Abstract
Introduction:
The maintenance of a stem cell pool is imperative to enable healing processes in the dental pulp tissue throughout life. As such, knowing mechanisms underlying stem cell self-renewal is critical to understand pulp pathophysiology and pulp regeneration. The purpose of this study was to evaluate the impact of stem cell factor (SCF) signaling through its receptor tyrosine kinase (c-Kit) on the self-renewal of human dental pulp stem cells (hDPSC).
Methods:
hDPSC were stably transduced with lentiviral vectors expressing shRNA-c-Kit or vector control. The impact of the SCF/c-Kit axis on hDPSC self-renewal was evaluated using a pulpsphere assay in low attachment conditions and by evaluating the expression of polycomb complex protein Bmi-1 (master regulator of self-renewal) by western blot and flow cytometry.
Results:
c-Kit-silenced hDPSC formed less pulpspheres when compared to hDPSC transduced with control vector (p<0.05). Evaluation of pulpsphere morphology revealed the presence of 3 distinct sphere types, i.e. holospheres, merospheres and paraspheres. While c-Kit silencing decreased the number of holospheres compared to control cells (p<0.05), it had no effect on the number of merospheres and paraspheres. Recombinant human stem cell factor (rhSCF) increased the number of holospheres (p<0.05) and induced dose-dependent Bmi-1 expression in hDPSC. As expected, the inductive capacity of rhSCF on Bmi-1 expression and fraction of Bmi-1-positive cells was inhibited when we silenced c-Kit in hDPSC.
Conclusion:
These results unveiled the role of SCF/c-Kit signaling on the self-renewal of hDPSC and suggested that this pathway enables long-term maintenance of stem cell pools in human dental pulps.
Keywords: Perivascular niche, Pulp Biology, Stemness, Regenerative Endodontics, Tissue Regeneration
INTRODUCTION
Stem cells are maintained in specialized niches where they are relatively quiescent until external signals (e.g. tissue wound) disrupt this equilibrium and drive their fate towards differentiation into cells that orchestrate tissue regeneration. The maintenance of a tissue-specific stem cell pool is critical for the function of human organs and most human tissues (except for enamel). Notably, it requires symmetric cell division through the process of self-renewal (1). However, very little is known about the process of stem cell self-renewal in the dental pulp tissue. We have postulated that understanding the biology of stem cell niches and maintenance of stem cell pools via self-renewal will provide insights into mechanisms that maintain dental pulp tissue homeostasis as well as provide information that can be exploited in Regenerative Endodontics (2). Here, we performed studies that evaluated the impact of SCF signaling through its c-Kit (3) on the self-renewal of hDPSC.
The long-term maintenance and ability of tissue self-repair requires the function of stem cell niches. Stem cells can be activated to replace terminally differentiated cells and to regulate healing responses (3–5). We have observed that stem cells are located around blood vessels in perivascular niches in human dental pulps (6). It is known that a key function of the perivascular niche is to maintain the survival and self-renewal of stem cells in physiological conditions (7) and in diseases such as cancer (8). As such, understanding the molecular crosstalk between the stem cells and the other cells from the perivascular niche (e.g. endothelial cells, fibroblasts) is critical to understand the pathobiology of tissue homeostasis and tissue response to injury.
It has been long described that the ability of growing spheres under ultra-low attachment and low-serum conditions is a common feature of physiological and pathological stem cells (9). Under these conditions, each sphere is derived from the clonal expansion of one stem cell while non-stem cells typically undergo apoptosis. Therefore, an increase in the number of spheres upon dissociation and in vitro passaging to a new ultra-low attachment plates is representative of the ability of these cells to self-renew, i.e. generate daughter stem cells upon division (9,10). We have recently observed that hDPSC cells form spheres and express higher levels of Bmi-1, an inducer of stem cell self-renewal (11,12), when cultured in ultra-low attachment plates when compared to hDPSC cultured in standard plates.
A seminal paper from the Morrison laboratory defined the role of endothelial cell-secreted factors in the maintenance of the hematopoietic stem cell niche (7). They showed that endothelial cell-secreted SCF signaling through its receptor c-Kit induces self-renewal, migration, survival of stem cells, and is required for maintenance of hematopoietic perivascular niches. Notably, c-Kit was shown to be a marker for a subpopulation of dental pulp progenitor cells (13,14). We know that hDPSC reside in the perivascular niche in close proximity to endothelial cells that can secrete SCF. We also know that hDPSC express c-Kit. However, we do not know the signaling pathway that induces Bmi-1 expression and self-renewal in hDPSC. Here, we hypothesized that SCF signaling through c-Kit induces Bmi-1 and maintains hDPSC self-renewal.
MATERIALS AND METHODS
Cell Culture:
We used hDPSC isolated from permanent teeth (gift from Dr. Songtao Shi), as described previously (15). hDPSC on passages 3–7 were cultured in alpha-MEM (Invitrogen; Carlsbad, CA, USA) supplemented with 15% fetal bovine serum (FBS; Invitrogen) and 1% penicillin/streptomycin (Invitrogen) at 37°C and 5% CO2. The cell culture medium was changed every 2 days in all experiments included here.
