Abstract
Objective
To describe the experience of obtaining a diagnosis of frontotemporal degeneration (FTD) for patients and caregivers.
Methods
Data came from a 2017 web-based survey of 698 FTD caregivers. Clinical characteristics and diagnostic experiences were described according to the phenotype of the patient with FTD (behavioral variant FTD, primary progressive aphasia, FTD with motor neuron disease, or progressive supranuclear palsy/corticobasal syndrome). Unadjusted and adjusted logistic regression analyses determined associations between patient with FTD and caregiver characteristics and (1) receiving a diagnosis >1 year after initial symptoms and (2) first receiving a non-FTD diagnosis.
Results
Mean age was 66 ± 9 years for patients with FTD and 61 ± 10 years for FTD caregivers. Forty-four percent of patients took more than 1 year; 65% saw 3 or more doctors; and 84% required 3 or more visits to establish an FTD diagnosis. Initial diagnosis was depression or other psychiatric condition in 21% of patients. Twenty-eight percent of caregivers and 26% of patients lost ≥11 work days seeking diagnosis. The majority of diagnoses (66%) were made by neurologists. Patient and caregiver age, having a spouse caregiver, rural residency, and mood changes as first symptom were associated with a longer time to receive FTD diagnosis. Caregivers frequently rated diagnosing doctors as good/excellent in knowledge of FTD but as inadequate/poor on knowledge of available community resources.
Conclusions
This study, which quantifies the patient with FTD and caregiver burden before receiving the FTD diagnosis, can inform clinical practice, interventions to address diagnostic delays, and programs and services to support patients/caregivers during and following the diagnosis.
Frontotemporal degeneration (FTD), a common cause of dementia in <60 year olds, affects more than 60,000 individuals in the United States.1–4 FTD is a diverse group of disorders with prominent features of language, personality, behavior, cognition, and/or motor dysfunction and can be divided into 4 predominant clinical phenotypes: behavioral variant FTD (bvFTD), primary progressive aphasia (PPA), FTD with motor neuron disease (FTD-MND), and Parkinson-plus movement disorders due to progressive supranuclear palsy (PSP) or corticobasal syndrome (CBS).5–9 All FTD phenotypes cause progressive loss of function and independence, leading to extraordinary social and financial burden compared with other neurodegenerative dementias such as Alzheimer disease (AD).10–13
Because FTD is less prevalent (15–22 per 100,00010) than more recognized neurodegenerative disorders such as AD, little is known about FTD diagnosis and management in communities outside of an academic medical center. Furthermore, little is known about caregiver-patient experiences in obtaining a diagnosis, FTD provider knowledge, or referrals services provided.
In conjunction with the Association for Frontotemporal Degeneration (AFTD) (theAFTD.org), caregivers of FTD patients were surveyed on the challenges, burdens, and barriers of obtaining a diagnosis and patient care. The survey's primary goal was to understand FTD caregiver experiences and collect important data to improve FTD detection, diagnosis, and management.
Few published studies focus on the diagnostic journey for dementia patients, particularly those with FTD. This study addresses this gap in the literature by characterizing the diagnosing doctor, patient's diagnostic experience, predictors of time to receive the FTD diagnosis, and predictors of initially receiving a non-FTD diagnosis.
Methods
After receiving a grant from the AFTD, the research team created a 250-question survey aimed at FTD caregivers (survey: tinyurl.com/y6cpy9aq). It was conducted in 3 waves using emails and social media, as well as AFTD's newsletter and web site. Designed in Qualtrics, the questionnaire was based on preexisting instruments and investigator-generated questions when no established questions were available. Questions assessed demographics, clinical symptoms, diagnosis, health economics, disease severity, caregiver burden, and activities of daily living. Additional details about the survey have been published previously.10,14 The survey was restricted to 698 of the 956 caregiver respondents who had nonmissing data on time to receive FTD diagnosis. Respondents missing time to receive diagnosis (n = 258) data were frequently missing data on other key variables (e.g., 189 were missing patient age, 190 were missing patient sex, and 174 were missing caregiver relationship to the patient).
Demographics were collected on the caregivers and patients, including age, sex, education, race, and ethnicity. The survey also collected information on the caregiver's relationship to the patient (spouse, child, or other) and living situation (living with the patient or living in an urban/suburban/rural area). Caregivers provided the FTD diagnosis (bvFTD, PPA, FTD-MND, PSP, CBS, or other), information on the first symptom (e.g., cognitive and motor), and details surrounding the diagnosis, such as work days lost while seeking the FTD diagnosis, type of diagnosing doctor (e.g., primary care), initial diagnosis received, years to receive diagnosis, number of doctors visited and doctor visits before receiving the FTD diagnosis, diagnostic tests ordered (e.g., MRI), and caregiver opinions on the diagnosing doctor (e.g., knowledge of FTD).
