Abstract
Background:
Dysphagia is one of the most common complications of surgical procedures in the anterior cervical spine, and can persist up to 2 years postoperatively. Corticosteroids are relatively safe and inexpensive for treating various inflammatory conditions. Perioperative corticosteroid administration for anterior cervical spine procedures may effectively minimize postoperative dysphagia, potentially leading to better outcomes, decreased readmission rates, and improved patient satisfaction. The purpose of this study was to determine the efficacy of perioperative corticosteroids in decreasing the severity and duration of dysphagia following single-level and multilevel anterior cervical spine procedures.
Methods:
Seventy-four patients undergoing elective anterior cervical surgical procedures for degenerative conditions were recruited. Patients with prior cervical procedures; with a diagnosis of fracture, malignancy, or infection; or requiring combined anterior-posterior procedures were excluded. Patients were randomized to perioperative intravenous dexamethasone or saline solution. Doses were administered before incision and at 8 and 16 hours postoperatively. Investigators and patients were blinded to the treatment throughout the study. Dysphagia outcomes were assessed with use of the Bazaz dysphagia scale and the Dysphagia Short Questionnaire (DSQ) at 1 day, 2 days, 1 week, 2 weeks, 1 month, 3 months, 6 months, and 12 months postoperatively. Statistical analysis was performed comparing means and standard deviations; significance was set at p < 0.05. Clinical outcomes were measured with use of the Quality of Life-12 and Neck Disability Index.
Results:
Sixty-four patients were included in the analysis. There were 49 anterior cervical discectomies and fusions, 8 corpectomies, 1 hybrid procedure (corpectomy and adjacent discectomy), and 6 single-level arthroplasties. Patients who received corticosteroids had significantly better dysphagia scores on both the Bazaz scale and DSQ at most time points up to 6 months postoperatively (p < 0.05). On subgroup analysis, patients with multilevel (≥2-level) fusion benefited significantly from corticosteroids on both scales, whereas those with single-level procedures did not. There were no short-term wound complications or infections, and length of stay and fusion rates were comparable.
Conclusions:
Perioperative administration of corticosteroids can reduce dysphagia symptoms following multilevel anterior cervical procedures. Benefit was noted immediately and up to 6 months postoperatively. There was no significant effect on short-term wound-healing, infection rates, length of stay, or fusion rates.
Level of Evidence:
Therapeutic Level I. See Instructions for Authors for a complete description of levels of evidence.
Dysphagia is one of the most common complications of one of the most commonly performed spinal surgical procedures. Postoperative dysphagia following anterior cervical spine procedures can have immediate and long-term impact for patients. Patients are typically hospitalized following many of these procedures, and dysphagia occurring immediately postoperatively can increase the length of stay for these patients. For patients in whom such a surgical procedure is planned as outpatient, dysphagia is a potential cause of admission or readmission. One recent meta-analysis found that dysphagia accounted for 62% of the complications reported for outpatient anterior cervical surgical procedures1. Moreover, the presence of dysphagia has been shown to significantly increase 1-year medical costs2. Although symptoms of postoperative dysphagia resolve over time in the majority of patients, there remains a percentage of patients who have substantial trouble swallowing even up to 2 years postoperatively3.
Although not completely understood, risk factors for postoperative dysphagia that have been widely reported include multilevel procedures, surgical procedures of the upper cervical spine, revision procedures, hardware prominence, increased surgical time, prior history of dysphagia, older age, and female sex. Proposed means for mitigating dysphagia include preoperative tracheal and esophageal traction exercises, reduction of endotracheal tube cuff pressure after placement of retractors, use of dynamic rather than self-retaining retractors, use of lower-profile instrumentation, a cervical approach that remains lateral to the omohyoid muscle in the upper cervical spine and medial to it at lower levels, avoidance of prolonged surgical time, and use of corticosteroids4.
Perioperative corticosteroids are known to be a safe and inexpensive pharmacologic treatment for various clinical conditions, including spinal cord compression, brain swelling, and respiratory conditions. A simple protocol for administration of perioperative corticosteroids for anterior cervical spine procedures may be effective in minimizing postoperative dysphagia, which could potentially lead to better patient outcomes, decreased readmission rates, and improved patient satisfaction. The purpose of the present study was to determine the efficacy of perioperative administration of intravenous (IV) corticosteroids in decreasing the severity and duration of dysphagia following anterior cervical spine surgical procedures.
