Abstract
Introduction
Numerous neuroimaging studies demonstrated an association between the apolipoprotein E (APOE) ε4 allele and resting‐state functional connectivity (rsFC) of regions within the default mode network (DMN), both in healthy populations and patients with AD. It remains unclear whether the APOE ε4 allele differentially affects the brain's functional network architecture across race/ethnicity.
Methods
We investigated rsFC within DMN subsystems in 170 APOE ε4 carriers compared to 387 APOE ε4 non‐carriers across three major racial/ethnic groups, including non‐Hispanic Whites (n = 166), non‐Hispanic Blacks (n = 185), and Hispanics (n = 206) from the Washington Heights‐Inwood Columbia Aging Project.
Results
Compared to APOE ε4 non‐carriers, APOE ε4 carriers had lower rsFC in temporal DMN, but only in non‐Hispanic Whites. Non‐Hispanic Black and Hispanic APOE ε4 carriers had slightly higher or similar rsFC compared with non‐Hispanic White APOE ε4 non‐carriers.
Discussion
These findings suggest that APOE ε4 modulates DMN rsFC differently in non‐Hispanic Whites compared with non‐Hispanic Blacks and Hispanics.
Keywords: APOE ε4 differences, brain aging, dementia, neuroimaging, racial/ethnic differences, resting‐state functional connectivity
1. BACKGROUND
Apolipoprotein E (APOE) ε4 is the most potent genetic risk factor of late‐onset Alzheimer's disease (AD). The increased risk for AD is two‐ to three‐fold in people with one copy of the ε4 allele and approximately 12‐fold in those with two copies of the ε4 allele compared to individuals with no copies of the ε4 allele. 1 Older non‐Hispanic Blacks and Hispanics, a rapidly growing segment of the U.S. population, bear a disproportionate burden of AD and cognitive impairment compared to non‐Hispanic Whites, 2 but the relationship between APOE ε4 and risk for AD is different across racial/ethnic groups. Despite higher APOE ε4 allele frequency in non‐Hispanic Blacks compared to non‐Hispanic Whites, most studies show a weaker association of APOE ε4 in non‐Hispanic Blacks and Hispanics with AD. 3 , 4 , 5 , 6
Resting‐state functional connectivity (rsFC), specifically within the default mode network (DMN), is vulnerable to the earliest stages of AD pathology, 7 , 8 , 9 which accumulates years before symptom onset, 10 , 11 suggesting the potential of rsFC as an early marker of synaptic and neuronal dysfunction in AD. Several studies also report that the APOE ε4 allele is associated with decreased DMN connectivity and function among cognitively normal older adults, 12 , 13 , 14 even in the absence of amyloid 15 and atrophy. 16 , 17 Nevertheless, previous studies investigating the relationship between APOE ε4 and rsFC have included predominantly white adults of European ancestry. 15 , 16
Studies also suggest that the DMN is organized around a set of interacting subsystems that comprise distributed association areas of the brain. 18 We hypothesized that because APOE ε4 demonstrates differential associations with AD risk across race/ethnicity, it would also affect DMN subsystems differently across racial/ethnic groups. Given the weaker association of APOE ε4 with the risk of AD in non‐Hispanic Blacks and Hispanics, we posited that the expected association between APOE ε4 and DMN rsFC would not be present or would be weaker in these groups compared with non‐Hispanic Whites. The current study examined whether race/ethnicity moderates the relationship between APOE ε4 and rsFC in subsystems of the DMN in a community‐based sample of racially/ethnically diverse older adults from the Washington Heights‐Inwood Columbia Aging Project (WHICAP). We predicted that APOE ε4 carriers would have reduced rsFC in the DMN, and this effect would be most prominent in non‐Hispanic Whites compared to non‐Hispanic Blacks and Hispanics.
2. METHODS
2.1. Participants
We selected participants from the WHICAP cohort, a community‐based study of cognitive aging and dementia in residents of northern Manhattan, New York. WHICAP participants were recruited in three waves, beginning in 1992, 1999, and 2009. Magnetic resonance imaging (MRI) was introduced into the WHICAP study in 2005 19 with 1.5T scanning; current analyses include participants recruited from the 2009 cohort from which 700 participants received functional scans with 3T MRI beginning in 2011. 20 Participants self‐identified with a race/ethnicity, including the following mutually exclusive categories: non‐Hispanic White, non‐Hispanic Black, Hispanic, or other. As the purpose of this study was to examine rsFC, only participants with complete resting‐state functional MRI (fMRI) sequences from the 2009 cohort, as well as cognitive, demographic, and APOE data, were considered for the analyses, resulting in a sample size of 579. Among these 579 participants, 22 (≈4%) met clinical criteria for AD, and an additional 107 (18%) individuals met criteria for mild cognitive impairment (MCI). We excluded participants who met criteria for dementia, and included participants meeting criteria for MCI (final n = 557). All participants gave written informed consent and received compensation for their participation. The Columbia University Medical Center Institutional Review Board approved this study.
