Abstract
Multiple and diverse psychotherapeutic or psychopharmacologic treatments effectively reduce symptoms for many patients with anxiety disorders, but the trajectory and magnitude of response vary considerably. This heterogeneity of treatment response has invigorated the search for biomarkers of treatment response in anxiety disorders, across the lifespan. In this review, we summarize evidence for biomarkers of treatment response in children, adolescents and adults with generalized, separation and social anxiety disorders as well as panic disorder. We then discuss the relationship between these biomarkers of treatment response and the pathophysiology of anxiety disorders. Finally, we provide context for treatment response biomarkers of the future, including neuronally-derived extracellular vesicles in anxiety disorders and discuss challenges that must be overcome prior to the debut of treatment response biomarkers in the clinic. A number of promising treatment response biomarkers have been identified, although there is an urgent need to replicate findings and to identify which biomarkers might guide clinicians in selecting from available treatments rather than just simply identifying patients who may be less likely to respond to a given intervention.
Keywords: generalized anxiety disorder, separation anxiety disorder, fMRI, pharmacogenomics
Introduction
Anxiety disorders are the most common mental health conditions across the lifespan. They typically begin in childhood and adolescence (Beesdo-Baum and Knappe, 2012; Beesdo et al., 2010) and inflict significant morbidity (Baxter et al., 2014; James et al., 2018). Moreover, these disorders—generalized anxiety disorder, social anxiety disorder, separation anxiety disorder, specific phobia, panic disorder and agoraphobia—have a lifetime prevalence close to 30% (Merikangas et al., 2010). Further, 50% percent of adults (Stein et al., 1999; Van Ameringen et al., 2001) and 40% of children and adolescents with anxiety disorders fail to respond to psychopharmacologic or psychotherapeutic treatments (RUPP 2001; Strawn et al., 2015; Walkup et al., 2008).
Current evidence-based treatment of anxiety disorders is all too often a trial-and-error process. Clinicians typically initiate a first-line treatment (i.e., selective serotonin reuptake inhibitors [SSRIs] and/or psychotherapy) (Roy-Byrne et al., 2010; Stein and Sareen, 2015; Strawn et al., 2018a; Strawn et al., 2020) and then wait 8-10 weeks (Connolly and Bernstein, 2007; Wehry et al., 2015), during which time they may observe improvement or intolerable side effects. The protracted interval between treatment initiation and response as well as the variable response rate (50-60% of patients improving) (Walkup et al., 2008) represents a significant shortcoming of SSRI and SNRI pharmacotherapy (Strawn et al., 2018a). There is limited data to predict, which patients with anxiety disorders will respond to which SSRIs, SNRIs or other interventions. This conundrum has spurred the search for treatment response biomarkers (Nasrallah, 2019). Given multiple evidence-based treatments for anxiety across psychopharmacologic classes (Strawn et al., 2018a) and psychotherapeutic modalities, and the high prevalence of these disorders, having reliable biomarkers for treatment response may shift treatment paradigms and herald an era of precision psychiatry—helping clinicians to pair the right treatments with the right patients.
Many studies of biomarkers of treatment response in anxiety disorders have focused on EEG, functional MRI (fMRI) and 1H magnetic resonance spectroscopy (MRS); however, many of these modalities are encumbered by site-to-site variability, are methodologically intense, or are not readily available for large-scale use. By contrast, molecular markers can be obtained in non-invasive or minimally invasive ways and offer an easily accessible precision medicine approach that will facilitate precise treatment selection and hence greatly improve outcome.
With this in mind, we sought to review the literature to identify potential biomarkers of treatment response to pharmacotherapy or psychotherapy in children, adolescents and adults with anxiety disorders. We cover the gamut of molecular disciplines in addition to neuroimaging and pharmacogenetics. We review inflammatory biomarkers, pharmacodynamic biomarkers, acid sensing markers and neurotropic biomarkers. We propose an approach to measuring the molecules described above in neuronally-derived extracellular vesicles (EVs), which carry RNA, protein and lipid signatures from the CNS to the soma and can be captured by minimally invasive means such as a simple phlebotomy.
1. Methods
For this unstructured review, searches were conducted in PubMed, PsychINFO, clinicaltrials.gov. Search results were compiled and reviewed to provide an overview of the current knowledge regarding treatment response biomarkers in anxiety disorders. For the PubMed search (inception through March 1, 2020), we used the following search strategy (anxiety OR social phobia OR social anxiety disorder OR SAD OR generalized anxiety disorder OR GAD OR separation anxiety disorder OR panic disorder*) AND (biomarker* OR fMRI or functional magnetic resonance imaging OR voxel based morphometry OR VBM or functional connectivity OR amygdala OR prefrontal cortex OR spectroscopy OR inflammat* OR cytokine* OR c-reactive protein OR CRP OR substance p OR norepinephrine OR serotonin OR GABA OR glutamate OR GLX OR CRH OR cortisol OR psychotherapy OR cognitive behavioral therapy OR CBT OR selective serotonin reuptake inhibitor OR SSRI OR selective serotonin norepinephrine reuptake inhibitor OR SNRI OR selective serotonin norepinephrine reuptake inhibitor OR fluoxetine OR fluvoxamine OR citalopram OR escitalopram OR fluoxetine OR paroxetine OR venlafaxine OR desvenlafaxine OR duloxetine OR vortioxetine OR vilazodone OR vilazodone OR levomilnacipran OR benzodiazepine OR clonazepam OR lorazepam OR diazepam OR tricyclic). The results of the search were then manually limited. For this unstructured review, we also attempted to highlight key findings and developments that have been published in the last 2 decades. The resulting information from these searches was reviewed by the authors to provide an overview of the current knowledge regarding treatment response biomarkers in anxiety disorders.
