Abstract
Background
Acinetobacter baumannii (A. baumannii) has become one of the most important opportunistic pathogens inducing nosocomial pneumonia and increasing mortality in critically ill patients recently. The interaction between A. baumannii infection and immune response can influence the prognosis of A. baumannii related pneumonia. The target of the present study was to investigate the role of immunodeficiency in A. baumannii induced pneumonia.
Methods
Male BALB/c mice were randomly divided into the normal immunity control (NIC) group, normal immunity infection (NIA) group, immune compromised control (CIC) group, and immune compromised infection (CIA) group (n = 15 for each group). Intraperitoneal injection of cyclophosphamide and intranasal instillation of A. baumannii solution were used to induce compromised immunity and murine pneumonia, respectively. The mice were sacrificed at 6 and 24 h later and the specimens were collected for further tests. Seven-day mortality of mice was also assessed.
Results
After A. baumannii stimulation, the recruitment of neutrophils in mice with normal immunity increased sharply (P = 0.030 at 6 h), while there was no significant raise of neutrophil counts in mice with compromised immune condition (P = 0.092 at 6 h, P = 0.772 at 24 h). The Th cell polarization presented with pulmonary interleukin (IL)-4 and interferon (IFN)-γ level in response to the A. baumannii in CIA group were significantly depressed in comparison with in NIA group (IFN-γ: P = 0.003 at 6 h; P = 0.001 at 24 h; IL-4: P < 0.001 at 6 h; P < 0.001 at 24 h). The pulmonary conventional dendritic cell accumulation was even found to be inhibited after A. baumannii infection in immunocompromised mice (P = 0.033). Correspondingly, A. baumannii associated pneumonia in mice with compromised immunity caused more early stage death, more severe histopathological impairment in lung.
Conclusion
A. baumannii could frustrate the immune response in immunocompromised conditions, and this reduced immune response is related to more severe lung injury and worse outcome in A. baumannii induced pneumonia.
Keywords: Acinetobacter baumannii, Compromised immunity, Dendritic cells, Helper T cell, Neutrophilic granulocytes, Pneumonia
Introduction
Acinetobacter baumannii (A. baumannii) which is an emerging nosocomial, opportunistic pathogen causing a wide range of clinical manifestations, has become a significant challenge in intensive care unit (ICU) patients. Although A. baumannii is considered to have limited virulence, the occurrence of multiple antimicrobial resistance and the biofilm formation enhance their spread and limit our ability to eliminate Acinetobacter species.[1] The prevalence of A. baumannii colonization/ infection in ICU was 1.23 to 4.35 cases/1000 patient-days causing a mortality of 27.8% to 36.5%.[2–4] Our own study showed that A. baumannii was the most common multi-drug resistant bacterium in ICU.[5] The most frequent clinical manifestations of A. baumannii infection in critically ill patients is pneumonia. The rate of pneumonia in A. baumannii infection ranges from 46.2% to 91.9%.[2,4,6] Likewise, A. baumannii has become one of the most important opportunistic pathogens in nosocomial pneumonia.[7] In China, its infection accounted for 25.8% of the hospital-acquired pneumonia (HAP) and 28.4% of the Gram-negative bacteria induced HAP.[8]
The specific structures of A. baumannii facilitate the biofilm formation and bacteria colonization on skin, conjunctiva, oral, respiratory, rectal, and genitourinary tracts of patients by promoting bacterial adherence to various surfaces.[9,10] The colonization rate in critically ill patients detected with A. baumannii positive is as high as 44.3%.[6] The colonization on the respiratory tract can advance to pneumonia preferentially in immunocompromised patients.[4,11,12] On the other side, A. baumannii was also found to be able to impair immune functions.[13,14] The interaction of host immunity and A. baumannii influents the severity of infection,[1,15] as well as the clinical outcome. Correspondingly, immunomodulatory therapeutics in patients with compromised immune status may be a beneficial strategy for treatment of Acinetobacter infections. However, it is dependent on a more complete understanding of their mutual effects.
As both innate and adaptive immune response are involved in the pathogenesis of A. baumannii induced pneumonia,[16–18] in the present study, we observed the response of the neutrophilic granulocytes, conventional dendritic cells (cDCs) and lymphocytes to A. baumannii in A. baumannii induced pneumonia mice model under different immune conditions to explore the relationship between the immune conditions and the lung injury induced by A. baumannii infection.
Methods
Animals
Eight- to 10-week-old BALB/c male mice were provided by the Laboratory Animal Center of Yangzhou University (Yangzhou, China). Mice were housed under specific pathogen-free conditions and were given free access to sterile water and certified mouse food. The experimental procedures involving animals in this study complied with the National Research Council's guidelines and were approved by the Care of Experimental Animals Committee of the Southeast University (No. 20140518002).
Experimental protocol
Mice were randomly divided into four groups (n = 15 for each group): the normal immunity control (NIC) group, the normal immunity with A. baumannii infection (NIA) group, the compromised immunity control (CIC) group and the compromised immunity with A. baumannii infection (CIA) group. Mice in CIC and CIA groups were intraperitoneally injected with 75 mg/kg cyclophosphamide (CTX, Jiangsu Hengrui Medicine, China) on the first day and 50 mg/kg CTX on the fourth day to induce immunocompromised model. Meanwhile, the mice in the NIC and NIA groups were injected intraperitoneally the same volume of saline. Subsequently, murine pneumonia in NIA and CIA group was induced on the fifth day by intranasal instillation of 50 μL bacterial solution containing 108 colony forming units/mL of A. baumannii (ATCC-19606, American Type Culture Collection, ATCC, Manassas, VA, USA) following anesthesia with 10% chloral hydrate. For the NIC and CIC groups, mice were administered with the same volume of saline intranasally. Five mice were sacrificed at 6 or 24 h, respectively after bacteria administration. The left lung was collected to detect the wet weight to dry weight ratio, the right superior lobe was preserved in liquid nitrogen to detect enzyme-linked immunosorbent assay (ELISA) and Western blotting subsequently, the right inferior lobe was fixed in formalin for subsequent hematoxylin and eosin staining, and the right middle lobe was separated to prepare for flow cytometry. The other five mice in each group were kept for evaluating the 7-day mortality.