RESEARCH IN CONTEXT
Systematic review: Review of the existing literature using PubMed revealed evidence that the apolipoprotein E (APOE) ε4 allele, one of the strongest genetic risk factors for Alzheimer's disease (AD), is associated with resting‐state functional connectivity (rsFC) in older adults without dementia. The vast majority of research, however, included predominantly non‐Hispanic White participants. APOE ε4 may be a predictor of rsFC in healthy older adults, but it is unclear whether this association differs across race/ethnicity.
Interpretation: Our findings suggest that APOE ε4 modulates rsFC in default mode network subsystems differently in non‐Hispanic Whites compared to non‐Hispanic Blacks and Hispanics.
Future directions: This study takes an essential step toward understanding sources of APOE ε4‐related disparities in AD risk. Future studies should identify sociocultural mechanisms, as well as the interplay between biological and sociocultural mechanisms, that may underlie these differences.
2.2. Measures
Participants were evaluated in their preferred language, English or Spanish, using a comprehensive neuropsychological battery that has been validated in both language groups. 21 Dementia diagnosis was adjudicated based on standardized criteria 22 , 23 , 24 at a diagnostic consensus conference, which included at least one neuropsychologist and one neurologist who reviewed medical, neuropsychological, and functional interview data. Diagnostic formulation did not include MRI data. MCI diagnosis was made as described previously. 25 Blood samples were collected, and the single nucleotide polymorphisms (SNPs) rs7412 and rs429358 in APOE (gene map locus 19q13.2) were genotyped with KASPar® PCR SNP genotyping system (LGC Genomics, Hoddesdon, Herts, UK) and subsequently categorized as APOE ε4 positive (APOE ε4+; n = 170; 161 heterozygote) or negative (APOE ε4–; n = 387) based on the presence of the ε4 allele.
2.3. MRI acquisition and processing
Participants were scanned on a 3T Philips Intera scanner at Columbia University between 2011 and 2019. Parameters for the T1‐weighted anatomical scans were: TR = 6.6 ms; TE = 3.0 ms; flip angle = 8°; field of view (FOV) = 256 × 200 × 165 mm3; in‐plane resolution = 1 × 1 mm2; slices = 165. Resting‐state functional time series was acquired using a gradient echo‐planar imaging (EPI) sequence with the following parameters TR = 2000 ms; TE = 20 ms; flip angle = 72°; FOV = 224 × 224 × 111 mm3; slice thickness = 3.0 mm; in‐plane resolution = 2 × 2 mm2; volumes = 285. Images were acquired in the axial orientation for a total acquisition time of 19 minutes. Scanning acquisition parameters for the EPI sequence changed for some participants (n = 211): TR = 2000 ms; TE = 30 ms; flip angle = 72°; FOV = 240 × 240 × 117 mm3; slice thickness = 3.0 mm; in‐plane resolution = 3 × 3 mm2; volumes = 200. Images were acquired in the axial orientation for a total acquisition time of 7 minutes. We created a dichotomous variable (“scan protocol”) to represent whether participants received the 7‐minute acquisition or the 19‐minute protocol. This indicator variable was later included as a covariate in our analysis to control for differences in scan protocol.