Means (and SDs) and frequencies (and percentages) were used to describe the sample's demographic, clinical, and diagnosis characteristics. Clinical characteristics and experiences were described separately by FTD phenotype (e.g., bvFTD). Unadjusted and adjusted logistic regression analyses determined the association between FTD patient and caregiver characteristics and receiving a diagnosis >1 year after initially presenting to a doctor (vs ≤1 year). Logistic regression models included patient and caregiver age, patient and caregiver sex, patient and caregiver education, caregiver relationship to patient (spouse or not spouse), caregiver's rural residence, caregiver not living with the patient, and initial symptoms (e.g., cognitive and behavioral). Unadjusted and adjusted logistic regression models also examined associations between patient and caregiver characteristics (including FTD subtype: bvFTD, PPA, and FTD-MND/PSP/CBS) and initially receiving a non-FTD diagnosis (vs initially receiving FTD diagnosis). An alpha = 0.05 was used to determine statistical significance.
Standard protocol approvals, registrations, and patient consents
The Florida Atlantic University's Institutional Review Board determined that the study was exempt.
Data availability
Anonymized data can be shared with qualified investigators on request.
Results
Sample characteristics
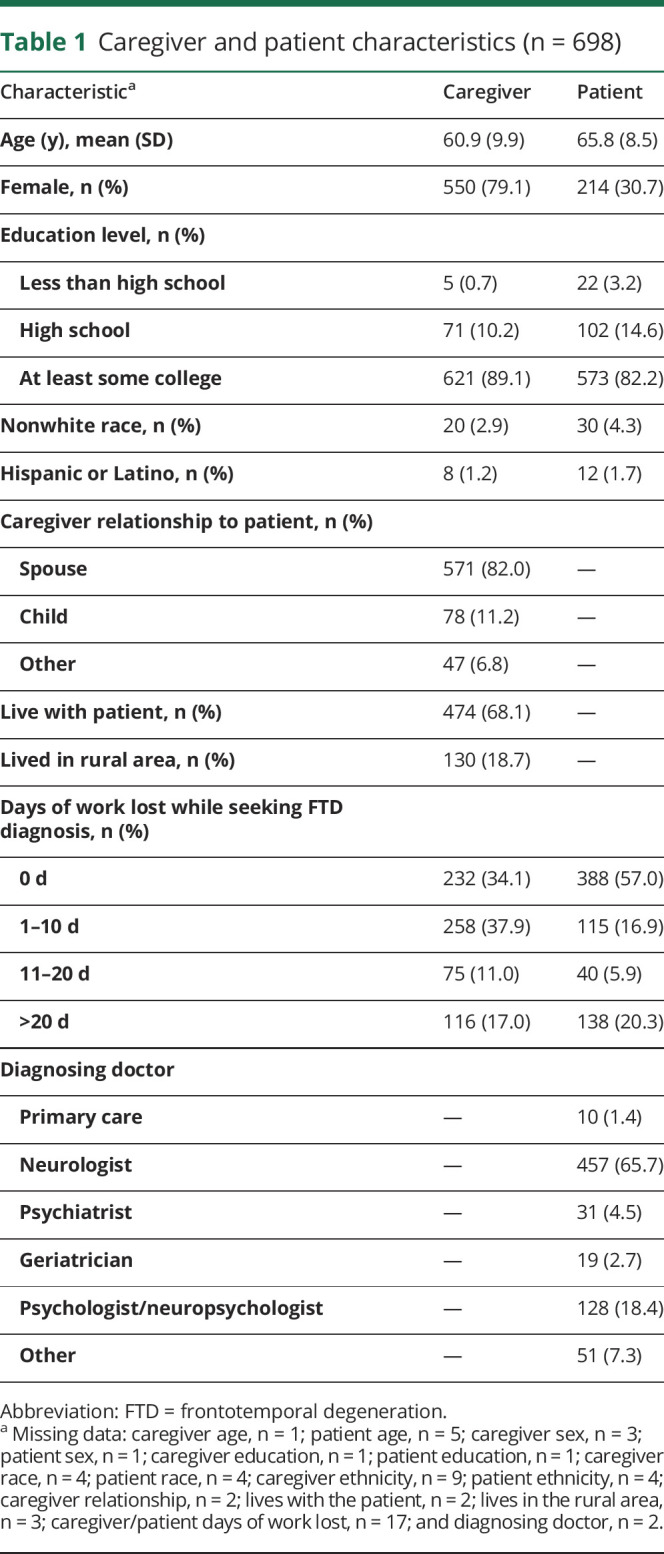
The patients with FTD were a mean age of 66 years (SD = 9), and the caregivers (spouse, child, or other) were a mean age of 61 years (SD = 10) (spouse caregiver age: mean = 63, SD = 8) (table 1). Most patients (82%) and caregivers (89%) had at least some college education. Eighty-two percent of caregivers were the patients' spouses, 68% lived with the patient, and 19% lived in a rural area. Twenty-eight percent of caregivers and 26% of patients lost ≥11 work days while seeking the diagnosis. Sixty-six percent of FTD diagnoses were made by neurologists and 18% by psychologists, with the remainder made by primary care physicians (1%), psychiatrists (5%), geriatricians (3%), or other clinicians (7%).