Materials and Methods
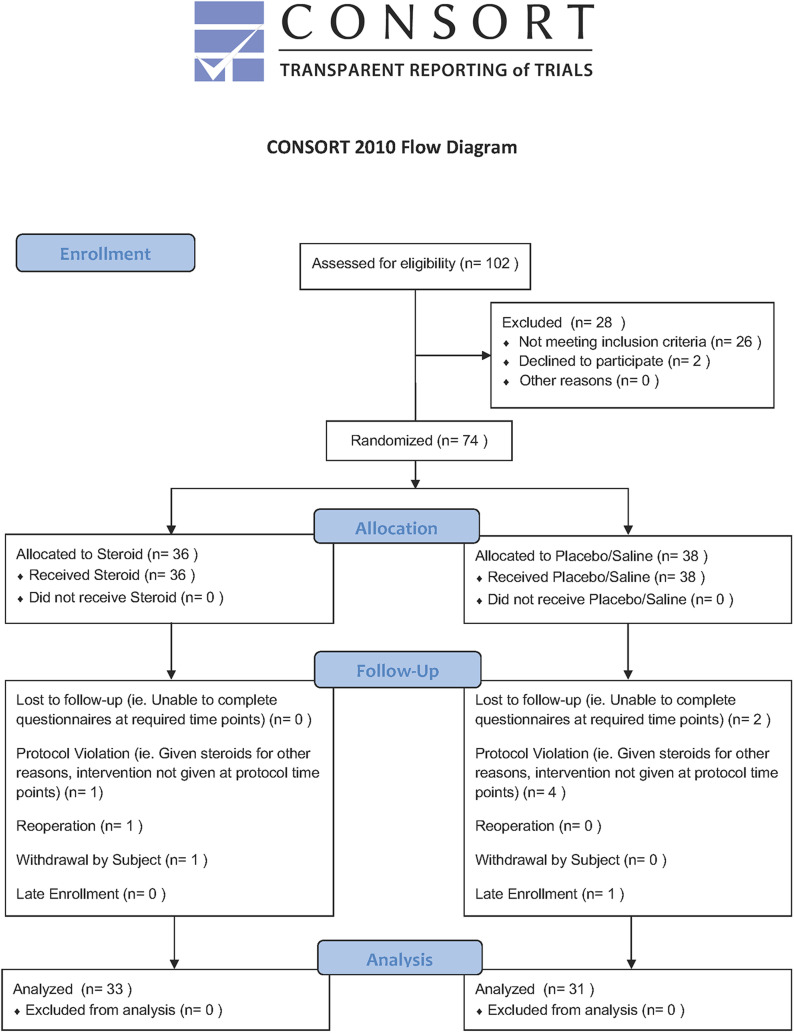
This study was a prospective, double-blinded, randomized controlled trial. Institutional review board approval was obtained, and the study was registered at ClinicalTrials.org (NCT02416934). All patients scheduled for elective anterior cervical spine procedures at our academic tertiary transfer hospital were considered for enrollment by the clinical trials manager (Fig. 1). Patients scheduled for revision procedures; those requiring combined anterior and posterior approaches; patients undergoing surgical procedures for traumatic, neoplastic, or infectious indications; and any patients already receiving or with medical contraindications to systemic corticosteroids were excluded.
Fig. 1.
CONSORT (Consolidated Standards of Reporting Trials) flow diagram.
After granting informed consent, patients were randomized to receive either perioperative IV dexamethasone or placebo. Randomization was performed with use of a computer spreadsheet random number generator. Utilizing the random number list, the investigational pharmacist assigned a code determining whether the patient would receive corticosteroid or placebo. The investigational pharmacist was the only individual who was unblinded to the treatment delivered. Patients receiving the corticosteroids were administered dexamethasone at a dose of 0.3 mg/kg preoperatively followed by 0.15 mg/kg every 8 hours for 2 doses. Patients randomized to receive placebo were administered an equivalent volume of saline solution at the same time points. Medications were mixed by the investigational pharmacy and delivered to the operating room with contents blinded to the treating anesthesiologist, surgeon, clinical trials manager, and patient. Preoperative dosages were administered by the anesthesiologist prior to incision. Postoperative dosages were administered by nurses in the inpatient units at 8 and 16 hours postoperatively. Order sets were created within the EPIC electronic medical record (EMR) system (EPIC Systems) to standardize the drug administration protocol for all enrolled patients. The process was overseen and coordinated by our clinical trials manager, who was also blinded to the treatment. Patient recruitment was initiated on institutional review board approval in July 2012 and continued until 64 patients completed 12 months of follow-up.
