Abstract
Background
The relationship between macrocirculation and microcirculation remains controversial. The loss of coherence between microcirculation and macrocirculation has already been found in late-stage sepsis shock. The objective of this study was to determine the earliest possible time of detecting the loss of coherence between microcirculation and macrocirculation in early-stage endotoxemic shock.
Methods
We randomized 24 female New Zealand white rabbits into two groups: endotoxemic shock group (n = 14) and control group (n = 10). Rabbits in the endotoxemic shock group were equipped with arterial and venous catheters and received an intravenous infusion of Escherichia coli lipopolysaccharide (LPS, 2 mg/kg over 10 min). Rabbits in the control group received the same dose of saline infusion. Microcirculatory perfusion parameters were assessed in the sublingual mucosa using sidestream dark-field video microscopy. Systemic hemodynamics and blood lactate levels were measured at baseline and over a 120-min period.
Results
Ninety minutes after completing LPS infusion, all animals in the endotoxemic shock group developed a hypodynamic septic condition, characterized by low cardiac output and increased systemic vascular resistance; 120 min after completing LPS infusion, the mean arterial pressure decreased by 25% (P = 0.01), confirming ongoing endotoxemic shock. However, significant decreases in sublingual microcirculatory parameters of small vessels (microvascular flow index, perfused vessel density, and proportion of small perfused vessels) were observed 30 min after completing LPS infusion (P = 0.01, for all), and threshold decreases of 30% were found 60 min after completing LPS infusion (P = 0.001, for all) in the endotoxemic shock group. Lactate levels significantly increased to more than 2 mm/L at 90 min and more than 4 mm/L at 120 min in the endotoxemic shock group (P = 0.02 and P = 0.01, respectively).
Conclusions
Changes in microcirculatory perfusion precede changes in macrocirculation and lactate levels in a rabbit model of endotoxemia shock. Microcirculation, macrocirculation, and oxygen metabolism are distinct in early-stage endotoxic shock.
Keywords: Shock, Endotoxemia, Microcirculation, Lactate
Background
Septic shock is a common disease in critical ill patients and remains associated with high morbidity and mortality.[1] Recent multicenter trials conducted in the United States[2] (Protocolized Care for Early Septic Shock), Australia and Asia[3] (Australasian Resuscitation in Sepsis Evaluation trial), and the United Kingdom[4] (Protocolized Management in Sepsis) have demonstrated that normalized macrocirculatory parameters fail to improve survival.[5] Global hemodynamic parameters such as cardiac output, blood pressure variables, and venous saturation are poorly reflective of tissue perfusion.[6] Therefore, normalizing the systemic circulation does not guarantee the restoration of microcirculatory perfusion and cellular O2 metabolism in the early stage of septic shock.[7] Many prior studies of microcirculation have suggested that microvascular perfusion appears to be relatively independent of global hemodynamic variables in the late stage of sepsis.[8–10] In particular, more researches have found that resuscitation results in a normalization of systemic hemodynamic variables but does not lead to a parallel improvement in microcirculatory perfusion and oxygenation.[11–13] Whether this separation can be found earlier, rather than after completion of macrocirculation resuscitation, is still a great challenge in clinical practice at present. However, the timing of the uncoupling of macro- and microcirculation in early-stage septic shock has not been clearly studied. Therefore, by monitoring sublingual microcirculation using sidestream dark-field imaging (SDF), we aimed to observe the changes in microvascular blood flow and macrocirculation during the early stage of endotoxemic shock. We hypothesized that microcirculatory abnormalities occur earlier than those in macrocirculation and that microcirculation, macrocirculation, and oxygen metabolism are distinct in the early stage of endotoxic shock.
Methods
We performed a randomized, open-label, controlled experimental study. This study was reviewed and approved by the Institutional Animals Experimentation Committee of Ruijin Hospital of the Shanghai Jiao Tong University School of Medicine (No. RJ-2015-025).
Study design
Twenty-four female specific pathogen-free New Zealand white rabbits (Harlan Netherlands BV, Horst, the Netherlands) with a mean body weight of 3.0 ± 0.3 kg were used in this study. The animals were quarantined 1 week before the start of the investigation to permit adaptation to environmental conditions. Animals were housed in pairs in large conventional cages in a light-controlled room maintained at 22 ± 1°C with a relative humidity of 55% ± 10% and received a standard diet of food pellets and water for consumption ad libitum.
Surgical procedure
All animals were anesthetized via intramuscular injection of ketamine (20 mg/kg, Hengrui Medicine, China), and a marginal ear vein was cannulated to ensure continuous intravenous anesthesia (4 mg·kg−1·h−1, midazolam; Hengrui Medicine) during the entire study protocol. The adequacy of anesthesia was assessed by monitoring heart rate (HR) and blood pressure responses to external noxious stimuli. Rabbits were block-randomized to the endotoxemic shock group or control group.
Catheters were advanced through the right internal jugular vein to administer drugs, and a thermodilution catheter (4F, 8 cm Pulsiocath PV2014L16; Pulsion Medical Systems, Munich, Germany) was inserted through the right femoral artery to measure blood pressure and to obtain blood gases. Normal saline containing 4 UI/mL of heparin (BBCA Pharmaceutical, China) was infused at a rate of 2 mL/h via the arterial line. Basal measurements were acquired after a stabilization period of at least 30 min.