The CONN toolbox, 26 which uses Statistical Parametric Mapping 12 software (www.fil.ion.ucl.ac.uk/spm/), was used to process the imaging data. We realigned each image to the mean of the time series, slice‐time corrected, skull‐stripped, normalized to Montreal Neurological Institute (MNI) space, and smoothed with a 6 mm full‐width half‐max kernel, and adjusted for movement artifacts. The resting‐state fMRI time series was corrected on a voxel‐by‐voxel basis with the anatomical component correction (aCompCor) method, which removes the principal components attributed to white matter and cerebrospinal fluid signals 27 and eliminates the need for a global signal regression. 28 , 29 Additional post‐processing of time series data was performed with the Artifact Detection Toolbox 30 to identify image frames at the subject level with extreme motion as outliers. We also excluded participants with greater than 3 mm movement, > 50% scrubbed volumes, or poor signal‐to‐noise data (determined from six subject‐specific motion parameters and their first derivatives; n = 99). Participants excluded from the rsFC analysis were similar to included participants with respect to cognitive status, race/ethnicity, sex/gender, age at scan, and APOE ε4 (data not shown). Frames identified as extreme motion outliers, as well as six subject‐specific motion parameters and their first derivatives, were also calculated as potential confounds in the denoising processing step. The residual blood‐oxygen‐level‐dependent (BOLD) time‐series in each voxel was band‐pass filtered at 0.009 to 0.08 Hz to focus on low‐frequency fluctuations. 31
2.4. Statistical analysis
The mean BOLD signal time course was used to compute regions of interest (ROI)‐to‐ROI connectivity between ROIs within the subsystems of the DMN, using the Power Atlas. 32 Subsystems of the DMN included frontal (eg, inferior, medial, superior, and middle frontal gyri and anterior cingulate), temporal (eg, inferior, middle, and superior temporal gyri, parahippocampal, and fusiform gyri), and occipito‐parietal (eg, precuneus, cingulate, angular gyri), totaling 55 regions (see Figure 1). We computed functional connectivity between each ROI within each subsystem using the Conn functional connectivity toolbox. The residual BOLD time‐series was extracted from gray matter voxels within the 55 ROIs in the preprocessed resting‐state fMRI data. After the temporal preprocessing of fMRI data, ROI‐to‐ROI functional connectivity analysis was performed by grouping voxels into the 55 ROIs defined into subsystems of the DMN, and estimating the correlation matrix for each subject by computing Pearson's correlation coefficients between each ROI time‐series and the time‐series of all other ROIs within the DMN subsystem. These correlation coefficients for each participant were converted to z‐values using Fisher's transform. False discovery rate (FDR; P < .05) was used to correct for multiple comparisons in calculating the significance of each ROI–ROI connection. 33 We then conducted separate 2 × 2 univariate analyses of variance (ANOVAs) within each DMN subsystem to compare the mean differences in rsFC strength, using non‐Hispanic white APOE ε4 non‐carriers as the reference group. Bonferroni correction was applied to correct for multiple comparison. Race/ethnicity and APOE ε4 status were between‐group factors while controlling for age, sex/gender, and scan protocol. We conducted a sensitivity analysis with and without MCI participants. Because the pattern of results remained the same, we reported results of the full sample for greater power. We also conducted stratified analyses by MCI status (Table S1 in supporting information) and scanning protocol (Table S2 in supporting information).
FIGURE 1.
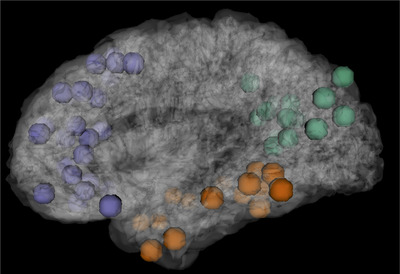
Subsystem of the default mode network, including frontal (purple), temporal (orange), and occipito‐parietal (green) regions. Each point represents a 5 mm spherical region‐of‐interest
3. RESULTS
3.1. Demographic characteristics
The sample included 170 APOE ε4 carriers and 387 APOE ε4 non‐carriers. Demographic details of the 557 participants included in the analysis, separated by APOE ε4 status, are shown in Table 1. Compared to non‐carriers, APOE ε4 carriers were younger, had more years of education, and were more likely to be non‐Hispanic Black. Compared to non‐Hispanic Whites, non‐Hispanic Blacks and Hispanics had lower cognitive performance scores, were less likely to be women, and were less likely to be diagnosed with MCI. There was no differential effect of APOE ε4 on any of the other demographic variables across race/ethnicity.
TABLE 1.
Sample characteristics
| Characteristics | APOE ε4+ (n = 170) | APOE ε4– (n = 387) | Total (n = 557) | F/X2 | η 2 |
|---|---|---|---|---|---|
| Age; M (SD) | 72.18 (5.03) | 73.47 (5.29) | 73.08 (5.24) | 7.19 ^ | .013 |
|
Education (years); M (SD) |
13.15 (4.29) | 11.99 (4.66) | 12.34 (4.58) | 7.67 ^ | .014 |
| Sex | .40 | — | |||
| % Women | 61.8 | 58.9 | 59.8 | — | — |
| Race/Ethnicity | 24.30 † | — | |||
| NH White | 28.2 | 30.5 | 29.8 | — | — |
| NH Black | 47.1 | 27.1 | 33.2 | — | — |
| Hispanic | 25.6 | 42.4 | 37.0 | — | — |
| % MCI positive | 20.6 | 18.6 | 19.2 | .30 | — |
| % MCI positive (by race/ethnicity) | NHW | NHB | Hispanic | 5.45 | — |
| 13.3 | 22.2 | 21.4 |
Abbreviations: APOE, apolipoprotein E; M, mean; MCI, mild cognitive impairment; NH, non‐Hispanic; SD, standard deviation.