Lentiviral-mediated gene silencing:
Gene silencing was performed with lentiviral vectors encoding shRNA constructs. Briefly, 293T cells were transiently co-transfected with lentivirus packaging vector psPAX2, PMD2 and shRNA-C (scrambled vector control) and shRNA-c-kit (Vector Core, University of Michigan) with calcium phosphate. We used supernatants containing the lentiviruses to infect passage 3 hDPSC overnight, and then cells were selected with 1 μg/mL puromycin (InVivogen; San Diego, CA, USA) for at least 1 week. Gene silencing efficiency was determined by western blot.
Pulpsphere assay:
hDPSC cells stably transduced with shRNA-c-Kit or shRNA-C were cultured in 6-well ultra-low attachment plates (Corning; Corning, NY, USA) for 10 days with α-MEM (Invitrogen) supplemented with 0 or 20 ng/ml rhSCF. For sphere passaging, cells were exposed to 0.25% trypsin for 5 minutes and then mechanically dissociated. Trypsin neutralizing solution (Lonza; Basel, Switzerland) was used to neutralize trypsin. Cells were counted, diluted to 1,500 per 3 ml, and then added to new 6-well ultra-low attachment plates. Colonies of 25 cells or more were considered spheres (10). The number of spheres per well was analyzed in the 1st and 2nd passage plates to verify the impact of treatment on hDPSC self-renewal. Data were obtained from triplicates and represent at least 3 independent experiments.
H&E staining for pulpspheres:
After culturing for 3–5 days, spheres were collected in a glass slide using a cytospin tank at 4°C at 1,500 rpm for 10 minutes. Once spheres were attached to the glass slide, hematoxylin and eosin was used to stain the cells, as follows: Spheres were covered with a drop of hematoxylin for 30 seconds, washed with distilled water then stained with eosin for 2 minutes. Slides were washed with tap water twice for 2 minutes then a coverslip was mounted. Images were analyzed using NIS Elements (Nikon; Tokyo, Japan).
Flow cytometry:
hDPSC were trypsinized, harvested and aliquoted at 106 cells/100 μl into FACS tubes. Cells were resuspended in fixation buffer (BD Biosciences; San Jose, CA, USA), incubated for 10 minutes and washed 2 times with PBS. Cells were then permeabilized with 250 μl of cold Perm Buffer III Phosflow (BD Biosciences) on ice for 30 minutes. Cells were resuspended in 100 μl Flow Cytometry Staining Buffer (BD Biosciences) in presence of Alexa Fluor 647 mouse anti-human Bmi-1 (BD Biosciences) or Alexa Fluor 647 mouse IgG1 (R&D Systems). Cells were mixed gently and incubated at 4°C for 30 minutes in the dark prior to flow cytometric analysis.
Western Blot:
Cells were lysed in NP40 buffer, proteins (20–50 μg/lane) were electrophoresed in SDS-polyacrylamide gel and transferred to nitrocellulose membranes (Protran; Whatman, Dassel, Germany). Membranes were incubated at 4°C overnight with primary antibodies, as follows: mouse anti-human SCF, mouse anti-human CD117/c-Kit, mouse anti-human Bmi-1, or mouse anti-human GAPDH (R&D Systems). SuperSignal West Pico Chemiluminescent Substrate (Pierce; Rockford, IL, USA) was used to visualize immunoreactive proteins. Here, we used dental pulp cells (DP) that outgrew from partially digested human pulp tissue specimens; periodontal ligament cells (PDL) cells obtained commercially (HPdLF, Lonza, Walkersville, USA); and Stem cells from Human Exfoliated Deciduous teeth (SHED; gift from Songtao Shi) as control cell types.
RT-PCR:
Total RNA was extracted with TRIzol reagent (Invitrogen) and RT-PCR reactions were performed with Superscripttm III one-step RT-PCR system with Platinum™ Taq DNA polymerase (Thermo Fisher Scientific; Waltham, MA, USA) according to manufacturer’s instructions. RNA was extracted from human dental pulp cells (DP), cells from the periodontal ligament (PDL), human dental pulp stem cells (hDPSC) and stem cells from exfoliated deciduous teeth (SHED). Primers were the following: c-Kit (sense 5’-tcatcgagtgtgatgggaaa-3’ and anti-sense 3’-cacgtttttgatggtgatgc-5’), SCF (sense 5′-ccgctctctttggatctcag-3’ and anti-sense 3′-gtgtggcataagggctcact-5’), BMI-1 (sense 5’-ccagggcttttcaaaaatga-3’ and anti-sense 3’-gcatcacagtcattgctgct-5’), and GAPDH (sense 5’-gaccccttcattgacctcaact-3’ and anti-sense 3’-accaccttcttgatgtcatc-5’).
Statistical analysis:
All in vitro experiments had a sample size of 3 (n=3) and were performed 3 independent times to verify reproducibility of the data. The numeric data obtained from gene expression, RT-PCR and flow cytometry were analyzed by ANOVA (p<0.05), with the Tukey’s test used as post-hoc. Statistical analyses were performed using GraphPad Prism (GraphPad Software; San Diego, CA, USA).
