Abstract
-
➢
Clinicians should exercise a high level of suspicion in at-risk patients (those who use corticosteroids, consume excessive alcohol, have sickle cell disease, etc.) in order to diagnose osteonecrosis of the femoral head in its earliest stage.
-
➢
Nonoperative treatment modalities have generally been ineffective at halting progression. Thus, nonoperative treatment is not appropriate in early stages when one is attempting to preserve the native joint, except potentially on rare occasions for small-sized, medially located lesions, which may heal without surgery.
-
➢
Joint-preserving procedures should be attempted in early-stage lesions to save the femoral head.
-
➢
Cell-based augmentation of joint-preserving procedures continues to show promising results, and thus should be considered as an ancillary treatment method that may improve clinical outcomes.
-
➢
The outcomes of total hip arthroplasty in the setting of osteonecrosis are excellent, with results similar to those in patients who have an underlying diagnosis of osteoarthritis.
The prevalence of osteonecrosis of the femoral head (ONFH) is increasing1-5. It is unclear whether this is a true increase or is from heightened awareness and diagnostic advancements. Nevertheless, ONFH mainly affects younger adults and accounts for approximately 10% of total hip arthroplasties (THAs) in the United States annually6,7. Factors that aid in understanding its pathogenesis and prevention, as well as preservation methods, can delay or prevent THAs. Fortunately, there has been substantial progress in research pertaining to the etiology, screening modalities, diagnosis, classification, and treatment of this disease. Notably, cell-based augmentation procedures have been increasingly reported. This review will serve as an update on nontraumatic ONFH. We evaluated peer-reviewed studies since 2015 to provide background on treatment based on the highest level of evidence, which can be used with the previous 3 Current Concepts Reviews on this topic8-10.
Etiology and Pathogenesis
Osteonecrosis is death of bone that may be associated with circulatory disruption from various factors. Corticosteroid use and excessive alcohol intake are associated with >80% of the cases10,11. These factors diminish femoral perfusion through mechanisms including vascular endothelial damage and microvascular thrombosis12-15. They also induce intramedullary adipogenesis16-19, which increases intraosseous pressure leading to venous stasis and arterial obstruction19,20. Corticosteroids can decrease osteoblast production, increase osteocyte apoptosis, and prolong the osteoclast lifespan15,21,22. Clinicians should exercise a high level of suspicion in at-risk patients (those who use corticosteroids, consume excessive alcohol, have sickle cell disease, etc.) in order to diagnose osteonecrosis of the femoral head in its earliest stage.
Weinstein et al.23 attempted to determine the pathogenetic sequence leading to ONFH in mice after prednisolone administration. Bone marrow analysis at 14 days revealed decreases in hypoxia-inducible factor-1α, vascular endothelial growth factor, and osteoblast count, and increased osteoclasts. Histologic analysis at 28 days revealed decreases in cancellous density, cortical width, and trabecular thickness. After 42 days, diffuse cancellous tissue necrosis and cortical architecture deterioration were noted.
Recent work has attempted to elucidate the pathogenetic mechanisms involving microRNAs, several of which regulate the differentiation of bone marrow mesenchymal stem cells (BM-MSCs) into adipogenic and osteogenic progenitor cells. In vitro analyses of BM-MSCs derived from humans24-27 and animals28-30 have identified various microRNAs as potential biomarkers and therapeutic targets. While this work is beyond the scope of this review, the interested reader is referred to several studies24-30.
Diagnosis and Prognosis
The diagnosis of ONFH typically involves radiographs and magnetic resonance imaging (MRI). MRI is up to 100% sensitive for this diagnosis31-34. The presence of subchondral fracture suggests disease progression and may help to define the treatment course. Computed tomography (CT) may be superior to MRI in detecting subchondral fractures35,36, but further studies are necessary to determine if the additional cost and radiation exposure are justified.
Successful treatment depends on accurate staging. There is no consensus regarding the best classification system since many have demonstrated limited interobserver and intraobserver reliability (Table I)37-40. Thus, treatment plans may differ on the basis of the system used, and the ability to compare results among studies is limited even when the same system is used. The Association Research Circulation Osseous (ARCO) classification system41 was developed to create a uniform classification tool for clinical trials by merging the Ficat42, Japanese Orthopaedic Association43, and Steinberg systems44, and has been recently revised. In the latest version, stage 0 was eliminated, stage III was subdivided into early (IIIA) and late (IIIB) stages depending on the degree of head depression (≤2 versus >2 mm), and acetabular involvement was incorporated into stage IV (Table II)45. These modifications result in a system that may be more practical for clinical and research-related applications. While it has not been formally validated, some of its radiographic features (e.g., head depression46-51) have been. Nevertheless, future studies will assess the validity and reproducibility of the most recent ARCO system.
TABLE I.
Reliability of Classification Systems for Osteonecrosis of the Femoral Head
| Study | Classification System* | Measure of Reliability | Result† |
| Schmitt-Sody et al.38 (2008) | Ficat | Interobserver reliability | 0.37 (0.23-0.70) |
| Intraobserver reliability | 0.50 (0.29-0.71) | ||
| ARCO | Interobserver reliability | 0.35 (0.06-0.56) | |
| Intraobserver reliability | 0.44 (0.26-0.56) | ||
| Smith et al.39 (1996) | Ficat | Interobserver reliability | 0.46 (0.30-0.67) |
| Intraobserver reliability | 0.59 (0.44-0.73) | ||
| Kay et al.40 (1994) | Ficat | Interobserver variability | 0.56 ± 0.01 |
| Intraobserver variability | 0.82 ± 0.16 |
ARCO = Association Research Circulation Osseous.
Data are presented as the mean kappa value (range) or mean kappa value and standard deviation. According to the guidelines of Svanholm et al.187, kappa values for reliability of <0.5 indicate poor agreement, those between 0.5 and 0.75 indicate fair agreement, and values of >0.75 indicate excellent agreement.
TABLE II.
| Stage | Description |
| I | Normal radiograph and abnormal MRI findings |
| II | No crescent sign, radiographic evidence of sclerosis, osteolysis, or focal osteoporosis |
| III | Subchondral fracture, fracture in the necrotic portion, and/or flattening of the femoral head on radiograph or CT scan |
| IIIA | Femoral head depression of ≤2 mm |
| IIIB | Femoral head depression of >2 mm |
| IV | Evidence of osteoarthritis, joint space narrowing, and degenerative acetabular change |
ARCO = Association Research Circulation Osseous, MRI = magnetic resonance imaging, and CT = computed tomography.
Studies have attempted to identify serum biomarkers that are associated with ONFH52-66. Among these, only 3 have determined the diagnostic performance by means of sensitivities, specificities, or positive and negative predictive values56,63,66. Without such analyses, significant associations of certain biomarkers with ONFH can only be considered speculative and should not be used for screening or diagnosis yet.
Nonoperative Therapy
ONFH typically follows a progressive course, with a majority of untreated lesions leading to collapse. Nonsurgical treatment modalities have generally been ineffective at halting progression (Table III). They are not appropriate in early stages when attempting to preserve the native joint, except for rarely encountered, small-sized, medially located lesions (<10%)67. Recent studies have evaluated the efficacy of pharmacological therapy including bisphosphonates68-78, anticoagulants79,80, vasodilators81, acetylsalicylic acid82, and lipid lowering agents (Table IV)83-86. Biophysical modalities including extracorporeal shockwave therapy87,88, pulsed electromagnetic fields89,90, and hyperbaric oxygen91,92 have also been investigated. However, studies have been small-scale, single-center, and of low-level evidence, often with inconclusive results. Therefore, these modalities remain experimental.
TABLE III.
Recommendation for Treatment of Osteonecrosis of the Femoral Head
| Treatment | Grade of Recommendation* |
| Operative | |
| Precollapse | |
| Core decompression | A |
| Multiple small-diameter drilling | A |
| Adjunctive bone-grafting | A |
| Cell-based therapy | B |
| Nonvascularized bone-grafting | B |
| Vascularized bone-grafting | B |
| Tantalum rod | C |
| Rotational osteotomy | B |
| Angular osteotomy | C |
| Postcollapse | |
| Total hip arthroplasty | A |
| Nonoperative | |
| Observation | I |
| Weight-bearing restriction | I |
| Bisphosphonates | I |
| Anticoagulants | I |
| Vasodilators | I |
| Acetylsalicylic acid | I |
| Extracorporeal shockwave therapy | I |
| Pulsed electromagnetic fields | I |
| Hyperbaric oxygen | I |
According to Wright188, grade A indicates good evidence (Level-I studies with consistent findings) for or against recommending intervention; grade B, fair evidence (Level-II or III studies with consistent findings) for or against recommending intervention; grade C, poor-quality evidence (Level-IV or V studies with consistent findings) for or against recommending intervention; and grade I, insufficient or conflicting evidence not allowing a recommendation for or against intervention.
TABLE IV.
Nonoperative Therapy*
| Study | Therapy Used | No. of Hips | Stage of Disease | Mean Follow-up (Range) (yr) | Hip Survivorship (%) |
| Level-I evidence | |||||
| Lee et al.75 (2015) | Bisphosphonate | 55 | Precollapse | 2 (NR) | 47 |
| Level-III evidence | |||||
| Gianakos et al.189 (2016) | Bisphosphonate | 40 | Precollapse | 2.1 (NR) | 47.5 |
| Albers et al.82 (2015) | Acetylsalicylic acid | 12 | Precollapse | 3.7 (NR) | 91.7 |
| Level-IV evidence | |||||
| Xie et al.190 (2018) | ESWT | 43 | Precollapse | 10.8 (10.1-11.5) | 81 |
| Algarni and Al Moallem191 (2018) | ESWT | 33 | Precollapse | 5 (2-9) | 95.3 |
| Glueck et al.79 (2015) | Anticoagulant | 9 | Precollapse | 9 (4-16) | 66 |
| Koren et al.91 (2015) | Hyperbaric Oxygen | 78 | Precollapse | 11.5 (NR) | 93 |
NR = not reported, and ESWT = extracorporeal shockwave therapy.
Operative Treatment
Core Decompression
For precollapse ONFH, core decompression (CD) procedures can be performed in an attempt to preserve the femoral head93. They have been used for >50 years and have been shown to outperform nonoperative management of precollapse lesions (Table V)94,95. A recent meta-analysis of 32 studies evaluating 2,441 hips determined that CD is a safe and effective treatment method with an overall success rate of 65% at a mean follow-up of 54.3 months (range, 2 to 228 months)96. Roth et al.97 performed a systematic review of 159 studies, which led the German consensus guidelines to recommend CD over nonoperative modalities for precollapse ONFH if the lesion size is <30% of the femoral head volume. There appears to be a consensus in the literature that CD is more effective than nonoperative management on the basis of a few older small-scale randomized studies95,98-101. However, to our knowledge, there are no recently performed, high-quality randomized trials, possibly because of a reluctance to assign patients to nonoperative treatments with the knowledge that CD may be superior.
TABLE V.
Core Decompression
| Study | Level of Evidence | Stage of Disease (no. of hips) | Mean Age (yr) | Mean Follow-up (yr) | Survivorship According to Stage* (%) | ||
| Precollapse | Postcollapse | Precollapse | Postcollapse | ||||
| Traditional core decompression | |||||||
| Lakshminarayana et al.110 (2019) | III | 36 | – | 30 | 4.46 | 75 | – |
| Nazal et al.192 (2019) | IV | 11 | – | 36.4 | 7 | 54.5 | – |
| Multiple drilling technique | |||||||
| Yin et al.193 (2016) | III | 26 | – | 41.6 | 3 | 58 | – |
| Haberal et al.107 (2018)† | IV | 14 | 16 | 43.3 | 2.9 | 100 | 87.5 |
| Core decompression with bone-grafting | |||||||
| Lakshminarayana et al.110 (2019) | III | 40 | – | 30 | 4.5 | 70 | – |
| Zeng et al.109 (2015) | III | 18 | – | 40.7 | 4.4 | 77.8 | – |
| Tantalum rod | |||||||
| Liu et al.194 (2015) | III | 26 | 31 | 35.4 | 5 | 79.3 | 41.9 |
| Hu et al.195 (2018) | IV | 43 | 29 | 44.5 | 2.2 | 92.9 | 86.7 |
| Lu et al.196 (2018) | IV | 57 | – | 40.7 | 4 | 84.6 | – |
Survivorship defined as no radiographic progression or need for further surgery.
Studies that did not describe radiographic outcomes were based only on the need for further surgery.
CD is typically performed under fluoroscopic guidance based on the lesion location depicted by MRI. The use of MRI for real-time 3-dimensional CD guidance is technically feasible, safe, and accurate102. While limited by additional costs and need for MRI-compatible instruments, the improved accuracy and decreased radiation with these techniques may be worthy of further evaluation. Irrigative cooling to prevent thermal necrosis should also be assessed, especially for patients who have hard or sclerotic bone (e.g., young men and patients with sickle-cell disease)103,104.
Some authors have reported using multiple small-diameter (3 to 8-mm) drilling rather than a large single core (Figs. 1-A and 1-B), as they may be less invasive and decrease the risk of fractures105-108. Attempts have been made to enhance the results of CD with bone grafts, synthetic bone substitutes, bone morphogenetic proteins, tantalum rods, or adjunctive cells (Table VI). There is no consensus regarding the optimal CD technique. The following sections describe recent studies on these variations.