P < .01.
P < .001.
3.2. Imaging results
Overall, the effect of APOE ε4 on rsFC differed across racial/ethnic groups (see Table 2). In temporal DMN, non‐Hispanic White APOE ε4 carriers compared to non‐Hispanic White non‐carriers had lower rsFC (β = –.01, [–.02, –.001]). Non‐Hispanic Black APOE ε4 carriers had greater rsFC compared to non‐Hispanic White non‐carriers (β = .02, [.005, .04]). Hispanic APOE ε4 carriers had similar rsFC compared to non‐Hispanic White non‐carriers (β = .01, [–.008, .03]). Connectivity was lower in Non‐Hispanic Blacks compared to non‐Hispanic Whites (β = ‐.01, [–.02, .004]) while Hispanics had similar rsFC compared to non‐Hispanic Whites (β = ‐.006, [–.02, .003]).
TABLE 2.
APOE ε4 on rsFC DMN subsystems across race/ethnicity
| 95% CI | |||
|---|---|---|---|
| B(SE) | Lower | Upper | |
| Frontal DMN | |||
| Age | 0.0002(.0002) | –0.0002 | .001 |
| Sex/Gender | .003(.003) | –.002 | .009 |
| Scan Protocol | .031(.003) | .025 | .037 † |
| APOE ε4+ | –.001(.006) | –.013 | .010 |
| Hispanic | –.005(.004) | –.013 | .003 |
| NH Black | –.0004(.005) | –.009 | .009 |
| Hispanic x APOE ε4+ | .001(.008) | –.015 | .017 |
| NH Black x APOE ε4+ | –.001(.008) | –.016 | .014 |
| Temporal DMN | |||
| Age | 0.0002(.0003) | –0.0003 | .001 |
| Sex/Gender | .001(.004) | –.006 | .008 |
| Scan protocol | .026(.004) | .019 | .033 † |
| APOE ε4+ | –.010(.007) | –.023 | .003 |
| Hispanic | –.007(.005) | –.016 | .003 |
| NH Black | –.012(.005) | –.022 | –.001* |
| Hispanic x APOE ε4+ | .011(.010) | –.008 | .030 |
| NH Black x APOE ε4+ | .024(.009) | .006 | .041 ^ |
| Occipito‐parietal DMN | |||
| Age | –0.00002(.0004) | –.001 | .001 |
| Sex/Gender | –0.002(.005) | –.010 | .008 |
| Scan protocol | .062(.005) | .052 | .072† |
| APOE ε4+ | –.002(.010) | –.021 | .017 |
| Hispanic | –.009(.007) | –.023 | .004 |
| NH Black | –.020(.008) | –.035 | –.005 ^ |
| Hispanic x APOE ε4+ | .015(.014) | –.011 | .042 |
| NH Black x APOE ε4+ | .006(.013) | –.019 | .031 |
Abbreviations: APOE, apolipoprotein E; B, unstandardized parameter estimate; CI, confidence interval; DMN, default mode network; NH, non‐Hispanic; SE, standard error.
Note: Underlined significant findings did not survive Bonferroni correction.
P < .05.
P < .01.
P < .001.
In occipito‐parietal DMN, rsFC differed across racial/ethnic groups where non‐Hispanic Blacks had lower connectivity compared to non‐Hispanic Whites (β = –.018, [–.03, –.004]) while Hispanics had similar connectivity compared to non‐Hispanic Whites (β = –.009, [–.02, .005]). APOE ε4 carriers had similar rsFC in occipito‐parietal DMN regions in non‐Hispanic Blacks (β = .005, [–.020, .030]) and Hispanics β = .018, [–.009, .044]) compared to non‐Hispanic White APOE ε4 non‐carriers.
In frontal DMN, rsFC was similar across racial/ethnic groups. APOE ε4 carriers also had similar rsFC in frontal DMN regions in non‐Hispanic Blacks and Hispanics compared to non‐Hispanic White APOE ε4 non‐carriers (see Figure 2). The pattern of results was similar after excluding MCI participants.
FIGURE 2.
