Abstract
Background:
Romosozumab is a bone-forming antibody that increases bone formation and decreases bone resorption. We conducted a double-blinded, randomized, phase-2, dose-finding trial to evaluate the effect of romosozumab on the clinical outcomes of open reduction and internal fixation of intertrochanteric or femoral neck hip fractures.
Methods:
Patients (55 to 94 years old) were randomized 2:3:3:3 to receive 3 subcutaneous injections of romosozumab (70, 140, or 210 mg) or a placebo postoperatively on day 1 and weeks 2, 6, and 12. The primary end point was the difference in the mean timed “Up & Go” (TUG) score over weeks 6 to 20 for romosozumab versus placebo. Additional end points included the time to radiographic evidence of healing and the score on the Radiographic Union Scale for Hip (RUSH).
Results:
A total of 332 patients were randomized: 243 to receive romosozumab (70 mg, n = 60; 140 mg, n = 93; and 210 mg, n = 90) and 89 to receive a placebo. Although TUG scores improved during the study, they did not differ significantly between the romosozumab and placebo groups over weeks 6 to 20 (p = 0.198). The median time to radiographic evidence of healing was 16.4 to 16.9 weeks across treatment groups. The RUSH scores improved over time across treatment groups but did not differ significantly between the romosozumab and placebo groups. The overall safety and tolerability profile of romosozumab was comparable with that of the placebo.
Conclusions:
Romosozumab did not improve the fracture-healing-related clinical and radiographic outcomes in the study population.
Level of Evidence:
Therapeutic Level I. See Instructions for Authors for a complete description of levels of evidence.
Hip fractures are a devastating clinical manifestation of osteoporosis. The almost 2 million hip fractures that occur each year in people older than 50 years are associated with substantial morbidity, excess mortality, and high health-care costs1-4. Almost all hip fractures are treated surgically. In the elderly, compromised mechanical and biological capacity, comorbidities, and possible complications make the management of hip fractures challenging, and the acceleration of fracture-healing is the desirable therapeutic outcome5. The systemic bone-forming agent teriparatide was investigated to assess its ability to accelerate fracture-healing, and while the results of a retrospective analysis were promising6, randomized controlled studies yielded inconclusive results7-9.
Romosozumab is a bone-forming antibody that increases bone formation and decreases bone resorption and is indicated to treat osteoporosis in postmenopausal women at high risk for fracture10. Romosozumab increased bone mineral density (BMD)11; reduced the prevalence of vertebral and clinical (a composite of nonvertebral and symptomatic vertebral) fractures (compared with a placebo)12; and, when followed by alendronate, reduced the risk of vertebral, nonvertebral, and hip fractures (compared with alendronate alone)13.
Preclinical studies showed that romosozumab enhances fracture-healing. Romosozumab significantly increased bone mass and strength at the fracture site in a closed femoral fracture model in rats by week 7 and in a fibular osteotomy model in cynomolgus monkeys by week 1014, promoted fracture-healing and increased bone strength in a mouse femoral osteotomy model by week 615, and increased the area of newly formed bone in a rat femoral osteotomy model by week 616.
On the basis of these preclinical data, we hypothesized that romosozumab would accelerate healing of hip fractures and improve physical functioning of human subjects. We conducted a phase-2, dose-finding trial to evaluate the effect of romosozumab administered over 12 weeks on the clinical outcomes of open reduction and internal fixation of intertrochanteric or femoral neck hip fractures.
Materials and Methods
Study Design
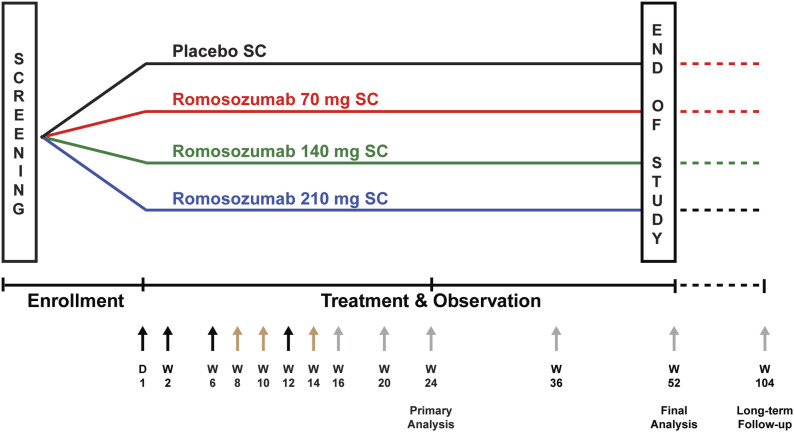
This phase-2, multicenter, international, randomized, double-blinded, placebo-controlled study enrolled patients with an acute, unilateral, low-energy hip fracture (sustained from a standing height or less) and treated with open reduction and internal fixation. The study was registered in ClinicalTrials.gov (NCT01081678). Dosing regimens were based on phase-1 single and multiple-dose studies that demonstrated a pharmacologic effect of romosozumab on bone formation markers17,18. An interactive voice response system was used to randomize patients 2:3:3:3 to receive 70, 140, or 210 mg of romosozumab or a placebo (Fig. 1); randomization was stratified into 7 strata by the type of fracture and fixation device and age. All participants and study personnel were blinded to the type of treatment.
Fig. 1.
Study schema. Randomization was stratified into 7 strata: (1) intertrochanteric fracture, sliding hip screw, 55 to 75 years old; (2) intertrochanteric fracture, sliding hip screw, ≥76 years old; (3) intertrochanteric fracture, intramedullary nail, 55 to 75 years old; (4) intertrochanteric fracture, intramedullary nail, ≥76 years old; (5) displaced femoral neck fracture, sliding hip screw; (6) displaced femoral neck fracture, cancellous screws; and (7) undisplaced femoral neck fracture. Within each stratum, patients were randomized using an allocation ratio of 2:3:3:3 to receive subcutaneous injections of romosozumab (70, 140, or 210 mg) or a placebo. The placebo group received 3 vials of placebo solution; the 70-mg group, 1 vial containing 70 mg of romosozumab and 2 vials of matched placebo solution; the 140-mg group, 2 vials each containing 70 mg of romosozumab and 1 vial of matched placebo solution; and the 210-group, 3 vials each containing 70 mg of romosozumab. Black arrows indicate study visits with administration of the investigational product, gray arrows indicate study visits without administration of the investigational product, and brown arrows indicate telephone visits. D = day, SC = subcutaneous, W = week.
