The skin is the outermost protective organ susceptible to damage by external injury and retains undesired scars after wound healing, which often diminishes the quality of life and causes economic burden. Periostin, an extracellular matrix component, is reportedly closely associated with skin wound healing and pathologic scar formation. It is expressed in various normal tissues, often associated with fibroblasts, and involved in remodeling of the extracellular matrix environment.[1,2] In some disease states, such as myocardial infarction, cancer, liver fibrosis, and pathologic scarring of the skin, periostin is reportedly a vital regulator of the progression of the disease and significantly over-expressed.[3] This article summarizes recent advancements in studies on the role of periostin in skin wound healing and pathologic scar formation.
Skin wound healing is a well-organized and highly coordinated physiologic phenomenon, activated immediately after skin injury, proceeding through spatiotemporally regulated secretion of several key mediators in the wound.[3,4] Cells related to repair are recruited by these mediators at the lesion, secrete more repair mediators, proliferate and differentiate to form granulation tissue, which finally contract and lead to wound closure[Figure 1].[5] Periostin is considered one of the key mediators of skin wound healing and is up-regulated after skin injury.[6,7] It is up-regulated after injury and peaks at 7 days in skin wound tissue. Immediately after skin injury, the infiltrated macrophages in the granulation tissue secrete large amount of transforming growth factor-β1 (TGF-β1), which initiate fibroblast migration and keratinocyte proliferation.[8] As an essential downstream effector of TGF-β superfamily signaling, periostin induces fibroblast activation via TGF-β1 in the wound and is regulated by TGF-β1.[4]
Figure 1.
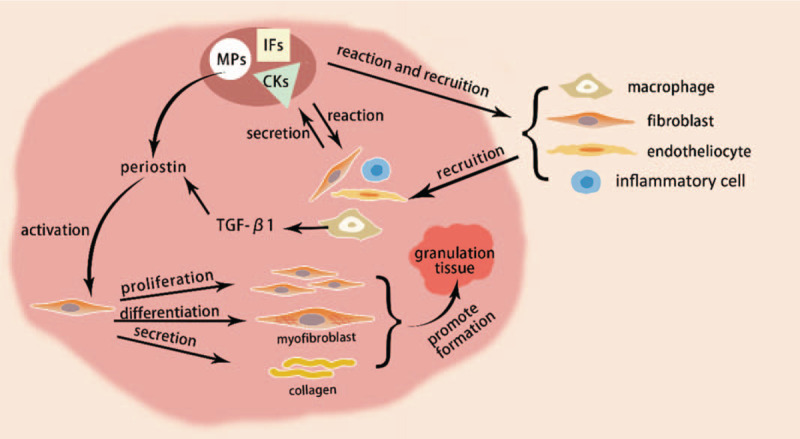
Roles of periostin in wound healing. Skin wound healing is activated immediately after injury, which initiated with spatiotemporally regulated secretion of several key mediators, like IFs, CKs, MPs. Various cells related to repair are recruited at the lesion under stimulation of these mediators and secrete more key mediators in reaction. Periostin, as a matricellular protein, plays important roles in wound healing. It can promote fibroblast proliferation, differentiation, migration and collagen synthesis, accelerate the formation of granulation tissue, and finally lead to wound closure. IFs: Inflammatory cytokines; CKs: Cytokines; MPs: Matricellular proteins; TGF-β1: Transforming growth factor-β1.