RESULTS
SCF signaling through c-Kit regulates hDPSC self-renewal
To begin to understand the impact of SCF signaling through c-Kit in dental pulp stem cells, we performed western blots (Fig. 1A) and RT-PCR (Fig. 1B) that demonstrated the expression of this ligand-receptor pair in dental pulp cells (DP), periodontal ligament cells (PDL), human dental pulp stem cells (hDPSC), and stem cells from exfoliated teeth (SHED). To study the role of c-Kit in SCF signaling, we generated c-Kit silenced hDPSC upon stable transduction of lentiviral vectors encoding shRNA-c-Kit (Fig. 1C–D). As gene silencing was more effective with shRNA clone “b” (Fig. 1D), this shRNA sequence was used throughout this work. Control cells were transduced with vectors encoding scramble shRNA sequences and used at the same passage as the shRNA-c-Kit transduced cells here, and throughout this project.
Figure 1:
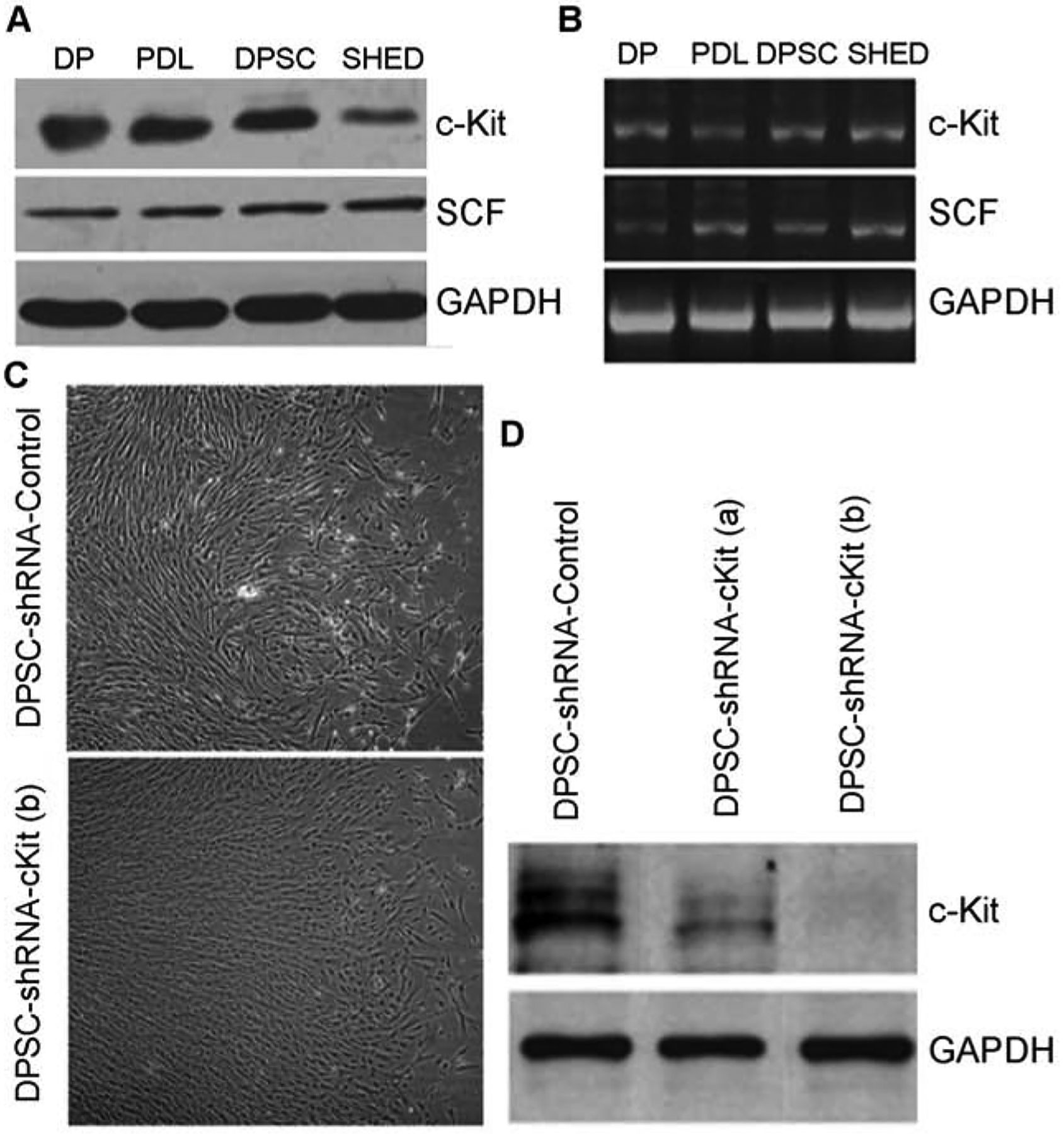
Baseline levels of SCF and c-Kit in DPSC. (A) Western Blot and (B) RT-PCR demonstrating baseline levels of SCF and c-Kit on Dental Pulp cells (DP), Periodontal Ligament Cells (PDL), Dental Pulp Stem Cells (DPSC) and Stem Cells from Exfoliated Deciduous Teeth (SHED). (C-D) Lentiviral mediated silencing of c-Kit in DPSC. (C) Morphology of DPSC-shRNA-c-Kit and DPSC-shRNA-Control cells in standard culture conditions. (D) Gene silencing efficiency determined by Western blot using two different shRNA sequences for c-Kit (a,b).