2. Results
2.1.1. Functional Neuroimaging Biomarkers of Treatment Response in Adults
Functional imaging can identify the effects of treatment on activity within structures that are implicated in the pathophysiology of anxiety disorders (Strawn et al., 2012). Identifying biomarkers based on functional activation in fMRI studies is based on the relationship between increased activation of several prefrontal structures and anxiety severity (Strawn et al., 2012). One of the first studies to leverage fMRI to predict treatment response revealed that pre-treatment activity of the anterior cingulate cortex and decreased amygdala activity were associated with the degree of venlafaxine-related improvement (Nitschke et al., 2009; Whalen et al., 2008). Several additional studies examined the potential utility of fMRI to predict response to psychotherapy. Fick and colleagues (2018) observed that pre-treatment dorsal anterior cingulate cortex activation predicted the degree of response to cognitive behavioral therapy (CBT) or escitalopram in adults with social anxiety disorder. In this study, activation patterns accurately identified 81% of responders/non-responders (Frick et al., 2018). A second study examining neurofunctional biomarkers of CBT response in social anxiety disorder, found that CBT decreased functional activity in prefrontal regions (dorsomedial, medial frontal gyrus) and insula and suggested that “pre-treatment cortical hyperactivity to social threat signals may serve as a prognostic indicator of CBT success” (Klumpp et al., 2013). A third study of CBT found that functional activation patterns in response to aversive facial stimuli, at baseline, predicted treatment response in adults with social anxiety disorder (Doehrmann et al., 2013). Last, a recent study that examined pre-treatment predictors of response to either sertraline or CBT in mixed anxiety disorders in a population of children, adolescents and young adults is discussed below (Kujawa et al., 2016). Taken together, these studies suggest that pre-treatment activity in structures within prefrontal-amygdala pathways predicts treatment response as does treatment-related change in the activity of these structures.
At this juncture, studies of anxious adults consistently implicate anterior cingulate cortex activity as a neurofunctional biomarker for anxiolytic treatments. This is not surprising for several reasons. First, the anterior cingulate modulates the activity of the amygdala—a structure that is hyperresponsive to threat and functionally hyperactivated in many patients with anxiety disorders (Figure 1). Second, the anterior cingulate cortex is hyperactivated (potentially a compensatory function) in patients with anxiety disorders and is hyperresponsive to anxiogenic challenges (e.g., administration of the neuropeptide cholecystokinin-tetrapeptide (CCK-4) (Leicht et al., 2013). Third, treatment with broad-spectrum anxiolytics (e.g., alprazolam) attenuates anterior cingulate cortex activation after CCK-4 administration increases functional connectivity between the anterior cingulate cortex and amygdala and reductions in CCK-4-driven activation predict the alprazolam-related improvement in anxiety (Leicht et al., 2013).
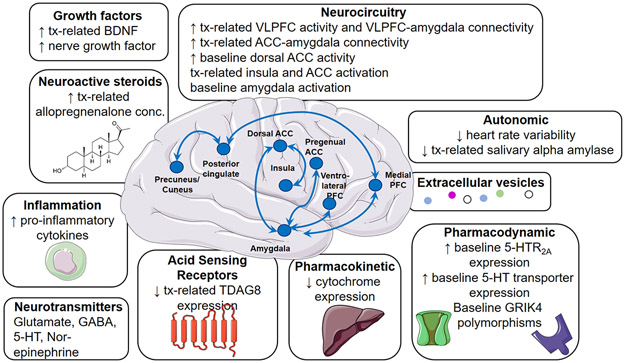
FIGURE 1: Current and emerging treatment response biomarkers in anxiety disorders.
Tx, treatment, BDNF, brain-derived neurotrophic factor, VLPFC, ventrolateral prefrontal cortex, ACC, anterior cingulate cortex, GABA, γ-aminobutyric acid, 5-HT, 5-hydroxytryptamine, TDAG8, t-cell death associated g-coupled protein receptor.
3.1.2. Functional Neuroimaging Biomarkers in Pediatric Anxiety Disorders
In pediatric anxiety disorders, one of the first fMRI studies to set the stage for identifying neurofunctional biomarkers of treatment response compared pre- and post-treatment brain activation in anxious youth receiving fluoxetine (n=7) or CBT (n=7). Both treatments increased ventrolateral prefrontal cortex activity (Maslowsky et al., 2010). Also, in anxious youth greater pre-treatment amygdala activation predicts the magnitude of CBT or fluoxetine-associated improvement (McClure et al., 2007). Several findings of the neurophysiology of treatment response in pediatric anxiety disorders emerge from studies conducted by Phan and colleagues in which children and adolescents (ages 9-19 years) with generalized, separation, and/or social anxiety disorder (as well as healthy controls) underwent fMRI approximately 12-13 weeks apart during which time they were treated with either CBT or sertraline (Burkhouse et al., 2018). In the most recent of these studies that leveraged an implicit threat task, anxious children, adolescents and young adults had reduced medial prefrontal cortex/anterior cingulate cortex activation, but effective treatment increased activation in this region. That some regions have increased functional activity in patients with anxiety and that effective treatments increase the activity of these regions is consistent with the notion that these regions—which are often regulatory—serve a compensatory function. Consistent with this notion, patients who had greater decreases in social anxiety/avoidance symptoms had greater increases in anterior angulate cortex activation (Burkhouse et al., 2018). In this sample, using a facial affect probe, increased activation of the dorsolateral prefrontal cortex and ventrolateral prefrontal cortex, as well as precentral/postcentral gyri, prior to treatment, predicted better improvement in anxiety symptoms regardless of whether patients received sertraline or CBT (Kujawa et al., 2016). A small study of mindfulness-based cognitive therapy for children (MBCT-C) in youth with generalized, social, and/or separation anxiety disorder who were at risk for developing bipolar disorder found, prior to and following 12 weeks of therapy, found that MBCT-C increased activation of the bilateral insula and anterior cingulate. In this study, treatment-related decreases in anxiety correlated with change in activation in the bilateral insula and anterior cingulate (Cotton et al., 2016). Taken together, these studies suggest that, in pediatric anxiety, hyperarousal in prefrontal-amygdala circuits predicts treatment response to both psychotherapy and SSRIs and is reduced by these treatments.