Previous studies have reported the positive function of periostin in promoting proliferation and differentiation of fibroblasts, keratinocytes, and mesenchymal cells in the wound, resulting in appropriate re-epithelialization and myofibroblast differentiation in the granulation tissue, thus gradually closing the wound.[1,6,9] Elliott et al[6] reported that full-thickness skin wounds were significantly larger in periostin-knockout mice than those in wild type mice at 5 and 7 days after wounding, and α-smooth muscle actin (α-SMA) was significantly down-regulated in wounds of periostin-deficient-mice. Moreover, Ontsuka et al[7] reported that the intervals for wound closure were significantly extended in periostin-knockout mice than those in wild type mice, suggesting that periostin could affect wound re-epithelialization. The mechanism underlying the role of periostin in promoting re-epithelialization was reported to be related to the secretion of interleukin (IL)-6. Cooperation of periostin and IL-1a can induce IL-6 production in fibroblasts by activating the nuclear factor kappa beta pathway and finally accelerating keratinocyte proliferation and differentiation, which is critical for re-epithelialization during wound healing.[9] In 2019, Elliott et al[10] inserted periostin- and connective tissue growth factor (CCN2)-containing scaffolds into full-thickness skin wound of diabetic mice. Compared to those untreated mice, addition of periostin- and CCN2-containing scaffolds suppresses neutrophil persistence, and increases the closure rate, mesenchymal cell infiltration, collagen density, and revascularization of wounds. Besides promoting wound healing itself, periostin also reportedly enhances the function of adipose-derived stem cells (ADSCs) in promoting wound healing. Qin et al[11] performed lentiviral transduction of the periostin gene into ADSCs to facilitate periostin secretion from ADSCs. Compared with unmodified control ADSCs, periostin-secreting ADSCs displayed better survival, greater paracrine signal-mediated tissue repair, and superior tissue repair properties under hypoxia.
As periostin plays vital roles in wound healing, studies have attempted to artificially express periostin through various methods to effectively promote skin wound healing. Yang et al[12] found that histamine could induce periostin production in mouse fibroblasts by activating extracellular signal-regulated kinase (ERK) 1/2 pathway. However, this modulating function only works on cells that express periostin and is not observed in periostin knockout mouse cells. Although periostin was demonstrated to promote skin wound healing, Nunomura et al[3] in 2018 reiterated that wound healing is not always faster at greater periostin levels. They reported that the skin wound healing was significantly delayed in periostin-transgenic mice. Since spatiotemporal expression of periostin is reportedly important to promote wound healing,[4] the results from Nunomura's study remind us that it is potentially infeasible to promote skin wound healing by simply up-regulating periostin, unlike regulated spatiotemporal expression of periostin, constitutive transgenic periostin expression may in turn interrupt normal wound closure. Further studies are required to investigate complex mechanisms underlying spatiotemporal expression of periostin.
After skin injury, fibroblasts are activated immediately, thus initiating the repair process. However, upon fibroblast over-activation, a series of pathologic processes would be initiated, including fibroblast over-proliferation, excessive extracellular matrix secretion, and excessive myofibroblast differentiation, and finally form pathologic scars.[2,13] Periostin expression is up-regulated in pathologic scar tissue in comparison with normal skin tissue. Qin's research group screened out periostin gene from the subtraction hybridization library of pathologic scars, and demonstrated that periostin gene expression displayed an increasing trend in normal skin, hypertrophic scars, and keloid tissue. Upon supplementation of fibroblast media with hydrocortisone, a scar inhibitor, they also found that periostin mRNA levels in keloid fibroblasts (KFs) and hypertrophic scar fibroblasts decreased significantly by 32% and 47%, respectively, and the proliferation and secretion viability of pathologic fibroblasts was weakened.[14] These findings together indicate a close association between periostin and pathologic scar formation, and suggest that regulation of periostin expression through effective methods may be a novel therapeutic method for pathologic scar.