Patients received 3 subcutaneous injections of romosozumab or a placebo postoperatively on day 1 and at weeks 2, 6, and 12. All patients took 50,000 IU of vitamin D once postoperatively and ≥1,000 mg of calcium and ≥800 IU of vitamin D daily from the time of screening to week 36. The timing of study visits is shown in Figure 1.
The primary end point was the timed “Up & Go” (TUG) score over weeks 6 to 20. Additional end points included the time to radiographic evidence of healing (defined as effacement of the fracture lines by newly formed bone along the cortices and within the trabecular bone on anteroposterior and lateral [or oblique] radiographs), the Radiographic Union Scale for Hip (RUSH) score, the Harris hip score (HHS), and hip pain on a visual analog scale (VAS).
The study was performed in accordance with the World Medical Association Declaration of Helsinki. The protocol was approved by the independent ethics committee or institutional review board at each site. All participants provided written informed consent.
Participants
Eligible patients were 55 to 95 years old and had a radiographically confirmed primary, acute, unilateral, low-energy intertrochanteric or femoral neck fracture amenable to repair by internal fixation. Exclusion criteria included severe lower-extremity osteoarthritis, a preinjury inability to rise independently from an armchair or walk 200 m, use of bone grafts or substitutes at the time of fracture fixation, major polytrauma or substantial axial trauma, and a pathological fracture or history of metabolic or bone disease (except osteoporosis). All eligibility criteria are listed in the Appendix.
Study Procedures
Anteroposterior and lateral (or oblique) radiographs of the proximal part of the femur were obtained at every clinic visit starting from week 2. The quality of surgical fixation and radiographic evidence of fracture-healing were determined by independent reviewers (orthopaedic/trauma surgeons and radiologists), blinded to treatment. Radiographic evaluation ended once healing was confirmed, except for mandatory radiographs at weeks 52 (end of study) and 104 (long-term follow-up).
The TUG test is a validated and reliable tool used to assess functional mobility of persons with impaired mobility19-22. Since TUG correlates well with activities of daily living, it was used to assess functional recovery in our study. Clinicians timed the patient while they stood up from a seated position in a chair, walked 3 m, turned around, walked back to the chair, and returned to the seated position. Study staff were trained on how to administer the TUG test via a training video.
The RUSH score is a validated and reliable tool developed to objectively assess hip (femoral neck) fracture-healing after surgical repair23-26. RUSH quantifies 10 measures of fracture-healing: cortical bridging and disappearance of the cortical fracture line in the anterior, posterior, medial, and lateral femoral neck regions and trabecular consolidation and disappearance of the trabecular fracture line (trabecular healing is indicated by consolidation of the matrix and disappearance of the fracture line). Each of the 10 healing measures are scored as 1, 2, or 3; a minimum total score of 10 indicates no healing, and a maximum total score of 30 indicates complete healing.
Adverse events were recorded at each study visit and coded using MedDRA (Medical Dictionary for Regulatory Activities), version 15.1. To determine the immunogenicity of romosozumab and its relationship to safety, blood samples taken on day 1 and weeks 6, 12, 20, 24, 36, and 52 were assessed for the presence of anti-romosozumab binding and neutralizing antibodies.
Statistical Analyses
The sample size calculation assumed that romosozumab would reduce the mean TUG scores over weeks 6 to 20 by 25% compared with the score associated with a placebo, which was approximately 36 seconds according to Ingemarsson et al.27. Allowing for a 20% withdrawal rate by week 24 and a type-I error of 5%, the calculation showed that approximately 90 patients per group (and 60 for the 70-mg group) would provide ≥80% power to detect differences between romosozumab and placebo with the use of a 2-sided t test.
A linear mixed-effects model was fit with log-transformed TUG scores as the dependent variable and treatment group, sex, prefracture community dwelling status, prefracture use of a walking aid, geographic region, quality of surgical fixation, visit week, and visit-by-treatment interaction as independent variables, stratified by the randomization strata.
The difference in the least-squares-mean (LSM) TUG scores over weeks 6 to 20 between the romosozumab and placebo groups was determined. Measurements obtained after unplanned revision surgery (indicative of poor healing) were assigned the visit-dependent worst value, which was imputed if a patient could not perform or complete the TUG test. Results based on log-transformed data were back-transformed to seconds.
For time to radiographic evidence of healing, a proportional-hazards model was fitted, adjusted for sex, prefracture community dwelling status, prefracture use of a walking aid, and quality of surgical fixation as independent covariates, stratified by the randomization strata. Patients were censored for unplanned revision surgery before radiographic evidence of healing. The estimate of the treatment effect was the hazard ratio (with 95% confidence interval [CI]) of romosozumab versus placebo with respect to time to revision-surgery-free healing28,29. The cumulative incidence function was determined for each treatment group.
Treatment differences in the total RUSH score were assessed using the van Elteren stratified rank test (adjusting for randomization strata) at each time point30. Missing scores were imputed using the last-observation-carried-forward approach.
The final analysis was conducted after completion of the week-52 assessments. Analyses of efficacy and safety were performed after unblinding and included all randomized patients who had received ≥1 dose of the investigational product.
Results
Patient Disposition and Baseline Characteristics
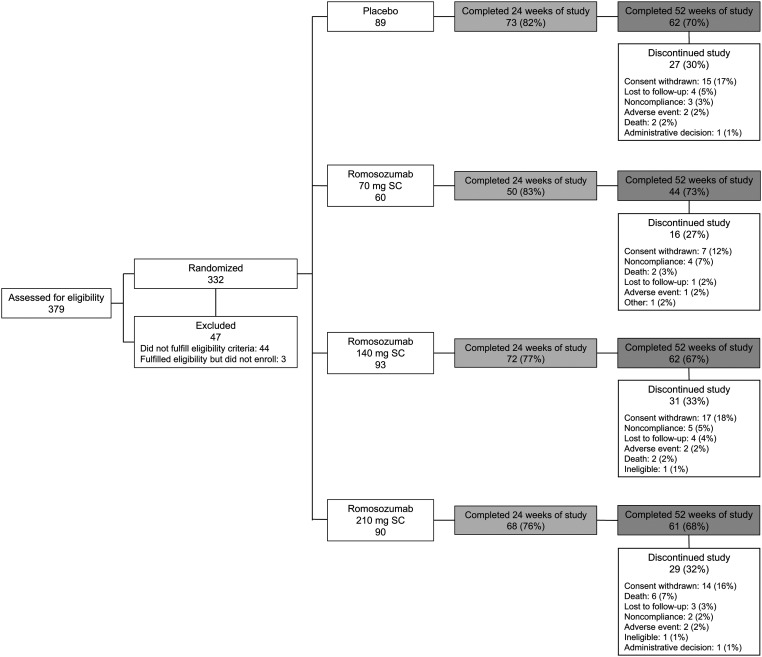
A total of 332 patients were randomized at 63 sites in 22 countries (see Appendix Table) between June 2010 and January 2013: 243 were randomized to receive romosozumab (70 mg, n = 60; 140 mg, n = 93; 210 mg, n = 90) and 89, to receive a placebo. Overall, 325 patients received ≥1 dose of the investigational product, and 263 (79.2%) and 229 (69.0%) completed 24 and 52 weeks of the study, respectively. Discontinuation rates and reasons for discontinuation were comparable among the treatment groups (Fig. 2).