2.2. Neurochemical biomarkers
2.2.1. Gamma-aminobutyric acid (GABA)
The major inhibitory neurotransmitter in the mammalian CNS, gamma-aminobutyric acid (GABA) has been a focus of biomarker discovery in anxiety disorders as these disorders ostensibly represent excitatory/inhibitory imbalance. GABA concentrations in cerebrospinal fluid (CSF) or measured indirectly with proton magnetic resonance spectroscopy (1H MRS) are lower in patients with anxiety disorders, specifically panic disorder (Goddard et al., 2001; Long et al., 2013), although GABA concentrations may not be reduced in all cortical regions (Hasler et al., 2009). Interestingly, higher CSF GABA concentrations predict improvement in anxiety and reductions in panic attacks (Rimón et al., 1995). Further, the SSRI, citalopram, which is commonly utilized to treat anxiety disorders, decreases GABA in the pregenual anterior cingulate cortex and this decrease predicts improvement in adults with MDD (Brennan et al., 2017). Despite these potentially promising findings, the GABAergic system is complex and therefore it is difficult to directly harness this system as a single neurochemical biomarker. Peripheral GABA concentrations may not reflect CNS concentrations but are decreased by IV benzodiazepines in adults with anxiety disorders, but plasma levels or the magnitude of these reductions do not predict change in anxiety symptoms (Roy-Byrne et al., 1992). A modicum of studies suggest that GABAergic biomarkers may predict treatment response or reflect target engagement, future studies must focus not just on GABA, but on the balance of excitatory and inhibitory transmission. So called “excitatory-inhibitory” imbalance has been examined in depressive and psychotic disorders (Lener et al., 2017), but to our knowledge has not been evaluated as a predictor of treatment response in anxiety disorders.
2.2.2. Glutamate
Using 1H MRS, several studies have observed glutamatergic abnormalities in adolescents and adults with generalized and social anxiety disorders (Howells et al., 2015; Phan et al., 2005; Strawn et al., 2013), although studies of adults with panic disorder have not observed glutamatergic differences (Hasler et al., 2009). To date, only one study has examined the potential value of 1H MRS measured glutamate (or glutamatergic indices) as a biomarker of treatment response in anxiety disorders. In adults with social anxiety disorder, levetiracetam increased whole brain GABA levels and significantly reduced whole brain glutamine, although the relationship of these neurochemical changes and improvement in anxiety was not assessed (Pollack et al., 2008). Like with noradrenergic measures (discussed in 2.2.3) and anti-adrenergic, glutamatergic biomarkers may provide specificity with regard to predicting response as anxiolytics with glutamatergic target engagement become more clinically available (Chojnacka-Wójcik et al., 2001; Palucha and Pilc, 2007; Taylor et al., 2018).
2.2.3. Norepinephrine
The catecholamine norepinephrine plays a critical role as one of the principal mediators of the mammalian response to stress and has long been implicated in the pathophysiology of anxiety disorders and posttraumatic stress disorder (Strawn and Geracioti, 2008). Additionally, the adrenergic receptors to which norepinephrine (and epinephrine) bind are targets of anxiolytic therapy and are divided into two major types, α and β adrenoreceptors. There are a number of relevant polymorphisms in the α and β receptors attenuate the effects of some β-agonists and antagonists, including mutations in the ADRB2 gene. While not studied as a biomarker in either pediatric or adult anxiety disorders, β-receptor agonists produce variable and heterogeneous effects in anxiety disorders (Litonjua et al., 2010). Thus, understanding whether this heterogeneity of treatment effect could be predicted by functionally-relevant polymorphisms in genes encoding alpha and beta receptors represents an important line of inquiry. Further, CNS and peripheral norepinephrine dynamics differ substantially (Peskind et al., 1986). Norepinephrine in plasma and in CSF are derived from largely disparate sources; therefore, dissociation between peripheral and CNS norepinephrine concentrations can occur. Results of serial CSF and plasma sampling studies indicate that plasma norepinephrine concentrations predict only about 20% of the CSF (i.e., CNS) norepinephrine concentrations (Geracioti et al., 1993). This results in significant challenges to measuring plasma norepinephrine as a biomarker for anxiety disorders.
