of Trauma and Reconstructive Surgery, University Hospital RWTH Aachen, Aachen, Germany; Martin Holst, TFS Trial Form Support, Hamburg, Germany; Abdul Assim Kamand, MD, Department of Trauma Surgery, Hochtaunus-Kliniken Bad Homburg, Bad Homburg, Germany; Christian Kleber, MD, PhD, University Centre for Orthopaedics and Trauma Surgery, Dresden, Germany; Kai Kronfeld, Interdisciplinary Center for Clinical Trials (IZKS), University Medical Center Mainz, Mainz, Germany; Ingo Marzi, MD, PhD, Department of Trauma, Hand and Reconstructive Surgery, University Hospital, Frankfurt, Germany; Simon Meier, MD, Department of Trauma, Hand and Reconstructive Surgery, University Hospital, Frankfurt, Germany; Michael Müller, MD, PhD, Department of Trauma Surgery, Clinical Center Bayreuth, Bayreuth, Germany; Lars Peter Müller, MD, PhD, Center for Orthopedic and Trauma Surgery, University Medical Center, Cologne, Germany; Thomas Nusselt, MD, Center for Trauma and Orthopedic Surgery, Gemeinschaftsklinikum Mittelrhein, Evangelischer Stift Koblenz, Germany; Eva Pfeifer, MD, Department of Trauma Surgery, Clinical Center Bayreuth, Bayreuth, Germany; Christian Ruckes, Interdisciplinary Center for Clinical Trials (IZKS), University Medical Center Mainz, Mainz, Germany; Lothar Rudig, MD, PhD, Department of Trauma Surgery, GPR-Klinikum, Rüsselsheim, Germany; Stephan Sehmisch, MD, PhD, Department of Trauma Surgery, Plastic and Reconstructive Surgery, University Medical Centre, Göttingen, Germany; Joseph Stollberg-Stollberg, MD, Department of Trauma Surgery, University of Muenster, Muenster, Germany; Stephan Uschok, MD, Center for Orthopedic and Trauma Surgery, University Medical Center, Cologne, Germany; Dominik Maximilian Vogt, MD, Department of Orthopedics and Traumatology, University Medical Centre Schleswig Holstein, Campus Lübeck, Lübeck, Germany; Guido Wanner, MD, PhD, Department of Trauma Surgery, Schwarzwald-Baar Klinikum, Villingen-Schwenningen, Germany; Erik Wilde, MD, Department of Orthopedics and Traumatology, University Medical Centre Schleswig Holstein, Campus Lübeck, Lübeck, Germany; Veit Winkelbach, MD, Department of Orthopedics and Trauma Surgery, Academic Teaching Hospital, Fulda, Germany; and Simon Zeitter, MD, Department of Trauma Surgery, BG Traumacenter Duisburg, Duisburg, Germany.
The authors gratefully acknowledge Arndt Felten, MD (Department of Radiology, Academic Teaching Hospital of the Universities Mainz and Heidelberg, Westpfalz-Clinics Kaiserslautern, Germany), who performed the radiographic assessment.
Footnotes
CERTiFy Study Group members are listed in a Note at the end of the article.
Disclosure: This study was funded by BONESUPPORT, the manufacturer of the CERAMENT bone void filler that was used in this study. The funding source had no role in the study design, data collection and analysis, decision to publish, or manuscript preparation. The Disclosure of Potential Conflicts of Interest forms are provided with the online version of the article (http://links.lww.com/JBJS/F631).
Contributor Information
Collaborators: Onays Al Sadi, Hagen Andruszkow, Charlotte Arand, Peter Biberthaler, Tim Danko, Michael Diefenbeck, Sven-Oliver Dietz, Jochen Franke, Holger Freischmidt, Stephan Frosch, Erol Gercek, Matthias Geyer, Martin Glombitza, Marc Hanschen, Matthias Hansen, Martin Henri Hessmann, Martijn Hofman, Martin Holst, Abdul Assim Kamand, Christian Kleber, Kai Kronfeld, Ingo Marzi, Simon Meier, Michael Müller, Lars Peter Müller, Thomas Nusselt, Eva Pfeifer, Christian Ruckes, Lothar Rudig, Stephan Sehmisch, Joseph Stollberg-Stollberg, Stephan Uschok, Dominik Maximilian Vogt, Guido Wanner, Erik Wilde, Veit Winkelbach, and Simon Zeitter
Data Sharing
A data-sharing statement is provided with the online version of the article (http://links.lww.com/JBJS/F632).