The primary outcome was change in swallowing as assessed with the Bazaz scale and the Dysphagia Short Questionnaire (DSQ); these data were collected preoperatively and at 1 day, 2 days, 1 week, 2 weeks, 1 month, 3 months, 6 months, and 1 year postoperatively. Both the Bazaz scale and DSQ are established tools for measurement of dysphagia based on patient-reported symptoms5,6. Patients also completed the Quality of Life-12 and Neck Disability Index questionnaires and a visual analog scale for pain preoperatively and at 3 months, 6 months, and 1 year postoperatively. Additional data collected included patient comorbidities, levels and types of surgical procedures, intraoperative fluids, estimated blood loss, types of retractors, type of postoperative bracing (if any), length of stay, and other surgically related complications. Osseous fusion was evaluated with use of flexion/extension radiographs at the last clinic visit and, if available, with use of computed tomography (CT) or magnetic resonance imaging (MRI). We considered fusion to be achieved if radiographs demonstrated <1 mm of interspinous motion between flexion and extension7 or if CT or MRI demonstrated clear evidence of bone bridging from end plate to end plate.
Statistical Analysis
A power analysis based on a minimal clinically important difference of 30% in primary outcome scores, an alpha of 0.05, and a beta of 0.2 estimated a need for 32 patients per group. Assuming a dropout rate of 10%, the total sample size target for the study was set at 70 patients.
Analysis of variance (ANOVA) and Fisher exact tests were utilized to compare differences in demographics between corticosteroid and placebo groups. The results reported come from a mixed-model ANOVA in which the random factor was subject nested within group. Both the group and time factors were significant. Because the group factor was significant, we used contrasts to examine significance at individual time points. The method is known as the Fisher protected t test. Differences in fusion rates between the 2 groups were compared with the Fisher exact test and chi-square analysis.
Results
A total of 74 patients were recruited, of whom 64 patients completed the 12-month follow-up and were included in the study (Fig. 1). There were no significant differences in patient demographics, comorbidities, or number of levels involved (Table I). There were 33 patients in the corticosteroid group and 31 in the placebo group. The most commonly performed procedure was anterior cervical discectomy and fusion (49 patients), followed by anterior corpectomy and disc arthroplasty (Table II).
TABLE I.
Patient Demographics
| Corticosteroid Group | Saline Solution Group | P Value | |
| Age* (yr) | 53.2 | 50.3 | 0.2886 |
| Sex† | 0.6147 | ||
| Female | 20 | 16 | |
| Male | 13 | 15 | |
| Smoking† | 0.6210 | ||
| Nonsmokers | 18 | 19 | |
| Smokers | 15 | 12 | |
| BMI* (kg/m2) | 30.1 | 32.0 | 0.3839 |
| ASA grade* | 2.6 | 2.4 | 0.2482 |
| No. of levels fused* | 1.7 | 1.5 | 0.2921 |
The values are given as the mean. BMI = body mass index, and ASA = American Society of Anesthesiologists.
The values are given as the count.
TABLE II.
Surgical Procedures*
| Corticosteroid Group | Saline Solution Group | |
| Single level (n = 33) | 15 | 18 |
| ACDF (n = 27) | 13 | 14 |
| Disc arthroplasty (n = 6) | 2 | 4 |
| Multilevel (n = 31) | 18 | 13 |
| ACDF (n = 22) | 11 | 11 |
| 2 levels (n = 16) | ||
| 3 levels (n = 6) | ||
| Corpectomy (n = 8) | 6 | 2 |
| Hybrid (n = 1) | 1 | 0 |
The values are given as the count. ACDF = anterior cervical discectomy and fusion, hybrid = ACDF with adjacent corpectomy.