Fig. 2.
Flow of patients through the study. SC = subcutaneous.
Baseline demographics and disease characteristics were generally balanced across the treatment groups; however, there was a higher percentage of women in the placebo group (75.3%) than in the total romosozumab group (66.3%) and a higher percentage of Asian patients in the 210-mg romosozumab group (21.1%) than in the other groups (11.7% to 13.5%) (Table I). Across the treatment groups, approximately 80% of the patients were classified as either healthy or having mild, systematic disease according to the American Society of Anesthesiologists (ASA) classification; 61.8% to 72.0% of the patients had an intertrochanteric hip fracture, 16.1% to 23.6% had an intertrochanteric hip fracture with extension into the subtrochanteric region, and 10.0% to 14.6% had a femoral neck fracture. Most patients were injured falling from a standing height or less. Most internal fixation implants were intramedullary nails (range across groups, 54.4% to 55.9%).
TABLE I.
Baseline Demographics and Disease Characteristics
| Placebo (N = 89) | Subcutaneous Romosozumab | ||||
| 70 mg (N = 60) | 140 mg (N = 93) | 210 mg (N = 90) | Total Romosozumab Group (N = 243) | ||
| Sex (no. [%]) | |||||
| Female | 67 (75.3) | 42 (70.0) | 64 (68.8) | 55 (61.1) | 161 (66.3) |
| Male | 22 (24.7) | 18 (30.0) | 29 (31.2) | 35 (38.9) | 82 (33.7) |
| Median age (range) (yr) | 78 (55-91) | 78.5 (55-94) | 79 (55-94) | 79 (55-93) | 79 (55-94) |
| Geriatric age group (no. [%]) | |||||
| ≥65 yr | 79 (88.8) | 52 (86.7) | 76 (81.7) | 79 (87.8) | 207 (85.2) |
| ≥75 yr | 54 (60.7) | 37 (61.7) | 56 (60.2) | 56 (62.2) | 149 (61.3) |
| Race (no. [%]) | |||||
| White | 77 (86.5) | 52 (86.7) | 81 (87.1) | 70 (77.8) | 203 (83.5) |
| Asian | 12 (13.5) | 7 (11.7) | 11 (11.8) | 19 (21.1) | 37 (15.2) |
| Black | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.1) | 1 (0.4) |
| Hispanic | 0 (0.0) | 1 (1.7) | 1 (1.1) | 0 (0.0) | 2 (0.8) |
| Geographic region (no. [%]) | |||||
| Eastern Europe | 27 (30.3) | 22 (36.7) | 41 (44.1) | 25 (27.8) | 88 (36.2) |
| Western Europe | 30 (33.7) | 15 (25.0) | 29 (31.2) | 27 (30.0) | 71 (29.2) |
| India | 10 (11.2) | 7 (11.7) | 10 (10.8) | 18 (20.0) | 35 (14.4) |
| North America | 14 (15.7) | 11 (18.3) | 4 (4.3) | 8 (8.9) | 23 (9.5) |
| Latin America | 5 (5.6) | 4 (6.7) | 6 (6.5) | 9 (10.0) | 19 (7.8) |
| Australia and New Zealand | 1 (1.1) | 1 (1.7) | 2 (2.2) | 2 (2.2) | 5 (2.1) |
| Other | 2 (2.2) | 0 (0.0) | 1 (1.1) | 1 (1.1) | 2 (0.8) |
| ASA classification* (no. [%]) | |||||
| Class I | 24 (27.0) | 19 (31.7) | 32 (34.4) | 32 (35.6) | 83 (34.2) |
| Class II | 49 (55.1) | 30 (50.0) | 43 (46.2) | 43 (47.8) | 116 (47.7) |
| Class III | 16 (18.0) | 9 (15.0) | 17 (18.3) | 14 (15.6) | 40 (16.5) |
| Class IV | 0 (0.0) | 2 (3.3) | 1 (1.1) | 1 (1.1) | 4 (1.6) |
| Mean body mass index (SD)† (kg/m2) | |||||
| Women | 25.0 (4.5) | 24.4 (3.5) | 23.6 (4.0) | 23.7 (3.4) | 23.9 (3.6) |
| Men | 24.7 (4.7) | 24.4 (2.9) | 25.2 (4.6) | 24.1 (4.2) | 24.5 (4.1) |
| Location of hip fracture (no. [%]) | |||||
| Intertrochanteric | 55 (61.8) | 41 (68.3) | 67 (72.0) | 62 (68.9) | 170 (70.0) |
| Intertrochanteric extending into subtrochanteric region | 21 (23.6) | 11 (18.3) | 15 (16.1) | 19 (21.1) | 45 (18.5) |
| Femoral neck | 13 (14.6) | 8 (13.3) | 11 (11.8) | 9 (10.0) | 28 (11.5) |
| Mechanism of injury (no. [%]) | |||||
| Fall from standing height or less | 76 (85.4) | 54 (90.0) | 82 (88.2) | 79 (87.8) | 215 (88.5) |
| Fall on stairs, steps, or curb | 8 (9.0) | 2 (3.3) | 9 (9.7) | 3 (3.3) | 14 (5.8) |
| Fall from ∼20 in (51 cm) | 3 (3.4) | 2 (3.3) | 2 (2.2) | 2 (2.2) | 6 (2.5) |
| Fall from higher than ∼20 in (51 cm) | 2 (2.2) | 2 (3.3) | 0 (0.0) | 4 (4.4) | 6 (2.5) |
| Spontaneous (stress) fracture | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (2.2) | 2 (0.8) |
| Method of internal fixation (no. [%]) | |||||
| Intramedullary nail | 49 (55.1) | 33 (55.0) | 52 (55.9) | 49 (54.4) | 134 (55.1) |
| Sliding hip screw | 31 (34.8) | 22 (36.7) | 32 (34.4) | 35 (38.9) | 89 (36.6) |
| Cancellous screws | 9 (10.1) | 5 (8.3) | 9 (9.7) | 6 (6.7) | 20 (8.2) |
Class I = healthy patient with no medical problems, Class II = mild systemic disease, Class III = severe systemic disease but not incapacitating, and Class IV = severe systemic disease that is a constant threat to life.
