Abstract
Objective
To describe pregnancies exposed to teriflunomide (TERIF) in women with multiple sclerosis (MS) in France over the period 2014–2016.
Methods
All 15- to 49-year-old women with MS in the national health insurance database were included. Pregnancies that had started between August 2014 and March 2016 were identified from their outcomes. Three groups according to treatment exposure were compared: TERIF, interferons (IFNs) or glatiramer acetate, and no medication.
Results
Among the 44,008 women with MS followed 24.5 months on average, 2,639 pregnancies were identified. There were 1,538 pregnancies (58.3%) that were not exposed to any MS treatment in accordance with the guidelines. A total of 673 pregnancies (25.5%) were exposed to IFN and/or glatiramer acetate, and possible or probable exposure to contra-indicated treatments was observed in 428 pregnancies (16.2%), of whom 47 pregnancies were exposed to TERIF. The annual incidence rate of pregnancies exposed to TERIF was 1.4 per 100 patient-years; i.e., 3 times less than the 2 control groups (5.6 and 4.7, respectively). The median exposure duration to TERIF was 45 days after conception. The outcomes comprised 23 live births, 22 abortions (3 times more than the 2 other groups), and 2 miscarriages. All newborns were healthy at birth.
Conclusions
Despite specific TERIF guidelines for pregnancy-related issues and the availability of alternative therapies, some pregnancies exposed to TERIF were identified. Most of the cases were because of the absence of the recommended accelerated elimination procedure and appeared to be mostly unplanned pregnancies that probably reflect a lack of effective contraception.
Epidemiology of multiple sclerosis (MS) shows that a high proportion of patients are women of childbearing age. The therapeutic arsenal has substantially expanded in the past 20 years with 10 disease-modifying treatments (DMTs) currently available to neurologists1 including teriflunomide (TERIF) (Aubagio; Sanofi Genzyme, Cambridge, MA).2,3 As animal studies have shown embryotoxic and teratogenic effects in rats and rabbits,4–6 it is contraindicated in pregnant women and its prescription must be accompanied by an effective contraception. Moreover, as its plasma concentration remains significant for 8 months on average, specific guidelines have been devised,7 and an accelerated elimination procedure (AEP) must be implemented in case of a desire to become pregnant.
If it is not possible to stop DMT during pregnancy because of high MS activity,8 interferons (IFN) and glatiramer acetate (GA) can be alternative therapies,9–11 as their in utero exposure has been shown to not entail risks for the fetus.12,13 Therefore, in light of these guidelines, exposure of pregnant women with MS to TERIF should be avoided. However, a retrospective analysis of all the participants in TERIF clinical trials14 allows identification of 70 exposed pregnancies. In terms of real-life exposure, at the time of study, the French pharmacovigilance was aware of 22 cases and at international level, 232 cases have been reported,15 but underreporting is strongly suspected in such spontaneous reporting systems.16
The aim of the present study was to describe pregnancies that have been exposed to TERIF in France using the French health insurance database.
Methods
Study design
This was a retrospective cohort study using prospectively collected data. Study period covered 29 months, from August 1, 2014 (3 months before TERIF became available in pharmacies in France) to December 31, 2016 (the most recent available data when the study was performed).
Data source
The study was performed using data derived from the French national health insurance database (“Système d'Information Inter-Régimes de l'Assurance-Maladie,” SNIIRAM) linked to the French hospital discharge database (“Programme de Médicalisation des Systèmes d'Information,” PMSI).17 This database covers nearly the entire French population (97% of the 65.3 million inhabitants) and contains exhaustive data on all reimbursements for health-related expenditures, including medicinal products and outpatient medical and nursing care prescribed or performed by health care professionals, as well as demographic data such as age, sex, area of residence, and vital status. The medical indication for outpatient reimbursements was not available although the patient's status for 100% reimbursement of care related to a severe and costly long-term disease (LTD) (MS being on this list) is recorded and categorized according to the International Classification of Diseases, 10th revision (ICD-10; i.e., “G35” for MS). For hospital admissions, detailed medical information is available for all admissions to French public and private hospitals, including the dates of hospital admission and discharge, the discharge diagnoses based on ICD-10 codes, and medical procedures performed during the hospital stay.
Study population
All 15-49 year-old women identified as having MS and who received at least one reimbursement from the French health insurance system over the study period (to ensure active coverage) were eligible for inclusion in this nationwide study.
The identification of MS was performed from January 1, 2010 to December 31, 2016 and based on a 3-criteria algorithm18: the presence of a LTD for MS, MS-related hospital admissions (principal, related, or associated diagnosis code = “G35” in medicine/surgery/obstetrics [MSO] hospitals as well as in postacute care and rehabilitation hospitals [REHAB]) or reimbursement for a MS-specific DMT (TERIF, GA, IFN, fingolimod, dimethyl fumarate, and natalizumab).
Women with a history of a procedure corresponding to a hysterectomy, interruption of tubal patency, or Essure (Bayer AG, Leverkusen, Germany) implant placement were excluded at that date.
For each included woman, the follow-up started on August 1, 2014 (start of the study) if all of the inclusion criteria were met and if not, at the latest between the MS identification date and the 15th birthday. It ended at the first occurrence of the following events: death, permanent sterilization, 50th birthday, or the end of the study on December 31, 2016.