Hematoxylin and eosin staining and lung injury scoring
The right inferior lobe was embedded in paraffin. Then the embedded blocks were sliced sagittally at 5 μm thickness. The sections were stained with hematoxylin and eosin. The severity of lung injury was semi-quantitatively evaluated according to the criteria of Smith et al.[19] Briefly, edema, alveolar, and interstitial inflammation and hemorrhage, atelectasis, necrosis, and hyaline membrane formation were each scored using a 0 to 4 point scale: 0, no injury; 1, injury in 25% of the field; 2, injury in 50%; 3, injury in 75%; and 4, injury throughout the field. The sum of scores reflected the extent of lung injury. Ten randomly selected high-power fields (400×) in each slide were analyzed by two investigators who were blinded to the mouse groups.
Lung wet/dry weight ratio
Lung edema was determined using the lung wet/dry weight ratio. Briefly, the whole left lung was removed, blotted, and weighed. Subsequently, the lung was placed in an oven at 60°C for 48 h to dry and then was reweighed. Wet/dry weight ratios were then calculated.
Neutrophilic granulocyte and lymphocyte counts
After sacrificing the mice, 0.5 mL blood was collected from the heart for neutrophilic granulocyte and lymphocyte counts using a hematology analyzer.
Lung cell isolation
Single-cell suspensions were obtained according to our previous protocol to analyze the phenotypes of lung cDCs.[20] Briefly, the right middle lobe was finely dissected into small pieces with scissors. It then was digested using type V collagenase (Sigma-Aldrich, St. Louis, MO, USA). Subsequently, digested lung debris were pressed through a 70-μm sterile mesh and were cleared of red cells with red blood cell lysis buffer to generate single-cell suspensions.
Flow cytometry
To analyze the accumulation and maturation of pulmonary cDCs with flow cytometry, the pulmonary single-cell suspensions were stained with monoclonal antibodies including phycoerythrin (PE)-labeled anti-CD11c monoclonal antibody (clone: REA754) (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany), Percpcy5.5-labeled anti-CD11b monoclonal antibody (clone: M1/70) (eBioscience, San Diego, CA, USA) and fluorescein isothiocyanate (FITC)-labeled anti-MHC II monoclonal antibody (clone: REA813) (MACS Phamingen) after blocking Fc binding. Phenotypic expression was acquired using the flow cytometer (FACS Calibur, BD) and analyzed by Flow Jo software (Treestar, San Carlos, USA). For each analysis, 100,000 events were collected. Cells in the single-cell suspensions isolated from the lung tissue with CD11c+ and CD11b+ were defined as cDCs. MHC II+ in CD11c+ and CD11b+ lung cells reflect the maturation of cDCs. Each sample derived from one mouse was analyzed one time.
ELISA
Level of interferon (IFN)-γ and interleukin (IL)-4 in lung homogenates were assessed by the commercialized sandwich ELISA kit (Cusabio Biotech Co., Ltd, China) strictly according to the instructions of the manufacturer. The concentrations of these cytokines were calculated from a standard curve and were expressed in picograms or nanograms/milligram lung tissue. Each sample was duplicated tested.
Western blotting
Total protein lysates were extracted from the right superior lobe using radio-immunpresipitering assay lysis buffer (Beyotime Institute of Biotechnology, China). Following separation by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, the proteins were electro-transferred to polyvinylidene fluoride membranes (Millipore, Bedford, USA). These were then blocked for 1 h at room temperature and were incubated at 4°C overnight with primary antibodies to GATA-3, T-bet, and β-actin (Santa Cruz Biotechnology, Santa Cruz, USA), respectively. On the following day, the immunoreactive bands were detected with a chemiluminescence imaging system (ChemiQ 4800mini, Ouxiang, China) after incubation with a horseradish peroxidase-conjugated secondary antibody (Zhongshan Golden Bridge Biotechnology, Beijing, China) for 1 h at room temperature. Each protein was repeated three times with protein collected from different mice.
Statistical analyses
Continuous variables were presented as the mean ± standard deviation and the median (interquartile range) based on the distribution of quantitative variables. Statistical analyses were performed using SPSS 12.0 (SPSS Inc., Chicago, IL, USA). Groups with normal distribution were compared using Student's t test or one-way analysis. Non-parametric tests of comparison were used for variables evaluated as not normally distributed. The differences in categorical variables were assessed using the χ2 test or the Fisher exact test.
Results
Induction of immunocompromised condition with CTX
The immunocompromised mice exhibited decreased appetite and activity, dullness and tarnished fur after CTX treatment. Compared with control mice in the NIC group, a significant reduction of neutrophilic granulocytes was observed in the CIC group (CIC vs. NIC: 62.00 ± 31.14 × 106/L vs. 374.00 ± 147.24 × 106/L at 6 h, P = 0.002; 62.00 ± 55.41 × 106/L vs. 462.50 ± 311.17 × 106/L at 24 h, P = 0.024) [Figure 1A]. There was also an inhibition of lymphocytes in CIC group compared with NIC group (470.00 ± 296.31 × 106/L vs. 1712.50 ± 776.80 × 106/L at 6 h, P = 0.043; 468.00 ± 296.77 × 106/L vs. 1560.00 ± 699.29 × 106/L at 24 h, P = 0.015) [Figure 1B]. The pulmonary cDC counts defined as CD11c+ and CD11b+ with flow cytometry was less in CIC group than in NIC group (0.37% ± 0.14% vs. 0.58% ± 0.12% at 6 h, P = 0.039; 0.46% ± 0.09% vs. 0.61% ± 0.07% at 24 h, P = 0.016) [Figure 2A], but there was no difference in the maturation percentage of cDCs (CD11c+, CD11b+, MHC II+) between these two groups (47.84% ± 13.78% vs. 41.06% ± 13.29% at 6 h, P = 0.451; 71.60% ± 16.41% vs. 57.74% ± 12.02% at 24 h, P = 0.241) [Figure 2B].
Figure 1.