SD = standard deviation.
Efficacy
TUG Scores by Visit
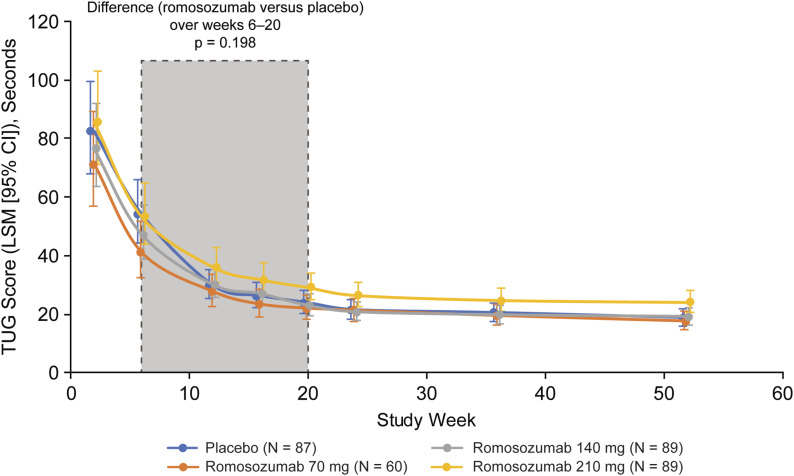
The LSM TUG scores improved from weeks 2 to 52 for each treatment group (Fig. 3). From weeks 2 to 20, the LSM TUG scores for the placebo and 70-mg, 140-mg, and 210-mg romosozumab groups improved from 82 to 24, 71 to 22, 77 to 23, and 86 to 29 seconds, respectively; the scores leveled off after week 20. There were no significant differences in the LSM TUG scores over weeks 6 to 20 between the romosozumab and placebo groups (primary end point, p = 0.198). At week 52, the LSM (and 95% CI) TUG score ratios (romosozumab:placebo) were 0.9 (0.7 to 1.2), 1.0 (0.8 to 1.3), and 1.3 (1.0 to 1.6) for the 70, 140, and 210-mg groups, respectively.
Fig. 3.
TUG scores by visit. Estimates are based on a linear mixed-effects model for repeated measures, adjusting for treatment, sex, prefracture community-dwelling status, prefracture walking aid use, geographic region (group 1: Greece, India, Italy, and Lithuania; group 2: Switzerland, Denmark, Estonia, Finland, Latvia, the Netherlands, Hungary, and New Zealand; group 3: Argentina, Australia, Belgium, Bulgaria, Canada, Germany, U.K., Hong Kong, Poland, and U.S.), quality of surgical fixation, visit, treatment-by-visit interaction, and randomization strata. Log-transformed scores were back-transformed to seconds using the exponential transformation. The results are presented as LSMs with 95% CIs. The p value is based on an F test of multilinear contrasts at weeks 6 to 20.
Time to Radiographic Evidence of Healing
The cumulative incidence function estimate of patients who had radiographic evidence of healing at weeks 24 and 52 was similar across treatment groups (range, 66.2% to 78.6% at week 24 and 89.1% to 93.2% at week 52; Table II). There were no apparent dose or treatment-group-related trends in the median time to radiographic evidence of healing (range, 16.4 to 16.9 weeks across groups) and no significant differences between the romosozumab and placebo groups (Table II). Nonunion was reported in 2 patients in the placebo group at week 52.
TABLE II.
Radiographic Evidence of Healing*
| Placebo (N = 87) | Subcutaneous Romosozumab | |||
| 70 mg (N = 60) | 140 mg (N = 89) | 210 mg (N = 89) | ||
| Patients with radiographic healing at wk 24 | ||||
| CIF estimate (95% CI) (%) | 73.2 (62.6-82.8) | 78.6 (66.7-88.5) | 72.8 (62.3-82.3) | 66.2 (55.1-77.1) |
| Patients with radiographic healing at wk 52 | ||||
| CIF estimate (95% CI) (%) | 93.2 (85.1-97.8) | 90.1 (79.5-96.6) | 93.1 (85.6-97.4) | 89.1 (79.9-95.3) |
| Median time to radiographic evidence of healing† | ||||
| CIF estimate (95% CI) (wk) | 16.4 (15.3-20.1) | 16.9 (12.9-20.3) | 16.6 (13.3-17.1) | 16.9 (13.3-20.9) |
| HR‡ (95% CI), p value | 1.1 (0.7-1.6), p = 0.79 | 1.1 (0.8-1.6), p = 0.62 | 1.1 (0.7-1.6), p = 0.76 | |
N = number of randomized patients who received ≥1 dose of investigational product. Data are presented as point estimates. CIF = cumulative incidence function, CI = confidence interval, and HR = hazard ratio.
From fracture fixation date.
HR is based on a Cox proportional-hazards model with treatment groups as the independent variable, stratified by randomization strata, and adjusted for sex, prefracture community dwelling status, use of prefracture walking aid, and quality of surgical fixation. An HR of >1 favors romosozumab.
When evaluated by subgroup (age, sex, fracture type, and fixation type), the results were consistent with the overall study population (data not shown).
