Abstract
Background
As Parkinson disease (PD) progresses, symptoms increase, quality of life (QoL) declines, and individuals may become homebound, often losing access to neurologic care. We aimed to determine whether facilitating expert in-home care could improve our understanding of disease progression, treatment options, and unmet needs in this vulnerable population, and whether such a model could mitigate decline in QoL.
Methods
Patients with PD meeting Medicare homebound criteria were eligible for quarterly interdisciplinary home visits over 12 months. Each visit entailed an evaluation by a movement disorders neurologist, social worker, and nurse, including history, examination, medication reconciliation, psychosocial evaluation, pharmacologic and nonpharmacologic management, and service referrals. Disease severity, as measured by the Unified Parkinson's Disease Rating Scale (UPDRS), and QoL using the Neuro-QoL were measured at visits 1 and 4.
Results
Of 27 enrolled patients, 23 completed 4 visits, with high retention and high patient- and caregiver-reported satisfaction. The mean age at baseline was 80.9 years (SD 7.8) with a mean total UPDRS of 65.0 (SD 20.0). After one year of home visits, total UPDRS worsened by a mean of 11.8 points (p < 0.01) without a change in any of 8 QoL domains (p = 0.19–0.95).
Conclusions
Homebound individuals with advanced PD receiving interdisciplinary home visits experienced no significant decline in QoL over 1 year, despite disease progression. Our findings highlight the disease severity and impaired QoL of the advanced, homebound PD population, and the potential for novel approaches to foster continuity of care.
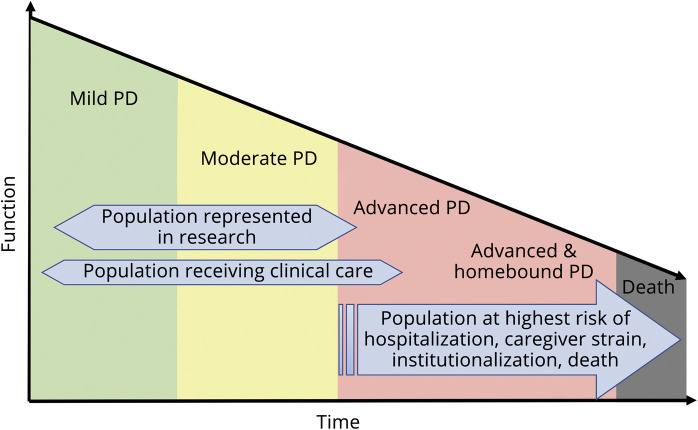
As Parkinson disease (PD) progresses, symptoms increase in number and severity.1 Individuals with advanced PD have a symptom burden comparable to patients with metastatic cancer.2 In both moderate and advanced PD, as motor and nonmotor symptoms worsen, quality of life (QoL) follows linearly along with worsening caregiver strain, both of which contribute to roughly 10–20,000 people becoming homebound each year in the US.3–5 Once homebound, this population loses access to expert care, leading to a surge in acute healthcare utilization, institutionalization, and excess morbidity.6,7 Simultaneously, this population is underrepresented in PD research (figure 1). In other complex elderly populations, modern adaptations of house calls have improved quality of care and limited functional decline.8,9 Most successful models have been interdisciplinary; however, PD implementations have been limited.10,11
Figure 1. Unmet need for care and research in the advanced, homebound PD population.
PD = Parkinson disease.
In 2014, we launched the Edmond J. Safra Interdisciplinary Home Visit Program for Advanced PD (HVP) to provide comprehensive care and outreach to homebound individuals with PD and related disorders. The HVP has been described elsewhere in detail.12 Here, we report on a prospective one-year cohort study of a subset of HVP participants with PD. Our objectives were to describe: retention in and satisfaction with the HVP over one year; disease progression of advanced, homebound individuals over time; and whether the HVP can stabilize QoL despite expected functional decline. For this prospective study, individuals with significant cognitive impairment were excluded due to limitations with self-report assessments.
Methods
Standard protocol approvals, registrations, and patient consents
This study was approved by the New York University School of Medicine Institutional Review Board. Written informed consent was obtained from all participants.
Study participants and recruitment
Patients were recruited from the Marlene and Paolo Fresco Institute for Parkinson's and Movement Disorders at New York University. In addition to chart review to identify patients, movement disorders neurologists directly referred potential participants who were at least 21 years old, primarily English-speaking, diagnosed with idiopathic PD,13 and had missed >1 recent appointment or had been hospitalized or temporarily institutionalized in the preceding year. Using Medicare homebound criteria—leaving the home “required a considerable and taxing effort”, and was either contraindicated or required the use of assistive devices, assistive transportation, or the aide of another person14—we asked referring providers for their assessment of the individual's homebound status at the time of referral.
To identify individuals most at risk of hospitalization or institutionalization, we required patients to have ≥1 of the following criteria, as judged by their neurologist: motor fluctuations, multimorbidity, medication mismanagement, mild cognitive impairment, depression, anxiety, recent increased falls, or suspected elder abuse, neglect, or caregiver burnout. Patients with possible or probable atypical parkinsonism were excluded. Additionally, we excluded individuals scoring ≤20 on the Folstein Mini-Mental State Examination (MMSE) at screening to avoid impaired capacity to consent in the absence of a caregiver,15 and inability to complete study assessments. Cognitive impairment did not preclude HVP participation,12 only prospective study participation. The catchment area included all 5 boroughs of New York City.