Regarding the potential of norepinephrine metabolism as a treatment response biomarker, SSRIs (fluoxetine and fluvoxamine) decrease CSF concentrations of the primary norepinephrine metabolite in humans, 5-methoxy-4-hydroxyphenylglycol (MHPG) and the primary 5-HT metabolite, 5-hydroxyindoleacetic acid (5-HIAA) (De Bellis et al., 1993a; Sheline et al., 1997). Additionally, increased salivary MHPG concentrations in depressed adults are associated with SSRI response, but not response to mirtazapine (Egami et al., 2013). Noradrenergic dynamics in adults with anxiety disorders have also been examined with challenge studies. The α2-adrenergic receptor antagonist yohimbine increases noradrenergic function and its administration to patients with panic disorder increases plasma MHPG more than in healthy individuals (Charney et al., 1992, 1984). Interestingly, in a placebo-controlled study of adults with panic disorder, fluvoxamine reduced yohimbine-induced anxiety but did not affect MHPG concentrations. It is of interest that the α2 agonist, guanfacine improves anxiety in pediatric patients with generalized, separation and social anxiety disorders (Strawn et al., 2017) and this raises the possibility that given the absence of SSRI-related effects on probes of noradrenergic activity, these probes could still have utility in assessing responsiveness to an anti-adrenergic medications.
3.2.4. Sympathetic-Parasympathetic Biomarkers
Heart rate variability, an end product of parasympathetic and sympathetic balance (or imbalance), has been explored over the past several decades as a predictor of SSRI response in adults with major depressive disorder. Only recently however, Kircanski and colleagues (2019) examined heart rate variability as a predictor of response to escitalopram, sertraline, and extended release venlafaxine in adults with major depressive disorder (MDD) (N=1008) and specifically examined its predictive value in patients with anxious depression (N=309). Pre-treatment heart rate variability, predicted response differently in anxious versus non-anxious depression. In anxious depression, patients with higher heart rate variability had greater improvement whereas those with lower heart rate variability had worse outcomes (Kircanski et al., 2019).
Salivary α-amylase has also been explored in children, adolescents and adults with anxiety disorders (Keeshin et al., 2015). Salivary α-amylase represents a widely used as a surrogate marker of autonomic nervous system tone (Granger et al., 2007). In this regard, both sympathetic and parasympathetic nervous system activity contribute to the secretion of α-amylase into saliva, although levels of salivary α-amylase primarily reflect sympathetic activity (Bosch et al. 2011). In patients with anxious depression, tricyclic antidepressants appear to increase salivary α-amylase concentrations.
2.3. Acid Sensing Molecular Markers in Anxiety Disorders
Disruption of acid-base homeostasis has been pathologically implicated in anxiety disorders and specifically in panic disorder (Vollmer et al., 2015). Recently, in a translational rodent model of panic disorder, abnormalities in the microglial acid sensing G-protein coupled receptor, T-cell death associated gene-8 (TDAG8) and the acid-sensing ion channel (ASIC) have been associated with panic physiology. In a small study of adolescents and young adults with panic disorder, relative TDAG8 mRNA expression was increased compared to healthy controls and correlated with the severity of panic disorder symptoms (Strawn et al., 2018b). Moreover, in patients who received treatment (primarily with SSRIs), TDAG8 mRNA expression was lower in those who achieved remission relative to those who did not (Strawn et al., 2018b).
These acid perturbations have been used to screen potentially effective treatments and include inhalation of CO2 and infusion of sodium lactate. Intravenous infusion of a 0.5 M sodium lactate (10 ml kg−1) produces panic attacks in vulnerable individuals and these attacks, like those elicited by CO2-inhallation phenomenologically mirror spontaneous panic attacks (that is, symptoms of dyspnea, generalized fear, a desire to flee and fear of losing control). Since the discovery that lactate infusion and CO2 challenge could elicit panic attacks in vulnerable individuals (i.e., patients with panic disorder), these paradigms have been used to screen SSRIs, GABAergic agents, CRH-1 antagonists, etc. as potentially efficacious treatments for panic disorder. However, despite this, these paradigms have not been successfully utilized as biomarkers of treatment response.
3.4. Neuroactive Steroids
Allopregnenolone (3α,5α -tetrahydroprogesterone), an endogeneous inhibitory pregnane neurosteroid and its exogeneous sibling, brexanolone, are derived from progesterone and are positive allosteric modulators at the GABAA receptor (Reddy, 2010; Zorumski et al., 2019). In adults, lactate and cholecystokinin-induced panic attacks are associated with decreases in neuroactive steroids, including allopregnanolone and 3α,5β-THP as well as increases in 3β,5α-THP (functional antagonistic isomer) contractions which is consistent with decreased GABAergic tone. Further, in adults with panic disorder, tiagabine, a GABA reuptake inhibitor, increases allopregnanolone concentrations (p=0.005) and the magnitude of this change predicts decreased panic symptoms (P. et al., 2009).
3.5. Neuroinflammatory biomarkers
Accumulating data suggest that cytokines and other pro-inflammatory molecules modulate neurocircuitry and neuronal processes; however, relatively little is known about effects of cytokines on neuronal communication and anxiety disorders, compared to depression (Hou and Baldwin, 2012). Further evidence for the potential causal role of increased inflammatory tone in anxiety comes from studies of patients with hepatitis C who have received the inflammatory cytokine, interferon-α. In these samples, up to 50% of patients develop anxiety symptoms in addition to depressive symptoms (Capuron et al., 2002). Furthermore, the development of anxiety and depressive symptoms were associated with the short allele of the serotonin transporter promotor polymorphism (Lotrich et al., 2009) and were prevented by pre-treatment with an SSRI (Musselman et al., 2001).
Administration of lipopolysaccharide, an immune activator to healthy adults results in increased anxiety. Interestingly in this study, individuals with “clinically significant anxiety” experienced increases in IL6 that were independent of depressive symptoms. An additional study that examined patients with GAD and coronary artery disease suggested an association between c-reactive protein (CRP) and anxiety symptoms (Bankier et al., 2009). Also, in patients with GAD, T-cell dysfunction has been observed which potentially increases susceptibility to infection and inflammatory/auto-immune disorders (Barros et al., 2011; Vieira et al., 2010). Thus, the relationship between anxiety and inflammation is complex and bidirectional which complicates studying inflammatory biomarkers of treatment response in anxiety.