Identification of pregnancies
Pregnancies were identified through their outcome by the hospital stay for birth or abortion (diagnosis and/or medical procedures) from hospital discharge databases or by reimbursement of either a nonhospital medical abortion or a home delivery.19
The 47 exposed cases received initial TERIF prescription by different doctors in different hospitals, in different French regions.
The date of the start of pregnancy was calculated using the gestational age indicated in the hospital records or by using the estimated start date as reported in the pregnancy declaration to the health insurance system. When unavailable, it was calculated using the median pregnancy duration in 2014 in France according to the pregnancy outcome.
Only pregnancies that started later than August 1, 2014 and before March 31, 2016 were counted. March was chosen to allow any pregnancy to reach 9-month duration by study end date, and not favor adverse outcomes to be collected.
Definition of exposure to TERIF and other DMTs
This immuno-active drug is administered orally once per day at a single dose in Europe (14 mg). TERIF and other DMTs were identified by their CIP13 code (TERIF's CIP13 code 3400927499890). For each medication reimbursement, the date and the quantity were available.
Because of TERIF's long half-life, it must be terminated at least 8 months prior to conception.4 Otherwise, an AEP is recommended using cholestyramine (8 g, 3 times per day for 11 days; i.e., dispensation of 66 packages of 4 g is expected for a complete procedure). Activated charcoal can also be used (50 g, twice per day for 11 days); however, as it is not reimbursed by the health insurance system, it could not be assessed in the present study. Two TERIF plasma concentration tests under the threshold of 0.02 mg/L with a 14-day interval must be obtained before conception can safely take place. As these laboratory tests are exclusively paid for by the company that markets TERIF and not by the health insurance system, this information was not available to us.
Three groups of patients were compared according to their drug exposure (at least 1 day) during pregnancy: exposed to TERIF, exposed to IFN or GA, or not exposed to any DMT. A pregnancy was considered as exposed to TERIF if the woman had at least one reimbursement (1) during pregnancy, or (2) in the 8-month period preceding the onset of pregnancy in the absence of a complete AEP. If TERIF was stopped without AEP, TERIF exposure remained ongoing, i.e., exposure duration does not mean current TERIF intake. A pregnancy was considered as exposed to IFN or GA if at least one reimbursement fell within the pregnancy period. If a woman was exposed to both TERIF and IFN or GA during pregnancy, she was included in the TERIF group.
We identified 5 profiles of TERIF exposure and found that the guidelines regarding pregnancy-related issues were not adhered to for most of the exposed pregnancies.
Other data
Regarding TERIF, we knew who the initial prescribers were. The circumstances of exposure to TERIF were also studied, by looking at the reimbursement of ovulation induction medications and folic acid (in the conception period, as recommended by French guidelines to prevent from neural tube defects20) to approximate the desire or the intention to become pregnant, and of oral contraceptives to approximate its absence.