References
- 1.Koval KJ, Helfet DL. Tibial plateau fractures: evaluation and treatment. J Am Acad Orthop Surg. 1995. March;3(2):86-94. [DOI] [PubMed] [Google Scholar]
- 2.Lachiewicz PF, Funcik T. Factors influencing the results of open reduction and internal fixation of tibial plateau fractures. Clin Orthop Relat Res. 1990. October;259:210-5. [PubMed] [Google Scholar]
- 3.Azi ML, Aprato A, Santi I, Kfuri M, Jr, Masse A, Joeris A. Autologous bone graft in the treatment of post-traumatic bone defects: a systematic review and meta-analysis. BMC Musculoskelet Disord. 2016. November 9;17(1):465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Myeroff C, Archdeacon M. Autogenous bone graft: donor sites and techniques. J Bone Joint Surg Am. 2011. December 7;93(23):2227-36. [DOI] [PubMed] [Google Scholar]
- 5.Silber JS, Anderson DG, Daffner SD, Brislin BT, Leland JM, Hilibrand AS, Vaccaro AR, Albert TJ. Donor site morbidity after anterior iliac crest bone harvest for single-level anterior cervical discectomy and fusion. Spine (Phila Pa 1976). 2003. January 15;28(2):134-9. [DOI] [PubMed] [Google Scholar]
- 6.Heneghan HM, McCabe JP. Use of autologous bone graft in anterior cervical decompression: morbidity & quality of life analysis. BMC Musculoskelet Disord. 2009. December 16;10(1):158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dimitriou R, Mataliotakis GI, Angoules AG, Kanakaris NK, Giannoudis PV. Complications following autologous bone graft harvesting from the iliac crest and using the RIA: a systematic review. Injury. 2011. September;42(Suppl 2):S3-15. Epub 2011 Jun 25. [DOI] [PubMed] [Google Scholar]
- 8.Sbitany H, Koltz PF, Waldman J, Girotto JA. Continuous bupivacaine infusion in iliac bone graft donor sites to minimize pain and hospitalization. Cleft Palate Craniofac J. 2010. May;47(3):293-6. [DOI] [PubMed] [Google Scholar]
- 9.Heikkilä JT, Kukkonen J, Aho AJ, Moisander S, Kyyrönen T, Mattila K. Bioactive glass granules: a suitable bone substitute material in the operative treatment of depressed lateral tibial plateau fractures: a prospective, randomized 1 year follow-up study. J Mater Sci Mater Med. 2011. April;22(4):1073-80. Epub 2011 Mar 23. [DOI] [PubMed] [Google Scholar]
- 10.Russell TA, Leighton RK; Alpha-BSM Tibial Plateau Fracture Study Group. Comparison of autogenous bone graft and endothermic calcium phosphate cement for defect augmentation in tibial plateau fractures. A multicenter, prospective, randomized study. J Bone Joint Surg Am. 2008. October;90(10):2057-61. [DOI] [PubMed] [Google Scholar]
- 11.Pernaa K, Koski I, Mattila K, Gullichsen E, Heikkila J, Aho A, Lindfors N. Bioactive glass S53P4 and autograft bone in treatment of depressed tibial plateau fractures - a prospective randomized 11-year follow-up. J Long Term Eff Med Implants. 2011;21(2):139-48. [DOI] [PubMed] [Google Scholar]
- 12.Meinberg EG, Agel J, Roberts CS, Karam MD, Kellam JF. Introduction: Fracture and dislocation classification compendium-2018. J Orthop Trauma. 2018. January;32(Suppl 1):S1-S170. [DOI] [PubMed] [Google Scholar]