Periostin reportedly affects pathologic scar formation by regulating the phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt) and ERK pathway and ras homolog gene family member A/Rho-associated protein kinase (RhoA/ROCK) pathway, which are necessary for the proliferation, differentiation, migration, invasion, and matrix collagen synthesis of pathologic scar fibroblasts.[6,13,15]In vitro studies by Crawford et al[2] assessed the effect of matrix-associated periostin on proliferation vitality and differentiation ability of fibroblasts isolated from hypertrophic scars and healthy skin, respectively. Results showed that periostin induced proliferation, α-SMA expression, and collagen contraction of hypertrophic scar-derived fibroblasts; however, it has no effect on normal dermal fibroblasts. When endogenous periostin is blocked by reagents, the number of proliferated fibroblasts and α-SMA-positive fibroblasts decreased markedly, and this inhibitory state was effectively alleviated through exogenous supplementation of periostin in the medium. Zhang et al[13] determined periostin function in KFs, showing similar results with periostin function on hypertrophic scar fibroblasts in Crawford's study and confirmed that periostin augmented the proliferation of KFs by activating the integrin αvβ3-PI3K/Akt pathway. In 2019, Maeda et al[15] reported a “vicious cycle” by which periostin was involved in pathologic scar formation. They found that IL-4 and IL-13 promote periostin expression and secretion in pathologic scars, and periostin in turn induces RhoA/ROCK pathway-mediated TGF-β1 secretion. Secreted TGF-β1 then induces further periostin production and secretion, thereby promoting abnormal scar formation. This founding indicated that inhibition of the RhoA/ROCK pathway may be potential therapeutic strategies to regulate periostin expression and reduce abnormal scar formation. Upon further elucidation of the mechanism underlying periostin-mediated promotion of pathologic scar formation, it has been possible to treat pathologic scars by regulating periostin expression. However, periostin expression is not a simple procedure, since it involves complex initiation and regulatory mechanisms, warranting more detailed elucidation of expression-level changes at different stages of scar formation.
Besides, periostin may potentially help scar fibroblasts survive under ischemia-hypoxia environment. Zhang et al[13] demonstrated that periostin was significantly up-regulated in KFs under hypoxia (2% O2), knockdown of periostin gene effectively decreased hypoxia-stimulated proliferation, collagen synthesis, migration, invasion, and Akt phosphorylation level of KFs; when supplement periostin in the growth medium of periostin cells, this phenomenon could be reversed. This result suggests a new hypothesis: periostin contributes to periostin-mediated pathologic scar development by promoting angiogenesis. Based on this hypothesis, Zhang et al[16] further used conditioned medium from KFs to culture HUVECs, reporting that periostin promoted the migration and vessel tubule formation of HUVECs by activating the ERK1/2 and FAK pathways, and this phenomenon exerted a dose-dependent effect with periostin. Furthermore, periostin promotes the secretion of important angiogenic cytokines in KFs, including vascular endothelial growth factor and angiopoietin 1.[16] This series of studies elucidate the potential role of periostin in promoting angiogenesis, such that scar fibroblasts can tolerate hypoxia, which otherwise deters the survival of normal skin fibroblasts. The angiogenic function of periostin allowed pathologic scar fibroblasts to maintain their constant growth and erosion of surrounding tissue, which may be one of the important breakthroughs for the study of pathologic scar interventional therapy. However, studies on the effect of periostin on angiogenesis in skin pathologic scar are limited, and the exact mechanisms underlying the involvement of periostin in the process of pathologic scar formation is still not fully understood, further studies are still warrant.
Funding
This work was supported by a grant from the National Natural Science Foundation of China (No. 81772090).
Conflicts of interest
None.
Footnotes
How to cite this article: Yin SL, Qin ZL, Yang X. Role of periostin in skin wound healing and pathologic scar formation. Chin Med J 2020;133:2236–2238. doi: 10.1097/CM9.0000000000000949