We also described the mode of delivery and data related to the newborn: sex, gestational age, length of the hospital birth stay, diagnosis and procedures performed during the birth stay if any, and the admission in an intensive care unit.
Statistical analysis
The incidence rate of pregnancies was calculated for the period between the August 1, 2014 and the March 31, 2016 in the 3 groups (TERIF, IFN-GA, and no treatment), overall and by age groups. Pregnancy outcomes, characteristics of pregnancies, deliveries, and newborns were compared between the 3 groups using χ2 tests (or Fisher's tests) and analysis of variance tests, as appropriate. The significance threshold was set at 5%. The statistical analyses were performed using SAS software.
Standard protocol approvals, registrations, and patient consents
The present study was conducted in accordance with the French legislation and after scientific validation from the French National Agency for Medicines and Health Products Safety.
Data availability
Data cannot be shared for legal reasons. Indeed, under French law and regulations, patient-level data from the French health insurance database cannot be made available.17
Results
Study population
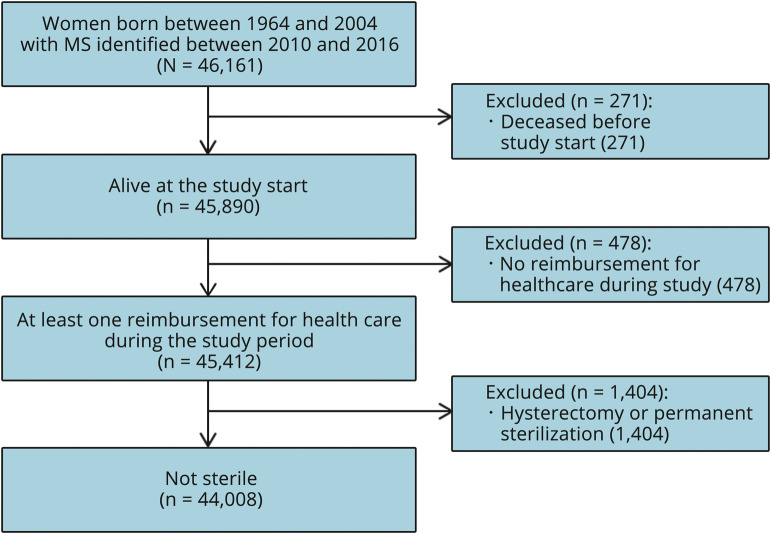
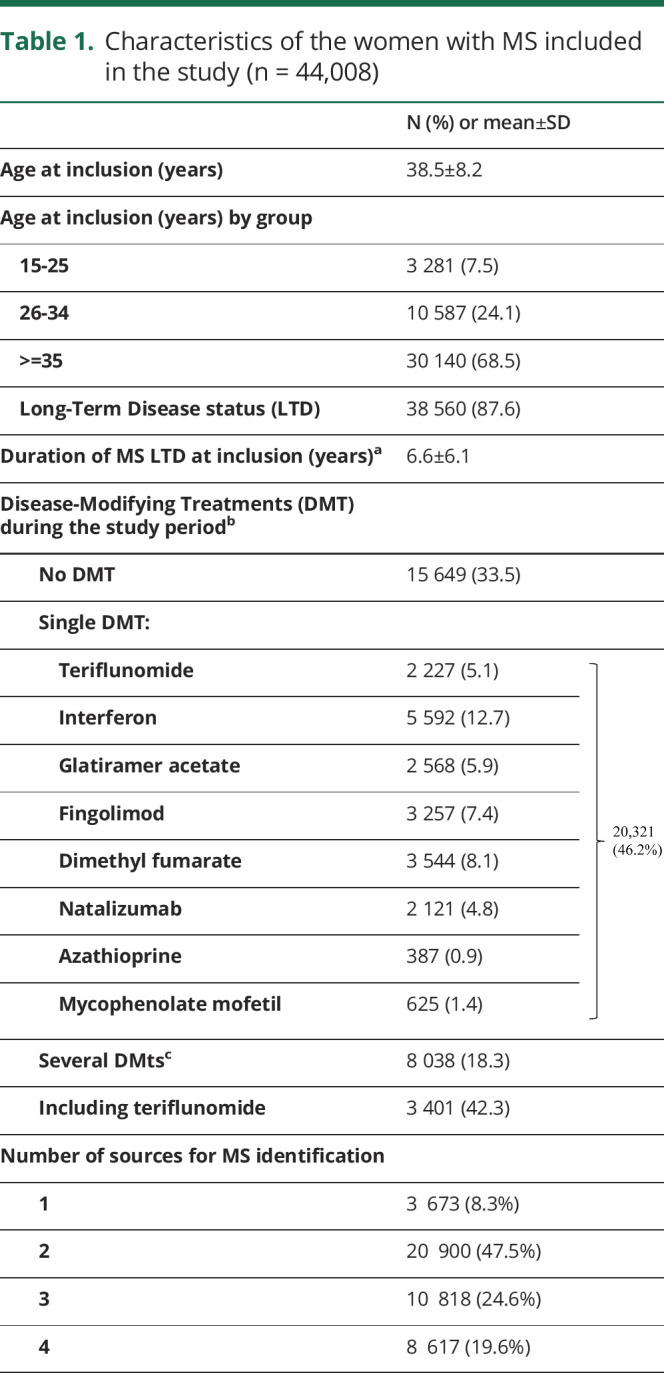
A total of 44,008 women were included (figure 1), with an average follow-up of 24.5 months (SD: 8.6). Their characteristics are presented in table 1.
Figure 1. Flowchart of the study population (n = 44,008).
MS = multiple sclerosis.
Table 1.
Characteristics of the women with MS included in the study (n = 44,008)
TERIF exposure
Over the 29-month period, 5,628 women with MS received TERIF at least once: 2,227 as the only treatment and 3,401 before or after another DMT. TERIF was started at a median age of 39.9 years. The mean number of TERIF packages was 12.8 (SD 8.6), which approximately represented one year of treatment. Half of the TERIF initial prescribers were hospital doctors (n = 2,978, 53%) and more than a third (n = 2,176, 39%) were private neurologists. Non-neurologist prescribers were identified for 3% (n = 188) of the initial prescriptions (general practitioners (79, 1.4%), neuropsychiatrists (23, 0.4%), obstetricians/gynecologists (11, 0.2%) and others) while the prescriber's specialty was not mentioned for the remaining 5%.
Pregnancies
A total of 2,639 pregnancies were identified over the period from August 1, 2014 to March 31, 2016, which correspond to an incidence rate of pregnancies in women with MS of 4.4 per 100 patient-years (7.6, 11.5, and 1.5 per 100 patient-years in <25 years, 25–35 years, and >35 years, respectively). The outcomes were 1,980 (75.0%) live births, 12 (0.5%) stillborn children, 438 (16.6%) voluntary or medical abortions, 127 (4.8%) miscarriages, 36 (1.4%) ectopic pregnancies, and 46 (1.7%) others (e.g., hydatidiform moles and other abnormal products of conception).
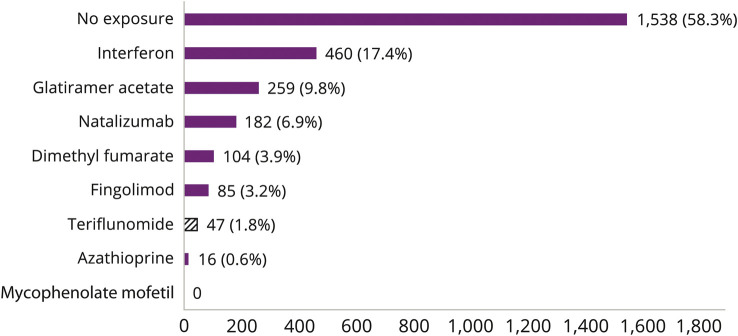
Overall, 1,538 (58.3%) pregnancies were not exposed to any DMT, 673 pregnancies (25.5%) were exposed to IFN and/or GA, and possible or probable exposure to contra-indicated DMTs (including TERIF) was observed in the 428 remaining pregnancies (16.2%) (figure 2).