Once potential patients were referred or identified through chart review, the team contacted the patient or caregiver. If eligibility criteria were confirmed and the individual amenable, we scheduled the initial visit and sent the Unified Parkinson's Disease Rating Scale parts I and II to be completed prior to the visit.16
Study visits
The team—comprised of a movement disorders neurologist, nurse, and social worker—traveled to the patient's home approximately every 4 months for a total of 4 visits over 1 year, becoming the patient's default care providers during that time and enacting clinical changes. Given its pilot nature, HVP funding supported the nurse and social worker's time and participants were not billed. The neurologist's time was supported by the institution. As the visit protocol is described elsewhere in detail,12 we will briefly summarize the visits here. The nurse gathered orthostatic vital signs, given the prevalence of orthostatic hypotension and then identified fall risks and safety hazards throughout the home.17 The nurse reviewed the UPDRS I and II to identify impairments in activities of daily living. Next, she conducted a real-time medication reconciliation, comparing prescription and over-the-counter medications to those listed in the most recent outpatient encounter, and identifying signs of nonadherence.
The social worker reviewed the patient's living situation and healthcare utilization, and conducted a psychosocial needs assessment of the patient and caregiver, if applicable. The neurologist posed UPDRS follow-up questions and inquired about additional advanced PD concerns (e.g., constipation, unintentional weight loss), medication efficacy, adverse effects, and adherence, before examining the patient. The social worker and neurologist initiated a conversation regarding the patient's goals of care and advance directives. The team formulated a comprehensive assessment and plan, including medication, dietary, and home safety recommendations and referrals. These were reviewed, documented on a health literacy-friendly after-visit template, and confirmed with teach-back. Following each visit, we sent a comprehensive note to all of the patient's healthcare providers. Two weeks postvisit, we called to review the progress and identify the additional concerns. Patients and caregivers were provided with contact information for the HVP team for interim needs and questions.
Study assessments
We evaluated feasibility based on retention in the HVP, defined by the number of eligible patients enrolled and completing 4 visits. We assessed patient and caregiver satisfaction at visit 4 using the Client Satisfaction Inventory-Short Form (CSI-SF), scored 0–100, where 100 is perfect.18
To evaluate disease progression, we documented the UPDRS subjective and objective scores and Hoehn and Yahr (HY) stage at each visit.19 At visits 1 and 4, we administered 8 QoL domains from the Neuro-QoL item banks deemed to be of particular importance in advanced PD, namely Stigma, Fatigue, Depression, Anxiety, Emotional and Behavioral Dyscontrol, Positive Affect and Well-being, Ability to Participate in Social Roles and Activities, and Satisfaction with Social Roles and Activities.20 Compared to PD-specific assessments in which a ceiling effect was anticipated, Neuro-QoL has been validated for use in PD populations, demonstrates responsiveness to change, and includes more questions and a broader scoring range pertinent to advanced PD.21 We did not include motor-focused domains due to anticipated ceiling effects. Each queried domain is reported as a T score with a mean of 50 and standard deviation of 10. Lower scores indicate less of the quantity measured.
As exploratory outcomes, we measured caregiver burden using the Multidimensional Caregiver Strain Index (MCSI) at visits 1 and 4.22 The MCSI is scored from 0 to 72; 20–29 indicates moderate strain and ≥30 indicates severe strain. We evaluated cognitive change with MMSE at visits 1 and 4, and tracked acute healthcare utilization as the combined number of emergency room and hospital visits in the 12 months prior to vs 12 months enrolled in the HVP, as measured by patient and caregiver report at each of the 4 home visits, combined with review of the institutional electronic medical record.
Data collection and statistical analysis
We entered data into a HIPAA-secure electronic database, with data exported to STATA 14 for analysis.23,24 Demographic characteristics were analyzed for normality and reported as mean and standard deviation, or median and interquartile range, as appropriate. We calculated the changes in UPDRS motor and total scores, HY, Neuro-QoL domains, MMSE, and MCSI between visits 1 and 4 using paired t tests for normally distributed data and Wilcoxon signed-rank test for nonparametrically distributed data. We analyzed the change in utilization rate using generalized estimating equation modeling with a Poisson distribution for count data. For all analyses, we used a two-tailed α of 0.05.
Data availability
Anonymized data not published herein will be shared with qualified investigators pending request and approval by appropriate institutional review boards.