Figs. 1-A and 1-B Small-diameter CD for ONFH. A trocar is introduced into the necrotic lesion using light mallet blows (Fig. 1-A) under fluoroscopic guidance (Fig. 1-B).
Fig. 1-A.
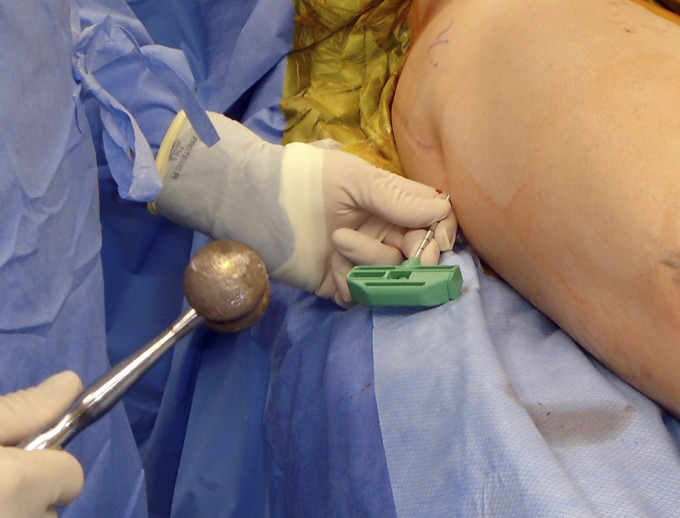
Fig. 1-B.
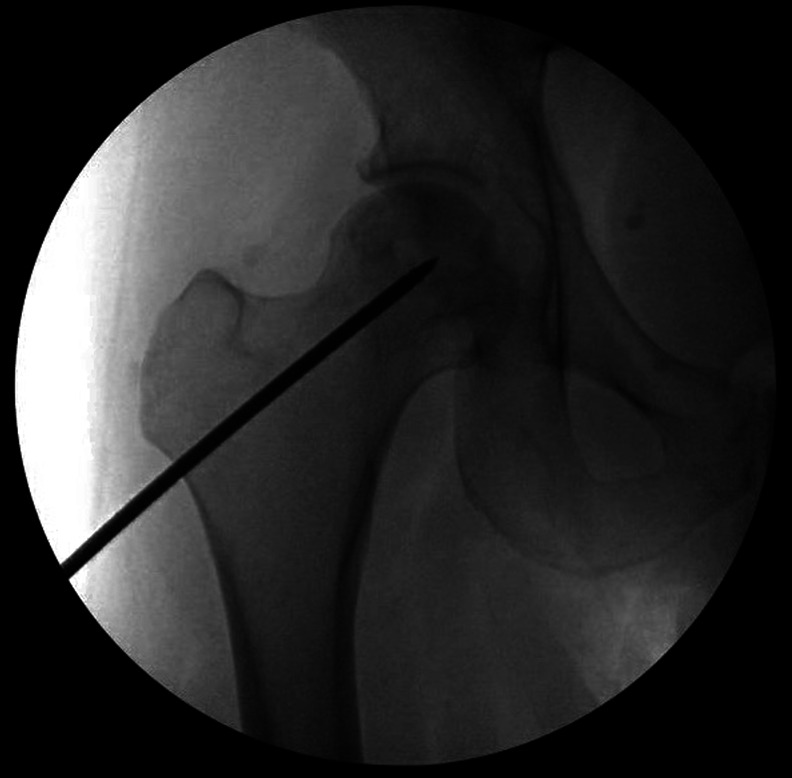
TABLE VI.
Advantages and Disadvantages of Adjunctive Therapies for Core Decompression
| Treatment | Advantages | Disadvantage(s) |
| Autologous strut-grafting |
|
|
| Autologous bone chips |
|
|
| Allogeneic bone-grafting |
|
|
| Synthetic bone substitute |
|
|
| Bone morphogenetic protein |
|
|
| Tantalum rod |
|
|
Adverse effects may include heterotopic ossification, wound complications, inflammatory reactions, induction of structurally abnormal bone, and osteoclast ossification.
Adjunctive Bone-Grafting
Bone-grafting is sometimes performed as an ancillary procedure for CD that may provide structural support and act as a scaffold for new bone formation93. Zeng et al.109 reviewed the cases of 18 patients who had bilateral ONFH (36 hips). CD with fibular bone allograft augmentation was performed on 1 hip, while THA was carried out on the contralateral side. After a mean follow-up of 53 months (range, 20 to 107 months), the mean Harris hip score (HHS) (and standard deviation) was 83.8 ± 17.9 points postoperatively compared with 61.6 ± 17.0 points preoperatively (p < 0.05), with 14 hips (78%) avoiding THA. Lakshminarayana et al.110 prospectively followed 36 hips treated with standard CD compared with 40 hips with precollapse lesions that underwent CD with fibular grafting. At the time of the final follow-up (mean, 53.5 months; range, 44 to 63 months), radiographic progression was noted in 9 hips (25%) that underwent CD alone and in 12 hips (30%) with fibular grafting. The authors concluded that CD with or without fibular grafting is efficacious for early-stage ONFH. Sallam et al.111 performed an inverted femoral head graft procedure by harvesting a cylindrical bone block from the femoral head and reinserting it in a reversed direction so that the cortical portion supported the subchondral bone. Compared with 34 hips treated with CD, the 33 hips treated with inverted femoral grafts demonstrated significantly improved 10-year survivorship (67.3% versus 37%; p = 0.046).
Small-Diameter Drilling
Small-diameter drilling may provide the same clinical benefit as CD105,112. Mont et al. percutaneously introduced 3-mm trephinations in 45 precollapse hips. After a mean follow-up of 2 years (range, 20 to 39 months), 32 hips (71%) had good to excellent HHS results106. Mohanty et al.113 compared multiple small-diameter drilling (33 hips) and autologous fibular strut-grafting (35 hips). Three-year survivorship analysis demonstrated that 26 hips (78.8%) with small-diameter drilling did not require THA compared with 33 hips (94.3%) that underwent bone-grafting (p > 0.05). The authors concluded that precollapse ONFH can be treated with small-diameter CD, while early postcollapse disease may derive more benefit from fibular strut-grafting. Many CD procedures with adjunctive cell-based therapy utilize small-diameter drilling and are discussed later114-116.
Adjunctive Cell-Based Treatment of CD
Cell-based augmentation of CD has recently gained substantial attention (Table VII). Hernigou and Beaujean117, in 2002, were the first, as far as we know, to report CD with autologous concentrated bone-marrow grafting. Since then, there have been many studies using various types of cell-based therapies. Piuzzi et al.118 reviewed the literature through 2016 to assess the benefits of cell-based treatments. Among 11 studies included in that review, 6 compared CD with adjunctive cell-based therapy and CD alone, 2 compared CD and adjunctive bone-grafting with and without cells, 2 compared tantalum rods with and without cells, and 1 study evaluated CD with bone-grafting compared with CD with cells. Overall, 24.5% (93) of 380 hips receiving cell therapies showed radiographic progression compared with 40% (98) of 245 hips of controls. Nine of 10 studies that described failure rates showed lower THA conversion rates with cell therapy (16%) than with controls (21%). All 10 studies that described patient-reported outcomes demonstrated improved results with cell therapies compared with controls. However, high levels of heterogeneity among the treatments were noted. Even when the same harvest procedure was used, the product was not consistent because of inherent differences among individuals119. Standardized practices for the investigation and reporting of cell-based therapies should be implemented to facilitate reproducibility.
TABLE VII.
Cell-Based Treatment*
| Study | No. of Hips | Stage of Disease | Type of Cell-Based Therapy | Comparison Group | Mean Follow-up (Range) (yr) | Hip Survivorship (%) |
| Level-III evidence | ||||||
| Hauzeur et al.114 (2018) | 38 | Precollapse and postcollapse | BMAC | CD alone | 2 | 34.8 for CD+BMAC, and 34.8 for CD alone |
| Hernigou et al.120 (2018) | 125 | Precollapse | BMC | CD alone | 25 (20-30) | 76 for CD+BM-MSC, and 24 for CD alone† |
| Houdek et al.123 (2018) | 35 | Precollapse | BMC+PRP | None | 3 (2-4) | 84 |
| Kang et al.115 (2018) | 106 | Precollapse and postcollapse | BMC | CD alone | 4.28 | 71.7 for CD+BM-MSC, and 51 for CD alone† |
| Cruz-Pardos et al.197 (2016) | 60 | Precollapse | BMAC | CD alone | 3 (2-6.6) for CD+BMAC, and 5.3 (2-14.3) for CD alone | 46 for CD+BMAC, and 47.4 for CD alone |
| Pepke et al.198 (2016) | 24 | Precollapse | BMC | CD alone | 2 (NR) | 64 for CD+BM-MSC, and 57 for CD alone‡ |
| Tabatabaee et al.199 (2015) | 28 | Precollapse and postcollapse | BMC | CD alone | 2 (NR) | 100 for CD+BM-MSC, and 78.6 for CD alone |
| Level-IV evidence | ||||||
| Talathi and Kamath125 (2018) | 43 | Precollapse | BMAC | None | 1.3 (NR) | 94 |
| Tomaru et al.200 (2017) | 31 | Precollapse | BMAC | None | 5.8 (2-6.9) | 90.3 |
| Chen et al.201 (2016) | 9 | Precollapse | hUC-MSC | None | 24 | NR |
| Kuroda et al.202 (2016) | 10 | Precollapse | rhFGF-2 | None | 1 (NR) | 90 |
| Persiani et al.203 (2015) | 31 | Precollapse and postcollapse | BMC | None | 3.1 (2-4) | 80 |
BMAC = bone marrow aspirate concentrate, CD = core decompression, BM-MSC = bone marrow mesenchymal stem cells, BMC = bone marrow cells, PRP = platelet-rich plasma, NR = not reported, hUC-MSC = human umbilical cord-derived mesenchymal stem cells, and rhFGF-2 = recombinant human fibroblast growth factor-2.
A significant difference was found between groups.
Based on radiographic evidence of disease progression.
Kang et al.115 compared the efficacy of CD with and without bone marrow aspirate concentrate (BMAC) augmentation in 106 hips (Fig. 2). At a mean follow-up of 4.3 years (range, 3 to 10 years), the BMAC group had lower THA conversion rates (49% versus 28.3%; p = 0.028). For patients who had precollapse disease, the failure rate was significantly improved with BMAC augmentation (50% versus 20%; p = 0.014). Those who had postcollapse lesions did not derive the same benefit. Similarly, other studies have found that BMAC augmentation of CD decreases the THA conversion rate in early-stage lesions but not advanced lesions114,115,120-122.
Fig. 2.
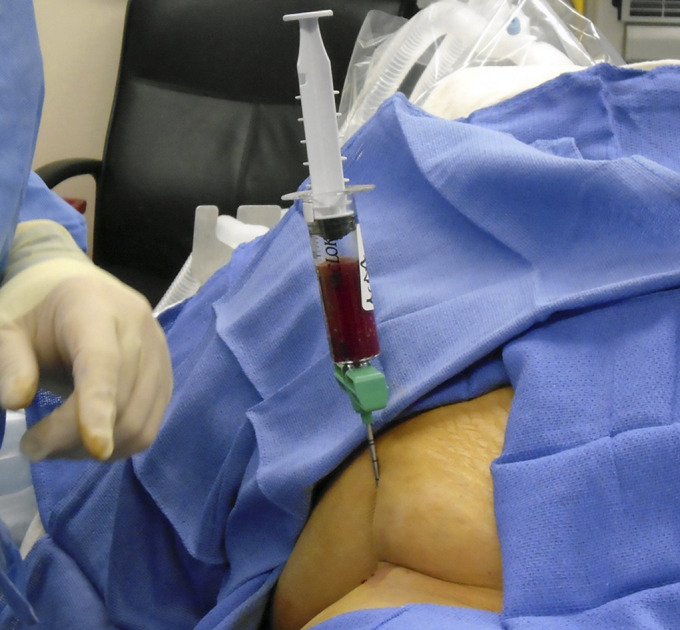
Aspiration of bone marrow from the iliac crest for subsequent processing and implantation following femoral head CD.
Hernigou et al.120 reported 30-year results of bilateral corticosteroid-associated precollapse ONFH. Larger lesions were treated with BMAC injection and smaller contralateral defects underwent CD alone. The cell-therapy group was approximately 3 times less likely to require THA (p < 0.0001). In another cohort, 35 precollapse hips underwent CD with ancillary BMAC plus platelet-rich plasma123. MRI analysis at a mean follow-up of 3 years (range, 2 to 4 years) demonstrated that 93% of the hips did not collapse. A meta-analysis of 14 randomized controlled trials evaluated CD with adjunctive cell-based therapy (n = 275) compared with CD alone (n = 265)124. Compared with CD alone, augmentation with cells was associated with significant improvements in the 24-month visual analog scale pain score (p = 0.028) and the need for THA (p = 0.007).
In summary, on the basis of the available literature, the results of cell-enhanced CD appear promising. However, factors that influence its success have yet to be elucidated (Table VIII). The lack of standardization with respect to quantitative and qualitative characterizations of harvest methods, processing, and transplantation of cells continues to present a challenge. Although the number of osteogenic progenitor cells has been shown to influence outcomes123,125, the minimum number of cells needed remains unknown. In fact, most of the cells in BMAC are not mesenchymal or vascular progenitor cells. Moreover, it remains unclear whether the BM-MSCs from patients with ONFH still have osteogenic potential25,126. For these reasons, it is important to establish standards for preparation and reporting for cell-based procedures.