The endotoxemic shock group was created via intravenous infusion of 2 mg/kg of Escherichia coli lipopolysaccharide (LPS, O55:B5; Sigma Chem Co., St. Louis, MO, USA) over 10 min. We set a threshold decrease of 25% in mean arterial pressure (MAP) as confirmation of ongoing endotoxemic shock.[14]
Hemodynamic parameters and measurements
HR and MAP were continuously monitored and recorded. The hemodynamic measurements were acquired from all rabbits using the dedicated indwelling arterial catheter and pulse indicator continuous cardiac output (PiCCO) device (Pulsion Medical Systems). This method was previously used in several animal studies.[15]
PiCCO monitoring was appropriately calibrated for pulse contour analysis at every measurement time point using two 3-mL bolus injections of 4°C normal saline. A third calibration injection was performed whenever a difference of >10% was observed between these measurements.
The cardiac index (CI), stroke volume index (SVI), and systemic vascular resistance index (SVRI) were recorded via trans-pulmonary dilution. The central venous pressure (CVP) was measured in the right external jugular vein. Hemodynamic parameter measurements were recorded every 30 min for a total of 120 min.
Microcirculation measurements
Microvascular blood flow imaging was performed using SDF technology (Microscan Video Microscope System, MicroVision Medical, Amsterdam, the Netherlands). The SDF technique was incorporated into a handheld video microscopy instrument and applied to the sublingual mucosa after gentle removal of saliva with a gauze swab. Microcirculation measurements were performed with the animals resting in a dorsally recumbent position.[16] The jaws were carefully separated and fixed in an open configuration using a 1.0-cm piece of cotton dental roll wedged between the right maxillary and mandibular first and second premolars. The tongue was then carefully rolled backwards to allow free access to the palate mucosal surface.
Without applying pressure and after gentle removal of saliva or secretions by isotonic saline-drenched gauze, steady images of at least 20 s were obtained using a portable computer and analog-to-digital video converter (ADVC110, Canopus Co., San Jose, CA, USA).[17] Microcirculation measurements were obtained pre-operatively (baseline); immediately post-operatively; and after 30, 60, 90, and 120 min.
The images were stored under a random number for later analysis using Automated Vascular Analysis 3.0 software (Microvision Medical Inc., San Jose, CA, USA). To compute the microvascular flow index (MFI), vessels with continuous flow were further divided into normal and sluggish. We performed an analysis[18] based on semiquantitative criteria that distinguished between no flow (0), intermittent flow (1), sluggish flow (2), and continuous flow (3) in individual vessels. The overall score was called the MFI and was the average of the individual values. The total vessel density was calculated as the number of vessels crossing defined gridlines divided by the total length of the lines. Small-vessel perfusion was defined as the proportion of small perfused vessels (PPV) and calculated as the number of capillaries continuously perfused divided by the total number of vessels of the same type. The analysis was restricted to vessels with diameters <20 μm, whereas the vessels of greater diameter were assessed only to rule out compression artifacts. The PPV was calculated as follows: 100 × ([total number of vessels − {no flow + intermittent flow}]/total number of vessels). Perfused vessel density (PVD) was calculated by multiplying the vessel density by the proportion of PPV.
Blood gas and lactate level measurements
Arterial blood samples were obtained at baseline and after every 30 min for a total of 120 min post-operatively for measurements of pH, PO2, PCO2, 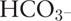 , and arterial lactate levels using a blood gas analyzer (ABL 500; Radiometer, Copenhagen, Denmark).
, and arterial lactate levels using a blood gas analyzer (ABL 500; Radiometer, Copenhagen, Denmark).
Statistical analysis
Data analysis was performed using SPSS 10.0 computer software (SPSS, Chicago, IL, USA). Data are expressed as mean ± standard deviation. Hemodynamic data were compared using analysis for repeated measurements (general linear model procedure with analysis of variance). The normal distribution of the data was assessed using the Shapiro-Wilk test. The differences between variables before and after volume expansion were evaluated using the paired Student's t test. We considered a P < 0.05 as statistically significant.
Results
In total, 24 rabbits were randomly assigned to either the endotoxemic shock group (n = 14, 3.1 ± 0.4 kg) or control group (n = 10, 3.1 ± 0.5 kg).
Macrovascular flow function change
Thirty minutes after completing LPS infusion, the CI had increased (P = 0.04) and MAP and SVRI had decreased compared to baseline values (P = 0.01 and P = 0.1, respectively). Sixty minutes after completing LPS infusion, the CI and SVI had increased compare to baseline (P = 0.01 and P = 0.03, respectively) and SVRI had decreased compared to baseline values (P = 0.01) [Figure 1]. Ninety minutes after completing LPS infusion, all the other animals in the endotoxemic group developed a hypodynamic septic condition, characterized by the low cardiac output and increased systemic vascular resistance [Table 1 and Figure 2]. Meanwhile, the MAP had decreased from 85 ± 2 to 71 ± 3 mmHg (P = 0.01). The MAP showed a threshold decrease of 25% (from 85 ± 2 to 64 ± 2 mmHg), which confirmed ongoing endotoxemic shock 120 min after initiating the infusion of LPS in all rabbits in the endotoxemic shock group.