- 13.Nusselt T, Hofmann A, Wachtlin D, Gorbulev S, Rommens PM. CERAMENT treatment of fracture defects (CERTiFy): protocol for a prospective, multicenter, randomized study investigating the use of CERAMENT™ BONE VOID FILLER in tibial plateau fractures. Trials. 2014. March 8;15(1):75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shaw KA, Griffith MS, Shaw VM, Devine JG, Gloystein DM. Harvesting autogenous cancellous bone graft from the anterior iliac crest. JBJS Essent Surg Tech. 2018. July 11;8(3):e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hettwer W, Horstmann PF, Bischoff S, Güllmar D, Reichenbach JR, Poh PSP, van Griensven M, Gras F, Diefenbeck M. Establishment and effects of allograft and synthetic bone graft substitute treatment of a critical size metaphyseal bone defect model in the sheep femur. APMIS. 2019. February;127(2):53-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nilsson M, Wang JS, Wielanek L, Tanner KE, Lidgren L. Biodegradation and biocompatability of a calcium sulphate-hydroxyapatite bone substitute. J Bone Joint Surg Br. 2004. January;86(1):120-5. [PubMed] [Google Scholar]
- 17.Abramo A, Geijer M, Kopylov P, Tägil M. Osteotomy of distal radius fracture malunion using a fast remodeling bone substitute consisting of calcium sulphate and calcium phosphate. J Biomed Mater Res B Appl Biomater. 2010. January;92(1):281-6. [DOI] [PubMed] [Google Scholar]
- 18.Iundusi R, Gasbarra E, D’Arienzo M, Piccioli A, Tarantino U. Augmentation of tibial plateau fractures with an injectable bone substitute: CERAMENT™. Three year follow-up from a prospective study. BMC Musculoskelet Disord. 2015. May 13;16(1):115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McNally MA, Ferguson JY, Lau ACK, Diefenbeck M, Scarborough M, Ramsden AJ, Atkins BL. Single-stage treatment of chronic osteomyelitis with a new absorbable, gentamicin-loaded, calcium sulphate/hydroxyapatite biocomposite: a prospective series of 100 cases. Bone Joint J. 2016. September;98-B(9):1289-96. [DOI] [PubMed] [Google Scholar]
- 20.Rasmussen PS. Tibial condylar fractures. Impairment of knee joint stability as an indication for surgical treatment. J Bone Joint Surg Am. 1973. October;55(7):1331-50. [PubMed] [Google Scholar]
- 21.Jerosch J, Castro WHM, Halm H, Assheuer J. Verlaufskontrolle mit unterschiedlichen bildgebenden verfahren über die einheilung von autogenen und allogenen knochentransplantaten. Orthop Prax. 1994;(30):377-83. [Google Scholar]
- 22. https://www.meddra.org/ International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. MedDRA (Medical Dictionary for Regulatory Activities) Accessed 2019 Nov 6.
- 23.Gagnier JJ. Patient reported outcomes in orthopaedics. J Orthop Res. 2017. October;35(10):2098-108. Epub 2017 Jun 13. [DOI] [PubMed] [Google Scholar]
- 24.Bikhchandani J. Enhanced recovery after surgery and its effects on patient reported outcomes. Surg Clin North Am. 2018. December;98(6):1129-35. Epub 2018 Sep 28. [DOI] [PubMed] [Google Scholar]
- 25.Ferguson RJ, Palmer AJ, Taylor A, Porter ML, Malchau H, Glyn-Jones S. Hip replacement. Lancet. 2018. November 3;392(10158):1662-71. [DOI] [PubMed] [Google Scholar]
- 26.Gallagher EJ, Liebman M, Bijur PE. Prospective validation of clinically important changes in pain severity measured on a visual analog scale. Ann Emerg Med. 2001. December;38(6):633-8. [DOI] [PubMed] [Google Scholar]
- 27.Jónsson BY, Mjöberg B. Porous titanium granules are better than autograft bone as a bone void filler in lateral tibial plateau fractures: a randomised trial. Bone Joint J. 2015. June;97-B(6):836-41. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
A data-sharing statement is provided with the online version of the article (http://links.lww.com/JBJS/F632).