RUSH Scores by Visit
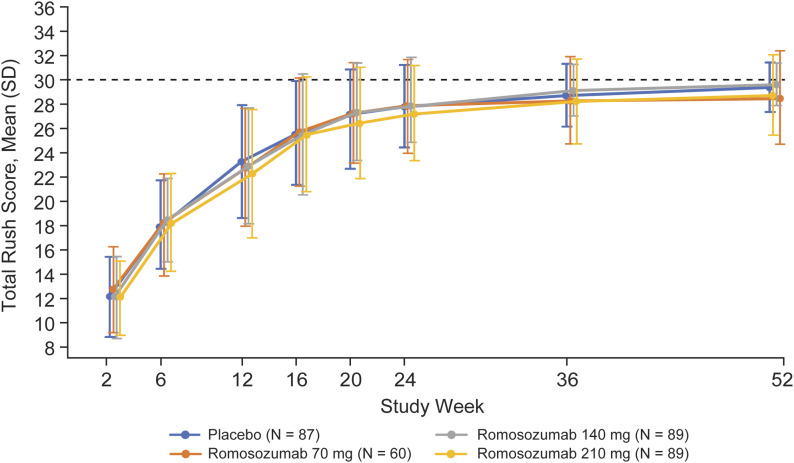
The RUSH scores improved over time across all treatment groups, plateauing between weeks 36 and 52 (Fig. 4). There were no significant differences in the RUSH scores between the romosozumab and placebo groups at any time. The mean total RUSH scores across treatment groups ranged from 28.2 to 29.1 at week 36 and from 28.5 to 29.6 at week 52.
Fig. 4.
RUSH scores by visit. N = number of randomized patients who received ≥1 dose of investigational product. The results are presented as the mean and standard deviation (SD). The dashed line represents the maximum total RUSH score (30 points).
HHS
The HHS improved over time for all of the romosozumab groups and the placebo group. The values were similar between the placebo group and all of the romosuzumab groups up to week 24. For weeks 36 and 52, the repeated-measures model indicated a significant difference for the 140-mg romosozumab group, favoring romosozumab compared with placebo. At week 36, the LSM (and 95% CI) was 86.8 (83.5 to 90.2) in the 140-mg romosozomab group and 80.3 (77.0 to 83.6) in the placebo group (p = 0.0062). At week 52, the LSM (and 95% CI) was 89.0 (85.9 to 92.1) in the 140-mg romosozomab group and 84.3 (81.3 to 87.4) in the placebo group (p = 0.0365). However, this was likely a chance finding, as the p values were not corrected for multiplicity and the 210-mg group did not show a significant difference.
VAS Hip Pain
The difference in the LSM VAS hip pain between the placebo group and individual romosozumab groups was not significant at any time point.
Safety
A total of 325 patients (87 in the placebo group and 238 in the total romosozumab group) received ≥1 dose of the investigational product over the 12-week dosing period and were included in the 52-week safety analysis. Sixty-nine patients (79.3%) in the placebo group and 157 (66.0%) in the total romosozumab group reported ≥1 adverse event that emerged during treatment (Table III). No trends were apparent in the pattern or types of adverse events across treatment groups; however, a higher percentage of patients in the romosozumab group than in the placebo group reported back pain (6.7% versus 0%) and arthralgia (5.9% versus 2.3%), and a lower percentage reported constipation (8.8% versus 12.6%), diarrhea (3.8% versus 9.2%), and pain in an extremity (0.8% versus 5.7%).
TABLE III.
Adverse Events*
| Placebo (N = 87) | Subcutaneous Romosozumab | ||||
| 70 mg (N = 60) | 140 mg (N = 89) | 210 mg (N = 89) | Total Romosozumab Group (N = 238) | ||
| Adverse events during treatment (no. [%]) | 69 (79.3) | 39 (65.0) | 54 (60.7) | 64 (71.9) | 157 (66.0) |
| Serious adverse events† (no. [%]) | 25 (28.7) | 9 (15.0) | 15 (16.9) | 26 (29.2) | 50 (21.0) |
| Acute myocardial infarction | 1 (1.1) | 0 (0.0) | 1 (1.1) | 2 (2.2) | 3 (1.3) |
| Pneumonia | 1 (1.1) | 0 (0.0) | 1 (1.1) | 2 (2.2) | 3 (1.3) |
| Cardiac arrest | 0 (0.0) | 1 (1.7) | 1 (1.1) | 1 (1.1) | 3 (1.3) |
| Hip fracture | 0 (0.0) | 0 (0.0) | 1 (1.1) | 2 (2.2) | 3 (1.3) |
| Postoperative wound infection | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (3.4) | 3 (1.3) |
| Cellulitis | 3 (3.4) | 0 (0.0) | 1 (1.1) | 1 (1.1) | 2 (0.8) |
| Acute pulmonary edema | 1 (1.1) | 0 (0.0) | 0 (0.0) | 2 (2.2) | 2 (0.8) |
| Cardiac failure | 0 (0.0) | 0 (0.0) | 1 (1.1) | 1 (1.1) | 2 (0.8) |
| Cerebrovascular accident | 0 (0.0) | 1 (1.7) | 0 (0.0) | 1 (1.1) | 2 (0.8) |
| Diverticulitis | 0 (0.0) | 0 (0.0) | 1 (1.1) | 1 (1.1) | 2 (0.8) |
| Lower respiratory tract infection | 0 (0.0) | 0 (0.0) | 1 (1.1) | 1 (1.1) | 2 (0.8) |
| Medical device complication | 0 (0.0) | 1 (1.7) | 0 (0.0) | 1 (1.1) | 2 (0.8) |
| Bacterial pneumonia | 0 (0.0) | 1 (1.7) | 0 (0.0) | 1 (1.1) | 2 (0.8) |
| Osteoarthritis | 2 (2.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Fatal adverse events‡ (no. [%]) | 2 (2.3) | 2 (3.3) | 2 (2.2) | 6 (6.7) | 10 (4.2) |
| Adverse events leading to discontinuation of investigational product (no. [%]) | 4 (4.6) | 2 (3.3) | 5 (5.6) | 3 (3.4) | 10 (4.2) |
| Adverse events leading to study discontinuation (no. [%]) | 2 (2.3) | 1 (1.7) | 2 (2.2) | 2 (2.2) | 5 (2.1) |
| Adverse events of interest§ (no. [%]) | |||||
| Hypersensitivity | 2 (2.3) | 1 (1.7) | 0 (0.0) | 2 (2.2) | 3 (1.3) |
| Hypocalcemia | 0 (0.0) | 0 (0.0) | 1 (1.1) | 0 (0.0) | 1 (0.4) |
| Injection-site reactions | 1 (1.1) | 1 (1.7) | 0 (0.0) | 1 (1.1) | 2 (0.8) |
| Hyperostosis# | 1 (1.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Malignancy | 1 (1.1) | 0 (0.0) | 1 (1.1) | 2 (2.2) | 3 (1.3) |
| Osteoarthritis | 3 (3.4) | 4 (6.7) | 5 (5.6) | 2 (2.2) | 11 (4.6) |
N = number of patients randomized who received ≥1 dose of investigational product.