TABLE VIII.
Advantages and Disadvantages of Bone Marrow Aspirate Concentrate
| Advantages | Disadvantages |
| Ease of availability | Increased cost and surgical time |
| Potential for multilineal differentiation (osteoblasts, chondrocytes, lipocytes, tenocytes, etc.) | Need for additional equipment |
| No risk of malignant transformation | Not osteoconductive |
| Free of ethical issues | Does not provide structural support |
| Can be combined with osteoconductive materials (e.g., various bone grafts). | Inherent differences in sample composition among individual patients |
| Need for additional surgical procedure | The number of osteogenic progenitor cells that are being implanted is unknown at the time of the procedure |
| Potential for harvest site morbidity |
Tantalum Rods
Tantalum rods may provide structural support following CD, but their results have not been optimal127-131. When survivorship was evaluated in 104 hips with ARCO grade-II or III ONFH that were managed with tantalum rods, the survival rate was 53% in 90 patients at a mean follow-up of 43 months (range, 1 to 78 months)132. Because of the increased complication rates in patients who undergo THA following tantalum rod failure133-135, this treatment modality has fallen out of favor.
Bone-Grafting
Various bone-grafting options exist, including nonvascularized or vascularized autologous bone harvested from the iliac crest46,50,136-140, fibula110,141-145, or femur as well as allogeneic sources146, and synthetic preparations147,148. The following sections review these options (Table IX).
TABLE IX.
Bone-Grafting
| Study | No. of Hips | Stage of Disease | Type of Graft | Other Implants and/or Procedures | Mean Follow-up (Range)* (yr) | Hip Survivorship (%) |
| Nonvascularized | ||||||
| Level-II evidence | ||||||
| Lin et al.160 (2018) | 16 | Precollapse and postcollapse | Iliac crest bone autograft | Graft loaded into lantern-shaped screw | 3 (NR) | 88 |
| Level-IV evidence | ||||||
| Yildiz et al.152 (2018) | 28 | Precollapse and postcollapse | Iliac crest bone autograft | – | 4.4 (2-6.7) | 71† |
| Sallam et al.111 (2017) | 71 | Precollapse and postcollapse | Inverted femoral head autograft | – | 7.9 (3-14) | |
| Vascularized | ||||||
| Level-III evidence | ||||||
| Feng et al.137 (2019) | 84 | Precollapse and postcollapse | Greater trochanter flap | Corticocancellous iliac bone graft | 9.7 (6-9) | 92.9 |
| Zhang et al.159 (2019) | 115 | Precollapse | Sartorius muscle-pedicled iliac bone | – | 2.6 (2-4) | 87.5† |
| 84 | Circumflex iliac deep bone flap | – | 88.2† | |||
| Zhao et al.140 (2017) | 2179 | Precollapse and postcollapse | Lateral femoral circumflex vessel-pedicled iliac bone | – | 12‡ (5-25) | 82 |
| Ünal et al.145 (2016) | 26 | Precollapse and postcollapse | Free vascularized fibular graft | – | 7.6 (5-9) | 76.9 |
| Level-IV evidence | – | |||||
| Xie et al.138 (2019) | 847§ | Precollapse and postcollapse | Lateral femoral circumflex vessel-pedicled iliac bone | – | 15 (5-25) | 89.1# |
| Cho et al.157 (2018) | 24 | Precollapse | Gluteus medius-pedicled greater trochanter flap | – | 6.2 (2-10) | 87.5# |
| Chen et al.136 (2016) | 64 | Precollapse and postcollapse | Sartorius muscle-pedicled iliac bone | – | 2.9 (2-4) | 81.3 |
NR = not reported.
No radiographic progression.
Median.
Traumatic cases excluded from analysis.
Avoided THA.
Nonvascularized Bone-Grafting
Nonvascularized fibular grafts, cortical strut grafts, or cancellous bone chips are viable options for the treatment of ONFH. Techniques for the implantation of these grafts include the Phemister technique109,113,149,150, the trapdoor procedure137,146,151, and the lightbulb technique (Fig. 3)51,152-154. In one of the first reports of the lightbulb procedure, which was published in 1994, Rosenwasser et al.155 followed 13 patients with Ficat stage-I, II, or III lesions. After a mean follow-up of 12 years (range, 10 to 15 years), 11 patients were symptom-free with minimal progression. Mont et al.156 performed the lightbulb technique in 21 hips by implanting demineralized bone matrix, bone morphogenetic protein-rich allograft bone chips, and a thermoplastic carrier. After a mean follow-up of 48 months (range, 36 to 55 months), 86% of the hips were clinically successful. Yildiz et al.152 performed the lightbulb procedure in 28 hips. After a mean follow-up of 52.6 months (range, 24 to 80 months), radiographic progression was seen in 28.6% of the hips and only 14.3% were converted to THA. While the lightbulb technique has yielded good outcomes, its disadvantages must be noted. Compared with placement of bone grafts through a CD track, the larger incision associated with this procedure renders it more invasive and technically demanding. In addition, surgeons should be prepared to perform THAs for hips with cartilage delamination, although this can be considered an advantage for the patient.
Fig. 3.
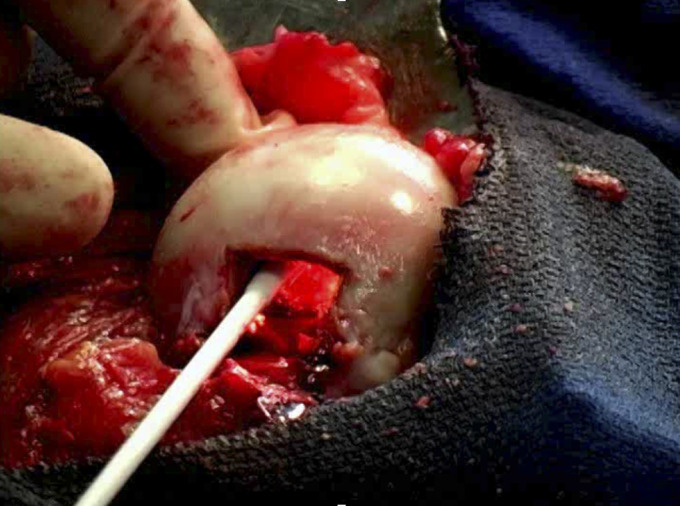
The lightbulb technique—creation of a cortical window at the femoral head-neck junction for evacuation of necrotic tissue and replacement with a bone graft.
Vascularized Bone-Grafting
Restoring blood supply to the necrotic lesion may be important for successful management of ONFH. Many types of vascularized bone grafts, including free vascularized fibular grafts (FVFGs), greater trochanter flaps, and various muscle-pedicled bone flaps, have been used successfully. Ünal et al.145 reviewed 26 hips that underwent FVFG. At a mean follow-up of 7.6 years (range, 5 to 9.2 years), the HHS was >80 points in 15 of 16 patients who had precollapse disease and in 6 of 7 patients who had postcollapse lesions. The efficacy of vascularized bone grafts derived from the ilium and greater trochanter has been evaluated in numerous studies, the results of which are outlined in Table IX46,50,111,136-140,145,152,157-160. Some disadvantages of vascularized bone-grafting (Table X) include its technically difficult and time-consuming nature, as it may require 2 surgical teams and many hours of surgery. In addition to concerns over the long-term patency of the anastomosis and viability of the graft161,162, there is potential for fracture since grafts are placed in the diaphyseal area. Furthermore, it is not only difficult to reach the lesion but ancillary nonvascularized bone grafts must also be used to fill in the remaining osteonecrotic void since the fibular graft supports only 1 area of the typically larger lesion. In addition, potential harvest-site morbidity includes flexor hallucis longus contracture, peroneal nerve injury, ankle instability, and gait alterations, which can approach a prevalence of 13% to 20%163,164.
TABLE X.
Advantages and Disadvantages of Various Vascularized Bone-Grafting Techniques
| Technique | Advantages | Disadvantages |
| Lateral femoral circumflex vessel-pedicled iliac bone | Minimal donor-site morbidity | Potential damage to lateral femoral cutaneous and ilioinguinal nerves |
| Corticocancellous, unicortical, or bicortical bone | ||
| Large amounts of cancellous bone can be harvested as additional graft material | ||
| Long, large-diameter pedicle facilitates blood flow | ||
| No need for microsurgery | ||
| Greater trochanter flap | No need for microsurgery | May not provide as much support as fibular grafts |
| Free vascularized fibular graft | Endosteal and periosteal blood supply | No cancellous bone |
| Dual blood supply allows for different osteotomies | Flexor hallucis longus contracture | |
| Cortical bone provides good structural support | Claw toe deformity | |
| Peroneal nerve injury | ||
| Gait alterations | ||
| Ankle instability | ||
| Sartorius muscle-pedicled iliac bone | No need for microsurgery | May not provide as much support as fibular grafts |
| Gluteus medius-pedicled greater trochanter flap | No need for microsurgery | May affect hip mobility |
| May not provide as much support as fibular grafts |
Osteotomy
Intertrochanteric or rotational osteotomies of the proximal part of the femur have been performed to shift the affected areas of the femoral head away from weight-bearing regions165,166. Transtrochanteric rotational osteotomies are commonly performed in Japan, while the intertrochanteric flexion-varus or extension-valgus variants are more commonly performed in Europe166-168. These procedures are less commonly used in the U.S. because they are difficult to perform, have variable results, and can only be used in a select group of patients who have small lesions that can be rotated away from the weight-bearing zone. Also, if they fail, they make subsequent conversion to THA more difficult169,170.
THA
THA has been the treatment of choice for symptomatic advanced-stage femoral head collapse, particularly when secondary acetabular changes are noted. Suitable candidates for THAs include patients who have large lesions with or without collapse or those who have cartilage delamination without apparent collapse. THA has been shown to yield excellent results in multiple studies with outcomes comparable with those for patients who have other diagnoses (Table XI). Due to the younger demographic of patients with ONFH, the long-term durability of THA is especially important.
TABLE XI.
Total Hip Arthroplasty*
| Study | Implant Description | Cohort Description | No. of Hips | Follow-up (Range)† (yr) | Age† (yr) | Mean Harris Hip Score (points) | Implant Survivorship (%) |
| Level-II evidence | |||||||
| Capone et al.175 (2017) | Cementless CoC | Age of <60 yr | 37 | 5.6 (3-10) | 52 (27-61) | 90 | 100 |
| Level-III evidence | |||||||
| Jo et al.204 (2018) | Cementless CoC | ARCO stage II vs. stage III vs. stage IV | 56 in stage II, 458 in !stage III, 336 in stage IV | 1 (NR) | 49 (16-84) | 98.5 for stage II, 96.6 for stage III, 95.9 for stage IV | NR |
| Miladi et al.176 (2018) | Cementless SS vs. cemented CS | - | 6 SS and 10 CS | 7 (1-18) | 46 (31-69) | 94 for SS, 92.6 for CS | 100 |
| Level-IV evidence | |||||||
| Suksathien and Sueajui171 (2019) | Cementless SS | - | 83 | 5.8 (5-7) | 44 (21-68) | 99.6 | 98.8 |
| Assi et al.205 (2018) | Cementless DMC | - | 30 | 4.3 (2-10) | 55 (25-90) | 98.7 | 100 |
| Martz et al.206 (2017) | Cementless DMC | Age of <55 yr | 40 | 10.8 ± 3.5 | 44 ± 8 | 95.7 | 100 |
| Swarup et al.207 (2017) | Various | Age of <35 yr | 204 | 14 (2-27) | 27.3 (13-35) | NR | 86 at 10 yr, 66 at 20 yr |
| Lim et al.208 (2016) | Cementless CoC | - | 53 | 5.3 (5-6) | 49 (20-80) | 97 | 100 |
CoC = ceramic on ceramic, NR = not reported, SS = short stem, CS = conventional stem, and DMC = dual-mobility cup.
Data are reported as the mean with the range in parentheses or the mean and standard deviation.
Short-stem femoral components are commonly used as an attempt to preserve metaphyseal bone171-176. Researchers have reported good short174, mid171,175, and long-term173 outcomes of these stems in patients with ONFH. Due to differences among available short-stem femoral components, it is difficult to draw conclusions regarding the optimal design. It has been suggested that short stems with diaphyseal anchorage do not increase the risk of aseptic loosening; however, in stems with metaphyseal fixation, preoperative MRI can ensure that the osteonecrotic lesion does not extend distal to the femoral neck177. Selection of the femoral prosthesis should be based on overall bone quality. A cemented stem may be appropriate in elderly patients who have very widened canals. Conversely, in some younger patients with sickle-cell disease who have sclerotic proximal femoral bone, an extensively coated stem with cementless fixation can be used. Likewise, the etiology of osteonecrosis should be considered when performing a THA. For instance, patients with sickle-cell disease often have bone infarcts within the femoral canal that may complicate femoral preparation and component positioning, and patients with a history of bone-grafting may have resultant defects in the femoral neck or intertrochanteric region. In these patients, intraoperative radiographs may be necessary to avoid varus placement of the components. Additionally, extra care should be taken when preparing the femoral and acetabular components (e.g., screw fixation) in patients who have soft bone (i.e., corticosteroid-associated osteoporosis).