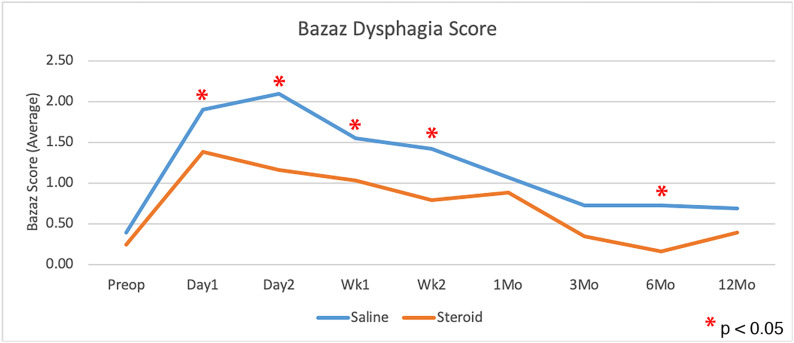
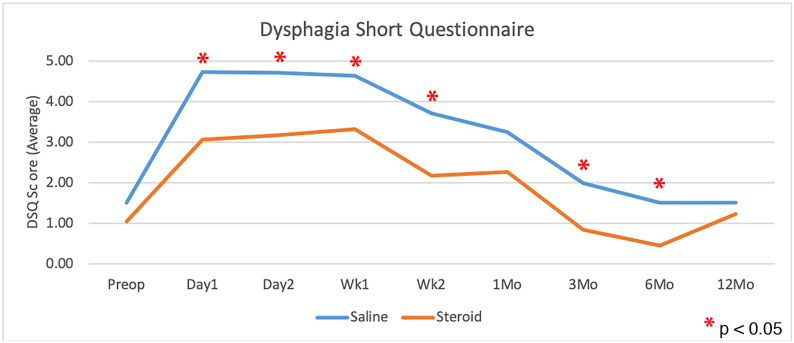
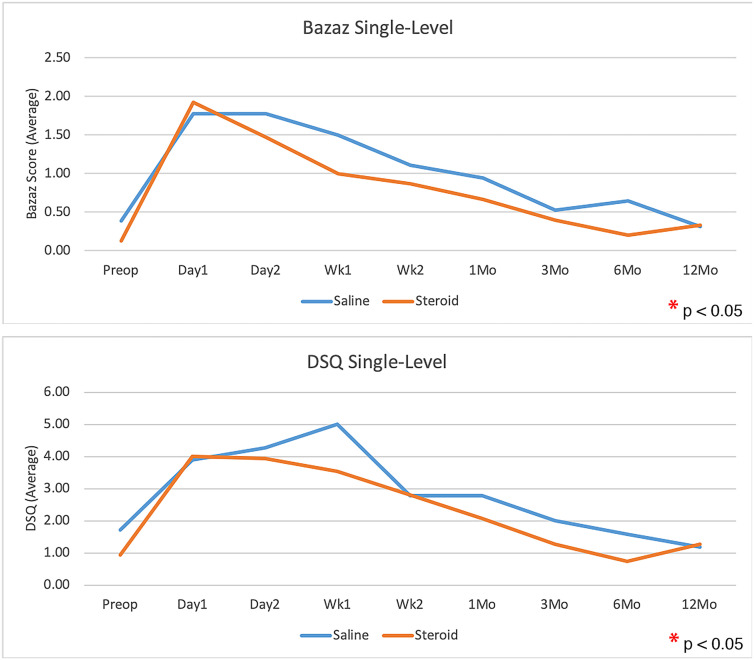
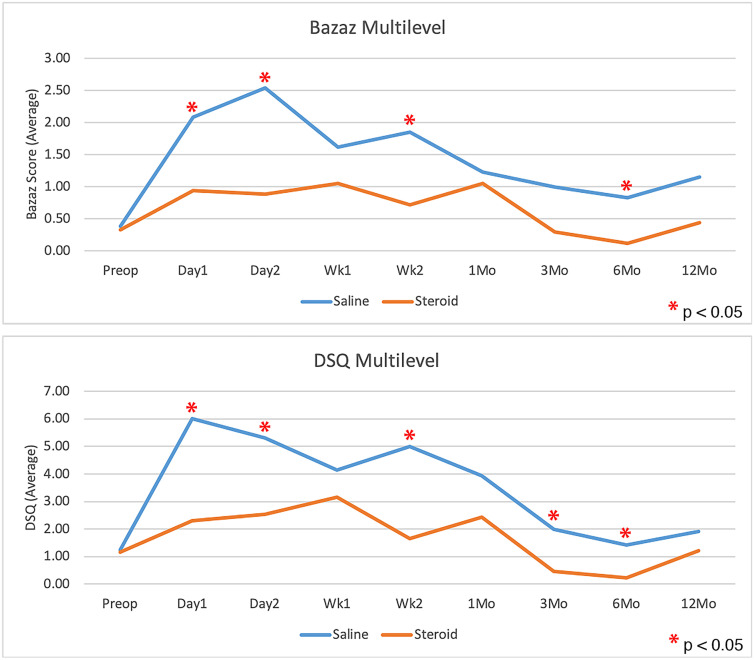
Patients who received corticosteroids had significantly less dysphagia than control patients, as measured with use of the Bazaz scale and DSQ, averaged over all time points (p = 0.0108 and p = 0.0202, respectively; Figs. 2 and 3). The largest difference appeared to be within the first 2 weeks, with the scores showing no significant difference at 1 year (Table III). There was no significant difference in dysphagia between single-level and multilevel procedures in patients who received corticosteroids or among those who received saline solution with the exception of a greater Bazaz dysphagia score on postoperative day 1 in single-level patients who received corticosteroids (p = 0.0480). The difference in DSQ at this time point did not reach significance (p = 0.0646). Patients who underwent single-level procedures showed no significant difference in dysphagia between corticosteroid and saline solution groups in overall average dysphagia (p = 0.3636 and p = 0.4698 for Bazaz and DSQ, respectively), or at any individual time point (Fig. 4). Corticosteroids significantly decreased dysphagia in patients with multilevel procedures overall (p = 0.0027 and p = 0.0044 for Bazaz and DSQ, respectively) and within the first 6 months after the surgical procedure (Fig. 5). Only 6 patients underwent arthroplasty procedures, and compared with all fusion patients, they demonstrated decreased dysphagia on postoperative day 2 only (p = 0.0174 and p = 0.0297 for Bazaz and DSQ, respectively). The small number of arthroplasty patients did not warrant statistical analysis of the impact of corticosteroids for this subgroup.
Fig. 2.