To evaluate the effect of c-Kit signaling on stemness, we performed the pulpsphere assay where hDPSC were cultured in ultra-low attachment plates (Fig. 2A). We noticed that c-Kit-silenced cells showed reduced sphere-forming capacity at both 3 and 5 days (p<0.05) of culture in low conditions (Fig. 2B). To evaluate the role of this signaling pathway on self-renewal, we trypsinized the 1st passage (primary) pulpspheres and passed them to new plates to generate 2nd passage (secondary) spheres. Remarkably, c-Kit-silenced hDPSC formed a few aggregates but were not able to form pulpspheres (Fig. 2B), demonstrating the major role of c-Kit silencing on the sphere-forming capacity of hDPSC. In parallel experiments, we tested the hypothesis that culture in excess rhSCF rescues the sphere-forming capacity of c-Kit-silenced hDPSC. Quantification of these data demonstrated that even in presence of high levels of rhSCF, c-Kit-silenced hDPSC showed fewer primary spheres and no secondary spheres (p<0.05) when compared to control hDPSC (Fig. 2C).
Figure 2:
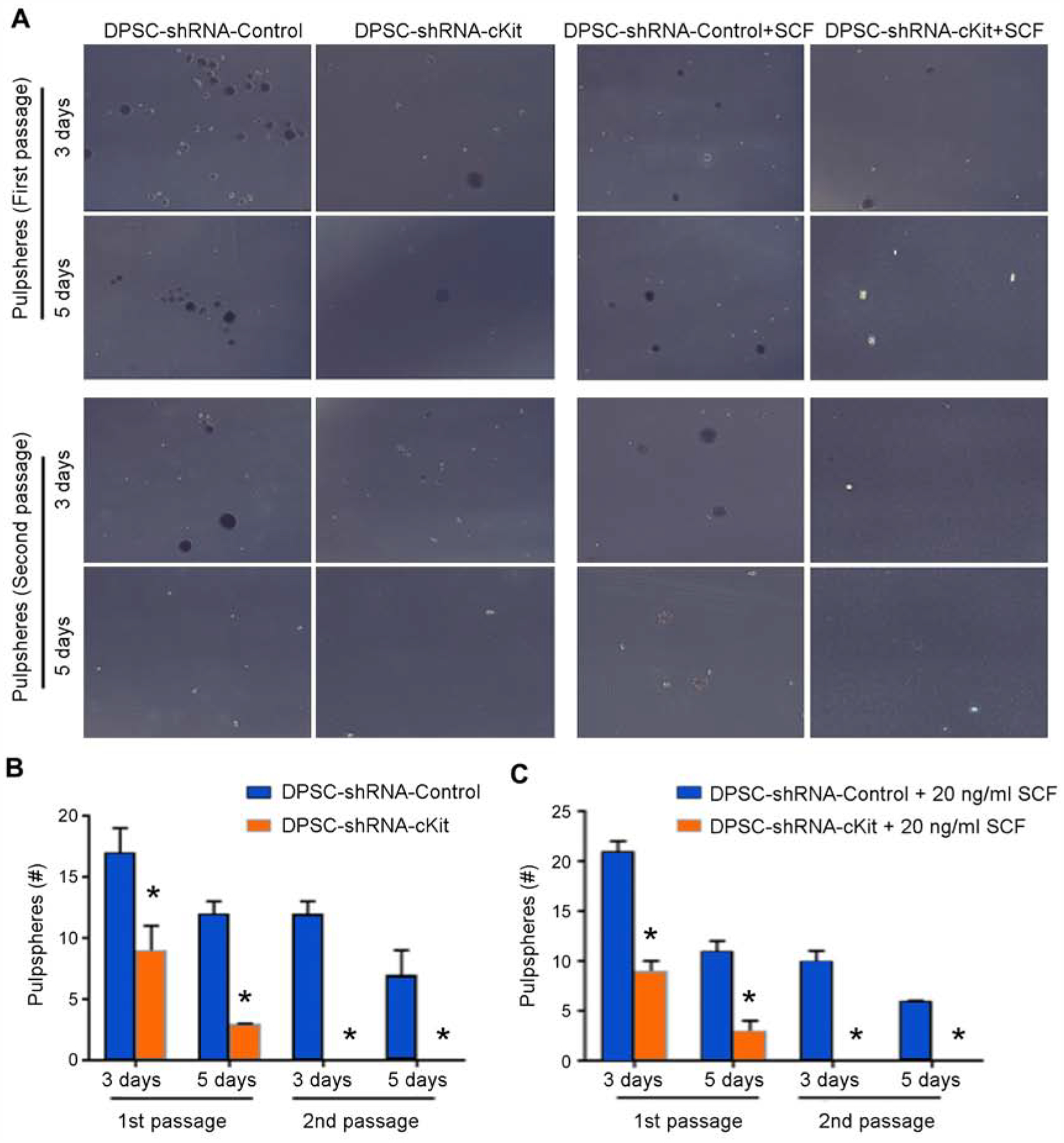
Dental pulpsphere formation assay. (A) Panel showing DPSC-shRNA-c-Kit and DPSC-shRNA-Control cells in the presence or absence of 20 ng/ml rhSCF in ultra-low attachment plates. (B) Graph showing pulpsphere counts of untreated DPSC-shRNA-c-Kit or DPSC-shRNA-Control cells at days 3 and 5 for both, primary and secondary passage spheres. (C) Graph showing pulpsphere counts of DPSC-shRNA-c-Kit and DPSC-shRNA-Control cells exposed to SCF at days 3 and 5 for both, primary and secondary passage spheres. Asterisks depict statistical significance at p<0.05.