Figure 2. Distribution of disease-modifying therapies exposure in pregnancies (n = 2,639).
Y-axis: disease-modifying therapies. X-axis: number of pregnancies exposed over the study period.
Pregnancies exposed to TERIF
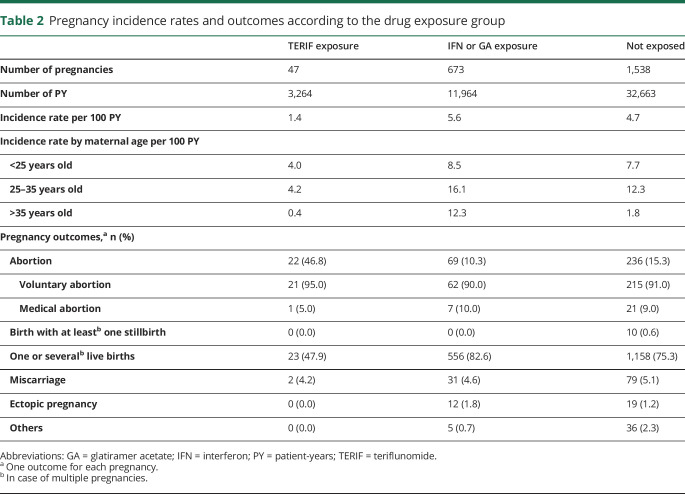
A total of 47 pregnancies were considered to be exposed to TERIF. The annual incidence rate of pregnancies exposed to TERIF was 3 to 4 times lower than that of the 2 other groups (table 2).
Table 2.
Pregnancy incidence rates and outcomes according to the drug exposure group
In 24 cases, TERIF was stopped in the 8-month period preceding conception but no AEP was performed (20 cases) or it was either incomplete (3 cases) or performed too late (starting less than 11 days before conception, 1 case). For the 23 other cases, pregnancy started before TERIF discontinuation. For 3 of them, a complete AEP has reduced the exposure duration of the exposure, while no procedure was started or performed in an incomplete way for the 20 others.
The median duration of TERIF exposure was 45 days (1st quartile, Q1: 30–3rd quartile, Q3: 220). The exposure occurred in the first trimester of pregnancy for 32 cases (69%) and lasted less than one month and 2 months for respectively 12 and 16 pregnancies. It lasted more than 7 months in 13 cases (27%) because of the absence of complete AEP at TERIF discontinuation.
Pregnancy outcomes
The 47 pregnancies exposed to TERIF resulted in 23 live births, 22 abortions, and 2 miscarriages (table 2). The proportion of abortions in the TERIF group was higher than in the 2 other groups (47% vs 10% and 15%, p < 0.0001). No stillbirths or ectopic pregnancies occurred in the TERIF group.
Circumstances of TERIF exposure
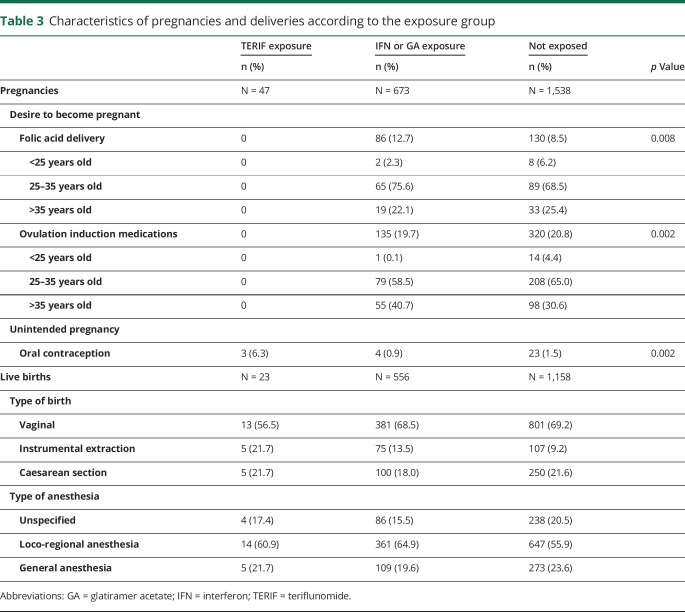
None of the pregnancies exposed to TERIF seem to be medically anticipated as no folic acid supplementation or medically assisted procreation procedures was found in the 47 cases (table 3). By contrast, these reimbursements were identified in about 30% of cases in the 2 other groups (folic acid in 12.7% [n = 86] and 8.5% [n = 130], and ovulation induction medications in 19.7% [n = 135] and 20.8% [n = 320], for the IFN-GA and the untreated groups, respectively).
Table 3.
Characteristics of pregnancies and deliveries according to the exposure group
Moreover, 6.4% of the patients exposed to TERIF (3 cases) received an oral contraception in the month prior to conception, which was higher than in the 2 other groups (0.9% in the IFN-GA group and 1.5% in the untreated group) and suggests unintended pregnancies.