Comparison of average Bazaz dysphagia scores for the saline control and corticosteroid groups preoperatively and at each time point postoperatively (single and multilevel procedures combined). *Denotes a significant difference.
Fig. 3.
Comparison of average DSQ scores for the saline control and corticosteroid groups preoperatively and at each time point postoperatively (single and multilevel surgeries combined). *Denotes a significant difference.
Fig. 4.
Bazaz and DSQ dysphagia scores for patients who underwent single-level procedures only, comparing the saline control group with the corticosteroid at each time point. No significant difference was found.
Fig. 5.
Bazaz (top) and DSQ dysphagia (bottom) scores for patients who underwent multilevel procedures only, comparing the saline control group with the corticosteroid at each time point. *Denotes a significant difference.
TABLE III.
Dysphagia Outcome Scores*
| Corticosteroid Group | Saline Solution Group | P Value | |
| Bazaz dysphagia score | |||
| Preop. | 0.24 ± 0.61 | 0.39 ± 0.76 | 0.5235 |
| Day 1 | 1.38 ± 1.35 | 1.90 ± 1.40 | 0.0169† |
| Day 2 | 1.16 ± 1.35 | 2.10 ± 1.33 | <0.0001† |
| Week 1 | 1.03 ± 1.29 | 1.55 ± 1.26 | 0.0227† |
| Week 2 | 0.79 ± 1.08 | 1.42 ± 1.23 | 0.0055† |
| 1 month | 0.88 ± 1.22 | 1.06 ± 1.09 | 0.4128 |
| 3 months | 0.34 ± 0.94 | 0.72 ± 1.00 | 0.1154 |
| 6 months | 0.16 ± 0.63 | 0.72 ± 1.07 | 0.0173† |
| 12 months | 0.39 ± 0.83 | 0.69 ± 1.17 | 0.1998 |
| DSQ | |||
| Preop. | 1.06 ± 1.41 | 1.52 ± 2.10 | 0.3808 |
| Day 1 | 3.06 ± 2.57 | 4.73 ± 3.06 | 0.0017† |
| Day 2 | 3.19 ± 3.20 | 4.71 ± 2.85 | 0.0043† |
| Week 1 | 3.33 ± 3.22 | 4.65 ± 3.74 | 0.0118† |
| Week 2 | 2.18 ± 2.17 | 3.71 ± 3.27 | 0.0034† |
| 1 month | 2.27 ± 2.92 | 3.26 ± 3.29 | 0.0584 |
| 3 months | 0.84 ± 1.71 | 2.00 ± 2.54 | 0.0331† |
| 6 months | 0.47 ± 1.50 | 1.52 ± 2.40 | 0.0324† |
| 12 months | 1.24 ± 2.72 | 1.52 ± 2.89 | 0.5216 |
The values are given as the mean and standard deviation.
Significant.