Figure 1.
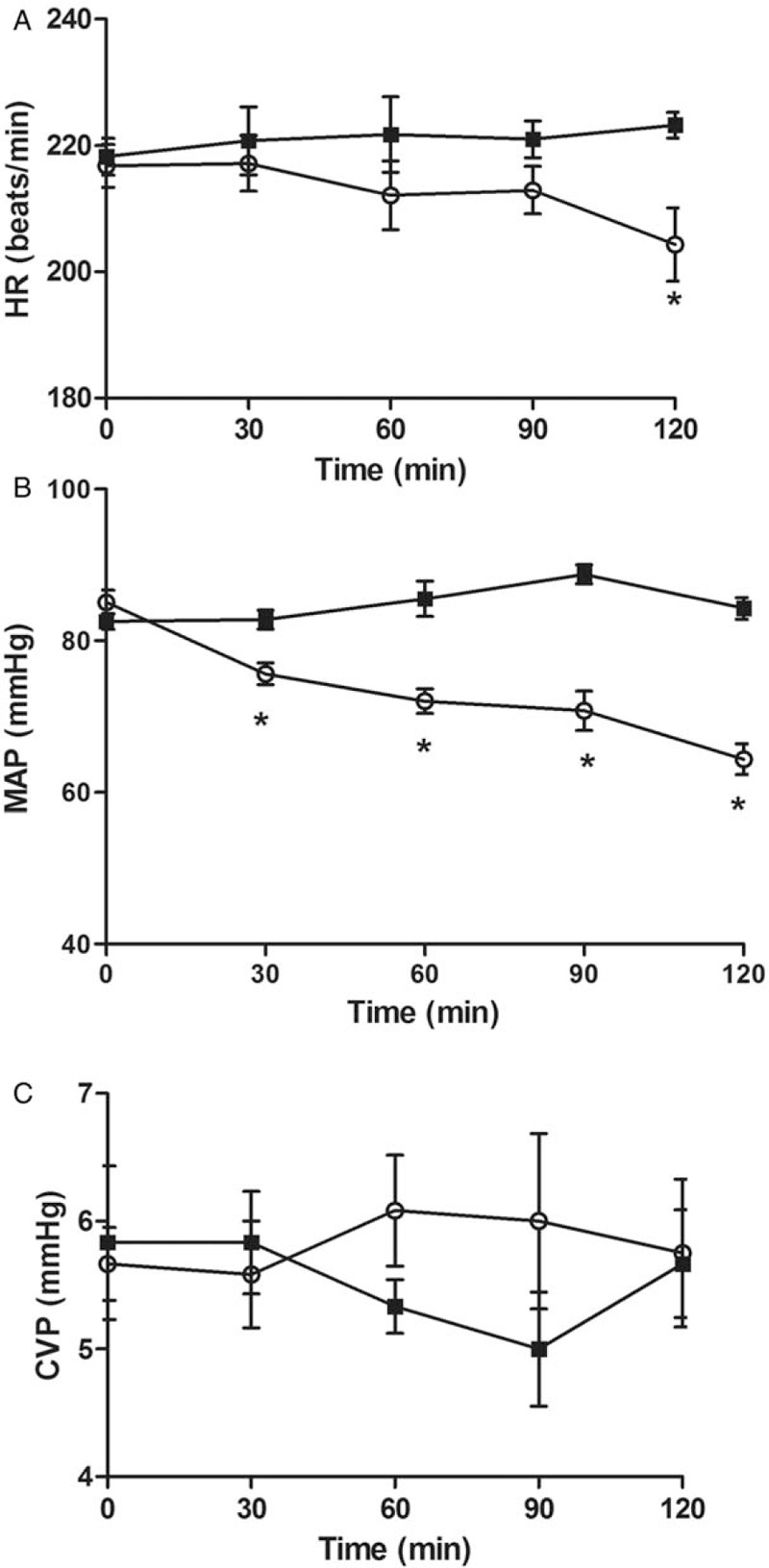
Differences in heart rate (HR) (A), Mean arterial pressure (MAP) (B), and Central venous pressure (CVP) (C), during endotoxemic shock and control groups. Values shown as mean ± standard deviation of all animals at each time point. ∗P < 0.05 vs. baseline.
Table 1.
Systemic hemodynamic variables over time in endotoxemic shock group (n = 14) and the control group (n = 10).
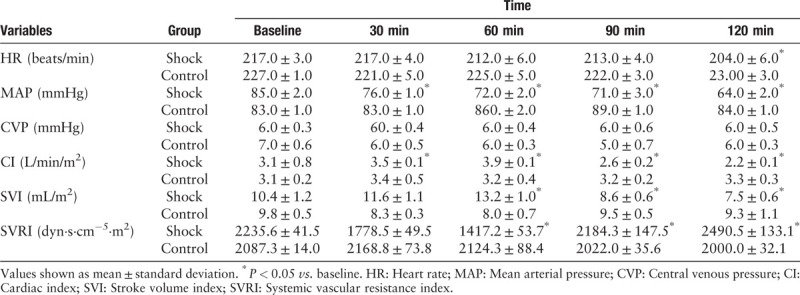
Figure 2.