Table 1.
Caregiver and patient characteristics (n = 698)
Experience obtaining the FTD diagnosis
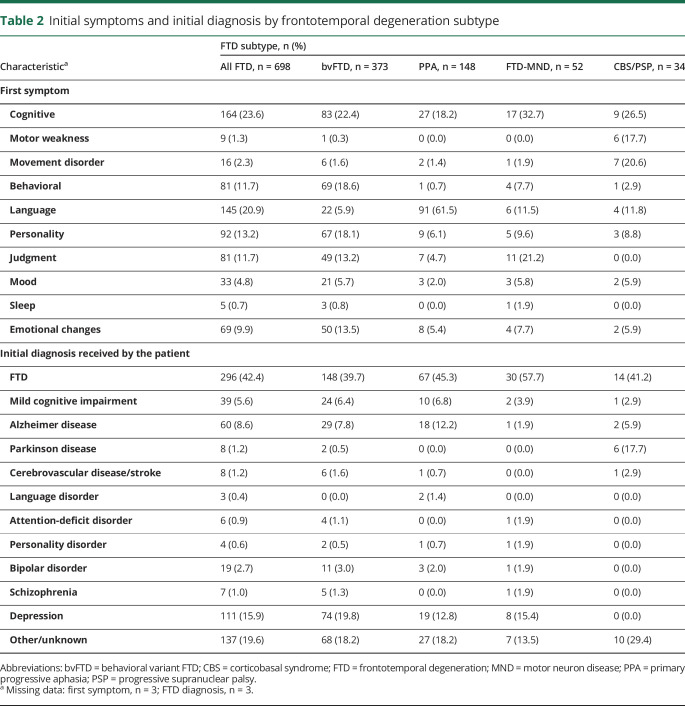
The majority of patients had diagnoses of bvFTD (53%) or PPA (21%), with smaller percentages diagnosed with FTD-MND (7%) or PSP/CBS (5%) (remaining had unspecified/unknown subtypes) (table 2). Cognitive changes were most often reported as the first symptom among patients with bvFTD (22%), FTD-MND (33%), and PSP/CBS (27%), and language was reported as the first symptom among 62% of patients with PPA. Among all patients, FTD was the initial diagnosis 42% of the time. Depression was the initial diagnosis 16% of the time, AD 9% of the time, mild cognitive impairment 6% of the time, and a psychiatric disorder (attention-deficit disorder, personality disorder, bipolar disorder, and schizophrenia) 5% of the time. Depression was initially diagnosed in 20% of bvFTD cases, 13% of PPA cases, and 15% of FTD-MND cases (0% in PSP/CBS). AD was initially diagnosed in 8% of bvFTD cases, 12% of PPA cases, 2% of FTD-MND cases, and 6% of PSP/CBS cases. Parkinson disease was initially diagnosed in a much higher percentage of PSP/CBS cases (18%) than bvFTD, PPA, and FTD-MND cases (<1%).
Table 2.
