Thrombolytic therapy within 4.5 h from symptom onset is a recognized effective and standard therapy for acute ischemic stroke (AIS), but some patients still have a poor clinical outcome. The evaluation and control of predictors for AIS poor clinical outcomes is integral to achieving optimal treatments, but the prognostic value of admission blood glucose (ABG) for this purpose is unclear and still under debate. ABG evaluated in patients without diabetes mellitus (DM) often suggests acute stress hyperglycemia, while ABG may have a close relationship with long-term blood glucose control in patients with DM. However, in most studies about the influence of ABG on the prognosis of recombinant tissue-type plasminogen activator (rtPA) therapy after AIS, patients were not classified into those with and without DM. The present study was designed to investigate the prognostic value of ABG for clinical outcomes of AIS after thrombolysis according to DM status in a Chinese population.
The Thrombolysis Implementation and Monitor of Acute Ischemic Stroke in China (TIMS-China) study comprised a prospective, multicenter, open-label, observational study conducted from January 2000 to November 2008 at 67 investigative sites in China. Patients who were treated with intravenous rtPA (Actilyse; Boehringer Ingelheim, Germany) within 4.5 h after ischemic stroke onset were recruited. All patients underwent a standard investigation protocol comprising blood tests, electrocardiography, magnetic resonance imaging, and cranial computed tomography (CT). Ethics committees at the participating hospitals approved data collection for the TIMS-China study. All patients or their legal representatives were fully informed of the study and provided their written consent before study entry.
We measured ABG of all patients at the emergency department before intravenous thrombolysis, recorded the metabolic parameters and stroke risk factors in our computer-based laboratory system. All patients underwent brain CT examination at admission. DM was defined as a history of DM or use of insulin and/or oral hypoglycemic agents. According to the American Diabetes Association guidelines, random glucose testing in an average adult produces values ranging from 80 to 140 mg/dL (4.4–7.8 mmol/L), with ≥200 mg/dL (11.1 mmol/L) considered to show diabetes. Herein, ABG ≥7.8 mmol/L was defined as hyperglycemia and ≥11.1 mmol/L was defined as severe hyperglycemia.
Primary outcome measurements included 90-day functional outcome and 90-day mortality. Functional dependence was defined by the modified Rankin Scale (mRS) score at 3 months and categorized as favorable (mRS score 0–2) or poor (mRS score 3–6). Secondary outcomes included early neurological deterioration (END) and 7-day mortality. END in AIS was defined according to a National Institutes of Health Stroke Scale score increase of ≥4 points between admission and at 24 h. Mortality included deaths from all causes. The safety evaluation was based on the incidence of symptomatic intracranial hemorrhage (sICH) at 36 h. sICH following rtPA administration was defined according to the modified Safe Implementation of Treatment in Stroke-Monitoring Study (SITS-MOST) definition.
The data were statistically analyzed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA). We conducted univariate comparisons between groups with and without a history of DM. Analysis of the χ2 test and variance was made to assess univariate differences between categorical and continuous variables. Measurement data expressed as means ± standard deviations were analyzed by t-test. A logistic regression model adjusted for potential confounding effects selected according to their significance in univariate analyses was used to assess the effects of a history of DM and ABG on clinical outcomes. P ≤ 0.05 was considered to be statistically significant Analyses were also conducted in patients with different ABG levels and diabetic and non-diabetic statuses.
Out of all 1440 patients in the TIMS-China registry, 1128 received treatment of intravenous rtPA within 4.5 h after onset. Forty-four patients were excluded for a lack of information on their history of DM (n = 10) or missing ABG levels (n = 34), so 1084 patients were analyzed [Supplementary Figure 1]. Baseline characteristics for the patients are given in Supplementary Tables 1 and 2. A DM history was found in 191 (17.62%) patients. In total, 726 (66.97%) patients had ABG <7.8 mmol/L, 257 (23.71%) had 7.8 to 11.1 mmol/L, and 101 (9.32%) had ABG ≥11.1 mmol/L.