In a 2019 study178, 461 hips with ONFH in 413 patients (mean age, 59 years; range, 24 to 94 years) who underwent THA were matched 1:1 to hips that only had osteoarthritis. No differences between the groups were reported at the time of the final follow-up (median, 10 years). The median HHS was 93 points (range, 27 to 100 points) in the ONFH group and 93 points (range, 43 to 100 points) in the osteoarthritis group. The 15-year revision rate was 6.6% in the ONFH group and 4.5% in the osteoarthritis group (hazard ratio = 1.8, p = 0.09). In another recent study179, 133 hips in 101 patients (mean age, 25 years; range, 16 to 54 years) with osteonecrosis because of sickle-cell disease underwent THA. After a mean follow-up of 14.6 years (range, 5 to 17 years), the mean Merle d’Aubigné score improved from 5.1 to 1.2 points in the pain subscale and from 2.2 to 4.8 points in the function domain. The authors reported an overall survivorship of 96.8% at the 10-year follow-up and 94.1% at 15 years.
Based on the available literature, it appears that the outcomes of THA in the setting of ONFH are similar to those for patients who have an underlying diagnosis of osteoarthritis. Despite the younger age of patients with ONFH compared with those who have isolated osteoarthritis, excellent long-term clinical results following THA have been demonstrated in this subset of patients.
Nevertheless, polyethylene wear in patients with ONFH who undergo THA remains a concern, likely because of the higher activity levels in younger patient populations180-182. Min et al.183 evaluated the long-term durability of 85 THAs with highly cross-linked polyethylene liners in patients with ONFH who were <50 years old (mean, 42 years; range, 25 to 49 years). At the time of the final follow-up evaluation (mean, 13.5 years; range, 10 to 17.3 years), all hips had wear levels below the osteolysis threshold (0.10 mm/yr)184,185, and it was found that age and activity level had no influence on the polyethylene wear. However, the authors of the current review stress the importance of including activity levels in THA survivorship analyses, particularly for patients who have ONFH. It has been suggested that at the time of THA, younger age is more predictive of higher activity levels in patients with osteoarthritis compared with other diagnoses186. Because of the variability in the activity level of patients with ONFH who undergo THA, including these data is critical for studies of these patients.
Because osteonecrosis is an end diagnosis, the underlying cause may independently influence THA outcomes. A major weakness of the literature on arthroplasty for ONFH is that patient diagnoses besides osteonecrosis are usually not delineated. To this end, these studies should attempt to stratify results by etiology rather than by reducing potentially distinct populations to a single group.
Overview
While our understanding of femoral head osteonecrosis continues to improve, the management of this disease remains difficult. Early diagnosis and prompt treatment are paramount, as postcollapse lesions are less amenable to joint-preserving techniques. Biologic augmentation of CD has shown promising results in providing symptomatic relief and slowing the natural progression of this disease, but more study is necessary. For postcollapse lesions, when ≤2 mm of head depression is present, preservation of the femoral head can be attempted with vascularized or nonvascularized bone-grafting. For late-stage lesions with >2 mm of head depression or acetabular involvement, and that have failed nonoperative management, THA remains the best option.
Footnotes
Investigation performed at Lenox Hill Hospital, New York, NY; Cleveland Clinic, Cleveland, Ohio; Stanford University Medical Center, Stanford, California; and Johns Hopkins University School of Medicine, Baltimore, Maryland
Disclosure: This work was supported by grant R34-AR073505-01A1 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the U.S. National Institutes of Health (L.C.J., S.B.G., and M.A.M.). The Disclosure of Potential Conflicts of Interest forms are provided with the online version of the article (http://links.lww.com/JBJS/F832).
References
- 1.Bergman J, Nordström A, Nordström P. Epidemiology of osteonecrosis among older adults in Sweden. Osteoporos Int. 2019. May;30(5):965-73. Epub 2019 Jan 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cooper C, Steinbuch M, Stevenson R, Miday R, Watts NB. The epidemiology of osteonecrosis: findings from the GPRD and THIN databases in the UK. Osteoporos Int. 2010. April;21(4):569-77. Epub 2009 Jun 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dima A, Pedersen AB, Pedersen L, Baicus C, Thomsen RW. Association of common comorbidities with osteonecrosis: a nationwide population-based case-control study in Denmark. BMJ Open. 2018. February 8;8(2):e020680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fukushima W, Fujioka M, Kubo T, Tamakoshi A, Nagai M, Hirota Y. Nationwide epidemiologic survey of idiopathic osteonecrosis of the femoral head. Clin Orthop Relat Res. 2010. October;468(10):2715-24. Epub 2010 Mar 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kang JS, Park S, Song JH, Jung YY, Cho MR, Rhyu KH. Prevalence of osteonecrosis of the femoral head: a nationwide epidemiologic analysis in Korea. J Arthroplasty. 2009. December;24(8):1178-83. Epub 2009 Jul 28. [DOI] [PubMed] [Google Scholar]
- 6.Mankin HJ. Nontraumatic necrosis of bone (osteonecrosis). N Engl J Med. 1992. May 28;326(22):1473-9. [DOI] [PubMed] [Google Scholar]
- 7.Moya-Angeler J, Gianakos AL, Villa JC, Ni A, Lane JM. Current concepts on osteonecrosis of the femoral head. World J Orthop. 2015. September 18;6(8):590-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mont MA, Jones LC, Hungerford DS. Nontraumatic osteonecrosis of the femoral head: ten years later. J Bone Joint Surg Am. 2006. May;88(5):1117-32. Erratum in: J Bone Joint Surg Am. 2006 Jul;88(7):1602. [DOI] [PubMed] [Google Scholar]
- 9.Mont MA, Cherian JJ, Sierra RJ, Jones LC, Lieberman JR. Nontraumatic osteonecrosis of the femoral head: where do we stand today? A ten-year update. J Bone Joint Surg Am. 2015. October 7;97(19):1604-27. [DOI] [PubMed] [Google Scholar]
- 10.Mont MA, Hungerford DS. Non-traumatic avascular necrosis of the femoral head. J Bone Joint Surg Am. 1995. March;77(3):459-74. [DOI] [PubMed] [Google Scholar]
- 11.Lespasio MJ, Sodhi N, Mont MA. Osteonecrosis of the hip: a primer. Perm J. 2019;23:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderson PA, Jeray KJ, Lane JM, Binkley NC. Bone health optimization: beyond Own the Bone: AOA critical issues. J Bone Joint Surg Am. 2019. August 7;101(15):1413-9. [DOI] [PubMed] [Google Scholar]
- 13.Gaddini GW, Grant KA, Woodall A, Stull C, Maddalozzo GF, Zhang B, Turner RT, Iwaniec UT. Twelve months of voluntary heavy alcohol consumption in male rhesus macaques suppresses intracortical bone remodeling. Bone. 2015. February;71:227-36. Epub 2014 Nov 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kahler-Quesada AM, Grant KA, Walter NAR, Newman N, Allen MR, Burr DB, Branscum AJ, Maddalozzo GF, Turner RT, Iwaniec UT. Voluntary chronic heavy alcohol consumption in male rhesus macaques suppresses cancellous bone formation and increases bone marrow adiposity. Alcohol Clin Exp Res. 2019. December;43(12):2494-503. Epub 2019 Oct 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weinstein RS. Clinical practice. Glucocorticoid-induced bone disease. N Engl J Med. 2011. July 7;365(1):62-70. [DOI] [PubMed] [Google Scholar]
- 16.Cui Q, Wang Y, Saleh KJ, Wang GJ, Balian G. Alcohol-induced adipogenesis in a cloned bone-marrow stem cell. J Bone Joint Surg Am. 2006. November;88(Suppl 3):148-54. [DOI] [PubMed] [Google Scholar]
- 17.Johnston JC, Haile A, Wang D, Ronnett G, Jones LC. Dexamethasone treatment alters function of adipocytes from a mesenchymal stromal cell line. Biochem Biophys Res Commun. 2014. September 5;451(4):473-9. Epub 2014 Jul 29. [DOI] [PubMed] [Google Scholar]
- 18.Sheng H, Sheng CJ, Cheng XY, Zhang G, Lee KM, Leung KS, Qu S, Qin L. Pathomorphological changes of bone marrow adipocytes in process of steroid-associated osteonecrosis. Int J Clin Exp Pathol. 2013. May 15;6(6):1046-50. [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y, Li Y, Mao K, Li J, Cui Q, Wang GJ. Alcohol-induced adipogenesis in bone and marrow: a possible mechanism for osteonecrosis. Clin Orthop Relat Res. 2003. May;410:213-24. [DOI] [PubMed] [Google Scholar]
- 20.Kawai K, Tamaki A, Hirohata K. Steroid-induced accumulation of lipid in the osteocytes of the rabbit femoral head. A histochemical and electron microscopic study. J Bone Joint Surg Am. 1985. June;67(5):755-63. [PubMed] [Google Scholar]
- 21.Tan G, Kang PD, Pei FX. Glucocorticoids affect the metabolism of bone marrow stromal cells and lead to osteonecrosis of the femoral head: a review. Chin Med J (Engl). 2012. January;125(1):134-9. [PubMed] [Google Scholar]
- 22.Houdek MT, Wyles CC, Packard BD, Terzic A, Behfar A, Sierra RJ. Decreased osteogenic activity of mesenchymal stem cells in patients with corticosteroid-induced osteonecrosis of the femoral head. J Arthroplasty. 2016. April;31(4):893-8. Epub 2015 Aug 29. [DOI] [PubMed] [Google Scholar]
- 23.Weinstein RS, Hogan EA, Borrelli MJ, Liachenko S, O’Brien CA, Manolagas SC. The pathophysiological sequence of glucocorticoid-induced osteonecrosis of the femoral head in male mice. Endocrinology. 2017. November 1;158(11):3817-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hao C, Yang S, Xu W, Shen JK, Ye S, Liu X, Dong Z, Xiao B, Feng Y. MiR-708 promotes steroid-induced osteonecrosis of femoral head, suppresses osteogenic differentiation by targeting SMAD3. Sci Rep. 2016. March 2;6:22599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang A, Ren M, Song Y, Wang X, Wang Q, Yang Q, Liu H, Du Z, Zhang G, Wang J. MicroRNA expression profiling of bone marrow mesenchymal stem cells in steroid-induced osteonecrosis of the femoral head associated with osteogenesis. Med Sci Monit. 2018. March 28;24:1813-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wei B, Wei W, Zhao B, Guo X, Liu S. Long non-coding RNA HOTAIR inhibits miR-17-5p to regulate osteogenic differentiation and proliferation in non-traumatic osteonecrosis of femoral head. PLoS One. 2017. February 16;12(2):e0169097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dai Z, Jin Y, Zheng J, Liu K, Zhao J, Zhang S, Wu F, Sun Z. MiR-217 promotes cell proliferation and osteogenic differentiation of BMSCs by targeting DKK1 in steroid-associated osteonecrosis. Biomed Pharmacother. 2019. January;109:1112-9. Epub 2018 Nov 6. [DOI] [PubMed] [Google Scholar]
- 28.Gu C, Xu Y, Zhang S, Guan H, Song S, Wang X, Wang Y, Li Y, Zhao G. miR-27a attenuates adipogenesis and promotes osteogenesis in steroid-induced rat BMSCs by targeting PPARγ and GREM1. Sci Rep. 2016. December 2;6:38491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liao W, Ning Y, Xu HJ, Zou WZ, Hu J, Liu XZ, Yang Y, Li ZH. BMSC-derived exosomes carrying microRNA-122-5p promote proliferation of osteoblasts in osteonecrosis of the femoral head. Clin Sci (Lond). 2019. September 19;133(18):1955-75. [DOI] [PubMed] [Google Scholar]
- 30.Zha X, Sun B, Zhang R, Li C, Yan Z, Chen J. Regulatory effect of microRNA-34a on osteogenesis and angiogenesis in glucocorticoid-induced osteonecrosis of the femoral head. J Orthop Res. 2018. January;36(1):417-24. Epub 2017 Jun 14. [DOI] [PubMed] [Google Scholar]
- 31.Hauzeur JP, Pasteels JL, Schoutens A, Hinsenkamp M, Appelboom T, Chochrad I, Perlmutter N. The diagnostic value of magnetic resonance imaging in non-traumatic osteonecrosis of the femoral head. J Bone Joint Surg Am. 1989. June;71(5):641-9. [PubMed] [Google Scholar]