Includes those that occurred in at least 2 patients in the total romosozumab or placebo group.
Fatal events were reported as death (day 273) and coronary artery hemorrhage (day 3) in the placebo group; respiratory failure (day 3) and cardiac arrest (day 72) in the 70-mg romosozumab group; cardiac arrest (day 14) and cardiac failure (day 187) in the 140-mg romosozumab group; and cardiac arrest (day 2), cardiorespiratory arrest (day 75), cerebrovascular accident (day 258), bacterial pneumonia (day 3), cardiac disorder (day 119), and acute respiratory failure (day 12) in the 210-mg romosozumab group.
Adverse events of interest at the time of this study were prospectively defined.
Reported as extraskeletal ossification.
Serious adverse events were reported for 25 (28.7%) of the patients in the placebo group and 50 (21.0%) in the total romosozumab group; no serious adverse event was reported for >3 patients in any group. Serious adverse events in the system order class of cardiac, vascular, and nervous system disorders were generally comparable between groups, except for cardiac disorders (placebo, 3.4%; total romosozumab group, 5%), most of which occurred during the follow-up period. Adverse events leading to discontinuation of use of the investigational product or participation in the study were comparable between treatment groups (Table III).
Adverse events of interest included hypersensitivity, hypocalcemia, injection-site reactions, hyperostosis, malignancy, and osteoarthritis; all were comparable among treatment groups (Table III). Of note, 1 patient (in the 210-mg romosozumab group) had a serious hypersensitivity adverse event of acute generalized exanthematous pustulosis on study day 2 that resolved with topical steroid treatment; 1 (140-mg group) had a non-serious adverse event of hypocalcemia (day 10) in the setting of congestive heart failure; 1 (210-mg group) had a serious adverse event of acute myeloid leukemia after the last injection; and 2 (placebo group) had serious adverse events of radiographically evident worsening of preexisting osteoarthritis, with 1 of them also having worsening of symptoms of preexisting osteoarthritis.
None of the patients who received romosozumab stopped using it because of injection-site reactions, and none of the adverse events were suggestive of osteonecrosis of the jaw or atypical femoral fracture. Ten (4.2%) of the patients in the total romosozumab group and 2 (2.3%) in the placebo group had fatal adverse events; none were considered related to the investigational product (Table III).
Anti-romosozumab binding antibodies were detected in 20 (9.4%) of 213 patients treated with romosozumab and were transient in 8 of them. The transient neutralizing antibodies to romosozumab were detected in 5 patients (2.3%) and did not appear to affect the safety profile of romosozumab.
Discussion
Although romosozumab was shown to increase bone formation, reduce bone resorption, improve BMD, and decrease fracture rates in postmenopausal women11,13, stimulation of bone formation associated with short-term romosozumab treatment did not significantly accelerate fracture-healing following hip fracture fixation in our study population. The TUG scores and median time to radiographic healing were similar across treatment groups and were within the range observed in other hip-fracture-fixation studies of patients with comparable demographics31-35. The RUSH scores at the end of the study period indicated that almost all fractures were sufficiently healed.
The prevalence of adverse events was comparable among the treatment groups and consistent with the type of events that would be expected in this population of mostly elderly patients. A slightly higher prevalence of cardiac serious adverse events was reported in the romosozumab groups, and while some were recorded during the active treatment phase, most occurred during the follow-up period. The heterogeneous nature of the reported events, their low number, and a 3:1 randomization may limit definitive interpretation. Two much larger pivotal fracture trials of women with postmenopausal osteoporosis showed discordant results with regard to the number of positively adjudicated cardiovascular serious adverse events12,13. While a higher number was observed in the romosozumab group in the trial comparing romosozumab with alendronate13, no difference was observed in the larger, 7,000-patient placebo-controlled trial12.
Similar to the current study, a pair of trials comparing teriparatide with a placebo showed no acceleration of hip fracture-healing in the teriparatide group8. In a study comparing the effects of teriparatide and risedronate on recovery after hip fractures7,9, patients in the teriparatide group completed the TUG test in a shorter time, but there was no significant difference in the time to radiographic evidence of healing; the authors noted that the TUG test was a secondary end point, and the results should be interpreted with caution7. The time to radiographic evidence of healing in the teriparatide versus risedronate study was approximately 12 weeks in both arms compared with 16 weeks in our study, probably because of differences in the study populations—the teriparatide study enrolled patients with low-trauma pertrochanteric hip fractures. The reason for the absence of accelerated healing, despite stimulation of bone formation, is unclear. In our study, patients were treated at sites carefully selected for high surgical standards of care, and they had an overall rapid improvement in their functional scores and radiographic signs of healing regardless of treatment group. The near-perfect RUSH scores at week 36 suggest that complete fracture-healing had occurred in most patients by that time.
Our study had several methodological strengths, including stratified randomization to reduce possible bias as well as the use of outcome measures proven to be valid and achievable in elderly populations and patients with hip fracture20,23,26. The study population, however, may not have been at sufficient risk for delayed healing to demonstrate benefit from an intervention for acceleration of fracture-healing.
Our study has some limitations. The TUG tests were performed locally and not recorded with videography; therefore, no central adjudication of the results was possible. Intertrochanteric fractures, the most common type of fracture in our study, are usually not complicated by issues with fracture union, and it is plausible that the lack of treatment effect in our study was due to the inclusion of these fracture types. In addition, we were unable to fully assess prefracture morbidity, but since this was a randomized study, outcomes were unlikely to have been confounded by presurgery imbalances. Finally, differences among the sites regarding patient instruction and encouragement for the TUG test, which is considered challenging in this patient population, are possible and may have skewed some of the results; a patient-reported outcome measure reflecting improvement in quality of life might have been a more appropriate end point.