SCF induces Bmi-1 expression
To begin to understand the mechanisms underlying the effect of SCF in hDPSC self-renewal, we exposed these cells to increasing concentrations of rhSCF and evaluated expression of Bmi-1 by Western blot. We observed that rhSCF caused a dose-dependent increase in Bmi-1 expression in control hDPSC (Fig. 3A). As expected, we did not observe changes in c-Kit and intracellular SCF in these cells exposed to rhSCF (Fig. 3A). In contrast, the inductive capacity of rhSCF on Bmi-1 expression was largely abrogated in c-Kit-silenced hDPSC (Fig. 3A), further demonstrating the impact of c-Kit signaling on rhSCF-mediated responses. In confirmatory experiments using an alternate method (i.e. flow cytometry), we observed that rhSCF induced a substantial increase (p<0.05) in the fraction of Bmi-1-positive control hDPSC (Fig. 3B). Interestingly, when c-Kit-silenced cells were examined by flow cytometry, we observed that rhSCF did increase the fraction of Bmi-1-positive cells, but not to the same extent as in control hDPSC (Fig. 3B).
Figure 3:
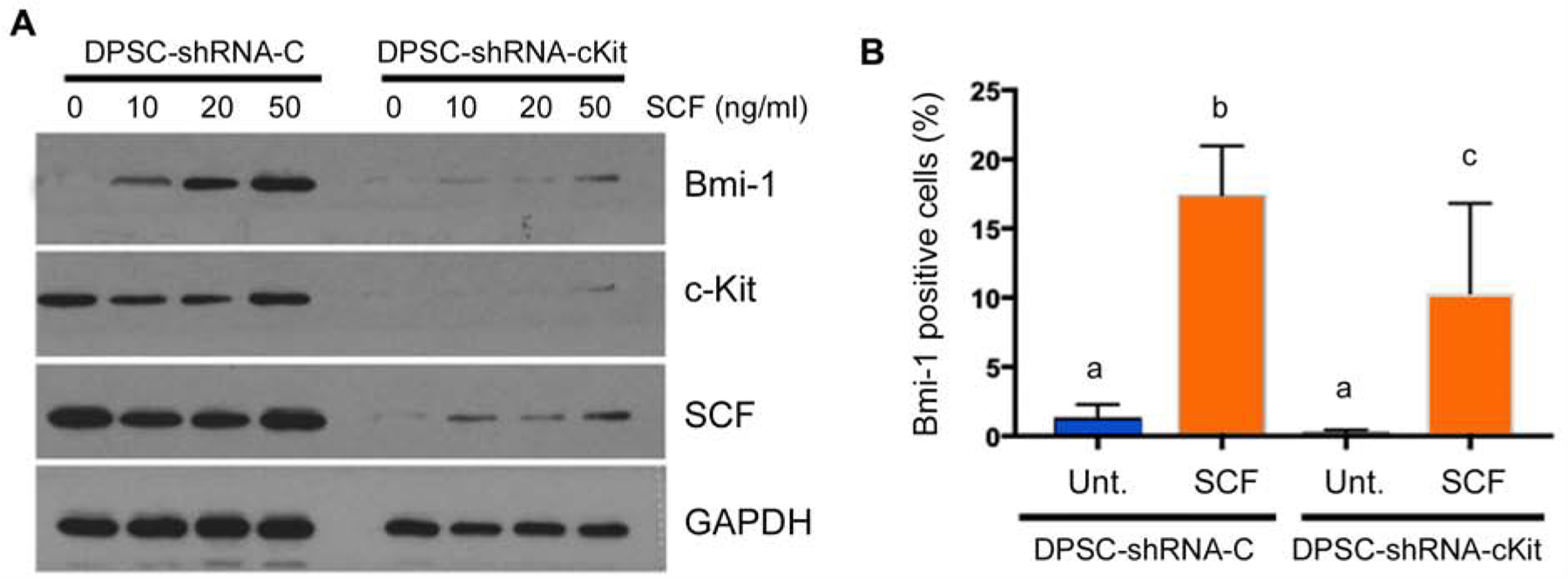
c-Kit silencing inhibits expression of Bmi-1, a master regulator of self-renewal. (A) Western blot showing expression of Bmi-1 in DPSC-shRNA-c-Kit or DPSC-shRNA-Control cells in presence of 0–50 ng/ml rhSCF. (B) Graph depicting flow cytometry data for the percentage of Bmi-1-positive DPSC-shRNA-c-Kit or DPSC-shRNA-Control cells upon treatment with 0 or 20 ng/ml rhSCF. Different low case letters indicate statistical significance at p<0.05.
Pulpsphere classification assay
To further understand the impact of SCF signaling through c-Kit on primary pulpspheres (Fig. 4A), we classified them according to Almeida and collaborators (16). They defined the following stem cell sphere types: A) Holosphere, where there is a more dense mass of cells and they are organized in a very round, uniform manner; B) Merospheres, where the stem cells are less organized and not as dense; and C) Paraspheres, where there is fewer cells and they are not organized in any specific shape (Fig. 4B). We observed that treatment with rhSCF increased the number of holospheres (p<0.05), but not the number of merospheres or paraspheres (p>0.05) (Fig. 4C). Notably, c-Kit silencing mediated a significant decrease in the number of holospheres (p>0.05) but had no inhibitory effect on the number of merospheres or paraspheres (Fig. 4C).