Univariate and multivariate analyses showed that a history of diabetes was related to an increased risk of sICH according to the SITS-MOST. sICH occurred in 3.66% and 1.46% of patients with and without diabetes, respectively (P = 0.016). A history of DM was associated with an elevated risk of END, 7-day mortality, 90-day functional outcome, and 90-day mortality, but the associations were not significant. When ABG levels were stratified by diagnostic threshold (<7.8 or ≥11.1 mmol/L), we noted that the risk of poor outcomes increased in a graded fashion as ABG increased. The risk of poor outcomes was significantly higher in the severe hyperglycemia group (END-adjusted odds ratio [OR], 2.23; 95% confidence interval [CI], 1.13–4.41, P = 0.020; and mRS >2-adjusted OR, 1.55; 95% CI, 1.00–2.41; P = 0.0490). Similar results were evident for deaths (7-day mortality-adjusted OR, 2.70; 95% CI, 1.32–5.54; P = 0.007 and 90-day mortality-adjusted OR, 2.31; 95% CI, 1.31–4.07; P = 0.004). The ABG level was not significantly associated with sICH according to the SITS-MOST. The adjusted OR per 1 standard deviation increase in the glucose level was 2.68 (95% CI, 0.84–8.58; P = 0.10) for the univariate analysis and 2.54 (95% CI, 0.77–8.35; P = 0.13) for the multivariate analysis [Supplementary Table 3].
Elevated ABG was an independent predictor of poor outcomes only among patients without DM where the risk exposure of poor clinical outcomes was significantly greater in those with severe hyperglycemia (END-adjusted OR, 4.082; 95% CI, 1.625–10.254; P = 0.0028). Similar results were observed in the mortality rate (7-day mortality-adjusted OR, 4.250; 95% CI, 1.591–11.352, P = 0.0038 and 90-day mortality-adjusted OR, 3.704; 95% CI, 1.647–8.326; P = 0.0015). sICH and 90-day functional outcome were not associated with ABG, and ABG was not significantly related to poor outcome or sICH in patients with diabetes and AIS [Table 1].
Table 1.
Effect of ABG on outcome after stroke thrombolysis with or without diabetes.
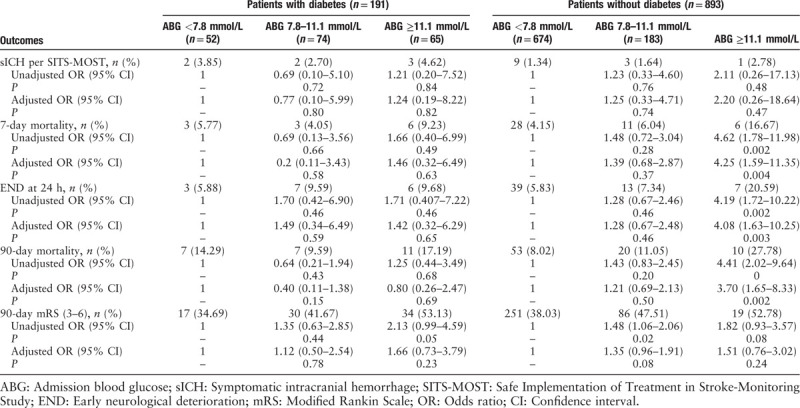
Our data show that elevated ABG is a predictor of poor outcomes in AIS patients with non-DM, not those with DM. Our results are in line with studies investigating the prognostic value of ABG on intracerebral hemorrhage, myocardial infarction, and other critical conditions.[1] The likely explanations for this are as follows: first, higher ABG in non-diabetes, usually defined as stress hyperglycemia, tends to occur in patients with critical illness. Second, diabetic patients with long-term exposure to increased glucose may have reduced the adverse effects of hyperglycemia on metabolism. Moreover, other cardiovascular risk factors, such as blood pressure and lipid levels, were required to maintain near-normal ranges in addition to glycemia control in the clinical treatment of diabetic patients.
Ischemic stroke patients with and without DM are likely to have different glucose cut-off values. Snarska et al[2] found that the ABG cut-off value for mortality prediction in AIS patients without DM was >113.5 mg/dL, compared with >210.5 mg/dL in those with DM. Farrokhnia et al[3] showed that the cut-off value of mean blood glucose >6.3 mmol/L predicted 30-day mortality in non-DM patients. However, in patients with diabetes, the corresponding value was 10.3 mmol/L. The ABG cut-off value for poor outcomes varies between non-diabetic and diabetic patients, which may explain why patients with AIS and diabetes with ABG ≥11.1 mmol/L had an elevated risk of mortality and neurological dysfunction in our study, but without statistical significance. Further studies are needed to investigate the ABG cut-off value for worse clinical outcome in patients with ischaemic stroke and diabetes.
sICH after thrombolytic therapy for AIS is a potentially devastating event incurring high mortality. ABG has been recognized as an independent risk factor for sICH after intravenous rtPA in ischemic stroke patients. However, the specific links between elevated ABG and sICH in patients with ischaemic stroke are still inconsistent. Some studies demonstrated that sICH is related to ABG in patients treated with rtPA and that a high ABG increases sICH.[4] However, this has not been shown in all observational studies of stroke.[5] We found that a history of diabetes rather than ABG was a predictor of sICH in patients with AIS. Our findings may be explained by the hypothesis that chronic microvascular injury rather than acute stress-induced hyperglycemia results in an increased risk of sICH. Glucose toxicity and pathophysiological changes caused by chronically elevated blood glucose play a major role in cerebral microvascular and macrovascular damage through metabolic and structural derangements.