Results
Enrollment
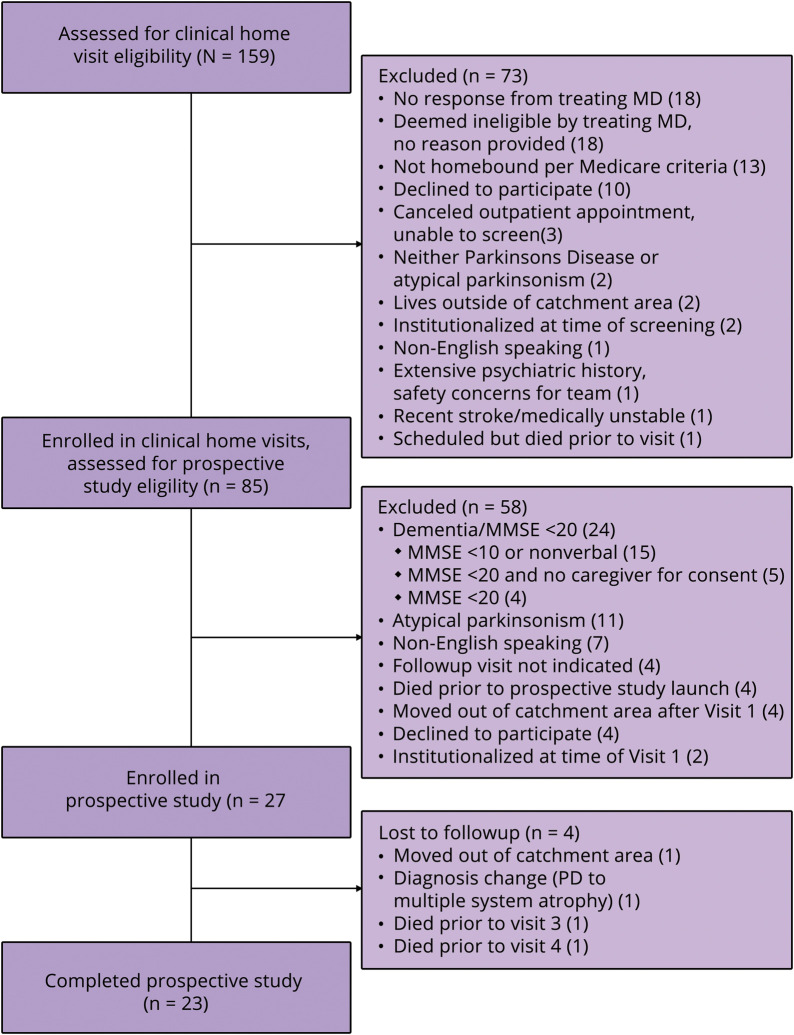
We assessed 159 individuals for home visit eligibility; 65 were directly referred by their neurologist and 94 identified via chart review with subsequent neurologist review. Figure 2 shows participant flow. Among 73 people excluded, the main reasons were: no response from the neurologist to the screening query (n = 18); deemed ineligible by neurologist without reason provided (n = 18); not homebound per Medicare criteria (n = 13); and patient declined participation (n = 10).
Figure 2. Study flow of participants.
MCSI = Multidimensional Caregiver Strain Index; PD = Parkinson disease.
Among the 85 individuals in the HVP, 58 were excluded from study participation. Eleven had atypical parkinsonism, 7 were non-English speakers, and 4 patients each: declined participation, planned to move following Visit 1, had no remaining needs after visit 1, or died prior to the prospective study launch. We excluded 24 individuals due to dementia. Although an MMSE ≤20 had been the a priori cutoff, 15 of the 24 had either an MMSE <10 or were nonverbal, and 5 had an MMSE below the cutoff, lacked capacity, and lacked a caregiver or proxy to consent. Four individuals were excluded solely for MMSE ≤20.
Retention and satisfaction
We enrolled 27 patients and 23 completed all 4 visits (85% retention). All 23 opted to remain in the HVP post-study, and 18 completed the CSI-SF after visit 4 (78% response rate). The median satisfaction scores among patients and caregivers were 97 (interquartile range [IQR] 90–100) and 98 (n = 11, IQR 94–100), respectively.
Patient characteristics at baseline
Table 1 demonstrates the characteristics of patients at visit 1, with a mean age of 80.9 years (SD 7.8) and mean PD duration of 10.5 years (SD 5.9). Over 44% were HY 4 (severe disease but able to stand unassisted) and 11.1% were HY 5 (wheelchair- or bedbound). The mean UPDRS motor score was 37.5 (SD 14.0), mean UPDRS total was 65.0 (SD 20.0), and mean MMSE was 26.8 (SD 2.5). Strikingly, 41% lived alone.
Table 1.
Baseline characteristics of home visit cohort
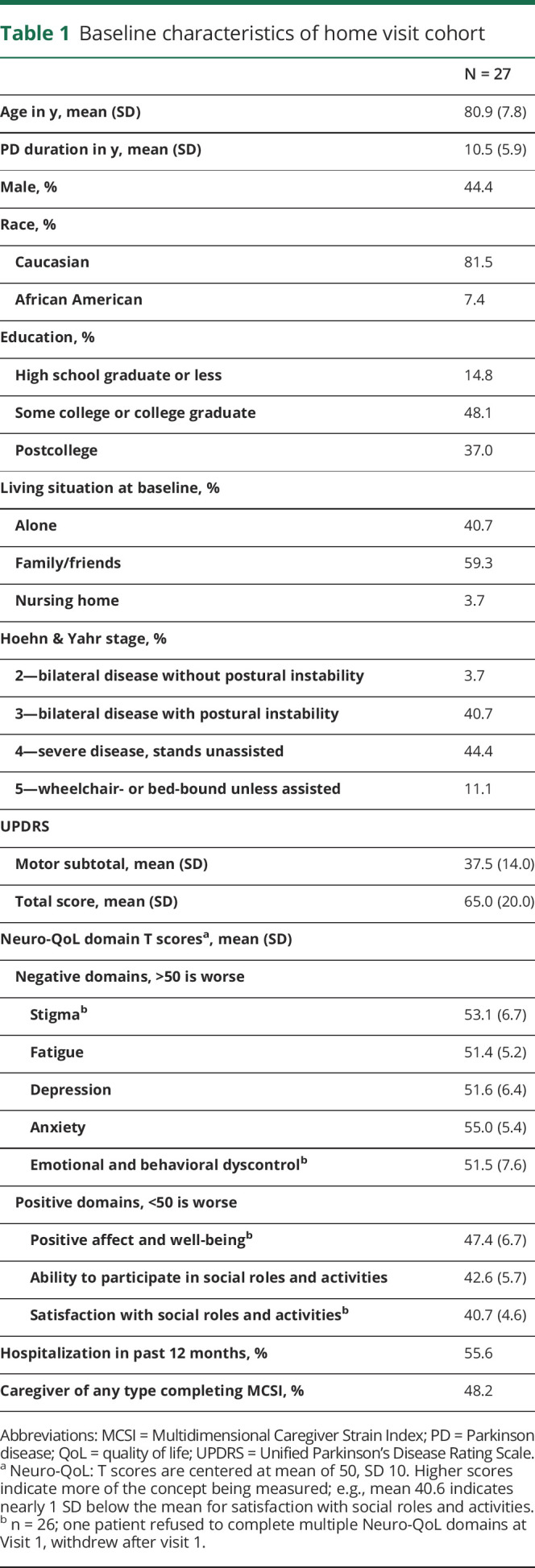
In all 8 domains of the Neuro-QoL, the cohort's mean was within 1 SD, or 10 points, of the standardized mean of 50. However, each domain was skewed toward poorer subjective performance (i.e., mean of 40.7 for satisfaction with social roles and activities, indicating nearly 1 SD below validation cohorts). Among 13 caregivers present for visit 1, the mean MCSI was 22.8 out of 72 (SD 16.5), indicating moderate strain.