References
- 1.Nikoloudaki G, Creber K, Hamilton DW. Wound healing and fibrosis: a contrasting role for periostin in skin and the oral mucosa. Am J Physiol Cell Physiol 2020; 318:C1065–C1077. doi: 10.1152/ajpcell.00035.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crawford J, Nygard K, Gan BS, O’Gorman DB. Periostin induces fibroblast proliferation and myofibroblast persistence in hypertrophic scarring. Exp Dermatol 2015; 24:120–126. doi: 10.1111/exd.12601. [DOI] [PubMed] [Google Scholar]
- 3.Nunomura S, Nanri Y, Ogawa M, Arima K, Mitamura Y, Yoshihara T, et al. Constitutive overexpression of periostin delays wound healing in mouse skin. Wound Repair Regen 2018; 26:6–15. doi: 10.1111/wrr.12616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou HM, Wang J, Elliott C, Wen W, Hamilton DW, Conway SJ. Spatiotemporal expression of periostin during skin development and incisional wound healing: lessons for human fibrotic scar formation. J Cell Commun Signal 2010; 4:99–107. doi: 10.1007/s12079-010-0090-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murota H, Lingli Y, Katayama I. Periostin in the pathogenesis of skin diseases. Cell Mol Life Sci 2017; 74:4321–4328. doi: 10.1007/s00018-017-2647-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elliott CG, Wang J, Guo X, Xu SW, Eastwood M, Guan J, et al. Periostin modulates myofibroblast differentiation during full-thickness cutaneous wound repair. J Cell Sci 2012; 125:121–132. doi: 10.1242/jcs.087841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ontsuka K, Kotobuki Y, Shiraishi H, Serada S, Ohta S, Tanemura A, et al. Periostin, a matricellular protein, accelerates cutaneous wound repair by activating dermal fibroblasts. Exp Dermatol 2012; 21:331–336. doi: 10.1111/j.1600-0625.2012.01454.x. [DOI] [PubMed] [Google Scholar]
- 8.Walker JT, McLeod K, Kim S, Conway SJ, Hamilton DW. Periostin as a multifunctional modulator of the wound healing response. Cell Tissue Res 2016; 365:453–465. doi: 10.1007/s00441-016-2426-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taniguchi K, Arima K, Masuoka M, Ohta S, Shiraishi H, Ontsuka K, et al. Periostin controls keratinocyte proliferation and differentiation by interacting with the paracrine IL-1alpha/IL-6 loop. J Invest Dermatol 2014; 134:1295–1304. doi: 10.1038/jid.2013.500. [DOI] [PubMed] [Google Scholar]
- 10.Elliott CG, Wang J, Walker JT, Michelsons S, Dunmore-Buyze J, Drangova M, et al. Periostin and CCN2 scaffolds promote the wound healing response in the skin of diabetic mice. Tissue Engineering Part A 2019; 25:1326–1339. doi: 10.1089/ten.tea.2018.0268. [DOI] [PubMed] [Google Scholar]
- 11.Qin J, Yuan F, Peng Z, Ye K, Yang X, Huang L, et al. Periostin enhances adipose-derived stem cell adhesion, migration, and therapeutic efficiency in Apo E deficient mice with hind limb ischemia. Stem Cell Res Ther 2015; 6:138.doi: 10.1186/s13287-015-0126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang L, Murota H, Serada S, Fujimoto M, Kudo A, Naka T, et al. Histamine contributes to tissue remodeling via periostin expression. J Invest Dermatol 2014; 134:2105–2113. doi: 10.1038/jid.2014.120. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Z, Nie F, Kang C, Chen B, Qin Z, Ma J, et al. Increased periostin expression affects the proliferation, collagen synthesis, migration and invasion of keloid fibroblasts under hypoxic conditions. Int J Mol Med 2014; 34:253–261. doi: 10.3892/ijmm.2014.1760. [DOI] [PubMed] [Google Scholar]
- 14.Song ZH, Qin ZL. Expression of periostin and the effect of hydrocortisone on it in human fibroblasts of scar. J Peking Univ Health Sci 2008; 40:301–305. doi: 10.3321/j.issn:1671-167X.2008.03.013. [PubMed] [Google Scholar]
- 15.Maeda D, Kubo T, Kiya K, Kawai K, Matsuzaki S, Kobayashi D, et al. Periostin is induced by IL-4/IL-13 in dermal fibroblasts and promotes RhoA/ROCK pathway-mediated TGF-beta1 secretion in abnormal scar formation. J Plast Surg Hand Surg 2019; 53:288–294. doi: 10.1080/2000656X.2019.1612752. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Z, Nie F, Chen X, Qin Z, Kang C, Chen B, et al. Upregulated periostin promotes angiogenesis in keloids through activation of the ERK?1/2 and focal adhesion kinase pathways, as well as the upregulated expression of VEGF and angiopoietin-1. Mol Med Rep 2015; 11:857–864. doi: 10.3892/mmr.2014.2827. [DOI] [PMC free article] [PubMed] [Google Scholar]