- 32.Pierce TP, Jauregui JJ, Cherian JJ, Elmallah RK, Mont MA. Imaging evaluation of patients with osteonecrosis of the femoral head. Curr Rev Musculoskelet Med. 2015. September;8(3):221-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang YZ, Cao XY, Li XC, Chen J, Zhao YY, Tian Z, Zheng W. Accuracy of MRI diagnosis of early osteonecrosis of the femoral head: a meta-analysis and systematic review. J Orthop Surg Res. 2018. July 4;13(1):167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zibis AH, Karantanas AH, Roidis NT, Hantes ME, Argiri P, Moraitis T, Malizos KN. The role of MR imaging in staging femoral head osteonecrosis. Eur J Radiol. 2007. July;63(1):3-9. Epub 2007 Jun 6. [DOI] [PubMed] [Google Scholar]
- 35.Stevens K, Tao C, Lee SU, Salem N, Vandevenne J, Cheng C, Neumann G, Valentin-Opran A, Lang P. Subchondral fractures in osteonecrosis of the femoral head: comparison of radiography, CT, and MR imaging. AJR Am J Roentgenol. 2003. February;180(2):363-8. [DOI] [PubMed] [Google Scholar]
- 36.Yeh LR, Chen CK, Huang YL, Pan HB, Yang CF. Diagnostic performance of MR imaging in the assessment of subchondral fractures in avascular necrosis of the femoral head. Skeletal Radiol. 2009. June;38(6):559-64. Epub 2009 Feb 21. [DOI] [PubMed] [Google Scholar]
- 37.Stöve J, Riederle F, Kessler S, Puhl W, Günther KP. [Reproducibility of radiological classification criteria of femur head necrosis]. Z Orthop Ihre Grenzgeb. 2001. Mar-Apr;139(2):163-7. German. [DOI] [PubMed] [Google Scholar]
- 38.Schmitt-Sody M, Kirchhoff C, Mayer W, Goebel M, Jansson V. Avascular necrosis of the femoral head: inter- and intraobserver variations of Ficat and ARCO classifications. Int Orthop. 2008. June;32(3):283-7. Epub 2007 Mar 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith SW, Meyer RA, Connor PM, Smith SE, Hanley EN., Jr Interobserver reliability and intraobserver reproducibility of the modified Ficat classification system of osteonecrosis of the femoral head. J Bone Joint Surg Am. 1996. November;78(11):1702-6. [DOI] [PubMed] [Google Scholar]
- 40.Kay RM, Lieberman JR, Dorey FJ, Seeger LL. Inter- and intraobserver variation in staging patients with proven avascular necrosis of the hip. Clin Orthop Relat Res. 1994. October;307:124-9. [PubMed] [Google Scholar]
- 41.Gardeniers JWM, Gosling-Garadeniers AC, Rijnen WHC. The ARCO staging system: generation and evolution since 1991. In: Koo KH. Mont MA Jones LC, editors. Osteonecrosis. New York: Springer; 2014. p 215. [Google Scholar]
- 42.Ficat RP. Idiopathic bone necrosis of the femoral head. Early diagnosis and treatment. J Bone Joint Surg Br. 1985. January;67(1):3-9. [DOI] [PubMed] [Google Scholar]
- 43.Sugano N, Atsumi T, Ohzono K, Kubo T, Hotokebuchi T, Takaoka K. The 2001 revised criteria for diagnosis, classification, and staging of idiopathic osteonecrosis of the femoral head. J Orthop Sci. 2002;7(5):601-5. [DOI] [PubMed] [Google Scholar]
- 44.Steinberg ME, Hayken GD, Steinberg DR. A quantitative system for staging avascular necrosis. J Bone Joint Surg Br. 1995. January;77(1):34-41. [PubMed] [Google Scholar]
- 45.Yoon BH, Mont MA, Koo KH, Chen CH, Cheng EY, Cui Q, Drescher W, Gangji V, Goodman SB, Ha YC, Hernigou P, Hungerford MW, Iorio R, Jo WL, Jones LC, Khanduja V, Kim HKW, Kim SY, Kim TY, Lee HY, Lee MS, Lee YK, Lee YJ, Nakamura J, Parvizi J, Sakai T, Sugano N, Takao M, Yamamoto T, Zhao DW. The 2019 revised version of Association Research Circulation Osseous staging system of osteonecrosis of the femoral head. J Arthroplasty. 2020. April;35(4):933-40. Epub 2019 Nov 27. [DOI] [PubMed] [Google Scholar]
- 46.Chen CC, Lin CL, Chen WC, Shih HN, Ueng SW, Lee MS. Vascularized iliac bone-grafting for osteonecrosis with segmental collapse of the femoral head. J Bone Joint Surg Am. 2009. October;91(10):2390-4. [DOI] [PubMed] [Google Scholar]
- 47.Kubo Y, Motomura G, Ikemura S, Sonoda K, Yamamoto T, Nakashima Y. Factors influencing progressive collapse of the transposed necrotic lesion after transtrochanteric anterior rotational osteotomy for osteonecrosis of the femoral head. Orthop Traumatol Surg Res. 2017. April;103(2):217-22. Epub 2016 Dec 23. [DOI] [PubMed] [Google Scholar]
- 48.Sugioka Y, Yamamoto T. Transtrochanteric posterior rotational osteotomy for osteonecrosis. Clin Orthop Relat Res. 2008. May;466(5):1104-9. Epub 2008 Mar 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang YS, Yin L, Lu ZD, Wu XJ, Liu HJ. Analysis of long-term outcomes of double-strut bone graft for osteonecrosis of the femoral head. Orthop Surg. 2009. February;1(1):22-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zeng YR, He S, Feng WJ, Li FL, Li J, Jian LY, Zeng JC, Fan YG. Vascularised greater trochanter bone graft, combined free iliac flap and impaction bone grafting for osteonecrosis of the femoral head. Int Orthop. 2013. March;37(3):391-8. Epub 2013 Jan 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zuo W, Sun W, Zhao D, Gao F, Su Y, Li Z. Investigating clinical failure of bone grafting through a window at the femoral head neck junction surgery for the treatment of osteonecrosis of the femoral head. PLoS One. 2016. June 10;11(6):e0156903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hao Y, Guo H, Xu Z, Qi H, Wang Y, Lu C, Liu J, Yuan P. The relationship between apolipoprotein genes polymorphisms and susceptibility to osteonecrosis of the femoral head: a meta-analysis. Lipids Health Dis. 2018. August 17;17(1):192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ma J, Guo W, Li Z, Wang B, Li S, Wang P. Hip osteonecrosis is associated with increased plasma IL-33 level. Mediators Inflamm. 2017;2017:1732638 Epub 2017 Jan 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marsh A, Schiffelers R, Kuypers F, Larkin S, Gildengorin G, van Solinge W, Hoppe C. Microparticles as biomarkers of osteonecrosis of the hip in sickle cell disease. Br J Haematol. 2015. January;168(1):135-8. Epub 2014 Sep 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Peng Y, Liu Y, Huang D, Huang W, Shao Z. Association of TNF-α-308(G/A) and -238(G/A) polymorphisms with non-traumatic osteonecrosis of the femoral head risks: a meta-analysis. Int Orthop. 2018. July;42(7):1711-21. Epub 2018 Mar 7. [DOI] [PubMed] [Google Scholar]
- 56.Chen XJ, Yang F, Chen ZQ, He MC, Hong GJ, Huang JY, Zhou YC, Qin YX, Wei QS, He W. Association of reduced sclerostin expression with collapse process in patients with osteonecrosis of the femoral head. Int Orthop. 2018. July;42(7):1675-82. Epub 2018 May 21. [DOI] [PubMed] [Google Scholar]
- 57.Chen Y, Zeng C, Zeng H, Zhang R, Ye Z, Xing B, Hu K, Li M, Cai DZ. Comparative serum proteome expression of the steroid-induced femoral head osteonecrosis in adults. Exp Ther Med. 2015. January;9(1):77-83. Epub 2014 Nov 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fang SH, Li YF, Jiang JR, Chen P. Relationship of α2-macroglobulin with steroid-induced femoral head necrosis: a Chinese population-based association study in Southeast China. Orthop Surg. 2019. June;11(3):481-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fukushima T, Hozumi A, Tomita M, Yonekura A, Miyata N, Miyamoto T, Taguchi K, Goto H, Tsuda K, Osaki M. Steroid changes adipokine concentration in the blood and bone marrow fluid. Biomed Res. 2016;37(3):215-20. [DOI] [PubMed] [Google Scholar]
- 60.Ghale-Noie ZN, Hassani M, Kachooei AR, Kerachian MA. High serum alpha-2-macroglobulin level in patients with osteonecrosis of the femoral head. Arch Bone Jt Surg. 2018. May;6(3):219-24. [PMC free article] [PubMed] [Google Scholar]
- 61.He M, Gong SD, Chen XJ, Yang F, Pang FX, Chen ZQ, Huang JY, Zhou YC, Qin YX, He W, Wei QS. Plasma C-terminal cross-linking telopeptide of type II collagen as a biomarker in advanced stages of femoral head osteonecrosis. Biomed Pharmacother. 2019. March;111:1213-20. Epub 2019 Jan 15. [DOI] [PubMed] [Google Scholar]
- 62.Li C, Shen L, Yang Y, Shuai B, Xu X, Ma C. Plasma ghrelin and von Willebrand Factor levels in patients with non-traumatic osteonecrosis of the femoral head. Hip Int. 2015. Jan-Feb;25(1):76-81. Epub 2014 Oct 19. [DOI] [PubMed] [Google Scholar]
- 63.Li T, Zhang Y, Wang R, Xue Z, Li S, Cao Y, Liu D, Niu Y, Mao X, Wang X, Li W, Guo Q, Guo M, Lin N, Chen W. Discovery and validation an eight-biomarker serum gene signature for the diagnosis of steroid-induced osteonecrosis of the femoral head. Bone. 2019. May;122:199-208. Epub 2019 Mar 8. [DOI] [PubMed] [Google Scholar]
- 64.Mao Z, Liu G, Chen JJ, Liu D, Xu MP, Zhao C, Yang HT, Yue YB. Serum α-melanocyte-stimulating hormone may act as a protective biomarker for non-traumatic osteonecrosis of the femoral head. Ann Clin Biochem. 2018. July;55(4):453-60. Epub 2017 Nov 8. [DOI] [PubMed] [Google Scholar]
- 65.Narayanan A, Khanchandani P, Borkar RM, Ambati CR, Roy A, Han X, Bhoskar RN, Ragampeta S, Gannon F, Mysorekar V, Karanam B, SM V, Sivaramakrishnan V. Avascular necrosis of femoral head: a metabolomic, biophysical, biochemical, electron microscopic and histopathological characterization. Sci Rep. 2017. September 6;7(1):10721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhu HY, Gao YC, Wang Y, Zhang CQ. Circulating exosome levels in the diagnosis of steroid-induced osteonecrosis of the femoral head. Bone Joint Res. 2016. June;5(6):276-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mont MA, Zywiel MG, Marker DR, McGrath MS, Delanois RE. The natural history of untreated asymptomatic osteonecrosis of the femoral head: a systematic literature review. J Bone Joint Surg Am. 2010. September 15;92(12):2165-70. [DOI] [PubMed] [Google Scholar]
- 68.Aya-ay J, Athavale S, Morgan-Bagley S, Bian H, Bauss F, Kim HK. Retention, distribution, and effects of intraosseously administered ibandronate in the infarcted femoral head. J Bone Miner Res. 2007. January;22(1):93-100. [DOI] [PubMed] [Google Scholar]
- 69.Hofstaetter JG, Wang J, Yan J, Glimcher MJ. The effects of alendronate in the treatment of experimental osteonecrosis of the hip in adult rabbits. Osteoarthritis Cartilage. 2009. March;17(3):362-70. Epub 2008 Sep 10. [DOI] [PubMed] [Google Scholar]
- 70.Little DG, Peat RA, Mcevoy A, Williams PR, Smith EJ, Baldock PA. Zoledronic acid treatment results in retention of femoral head structure after traumatic osteonecrosis in young Wistar rats. J Bone Miner Res. 2003. November;18(11):2016-22. [DOI] [PubMed] [Google Scholar]
- 71.Kang P, Pei F, Shen B, Zhou Z, Yang J. Are the results of multiple drilling and alendronate for osteonecrosis of the femoral head better than those of multiple drilling? A pilot study. Joint Bone Spine. 2012. January;79(1):67-72. Epub 2011 Jul 13. [DOI] [PubMed] [Google Scholar]
- 72.Lai KA, Shen WJ, Yang CY, Shao CJ, Hsu JT, Lin RM. The use of alendronate to prevent early collapse of the femoral head in patients with nontraumatic osteonecrosis. A randomized clinical study. J Bone Joint Surg Am. 2005. October;87(10):2155-9. [DOI] [PubMed] [Google Scholar]
- 73.Agarwala S, Shah SB. Ten-year follow-up of avascular necrosis of femoral head treated with alendronate for 3 years. J Arthroplasty. 2011. October;26(7):1128-34. Epub 2011 Jan 21. [DOI] [PubMed] [Google Scholar]
- 74.Chen CH, Chang JK, Lai KA, Hou SM, Chang CH, Wang GJ. Alendronate in the prevention of collapse of the femoral head in nontraumatic osteonecrosis: a two-year multicenter, prospective, randomized, double-blind, placebo-controlled study. Arthritis Rheum. 2012. May;64(5):1572-8. [DOI] [PubMed] [Google Scholar]
- 75.Lee YK, Ha YC, Cho YJ, Suh KT, Kim SY, Won YY, Min BW, Yoon TR, Kim HJ, Koo KH. Does zoledronate prevent femoral head collapse from osteonecrosis? A prospective, randomized, open-label, multicenter study. J Bone Joint Surg Am. 2015. July 15;97(14):1142-8. [DOI] [PubMed] [Google Scholar]
- 76.Agarwala S, Banavali SD, Vijayvargiya M. Bisphosphonate combination therapy in the management of postchemotherapy avascular necrosis of the femoral head in adolescents and young adults: a retrospective study from India. J Glob Oncol. 2018. September;4:1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yuan HF, Guo CA, Yan ZQ. The use of bisphosphonate in the treatment of osteonecrosis of the femoral head: a meta-analysis of randomized control trials. Osteoporos Int. 2016. January;27(1):295-9. Epub 2015 Sep 14. [DOI] [PubMed] [Google Scholar]