Figure 4:
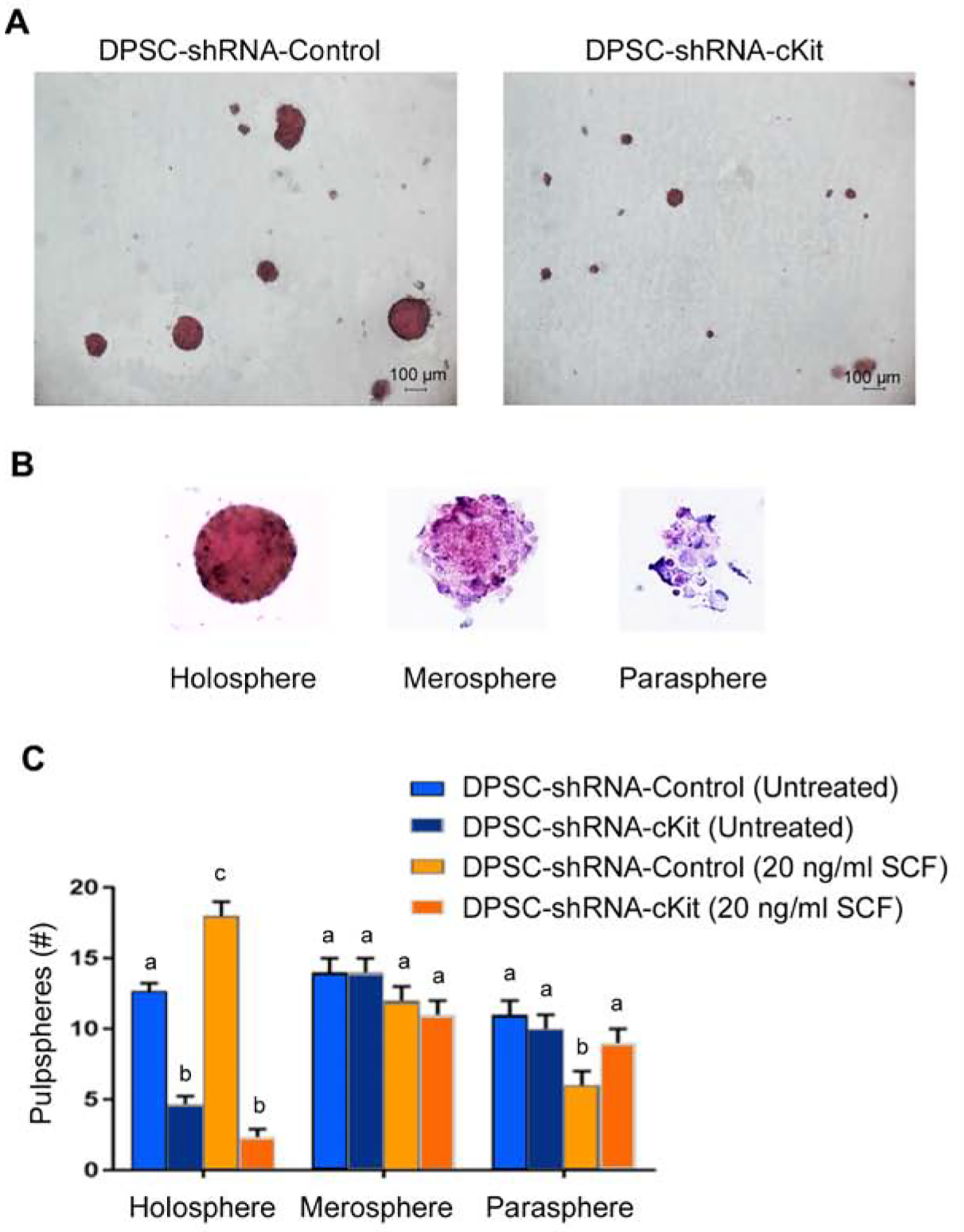
Classification of the dental pulpspheres according to shape/size. (A) Visualization of the pulpspheres generated by in DPSC-shRNA-c-Kit or DPSC-shRNA-Control cells in low attachment conditions. (B) Classification of the spheres as holospheres, merospheres and paraspheres16. (C) Graph depicting the number of holospheres, merospheres or paraspheres generated by DPSC-shRNA-c-Kit or DPSC-shRNA-Control cells treated with 0 or 20 ng/ml rhSCF. Different low case letters indicate statistical significance at p<0.05.
DISCUSSION
The ability of the dental pulp to heal and regenerate upon differentiation of new odontoblast-like cells (17,18) suggested that this tissue contained stem cells. These initial observations were confirmed by the seminal publication from Songtao Shi’s group that demonstrated the existence and function of hDPSC (15). Our group showed that stem cells from the dental pulp differentiate into tubular dentin-making odontoblasts (19,20) and functional blood vessels that anastomize with the host vasculature (19,21,22). These results, together with many other studies from laboratories worldwide, demonstrated the multipotency of dental pulp stem cells. However, little is known about the other major stem cell hallmark, i.e. the capacity of maintaining a stem cell pool through the process of self-renewal. Here, we unveiled the function of SCF signaling through c-Kit in the induction of self-renewal of dental pulp stem cells.