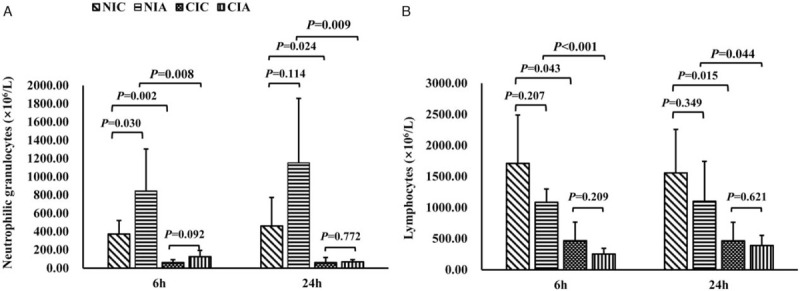
Neutrophilic granulocytes and lymphocytes in mice of A. baumannii-induced pneumonia with different immune conditions. Blood neutrophilic granulocytes (A) and lymphocytes (B) in mice with or without A. baumannii instillation in normal or compromised immune conditions at 6 and 24 h were analyzed. n = 5 for each group at each time point. CIA: Immune compromised infection; CIC: Immune compromised control; NIA: Normal immunity infection; NIC: Normal immunity control.
Figure 2.
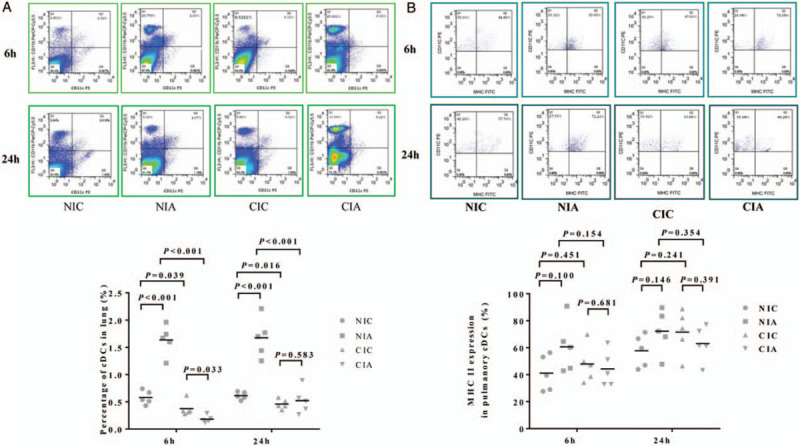
The effect of A. baumannii instillation on the quantity and maturation of pulmonary cDCs in mice with different immune conditions. (A) The percentage of cDCs (CD11c+ and CD11b+) in the single-cell suspensions isolated from the lung tissue in each group was analyzed using flow cytometry. (B) The percentage of mature pulmonary cDCs marked with MHCII+ in CD11c+ and CD11b+ was examined using flow cytometric analysis. n = 5 for each group at each time point. cDCs: Conventional dendritic cells; CIA: Immune compromised infection; CIC: Immune compromised control; NIA: Normal immunity infection; NIC: Normal immunity control.
Effect of A. baumannii on neutrophilic granulocytes in mice with different immune conditions
In mice with normal immunity, the neutrophilic granulocyte counts after A. baumannii stimulation (NIA) increased about 2.5 to 2.8 times compared with NIC mice (846.00 ± 459.71×106/L vs. 374.00 ± 147.24 × 106/L at 6 h, P = 0.030; 1154.00 ± 704.61 × 106/L vs. 462.50 ± 311.17 × 106/L at 24 h, P = 0.114). However, there was no significant raise of neutrophil counts in mice with compromised immune condition at 6 or 24 h of bacteria activation (CIA vs. CIC: 126.00 ± 68.04 × 106/L vs. 62.00 ± 31.14 × 106/L at 6 h, P = 0.092; 70.00 ± 22.36 × 106/L vs. 62.00 ± 55.41 × 106/L at 24 h, P = 0.772). Additionally, as for the neutrophilic granulocytes after bacteria stimulation in mice with different immune condition, it was found that neutrophilic granulocytes in mice with compromised immune condition (CIA) were significantly less than neutrophilic granulocytes in mice with normal immune condition (NIA) (126.00 ± 68.04 × 106/L vs. 846.00 ± 459.71 × 106/L at 6 h, P = 0.008; 70.00 ± 22.36 × 106/L vs. 1154.00 ± 704.61 × 106/L at 24 h, P = 0.009) [Figure 1A].
Effect of A. baumannii on lymphocytes in mice with different immune conditions
There was no significant difference in lymphocyte counts between mice in NIC group and NIA group (1712.50 ± 776.80 × 106/L vs. 1087.50 ± 212.35 × 106/L at 6 h, P = 0.207; 1560.00 ± 699.29 × 106/L vs. 1102.00 ± 644.26 × 106/L at 24 h, P = 0.349) or between mice in CIC and CIA group (470.00 ± 296.31 × 106/L vs. 255.00 ± 90.37 × 106/L at 6 h, P = 0.209; 468.00 ± 296.77 × 106/L vs. 390.0 ± 163.71 × 106/L at 24 h, P = 0.621) [Figure 1B]. When we compare lymphocytes after bacteria stimulation in mice with different immune conditions, much less lymphocytes were observed in mice with compromised immune condition (CIA) than in mice with normal immune condition (NIA) (255.00 ± 90.37 × 106/L vs. 1087.50 ± 212.35 × 106/L at 6 h, P < 0.001; 390.00 ± 163.71 × 106/L vs. 1102.00 ± 644.26 × 106/L at 24 h, P = 0.044).
Accumulation and maturation of pulmonary cDCs in A. baumannii induced pneumonia mice with different immune conditions
A. baumannii instillation increased the accumulation of pulmonary cDCs in mice with normal immunity (NIA group) compared with the control mice in NIC group at either 6 h (1.64% ± 0.27% vs. 0.58% ± 0.12%, P < 0.001) or 24 h (1.67% ± 0.36% vs. 0.61% ± 0.07%, P < 0.001). However, there was no significant difference in pulmonary cDCs rate in the mice with compromised immunity (CIA group) and the control mice of CIC group at 6 h (0.18% ± 0.07% vs. 0.37% ± 0.14%, P = 0.033) or 24 h (0.52% ± 0.24% vs. 0.46% ± 0.09, P = 0.583) [Figure 2A].