Initial symptoms and initial diagnosis by frontotemporal degeneration subtype
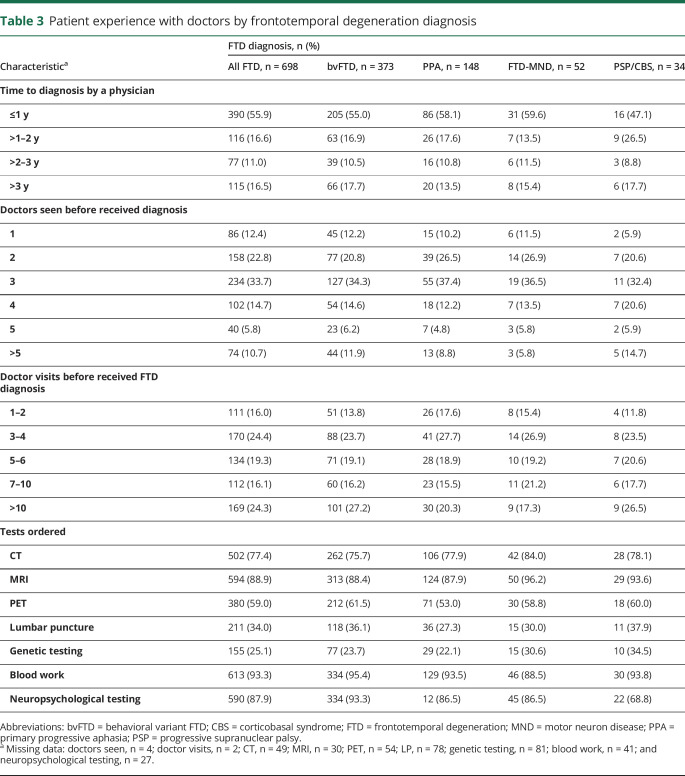
Altogether, 56% received their FTD diagnosis <1 year after initial symptoms, whereas 17% received the diagnosis 1–2 years after initial symptoms, 11% received their diagnosis 2–3 years after initial symptoms, and 17% received their diagnosis over 3 years after the initial symptoms (table 3). Time to receive the FTD diagnosis after initial symptoms did not differ substantially depending on FTD subtype. However, patients with caregivers living in rural areas less frequently (47%) received their FTD diagnosis within a year compared with those living in nonrural areas (58%) (data not shown). Only 12% of patients were diagnosed with FTD by the first doctor they visited. Fifty-six percent of patients went to 2–3 doctors, 30% went to 4–5 doctors, and 11% went to >5 doctors before receiving their FTD diagnosis. Sixteen percent of patients received their FTD diagnosis within their first 2 doctor visits. In contrast, the FTD diagnosis was not received until the third or fourth visit among 24%, the fifth or sixth visit among 19%, and 40% had ≥7 doctor visits before receiving their FTD diagnosis. The number of doctors seen and the number of doctor visits before receiving the FTD diagnosis were similar regardless of FTD subtype.
Table 3.
Patient experience with doctors by frontotemporal degeneration diagnosis
Fifty-six percent of patients went to 2–3 doctors, 30% went to 4–5 doctors, and 11% went to >5 doctors before receiving their FTD diagnosis.
MRI (89%), CT (77%), blood work (93%), and neuropsychological testing (88%) were frequently ordered for the diagnostic evaluation (table 3). Fluorodeoxyglucose-PET (FDG-PET) scans are approved for the differentiation between FTD and AD, with 59% of patients having an FDG-PET as part of their diagnostic workup. Lumbar punctures (34%) and genetic testing (25%) were less frequently ordered. Neuropsychological testing was used less frequently among patients with PSP/CBS (69%) than patients with bvFTD (93%), PPA (87%), or FTD-MND (87%), with no other substantial differences in tests by subtype.
Caregiver perception of physician knowledge of FTD
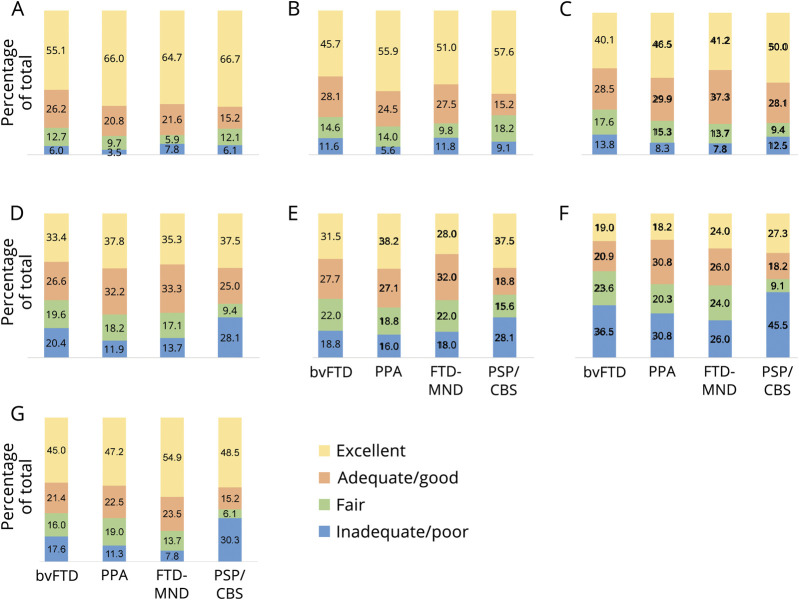
Caregivers reported that diagnosing doctors were inadequate/poor at having knowledge of FTD and its subtypes 4%–8% of the time (depending on subtype), explaining the diagnosis 6%–12% of the time, explaining the symptoms 8%–14% of the time, explaining disease progression 12%–28% of the time, explaining treatment options 16%–28% of the time, having knowledge of community resources 26%–46% of the time, and being sensitive to what the family was going through 8%–30% of the time (figure). In contrast, caregivers reported diagnosing doctors were excellent at having knowledge of FTD and its subtypes 55%–67% of the time (depending on subtype), explaining the diagnosis 46%–58% of the time, explaining the symptoms 40%–50% of the time, explaining disease progression 33%–38% of the time, explaining treatment options 28%–38% of the time, having knowledge of community resources 18%–27% of the time, and being sensitive to what the family was going through 45%–55% of the time.