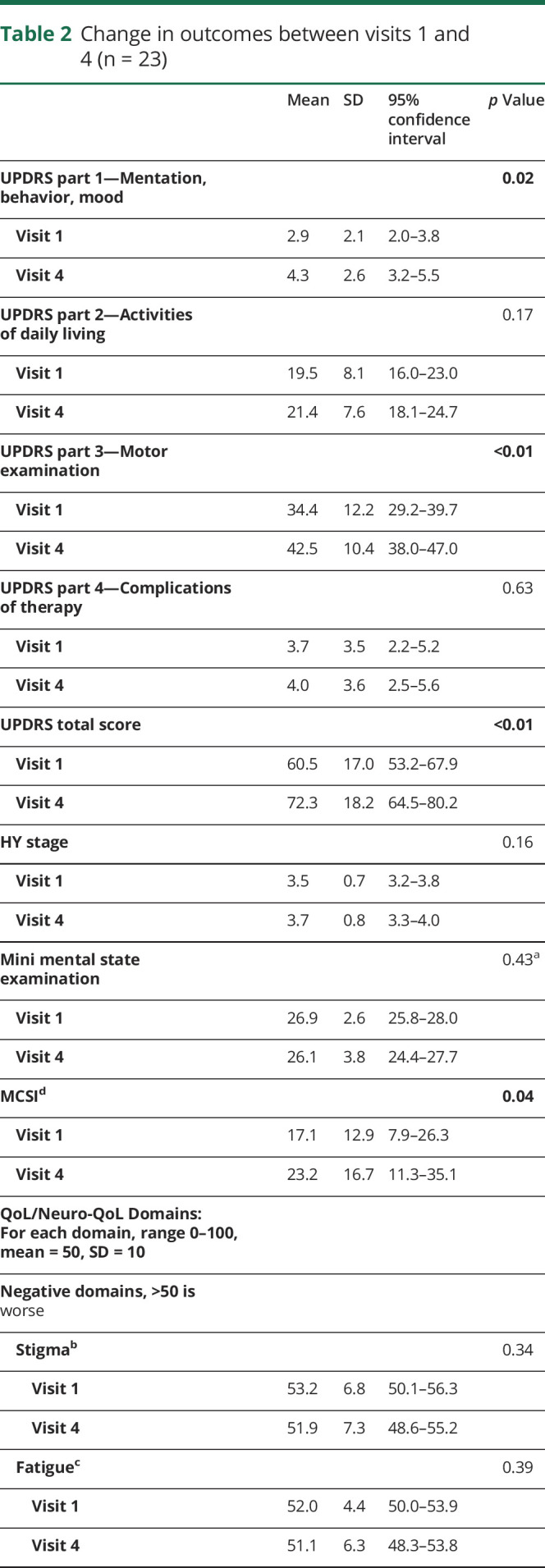
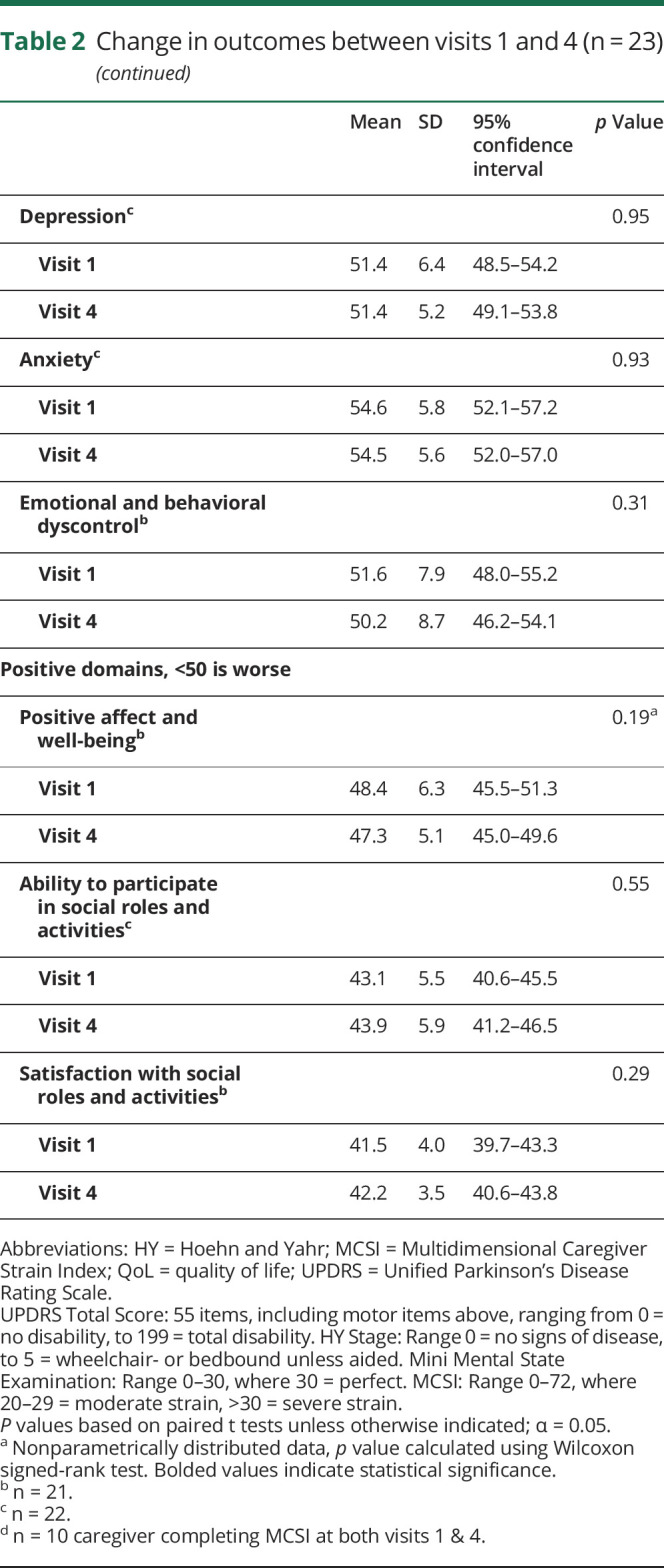
Change in outcomes over 1 year
As shown in table 2, mean UPDRS mentation score worsened from 2.9 to 4.3 (p = 0.02), motor score from 34.4 to 42.5 (p < 0.001), and mean UPDRS total from 60.5 to 72.3 (p < 0.001). Despite disease progression, we found no significant changes in any QoL domains studied (p = 0.19–0.95).
Table 2.
Change in outcomes between visits 1 and 4 (n = 23)
Caregiver strain
Among the 10 caregivers completing the MCSI at visits 1 and 4, strain significantly increased from mild to moderate (17.1 vs 23.2, p = 0.04). Furthermore, among the 3 caregivers who withdrew from the study and as such, did not complete a visit 4 MCSI (2 due to death, one due to relocation), the median visit 1 MCSI was 42 (range 29–55), indicating that they were under severe strain, significantly skewing baseline results.
Healthcare utilization
Over 55% of patients had been hospitalized in the 12 months prior to enrolling in the HVP. Despite disease progression and isolation, healthcare utilization remained the same (p = 0.15) and none of the patients were institutionalized during the study.
Discussion
We identified a unique cohort typically lost to clinical care and research—homebound individuals with advanced PD—and followed them via interdisciplinary home visits. To our knowledge, this is one of the oldest and most disabled PD populations prospectively studied, with a mean age of nearly 81 years and UPDRS total of 65 at study entry. Home visits were met with high satisfaction and retention. The scores on NeuroQoL, however, indicate poor baseline QoL across all domains, but with disability progression over 1 year, denoted by clinically important differences on the UPDRS,25 QoL did not significantly decline.