Different profiles of TERIF exposure and outcomes
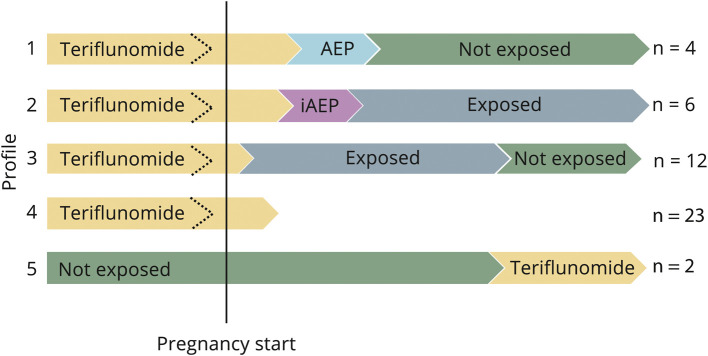
The 47 exposed cases received initial TERIF prescription by different doctors in different hospitals, in different French regions. The analysis of the 47 exposed cases allowed 5 different profiles of TERIF exposure to be identified (figure 3).
Figure 3. Five profiles of pregnancies exposed to TERIF (n = 47).
Vertical line represents pregnancy start. Dotted arrows represent potential TERIF discontinuation before pregnancy start. Profile 1: n = 4; Profile 2: n = 6; Profile 3: n = 12; Profile 4: n = 23; Profile 5: n = 2. AEP = accelerated elimination procedure; exposed = patient still exposed to TERIF after its prescription was stopped; iAEP = incomplete accelerated elimination procedure; not exposed = patient no more exposed to TERIF; TERIF = teriflunomide.
Profile 1 refers to pregnancies started when TERIF was either ongoing or stopped, and a complete AEP was carried out in the first 2 months of pregnancies, and the pregnancies were pursued without further exposure to TERIF. Four pregnancies matched this profile with a median duration of exposure to TERIF of 32.5 days (Q1: 27.0–Q3: 39.5) and they led to 4 live births. TERIF was stopped before pregnancy onset for one of them.
Profile 2 refers to women for whom an AEP was performed but the quantity dispensed was not sufficient (50 of the 66 packages recommended for treatment) to be considered as complete. The women were, therefore, considered to still be exposed even if TERIF was stopped. Six pregnancies matched this profile with a median duration of exposure to TERIF of 232 days (Q1: 100–Q3: 250) and they led to 5 live births and one miscarriage. For 3 women, TERIF was discontinued before pregnancy onset and for the 3 others, it was not.
Profile 3 refers to women exposed to TERIF during several months of the pregnancy, because of the fact that no elimination procedure was performed. Twelve pregnancies matched this profile with a median duration of exposure to TERIF of 232 days (Q1: 222.5–Q3: 239.5) and they led to 12 live births. TERIF was stopped in the 8 months before pregnancy onset for 7 cases.
Profile 4 refers to pregnancies started when treatment with TERIF was either ongoing or stopped, and for which no elimination procedure was undertaken and the pregnancy was terminated at an early stage. Twenty-three pregnancies matched this profile and they led to 22 abortions (21 voluntary and 1 medical abortions) and 1 miscarriage. For 13 cases, TERIF was stopped before pregnancy onset.
Profile 5 refers to a totally different situation, as it concerned women who received TERIF at the end of the pregnancy with a unique package being dispensed in the last trimester. These women were considered to be exposed according to our definition, although it probably reflects an anticipated prescription to resume TERIF in the postpartum period. The 2 pregnancies that matched this profile led to 2 live births. Liver function tests recommended at TERIF initiation were not carried out in one case and carried out several weeks after birth in the other case, which reinforces our hypothesis that these 2 cases were false positive cases of exposed pregnancies.
Characteristics of the deliveries and health of the newborns
The rate of vaginal delivery for pregnancies exposed to TERIF was lower than in the 2 other groups (although not significant, p = 0.4) (table 3), whereas the rate of instrumental extractions was higher (p = 0.005).
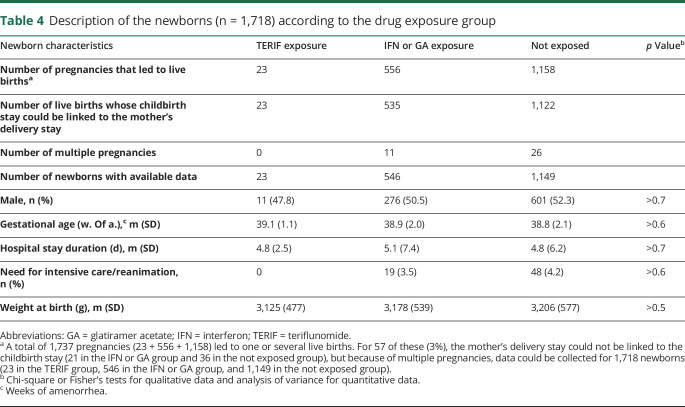
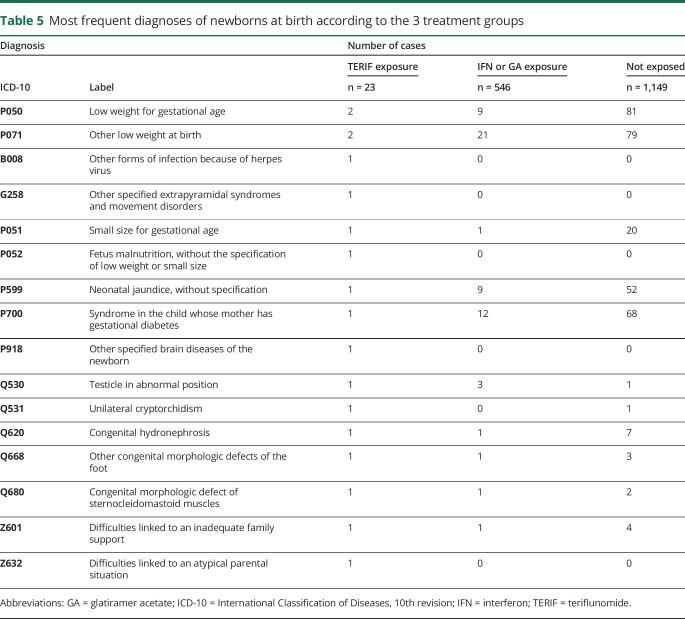
For the 1,737 exposed pregnancies that led to a live birth, data for 1,680 (96.7%) birth stays, including the 23 exposed cases to TERIF, were available. In total, data could be collected for 1,718 newborns owing to multiple pregnancies (table 4). All newborns characteristics were comparable between the 3 treatment groups. In the TERIF group, no intensive care was needed for the newborns and the diagnoses made at birth were diverse and nonspecific (table 5).