The number of levels operated on, operative time, IV fluids, and estimated blood loss had no effect on dysphagia. There were no early postoperative complications. There was no significant difference in Quality of Life-12, Neck Disability Index, or visual analog scale pain scores between groups. The average length of stay was 1.26 days, with a nonsignificant trend toward decreased length of stay in the corticosteroid group (1.18 compared with 1.35 days). Excluding patients who underwent arthroplasty, fusion rates were 78% for the corticosteroid group and 75% for the control group, which were not significantly different.
Discussion
We found that a 3-dose regimen of perioperative IV dexamethasone was associated with significantly less dysphagia as measured with use of 2 different dysphagia measurement tools. Moreover, the data showed no deleterious effects of perioperative corticosteroids with regard to medical or surgical complications or fusion. Quality of life, pain, and disability assessments were similar in both groups of patients.
The findings of the present study are consistent with those of prior studies of perioperative IV corticosteroids. In the study by Song et al., 20 patients undergoing multilevel anterior cervical discectomy and fusion were administered 4 doses of methylprednisolone during the first 24 hours postoperatively and were compared with 20 patients not receiving corticosteroids8. The authors used the Bazaz scale, administered for 5 days postoperatively, reporting decreased severity of dysphagia in the corticosteroid group starting on the second postoperative day, which was maintained through the 5 days of evaluation. Unfortunately, the authors did not conduct any longer-term follow-up. In another study, Jeyamohan et al. prospectively randomized 112 patients undergoing multilevel anterior cervical fusion to receive corticosteroids (dexamethasone administered once intraoperatively, then 4 times postoperatively over the subsequent 24 hours) or saline solution9. The authors evaluated dysphagia with use of the functional outcome swallowing scale, and also evaluated potential impact on spinal fusion at multiple time points from 1 month to 2 years postoperatively. The authors found significantly decreased dysphagia in the corticosteroid group at 1 month postoperatively, although both groups had similar rates of dysphagia at all subsequent follow-up time points. Additionally, the authors noted that although the corticosteroid group had significantly lower fusion rates at 6 months, there was no difference in fusion rates between groups by 12 months postoperatively9.
Local administration of corticosteroids has also been advocated as a method to reduce dysphagia. Lee et al. found that the application of triamcinolone and morselized collagen sponge in the retropharyngeal space reduced odynophagia (assessed with use of a visual analog pain scale) and radiographic soft-tissue swelling up to 2 weeks postoperatively10. With use of a large insurance-based database, Cancienne et al. evaluated the impact of local corticosteroid administration on dysphagia in patients undergoing long (≥3 levels) or short (1 to 2 levels) anterior cervical fusions11. The authors reported a lower incidence of dysphagia and shorter length of stay in patients undergoing longer fusions who received local corticosteroids compared with those who did not, with no difference in wound complications or infection rate. Those results, however, are tempered by the inherent limitations of large administrative database studies, which rely on accurate diagnosis and procedure coding. Koreckij et al. retrospectively reviewed 44 patients undergoing multilevel anterior cervical fusions, half of whom received methylprednisolone applied to an absorbable collagen sponge and placed in the retropharyngeal space before closure12. The authors noted lower incidence of dysphagia as measured with Bazaz and Eating Assessment Tool-10 scores at 6 weeks and 3 months postoperatively. In a randomized, prospective study, Edwards et al. reported lower incidence and severity of dysphagia in patients who received local corticosteroids (methylprednisolone on a collagen sponge) following anterior cervical fusion with use of recombinant human bone morphogenetic protein-2 (rhBMP-2)13. In a recent study, Jenkins et al. showed lower rates of dysphagia in patients who received either local corticosteroids (triamcinolone) or IV corticosteroids (dexamethasone) compared with control patients, with the local administration group appearing to have lower incidence of “severe” dysphagia in the first 2 weeks14. Despite improved dysphagia, complications have been reported with local corticosteroid administration in these patients. Lee et al. reported 2 cases of delayed esophageal perforation following retropharyngeal corticosteroid application, 1 of which occurred only 2 months postoperatively15.