Our findings are noteworthy for 2 reasons: first, the progression in such an advanced PD population has not previously been reported, as these patients are typically lost to follow-up. A recent, cross-sectional study of 76 German patients with advanced PD (mean HY 4.0, SD 0.7, range 3–5) using the newer Movement Disorder Society (MDS)-UPDRS scale reported a mean MDS-UPDRS part 2 score of 31.4, and part 3 motor score of 60.8 in their population.3 Converting our patients' UPDRS to MDS-UPDRS scores based on published formulas to facilitate comparison,26 our population's mean MDS-UPDRS part 2 score increased from 24 to 25 across visits, and MDS-UPDRS part 3 score increased from 43 to 53, suggesting our populations were roughly comparable. In that population, baseline QoL was extremely poor; however, no longitudinal data were provided. Second, the observed decline in function would be predicted to accompany a proportionate decline in QoL on various PD-specific and general QoL assessments, as the latter is closely and linearly correlated with impairments in activities of daily living and mobility, as measured by the UPDRS and MDS-UPDRS parts 2 and 3.3,4,27 In an observational, longitudinal study of a heterogeneous population including advanced PD and atypical parkinsonism, Higginson et al.28 demonstrated attrition of 36% and significant worsening of QoL and symptom burden over 1 year. However, differences in QoL assessments and the lack of UPDRS or MDS-UPDRS scores in that noninterventional study limit direct comparison with our population. Whether or not the decline the UK investigators and our team observed is the natural course of the disease or has been altered by observation—in the former case—or intervention—in the latter—requires future controlled studies. If either are true, our results may be biased toward the null, and progression in patients truly estranged from care may be worse.