Very few studies have examined inflammatory markers as predictors of treatment response in anxiety disorders. In one of these rare studies, changes in circulating concentrations of interleukin (IL)-6, IL8, IL-10, IL-18 and TNF-β have been observed (Vogelzangs et al., 2016). Also, in the Netherlands Study of Depression and Anxiety (NESDA), baseline inflammatory markers and lipopolysaccharide-induced cytokine secretion were increased in patients with mixed anxiety and depressive symptoms and strongly correlated with anxiety severity (p<0.001) (Vogelzangs et al., 2016). In children and adolescents with depressive or anxiety disorders (aged 9-18 years, N=41), plasma tumor necrosis factor TNF-α, interleukin (IL)-6, and IL-1β were examined with regard to SSRI treatment. SSRIs decreased TNF-α concentrations (p=0.037). Further, and of high clinical interest, elevated concentrations of these proinflammatory cytokines were associated with SSRI response. Also, higher levels of TNF-α, IL-6, and IL-1β might predict nonresponse to fluoxetine treatment in children (Amitai et al., 2016). An additional treatment study, albeit in patients with MDD, suggests that inflammatory markers could serve as biomarkers of treatment response for anxiety symptoms. In this placebo- controlled trial, infliximab, a monoclonal antibody that inhibits TNF improved both anxiety and depressive symptoms but did so only in patients with the highest inflammatory tone (as measured by CRP concentrations (i.e., CRP concentrations >5) (Raison et al., 2013). The dearth of inflammatory biomarker studies in anxiety disorders contrasts sharply with work in depressive disorders wherein two large, prospective randomized controlled trials studies suggested that inflammatory tone (as reflected by CRP) predicts differential response to noradrenergic agents vs SSRIs. To date, no studies have examined the ability of inflammatory markers to guide medication selection in anxiety disorders.
3.6. Adrenocortical biomarkers
Stoked by research in adults with major depressive disorder and posttraumatic stress disorder, there has been considerable interest in adrenocortical products as biomarkers of treatment response in anxiety disorders. Corticotropin releasing hormone (CRH)—the principal CNS effector of the organismic response to stress, stress-related conditions, particularly depressive and anxiety syndromes—has been implicated in the pathophysiology of affective and anxiety disorders for nearly four decades (Geracioti et al., 1997; Schulte et al., 1984; Wong et al., 2000). Additionally, markers of HPA-axis dysregulation often normalize following treatment with SSRIs (De Bellis et al., 1993b), tricyclic antidepressants (Heuser et al., 1998, 1996) in adults with major depressive disorder.
In anxiety disorders, examinations of hypothalamic-pituitary-adrenal (HPA) axis function focus almost exclusively on the end adrenocortical product, cortisol. Cross sectional studies of adults with anxiety disorders suggest dysregulation of cortisol release in older adults (Hek et al., 2013) and in youth with posttraumatic stress disorder (Keeshin et al., 2014), which shares many characteristics of anxiety disorders. In a placebo-controlled trial of escitalopram in older adults with GAD, escitalopram treatment reduces peak and total cortisol concentrations and decreases in cortisol predicted improvements in anxiety. Further, genetic variability in the serotonin transporter promoter polymorphism predicts treatment-related cortisol changes (Lenze et al., 2011). In adults with panic disorder, blunted adrenocorticotropic hormone and cortisol secretion in response to corticotropin-releasing hormone (CRH) stimulation normalize following 12 weeks of open-label alprazolam. Interestingly, patients with greater baseline dysregulation of the hypothalamic-pituitary-adrenal axis had worse outcomes (Curtis et al., 1997). Finally, regarding the viability of cortisol or other HPA axis elaborants as biomarkers of psychotherapy response, a recent meta-analysis (k=6, N≅275), of basal cortisol concentrations did not predict improvement nor did higher cortisol during exposure-based sessions—which are thought to be critical for anxiety-related improvement—predict improvement (Fischer and Cleare, 2017).
3.7. Neurotrophic Factors
While bran derived neurotrophic factor (BDNF) and glial cell line-derived neurotrophic factors (GDNF) are intimately linked to neuroplasticity (Mattson, 2008) and implicated in lower animal models of anxiety, only in recent years have they been examined in patients with anxiety disorders. Seen as a “brain fertilizer” because it promotes the growth of new neurons and maintains central and peripheral neuronal integrity, BDNF expression increases in response to exercise, caloric restriction and low carbohydrate/low fat diet and is associated with better cognition and decreased anxiety (Gyorkos et al., 2019; Meeusen, 2014; Stranahan et al., 2009). In Han Chinese patients with GAD who were treated with venlafaxine or escitalopram, serum BDNF and GDNF levels were lower compared to healthy individuals and BDNF and GDNF concentrations inversely correlated with Hamilton Anxiety Scale scores. However, post-treatment, neither BDNF or GDNF levels predicted treatment response or remission (Shen et al., 2019). Additionally, in a large placebo-controlled study of duloxetine in adults with GAD, treatment increased plasma BDNF concentrations and patients who responded or remitted had greater increases in BDNF concentrations compared to patients who failed to respond (Ball et al., 2013). Finally, in adults with GAD, serum nerve growth factor concentrations increased following CBT (compared to healthy subjects, p<0.005) and anxious patients with the smallest decreases in anxiety had the lowest increases in nerve growth factor concentrations (M.C. et al., 2007). While the association with BDNF and depression is more clearly established, the role BDNF plays in anxiety seems less clear with some studies suggesting an association with reduced BDNF in various regions of the brain with increase anxiety but other studies that fail find such correlations.