As for the cDC maturation, A. baumannii instillation had the trend to increase the maturation of pulmonary cDCs in mice with normal immunity (NIA group) compared with the control mice in NIC group, but no statistically significant difference was found (60.68% ± 19.34% vs. 41.06% ± 13.29% at 6 h, P = 0.100; or 72.24% ± 16.15% vs. 57.74% ± 12.02% at 24 h, P = 0.146). Similarly, there was no significant difference between CIA and CIC groups at neither 6 h (44.20% ± 13.16% vs. 47.84% ± 13.78%, P = 0.681) nor 24 h (63.08% ± 13.11% vs. 71.60% ± 16.41%, P = 0.391) [Figure 2B].
Polarization of Th cells after A. baumannii infection under different immune conditions
To assess Th cell polarization, the expression of T-bet protein, a Th1-inducing transcription factor and the expression of GATA-3, a Th2-related transcription factor at 24 h were examined by Western blotting. Also, the production of IFN-γ and IL-4, the Th1 and Th2 related cytokine respectively, in mouse lung at 6 and 24 h were evaluated using ELISA. There was no significant difference in the expression of these proteins between NIC and CIC groups (the relative expression of T-bet: 0.14 ± 0.04 vs. 0.19 ± 0.10, P = 0.299; the relative expression of GATA-3: 0.26 ± 0.04 vs. 0.32 ± 0.04, P = 0.139). Although we found that T-bet and GATA-3 increased after A. baumannii infection with both normal (the relative expression of T-bet in NIA vs. NIA: 0.14 ± 0.04 vs. 0.65 ± 0.09, P = 0.001; the relative expression of GATA-3 in NIC vs. NIA: 0.27 ± 0.04 vs. 0.87 ± 0.20, P = 0.007) and compromised immunity (the relative expression of T-bet in CIC vs. CIA: 0.19 ± 0.10 vs. 0.43 ± 0.03, P = 0.042; the relative expression of GATA-3 in CIC vs. CIA: 0.32 ± 0.04 vs. 0.64 ± 0.12, P = 0.014), the increment among mice with normal immunity was more significant than mice with compromised immunity (the relative expression of T-bet in NIA vs. CIA: 0.65 ± 0.09 vs. 0.43 ± 0.03, P = 0.044; the relative expression of GATA-3 in NIA vs. CIA: 0.87 ± 0.20 vs. 0.64 ± 0.12, P = 0.248) [Figure 3A].
Figure 3.
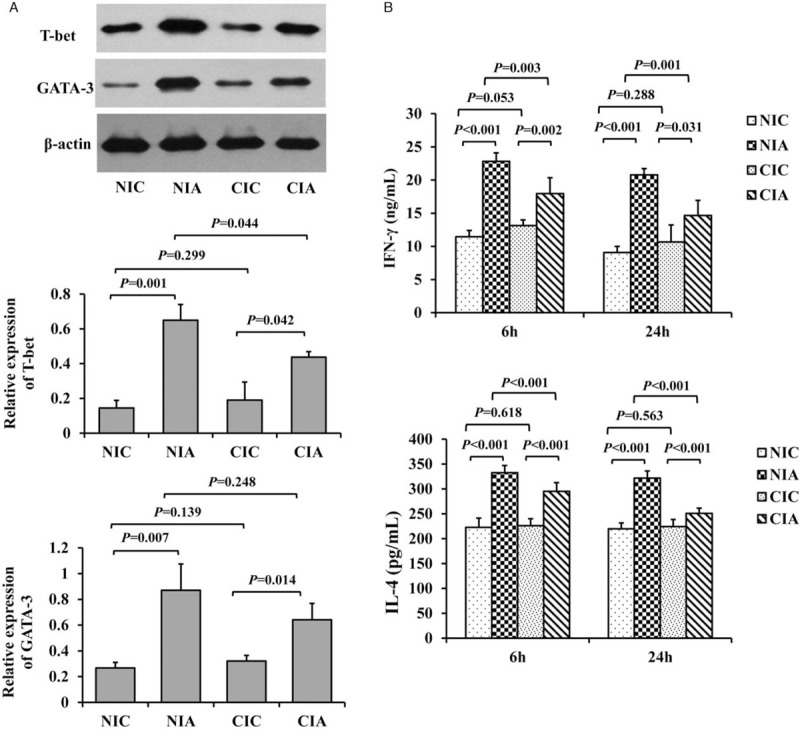
Th cell polarization in mice of A. baumannii-induced pneumonia with different immune conditions. (A) The expression of T-bet, a Th1-inducing transcription factor and GATA-3, a Th2 related transcription factor in mice receiving or not the instillation of A. baumannii with different immune conditions at 6 and 24 h were examined by Western blotting (n = 5 for each group at each time point). (B) Pulmonary Th1- and Th2-associated cytokines, IFN-γ and IL-4 respectively, with or without the induction of A. baumannii-associated pneumonia in mice with different immune conditions at 6 or 24 h were examined by ELISA tests. n = 5 for each group at each time point. CIA: Immune compromised infection; CIC: Immune compromised control; ELISA: Enzyme-linked immunosorbent assay; IFN: Interferon; IL: Interleukin; NIA: Normal immunity infection; NIC: Normal immunity control.
In consistent with the results of T-bet and GATA-3 proteins, no changes in IFN-γ and IL-4 level between NIC and CIC group were observed (IFN-γ: 11.45 ± 0.95 vs. 12.71 ± 0.80 ng/mL at 6 h, P = 0.053; 9.09 ± 0.91 vs. 10.67 ± 2.56 ng/mL at 24 h, P = 0.228; IL-4: 222.74 ± 18.84 vs. 226.47 ± 13.72 pg/mL at 6 h, P = 0.618; 220.10 ± 11.53 vs. 224.33 ± 14.30 pg/mL at 24 h, P = 0.563). Both the levels of IFN-γ and IL-4 were significantly increased at 6 and 24 h after A. baumannii challenge in mice with normal (IFN-γ in NIC vs. NIA: 11.45 ± 0.95 vs. 22.83 ± 1.28 ng/mL at 6 h, P < 0.001; 9.09 ± 0.91 vs. 20.79 ± 0.94 ng/mL at 24 h, P < 0.001; IL-4 in NIC vs. NIA: 222.74 ± 18.84 vs. 332.68 ± 14.63 pg/mL at 6 h, P < 0.001; 220.10 ± 11.53 vs. 271.56 ± 10.85 pg/mL at 24 h, P < 0.001) or compromised immunity and the IL-4 and IFN-γ level were higher in the NIA group than those in the CIA group (IFN-γ in CIC vs. CIA: 12.71 ± 0.80 vs. 17.95 ± 2.40 ng/mL at 6 h, P = 0.002; 10.67 ± 2.56 vs. 14.67 ± 2.27 ng/mL at 24 h, P = 0.031; IL-4 in CIC vs. CIA: 226.47 ± 13.72 vs. 295.36 ± 17.49 pg/mL at 6 h, P < 0.001; 224.33 ± 14.30 vs. 251.03 ± 10.28 pg/mL at 24 h, P < 0.001). However, the increase of IL-4 and IFN-γ in mice with normal immunity were much higher than in the immunocompromised mice (IFN-γ in NIA vs. CIA: 22.83 ± 1.28 vs. 17.95 ± 2.40 ng/mL at 6 h, P = 0.003; 20.79 ± 0.94 vs. 14.67 ± 2.27 ng/mL at 24 h, P = 0.001; IL-4 in NIA vs. CIA: 332.68 ± 14.63 vs. 295.36 ± 17.49 pg/mL at 6 h, P < 0.001; 271.56 ± 10.85 vs. 251.03 ± 10.28 pg/mL at 24 h, P < 0.001) [Figure 3B].