Prior home visit studies in PD have varied in implementation, population, and outcomes. A 6-month intervention in the UK involving 2 home visits from a nurse practitioner yielded referral provisions without change in function.29 Three additional nurse- or physician-only interventions were limited by minimal description of the study population, intervention, or outcomes.11,30,31 Through the HVP described here, we demonstrate the feasibility of recruiting, describing, and delivering multifaceted expertise to this underrepresented population. Furthermore, the stabilization of QoL in contrast to functional decline highlights a possible window of opportunity for impactful care despite advanced disease.
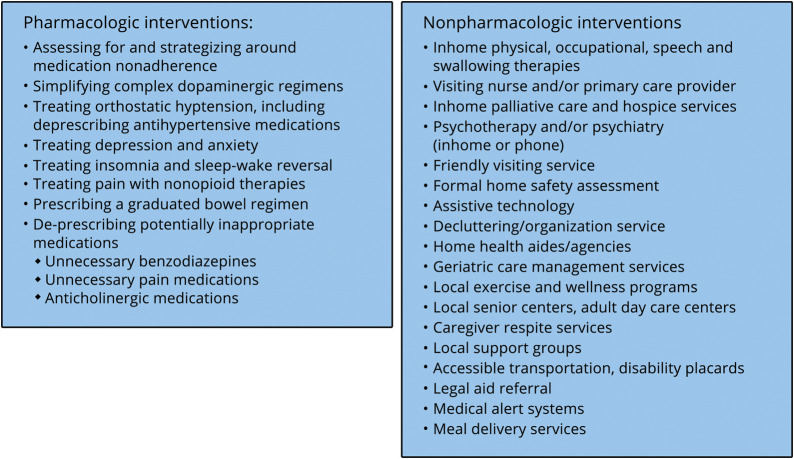
In addition to the proven benefits of neurologic and interdisciplinary care,10,32 we hypothesize that stabilization of QoL may be due to several features of this model. First, in-person, comprehensive medication reconciliation allowed our team to identify contraindications, interactions, and opportunities for improving adherence. For example, numerous patients reported frequent falls or lightheadedness, with objective orthostatic hypotension. Yet many were prescribed multi-antihypertensive regimens, possibly because their homebound status decreased contact with other physicians. Through identification of potentially inappropriate medications, regimens can be simplified, symptoms ameliorated, and adherence improved.33,34 Second, we employed common, practical interventions shown in figure 3, including frequent referrals to in-home therapies and community services, to proactively address issues before crises arose. In our retrospective analysis of the first 272 HVP visits, referrals were made at 92% of visits.12 Third, our team focused on patient-centered, health literacy-friendly35 counseling and resources. Despite progressive disability, the practical, palliative interventions may have buoyed patients' abilities to participate in and derive satisfaction from their social roles and activities, and likely contributed to satisfaction, retention, and QoL.
Figure 3. Common interventions used in the home visit program.
As the first iteration of this model, we recognize many limitations. This was intended as a first-pass, proof-of-concept model, with numerous resources and all team members attending each visit. We presupposed that if this population proved reachable by and amenable to such visits, and if the model demonstrated potential benefit, subsequent iterations would be honed for efficiency and cost-effectiveness. Second, this pilot study was small, lacked a control group, and was underpowered to detect significant changes in utilization and caregiver strain. A recent PD mortality study of over 17,000 patients used a 6 months timeframe, during which significant differences in mortality, utilization, and caregiver strain were detected.36 Yet their survival curves support our concern that follow-up beyond 1 year may be biased by attrition and comorbidities, substantiating our 12-month duration. While the NeuroQoL queries multiple domains and has been used in PD and other advanced movement disorders,37 it is both newer and not PD-specific, substantial drawbacks that limit comparisons with other interventions.
Whereas it might be assumed that community-dwelling individuals with PD have either minimal cognitive impairment or a caregiver, this was not always the case. By excluding individuals with significant cognitive impairment from this prospective study, we missed some of the most vulnerable, advanced patients otherwise enrolled in our HVP, limiting generalizability. We justified this trade-off as we sought to ensure that all participants had the capacity to consent, particularly those without proxy decision makers (41%). Furthermore, of 85 individuals assessed for study eligibility, only 5% were excluded due to MMSE ≤20 who might otherwise have been included.
The geographic location of New York City limits the generalizability of our findings given regional differences in the availability of movement disorders specialists, in-home therapies, services, and transportation. Finally, the absence of time and billing pressure cannot be discounted, and we lack detailed cost data on healthcare utilization and HVP team effort in this pilot. We are addressing all of the above with the next iteration of this model, with matched controls, validated and PD-specific patient- and caregiver-reported outcomes, and detailed utilization and team effort data, in a new cohort outside of New York without cognitive exclusion criteria. These iterations include strategically deployed telemedicine allowing the neurologist to join remotely. Additionally, we are piloting interventions to mitigate caregiver strain.
Despite the limitations, our results illuminate the feasibility of reaching these individuals, their satisfaction with the HVP, and the severity of symptoms, QoL, and level of unmet need of homebound patients with advanced PD. By providing comprehensive expert care, we may intervene on the mean 12-point annual functional decline and stabilize the decline in QoL that otherwise have been a foregone conclusion. Current and future studies are necessary to create an efficient, patient-centered model primed for broader implementation that can improve access to and quality of care for this population. Consequently, such a model may facilitate research beyond the outpatient setting, allowing for enhanced retention and better representation of the PD spectrum. The onus is on healthcare and research teams to acknowledge, account for, and actively seek out this population.