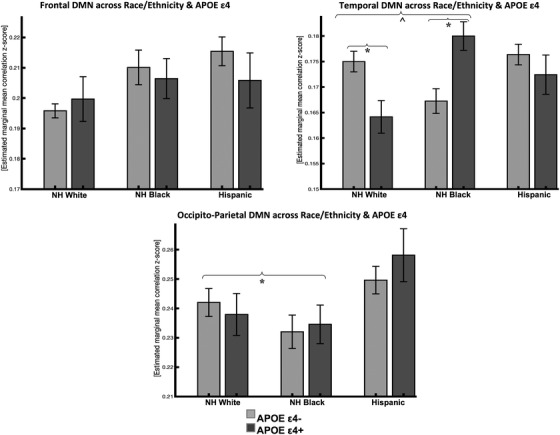
Resting‐state functional connectivity in DMN subsystems across race/ethnicity and APOE e4 status (95% confidence interval error bars). Abbreviations: NH, non‐Hispanic; DMN, default mode network; APOE, apolipoprotein E.
* P < .016 Bonferroni corrected; ^ P < .05 uncorrected
4. DISCUSSION
We used resting‐state functional neuroimaging to investigate the association between APOE ε4 and functional connectivity within DMN subsystems in a sample of racially and ethnically diverse older adults. Compared to non‐Hispanic Whites, non‐Hispanic Blacks showed lower rsFC in temporal and occipito‐parietal DMN subsystems. APOE ε4 carriers had lower rsFC in the temporal DMN subsystem, but only in non‐Hispanic Whites. In contrast, APOE ε4 carriers and non‐carriers had either slightly higher or similar connectivity in non‐Hispanic Blacks and Hispanics, respectively. The findings among non‐Hispanic White participants are consistent with previous studies that showed lower connectivity in predominantly non‐Hispanic White APOE ε4 carriers. 15 , 16 Our findings are the first, however, to shed light on the differing effects of APOE ε4 status on rsFC of the DMN across three major racial/ethnic groups.
Previous studies in non‐Hispanic White APOE ε4 older adults showed a more considerable decline in rsFC in DMN subsystems compared to APOE ε4 non‐carriers as they age 16 as well as in young (i.e., mean age 28 years) APOE ε4 carriers. 34 Similar DMN regions showed the highest resting metabolic activity in young adults and were also the same regions with earliest amyloid beta (Aβ) deposition in a previous study of older adults with AD. 35 These findings, given previous studies showing higher Aβ burden in cognitively normal APOE ε4 carriers, 36 suggest that increased resting metabolism in young adulthood within DMN subsystems may provide regional conditions that are conducive to amyloid deposition in older adults.
In addition to the existing literature showing an association between APOE ε4 and amyloid, APOE ε4 may operate through both Aβ‐dependent and Aβ‐independent pathways. For example, APOE in the brain is primarily expressed by astrocytes and microglia, 37 cell types that are now widely appreciated to play critical roles in the pathogenesis of AD. 38 , 39 APOE ε4 disrupts normal glial cell biology, intersecting with changes that occur during healthy aging to ultimately cause neurodegeneration and cognitive decline. 40 , 41 , 42 A recent study by Montagne et al. 43 presented evidence linking APOE ε4 to blood‐brain barrier (BBB) breakdown. Specifically, cognitively normal APOE ε4 carriers had a leaky BBB in the hippocampus and medial temporal lobe regions and this effect was worse in APOE ε4 carriers with MCI. Others show that APOE ε4 carriers who subsequently develop AD have proteins leaked through the BBB, demonstrating that the integrity of the barrier is lost before cognitive decline. 44 Disruption of rsFC in temporal brain regions, as observed in our study, suggests a potential pathway through which APOE ε4 is linked to AD risk. Overall, our results demonstrated differential effects of APOE ε4 on rsFC networks across race/ethnicity, suggesting that APOE ε4‐related brain dysfunction is more salient in non‐Hispanic Whites compared to non‐Hispanic Blacks and Hispanics.
In our sample, compared to non‐Hispanic Whites, non‐Hispanic Blacks showed lower rsFC in temporal and occipito‐parietal DMN regions, irrespective of APOE ε4 status. Non‐Hispanic Black APOE ε4 carriers also showed higher rsFC in temporal DMN compared to non‐Hispanic White non‐carriers. Because APOE status does not explain differences in rsFC across racial/ethnic groups and the differential effects of APOE ε4 across race/ethnicity, we suspect that those factors may be vascular in nature, but as this was not the focus of the current analysis, future studies will further examine these concepts. For example, genetic risk variants reported for AD in Blacks include ABCA7, 45 TREM2, 46 and SLC10A2. 47 One or more of these variants may attenuate the effect of APOE ε4 such that non‐Hispanic Blacks and Hispanics have less APOE ε4‐associated risk for AD than do non‐Hispanic Whites. The findings showing increased rsFC associated with APOE ε4 among non‐Hispanic Blacks also raise the possibility of a protective role in this group.