Table 4.
Description of the newborns (n = 1,718) according to the drug exposure group
Table 5.
Most frequent diagnoses of newborns at birth according to the 3 treatment groups
Discussion
The present nationwide population-based cohort study highlights that, despite the availability of specific guidelines regarding pregnancy-related issues, 47 pregnancies were exposed to TERIF within the first 20 months of its availability in France, among a total of 5,628 women with MS of childbearing age who initiated this treatment.
We identified 5 profiles of TERIF exposure and found that the guidelines regarding pregnancy-related issues were not adhered to for most of the exposed pregnancies. Half of pregnancies occurred while TERIF was ongoing and half while TERIF was stopped, but with a high probability of plasma concentrations being significant. Indeed, the AEP was often found to be lacking or performed in an incomplete manner. Almost half of the exposed pregnancies led to an abortion (46.8%). This rate was much higher than for the 2 other groups (10.3% and 15.3%) and in the general French population (19.0%),19 but similar to that from TERIF clinical trials.14
Our results are in line with the small amount of data collected in real-life settings and confirmed the under-notification in pharmacovigilance systems based on spontaneous notification. Regarding the 22 cases known from the national pharmacovigilance system at study start (unpublished), AEP was reported only in 9 cases (40.9%). It was notified that pregnancy was intended in 2 cases, unintended in 8 cases, and the status was unknown in 12 cases.
In the Danish prospective cohort study,21 31 pregnancies were identified, 13 women with MS and 18 of partners to a man treated with TERIF. Among the 13 pregnant women treated with TERIF, 11 (85%) chose an elective abortion and the 2 others resulted in live births, with normal gestation time, normal birth weight, and no congenital malformations.
In the international report presented at ECTRIMS in 2017,15 232 exposed pregnancies were identified, 62 being clinical study cases and 170 being postmarketing cases, of which respectively 62 and 67 had documented outcomes. AEP was performed in half of the cases (27/62 clinical study cases [43.5%] and 33/67 postmarketing cases [49.3%]). Outcomes were live birth in 22/62 (35.5%) and 30/67 (44.8%), spontaneous abortion in 8/62 (12.9%) and 19/67 (28.3%), elective abortion in 30/62 (48.4%) and 17/67 (25.4%), and ectopic pregnancy in 2/62 (3.2%) and fetal death in 1/67 (1.5%). Three structural abnormalities have been reported, but there was no increased frequency of birth defects.
It should be reminded that TERIF is the main active substance of leflunomide, used in the treatment of rheumatoid arthritis. A total of 2.4 million patient-years of postmarketing surveillance are available and have not shown to be a human teratogen.22 However, the limited number of exposed pregnancies suggests being prudent.
As mentioned earlier, it seems that none of the pregnancies exposed to TERIF in the present study were medically anticipated as there was no mention of medically assisted procreation or folic acid supplementation. However, according to reproductive health experts, folic acid supplementation before conception is not systematically carried out in the French general population.20 Moreover, it may not be reimbursed (if bought without any physician prescription or if integrated into food supplementation mixtures) and then is not systematically available in the database. For these 2 reasons, folic acid use cannot be considered as a good marker of the desire of pregnancy. Only 3 cases reported oral contraceptives but it should be kept in mind that most of them are not reimbursed in France and thus cannot be identified in the health insurance database. We also looked for intrauterine devices and found 8 insertion acts among the 47 TERIF cases in the 5 years preceding the onset of pregnancy. However, this information was not selected, as removal of such devices cannot be identified in the database and may lead to the overestimation of women really using this contraceptive method. A large number of unintended pregnancies are compatible with the high rate of abortions and the lack of a peak in the frequency of pregnancies in women between 25 and 35 years old. It also reflects that guidelines that advocate effective contraception for TERIF-treated women are probably not adhered to properly. However, the high rate of abortions may also be a reflection of the decision made by the women and their partner when they found out that the fetus was exposed to a teratogenic drug.