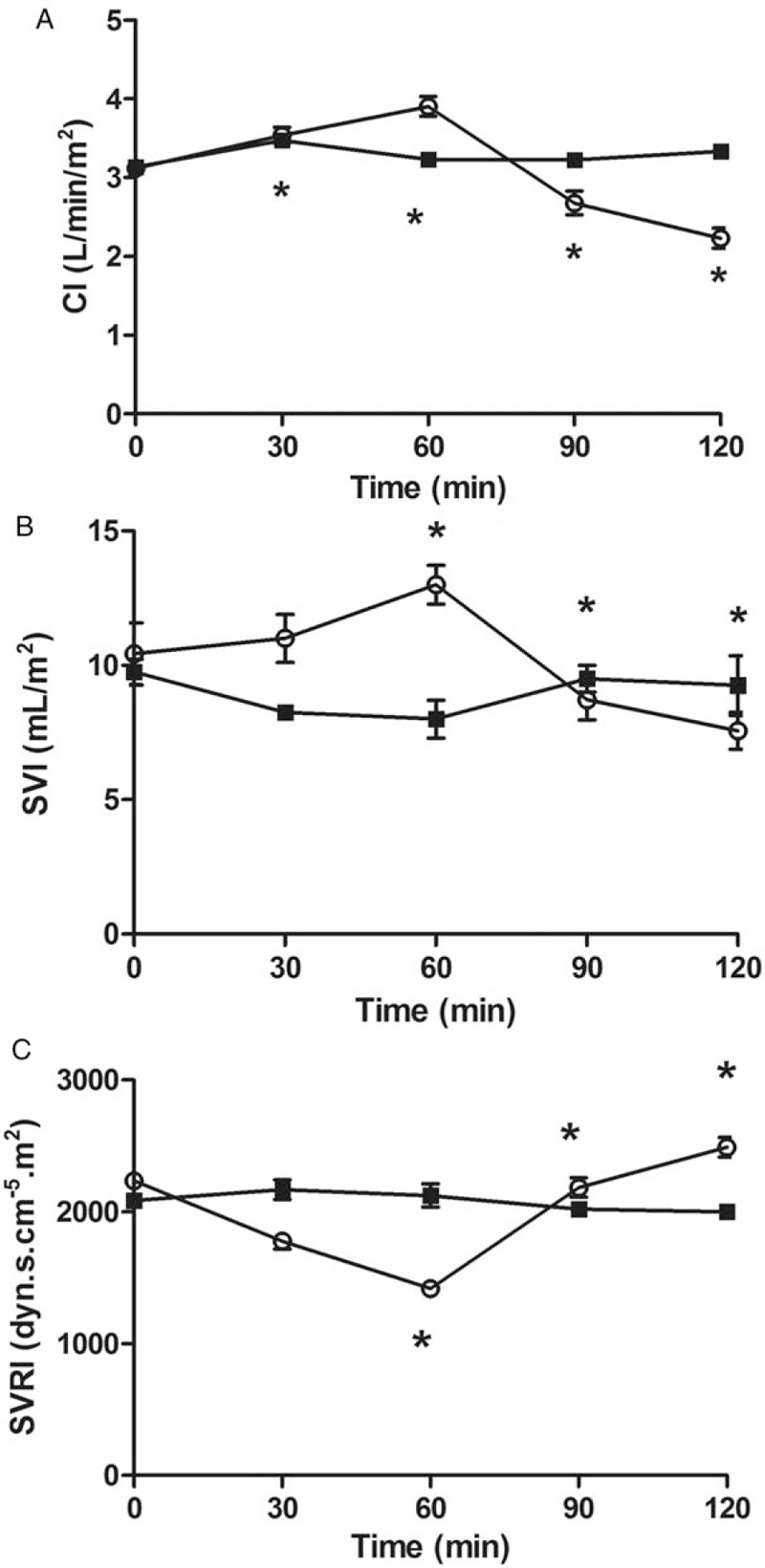
Differences in cardiac index (CI) (A), Stroke volume index (SVI) (B), and Systemic vascular resistance index (SVRI) (C), during endotoxemic shock and control groups. Values shown as mean ± standard deviation of all animals at each time point. ∗P < 0.05 vs. baseline.
The HR and CVP stabilized at levels that were not significantly different at any time (P > 0.05 for all). We observed no significant changes in any variable in the control group.
Microvascular flow function change
Sublingual microcirculation parameters of the small vessels are presented in Table 2. Significant decreases in microcirculatory parameters (MFI, PVD, and PPV) were observed 30 min after completing LPS infusion in the endotoxemic shock group (P = 0.01 for all). Compared with baseline, microcirculatory parameters (MFI, PVD, PPV) decreased to 28%, 40%, and 33%, respectively, after completing LPS infusion in the endotoxemic shock group (P = 0.001 for all) [Figure 3].
Table 2.
Microcirculatory variables over time in endotoxemic shock (n = 14) and control (n = 10) groups.
Figure 3.