Effect of A. baumannii on lung impairment and mortality among mice under different immune conditions
The pulmonary histopathology of each group after 6 or 24 h of administration with bacterium or saline was analyzed. We found that control mice in the NIC and CIC groups had relatively normal lung tissue, while the A. baumannii infection resulted in a thickened alveolar wall, alveolar and interstitial inflammatory cell infiltration, alveolar exudates and edema 6 h later. The pulmonary histopathology was exacerbated at 24 h with more significant inflammatory cell infiltration and hemorrhage. The Smith score for quantification of the lung injury was also raised in NIA or CIA group at 6 or 24 h compared with their control mice (NIC vs. NIA: 2.52 ± 0.19 vs. 3.22 ± 0.38 at 6 h, P = 0.007; 2.66 ± 0.15 vs. 5.02 ± 0.65 at 24 h, P < 0.001; CIC vs. CIA: 2.64 ± 0.11 vs. 3.36 ± 0.24 at 6 h, P < 0.001; 2.80 ± 0.24 vs. 8.08 ± 0.55, P < 0.001). Comparatively, the extent of lung injury was much more serious in CIA group at 24 h compared with lung injury in NIA group (3.22 ± 0.38 vs. 3.36 ± 0.24 at 6 h, P = 0.509; 5.02 ± 0.65 vs. 8.08 ± 0.55 at 24 h, P < 0.001) [Figure 4A].
Figure 4.
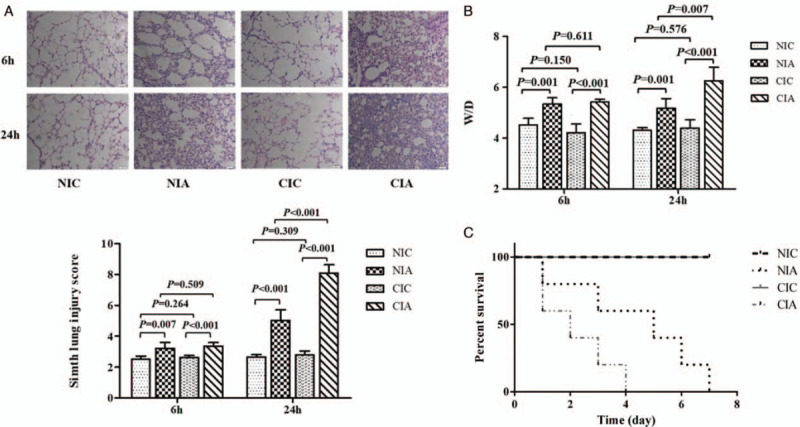
The effect of A. baumannii instillation on the pulmonary histopathology, pulmonary permeability, and mortality among mice with different immune conditions. (A) Hematoxylin and eosin staining and Smith score analysis to evaluate the histopathological changes in lung tissues from mice in each experimental group were performed at 6 and 24 h after A. baumannii administration (original magnification ×200). (B) Lung edema was measured by the ratio of lung wet weight to dry weight (W/D) at 6 and 24 h of the induction of A. baumannii-associated pneumonia in mice with different immune conditions. n = 5 for each group at each time point. (C) The mortalities of mice in different experimental groups were analyzed seven days after the administration with A. baumannii (n = 5 for each group). Comparing the NIA and CIA group, the hazard ratio is 0.37 (95% confidence interval, 0.05–0.85; P = 0.066). CIA: Immune compromised infection; CIC: Immune compromised control; NIA: Normal immunity infection; NIC: Normal immunity control.
Lung wet/dry weight ratio was calculated to evaluate permeability of pulmonary capillary and the injury of lung tissue. We found that the control mice with normal or compromised immunity had similar wet/dry ratio (NIC vs. CIC: 4.52 ± 0.26 vs. 4.22 ± 0.34 at 6 h, P = 0.150; 4.31 ± 0.10 vs. 4.40 ± 0.32 at 24 h, P = 0.576), but after infection with A. baumannii, the wet/dry weight ratio was significantly higher at either 6 or 24 h (CIA or NIA group) than their respective control (NIC vs. NIA: 4.52 ± 0.26 vs. 5.35 ± 0.24 at 6 h, P = 0.001; 4.31 ± 0.10 vs. 5.18 ± 0.38 at 24 h, P = 0.001; CIC vs. CIA: 4.22 ± 0.34 vs. 5.42 ± 0.11 at 6 h, P < 0.001; 4.40 ± 0.32 vs. 6.25 ± 0.54 at 24 h, P < 0.001). The increase seemed most significant in CIA group than in NIA group especially at 24 h (5.35 ± 0.24 vs. 5.42 ± 0.11 at 6 h, P = 0.611; 5.18 ± 0.38 vs. 6.25 ± 0.54 at 24 h, P = 0.007) [Figure 4B].
Additionally, we also assessed the seven-day mortality among these groups. Seven-day mortality of the mice in NIC and CIC group were 0% and 100% in NIA and CIA group. However, after A. baumannii infection, the immunocompromised mice (CIA group) all died within four days, while only 2/5 mice died within 4 days in the NIA group. It seemed that the infected mice with compromised immunity died earlier and faster than mice with normal immunity. Comparing the NIA and CIA group, the hazard ratio is 0.37 (95% confidence interval, 0.05–0.85; P = 0.066) [Figure 4C].