The present prospective, randomized, placebo-controlled, double-blinded study found that administration of perioperative IV corticosteroids reduced dysphagia without compromising fusion or clinical outcomes. Two different established patient-reported dysphagia measurement tools were utilized. Unlike previous studies, improvement in dysphagia symptoms was noted beginning on the first postoperative day, which remained at 6 months postoperatively. At 1 year postoperatively, there was no significant difference in dysphagia symptoms, radiographic fusion rate, or clinical outcomes between groups. Also unlike prior studies, both single-level and multilevel procedures were included. Although the incidence of dysphagia generally increases as the number of levels operated on increases, there is still a percentage of patients undergoing single-level procedures who will experience dysphagia16. Although we found no difference for single-level procedures, the present data suggest that perioperative IV dexamethasone is effective in reducing dysphagia in multilevel procedures.
This study is not without limitations; most notably, we included a wide array of surgical techniques (discectomy, corpectomy, and arthroplasty) as well as both single-level and multilevel procedures. Some studies have suggested that arthroplasty may result in a lower incidence of dysphagia than fusion17, although other studies have suggested otherwise18. Although multilevel procedures are a documented risk factor for dysphagia, the incidence of dysphagia after single-level surgical procedures is not zero16. The effect size in single-level procedures, however, may be smaller; therefore, this study is underpowered to adequately assess for dysphagia reduction in single-level anterior cervical surgical procedures. We believe, however, that our randomization process effectively eliminated the impact of these confounding variables, with a similar distribution of levels and techniques in both groups. Moreover, we believe that the inclusion of a varied surgical population more accurately represents clinical practice, thereby making the findings more broadly applicable.
One of the challenges in assessing dysphagia is that no “gold standard” patient-reported assessment tool exists. The questionnaires we chose are fairly easy for patients to complete and represent a contemporaneous self-assessment of swallowing. Although widely used, the Bazaz scale has not been independently validated; on the other hand, the DSQ has been6. Nevertheless, the use of multiple classification systems has been advocated by other authors to improve the overall assessment of dysphagia19.
In conclusion, we found that the perioperative administration of IV dexamethasone led to significantly lower incidence of dysphagia up to 6 months after an anterior cervical surgical procedure, particularly in multilevel patients. The present data support the use of perioperative corticosteroids in these patients, which represents a relatively inexpensive, safe treatment option that improved dysphagia without compromising clinical or radiographic outcomes.
Acknowledgments
Note: The authors thank Gerald Hobbs, PhD, for his statistical expertise assisting in both study design as well as data analysis. The authors thank Sheila Rye, clinical trials manager, for her significant efforts in every phase of the active study to ensure its completion and success.
Footnotes
Investigation performed at the Department of Orthopaedic Surgery, West Virginia University, Morgantown, West Virginia
Disclosure: This study was supported by a Cervical Spine Research Society 21st Century Development Grant. On the Disclosure of Potential Conflicts of Interest forms, which are provided with the online version of the article, one or more of the authors checked “yes” to indicate that the author had other relationships or activities that could be perceived to influence, or have the potential to influence, what was written in this work (http://links.lww.com/JBJS/F502).
Data Sharing
A data-sharing statement is provided with the online version of the article (http://links.lww.com/JBJS/F504).
References