SCF is a powerful chemokine that binds to the c-Kit receptor and induces progenitor cell recruitment and self-renewal in hematopoietic perivascular niches (7). Pan and collaborators showed that SCF stimulation induces migration, angiogenesis and collagen remodeling by dental pulp progenitor cells (23). Here, we observed that dental pulp stem cells of permanent and primary teeth express SCF and c-Kit raising the possibility of a functional role for SCF signaling through c-Kit in the regulation of stem cell self-renewal in dental pulp tissue.
In attempt to mimic the in vivo microenvironment for stem cells, several 3-dimensional (3D) culture systems have been developed. The formation of a 3D sphere, a spherical cluster of cells formed by self-renewal capacity of stem cells under low attachment, is one of the most widely used techniques for in vitro studies of stem cells (24,25). In general, stem cell spheres also have a greater multilineage differentiation capacity compared with their corresponding monolayer cells, suggesting that 3D sphere culture may enable the maintenance of stemness properties (26–28). In our study, DPSC-shRNA-c-Kit cells formed few primary spheres in serum-free, low attachment conditions, and did not generate any secondary spheres upon passaging to new plates. In contrast, vector control cells formed primary and secondary spheres upon passaging. The ability to form secondary spheres demonstrates the ability of these cells to self-renew, i.e. generate daughter stem cells upon symmetric division (9,10). These data gave us the first indication that SFC/c-Kit signaling is involved in the maintenance of stem cell pools in the dental pulp.
Seminal studies have demonstrated a correlation between stem cell properties and the morphology of colonies generated by single cells from hair follicles (29), epidermal keratinocytes (30), and head and neck cancer (16,31). To further understand the impact of c-Kit signaling on the stemness of hDPSC, we classified the pulpspheres according to their shape and size according to a method used by the Castilho laboratory to study distinct populations of head and neck cancer stem cells (16). Interestingly, we observed here that c-Kit-silenced hDPSC generated fewer holospheres, when compared to vector control hDPSC. In contrast, the number of merospheres and paraspheres remained the same. It is believed that holospheres represent a purer population of stem cells in cancer models. As such, we postulate that SCF signaling through c-Kit may have more impact on holospheres than in other sphere sub-types because true stem cells are more “addicted” to this pathway. However, further studies are necessary to fully address this hypothesis.
It has been shown that Bmi-1 is necessary for efficient self-renewal of hematopoietic stem cells, as well as adult peripheral and central nervous system neural stem cells (11,12). We observed here that Bmi-1 expression and the fraction of Bmi-1-positive cells are increased when hDPSC are exposed to SCF and that c-Kit silencing partially abrogated these responses. These data support our pulpsphere assay findings, as Bmi-1 is considered a master regulator of stemness and self-renewal (11). Considering the fact that stem cells reside in the perivascular niche in human pulps (6), the data presented here indicate that a crosstalk initiated by endothelial cell-derived SCF signaling through its receptor c-Kit triggers Bmi-1 expression in dental pulp stem cells. Such pathway might play a key role in the maintenance of a stem cell pool in the dental pulp tissue throughout the life of the tooth. Testing of this hypothesis through the use of in vivo models is ongoing work in our laboratory.
Statement of Clinical Significance:
The studies presented here unveiled a signaling pathway that regulate the maintenance of stem cell pools in the dental pulp tissue. The presence of stem cells is necessary for dental pulp healing and replacement of dead odontoblasts throughout the life of the tooth.
Acknowledgements:
This work was funded by grant R01-DE21410 from NIH/NIDCR (JEN). We thank Dr. Songtao Shi (University of Pennsylvania) for the gift of DPSC and SHED cells. The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interests Statement: The authors deny any conflict of interest related to this study.