- 78.Li D, Yang Z, Wei Z, Kang P. Efficacy of bisphosphonates in the treatment of femoral head osteonecrosis: a PRISMA-compliant meta-analysis of animal studies and clinical trials. Sci Rep. 2018. January 23;8(1):1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Glueck CJ, Freiberg RA, Wissman R, Wang P. Long term anticoagulation (4-16 years) stops progression of idiopathic hip osteonecrosis associated with familial thrombophilia. Adv Orthop. 2015;2015:138382 Epub 2015 Jan 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Guo P, Gao F, Wang Y, Zhang Z, Sun W, Jiang B, Wang B, Li Z. The use of anticoagulants for prevention and treatment of osteonecrosis of the femoral head: a systematic review. Medicine (Baltimore). 2017. April;96(16):e6646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Claßen T, Becker A, Landgraeber S, Haversath M, Li X, Zilkens C, Krauspe R, Jäger M. Long-term clinical results after Iloprost treatment for bone marrow edema and avascular necrosis. Orthop Rev (Pavia). 2016. March 31;8(1):6150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Albers A, Carli A, Routy B, Harvey EJ, Séguin C. Treatment with acetylsalicylic acid prevents short to mid-term radiographic progression of nontraumatic osteonecrosis of the femoral head: a pilot study. Can J Surg. 2015. June;58(3):198-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pritchett JW. Statin therapy decreases the risk of osteonecrosis in patients receiving steroids. Clin Orthop Relat Res. 2001. May;386:173-8. [DOI] [PubMed] [Google Scholar]
- 84.Jiang Y, Zhang Y, Zhang H, Zhu B, Li P, Lu C, Xu Y, Chen W, Lin N. Pravastatin prevents steroid-induced osteonecrosis in rats by suppressing PPARγ expression and activating Wnt signaling pathway. Exp Biol Med (Maywood). 2014. March;239(3):347-55. Epub 2014 Feb 7. [DOI] [PubMed] [Google Scholar]
- 85.Cui Q, Wang GJ, Su CC, Balian G. The Otto Aufranc Award. Lovastatin prevents steroid induced adipogenesis and osteonecrosis. Clin Orthop Relat Res. 1997. November;344:8-19. [PubMed] [Google Scholar]
- 86.Ajmal M, Matas AJ, Kuskowski M, Cheng EY. Does statin usage reduce the risk of corticosteroid-related osteonecrosis in renal transplant population? Orthop Clin North Am. 2009. April;40(2):235-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hao Y, Guo H, Xu Z, Qi H, Wang Y, Lu C, Liu J, Yuan P. Meta-analysis of the potential role of extracorporeal shockwave therapy in osteonecrosis of the femoral head. J Orthop Surg Res. 2018. July 3;13(1):166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ding H, Wang S, Feng H, Xu Y, Yan J, Duan X, Xing G. Clinical efficacy of individual extracorporeal shockwave treatment. Orthopade. 2019. July;48(7):610-7. [DOI] [PubMed] [Google Scholar]
- 89.Al-Jabri T, Tan JYQ, Tong GY, Shenoy R, Kayani B, Parratt T, Khan T. The role of electrical stimulation in the management of avascular necrosis of the femoral head in adults: a systematic review. BMC Musculoskelet Disord. 2017. July 28;18(1):319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Massari L, Fini M, Cadossi R, Setti S, Traina GC. Biophysical stimulation with pulsed electromagnetic fields in osteonecrosis of the femoral head. J Bone Joint Surg Am. 2006. November;88(Suppl 3):56-60. [DOI] [PubMed] [Google Scholar]
- 91.Koren L, Ginesin E, Melamed Y, Norman D, Levin D, Peled E. Hyperbaric oxygen for stage I and II femoral head osteonecrosis. Orthopedics. 2015. March;38(3):e200-5. [DOI] [PubMed] [Google Scholar]
- 92.Bosco G, Vezzani G, Mrakic Sposta S, Rizzato A, Enten G, Abou-Samra A, Malacrida S, Quartesan S, Vezzoli A, Camporesi E. Hyperbaric oxygen therapy ameliorates osteonecrosis in patients by modulating inflammation and oxidative stress. J Enzyme Inhib Med Chem. 2018. December;33(1):1501-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zalavras CG, Lieberman JR. Osteonecrosis of the femoral head: evaluation and treatment. J Am Acad Orthop Surg. 2014. July;22(7):455-64. [DOI] [PubMed] [Google Scholar]
- 94.Mont MA, Carbone JJ, Fairbank AC. Core decompression versus nonoperative management for osteonecrosis of the hip. Clin Orthop Relat Res. 1996. March;324:169-78. [DOI] [PubMed] [Google Scholar]
- 95.Stulberg BN, Davis AW, Bauer TW, Levine M, Easley K. Osteonecrosis of the femoral head. A prospective randomized treatment protocol. Clin Orthop Relat Res. 1991. July;268:140-51. [PubMed] [Google Scholar]
- 96.Hua KC, Yang XG, Feng JT, Wang F, Yang L, Zhang H, Hu YC. The efficacy and safety of core decompression for the treatment of femoral head necrosis: a systematic review and meta-analysis. J Orthop Surg Res. 2019. September 11;14(1):306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Roth A, Beckmann J, Bohndorf K, Fischer A, Heiß C, Kenn W, Jäger M, Maus U, Nöth U, Peters KM, Rader C, Reppenhagen S, Smolenski U, Tingart M, Kopp I, Sirotin I, Breusch SJ. S3-Guideline non-traumatic adult femoral head necrosis. Arch Orthop Trauma Surg. 2016. February;136(2):165-74. Epub 2015 Dec 14. [DOI] [PubMed] [Google Scholar]
- 98.Aaron RK, Lennox D, Bunce GE, Ebert T. The conservative treatment of osteonecrosis of the femoral head. A comparison of core decompression and pulsing electromagnetic fields. Clin Orthop Relat Res. 1989. December;249:209-18. [PubMed] [Google Scholar]
- 99.Hong YC, Zhong HM, Lin T, Shi JB. Comparison of core decompression and conservative treatment for avascular necrosis of femoral head at early stage: a meta-analysis. Int J Clin Exp Med. 2015. April 15;8(4):5207-16. [PMC free article] [PubMed] [Google Scholar]
- 100.Koo KH, Kim R, Ko GH, Song HR, Jeong ST, Cho SH. Preventing collapse in early osteonecrosis of the femoral head. A randomised clinical trial of core decompression. J Bone Joint Surg Br. 1995. November;77(6):870-4. [PubMed] [Google Scholar]
- 101.Neumayr LD, Aguilar C, Earles AN, Jergesen HE, Haberkern CM, Kammen BF, Nancarrow PA, Padua E, Milet M, Stulberg BN, Williams RA, Orringer EP, Graber N, Robertson SM, Vichinsky EP; National Osteonecrosis Trial in Sickle Cell Anemia Study Group. Physical therapy alone compared with core decompression and physical therapy for femoral head osteonecrosis in sickle cell disease. Results of a multicenter study at a mean of three years after treatment. J Bone Joint Surg Am. 2006. December;88(12):2573-82. [DOI] [PubMed] [Google Scholar]
- 102.Kerimaa P, Väänänen M, Ojala R, Hyvönen P, Lehenkari P, Tervonen O, Blanco Sequeiros R. MRI-guidance in percutaneous core decompression of osteonecrosis of the femoral head. Eur Radiol. 2016. April;26(4):1180-5. Epub 2015 Aug 1. [DOI] [PubMed] [Google Scholar]
- 103.Carpenter RD, Sigurdsson S, Zhao S, Lu Y, Eiriksdottir G, Sigurdsson G, Jonsson BY, Prevrhal S, Harris TB, Siggeirsdottir K, Guðnason V, Lang TF. Effects of age and sex on the strength and cortical thickness of the femoral neck. Bone. 2011. April 1;48(4):741-7. Epub 2010 Dec 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kosaraju V, Harwani A, Partovi S, Bhojwani N, Garg V, Ayyappan S, Kosmas C, Robbin M. Imaging of musculoskeletal manifestations in sickle cell disease patients. Br J Radiol. 2017. May;90(1073):20160130 Epub 2017 Mar 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Brown PJ, Mannava S, Seyler TM, Plate JF, Van Sikes C, Stitzel JD, Lang JE. Multiple small diameter drillings increase femoral neck stability compared with single large diameter femoral head core decompression technique for avascular necrosis of the femoral head. Surg Technol Int. 2016. October 26;29:247-54. [PubMed] [Google Scholar]
- 106.Mont MA, Ragland PS, Etienne G. Core decompression of the femoral head for osteonecrosis using percutaneous multiple small-diameter drilling. Clin Orthop Relat Res. 2004. December;429:131-8. [DOI] [PubMed] [Google Scholar]
- 107.Haberal B, Şahin O, Şimşek EK, Mahmuti A, Tuncay IC. Outcomes for core decompression with multiple drilling of the osteonecrosis of the femoral head in patients with solid organ transplantation. Eklem Hastalik Cerrahisi. 2018. December;29(3):159-64. [DOI] [PubMed] [Google Scholar]
- 108.Li J, Li ZL, Zhang H, Su XZ, Wang KT, Yang YM. Long-term outcome of multiple small-diameter drilling decompression combined with hip arthroscopy versus drilling alone for early avascular necrosis of the femoral head. Chin Med J (Engl). 2017. June 20;130(12):1435-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zeng Y, Qi X, Feng W, Li J, Li F, Zeng J, Yi C, Chen J. One-sided hip-preserving and concurrent contralateral total hip arthroplasty for the treatment of bilateral osteonecrosis of the femoral head in different stages: short-medium term outcomes. BMC Musculoskelet Disord. 2015. June 5;16:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lakshminarayana S, Dhammi IK, Jain AK, Bhayana H, Kumar S, Anshuman R. Outcomes of core decompression with or without nonvascularized fibular grafting in avascular necrosis of femoral head: short term followup study. Indian J Orthop. 2019. May-Jun;53(3):420-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sallam AA, Imam MA, Salama KS, Mohamed OA. Inverted femoral head graft versus standard core decompression in nontraumatic hip osteonecrosis at minimum 3 years follow-up. Hip Int. 2017. February 21;27(1):74-81. Epub 2017 Jan 24. [DOI] [PubMed] [Google Scholar]
- 112.Cilla M, Checa S, Preininger B, Winkler T, Perka C, Duda GN, Pumberger M. Femoral head necrosis: a finite element analysis of common and novel surgical techniques. Clin Biomech (Bristol, Avon). 2017. October;48:49-56. Epub 2017 Jul 6. [DOI] [PubMed] [Google Scholar]
- 113.Mohanty SP, Singh KA, Kundangar R, Shankar V. Management of non-traumatic avascular necrosis of the femoral head-a comparative analysis of the outcome of multiple small diameter drilling and core decompression with fibular grafting. Musculoskelet Surg. 2017. April;101(1):59-66. Epub 2016 Oct 18. [DOI] [PubMed] [Google Scholar]
- 114.Hauzeur JP, De Maertelaer V, Baudoux E, Malaise M, Beguin Y, Gangji V. Inefficacy of autologous bone marrow concentrate in stage three osteonecrosis: a randomized controlled double-blind trial. Int Orthop. 2018. July;42(7):1429-35. Epub 2017 Oct 7. [DOI] [PubMed] [Google Scholar]
- 115.Kang JS, Suh YJ, Moon KH, Park JS, Roh TH, Park MH, Ryu DJ. Clinical efficiency of bone marrow mesenchymal stem cell implantation for osteonecrosis of the femoral head: a matched pair control study with simple core decompression. Stem Cell Res Ther. 2018. October 25;9(1):274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Novais EN, Sankar WN, Wells L, Carry PM, Kim YJ. Preliminary results of multiple epiphyseal drilling and autologous bone marrow implantation for osteonecrosis of the femoral head secondary to sickle cell disease in children. J Pediatr Orthop. 2015. December;35(8):810-5. [DOI] [PubMed] [Google Scholar]
- 117.Hernigou P, Beaujean F. Treatment of osteonecrosis with autologous bone marrow grafting. Clin Orthop Relat Res. 2002. December;405:14-23. [DOI] [PubMed] [Google Scholar]
- 118.Piuzzi NS, Chahla J, Schrock JB, LaPrade RF, Pascual-Garrido C, Mont MA, Muschler GF. Evidence for the use of cell-based therapy for the treatment of osteonecrosis of the femoral head: a systematic review of the literature. J Arthroplasty. 2017. May;32(5):1698-708. Epub 2017 Jan 12. [DOI] [PubMed] [Google Scholar]
- 119.Henrich D, Nau C, Kraft SB, Zollfrank M, Kontradowitz K, Oppermann E, Schultheiss J, Meier S, Frank J, Marzi I, Seebach C. Effect of the harvest procedure and tissue site on the osteogenic function of and gene expression in human mesenchymal stem cells. Int J Mol Med. 2016. April;37(4):976-88. Epub 2016 Feb 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hernigou P, Dubory A, Homma Y, Guissou I, Flouzat Lachaniette CH, Chevallier N, Rouard H. Cell therapy versus simultaneous contralateral decompression in symptomatic corticosteroid osteonecrosis: a thirty year follow-up prospective randomized study of one hundred and twenty five adult patients. Int Orthop. 2018. July;42(7):1639-49. Epub 2018 May 9. [DOI] [PubMed] [Google Scholar]