- 1.Ban D, Liu Y, Cao T, Feng S. Safety of outpatient anterior cervical discectomy and fusion: a systematic review and meta-analysis. Eur J Med Res. 2016. August 31;21(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonilha HS, Simpson AN, Ellis C, Mauldin P, Martin-Harris B, Simpson K. The one-year attributable cost of post-stroke dysphagia. Dysphagia. 2014. October;29(5):545-52. Epub 2014 Jun 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shriver MF, Lewis DJ, Kshettry VR, Rosenbaum BP, Benzel EC, Mroz TE. Dysphagia rates after anterior cervical diskectomy and fusion: a systematic review and meta-analysis. Global Spine J. 2017. February;7(1):95-103. Epub 2017 Feb 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Joaquim AF, Murar J, Savage JW, Patel AA. Dysphagia after anterior cervical spine surgery: a systematic review of potential preventative measures. Spine J. 2014. September 1;14(9):2246-60. Epub 2014 Mar 21. [DOI] [PubMed] [Google Scholar]
- 5.Bazaz R, Lee MJ, Yoo JU. Incidence of dysphagia after anterior cervical spine surgery: a prospective study. Spine (Phila Pa 1976). 2002. November 15;27(22):2453-8. [DOI] [PubMed] [Google Scholar]
- 6.Skeppholm M, Ingebro C, Engström T, Olerud C. The Dysphagia Short Questionnaire: an instrument for evaluation of dysphagia: a validation study with 12 months’ follow-up after anterior cervical spine surgery. Spine (Phila Pa 1976). 2012. May 15;37(11):996-1002. [DOI] [PubMed] [Google Scholar]
- 7.Rhee JM, Chapman JR, Norvell DC, Smith J, Sherry NA, Riew KD. Radiological determination of postoperative cervical fusion: a systematic review. Spine (Phila Pa 1976). 2015. July 1;40(13):974-91. [DOI] [PubMed] [Google Scholar]
- 8.Song KJ, Lee SK, Ko JH, Yoo MJ, Kim DY, Lee KB. The clinical efficacy of short-term steroid treatment in multilevel anterior cervical arthrodesis. Spine J. 2014. December 1;14(12):2954-8. Epub 2014 Jun 12. [DOI] [PubMed] [Google Scholar]
- 9.Jeyamohan SB, Kenning TJ, Petronis KA, Feustel PJ, Drazin D, DiRisio DJ. Effect of steroid use in anterior cervical discectomy and fusion: a randomized controlled trial. J Neurosurg Spine. 2015. August;23(2):137-43. Epub 2015 May 1. [DOI] [PubMed] [Google Scholar]
- 10.Lee SH, Kim KT, Suk KS, Park KJ, Oh KI. Effect of retropharyngeal steroid on prevertebral soft tissue swelling following anterior cervical discectomy and fusion: a prospective, randomized study. Spine (Phila Pa 1976). 2011. December 15;36(26):2286-92. [DOI] [PubMed] [Google Scholar]
- 11.Cancienne JM, Werner BC, Loeb AE, Yang SS, Hassanzadeh H, Singla A, Shen FH, Shimer AL. The effect of local intraoperative steroid administration on the rate of postoperative dysphagia following ACDF: a study of 245,754 patients. Spine (Phila Pa 1976). 2016. July 1;41(13):1084-8. [DOI] [PubMed] [Google Scholar]
- 12.Koreckij TD, Davidson AA, Baker KC, Park DK. Retropharyngeal steroids and dysphagia following multilevel anterior cervical surgery. Spine (Phila Pa 1976). 2016. May;41(9):E530-4. [DOI] [PubMed] [Google Scholar]
- 13.Edwards CC, 2nd, Dean C, Edwards CC, Phillips D, Blight A. Can dysphagia following anterior cervical fusions with rhBMP-2 be reduced with local depomedrol application?: a prospective, randomized, placebo-controlled, double-blind trial. Spine (Phila Pa 1976). 2016. April;41(7):555-62. [DOI] [PubMed] [Google Scholar]
- 14.Jenkins TJ, Nair R, Bhatt S, Rosenthal BD, Savage JW, Hsu WK, Patel AA. The effect of local versus intravenous corticosteroids on the likelihood of dysphagia and dysphonia following anterior cervical discectomy and fusion: a single-blinded, prospective, randomized controlled trial. J Bone Joint Surg Am. 2018. September 5;100(17):1461-72. [DOI] [PubMed] [Google Scholar]
- 15.Lee SH, Mesfin A, Riew KD. Delayed esophageal perforation after anterior cervical fusion and retropharyngeal steroid use: a report of two cases. Spine J. 2015. October 1;15(10):e75-80. Epub 2015 Jun 28. [DOI] [PubMed] [Google Scholar]
- 16.Wu B, Song F, Zhu S. Reasons of dysphagia after operation of anterior cervical decompression and fusion. Clin Spine Surg. 2017. June;30(5):E554-9. [DOI] [PubMed] [Google Scholar]
- 17.McAfee PC, Cappuccino A, Cunningham BW, Devine JG, Phillips FM, Regan JJ, Albert TJ, Ahrens JE. Lower incidence of dysphagia with cervical arthroplasty compared with ACDF in a prospective randomized clinical trial. J Spinal Disord Tech. 2010. February;23(1):1-8. [DOI] [PubMed] [Google Scholar]
- 18.Anderson PA, Sasso RC, Riew KD. Comparison of adverse events between the Bryan artificial cervical disc and anterior cervical arthrodesis. Spine (Phila Pa 1976). 2008. May 20;33(12):1305-12. [DOI] [PubMed] [Google Scholar]
- 19.Rosenthal BD, Nair R, Hsu WK, Patel AA, Savage JW. Dysphagia and dysphonia assessment tools after anterior cervical spine surgery. Clin Spine Surg. 2016. November;29(9):363-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
A data-sharing statement is provided with the online version of the article (http://links.lww.com/JBJS/F504).