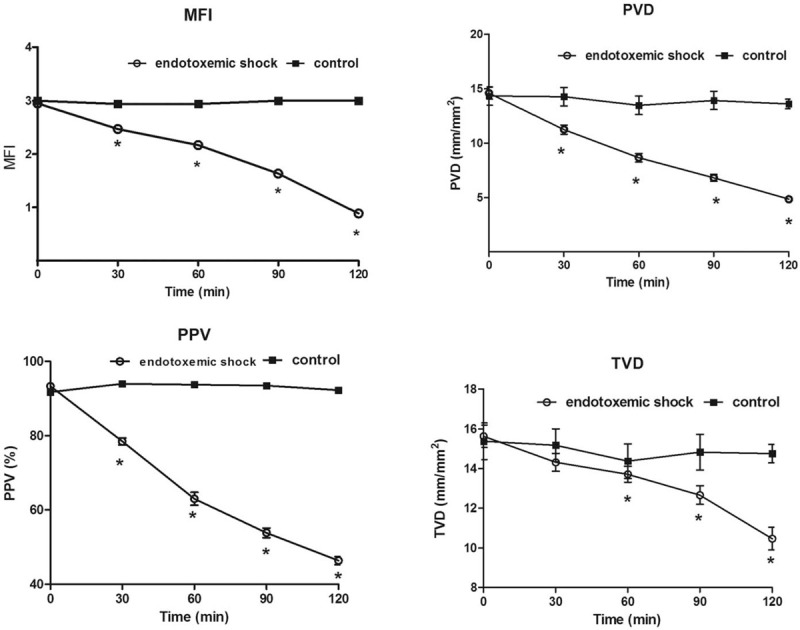
Changes in microvascular flow index (MFI), proportion of small perfused vessels (PPV), perfused vessel density (PVD), total vessel density (TVD) between 0 and 120 min. Values shown as mean ± standard deviation of all animals at each time point. ∗P < 0.05 vs. baseline.
Blood gas and lactate level changes
Lactate levels increased to over 2 mmol/L at 60 min and significantly increased to over 4 mmol/L at 120 min in the endotoxemic shock group (P = 0.02 and P = 0.01, respectively). Arterial pH and 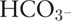 levels decreased significantly after 90 min in the endotoxemic shock group compared to those at baseline (P = 0.02 and P = 0.01, respectively). Table 3 shows the arterial blood gas and metabolic variables in both the endotoxemic shock and control groups.
levels decreased significantly after 90 min in the endotoxemic shock group compared to those at baseline (P = 0.02 and P = 0.01, respectively). Table 3 shows the arterial blood gas and metabolic variables in both the endotoxemic shock and control groups.
Table 3.
Blood gases and metabolic variables over time in endotoxemic shock (n = 14) and control (n = 10) groups.
Correlations among systemic hemodynamic variables, lactate levels, and sublingual microcirculation
None of the microcirculation variables were directly correlated to MAP levels at all time points. None of the microcirculation variables were directly correlated to lactate levels at all time points.
Discussion
The main results of the present study are that LPS infusion-induced endotoxemic shock in a rabbit model caused a reduction in the MFI, PVD, and PPV in the sublingual microcirculation beginning at 30 min post-completion of infusion of LPS. However, the MAP decreased to 25% of that at baseline 120 min after completing LPS infusion; compared with microcirculation, macrocirculation was delayed by about 90 min. Moreover, the alteration in lactate levels occurred later (60 min after completing LPS infusion) than the changes in the microcirculation occurred (30 min after completing LPS infusion) in our study. These results are consistent with the hypothesis that microcirculatory abnormalities occur earlier than systemic hemodynamic abnormalities; microcirculation, macrocirculation, and oxygen metabolism were distinct in the early stage of endotoxic shock.
The main purpose of this study was to determine whether the separation of macrocirculation and microcirculation occurs in the early stage of sepsis without macrocirculatory hemodynamic disorder and fluid resuscitation. A concept referred to as the “loss of hemodynamic coherence”[19] means that alterations in either microvascular perfusion or in oxygen utilization have not been improved by optimization of systemic hemodynamics, and thus, hemodynamic coherence between the macrocirculation and microcirculation has been eroded. Nevertheless, a study of the loss of hemodynamic coherence mainly focused on the late stage of shock or completion of macro-resuscitation. Trzeciak et al[20] found more impairments in the indices of microcirculatory perfusion in severe sepsis and septic shock in non-survivors compared with survivors. De Backer et al[8] found that the total vascular density, density of perfused small vessels, and PPV were higher in a late-stage sub-group than in an early-stage sub-group. Van et al[21] found that fluid resuscitation applied following endotoxemic shock is not successful in restoring microcirculatory perfusion to baseline, despite the normalization of cardiac output and MAP. Dubin et al[22] found that systemic and intestinal hemodynamics and sublingual and serosal microcirculation are restored, but the intestinal villi remain hypoperfused during the resuscitation phase in sheep in endotoxic shock. In contrast with these reports, we observed that in the early stage of endotoxic shock, the microcirculation shows clear evidence of dysfunction, even in the absence of any changes in static hemodynamic parameters, and there was no resuscitation treatment.