Figure. Caregiver evaluation of the diagnosing doctor by frontotemporal degeneration subtype.
Percentage of caregivers rating the diagnosing doctor as inadequate/poor, fair, adequate/good, or excellent, by: (A) knowledge of FTD and subtypes; (B) ability to explain the diagnosis; (C) ability to explain the symptoms; (D) ability to explain disease progression; (E) ability to explain treatment options; (F) knowledge of community resources; and (G) sensitivity to what the family was going through. bvFTD = behavioral variant; CBS = corticobasal syndrome; FTD-MND = frontotemporal degeneration with motor neuron disease; PPA = primary progressive aphasia; PSP = progressive supranuclear palsy.
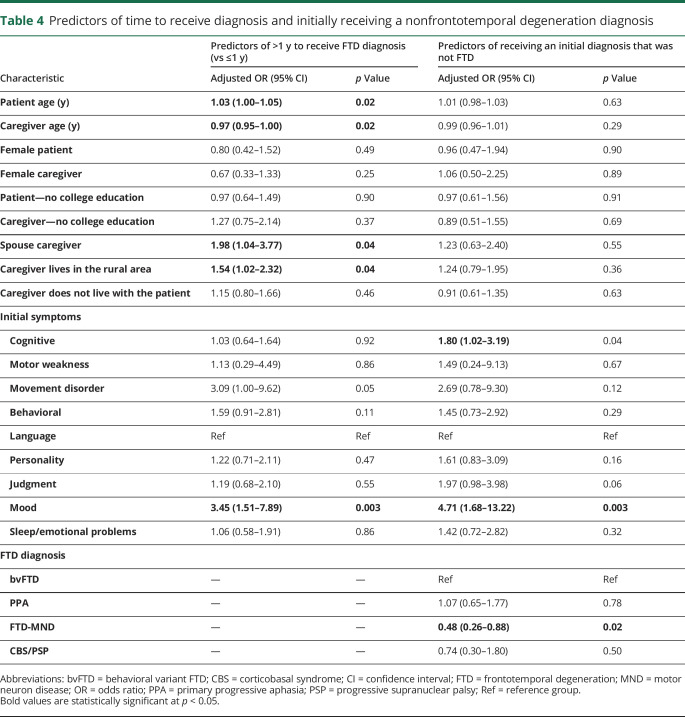
In the unadjusted analysis, predictors of receiving the FTD diagnosis >1 year (vs ≤1 year) following initial symptom presentation included caregiver's rural residence (odds ratio [OR]: 1.55; 95% confidence interval [CI]: 1.06–2.28) and having a movement disorder (OR: 3.40; 95% CI: 1.12–10.23) or mood changes as the first FTD symptom (OR: 3.55; 95% CI: 1.57–8.01) (data not shown). In the adjusted analysis, increasing patient age was associated with increased odds of >1 year to receive the FTD diagnosis, and increasing caregiver age was associated with decreased odds of >1 year to receive the FTD diagnosis (table 4). Having a spouse caregiver, caregiver's rural residence, and having mood changes as the first FTD symptom were associated with increased odds of >1 year to receive the FTD diagnosis. Having a movement disorder as the initial presenting symptom was a borderline significant predictor (p = 0.05).
Table 4.
Predictors of time to receive diagnosis and initially receiving a nonfrontotemporal degeneration diagnosis
In the unadjusted analysis, changes in cognition (OR: 1.66; 95% CI: 1.04–2.63) and mood (OR: 4.29; 95% CI: 1.74–10.55) as the initial symptom were associated with increased odds of initially receiving diagnoses other than FTD, whereas those diagnosed with FTD-MND were less likely to initially receive a non-FTD diagnosis (OR: 0.49; 95% CI: 0.27–0.88). These same variables were the only predictors in the adjusted model (table 4).