Discussion
Based on these results, a reduced immune response to A. baumannii instillation could be found in mice with compromised immune condition than mice with normal immunity. As expected, the compromised immunity was related to more severe lung injury and worse outcome in mice of A. baumannii pneumonia.
CTX was intraperitoneally administered in our study to induce immune deficiency in mice. CTX is a kind of alkylating agent which has been widely used as an immunosuppressive drug to inactivate the rapidly cycling immune cell population in some other investigations.[21,22] The relatively moderate dose of CTX used in our study was verified to be able to induce granulopenia, while also avoided increased mortality of mice receiving CTX administration within seven days. Besides neutrophlic granulocytes, CTX also took negative effect on lymphocytes and dendritic cells.
Neutrophils play a critical role in host resistance to respiratory A. baumannii infection. Early recruitment of neutrophils which take responsibilities in rapidly and continuously engulfing and killing bacteria is critical for initiating an efficient host defense against respiratory A. baumannii infection.[23,24] It was found that neutropenia was related to inhibited bacteria clearance, alteration of pro-inflammatory cytokine release thus leading to more severe microbial disease in A. baumannii infection.[25,26] In our study, at 6 h of A. baumannii administration, the recruitment of peripheral neutrophils sharply rose in mice with normal immunity. However, no changes in the neutrophil counts between the immunocompromised mice receiving or not receiving A. baumannii. The interactions between neutrophils and A. baumannii remain largely unknown. Some recent studies revealed that A. baumannii had negative effects on neutrophils. In the study of Kamoshida et al,[27] it was found that A. baumannii suppressed the adhesion ability of neutrophils through suppression of the surface expression of CD11a, thereby inhibiting PMA-induced NET formation. Another study found that A. baumannii could influence the neutrophil chemotaxis through the accumulation of phenylacetate.[28] The negative effects of A. baumannii on neutrophils might become more evident in compromised immune conditions. Still, it was hard to totally exclude the toxicity of CTX.
cDCs are specialized in the processing and presentation of antigens. They also activate the adaptive immune response.[29,30] Pulmonary cDCs mainly reside in the airway epithelium, alveolar septa or around pulmonary vessels with immature phenotypes.[31] In response to infection/inflammation, cDCs accumulate rapidly in lung, in some circumstances peaking as early as 2 h after challenge.[32,33] DCs play a positive role in the defensive immune response in infections resulting from multiple pathogenic bacteria.[34,35] For example, after fungal infection, DCs capture the fungal particles and present antigens to T cells, which induce a specific Th1 adaptive immune response to fungus.[36–39] Pene et al[40] found that intratracheal administration of DC in mice with compromised immunity infected with Pseudomonas aeruginosa could significantly improve the survival of these mice. The similar results found by Bohannon et al[41] indicated that DC could accelerate the clearance of Pseudomonas aeruginosa in burned mice. cDCs are also involved in the immune response to A. baumannii. Once mobilized to the lung, DCs sample incoming antigens and undergo the maturation process which is phenotypically characterized by the upregulation of cell-surface MHC II and co-stimulatory molecule expression (eg, CD80, CD86),[31] and elicited a specific Th1 response to enhance the immunity to A. baumannii.[18] In a recent study, it was found that the outer membrane vesicle from an A. baumannii clinical strain could activate bone marrow-derived DC to promote Th2 activity.[42] In our study, the finding that a rapid accumulation and maturation of cDCs in the lung of mice with normal immunity after A. baumannii stimulation suggested that cDCs were involved in the immune response to A. baumannii.
However, we noticed a significant inhibition of cDC counts presented after the induction of A. baumannii related pneumonia in immunocompromised mice. This may be explained by the virulence of A. baumannii to DCs. According to the study of Lee et al,[14] higher concentrations of A. baumannii could attack the mitochondria of DCs, thus causing apoptosis and necrosis of DCs as well as inhibition of both innate and adaptive immunity through the large quantities of reactive oxygen species produced.
Adaptive immunity is also involved in the immune response to A. baumannii. Upon receiving signals through the binding of antigen to the T-cell receptor in the presence of polarizing cytokines, naive Th precursor cells differentiate into Th1, Th2, and so on.[43] Interestingly, in our study, the lymphocyte counts had a decreased trend after A. baumannii instillation in mice with either normal or compromised immunity. It seems that the innate immunity may take a more critical role in the antimicrobial immune response to A. baumannii. The role of the adaptive immunity in A. baumannii infection as well as the effect of A. baumannii on adaptive immunity were rarely concerned. In our study, CTX administration did not influence significantly the Th cells polarization. However, the activation of either Th1 or Th2 polarization after A. baumannii stimulation was not as evident as in mice with normal immunity. The impairment of both Th1 and Th2 adaptive immunity in response to A. baumannii stimulation may be resulted from the compromised activity of cDCs.
There were some limitations to this paper. First, we used standard strains of A. baumannii, which may be different from the multi-drug resistant A. baumannii confronted in the clinic. Accordingly, the results may not fully match actual clinical situations. Second, we used CTX to induce the immunocompromised conditions, which had a wide inhibited effect on various immune cells. It was hard to clarify whether the reduced immune response to A. baumannii could be explained by the virulence of A. baumannii on immune cells under deficient immune condition or the negative effect of immunodeficiency induced by CTX. Further exploration about the specific effect and mechanisms were lack in this study which should be carried out in the upcoming investigation.
In summary, we may come to the conclusion that A. baumannii infection may induce an inhibited immune response in compromised immune conditions, and this reduced immune response is related to more severe lung injury and worse outcome in A. baumannii induced pneumonia. Further studies are needed to clarify the mutual effects of A. baumannii on certain immune cells and the underling mechanisms.
Funding
This project was supported by grants from the National Natural Science Foundations of China (No. 81300060), Specialized Research Fund for the Doctoral Program of Higher Education of China (No. 20130092120070).
Conflicts of interest
None.