We believe that identifying the inconsistency in the early stage of shock has more clinical significance than in the late stage or after resuscitation because it can help us adjust the appropriate treatment direction, rather than possibly aggravating the disease. If loss of hemodynamic coherence occurs, restoring macrocirculation (fluid resuscitation and vasoactive drugs) will not improve microcirculation and oxygen metabolism and may even aggravate their deterioration. For example, fluid resuscitation increases the risk of over-resuscitation, resulting in an increase in diffusion distance between the capillaries and tissue cells.[23] In addition, vasoactive medications can overwhelm endogenous receptor-mediated vasoregulation, further contributing to the loss of hemodynamic coherence.[24] Considering inappropriate fluid resuscitation or unreasonable use of vasoactive drugs may lead to more microcirculatory disorders; thus, it is very important to rebuild coherence between macrocirculation and microcirculation in the early stage of septic shock.[25] Boerma et al[26] identified that time is an important parameter in the establishment of coherences. In the early stages of sepsis, there is no coherence between intestinal and sublingual microcirculation, but after 3 days, when the septic insult generalizes, there is coherence between sublingual and intestinal microcirculation.
It is well established that hyperlactemia may reflect inadequate perfusion. However, the relationship between lactate levels and microcirculatory perfusion during circulatory shock is puzzling. Filbin et al[27] found that microcirculatory abnormalities are correlated with elevated serum lactate levels in patients with normotensive sepsis. Yeh et al[28] demonstrated a correlation between early sublingual small vessel density and late blood lactate levels in critically ill surgical patients. Hernandez et al[29] found that the presence of hyperlactatemia and high norepinephrine requirements increases the odds of identifying a severe underlying microvascular dysfunction during sublingual microcirculatory assessments in patients with septic shock. Conversely, Vellinga et al[30] identified that microcirculatory flow abnormalities do not differ between patients with and without mildly elevated lactate levels. Patients with a capillary MFI <2.6 tend to have higher lactate levels than those in patients with a higher MFI. In our study, hyperlactemia of greater than 4.0 mmol/L was observed at the onset of septic shock 120 min after completing LPS infusion; in contrast, microcirculatory variables (MFI, PVD, and PPV) were perturbed 60 min after completing LPS infusion. Meanwhile, we found no significant correlations between any microcirculatory perfusion variables, such as MFI, PVD, PPV, and lactate levels. Although lactate concentrations are relatively easy to measure at the bedside, there may be some drawbacks. For example, lactate represents a downstream metabolic product, the origin of which may not always reflect a lack of organ perfusion; thus, increased glycolysis may exceed the capacity to metabolize pyruvate and thereby increase lactate levels.[31] Therefore, the detection of abnormal changes in the microcirculation is more valuable than the detection of changes in lactate levels in the early stage.
Our study has some limitations, which warrant discussion. Although LPS has been used extensively in models of septic shock, the endotoxemia model is an imprecise replication of human septic shock.[32] The presence of LPS in the bloodstream causes fever, disseminated intravascular coagulation, hypotension, multiple organ failure, and in severe cases, septic shock and death.[33] The LPS-induced response is highly reproducible, opening a defined and stable window of opportunity in which interventions can be tested.[34] The 120-min length of evaluation is short, and the endotoxic model does not reflect hyperdynamic septic shock. However, the number of variables monitored logistically required a significant amount of time; thus, longer observation periods might have produced different results. Indeed, we lacked oxygenation monitoring, but there were basic oxygenation measurements, that is, arterial oxygen saturation and arterial blood lactate levels. Although these were insufficient to analyze the problem of oxygen metabolism in the sepsis pathogenesis, they should suffice for the goal we aimed to achieve. There were limits to the number of animals in each group, along with the use of only one sex (females) and the lack of use of antibiotics. However, as with our previous work, we were able to detect statistical differences between groups. Finally, sublingual microcirculation may not necessarily represent the perfusion of other regions, such as the splanchnic area. Thus, the data obtained from the sublingual region may not represent other regions of the body.
During the early phase of endotoxemia shock in a rabbit model, changes in microcirculatory perfusion precede changes in the macrocirculation and lactate levels. Microcirculation, macrocirculation, and oxygen metabolism are distinct in the early stage of endotoxic shock.
Conflicts of interest
None.