From our survey of almost 700 FTD caregivers, we found that patients and caregivers experience great frustration and burden in the time and resources required to receive the FTD diagnosis.
Discussion
From our survey of almost 700 FTD caregivers, we found that patients and caregivers experience great frustration and burden in the time and resources required to receive the FTD diagnosis. In searching for the diagnosis, approximately 25% of caregivers and patients lost ≥11 work days, almost half of patients were diagnosed >1 year after first symptoms, 65% of patients visited ≥3 doctors before obtaining the diagnosis (10% visited >5 doctors), and almost 25% of patients had >10 doctor visits before receiving the diagnosis. Delays in diagnosis occurred despite changes in language, personality, judgment, behavior, and emotion being reported as the first symptoms 67% of the time and motor/movement changes as the first symptoms 4% of the time. Therefore, although most patients displayed common FTD symptoms at initial presentation, they experienced burdensome delays in being diagnosed. Individuals with this form of early-onset dementia and their caregivers/spouses may be less likely to seek early medical attention due to the behavioral and personality manifestations of the disease and young onset.15 Studies have demonstrated a considerably longer time to receive the dementia diagnosis among early-onset vs late-onset cases, particularly for FTD,16,17 lost time that should be spent providing targeted care and support to patients and caregivers. In addition, the delay in receiving the correct diagnosis results in financial and psychological strain for patients and caregivers.15
Patients experiencing >10 doctor visits before diagnosis incur ≥$2,500 in medical expenditures for the visits alone (estimated from fairhealthconsumer.org figures), along with additional expenses for associated tests or procedures. In addition to high medical costs, it has been estimated that families experience a $25,000–$40,000 decrease in median household income in the year before FTD diagnosis10 attributable to curtailing work. Reducing delays in diagnosis in tandem with improved support services could decrease the burden to families in the period surrounding diagnosis.
Caregiver ratings of the diagnosing doctor were mixed. A majority of doctors were reported to have adequate/excellent FTD knowledge and were adequate/excellent in their abilities to explain the diagnosis and symptoms and their sensitivity to what the family was going through. However, only approximately half of doctors were rated as adequate/excellent in explaining disease progression and treatment options. In addition, fewer than half were rated as knowledgeable about available community resources.
These findings align with another study finding that family caregivers of patients with FTD were more dissatisfied with information provided about the disease, counseling, and follow-up compared with that reported by caregivers of patients with AD.18 A separate study of patients with dementia and caregivers reported that communication and provision of information during the diagnostic process was variable.19 Some of these deficiencies noted by caregivers in our study could be attributed to 34% of diagnoses made by non-neurologists, who may have limited knowledge in some areas in which doctors were rated. The negative ratings could also relate to self-perceptions or negative connotations with the information provided by the doctor (e.g., dissatisfaction with limited treatment options) or lack of community resources with which to refer patients. In addition, doctors diagnosing FTD have noted limitations in the support services available to patients before and after the diagnosis, noting that provision of adequate information during the diagnostic visit is limited by factors such as no pre-established relationship with the patient and the need for sensitivity when both patient and caregiver are present.20 Nonetheless, assessing caregiver perceptions is very important for informing practice in the diagnosis and care of patients with FTD and for reducing caregiver burden. In particular, the availability and accessibility of community resources is crucial for caregivers of patients with FTD, as the majority are spouses who have been shown to experience identity crises and social isolation due to behavioral manifestations and stigma of the disease.21,22
Patient and caregiver age, having a spouse caregiver, caregiver's rural residence, and mood changes as initial symptom were predictors of taking >1 year to receive the FTD diagnosis. As FTD is typically an early-onset dementia, clinicians may experience difficulty distinguishing FTD from other possible conditions that increase with patient age. On the other hand, increasing caregiver age may be associated with a shorter time until diagnosis because older caregivers have fewer childrearing and work responsibilities compared with younger caregivers and thus have more time to dedicate to obtaining the diagnosis. It is unclear why those with spouse caregivers would be associated with taking >1 year until diagnosis. However, it is possible that spouses are more likely to request second opinions or initially downplay some of the more prominent initial symptoms of disease, lengthening the time to receive the diagnosis. Living in a rural area can present logistic and financial difficulties in receiving adequate and timely health care and in our sample was associated with increased time to receive the FTD diagnosis. Last, patients who initially demonstrated mood symptoms were more likely to receive their FTD diagnosis >1 year after initial presentation. Consistent with this finding, of the 402 patients initially receiving a non-FTD diagnosis, 28% were diagnosed with depression. Therefore, mood changes as the initial presenting symptom appear to increase time to receive the FTD diagnosis.