This phase-2 dose-finding study did not identify a difference with respect to its primary end point and adds to published evidence7-9 failing to show acceleration of fracture-healing with the use of bone-forming agents at the doses and schedules tested in the respective study populations. The quality of the surgical devices and methods used at the academic centers in our study likely outweighed any effect of romosozumab on fracture-healing. Future studies should focus on augmentation of fracture repair when fracture-healing is at risk or potentially delayed or compromised.
Appendix
Supporting material provided by the authors is posted with the online version of this article as a data supplement at jbjs.org (http://links.lww.com/JBJS/F703).
Acknowledgments
Note: The authors thank Cassandra Milmont for statistical input. Medical writing support for this paper was funded by Amgen, Inc. and provided by Kathryn Boorer, PhD, of KB Scientific Communications, LLC.
Footnotes
Disclosure: This study was funded by Amgen, Inc. and UCB Pharma. On the Disclosure of Potential Conflicts of Interest forms, which are provided with the online version of the article, one, or more of the authors checked “yes” to indicate that the author had a relevant financial relationship in the biomedical arena outside the submitted work (including employment with Amgen Inc., the sponsor of the study) (http://links.lww.com/JBJS/F702).
Data Sharing
A data-sharing statement is provided with the online version of the article (http://links.lww.com/JBJS/F704).
References
- 1.Odén A, McCloskey EV, Johansson H, Kanis JA. Assessing the impact of osteoporosis on the burden of hip fractures. Calcif Tissue Int. 2013. January;92(1):42-9. Epub 2012 Nov 8. [DOI] [PubMed] [Google Scholar]
- 2.Johnell O, Kanis JA. An estimate of the worldwide prevalence, mortality and disability associated with hip fracture. Osteoporos Int. 2004. November;15(11):897-902. Epub 2004 May 4. [DOI] [PubMed] [Google Scholar]
- 3.Bentler SE, Liu L, Obrizan M, Cook EA, Wright KB, Geweke JF, Chrischilles EA, Pavlik CE, Wallace RB, Ohsfeldt RL, Jones MP, Rosenthal GE, Wolinsky FD. The aftermath of hip fracture: discharge placement, functional status change, and mortality. Am J Epidemiol. 2009. November 15;170(10):1290-9. Epub 2009 Oct 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.International Osteoporosis Foundation. Facts and statistics.Accessed2019Sep13. Accessed 2019 Sep 13 https://www.iofbonehealth.org/facts-statistics#category-16 [Google Scholar]
- 5.Kanakaris NK, West RM, Giannoudis PV. Enhancement of hip fracture healing in the elderly: evidence deriving from a pilot randomized trial. Injury. 2015. August;46(8):1425-8. [DOI] [PubMed] [Google Scholar]
- 6.Huang TW, Chuang PY, Lin SJ, Lee CY, Huang KC, Shih HN, Lee MS, Hsu RW, Shen WJ. Teriparatide improves fracture healing and early functional recovery in treatment of osteoporotic intertrochanteric fractures. Medicine (Baltimore). 2016. May;95(19):e3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aspenberg P, Malouf J, Tarantino U, García-Hernández PA, Corradini C, Overgaard S, Stepan JJ, Borris L, Lespessailles E, Frihagen F, Papavasiliou K, Petto H, Caeiro JR, Marin F. Effects of teriparatide compared with risedronate on recovery after pertrochanteric hip fracture: results of a randomized, active-controlled, double-blind clinical trial at 26 weeks. J Bone Joint Surg Am. 2016. November 16;98(22):1868-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhandari M, Jin L, See K, Burge R, Gilchrist N, Witvrouw R, Krohn KD, Warner MR, Ahmad QI, Mitlak B. Does teriparatide improve femoral neck fracture healing: results from a randomized placebo-controlled trial. Clin Orthop Relat Res. 2016. May;474(5):1234-44. Epub 2016 Mar 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malouf-Sierra J, Tarantino U, García-Hernández PA, Corradini C, Overgaard S, Stepan JJ, Borris L, Lespessailles E, Frihagen F, Papavasiliou K, Petto H, Aspenberg P, Caeiro JR, Marin F. Effect of teriparatide or risedronate in elderly patients with a recent pertrochanteric hip fracture: final results of a 78-week randomized clinical trial. J Bone Miner Res. 2017. May;32(5):1040-51. Epub 2017 Jan 26. [DOI] [PubMed] [Google Scholar]
- 10.Amgen Inc. EVENITY™ (romosozumab-aqqg) injection, for subcutaneous use. 2019. April Accessed 2019 Sep 4. https://www.pi.amgen.com/∼/media/amgen/repositorysites/pi-amgen-com/evenity/evenity_pi_hcp_english.ashx [Google Scholar]
- 11.McClung MR, Grauer A, Boonen S, Bolognese MA, Brown JP, Diez-Perez A, Langdahl BL, Reginster JY, Zanchetta JR, Wasserman SM, Katz L, Maddox J, Yang YC, Libanati C, Bone HG. Romosozumab in postmenopausal women with low bone mineral density. N Engl J Med. 2014. January 30;370(5):412-20. Epub 2014 Jan 1. [DOI] [PubMed] [Google Scholar]
- 12.Cosman F, Crittenden DB, Adachi JD, Binkley N, Czerwinski E, Ferrari S, Hofbauer LC, Lau E, Lewiecki EM, Miyauchi A, Zerbini CA, Milmont CE, Chen L, Maddox J, Meisner PD, Libanati C, Grauer A. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med. 2016. October 20;375(16):1532-43. Epub 2016 Sep 18. [DOI] [PubMed] [Google Scholar]
- 13.Saag KG, Petersen J, Brandi ML, Karaplis AC, Lorentzon M, Thomas T, Maddox J, Fan M, Meisner PD, Grauer A. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med. 2017. October 12;377(15):1417-27. Epub 2017 Sep 11. [DOI] [PubMed] [Google Scholar]
- 14.Ominsky MS, Li C, Li X, Tan HL, Lee E, Barrero M, Asuncion FJ, Dwyer D, Han CY, Vlasseros F, Samadfam R, Jolette J, Smith SY, Stolina M, Lacey DL, Simonet WS, Paszty C, Li G, Ke HZ. Inhibition of sclerostin by monoclonal antibody enhances bone healing and improves bone density and strength of nonfractured bones. J Bone Miner Res. 2011. May;26(5):1012-21. [DOI] [PubMed] [Google Scholar]