Regarding the delivery, the rate of instrumental extractions was higher for pregnancies exposed to TERIF than for the 2 other groups, with no clear explanation. In the general population, instrumental extraction is involved in approximately 12% of deliveries while caesareans amount to 20% of all deliveries.23
Regarding the TERIF initial prescriptions, about 3% were not prescribed by a neurologist while its prescription is restricted to this medical specialty.4 Errors in data entry are strongly suspected, but misuse by other specialists cannot be excluded.
We had the opportunity to study the risk and occurrence from exposed pregnancies since TERIF became available in France and to follow it over time. The number of exposed pregnancies did not decrease over time. Moreover, 19 additional pregnancies between April 1, 2016 and December 31, 2016 were identified as having been exposed to TERIF, but they were not analyzed because they occurred after the end of the study period (the inclusion of cases that started after this date would have led to an overestimation of the proportion of adverse pregnancy outcomes). Considering the 47 exposed cases, the 19 additional cases, and the 2 probably misclassified cases (profile 5), there may be a total of 64 TERIF-exposed pregnancies in 2 years in France.
The use of medico-administrative data in the present study implies some limitations. First of all, only data for reimbursed drugs were available; treatments that were not reimbursed by the health insurance system could not be studied and taken into account. This bias may apply to oral contraceptives, intrauterine devices, folic acid, and activated charcoal. According to the experts, cholestyramine is used much more widely than charcoal, so the use of the AEP may only be slightly underestimated. Regarding the AEP, we can assume that it could have been stopped prematurely in some cases because of a dosage of TERIF concentration under the threshold considered as significant (<0.02 mg/L), but these dosages and their results are not available as they were generated by the pharmaceutical company that markets TERIF. Nevertheless, the initial quantity dispensed by the pharmacist should be sufficient to cover the treatment period, irrespective of the intended control dosage.
Furthermore, the dispensing of a drug by a pharmacist does not necessarily equate with effective use of the treatment by the patient, which means that the length of TERIF exposure is only an estimation of the true exposure and it may be overestimated in case the woman did not take all of the pills in the package.
Despite these limitations, this data source also has several strengths. Firstly, it allows for studies to be conducted on exhaustive populations at a national scale, without any selection or recruitment bias, with data collection fully independent of self-declaration by individuals (either patients or health care providers), thereby avoiding the risk of underreporting or memory bias, as often found in pharmacovigilance system or in postmarketing studies.
Regarding data quality, the dates for the onset of pregnancy were mostly calculated using the gestational age specified in the hospital records (97.5%), 2.0% was estimated from the pregnancy declaration, and only 0.5% was calculated using the national median pregnancy duration, which suggest a good reliability in the dating.
Moreover, ascertainment of medication use based on claims data is independent of maternal or infant outcomes, which avoids parental recall bias.19 Therefore, use of medico-administrative databases could be generalized for monitoring pregnancy-related issues in relation to exposure to other drugs and could increase the comparability of studies conducted in different countries.
In conclusion, health care providers (primarily neurologists, but also pharmacists and general practitioners) should be reminded of the guidelines that stipulate combining TERIF with an effective contraception and the need to systematically carry out an AEP when the treatment is stopped because the patient wishes to become pregnant. Patients also have a role to play by informing their physician of their intentions regarding pregnancy. Lastly, there is no specific signal regarding newborns’ health after TERIF exposure, but it is essential to continue to monitor the number of exposed pregnancies and the children's health over the long term to better document any potential adverse health outcomes linked to in utero exposure to TERIF.
TAKE-HOME POINTS
→ Despite existing contraindications during pregnancy, 47 TERIF-exposed pregnancies were identified in a French nationwide cohort of women with MS, within the first 20 months following its market availability.
→ Half of the pregnancies occurred while TERIF was ongoing and the other half while TERIF was stopped.
→ Most of the cases were considered as TERIF-exposed because of problems in the recommended AEP (either lacking or performed in an incomplete manner).
→ Almost half of the TERIF-exposed pregnancies led to an abortion, without knowing whether this large proportion reflects unplanned pregnancies or consequences of the exposure.
→ Health care providers as well as patients with MS should be reminded of the TERIF guidelines regarding pregnancy-related issues.
Appendix. Authors
Study funding
The study was funded by the French National Agency for Medicines and Health Products Safety (“Agence Nationale de Sécurité du Médicament,” ANSM).
Disclosure
The author(s) declare that there are no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. A. Barataud-Reilhac reports no disclosures. S. Kerbrat reports no disclosures. J. Roux reports no disclosures. A. Guilleux reports no disclosures. E. Polard reports no disclosures. E. Leray reports consulting and lecture fees from Genzyme, which manufactures the drug that is evaluated in this study, but this work was strictly limited to epidemiology and prognosis of multiple sclerosis, outside drug effectiveness or safety. E. Leray also reports consulting and lecture fees or travel grants from Biogen, Genzyme, MedDay Pharmaceuticals, Merck, Novartis, and Roche. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
References
- 1.Dobson R, Giovannoni G. Multiple sclerosis - a review. Eur J Neurol 2019;26:27–40. [DOI] [PubMed] [Google Scholar]
- 2.O'Connor P, Wolinsky JS, Confavreux C, et al. Randomized trial of oral teriflunomide for relapsing multiple sclerosis. N Engl J Med 2011;365:1293–1303. [DOI] [PubMed] [Google Scholar]
- 3.Confavreux C, O'Connor P, Comi G, et al. Oral teriflunomide for patients with relapsing multiple sclerosis (TOWER): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol 2014;13:247–256. [DOI] [PubMed] [Google Scholar]
- 4.European Medicines Agency—find medicine—Aubagio [Internet]. Available at: ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/002514/human_med_001645.jsp&mid=WC0b01ac058001d124. Accessed May 30, 2018.