References
- 1.Beerman I, Rossi DJ. Epigenetic control of stem cell potential during homeostasis, aging, and disease. Cell Stem Cell 2015;16:613–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oh M, Nör JE. The perivascular niche and self-renewal of stem cells. Front Physiol 2015;6:367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yarden Y, Kuang WJ, Yang-Feng T, Coussens L, Munemitsu S, Dull TJ et al. Human proto-oncogene c-kit: a new cell surface receptor tyrosine kinase for an unidentified ligand. EMBO J 1987;6:3341–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robin C, Bollerot K, Mendes S, Haak E, Crisan M, Cerisoli F et al. Human placenta is a potent hematopoietic niche containing hematopoietic stem progenitor cells throughout development. Cancer Stem Cell 2009;5:385–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yagi H, Soto-Gutierrez A, Parekkadan B, Kitagawa Y, Tompkins RG, Kobayashi N. et al. Mesenchymal stem cells: mechanisms of immunomodulation and homing. Cell Transplantation 2010;19:667–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Machado CV, Passos ST, Campos TM, Bernardi L, Vilas-Boas DS, Nör JE et al. The dental pulp stem cell niche based on aldehyde dehydrogenase 1 expression. Int Endod J 2016;49:755–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ding L, Saunders TL, Enikolopov G, Morrison SJ. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature 2012;481:457–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ritchie KE, Nör JE. Perivascular stem cell niche in head and neck cancer. Cancer Lett 2013;338:41–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dontu G, Abdallah WM, Foley JM, Jackson KW, Clarke MF, Kawamura MJ et al. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev 2003;17:1253–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krishnamurthy S, Nör JE. Orosphere assay: a method for propagation of head and neck cancer stem cells. Head Neck 2013;35:1015–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park IK, Qian D, Kiel M, Becker MW, Pihalja M, Weissman IL et al. Bmi-1 is required for maintenance of adult self-renewing haematopoietic stem cells. Nature 2003;423:302–5. [DOI] [PubMed] [Google Scholar]
- 12.Molofsky AV, Pardal R, Iwashita T, Park IK, Clarke MF, Morrison SJ. Bmi-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation. Nature 2003;425:962–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balic A, Mina M. Characterization of progenitor cells in pulps of murine incisors. J Dent Res 2010;89:1287–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ishkitiev N, Yaegaki K, Kozhuharova A, Tanaka T, Okada M, Mitev V et al. Pancreatic differentiation of human dental pulp CD117⁺ stem cells. Regen Med 2013;8:597–612. [DOI] [PubMed] [Google Scholar]
- 15.Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSC) in vitro and in vivo. Proc Natl Acad Sci USA 2000;97:13625–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Almeida LO, Guimarães DM, Squarize CH, Castilho RM. Profiling the behavior of distinct populations of head and neck cancer stem cells. Cancers 2016;8(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fitzgerald M, Chiego DJ Jr, Heys DR. Autoradiographic analysis of odontoblast replacement following pulp exposure in primate teeth. Arch Oral Biol 1990;35:707–15. [DOI] [PubMed] [Google Scholar]
- 18.Lesot H, Begue-Kirn C, Kubler MD, Meyer JM, Smith AJ, Cassidy N et al. Experimental induction of odontoblast differentiation and stimulation during reparative processes. Cells Mat 1993;3:201–17. [Google Scholar]
- 19.Sakai VT, Zhang Z, Dong Z, Neiva KG, Machado MA, Shi S et al. SHED differentiate into functional odontoblasts and endothelium. J Dent Res 2010;89:791–6. [DOI] [PubMed] [Google Scholar]
- 20.Casagrande L, Demarco FF, Zhang Z, Araujo FB, Shi S, Nör JE. Dentin-derived DMP-2 and odontoblast differentiation. J Dent Res 2010;89:603–8. [DOI] [PubMed] [Google Scholar]
- 21.Bento LW, Zhang Z, Imai A, Nör F, Dong Z, Shi S et al. Endothelial differentiation of SHED requires MEK1/ERK signaling. J Dent Res 2013;91:51–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Z, Nör F, Oh M, Cucco C, Shi S, Nör JE. Wnt/β-catenin signaling determines the vasculogenic fate of post-natal mesenchymal stem cells. Stem Cells 2016;34:1576–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pan S, Dangaria S, Gopinathan G, Yan X, Lu X, Kolokythas A et al. SCF promotes dental pulp progenitor migration, neovascularization, and collagen remodeling - potential applications as a homing factor in dental pulp regeneration. Stem Cells Rev Rep 2013;9:655–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamamoto M, Kawashima N, Takashino N, Koizumi Y, Takimoto K, Suzuki N et al. Three-dimensional spheroid culture promotes odonto/osteoblastic differentiation of dental pulp cells. Arch Oral Biol 2014;59:310–7. [DOI] [PubMed] [Google Scholar]
- 25.Knight E, Przyborski S. Advances in 3D cell culture technologies enabling tissue-like structures to be created in vitro. J Anat 2015;227:746–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee EJ, Park SJ, Kang SK, Kim GH, Kang HJ, Lee SW et al. Spherical bullet formation via E-cadherin promotes therapeutic potency of mesenchymal stem cells derived from human umbilical cord blood for myocardial infarction. Mol Ther 2012;20:1424–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frith JE, Thomson B, Genever PG. Dynamic three-dimensional culture methods enhance mesenchymal stem cell properties and increase therapeutic potential. Tissue Eng Part C 2010;16:735–49. [DOI] [PubMed] [Google Scholar]
- 28.Li Y, Guo G, Li L, Chen F, Bao J, Shi Y et al. Three-dimensional spheroid culture of human umbilical cord mesenchymal stem cells promotes cell yield and stemness maintenance. Cell Tissue Res 2015;360:297–307. [DOI] [PubMed] [Google Scholar]
- 29.Rochat A, Kobayashi K, Barrandon Y. Location of stem cells of human hair follicles by clonal analysis. Cell 1994;76:1063–73. [DOI] [PubMed] [Google Scholar]
- 30.Papini S, Cecchetti D, Campani D, Fitzgerald W, Grivel JC, Chen S et al. Isolation and clonal analysis of human epidermal keratinocyte stem cells in long-term culture. Stem Cells 2003;21:481–94. [DOI] [PubMed] [Google Scholar]
- 31.Locke M, Heywood M, Fawell S, Mackenzie ICl. Retention of intrinsic stem cell hierarchies in carcinoma-derived cell lines. Cancer Res 2005;65:8944–50. [DOI] [PubMed] [Google Scholar]