Early-onset dementia including FTD is frequently misdiagnosed initially in up to 30%–50% of cases.23 Depression was the most common initial diagnosis among those first receiving a non-FTD diagnosis, in all FTD phenotypes except PSP/CBS. The second most common initial diagnosis was AD among those with bvFTD and PPA and Parkinson disease among those with FTD-MND. The predictors of being initially misdiagnosed included initially presenting with cognitive or mood changes and ultimately receiving an FTD-MND diagnosis. These predictors are consistent and represent distinguishing symptoms of the initial misdiagnoses that were received.
MRI, CT, neuropsychological testing, and blood work were routine in the diagnostic workup for the vast majority of the patients with FTD. FDG-PET scans, approved to distinguish between AD and FTD, were performed less often. PET use may increase in the future provided new tau tracers become more sensitive and specific to FTD, and similarly, lumbar punctures may increase in use if CSF biomarkers for FTD become available. With the advent of new biomarkers, it is hoped that the time to receive the correct diagnosis will be substantially reduced for patients with FTD.
Few studies have been published on the diagnostic experience of patients with dementia and their caregivers, and the limited studies that are peripherally related often have been qualitative, have small samples, and do not focus on FTD.15,19,21,24–27 A major strength of this study is its quantitative nature and large sample size, which allowed a characterization of the diagnostic experience by FTD phenotype. Another strength of the study is the unique information it provides regarding the period surrounding the diagnosis. A limitation of the study is that it was not population based, and therefore, the findings may be limited in their generalizability, but the large sample size helps reduce these concerns, given the rarity of this dementia type. Generalizability is also limited because the sample was highly educated and primarily of white race. Future studies are needed to evaluate the diagnostic journey across the spectrum of demographic characteristics that may influence FTD diagnosis, including among racially/ethnically underrepresented populations such as Hispanics and African Americans. The exclusion of 258 respondents missing data on time to receive FTD diagnosis may have biased the results. The study is based on caregiver self-reporting and thus may be affected by reporting bias or error, although many of the results and findings were consistent with what would be expected. In addition, no further details were collected on the subspecialty of the diagnosing neurologists, which may have provided details to help explain initial misdiagnosis or longer time to FTD diagnosis.
This study provides a fresh perspective on the experiences of patients with FTD and their caregivers by focusing on the period leading up to and during the time of the diagnosis. Unlike previous studies that have been qualitative in nature, our study provides a quantification of the burdens surrounding the period of diagnosis and of the caregiver experiences with the diagnosing doctor. Studies such as this can help inform clinical practice, the education of health professionals who diagnose and treat patients with FTD, possible interventions to reduce the time to FTD diagnosis, and programs and services to support patients and caregivers during and following the diagnosis.
Acknowledgment
The authors acknowledge Susan Dickinson MSGC, Nadine Tatton, PhD, and Sharon Denny, MA, for their support and input in crafting the caregiver survey and understanding how the results may affect patients with FTD and caregivers.
Appendix. Authors
Study funding
This study was supported by the Association for Frontotemporal Degeneration.
Disclosure
L.M. Besser reports no disclosures. J.E. Galvin reports research support from the NIH, Michael J. Fox Foundation, Harry T. Mangurian Foundation, and Albert Trust. He directs clinical trials for Biogen and Novartis. He receives licensing fees from Biogen, Roche, Eli Lilly, Quintiles, Roobrik, Continuum Clinical, and Langland. He receives consulting fees from Biogen, Eisai, Bracket, and Medavante. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
References
- 1.Knopman DS, Roberts RO. Estimating the number of persons with frontotemporal lobar degeneration in the US population. J Mol Neurosci 2011;45:330–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perry DC, Miller BL. Frontotemporal dementia. Semin Neurol 2013;33:336–341. [DOI] [PubMed] [Google Scholar]
- 3.Ratnavalli E, Brayne C, Dawson K, Hodges JR. The prevalence of frontotemporal dementia. Neurology 2002;58:1615–1621. [DOI] [PubMed] [Google Scholar]