3.8. Pharmacodynamic biomarkers of treatment response
Mutations in glutamatergic genes (i.e., GRIK4) have been associated with sertraline response in large samples of children and adolescents with generalized, separation and social anxiety disorders (Sakolsky et al., 2010). Polymorphisms in the 5-HT2A receptor gene, HTR2A (rs6313) are associated with sertraline dosing (p=0.011) in naturalistic studies of adolescents with anxiety (and depressive) disorders (Poweleit et al., 2019). Specifically, patients with G alleles had better treatment response and required lower sertraline doses to achieve response both in adolescence and adulthood. This finding is true not only for sertraline, but with other SSRIs as well. In a double-blind, placebo-controlled trial of pediatric patients treated with escitalopram, those who were homozygous for the G allele of the HTR2A gene (consistent with lower expression) had reduced treatment response compared to those patients who had at least one A allele. Finally, patients who were homozygous for a short allele of the serotonin transporter promotor polymorphism, SLC6A4, have a reduced magnitude and trajectory of treatment response compared to those who have a long allele (Strawn et al., 2019b). Finally, one study of patients with anxiety disorders which randomized individuals to a commercial pharmacogenetic test which included 10 genes (CYP1A2, CYP2C9, CYP2C19, CYP2D6, CYP3A4, CYP3A5, SLC6A4, COMT, HTR2A, and MTHFR) found that patients in primary care settings with anxiety symptoms who received pharmacogenetically guided care had greater response rates measured by improvements in HAM-A scores following 8 and 12 weeks of treatment (p=0.04; OR: 1.76 [1.03–2.99]) (Bradley et al., 2018). As genetic testing becomes increasingly common, and given that genetic factors may account for nearly one quarter of the variability in antidepressant response (Eichelbaum et al., 2006), it will be critical that recommendations that are derived from pharmacogenetic testing panels be harmonized. In this regard, medication recommendations are often not in agreement among commercial pharmacogenomic-based tools (Bousman and Dunlop, 2018) which puts into question the full utility of these tools at this point in time. One utility of pharmacogenetic testing is that they predict how people will respond to various antidepressants in a disease agnostic fashion, which is noteworthy given that in anxiety disorders, in some populations, SSRIs may outperform SNRIs (Locher et al., 2017; J.R. Strawn et al., 2018, 2015), may be associated with differential tolerability (Strawn et al., 2019a) and may have different dose-response relationships (Jakubovski et al., 2019).
3.9. Current limitations of treatment response biomarkers in anxiety disorders
Despite recent discoveries of treatment response biomarker in anxiety and related disorders, several recent limitations have been identified. Most biomarkers to date—other than neuroimaging-based biomarkers—are obtained or assessed in the periphery (i.e., plasma, serum, saliva, urine). However, the relationship between central and peripheral concentrations or regulation of these measures is remarkably complex and they may be differentially regulated or be produced from different origins. As an example of this, in the periphery, 5-HT is primarily derived from platelets (Endo et al., 2000) and the enterochromaffin cells of the gut. Studies of the serotonin metabolite, 5-HIAA illustrate the problem of relying on measures from only one compartment. In this regard, we previously demonstrated in both anxious and healthy adults that while central and peripheral 5-HIAA co-vary within individuals, among individuals, there is no relationship between cerebrospinal fluid 5-HIAA and plasma concentrations (Strawn et al., 2002). A second key limitation of peripherally derived biomarkers that are used as proxies for central dynamics of the same biomarker is that the disease process itself may result in dissociation of normal physiologic relationships. For example, peripheral hemodynamic measures have been used a reflection of noradrenergic tone and we have previously observed this relationship in healthy adults; however, in patients with posttraumatic stress disorder, hemodynamic measures are completely dissociated from central noradrenergic tone (Strawn et al., 2004). Another significant limitation is the systematic variability in measurements, particularly with regard to neuroimaging-based biomarkers of treatment response.
3.10. Emerging technology for predicting treatment response in anxiety disorders—Extracellular Vesicles
CNS-derived extracellular vesicles (EVs) represent a unique vehicle for biomarker discovery that may inform both the SSRI mechanism of action and molecular pathophysiology of anxiety disorders (Beninson et al., 2014). As small (40-1000 nm) lipid membrane-bound vesicles that reflect their tissue of origin (van der Pol et al., 2012; Yáñez-Mó et al., 2015), EVs dynamically respond to internal and external stimuli (van der Pol et al., 2012; Yáñez-Mó et al., 2015) (Figure 2). As such, EVs relay molecular signals between adjacent and distant cells by entering peripheral biofluids and transporting cargoes (e.g., RNA, proteins, lipids and metabolites) from one tissue to another—these cargoes may have diagnostic and prognostic utility (Thompson et al., 2016; Tsilioni et al., 2014). CNS-derived EVs that can be obtained in peripheral blood may expand our capacity to examine molecular messages generated in neurons, astrocytes, microglia, and oligodendrocytes in the brain; they may help us to hear “which cell type is saying what” i.e. ascertaining signals from the specific brain cell types to harness potential biomarkers. As an example, neuronally-derived EVs have been isolated using immunoabsorptive techniques that leverage L1CAM(+), an adhesive molecule, primarily expressed in neurons. L1CAM(+) EVs, are enriched in cargoes of neuronal origin and provide a “window” into molecular processes in the brain by way of peripheral biofluids (Mustapic et al., 2017). Utilizing L1CAM(+) EVs can help accelerate investigations of EV biology in psychiatric disorders and in finding treatment response biomarkers for a variety of CNS disorders (Raghavan, 2017).