- 121.Hernigou P, Rigoulot G, Auregan JC, Housset V, Bastard C, Dubory A, Lachaniette CHF. Unusual indication of cell therapy for hip osteonecrosis after pregnancy. SICOT J. 2018;4:46 Epub 2018 Nov 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Papakostidis C, Tosounidis TH, Jones E, Giannoudis PV. The role of “cell therapy” in osteonecrosis of the femoral head. A systematic review of the literature and meta-analysis of 7 studies. Acta Orthop. 2016. February;87(1):72-8. Epub 2015 Jul 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Houdek MT, Wyles CC, Collins MS, Howe BM, Terzic A, Behfar A, Sierra RJ. Stem cells combined with platelet-rich plasma effectively treat corticosteroid-induced osteonecrosis of the hip: a prospective study. Clin Orthop Relat Res. 2018. February;476(2):388-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wang Z, Sun QM, Zhang FQ, Zhang QL, Wang LG, Wang WJ. Core decompression combined with autologous bone marrow stem cells versus core decompression alone for patients with osteonecrosis of the femoral head: a meta-analysis. Int J Surg. 2019. September;69:23-31. Epub 2019 Jul 10. [DOI] [PubMed] [Google Scholar]
- 125.Talathi NS, Kamath AF. Autologous stem cell implantation with core decompression for avascular necrosis of the femoral head. J Clin Orthop Trauma. 2018. Oct-Dec;9(4):349-52. Epub 2018 Jun 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hernigou P, Daltro G, Hernigou J. Hip osteonecrosis: stem cells for life or behead and arthroplasty? Int Orthop. 2018. July;42(7):1425-8. Epub 2018 Jun 8. [DOI] [PubMed] [Google Scholar]
- 127.Nadeau M, Séguin C, Theodoropoulos JS, Harvey EJ. Short term clinical outcome of a porous tantalum implant for the treatment of advanced osteonecrosis of the femoral head. Mcgill J Med. 2007. January;10(1):4-10. [PMC free article] [PubMed] [Google Scholar]
- 128.Shuler MS, Rooks MD, Roberson JR. Porous tantalum implant in early osteonecrosis of the hip: preliminary report on operative, survival, and outcomes results. J Arthroplasty. 2007. January;22(1):26-31. [DOI] [PubMed] [Google Scholar]
- 129.Tanzer M, Bobyn JD, Krygier JJ, Karabasz D. Histopathologic retrieval analysis of clinically failed porous tantalum osteonecrosis implants. J Bone Joint Surg Am. 2008. June;90(6):1282-9. [DOI] [PubMed] [Google Scholar]
- 130.Tsao AK, Roberson JR, Christie MJ, Dore DD, Heck DA, Robertson DD, Poggie RA. Biomechanical and clinical evaluations of a porous tantalum implant for the treatment of early-stage osteonecrosis. J Bone Joint Surg Am. 2005;87(Suppl 2):22-7. [DOI] [PubMed] [Google Scholar]
- 131.Veillette CJ, Mehdian H, Schemitsch EH, McKee MD. Survivorship analysis and radiographic outcome following tantalum rod insertion for osteonecrosis of the femoral head. J Bone Joint Surg Am. 2006. November;88(Suppl 3):48-55. [DOI] [PubMed] [Google Scholar]
- 132.Ma J, Sun W, Gao F, Guo W, Wang Y, Li Z. Porous tantalum implant in treating osteonecrosis of the femoral head: still a viable option? Sci Rep. 2016. June 21;6:28227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cheng Q, Tang JL, Gu JJ, Guo KJ, Guo WS, Wang BL, Zhao FC. Total hip arthroplasty following failure of tantalum rod implantation for osteonecrosis of the femoral head with 5- to 10-year follow-up. BMC Musculoskelet Disord. 2018. August 16;19(1):289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lee GW, Park KS, Kim DY, Lee YM, Eshnazarov KE, Yoon TR. Results of total hip arthroplasty after core decompression with tantalum rod for osteonecrosis of the femoral head. Clin Orthop Surg. 2016. March;8(1):38-44. Epub 2016 Feb 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Olsen M, Lewis PM, Morrison Z, McKee MD, Waddell JP, Schemitsch EH. Total hip arthroplasty following failure of core decompression and tantalum rod implantation. Bone Joint J. 2016. September;98-B(9):1175-9. [DOI] [PubMed] [Google Scholar]
- 136.Chen X, Tan X, Gao S, Zhang X, Li J, Liu Y. Sartorius muscle-pedicle bone graft for osteonecrosis of the femoral head. Int Orthop. 2016. July;40(7):1417-25. Epub 2015 Jul 15. [DOI] [PubMed] [Google Scholar]
- 137.Feng W, Chen J, Wu K, Lu L, Deng P, Ye P, Cao H, Li J, Zeng J, Jie K, Qi X, Zeng Y. A comparative study of cortico-cancellous iliac bone graft with or without the combination of vascularized greater trochanter flap for the management of femoral head osteonecrosis: a minimum 6 years follow-up. BMC Musculoskelet Disord. 2019. June 22;20(1):298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Xie H, Wang B, Tian S, Liu B, Qin K, Zhao D. Retrospective long-term follow-up survival analysis of the management of osteonecrosis of the femoral head with pedicled vascularized iliac bone graft transfer. J Arthroplasty. 2019. August;34(8):1585-92. Epub 2019 Apr 2. [DOI] [PubMed] [Google Scholar]
- 139.Yang F, Wei Q, Chen X, Hong G, Chen Z, Chen Y, He W. Vascularized pedicle iliac bone grafts as a hip-preserving surgery for femur head necrosis: a systematic review. J Orthop Surg Res. 2019. August 27;14(1):270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhao D, Xie H, Xu Y, Wang Y, Yu A, Liu Y, Wang A, He W, Wang X, Li Z, Sun W, Tian S, Wang B, Liu B. Management of osteonecrosis of the femoral head with pedicled iliac bone flap transfer: a multicenter study of 2190 patients. Microsurgery. 2017. November;37(8):896-901. Epub 2017 Aug 14. [DOI] [PubMed] [Google Scholar]
- 141.Cao L, Guo C, Chen J, Chen Z, Yan Z. Free Vascularized fibular grafting improves vascularity compared with core decompression in femoral head osteonecrosis: a randomized clinical trial. Clin Orthop Relat Res. 2017. September;475(9):2230-40. Epub 2017 May 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kim SY, Kim YG, Kim PT, Ihn JC, Cho BC, Koo KH. Vascularized compared with nonvascularized fibular grafts for large osteonecrotic lesions of the femoral head. J Bone Joint Surg Am. 2005. September;87(9):2012-8. [DOI] [PubMed] [Google Scholar]
- 143.Plakseychuk A. CORR Insights®: free vascularized fibular grafting improves vascularity compared with core decompression in femoral head osteonecrosis: a randomized clinical trial. Clin Orthop Relat Res. 2017. September;475(9):2241-4. Epub 2017 Jun 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Plakseychuk AY, Kim SY, Park BC, Varitimidis SE, Rubash HE, Sotereanos DG. Vascularized compared with nonvascularized fibular grafting for the treatment of osteonecrosis of the femoral head. J Bone Joint Surg Am. 2003. April;85(4):589-96. [DOI] [PubMed] [Google Scholar]
- 145.Ünal MB, Cansu E, Parmaksızoğlu F, Cift H, Gürcan S. Treatment of osteonecrosis of the femoral head with free vascularized fibular grafting: results of 7.6-year follow-up. Acta Orthop Traumatol Turc. 2016. October;50(5):501-6. Epub 2016 Nov 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Meyers MH, Jones RE, Bucholz RW, Wenger DR. Fresh autogenous grafts and osteochondral allografts for the treatment of segmental collapse in osteonecrosis of the hip. Clin Orthop Relat Res. 1983. April;174:107-12. [PubMed] [Google Scholar]
- 147.Zhao P, Hao J. Analysis of the long-term efficacy of core decompression with synthetic calcium-sulfate bone grafting on non-traumatic osteonecrosis of the femoral head. Med Sci (Paris). 2018. October;34 Focus issue F1:43-6. Epub 2018 Nov 7. [DOI] [PubMed] [Google Scholar]
- 148.Papachristos IV, Rankine J, Giannoudis PV. Hip arthrodiastasis combined with core decompression and diamond concept for postcollapse femoral head avascular necrosis. BMJ Case Rep. 2019. August 13;12(8):e231081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Phemister DB. Treatment of the necrotic head of the femur in adults. J Bone Joint Surg Am. 1949. January;31A(1):55-66. [PubMed] [Google Scholar]
- 150.Keizer SB, Kock NB, Dijkstra PD, Taminiau AH, Nelissen RG. Treatment of avascular necrosis of the hip by a non-vascularised cortical graft. J Bone Joint Surg Br. 2006. April;88(4):460-6. [DOI] [PubMed] [Google Scholar]
- 151.Mont MA, Einhorn TA, Sponseller PD, Hungerford DS. The trapdoor procedure using autogenous cortical and cancellous bone grafts for osteonecrosis of the femoral head. J Bone Joint Surg Br. 1998. January;80(1):56-62. [DOI] [PubMed] [Google Scholar]
- 152.Yildiz C, Erdem Y, Koca K. Lightbulb technique for the treatment of osteonecrosis of the femoral head. Hip Int. 2018. May;28(3):272-7. Epub 2017 Nov 15. [DOI] [PubMed] [Google Scholar]
- 153.Ganz R, Büchler U. Overview of attempts to revitalize the dead head in aseptic necrosis of the femoral head—osteotomy and revascularization. Hip. 1983:296-305. [PubMed] [Google Scholar]
- 154.Seyler TM, Marker DR, Ulrich SD, Fatscher T, Mont MA. Nonvascularized bone grafting defers joint arthroplasty in hip osteonecrosis. Clin Orthop Relat Res. 2008. May;466(5):1125-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Rosenwasser MP, Garino JP, Kiernan HA, Michelsen CB. Long term followup of thorough debridement and cancellous bone grafting of the femoral head for avascular necrosis. Clin Orthop Relat Res. 1994. September;306:17-27. [PubMed] [Google Scholar]
- 156.Mont MA, Etienne G, Ragland PS. Outcome of nonvascularized bone grafting for osteonecrosis of the femoral head. Clin Orthop Relat Res. 2003. December;417:84-92. [DOI] [PubMed] [Google Scholar]
- 157.Cho KJ, Park KS, Yoon TR. Muscle pedicle bone grafting using the anterior one-third of the gluteus medius attached to the greater trochanter for treatment of Association Research Circulation Osseous stage II osteonecrosis of the femoral head. Int Orthop. 2018. October;42(10):2335-41. Epub 2018 Feb 24. [DOI] [PubMed] [Google Scholar]
- 158.Yu K, Xu Y, Tan H, He X, Cai D, Zhou T, Luo H, Duan J. [Clinical application of three dimensional printed navigation templates for treatment of osteonecrosis of femoral head with pedicled iliac bone graft]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2016. March;30(3):373-7. Chinese. [PubMed] [Google Scholar]
- 159.Zhang L, Fan Y, Zhang Y, Chen X, Liu Y. Comparison of sartorius muscle-pedicle and circumflex iliac deep bone flap grafts in the treatment of early non-traumatic osteonecrosis of femoral head in young adults. Acta Orthop Traumatol Turc. 2019. July;53(4):255-9. Epub 2019 Apr 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Lin D, Wang L, Yu Z, Luo D, Zhang X, Lian K. Lantern-shaped screw loaded with autologous bone for treating osteonecrosis of the femoral head. BMC Musculoskelet Disord. 2018. September 5;19(1):318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.González Della Valle A, Bates J, Di Carlo E, Salvati EA. Failure of free vascularized fibular graft for osteonecrosis of the femoral head: a histopathologic study of 6 cases. J Arthroplasty. 2005. April;20(3):331-6. [DOI] [PubMed] [Google Scholar]
- 162.Meloni MC, Hoedemaeker WR, Fornasier V. Failed vascularized fibular graft in treatment of osteonecrosis of the femoral head. A histopathological analysis. Joints. 2016. June 13;4(1):24-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Barla M, Polirsztok E, Peltié E, Jouve JL, Legré R, Dautel G, Barbary S, Journeau P. Free vascularised fibular flap harvesting in children: an analysis of donor-site morbidity. Orthop Traumatol Surg Res. 2017. November;103(7):1109-13. Epub 2017 May 31. [DOI] [PubMed] [Google Scholar]
- 164.Houdek MT, Bayne CO, Bishop AT, Shin AY. The outcome and complications of vascularised fibular grafts. Bone Joint J. 2017. January;99-B(1):134-8. [DOI] [PubMed] [Google Scholar]
- 165.Sugano N, Ohzono K, Masuhara K, Takaoka K, Ono K. Prognostication of osteonecrosis of the femoral head in patients with systemic lupus erythematosus by magnetic resonance imaging. Clin Orthop Relat Res. 1994. August;(305):190-9. [PubMed] [Google Scholar]