Acknowledgment
This work and the broader Home Visit Program were made possible by generous funding from the Edmond J. Safra Philanthropic Foundation. The authors would like to acknowledge the contributions of the movement disorders fellows participating in the home visit team over several years, including Britt Stone, MD, Arash Fazl, MD PhD, and Mia Ko, DO. The authors would like to acknowledge Amy Lemen, MA LCSW, and Geraldine Dacpano, MPH, for their work on the home visit program. Finally, the authors extend their gratitude to the patients and caregivers who welcomed this program and team into their lives and homes.
Appendix. Authors
Study funding
This work was supported by the Edmond J. Safra Philanthropic Foundation; the Parkinson Foundation; Parkinson Alliance; the Doris Duke Charitable Foundation Fund to Retain Clinical Scientists; the Feldstein Medical Foundation; and the National Institutes of Health (2L30NS084235-02, 2L30NS084235-03, 2L30NS084235-04; 1K23NS097615). None of the funding sources had involvement in the collection, analysis, or interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Disclosure
J.E. Fleisher has received research support from the NIH/NINDS (K23NS097615, L30SN084235, U01NS100610), Feldstein Medical Foundation, Doris Duke Charitable Foundation Fund to Retain Clinical Scientists, CurePSP, private philanthropy, and Biogen. She has received honoraria from the Parkinson Foundation and Michael J. Fox Foundation, and has received royalties from UpToDate. M.M. Sweeney reports no disclosures. S. Oyler reports no disclosures. T. Meisel reports no disclosures. N. Friede reports no disclosures. A. Di Rocco has received research support from the Parkinson Alliance, Parkinson Foundation, and Edmond J. Safra Foundation. J. Chodosh has received research support from the National Institutes of Health, New York State Department of Health, and Independence at Home, Long Beach, CA. J. Chodosh has received honoraria from Gerontological Society of America and he serves on the advisory board of Aging in New York Fund. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
TAKE-HOME POINTS
→ Individuals with advanced PD are at risk of becoming homebound and losing access to neurologic care.
→ Retention, patient satisfaction, and caregiver satisfaction were high in a pilot interdisciplinary HVP for people with advanced PD.
→ In homebound patients with advanced PD, QoL did not parallel the downward trajectory of disability over 1 year.
References
- 1.Hely MA, Reid WG, Adena MA, Halliday GM, Morris JG. The Sydney multicenter study of Parkinson's disease: the inevitability of dementia at 20 years. Mov Disord 2008;23:837–844. [DOI] [PubMed] [Google Scholar]
- 2.Miyasaki JM, Long J, Mancini D, et al. Palliative care for advanced Parkinson disease: an interdisciplinary clinic and new scale, the ESAS-PD. Parkinsonism Relat Disord 2012;18(suppl 3):S6–S9. [DOI] [PubMed] [Google Scholar]
- 3.Klietz M, Tulke A, Müschen LH, et al. Impaired quality of life and need for palliative care in a German cohort of advanced Parkinson's disease patients. Front Neurol 2018;9:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martinez-Martin P, Rodriguez-Blazquez C, Forjaz MJ, et al. Relationship between the MDS-UPDRS domains and the health-related quality of life of Parkinson's disease patients. Eur J Neurol 2014;21:519–524. [DOI] [PubMed] [Google Scholar]
- 5.Oguh O, Kwasny M, Carter J, Stell B, Simuni T. Caregiver strain in Parkinson's disease: National Parkinson Foundation Quality Initiative study. Park Relat Disord 2013;19:975–979. [DOI] [PubMed] [Google Scholar]
- 6.Kaltenboeck A, Johnson SJ, Davis MR, et al. Direct costs and survival of medicare beneficiaries with early and advanced Parkinson's disease. Park Relat Disord 2012;18:321–326. [DOI] [PubMed] [Google Scholar]
- 7.Hassan A, Wu SS, Schmidt P, et al. High rates and the risk factors for emergency room visits and hospitalization in Parkinson's disease. Parkinsonism Relat Disord 2013;19:949–954. [DOI] [PubMed] [Google Scholar]
- 8.Edes T, Kinosian B, Vuckovic NH, Nichols LO, Becker MM, Hossain M. Better access, quality, and cost for clinically complex veterans with home-based primary care. J Am Geriatr Soc 2014;62:1954–1961. [DOI] [PubMed] [Google Scholar]
- 9.Kono A, Izumi K, Yoshiyuki N, Kanaya Y, Rubenstein LZ. Effects of an updated preventive home visit program based on a systematic structured assessment of care needs for ambulatory frail older adults in Japan: a randomized controlled trial. J Gerontol A Biol Sci Med Sci 2016;71:1631–1637. [DOI] [PubMed] [Google Scholar]
- 10.Boult C, Green AF, Boult LB, Pacala JT, Snyder C, Leff B. Successful models of comprehensive care for older adults with chronic conditions: evidence for the Institute of Medicine's “retooling for an aging America” report. J Am Geriatr Soc 2009;57:2328–2337. [DOI] [PubMed] [Google Scholar]
- 11.Vickers LF, O'Neill CM. An interdisciplinary home healthcare program for patients with Parkinson's disease. Rehabil Nurs 1998;23:286–289, 299. [DOI] [PubMed] [Google Scholar]
- 12.Fleisher J, Barbosa W, Sweeney MM, et al. Interdisciplinary home visits for individuals with advanced Parkinson's disease and related disorders. J Am Geriatr Soc 2018, 66:1226–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 1992;55:181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pub 100-02 medicare benefit policy: clarification of the confined to the home definition in chapter 15, covered medical and other health services, of the medicare benefit policy manual. 2014. Available at: cms.gov/Regulations-and-Guidance/Guidance/Transmittals/downloads/R192BP.pdf. Accessed August 15, 2015.