Hispanic APOE ε4 carriers compared to non‐Hispanic White non‐carriers had similar rsFC in DMN subsystems. One explanation for this finding is the admixed nature of Hispanics in our sample. Previous studies showed that populations of European ancestry have a higher APOE ε4 risk compared to those of African ancestry 48 , 49 as well as compared to Hispanics. 50 We speculate that if rsFC is a sensitive marker of brain dysfunction, our results suggest that APOE ε4‐related brain dysfunction is more salient in non‐Hispanic whites compared to non‐Hispanic Blacks and Hispanics. While one pathway for developing AD among non‐Hispanic Whites is through APOE ε4 affecting rsFC, this pathway may be less operative in this cohort of older non‐Hispanic Black and Hispanic adults.
This study is limited by its cross‐sectional design, which precludes us from examining the relationship between rsFC and progression to AD. Future studies should examine rsFC in APOE ε4 carriers and non‐carriers across the lifespan and across racially/ethnically and socioeconomically diverse older adults to determine the different factors that may predict AD across diverse populations. Longitudinal studies will be essential to help plan the optimal timing for potential interventional studies in individuals at risk for symptomatic AD. Further, longitudinal studies will be necessary to define the time course of any alterations in rsFC, both those that appear independent of Aβ and, particularly, those that appear to progress to neuronal dysfunction and damage. Though reliable, our effects are small. Given that our study is the first to assess the association between APOE ε4 and DMN rsFC across race/ethnicity, it is critical for future work to test the impact of these differences on clinical outcomes. Future work will examine the extent to which APOE ε4 and race/ethnicity, and rsFC, are associated with rate of cognitive decline and disease states.
Future studies should also take a more in‐depth look at racial/ethnic differences in gene–gene and gene–environmental interactions with APOE ε4 because this polymorphism has less of an effect on racial/ethnic minorities. Thus, other factors (e.g., sociocultural, biological, behavioral) may be driving the differences we see in rsFC and the disparity in AD among racial/ethnic minorities. Future research should also explore these interactions across various biomarkers, which may lead to novel, population‐specific therapeutics, and risk predictions.
Though the APOE ε4 allele is the most well‐established genetic risk factor for AD, studies show a weak association of APOE ε4 and AD risk among racial/ethnic minority populations. Our findings show racial/ethnic differences in the association between APOE ε4 and rsFC of the DMN, where non‐Hispanic White APOE ε4 carriers have lower rsFC in AD‐related regions of the DMN. It is still critical that future studies examine how race/ethnicity modifies the risk and expression of AD to understand race‐dependent biological mechanisms of AD.
CONFLICTS OF INTEREST
The authors have no conflicts of interest related to this study.
Supporting information
Supporting Information.
Supporting Information.
ACKNOWLEDGMENTS
Data collection and sharing for this project was supported by the Washington Heights‐Inwood Columbia Aging Project (WHICAP, PO1AG07232, R01AG03721, RF1AG054023) funded by the National Institute on Aging (NIA). Additional funding to support the imaging data collected as WHICAP substudies was provided by NIA grants (R56AG034189, R01AG034189, R01AG054520, R01AG028786, RF1AG058067, RF1AG054070). WHICAP investigators have reviewed this manuscript for scientific content and consistency of data interpretation with previous WHICAP study publications. We acknowledge the WHICAP study participants and the WHICAP research and support staff for their contributions to this study.