- 5.Lu E, Wang BW, Alwan S, et al. A review of safety-related pregnancy data surrounding the oral disease-modifying drugs for multiple sclerosis. CNS Drugs 2014;28:89–94. [DOI] [PubMed] [Google Scholar]
- 6.Amato MP, Portaccio E. Fertility, pregnancy and childbirth in patients with multiple sclerosis: impact of disease-modifying drugs. CNS Drugs 2015;29:207–220. [DOI] [PubMed] [Google Scholar]
- 7.Bodiguel E, Bensa C, Brassat D, et al. Multiple sclerosis and pregnancy. Rev Neurol (Paris) 2014;170:247–265. [DOI] [PubMed] [Google Scholar]
- 8.Vukusic S, Marignier R. Multiple sclerosis and pregnancy in the “treatment era”. Nat Rev Neurol 2015;11:280–289. [DOI] [PubMed] [Google Scholar]
- 9.Amato MP, Portaccio E, Ghezzi A, et al. Pregnancy and fetal outcomes after interferon-β exposure in multiple sclerosis. Neurology 2010;75:1794–1802. [DOI] [PubMed] [Google Scholar]
- 10.Weber-Schoendorfer C, Schaefer C. Multiple sclerosis, immunomodulators, and pregnancy outcome: a prospective observational study. Mult Scler J 2009;15:1037–1042. [DOI] [PubMed] [Google Scholar]
- 11.Giannini M, Portaccio E, Ghezzi A, et al. Pregnancy and fetal outcomes after Glatiramer Acetate exposure in patients with multiple sclerosis: a prospective observational multicentric study. BMC Neurol 2012;12:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vaughn C, Bushra A, Kolb C, Weinstock-Guttman B. An update on the use of disease-modifying therapy in pregnant patients with multiple sclerosis. CNS Drugs 2018;32:161–178. [DOI] [PubMed] [Google Scholar]
- 13.Rommer PS, Zettl UK. Managing the side effects of multiple sclerosis therapy: pharmacotherapy options for patients. Expert Opin Pharmacother 2018;19:483–498. [DOI] [PubMed] [Google Scholar]
- 14.Kieseier BC, Benamor M. Pregnancy outcomes following maternal and paternal exposure to TERIF during treatment for relapsing–remitting multiple sclerosis. Neurol Ther 2014;3:133–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vukusic S, Coyle PK, Jurgensen S, et al. Pregnancy outcomes in patients with MS treated with teriflunomide: clinical study and postmarketing data (P.4.361). Neurol Adv Commun 2018;90:202563 Available at: onlinelibrary.ectrimscongress.eu/ectrims/2017/ACTRIMS-ECTRIMS2017/202563/sandra.vukusic.pregnancy.outcomes.in.patients.with.ms.treated.with.html. [Google Scholar]
- 16.Biagi C, Montanaro N, Buccellato E, Roberto G, Vaccheri A, Motola D. Underreporting in pharmacovigilance: an intervention for Italian GPs (Emilia-Romagna region). Eur J Clin Pharmacol 2013;69:237–244. [DOI] [PubMed] [Google Scholar]
- 17.Tuppin P, Rudant J, Constantinou P, et al. Value of a national administrative database to guide public decisions: from the système national d'information interrégimes de l'Assurance Maladie (SNIIRAM) to the système national des données de santé (SNDS) in France. Rev Epidemiol Sante Publique 2017;65(suppl 4):S149–S167. [DOI] [PubMed] [Google Scholar]
- 18.Foulon S, Maura G, Dalichampt M, et al. Prevalence and mortality of patients with multiple sclerosis in France in 2012: a study based on French health insurance data. J Neurol 2017;264:1185–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blotière P-O, Weill A, Dalichampt M, et al. Development of an algorithm to identify pregnancy episodes and related outcomes in health care claims databases: an application to antiepileptic drug use in 4.9 million pregnant women in France. Pharmacoepidemiol Drug Saf 2018;27:763–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Botto LD, Lisi A, Robert-Gnansia E, et al. International retrospective cohort study of neural tube defects in relation to folic acid recommendations: are the recommendations working? BMJ 2005;330:571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andersen JB, Moberg JY, Spelman T, Magyari M. Pregnancy outcomes in men and women treated with teriflunomide: a population-based nationwide register study. Front Immunol 2018;9:2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Østensen M, Förger F. How safe are anti-rheumatic drugs during pregnancy?. Curr Opin Pharmacol 2013;13:470–475. [DOI] [PubMed] [Google Scholar]
- 23.Quantin C, Cottenet J, Vuagnat A, et al. Quality of perinatal statistics from hospital discharge data: comparison with civil registration and the 2010 National Perinatal Survey [in French]. J Gynecol Obstet Biol Reprod (Paris) 2014;43:680–690. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data cannot be shared for legal reasons. Indeed, under French law and regulations, patient-level data from the French health insurance database cannot be made available.17