Footnotes
How to cite this article: Zhang H, Li L, Wu J, Qu HP, Tang YQ, Chen DC. Time of dissociation between microcirculation, macrocirculation, and lactate levels in a rabbit model of early endotoxemic shock. Chin Med J 2020;133:2153–2160. doi: 10.1097/CM9.0000000000000887
References
- 1.Fleischmann C, Scherag A, Adhikari NK, Hartog CS, Tsaganos T, Schlattmann P, et al. Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am J Respir Crit Care Med 2016; 193:259–272. doi: 10.1164/rccm.201504-0781OC. [DOI] [PubMed] [Google Scholar]
- 2.Yealy DM, Kellum JA, Huang DT, Barnato AE, Weissfeld LA, Pike F, et al. A randomized trial of protocol-based care for early septic shock. N Engl J Med 2014; 370:1683–1693. doi: 10.1056/NEJMoa1401602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Delaney AP, Peake SL, Bellomo R, Cameron P, Holdgate A, Howe B, et al. The Australasian resuscitation in sepsis evaluation (ARISE) trial statistical analysis plan. Crit Care Resusc 2013; 15:162–171. doi: 10.1111/1742-6723.12116. [PubMed] [Google Scholar]
- 4.Mouncey PR, Osborn TM, Power GS, Harrison DA, Sadique MZ, Grieve RD, et al. Protocolised management in sepsis (ProMISe): a multicentre randomised controlled trial of the clinical effectiveness and cost-effectiveness of early, goal-directed, protocolised resuscitation for emerging septic shock. Health Technol Assess 2015; 19:1–150. doi: 10.3310/hta19970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Osborn TM. Severe sepsis and septic shock trials (ProCESS, ARISE, ProMISe): what is optimal resuscitation? Crit Care Clin 2017; 33:323–344. doi: 10.1016/j.ccc.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 6.Asfar P, Meziani F, Hamel JF, Grelon F, Megarbane B, Anguel N, et al. High versus low blood-pressure target in patients with septic shock. N Engl J Med 2014; 370:1583–1593. doi: 10.1056/NEJMoa1312173. [DOI] [PubMed] [Google Scholar]
- 7.Weil MH, Tang W. Welcoming a new era of hemodynamic monitoring: expanding from the macro to the microcirculation. Crit Care Med 2007; 35:1204–1205. doi: 10.1097/01.CCM.0000259169.33624.D2. [DOI] [PubMed] [Google Scholar]
- 8.De Backer D, Donadello K, Sakr Y, Ospina-Tascon G, Salgado D, Scolletta S, et al. Microcirculatory alterations in patients with severe sepsis: impact of time of assessment and relationship with outcome. Crit Care Med 2013; 41:791–799. doi: 10.1097/CCM.0b013e3182742e8b. [DOI] [PubMed] [Google Scholar]
- 9.Edul VS, Enrico C, Laviolle B, Vazquez AR, Ince C, Dubin A. Quantitative assessment of the microcirculation in healthy volunteers and in patients with septic shock. Crit Care Med 2012; 40:1443–1448. doi: 10.1097/CCM.0b013e31823dae59. [DOI] [PubMed] [Google Scholar]
- 10.Mesquida J, Espinal C, Gruartmoner G, Masip J, Sabatier C, Baigorri F, et al. Prognostic implications of tissue oxygen saturation in human septic shock. Intensive Care Med 2012; 38:592–597. doi: 10.1007/s00134-012-2491-6. [DOI] [PubMed] [Google Scholar]
- 11.Trzeciak S, McCoy JV, Phillip DR, Arnold RC, Rizzuto M, Abate NL, et al. Early increases in microcirculatory perfusion during protocol-directed resuscitation are associated with reduced multi-organ failure at 24 h in patients with sepsis. Intensive Care Med 2008; 34:2210–2217. doi: 10.1007/s00134-008-1193-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ospina-Tascon G, Neves AP, Occhipinti G, Donadello K, Buchele G, Simion D, et al. Effects of fluids on microvascular perfusion in patients with severe sepsis. Intensive Care Med 2010; 36:949–955. doi: 10.1007/s00134-010-1843-3. [DOI] [PubMed] [Google Scholar]
- 13.Pranskunas A, Koopmans M, Koetsier PM, Pilvinis V, Boerma EC. Microcirculatory blood flow as a tool to select ICU patients eligible for fluid therapy. Intensive Care Med 2013; 39:612–619. doi: 10.1007/s00134-012-2793-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wiel E, Pu Q, Leclerc J, Corseaux D, Bordet R, Lund N, et al. Effects of the angiotensin-converting enzyme inhibitor perindopril on endothelial injury and hemostasis in rabbit endotoxic shock. Intensive Care Med 2004; 30:1652–1659. doi: 10.1007/s00134-004-2198-4. [DOI] [PubMed] [Google Scholar]
- 15.Arnemann PH, Hessler M, Kampmeier T, Morelli A, Van Aken HK, Westphal M, et al. Comparison of an automatic analysis and a manual analysis of conjunctival microcirculation in a sheep model of haemorrhagic shock. Intensive Care Med Exp 2016; 4:37.doi: 10.1186/s40635-016-0110-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Milstein DM, Lindeboom JA, Ince C. Intravital sidestream dark-field (SDF) imaging is used in a rabbit model for continuous noninvasive monitoring and quantification of mucosal capillary regeneration during wound healing in the oral cavity: a pilot study. Arch Oral Biol 2010; 55:343–349. doi: 10.1016/j.archoralbio.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 17.Goedhart PT, Khalilzada M, Bezemer R, Merza J, Ince C. Sidestream dark field (SDF) imaging: a novel stroboscopic LED ring-based imaging modality for clinical assessment of the microcirculation. Opt Express 2007; 15:15101–15114. doi: 10.1364/oe.15.015101. [DOI] [PubMed] [Google Scholar]