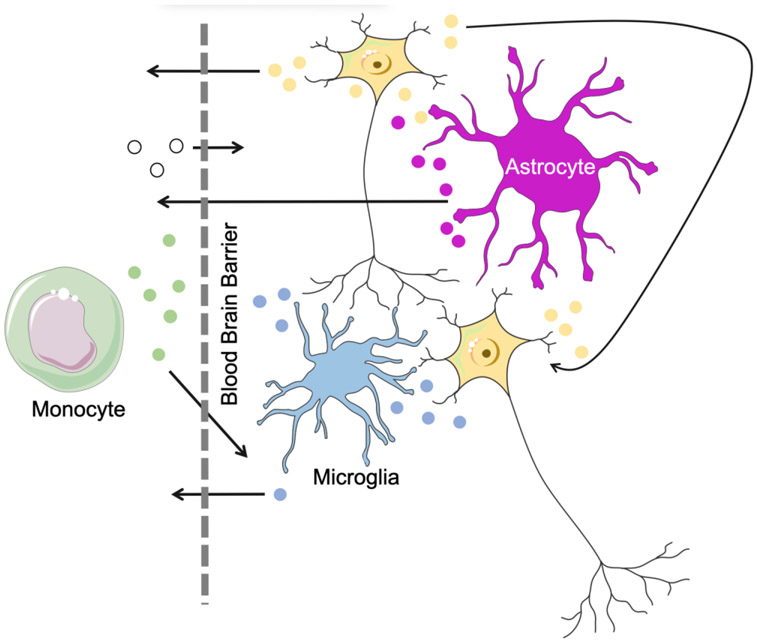
FIGURE 2: Extracellular vesicle (EV) trafficking.
EVs function as a mode of communication between cells within the CNS (right) and between the CNS and periphery (left). Unlike large proteins and many other molecular entities, EVs readily cross the blood brain barrier (dotted line). They represent an emerging biomarker of CNS processes that can be easily accessed via peripheral blood sampling.
3.10.1. Application of emerging biomarker technology: BDNF Expression in Neuronally-Derived EVs
A good example of the utility of brain derived EVs for biomarker research in anxiety and other CNS disorders can be seen with BDNF which h already been studied in relationship to treatment response (see section 3.7.). A common single nucleotide polymorphism in the BDNF gene causes a substitution of valine (val) to methionine (met). Importantly, 25% of Caucasians and an even larger percentage of Asians have this met/met polymorphism which is associated with an increased risk to anxiety and depression. Mice in which the BDNF met/met alleles have been inserted also demonstrate an increase in anxiety and depression. In these BDNF met/met mutant mice, administering SSRI during adolescence, seems to affect a critical developmental window. SSRI administration at that developmental stage increases BDNF and aborts the expected development of anxiety later in adulthood (Duman, 2017). Since neuronally derived EVs contain BDNF (Suire et al., 2017), we can use them to test CNS BDNF levels in plasma of adolescents with anxiety disorders as they are expressed in neurons and combine that information with genomic testing of the BDNF alleles. We can also test the finding in BDNF met/met adolescent mice that demonstrate a critical window of vulnerability in adolescence. Treating adolescents at a potentially biologically sensitive period, while measuring CNS BDNF levels, have the potential to protect them from further progression of anxiety symptoms in adulthood, and can help us elucidate a mechanism of action suggested in animal studies.
Neuronally-derived EVs can facilitate the translation of biomarkers from basic science and animal models of anxiety to the clinic. As an example, knockout prion protein (PrP) mice display anxiety-like behaviors and have increased in 5-HT receptors expression [93, 94]. PrPs are expressed mainly in the CNS and secreted in neuronally derived EVs. Studying PrP expression in neuronally-derived EVs in response to SSRIs may identify novel treatment response biomarkers. This is just one example of how examination of EV cargoes can be used to test hypotheses related to both pathophysiology and treatment response. Over the past few decades, despite accumulating molecular knowledge about pre-clinical anxiety, considerable barriers remain in translating this knowledge to clinical research. Neuronally-derived EVs are poised to bridge this gap.
4. Discussion
While the psychopharmacologic armamentarium for anxiety disorders, response to pharmacotherapy and psychotherapy varies and precision medicine tools are urgently needed. Given that anxiety disorders are among the most prevalent causes of morbidity globally (James et al., 2018), improving treatment outcomes—through the use of treatment response biomarkers—will enable better and more effective treatments for a substantial portion of the population.
By non-invasively measuring molecular signals in the CNS, we can discover biomarkers that are linked to pathophysiology and target engagement. CNS derived biomarkers that can be obtained peripherally (i.e., in blood samples) may expand our capacity to examine molecular messages from neurons, astrocytes, microglia, and oligodendrocytes. This enables us to identify which signals are released into the circulation by which cells and how to use these signals to generate translational clinical trials for biologically-targeted treatments for anxiety that include biomarker discovery and assess target engagement. Further, as we become skilled at accessing CNS molecular signals, we must learn how these biomarkers interact across compartments (e.g., peripheral blood, urine, CNS). The collection of peripheral and CNS biomarkers reviewed herein could be integrated into a single biomarker panel. Having a myriad of molecules, some that originate in the CNS and some in the periphery may increase the reliability of such panels. A host of inflammatory molecules are associated with anxiety and with treatment response, but may lack specificity, since inflammation can be triggered by many different factors. Using such inflammatory biomarkers in conjunction with biomarkers from other pathways, such as BDNF, could enhance the sensitivity and specificity of a treatment response biomarker panel.