- 166.Mont MA, Fairbank AC, Krackow KA, Hungerford DS. Corrective osteotomy for osteonecrosis of the femoral head. J Bone Joint Surg Am. 1996. July;78(7):1032-8. [DOI] [PubMed] [Google Scholar]
- 167.Sugioka Y, Hotokebuchi T, Tsutsui H. Transtrochanteric anterior rotational osteotomy for idiopathic and steroid-induced necrosis of the femoral head. Indications and long-term results. Clin Orthop Relat Res. 1992. April;277:111-20. [PubMed] [Google Scholar]
- 168.Sugioka Y, Katsuki I, Hotokebuchi T. Transtrochanteric rotational osteotomy of the femoral head for the treatment of osteonecrosis. Follow-up statistics. Clin Orthop Relat Res. 1982. September;169:115-26. [PubMed] [Google Scholar]
- 169.Osawa Y, Seki T, Morita D, Takegami Y, Okura T, Ishiguro N. Total hip arthroplasty after transtrochanteric rotational osteotomy for osteonecrosis of the femoral head: a mean 10-year follow-up. J Arthroplasty. 2017. October;32(10):3088-92. Epub 2017 May 18. [DOI] [PubMed] [Google Scholar]
- 170.Shigemura T, Yamamoto Y, Murata Y, Sato T, Tsuchiya R, Mizuki N, Toki Y, Wada Y. Total hip arthroplasty after failed transtrochanteric rotational osteotomy for osteonecrosis of the femoral head: a systematic review and meta-analysis. Orthop Traumatol Surg Res. 2018. December;104(8):1163-70. Epub 2018 Oct 4. [DOI] [PubMed] [Google Scholar]
- 171.Suksathien Y, Sueajui J. Mid-term results of short stem total hip arthroplasty in patients with osteonecrosis of the femoral head. Hip Int. 2019. November;29(6):603-8. Epub 2018 Dec 11. [DOI] [PubMed] [Google Scholar]
- 172.Schnurr C, Loucif A, Patzer T, Schellen B, Beckmann J, Eysel P. Short stem survival after osteonecrosis of the femoral head. Arch Orthop Trauma Surg. 2018. April;138(4):573-9. Epub 2018 Feb 3. [DOI] [PubMed] [Google Scholar]
- 173.Kim YH, Park JW. Ultra-short anatomic uncemented femoral stem and ceramic-on-ceramic bearing in patients with idiopathic or ethanol-induced femoral head osteonecrosis. J Arthroplasty. 2020. January;35(1):212-8. Epub 2019 Aug 28. [DOI] [PubMed] [Google Scholar]
- 174.Gallart X, Fernández-Valencia JA, Ríos G, Bori G, Riba J, Muñoz-Mahamud E, Combalía A. Early clinical and radiological outcomes for the Taperloc Complete Microplasty stem. Eur J Orthop Surg Traumatol. 2019. April;29(3):619-24. Epub 2018 Nov 8. [DOI] [PubMed] [Google Scholar]
- 175.Capone A, Bienati F, Torchia S, Podda D, Marongiu G. Short stem total hip arthroplasty for osteonecrosis of the femoral head in patients 60 years or younger: a 3- to 10-year follow-up study. BMC Musculoskelet Disord. 2017. July 17;18(1):301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Miladi M, Villain B, Mebtouche N, Bégué T, Aurégan JC. Interest of short implants in hip arthroplasty for osteonecrosis of the femoral head: comparative study “uncemented short” vs “cemented conventional” femoral stems. Int Orthop. 2018. July;42(7):1669-74. Epub 2018 May 15. [DOI] [PubMed] [Google Scholar]
- 177.Floerkemeier T, Budde S, Gronewold J, Radtke K, Ettinger M, Windhagen H, von Lewinski G. Short-stem hip arthroplasty in osteonecrosis of the femoral head. Arch Orthop Trauma Surg. 2015. May;135(5):715-22. Epub 2015 Mar 24. [DOI] [PubMed] [Google Scholar]
- 178.Hart A, Janz V, Trousdale RT, Sierra RJ, Berry DJ, Abdel MP. Long-term survivorship of total hip arthroplasty with highly cross-linked polyethylene for osteonecrosis. J Bone Joint Surg Am. 2019. September 4;101(17):1563-8. [DOI] [PubMed] [Google Scholar]
- 179.Ilyas I, Alrumaih HA, Rabbani S. Noncemented total hip arthroplasty in sickle-cell disease: long-term results. J Arthroplasty. 2018. February;33(2):477-81. Epub 2017 Sep 14. [DOI] [PubMed] [Google Scholar]
- 180.Kinkel S, Wollmerstedt N, Kleinhans JA, Hendrich C, Heisel C. Patient activity after total hip arthroplasty declines with advancing age. Clin Orthop Relat Res. 2009. August;467(8):2053-8. Epub 2009 Feb 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sechriest VF, 2nd, Kyle RF, Marek DJ, Spates JD, Saleh KJ, Kuskowski M. Activity level in young patients with primary total hip arthroplasty: a 5-year minimum follow-up. J Arthroplasty. 2007. January;22(1):39-47. [DOI] [PubMed] [Google Scholar]
- 182.Silva M, Shepherd EF, Jackson WO, Dorey FJ, Schmalzried TP. Average patient walking activity approaches 2 million cycles per year: pedometers under-record walking activity. J Arthroplasty. 2002. September;17(6):693-7. [DOI] [PubMed] [Google Scholar]
- 183.Min BW, Cho CH, Son ES, Lee KJ, Lee SW, Song KS. Highly cross-linked polyethylene in total hip arthroplasty in patients younger than 50 years with osteonecrosis of the femoral head: a minimum of 10 years of follow-up. J Arthroplasty. 2020. March;35(3):805-10. Epub 2019 Oct 15. [DOI] [PubMed] [Google Scholar]
- 184.Min BW, Song KS, Bae KC, Cho CH, Lee KJ, Kim HJ. Second-generation cementless total hip arthroplasty in patients with osteonecrosis of the femoral head. J Arthroplasty. 2008. September;23(6):902-10. Epub 2008 Mar 4. [DOI] [PubMed] [Google Scholar]
- 185.Shon WY, Gupta S, Biswal S, Han SH, Hong SJ, Moon JG. Pelvic osteolysis relationship to radiographs and polyethylene wear. J Arthroplasty. 2009. August;24(5):743-50. Epub 2008 Jun 13. [DOI] [PubMed] [Google Scholar]
- 186.Keeney JA, Nunley RM, Baca GR, Clohisy JC. Are younger patients undergoing THA appropriately characterized as active? Clin Orthop Relat Res. 2015. March;473(3):1083-92. Epub 2014 Sep 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Svanholm H, Starklint H, Gundersen HJ, Fabricius J, Barlebo H, Olsen S. Reproducibility of histomorphologic diagnoses with special reference to the kappa statistic. APMIS. 1989. August;97(8):689-98. [DOI] [PubMed] [Google Scholar]
- 188.Wright JG. Revised grades of recommendation for summaries or reviews of orthopaedic surgical studies. J Bone Joint Surg Am. 2006. May;88(5):1161-2. [DOI] [PubMed] [Google Scholar]
- 189.Gianakos AL, Moya-Angeler J, Duggal S, Zambrana L, Fields KG, Mintz DN, Cornell CN, Lane JM. The efficacy of bisphosphonates with core decompression and mesenchymal stem cells compared with bisphosphonates alone in the treatment of osteonecrosis of the hip: a retrospective study. HSS J. 2016. July;12(2):137-44. Epub 2016 Mar 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Xie K, Mao Y, Qu X, Dai K, Jia Q, Zhu Z, Yan M. High-energy extracorporeal shock wave therapy for nontraumatic osteonecrosis of the femoral head. J Orthop Surg Res. 2018. February 2;13(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Algarni AD, Al Moallem HM. Clinical and radiological outcomes of extracorporeal shock wave therapy in early-stage femoral head osteonecrosis. Adv Orthop. 2018. August 19;2018:7410246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Nazal MR, Parsa A, Martin SD. Mid-term outcomes of arthroscopic-assisted core decompression of precollapse osteonecrosis of femoral head-minimum of 5 year follow-up. BMC Musculoskelet Disord. 2019. October 15;20(1):448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Yin H, Yuan Z, Wang D. Multiple drilling combined with simvastatin versus multiple drilling alone for the treatment of avascular osteonecrosis of the femoral head: 3-year follow-up study. BMC Musculoskelet Disord. 2016. August 15;17(1):344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Liu Y, Su X, Zhou S, Wang L, Wang C, Liu S. A modified porous tantalum implant technique for osteonecrosis of the femoral head: survivorship analysis and prognostic factors for radiographic progression and conversion to total hip arthroplasty. Int J Clin Exp Med. 2015. February 15;8(2):1918-30. [PMC free article] [PubMed] [Google Scholar]
- 195.Hu R, Lei P, Li B, Liu H, Yang X, Wen T, Hu Y, Tian X. Real-time computerised tomography assisted porous tantalum implant in ARCO stage I-II non-traumatic osteonecrosis of the femoral head: minimum five-year follow up. Int Orthop. 2018. July;42(7):1535-44. Epub 2018 Mar 27. [DOI] [PubMed] [Google Scholar]
- 196.Lu Y, Lu X, Li M, Chen X, Liu Y, Feng X, Yu J, Zhang C, Niu D, Wang S, Wang Z, Lu J. Minimally invasive treatment for osteonecrosis of the femoral head with angioconductive bioceramic rod. Int Orthop. 2018. July;42(7):1567-73. Epub 2018 Apr 10. [DOI] [PubMed] [Google Scholar]
- 197.Cruz-Pardos A, Garcia-Rey E, Ortega-Chamarro JA, Duran-Manrique D, Gomez-Barrena E. Mid-term comparative outcomes of autologous bone-marrow concentration to treat osteonecrosis of the femoral head in standard practice. Hip Int. 2016. September 29;26(5):432-7. Epub 2016 Apr 21. [DOI] [PubMed] [Google Scholar]
- 198.Pepke W, Kasten P, Beckmann NA, Janicki P, Egermann M. Core decompression and autologous bone marrow concentrate for treatment of femoral head osteonecrosis: a randomized prospective study. Orthop Rev (Pavia). 2016. March 21;8(1):6162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Tabatabaee RM, Saberi S, Parvizi J, Mortazavi SM, Farzan M. Combining concentrated autologous bone marrow stem cells injection with core decompression improves outcome for patients with early-stage osteonecrosis of the femoral head: a comparative study. J Arthroplasty. 2015. September;30(9)(Suppl):11-5. Epub 2015 Jun 19. [DOI] [PubMed] [Google Scholar]
- 200.Tomaru Y, Yoshioka T, Sugaya H, Aoto K, Wada H, Akaogi H, Yamazaki M, Mishima H. Hip preserving surgery with concentrated autologous bone marrow aspirate transplantation for the treatment of asymptomatic osteonecrosis of the femoral head: retrospective review of clinical and radiological outcomes at 6 years postoperatively. BMC Musculoskelet Disord. 2017. July 6;18(1):292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Chen C, Qu Z, Yin X, Shang C, Ao Q, Gu Y, Liu Y. Efficacy of umbilical cord-derived mesenchymal stem cell-based therapy for osteonecrosis of the femoral head: a three-year follow-up study. Mol Med Rep. 2016. November;14(5):4209-15. Epub 2016 Sep 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Kuroda Y, Asada R, So K, Yonezawa A, Nankaku M, Mukai K, Ito-Ihara T, Tada H, Yamamoto M, Murayama T, Morita S, Tabata Y, Yokode M, Shimizu A, Matsuda S, Akiyama H. A pilot study of regenerative therapy using controlled release of recombinant human fibroblast growth factor for patients with pre-collapse osteonecrosis of the femoral head. Int Orthop. 2016. August;40(8):1747-54. Epub 2015 Dec 29. [DOI] [PubMed] [Google Scholar]
- 203.Persiani P, De Cristo C, Graci J, Noia G, Gurzì M, Villani C. Stage-related results in treatment of hip osteonecrosis with core-decompression and autologous mesenchymal stem cells. Acta Orthop Belg. 2015. September;81(3):406-12. [PubMed] [Google Scholar]
- 204.Jo WL, Lee YK, Ha YC, Kim TY, Koo KH. Delay of total hip arthroplasty to advanced stage worsens post-operative hip motion in patients with femoral head osteonecrosis. Int Orthop. 2018. July;42(7):1599-603. Epub 2018 Apr 26. [DOI] [PubMed] [Google Scholar]
- 205.Assi C, Kheir N, Samaha C, Kouyoumjian P, Yammine K. Early results of total hip arthroplasty using dual-mobility cup in patients with osteonecrosis of the femoral head. SICOT J. 2018;4:4 Epub 2018 Feb 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Martz P, Maczynski A, Elsair S, Labattut L, Viard B, Baulot E. Total hip arthroplasty with dual mobility cup in osteonecrosis of the femoral head in young patients: over ten years of follow-up. Int Orthop. 2017. March;41(3):605-10. Epub 2016 Nov 25. [DOI] [PubMed] [Google Scholar]
- 207.Swarup I, Shields M, Mayer EN, Hendow CJ, Burket JC, Figgie MP. Outcomes after total hip arthroplasty in young patients with osteonecrosis of the hip. Hip Int. 2017. May 12;27(3):286-92. Epub 2017 Jan 31. [DOI] [PubMed] [Google Scholar]
- 208.Lim SJ, Kim SM, Kim DW, Moon YW, Park YS. Cementless total hip arthroplasty using Biolox®delta ceramic-on-ceramic bearing in patients with osteonecrosis of the femoral head. Hip Int. 2016. Mar-Apr;26(2):144-8. Epub 2016 Feb 5. [DOI] [PubMed] [Google Scholar]