- 18.Guerci P, Tran N, Menu P, Losser MR, Meistelman C, Longrois D. Impact of fluid resuscitation with hypertonic-hydroxyethyl starch versus lactated ringer on hemorheology and microcirculation in hemorrhagic shock. Clin Hemorheol Microcirc 2014; 56:301–317. doi: 10.3233/CH-141663. [DOI] [PubMed] [Google Scholar]
- 19.Ince C. Hemodynamic coherence and the rationale for monitoring the microcirculation. Crit Care 2015; 19: Suppl 3: S8.doi: 10.1186/cc14726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trzeciak S, Dellinger RP, Parrillo JE, Guglielmi M, Bajaj J, Abate NL, et al. Early microcirculatory perfusion derangements in patients with severe sepsis and septic shock: relationship to hemodynamics, oxygen transport, and survival. Ann Emerg Med 2007; 49:91–98. doi: 10.1016/j.annemergmed.2006.08.02.1. [DOI] [PubMed] [Google Scholar]
- 21.van Genderen ME, Klijn E, Lima A, de Jonge J, Sleeswijk VS, Voorbeijtel J, et al. Microvascular perfusion as a target for fluid resuscitation in experimental circulatory shock. Crit Care Med 2014; 42:e96–e105. doi: 10.1097/CCM.0b013e3182a63fbf. [DOI] [PubMed] [Google Scholar]
- 22.Dubin A, Edul VS, Pozo MO, Murias G, Canullan CM, Martins EF, et al. Persistent villi hypoperfusion explains intramucosal acidosis in sheep endotoxemia. Crit Care Med 2008; 36:535–542. doi: 10.1097/01.CCM.0000300083.74726.43. [DOI] [PubMed] [Google Scholar]
- 23.He H, Long Y, Zhou X, Wang X, Zhang H, Chai W, et al. Oxygen-flow-pressure targets for resuscitation in critical hemodynamic therapy. Shock 2018; 49:15–23. doi: 10.1097/SHK.0000000000000929. [DOI] [PubMed] [Google Scholar]
- 24.Dunser MW, Ruokonen E, Pettila V, Ulmer H, Torgersen C, Schmittinger CA, et al. Association of arterial blood pressure and vasopressor load with septic shock mortality: a post hoc analysis of a multicenter trial. Crit Care 2009; 13:R181.doi: 10.1186/cc8167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ince C. Hemodynamic coherence and the rationale for monitoring the microcirculation. Crit Care 2015; 19: Suppl 3: S8.doi: 10 1186/cc14726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boerma EC, van der Voort PH, Spronk PE, Ince C. Relationship between sublingual and intestinal microcirculatory perfusion in patients with abdominal sepsis. Crit Care Med 2007; 35:1055–1060. doi: 10.1097/01.CCM.0000259527.89927.F9. [DOI] [PubMed] [Google Scholar]
- 27.Filbin MR, Hou PC, Massey M, Barche A, Kao E, Bracey A, et al. The microcirculation is preserved in emergency department low-acuity sepsis patients without hypotension. Acad Emerg Med 2014; 21:154–162. doi: 10.1111/acem.12314. [DOI] [PubMed] [Google Scholar]
- 28.Yeh YC, Wang MJ, Chao A, Ko WJ, Chan WS, Fan SZ, et al. Correlation between early sublingual small vessel density and late blood lactate level in critically ill surgical patients. J Surg Res 2013; 180:317–321. doi: 10.1016/j.jss.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 29.Hernandez G, Boerma EC, Dubin A, Bruhn A, Koopmans M, Edul VK, et al. Severe abnormalities in microvascular perfused vessel density are associated to organ dysfunctions and mortality and can be predicted by hyperlactatemia and norepinephrine requirements in septic shock patients. J Crit Care 2013; 28:538–539. doi: 10.1016/j.jcrc.2012.11.022. [DOI] [PubMed] [Google Scholar]
- 30.Vellinga N, Boerma EC, Koopmans M, Donati A, Dubin A, Shapiro NI, et al. Mildly elevated lactate levels are associated with microcirculatory flow abnormalities and increased mortality: a microSOAP post hoc analysis. Crit Care 2017; 21:255.doi: 10.1186/s13054-017-1842-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haas SA, Lange T, Saugel B, Petzoldt M, Fuhrmann V, Metschke M, et al. Severe hyperlactatemia, lactate clearance and mortality in unselected critically ill patients. Intensive Care Med 2016; 42:202–210. doi: 10.1007/s00134-015-4127-0. [DOI] [PubMed] [Google Scholar]
- 32.Libert C, Ayala A, Bauer M, Cavaillon JM, Deutschman C, Frostell C, et al. Part II: minimum quality threshold in preclinical sepsis studies (MQTiPSS) for types of infections and organ dysfunction endpoints. Shock 2019; 51:23–32. doi: 10.1097/SHK.0000000000001242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Post LO, Farrell DE, Cope CV, Baker JD, Myers MJ. The effect of endotoxin and dexamethasone on enrofloxacin pharmacokinetic parameters in swine. J Pharmacol Exp Ther 2003; 304:889–895. doi: 10.1124/jpet.102.042416. [DOI] [PubMed] [Google Scholar]
- 34.Remick DG, Newcomb DE, Bolgos GL, Call DR. Comparison of the mortality and inflammatory response of two models of sepsis: lipopolysaccharide vs. cecal ligation and puncture. Shock 2000; 13:110–116. doi: 10.1097/00024382-200013020-00004. [DOI] [PubMed] [Google Scholar]