Turney IC, Chesebro AG, Rentería MA, et al. APOE ε4 and resting‐state functional connectivity in racially/ethnically diverse older adults. Alzheimer's Dement. 2020;12:e12094 10.1002/dad2.12094
REFERENCES
- 1. Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261(5123):921‐923. [DOI] [PubMed] [Google Scholar]
- 2. Mayeda ER, Glymour MM, Quesenberry CP, Whitmer RA. Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimers Dement. 2016;12(3):216‐224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Farrer LA, Cupples LA, Haines JL, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein e genotype and Alzheimer disease: a meta‐analysis. JAMA. 1997;278(16):1349‐1356. [PubMed] [Google Scholar]
- 4. Maestre G, Ottman R, Stern Y, et al. Apolipoprotein E and Alzheimer's disease: ethnic variation in genotypic risks. Ann Neurol. 1995;37(2):254‐259. [DOI] [PubMed] [Google Scholar]
- 5. Pericak‐Vance M, Rajabli F, Feliciano‐Astacio B, etal. African haplotypic background mitigates the effect of apoe ε4 risk allele in Alzheimer disease. J Neurol Sci. 2017;381:1137. [Google Scholar]
- 6. Tang MX, Maestre G, Tsai WY, et al. Relative risk of Alzheimer disease and age‐at‐onset distributions, based on APOE genotypes among elderly African Americans, Caucasians, and Hispanics in New York City. Am J Hum Genet. 1996;58(3):574‐584. [PMC free article] [PubMed] [Google Scholar]
- 7. Greicius MD, Srivastava G, Reiss AL, Menon V. Default‐mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci USA. 2004;101(13):4637‐4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hedden T, Van Dijk KRA, Becker JA, et al. Disruption of functional connectivity in clinically normal older adults harboring amyloid burden. J Neurosci. 2009;29(40):12686‐12694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sheline YI, Raichle ME, Snyder AZ, et al. Amyloid plaques disrupt resting state default mode network connectivity in cognitively normal elderly. Biol Psychiatry. 2010;67(6):584‐587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chhatwal JP, Schultz AP, Johnson K, et al. Impaired default network functional connectivity in autosomal dominant Alzheimer disease. Neurology. 2013;81(8):736‐744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Quiroz YT, Schultz AP, Chen K, et al. Brain Imaging and blood biomarker abnormalities in children with autosomal dominant Alzheimer disease: a cross‐sectional study. JAMA Neurol. 2015;72(8):912‐919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fleisher AS, Sherzai A, Taylor C, Langbaum JBS, Chen K, Buxton RB. Resting‐state BOLD networks versus task‐associated functional MRI for distinguishing Alzheimer's disease risk groups. Neuroimage. 2009;47(4):1678‐1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen Y, Chen K, Zhang J, et al. Disrupted functional and structural networks in cognitively normal elderly subjects with the apoe ɛ4 allele. Neuropsychopharmacology. 2015;40(5):1181‐1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Matura S, Prvulovic D, Butz M, et al. Recognition memory is associated with altered resting‐state functional connectivity in people at genetic risk for Alzheimer's disease. Eur J Neurosci. 2014;40(7):3128‐3135. [DOI] [PubMed] [Google Scholar]
- 15. Sheline YI, Morris JC, Snyder AZ, et al. APOE4 allele disrupts resting state FMRI connectivity in the absence of amyloid plaques or decreased CSF Aβ42. J Neurosci. 2010;30(50):17035‐17040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Machulda MM, Jones DT, Vemuri P, et al. Effect of APOE ε4 status on intrinsic network connectivity in cognitively normal elderly subjects. Arch Neurol. 2011;68(9):1131‐1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chiesa PA, Cavedo E, Vergallo A, et al. Differential default mode network trajectories in asymptomatic individuals at risk for Alzheimer's disease. Alzheimer's & Dementia. 2019;15(7):940‐950. [DOI] [PubMed] [Google Scholar]
- 18. Buckner RL, Andrews‐Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124(1):1‐38. [DOI] [PubMed] [Google Scholar]
- 19. Brickman AM, Schupf N, Manly JJ, et al. Brain morphology in elderly African Americans, Caribbean Hispanics, and Caucasians from Northern Manhattan. Arch Neurol. 2008;65(8):1053‐1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brickman AM, Tosto G, Gutierrez J, et al. An MRI measure of degenerative and cerebrovascular pathology in Alzheimer disease. Neurology. 2018;91(15):e1402‐e1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Siedlecki KL, Manly JJ, Brickman AM, Schupf N, Tang M‐X, Stern Y. Do neuropsychological tests have the same meaning in spanish speakers as they do in english speakers? Neuropsychology. 2010;24(3):402‐411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. American Psychiatric Association . Diagnostic and statistical manual of mental disorders (4th ed., text rev.), Washington, DC: Author: Published online 2000. Accessed September 19, 2019. [Google Scholar]
- 23. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS‐ADRDA work group under the auspices of department of health and human services task force on Alzheimer's disease. Neurology. 1984;34(7):939‐944. [DOI] [PubMed] [Google Scholar]