- 15.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 16.Fahn S, Elton RJ, Committee UD. The unified Parkinson's disease rating scale. In: Fahn S, Marsden CD, Calne DB, Goldstein M, editors. Recent Developments in Parkinson's Disease. vol 2 Florham Park, NJ: Macmillan Healthcare Information; 1987:153–163; 293-304. [Google Scholar]
- 17.Francois C, Biaggioni I, Shibao C, et al. Fall-related healthcare use and costs in neurogenic orthostatic hypotension with Parkinson's disease. J Med Econ 2017;20:525-532. [DOI] [PubMed] [Google Scholar]
- 18.McMurtry SL, Hudson WW. The client satisfaction inventory: results of an initial validation study. Res Soc Work Pract 2000;10:644–663. [Google Scholar]
- 19.Goetz CG, Poewe W, Rascol O, et al. Movement disorder society task force report on the Hoehn and Yahr staging scale: status and recommendations. Mov Disord 2004;19:1020–1028. [DOI] [PubMed] [Google Scholar]
- 20.Cella D, Lai J-S, Nowinski CJ, et al. Neuro-QOL: brief measures of health-related quality of life for clinical research in neurology. Neurology 2012;78:1860–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nowinski CJ, Siderowf A, Simuni T, Wortman C, Moy C, Cella D. Neuro-QoL health-related quality of life measurement system: validation in Parkinson's disease. Mov Disord 2016;31:725–733. [DOI] [PubMed] [Google Scholar]
- 22.Stull DE. The multidimensional caregiver strain index (MCSI): its measurement and structure. J Clin Geropsychology 1996;2:175–196. [Google Scholar]
- 23.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inf 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.StataCorp. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP; 2015. [Google Scholar]
- 25.Shulman LM, Gruber-Baldini AL, Anderson KE, Fishman PS, Reich SG, Weiner WJ. The clinically important difference on the unified Parkinson's disease rating scale. Arch Neurol 2010;67:64–70. [DOI] [PubMed] [Google Scholar]
- 26.Goetz CG, Stebbins GT, Tilley BC. Calibration of unified Parkinson's disease rating scale scores to Movement Disorder Society-unified Parkinson's disease rating scale scores. Mov Disord 2012;27:1239–1242. [DOI] [PubMed] [Google Scholar]
- 27.Rahman S, Griffin HJ, Quinn NP, Jahanshahi M. Quality of life in Parkinson's disease: the relative importance of the symptoms. Mov Disord 2008;23:1428–1434. [DOI] [PubMed] [Google Scholar]
- 28.Higginson IJ, Gao W, Saleem TZ, et al. Symptoms and quality of life in late stage Parkinson syndromes: a longitudinal community study of predictive factors. PLoS One 2012;7:e46327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jahanshahi M, Brown RG, Whitehouse C, Quinn N, Marsden CD. Contact with a nurse practitioner: a short-term evaluation study in Parkinson's disease and dystonia. Behav Neurol 1994;7:189–196. [DOI] [PubMed] [Google Scholar]
- 30.Hurwitz A. Home visiting by nursing students to patients with Parkinson's disease. J Neurosci Nurs 1986;18:344–348. [DOI] [PubMed] [Google Scholar]
- 31.Hack N, Akbar U, Monari EH, et al. Person-centered care in the home setting for Parkinson's disease: operation house call quality of care pilot study. Parkinsons Dis 2015;2015:639494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Willis AW, Schootman M, Evanoff BA, Perlmutter JS, Racette BA. Neurologist care in Parkinson disease: a utilization, outcomes, and survival study. Neurology 2011;77:851–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lavan AH, Gallagher P, Parsons C, O'Mahony D. STOPPFrail (Screening Tool of Older Persons Prescriptions in Frail adults with limited life expectancy): consensus validation. Age Ageing 2017;46:600–607. [DOI] [PubMed] [Google Scholar]
- 34.Guiding principles for the care of older adults with multimorbidity: an approach for clinicians. Guiding principles for the care of older adults with multimorbidity: an approach for clinicians: American Geriatrics Society Expert Panel on the Care of Older Adults with Multimorbidity. J Am Geriatr Soc 2012;60:E1–E25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fleisher JE, Shah K, Fitts W, Dahodwala N. Associations and implications of low health literacy in Parkinson's disease. Mov Disord Clin Pract 2016;3:250–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hirdes JP, Poss JW, Mitchell L, Korngut L, Heckman G. Use of the interRAI CHESS scale to predict mortality among persons with neurological conditions in three care settings. PLoS One 2014;9:e99066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carlozzi NE, Victorson D, Sung V, et al. Hd-PRO-TRIADTM validation: a patient-reported instrument for the symptom triad of Huntington's disease. Tremor Hyperkinetic Mov 2014;4:223. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data not published herein will be shared with qualified investigators pending request and approval by appropriate institutional review boards.