- 4.Snowden JS, Neary D, Mann DM. Frontotemporal dementia. Br J Psychiatry 2002;180:140–143. [DOI] [PubMed] [Google Scholar]
- 5.Rascovsky K, Hodges JR, Knopman D, et al. . Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 2011;134:2456–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mesulam MM. Primary progressive aphasia: a language-based dementia. N Engl J Med 2003;349:1535–1542. [DOI] [PubMed] [Google Scholar]
- 7.Brooks BR, Miller RG, Swash M, Munsat TL; World Federation of Neurology Research Group on Motor Neuron Diseases. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Mot Neuron Disord 2000;1:293–299. [DOI] [PubMed] [Google Scholar]
- 8.Armstrong MJ, Litvan I, Lang AE, et al. . Criteria for the diagnosis of corticobasal degeneration. Neurology 2013;80:496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bensimon G, Ludolph A, Agid Y, et al. . Riluzole treatment, survival and diagnostic criteria in Parkinson plus disorders: the NNIPPS study. Brain 2009;132:156–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galvin JE, Howard DH, Denny SS, Dickinson S, Tatton N. The social and economic burden of frontotemporal degeneration. Neurology 2017;89:2049–2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Besser LM, Galvin JE. Perceived burden among caregivers of patients with frontotemporal degeneration in the United States. Int Psychogeriatr 2018;6:1–11. [DOI] [PubMed] [Google Scholar]
- 12.Riedijk SR, De Vugt ME, Duivenvoorden HJ, et al. . Caregiver burden, health-related quality of life and coping in dementia caregivers: a comparison of frontotemporal dementia and Alzheimer's disease. Dement Geriatr Cogn Disord 2006;22:405–412. [DOI] [PubMed] [Google Scholar]
- 13.Diehl-Schmid J, Schmidt EM, Nunnemann S, et al. . Caregiver burden and needs in frontotemporal dementia. J Geriatr Psychiatry Neurol 2013;26:221–229. [DOI] [PubMed] [Google Scholar]
- 14.Leggett AN, Zarit S, Taylor A, Galvin JE. Stress and burden among caregivers of patients with Lewy body dementia. Gerontologist 2011;51:76–85. [DOI] [PubMed] [Google Scholar]
- 15.Armari E, Jarmolowicz A, Panegyres PK. The needs of patients with early onset dementia. Am J Alzheimers Dis Other Demen 2013;28:42–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Vliet D, de Vugt ME, Bakker C, et al. . Time to diagnosis in young-onset dementia as compared with late-onset dementia. Psychol Med 2013;43:423–432. [DOI] [PubMed] [Google Scholar]
- 17.Rasmussen H, Hellzen O, Stordal E, Enmarker I. Family caregivers experiences of the pre-diagnostic stage in frontotemporal dementia. Geriatr Nurs 2019;40:246–251. [DOI] [PubMed] [Google Scholar]
- 18.Rosness TA, Haugen PK, Engedal K. Support to family carers of patients with frontotemporal dementia. Aging Ment Health 2008;12:462–466. [DOI] [PubMed] [Google Scholar]
- 19.Manthorpe J, Samsi K, Campbell S, et al. . From forgetfulness to dementia: clinical and commissioning implications of diagnostic experiences. Br J Gen Pract 2013;63:e69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bailey C, Dooley J, McCabe R. “How do they want to know?” Doctors' perspectives on making and communicating a diagnosis of dementia. Dementia (London) 2018:1471301218763904. doi: 10.1177/1471301218763904. [DOI] [PubMed] [Google Scholar]
- 21.Ducharme F, Kergoat MJ, Antoine P, Pasquier F, Coulombe R. The unique experience of spouses in early-onset dementia. Am J Alzheimers Dis Other Demen 2013;28:634–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Massimo L, Evans LK, Benner P. Caring for loved ones with frontotemporal degeneration: the lived experiences of spouses. Geriatr Nurs 2013;34:302–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Werner P, Stein-Shvachman I, Korczyn AD. Early onset dementia: clinical and social aspects. Int Psychogeriatr 2009;21:631–636. [DOI] [PubMed] [Google Scholar]
- 24.Merrilees J, Ketelle R. Advanced practice nursing: meeting the caregiving challenges for families of persons with frontotemporal dementia. Clin Nurse Spec 2010;24:245–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Vliet D, de Vugt ME, Bakker C, et al. . Caregivers' perspectives on the pre-diagnostic period in early onset dementia: a long and winding road. Int Psychogeriatr 2011;23:1393–1404. [DOI] [PubMed] [Google Scholar]
- 26.Galvin JE, Duda JE, Kaufer DI, Lippa CF, Taylor A, Zarit SH. Lewy body dementia: the caregiver experience of clinical care. Parkinsonism Relat Disord 2010;16:388–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luscombe G, Brodaty H, Freeth S. Younger people with dementia: diagnostic issues, effects on carers and use of services. Int J Geriatr Psychiatry 1998;13:323–330. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data can be shared with qualified investigators on request.