- 24. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the national institute on aging‐Alzheimer's association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):263‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Manly JJ, Tang M‐X, Schupf N, Stern Y, Vonsattel J‐PG, Mayeux R. Frequency and course of mild cognitive impairment in a multiethnic community. Ann Neurol. 2008;63(4):494‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Whitfield‐Gabrieli S, Nieto‐Castanon A . Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2(3):125‐141. [DOI] [PubMed] [Google Scholar]
- 27. Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage. 2007;37(1):90‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chai XJ, Castañón AN, Öngür D, Whitfield‐Gabrieli S. Anticorrelations in resting state networks without global signal regression. Neuroimage. 2012;59(2):1420‐1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: are anti‐correlated networks introduced? Neuroimage. 2009;44(3):893‐905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mazaika PK, Hoeft F, Glover GH, Reiss AL. Methods and software for fmri analysis of clinical subjects. Neuroimage. 2009;47(S58). [Google Scholar]
- 31. Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102(27):9673‐9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Power JD, Cohen AL, Nelson SM, et al. Functional network organization of the human brain. Neuron. 2011;72(4):665‐678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Benjamini Y, Hochberg Y. Controlling the false discovery rate ‐ a practical and powerful approach. J R Stat Soc Ser B‐Stat Methodol. 1995;57(1):289‐300. [Google Scholar]
- 34. Filippini N, MacIntosh BJ, Hough MG, et al. Distinct patterns of brain activity in young carriers of the APOE‐ε4 allele. Proc Natl Acad Sci. 2009;106(17):7209‐7214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Buckner RL, Snyder AZ, Shannon BJ, et al. Molecular, structural, and functional characterization of Alzheimer's disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25(34):7709‐7717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Reiman EM, Chen K, Liu X, et al. Fibrillar amyloid‐beta burden in cognitively normal people at 3 levels of genetic risk for Alzheimer's disease. Proc Natl Acad Sci USA. 2009;106(16):6820‐6825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Boyles JK, Pitas RE, Wilson E, Mahley RW, Taylor JM. Apolipoprotein E associated with astrocytic glia of the central nervous system and with nonmyelinating glia of the peripheral nervous system. J Clin Invest. 1985;76(4):1501‐1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ferris HA, Perry RJ, Moreira GV, Shulman GI, Horton JD, Kahn CR. Loss of astrocyte cholesterol synthesis disrupts neuronal function and alters whole‐body metabolism. Proc Natl Acad Sci USA. 2017;114(5):1189‐1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang L, Schuster GU, Hultenby K, Zhang Q, Andersson S, Gustafsson J‐A. Liver X receptors in the central nervous system: from lipid homeostasis to neuronal degeneration. Proc Natl Acad Sci USA. 2002;99(21):13878‐13883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Liu L, MacKenzie KR, Putluri N, Maletić‐Savatić M, Bellen HJ. The glia‐neuron lactate shuttle and elevated ros promote lipid synthesis in neurons and lipid droplet accumulation in glia via APOE/D. Cell Metab. 2017;26(5):719‐737.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liu L, Zhang K, Sandoval H, et al. Glial lipid droplets and ROS induced by mitochondrial defects promote neurodegeneration. Cell. 2015;160(1‐2):177‐190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. McDougall M, Choi J, Magnusson K, Truong L, Tanguay R, Traber MG. Chronic vitamin E deficiency impairs cognitive function in adult zebrafish via dysregulation of brain lipids and energy metabolism. Free Radic Biol Med. 2017;112:308‐317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Montagne A, Barnes SR, Sweeney MD, et al. Blood‐brain barrier breakdown in the aging human hippocampus. Neuron. 2015;85(2):296‐302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Profaci CP, Munji RN, Pulido RS, Daneman R. The blood–brain barrier in health and disease: important unanswered questions. J Exp Med. 2020;217(4):e20190062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Reitz C, Jun G, Naj A, et al. Variants in the ATP‐binding cassette transporter (ABCA7), apolipoprotein E ϵ4,and the risk of late‐onset Alzheimer disease in African Americans. JAMA. 2013;309(14):1483‐1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jin SC, Carrasquillo MM, Benitez BA, et al. TREM2 is associated with increased risk for Alzheimer's disease in African Americans. Molecular Neurodegeneration. 2015;10(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mez J, Chung J, Jun G, et al. Two novel loci, COBL and SLC10A2, for Alzheimer's disease in African Americans. Alzheimers Dement. 2017;13(2):119‐129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rajabli F, Feliciano BE, Celis K, et al. Ancestral origin of ApoE ε4 Alzheimer disease risk in puerto rican and African American populations. PLos Genet. 2018;14(12):e1007791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Weuve J, Barnes LL, Mendes de Leon CF, et al. Cognitive aging in black and white americans: cognition, cognitive decline, and incidence of Alzheimer disease dementia. Epidemiology. 2018;29(1):151‐159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Campos M, Edland SD, Peavy GM. An Exploratory study of APOE‐ε4 genotype and risk of Alzheimer's disease in mexican hispanics. J Am Geriatr Soc. 2013;61(6):1038‐1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information.
Supporting Information.