Footnotes
How to cite this article: Liu AR, Du WJ, Xie JF, Xu JY, Huang YZ, Qiu HB, Yang Y. Role of immunodeficiency in Acinetobacter baumannii associated pneumonia in mice. Chin Med J 2020;133:2161–2169. doi: 10.1097/CM9.0000000000001027
References
- 1.Mortensen BL, Skaar EP. Host-microbe interactions that shape the pathogenesis of Acinetobacter baumannii infection. Cell Microbiol 2012; 14:1336–1344. doi: 10.1111/j.1462-5822.2012.01817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garnacho-Montero J, Gutierrez-Pizarraya A, Diaz-Martin A, Cisneros-Herreros JM, Cano ME, Gato E, et al. Acinetobacter baumannii in critically ill patients: molecular epidemiology, clinical features and predictors of mortality. Enferm Infecc Microbiol Clin 2016; 34:551–558. doi: 10.1016/j.eimc.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 3.Ju MH, Yao YL, Du CL, Chen S, Song YL. Subsequent multidrug-resistant bacteremia is a risk factor for short-term mortality of patients with ventilator-associated pneumonia caused by Acinetobacter baumannii in intensive care unit: a multicenter experience. Chin Med J 2018; 131:361–363. doi: 10.4103/0366-6999.223859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xiao D, Wang L, Zhang D, Xiang D, Liu Q, Xing X. Prognosis of patients with Acinetobacter baumannii infection in the intensive care unit: a retrospective analysis. Exp Ther Med 2017; 13:1630–1633. doi: 10.3892/etm.2017.4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xie J, Ma X, Huang Y, Mo M, Guo F, Yang Y, et al. Value of American Thoracic Society guidelines in predicting infection or colonization with multidrug-resistant organisms in critically ill patients. PLoS One 2014; 9:e89687.doi: 10.1371/journal.pone.0089687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Villar M, Cano ME, Gato E, Garnacho-Montero J, Miguel Cisneros J, Ruiz de Alegria C, et al. Epidemiologic and clinical impact of Acinetobacter baumannii colonization and infection: a reappraisal. Medicine (Baltimore) 2014; 93:202–210. doi: 10.1097/MD.0000000000000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peleg AY, Hooper DC. Hospital-acquired infections due to gram-negative bacteria. N Engl J Med 2010; 362:1804–1813. doi: 10.1056/NEJMra0904124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feng DY, Zhou YQ, Zou XL, Zhou M, Wu WB, Chen XX, et al. Factors influencing mortality in hospital-acquired pneumonia caused by Gram-negative bacteria in China. J Infect Public Health 2019; 12:630–633. doi: 10.1016/j.jiph.2019.02.014. [DOI] [PubMed] [Google Scholar]
- 9.Li FJ, Starrs L, Burgio G. Tug of war between Acinetobacter baumannii and host immune responses. Pathog Dis 2018; 76:ftz004.doi: 10.1093/femspd/ftz004. [DOI] [PubMed] [Google Scholar]
- 10.Espinal P, Marti S, Vila J. Effect of biofilm formation on the survival of Acinetobacter baumannii on dry surfaces. J Hosp Infect 2012; 80:56–60. doi: 10.1016/j.jhin.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 11.Rice LB. Progress and challenges in implementing the research on ESKAPE pathogens. Infect Control Hosp Epidemiol 2010; 31: Suppl 1: S7–S10. doi: 10.1086/655995. [DOI] [PubMed] [Google Scholar]
- 12.Sengstock DM, Thyagarajan R, Apalara J, Mira A, Chopra T, Kaye KS. Multidrug-resistant Acinetobacter baumannii: an emerging pathogen among older adults in community hospitals and nursing homes. Clin Infect Dis 2010; 50:1611–1616. doi: 10.1086/652759. [DOI] [PubMed] [Google Scholar]
- 13.Kim CH, Jeong YJ, Lee J, Jeon SJ, Park SR, Kang MJ, et al. Essential role of toll-like receptor 4 in Acinetobacter baumannii-induced immune responses in immune cells. Microb Pathog 2013; 54:20–25. doi: 10.1016/j.micpath.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 14.Lee JS, Choi CH, Kim JW, Lee JC. Acinetobacter baumannii outer membrane protein A induces dendritic cell death through mitochondrial targeting. J Microbiol 2010; 48:387–392. doi: 10.1007/s12275-010-0155-1. [DOI] [PubMed] [Google Scholar]
- 15.Luo G, Lin L, Ibrahim AS, Baquir B, Pantapalangkoor P, Bonomo RA, et al. Active and passive immunization protects against lethal, extreme drug resistant-Acinetobacter baumannii infection. PLoS One 2012; 7:e29446.doi: 10.1371/journal.pone.0029446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsuchiya T, Nakao N, Yamamoto S, Hirai Y, Miyamoto K, Tsujibo H. NK1.1(+) cells regulate neutrophil migration in mice with Acinetobacter baumannii pneumonia. Microbiol Immunol 2012; 56:107–116. doi: 10.1111/j.1348-0421.2011.00402.x. [DOI] [PubMed] [Google Scholar]
- 17.Qiu H, KuoLee R, Harris G, Van Rooijen N, Patel GB, Chen W. Role of macrophages in early host resistance to respiratory Acinetobacter baumannii infection. PLoS One 2012; 7:e40019.doi: 10.1371/journal.pone.0040019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee JS, Lee JC, Lee CM, Jung ID, Jeong YI, Seong EY, et al. Outer membrane protein A of Acinetobacter baumannii induces differentiation of CD4+ T cells toward a Th1 polarizing phenotype through the activation of dendritic cells. Biochem Pharmacol 2007; 74:86–97. doi: 10.1016/j.bcp.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 19.Smith KM, Mrozek JD, Simonton SC, Bing DR, Meyers PA, Connett JE, et al. Prolonged partial liquid ventilation using conventional and high-frequency ventilatory techniques: gas exchange and lung pathology in an animal model of respiratory distress syndrome. Crit Care Med 1997; 25:1888–1897. doi: 10.1097/00003246-199711000-00030. [DOI] [PubMed] [Google Scholar]
- 20.Liu J, Zhang PS, Yu Q, Liu L, Yang Y, Qiu HB. Kinetic and distinct distribution of conventional dendritic cells in the early phase of lipopolysaccharide-induced acute lung injury. Mol Biol Rep 2012; 39:10421–10431. doi: 10.1007/s11033-012-1921-4. [DOI] [PubMed] [Google Scholar]