- 15.Cui L, Cheng H, Song C, Li C, Simonet WS, Ke HZ, Li G. Time-dependent effects of sclerostin antibody on a mouse fracture healing model. J Musculoskelet Neuronal Interact. 2013. June;13(2):178-84. [PubMed] [Google Scholar]
- 16.Suen PK, He YX, Chow DH, Huang L, Li C, Ke HZ, Ominsky MS, Qin L. Sclerostin monoclonal antibody enhanced bone fracture healing in an open osteotomy model in rats. J Orthop Res. 2014. August;32(8):997-1005. Epub 2014 Apr 30. [DOI] [PubMed] [Google Scholar]
- 17.Padhi D, Allison M, Kivitz AJ, Gutierrez MJ, Stouch B, Wang C, Jang G. Multiple doses of sclerostin antibody romosozumab in healthy men and postmenopausal women with low bone mass: a randomized, double-blind, placebo-controlled study. J Clin Pharmacol. 2014. February;54(2):168-78. Epub 2013 Dec 11. [DOI] [PubMed] [Google Scholar]
- 18.Padhi D, Jang G, Stouch B, Fang L, Posvar E. Single-dose, placebo-controlled, randomized study of AMG 785, a sclerostin monoclonal antibody. J Bone Miner Res. 2011. January;26(1):19-26. [DOI] [PubMed] [Google Scholar]
- 19.Kristensen MT, Foss NB, Kehlet H. Timed “Up & Go” test as a predictor of falls within 6 months after hip fracture surgery. Phys Ther. 2007. January;87(1):24-30. Epub 2006 Dec 1. [DOI] [PubMed] [Google Scholar]
- 20.Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991. February;39(2):142-8. [DOI] [PubMed] [Google Scholar]
- 21.Lin MR, Hwang HF, Hu MH, Wu HD, Wang YW, Huang FC. Psychometric comparisons of the Timed Up and Go, one-leg stand, functional reach, and Tinetti balance measures in community-dwelling older people. J Am Geriatr Soc. 2004. August;52(8):1343-8. [DOI] [PubMed] [Google Scholar]
- 22.Steffen TM, Hacker TA, Mollinger L. Age- and gender-related test performance in community-dwelling elderly people: Six-Minute Walk Test, Berg Balance Scale, Timed Up & Go Test, and gait speeds. Phys Ther. 2002. February;82(2):128-37. [DOI] [PubMed] [Google Scholar]
- 23.Chiavaras MM, Bains S, Choudur H, Parasu N, Jacobson J, Ayeni O, Petrisor B, Chakravertty R, Sprague S, Bhandari M. The Radiographic Union Score for Hip (RUSH): the use of a checklist to evaluate hip fracture healing improves agreement between radiologists and orthopedic surgeons. Skeletal Radiol. 2013. August;42(8):1079-88. Epub 2013 Apr 7. [DOI] [PubMed] [Google Scholar]
- 24.Bhandari M, Chiavaras M, Ayeni O, Chakraverrty R, Parasu N, Choudur H, Bains S, Sprague S, Petrisor B; Assessment Group for Radiographic Evaluation and Evidence (AGREE) Study Group (AGREE Investigators Writing Committee). Assessment of radiographic fracture healing in patients with operatively treated femoral neck fractures. J Orthop Trauma. 2013. September;27(9):e213-9. [DOI] [PubMed] [Google Scholar]
- 25.Bhandari M, Chiavaras MM, Parasu N, Choudur H, Ayeni O, Chakravertty R, Bains S, Hak A, Sprague S, Petrisor B. Radiographic Union Score for Hip substantially improves agreement between surgeons and radiologists. BMC Musculoskelet Disord. 2013. February 25;14:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frank T Osterhoff G Sprague S Garibaldi A Bhandari M Slobogean GP; FAITH Investigators. The Radiographic Union Score for Hip (RUSH) identifies radiographic nonunion of femoral neck fractures. Clin Orthop Relat Res. 2016 Jun;474(6):1396-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ingemarsson AH, Frändin K, Mellström D, Möller M. Walking ability and activity level after hip fracture in the elderly—a follow-up. J Rehabil Med. 2003. March;35(2):76-83. [DOI] [PubMed] [Google Scholar]
- 28.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496-509. [Google Scholar]
- 29.Prentice RL, Kalbfleisch JD, Peterson AV, Jr, Flournoy N, Farewell VT, Breslow NE. The analysis of failure times in the presence of competing risks. Biometrics. 1978. December;34(4):541-54. [PubMed] [Google Scholar]
- 30.van Elteren PH. On the combination of independent two-sample test of Wilcoxon. Bull Int Stat Inst. 1960;37:351-61. [Google Scholar]
- 31.Reindl R, Harvey EJ, Berry GK, Rahme E; Canadian Orthopaedic Trauma Society (COTS). Intramedullary versus extramedullary fixation for unstable intertrochanteric fractures: a prospective randomized controlled trial. J Bone Joint Surg Am. 2015. December 2;97(23):1905-12. [DOI] [PubMed] [Google Scholar]
- 32.Matre K, Vinje T, Havelin LI, Gjertsen JE, Furnes O, Espehaug B, Kjellevold SH, Fevang JM. TRIGEN INTERTAN intramedullary nail versus sliding hip screw: a prospective, randomized multicenter study on pain, function, and complications in 684 patients with an intertrochanteric or subtrochanteric fracture and one year of follow-up. J Bone Joint Surg Am. 2013. February 6;95(3):200-8. [DOI] [PubMed] [Google Scholar]
- 33.Boese CK, Buecking B, Schwarting T, Debus F, Ruchholtz S, Bliemel C, Frink M, Lechler P. The influence of pre-existing radiographic osteoarthritis on functional outcome after trochanteric fracture. Int Orthop. 2015. July;39(7):1405-10. Epub 2015 Jan 21. [DOI] [PubMed] [Google Scholar]
- 34.Herrera A, Domingo LJ, Calvo A, Martínez A, Cuenca J. A comparative study of trochanteric fractures treated with the Gamma nail or the proximal femoral nail. Int Orthop. 2002;26(6):365-9. Epub 2002 Jul 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu F, Liu G, Shao HG, Wang YJ, Li RQ, Yang HL, Geng DC, Xu YZ. Treatment of femoral neck fracture with percutaneous compression plate: preliminary results in 74 patients. Orthop Surg. 2015. May;7(2):132-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
A data-sharing statement is provided with the online version of the article (http://links.lww.com/JBJS/F704).