Because of multiple confounding factors in the AIS patient population, the relationship between ABG and diabetes with clinical outcomes has varied considerably among studies. The major limitation of our study was the use of retrospective data. For example, we only investigated ABG, with no data for fasting blood glucose or post-prandial blood glucose at 2-h, and without hemoglobin A1c data. Additionally, our diagnosis was based on a history of previous diagnoses of diabetes as well as patient medical records, medical histories, and medical treatments. This may have resulted in missed diagnoses, especially for new patients with diabetes who were previously undiagnosed. Moreover, no data were collected on the duration of diabetes or type of treatment. Larger prospective trials are needed to directly investigate these blood glucose parameters.
In summary, elevated ABG (≥11.1 mmol/L) was an independent predictor of poor clinical outcomes after thrombolysis for AIS in non-diabetic patients instead of diabetic ones, while a history of DM independently correlated with an increased risk of thrombolysis-related sICH. Our observations should help improve the management of AIS patients. In accordance with our findings, we emphasize the importance of better glycemic control by primary prevention, and suggest that attention should be paid to control sICH when thrombolysis is given to DM patients.
Funding
This study was supported by grants from the Ministry of Science and Technology of the People's Republic of China (No. 2015BAI12B04), the National Natural Science Foundation of China (No. 31672375), Beijing Municipal Science and Technology Commission (D151100002015001), Beijing Natural Science Foundation (7182049), Basic-Clinical Research Cooperation Funding of Capital Medical University (17JL34), and Beijing Municipal Administration of Hospitals’ Youth Programme (QML20160501).
Conflicts of interest
None.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
How to cite this article: Fang HJ, Pan YS, Wang YJ, Wang CX, Wang YL, Zhong LY. Prognostic value of admission hyperglycemia on outcomes of thrombolysis in ischemic stroke patients with or without diabetes. Chin Med J 2020;133:2244–2246. doi: 10.1097/CM9.0000000000001005
Hong-Juan Fang and Yue-Song Pan contributed equally to this research.
References
- 1.Kim S, Na SJ, Park TK, Lee JM, Song YB, Choi JO, et al. Prognostic value of admission blood glucose level in critically ill patients admitted to cardiac intensive care unit according to the presence or absence of diabetes mellitus. J Korean Med Sci 2019; 34:e70.doi: 10.3346/jkms.34.e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Snarska KK, Bachorzewska-Gajewska H, Kapica-Topczewska K, Drozdowski W, Chorazy M, Kulakowska A, et al. Hyperglycemia and diabetes have different impacts on outcome of ischemic and hemorrhagic stroke. Arch Med Sci 2017; 13:100–108. doi: 10.5114/aoms.2016.61009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farrokhnia N, Bjork E, Lindback J, Terent A. Blood glucose in acute stroke, different therapeutic targets for diabetic and non-diabetic patients? Acta Neurol Scand 2005; 112:81–87. doi: 10.1111/j.1600-0404.2005.00440.x. [DOI] [PubMed] [Google Scholar]
- 4.Saqqur M, Shuaib A, Alexandrov AV, Sebastian J, Khan K, Uchino K. The correlation between admission blood glucose and intravenous rt-PA-induced arterial recanalization in acute ischemic stroke: a multi-centre TCD study. Int J Stroke 2015; 10:1087–1092. doi: 10.1111/ijs.12517. [DOI] [PubMed] [Google Scholar]
- 5.Ahmed N, Davalos A, Eriksson N, Ford GA, Glahn J, Hennerici M, et al. Association of admission blood glucose and outcome in patients treated with intravenous thrombolysis: results from the Safe Implementation of Treatments in Stroke International Stroke Thrombolysis Register (SITS-ISTR). Arch Neurol 2010; 67:1123–1130. doi: 10.1001/archneurol.2010.210. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