- 21.Ikezawa Y, Nakazawa M, Tamura C, Takahashi K, Minami M, Ikezawa Z. Cyclophosphamide decreases the number, percentage and the function of CD25+ CD4+ regulatory T cells, which suppress induction of contact hypersensitivity. J Dermatol Sci 2005; 39:105–112. doi: 10.1016/j.jdermsci.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 22.Shen RN, Wu B, Lu L, Kaiser HE, Broxmeyer HE. Recombinant human interleukin-1 alpha: a potent bio-immunomodifier in vivo in immunosuppressed mice induced by cyclophosphamide, retroviral infection and surgical stress. In Vivo 1994; 8:59–63. [PubMed] [Google Scholar]
- 23.Lazaro-Diez M, Chapartegui-Gonzalez I, Redondo-Salvo S, Leigh C, Merino D, Segundo DS, et al. Human neutrophils phagocytose and kill Acinetobacter baumannii and A. pittii. Sci Rep 2017; 7:4571.doi: 10.1038/s41598-017-04870-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pires S, Parker D. Innate immune responses to Acinetobacter baumannii in the airway. J Interferon Cytokine Res 2019; 39:441–449. doi: 10.1089/jir.2019.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grguric-Smith LM, Lee HH, Gandhi JA, Brennan MB, DeLeon-Rodriguez CM, Coelho C, et al. Neutropenia exacerbates infection by Acinetobacter baumannii clinical isolates in a murine wound model. Front Microbiol 2015; 6:1134.doi: 10.3389/fmicb.2015.01134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pires S, Peignier A, Seto J, Smyth DS, Parker D. Biological sex influences susceptibility to Acinetobacter baumannii pneumonia in mice. JCI Insight 2020; 5.doi: 10.1172/jci.insight.132223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kamoshida G, Kikuchi-Ueda T, Nishida S, Tansho-Nagakawa S, Ubagai T, Ono Y. Pathogenic bacterium Acinetobacter baumannii inhibits the formation of neutrophil extracellular traps by suppressing neutrophil adhesion. Front Immunol 2018; 9:178.doi: 10.3389/fimmu.2018.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhuiyan MS, Ellett F, Murray GL, Kostoulias X, Cerqueira GM, Schulze KE, et al. Acinetobacter baumannii phenylacetic acid metabolism influences infection outcome through a direct effect on neutrophil chemotaxis. Proc Natl Acad Sci U S A 2016; 113:9599–9604. doi: 10.1073/pnas.1523116113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature 1998; 392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 30.Mellman I, Steinman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell 2001; 106:255–258. doi: 10.1016/s0092-8674(01)00449-4. [DOI] [PubMed] [Google Scholar]
- 31.Vermaelen K, Pauwels R. Pulmonary dendritic cells. Am J Respir Crit Care Med 2005; 172:530–551. doi: 10.1164/rccm.200410-1384SO. [DOI] [PubMed] [Google Scholar]
- 32.McWilliam AS, Nelson D, Thomas JA, Holt PG. Rapid dendritic cell recruitment is a hallmark of the acute inflammatory response at mucosal surfaces. J Exp Med 1994; 179:1331–1336. doi: 10.1084/jem.179.4.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu K, Victora GD, Schwickert TA, Guermonprez P, Meredith MM, Yao K, et al. In vivo analysis of dendritic cell development and homeostasis. Science 2009; 324:392–397. doi: 10.1126/science.1170540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bedoui S, Kupz A, Wijburg OL, Walduck AK, Rescigno M, Strugnell RA. Different bacterial pathogens, different strategies, yet the aim is the same: evasion of intestinal dendritic cell recognition. J Immunol 2010; 184:2237–2242. doi: 10.4049/jimmunol.0902871. [DOI] [PubMed] [Google Scholar]
- 35.Constantino J, Gomes C, Falcao A, Neves BM, Cruz MT. Dendritic cell-based immunotherapy: a basic review and recent advances. Immunol Res 2017; 65:798–810. doi: 10.1007/s12026-017-8931-1. [DOI] [PubMed] [Google Scholar]
- 36.Wuthrich M, Deepe GS, Jr, Klein B. Adaptive immunity to fungi. Annu Rev Immunol 2012; 30:115–148. doi: 10.1146/annurev-immunol-020711-074958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brown GD. Innate antifungal immunity: the key role of phagocytes. Annu Rev Immunol 2011; 29:1–21. doi: 10.1146/annurev-immunol-030409-101229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramirez-Ortiz ZG, Means TK. The role of dendritic cells in the innate recognition of pathogenic fungi (A. fumigatus, C. neoformans and C. albicans). Virulence 2012; 3:635–646. doi: 10.4161/viru.22295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li LY, Zhang HR, Jiang ZL, Chang YZ, Shao CZ. Overexpression of dendritic cell-specific intercellular adhesion molecule-3-grabbing nonintegrin in dendritic cells protecting against aspergillosis. Chin Med J 2018; 131:2575–2582. doi: 10.4103/0366-6999.244103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pene F, Zuber B, Courtine E, Rousseau C, Ouaaz F, Toubiana J, et al. Dendritic cells modulate lung response to Pseudomonas aeruginosa in a murine model of sepsis-induced immune dysfunction. J Immunol 2008; 181:8513–8520. doi: 10.4049/jimmunol.181.12.8513. [DOI] [PubMed] [Google Scholar]
- 41.Bohannon J, Cui W, Sherwood E, Toliver-Kinsky T. Dendritic cell modification of neutrophil responses to infection after burn injury. J Immunol 2010; 185:2847–2853. doi: 10.4049/jimmunol.0903619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cai W, Kesavan DK, Cheng J, Vasudevan A, Wang H, Wan J, et al. Vesicle-mediated dendritic cell activation in Acinetobacter baumannii clinical isolate, which contributes to Th2 response. J Immunol Res 2019; 2019:2835256.doi: 10.1155/2019/2835256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jankovic D, Liu Z, Gause WC. Th1- and Th2-cell commitment during infectious disease: asymmetry in divergent pathways. Trends Immunol 2001; 22:450–457. doi: 10.1016/s1471-4906(01)01975-5. [DOI] [PubMed] [Google Scholar]