How we should collect biomarkers represents an important area of future research. Biomarker assays for analytes that are used in clinical care are now available for point-of-care testing and some genomic markers are now being collected by patients outside of clinic visits. Beyond this, wearables have come of age in the last two decades and are now being used to perform sweat analysis, assess volatile organic compounds in skin (Piro et al., 2019) in addition to monitoring of general physiology. Such wearable technology which is non-invasive, miniaturized, and available for ambulatory patients also may allow for repeated sampling or even continuous monitoring of analytes. One such system provides continuous measurement of glucose, pH, temperature and lactate (Yokus et al., 2020), which as described above may be particularly relevant for patients with panic disorder. These measures that can be provided in real-time (Gratch et al., 2020; Robinaugh et al., 2020) may also allow continuous biomarker monitoring to be coupled with symptomatic tracking in the same way that cardiologists monitor intrathoracic impedance, brain naturetic peptide and clinical symptoms to improve treatment outcomes for patients with heart failure (Ypenburg et al., 2007).
In this review, we surveyed biomarkers in children, adolescents, adults and older adults. However, these relative utility, expression and physiology of these biomarkers may change over the lifespan. In addition the systems which they represent may also change over the course of as patients age. Perhaps the best example of this is the GABAergic system and the cytochrome P450 system. Benzodiazepines do not appear to be as effective in children with anxiety relative to adults and children require larger doses of benzodiazepines (on mg/kg basis) compared to adults. These differences may be related to differences in biodistribution and metabolism as well as changes in receptor density and subunit composition which ultimately affects binding affinity. In fact, subcortical regions do not reach adult VD for flumazenil until 14-18 years of age whereas cortical regions may not achieve adult VD until age 22 (Chugani et al., 2001). Therefore, simply assessing GABA levels without taking into consideration development/age might not accurately reflect GABA-related biomarkers. Analogously, cytochrome activity tends to vary across development and for CYP2C19 (discussed earlier), children may have higher levels whereas activity tends to decrease after approximately age 65 (Hicks et al., 2015) underscoring the need to consider age and development when incorporating a pharmacokinetic biomarker of treatment response into practice.
Many patients with anxiety do not receive adequate treatment, in terms of medication dose (or exposure) or duration of treatment; partial treatments are common, and patients often fail to reach full remission. Having biomarkers for treatment response that guide clinicians will almost certainly improve outcomes. This is especially important for non-psychiatric clinicians who do not receive special training in assessing anxiety, although it is noteworthy that these same non-psychiatric clinicians commonly integrate clinical assessment and biomarkers for other disorders such as atrial fibrillation, dyslipidemia, and treatment of acute otitis media. Thus, with psychiatric biomarkers of treatment response, future studies must examine the relationship between these biomarkers as well as genetic and clinical/demographic factors. More sophisticated precision medicine approaches will undoubtedly examine biomarkers in concert with clinical and demographic factors as well as prior treatment as we have done in non-psychiatric specialties. For example, when approaching a patient who is at risk for developing atrial fibrillation, the CHAD2 score guides treatment based on risk and this risk determination includes diagnosis (e.g., congestive heart failure, hypertension) as well as demographic factors (age >75 years) and history (prior history of cerebrovascular accident or transient ischemic attack) (Gage et al., 2001). An analogous score could consider the primary disorder, several biomarkers described in this review, age, etc. and guide the clinician to select the most effective pharmacotherapy, psychotherapy or combination for that patient.
5. Conclusion
The current state of treatment response biomarkers in anxiety disorders across development is promising. Emerging potential biomarkers are diverse in origin and function. They span the CNS and periphery and touch respiratory, inflammatory, cardiovascular and neuronal functions. Treatment response biomarkers are relevant to both psychopharmacologic and psychotherapeutic interventions. In fact, psychotherapy has a profound effect on the brain and changes brain function and these changes—whether probed centrally or peripherally-- can be leveraged as treatment response biomarkers to predict which psychotherapy before beginning treatment. With the advent of multiple evidence-based treatments for a myriad of disorders, precision tools to direct patients to the treatment that will work best in for them will revolutionize the field and help in improving outcomes and lessening long-term morbidity of mental illness. Several lessons must be kept in mind as we continue to search for novel, anxiety-specific biomarkers of treatment response.
The degree to which potential biomarkers reflect changes in pathophysiology should be elucidated.
Concordance between central and peripheral compartments must be considered and characterized as new biomarkers are identified.
Ontogenic aspects of treatment-response biomarkers must be understood, particularly given that treatment tolerability and efficacy varies across the lifespan.
The degree to which biomarkers reflect target engagement is critical to identifying “which treatment works for which patient.”
How biomarkers are differentially affected by the modality of treatment (e.g., psychotherapy or psychopharmacology)
Acknowledgments:
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Development (NICHD) through Grant R01HD098757 and by the Yung Family Foundation. The authors appreciate assistance from Mrs. Sara Varney, BS and Mrs. Ashley Specht, BBA (Anxiety Disorders Research Program at the University of Cincinnati) for technical assistance with the preparation of this manuscript.
abbreviations:
- SSRI, SRI
selective serotonin reuptake inhibitor
- SNRI
selective serotonin norepinephrine reuptake inhibitor
Footnotes
Disclosures: Dr. Strawn has received research support from the National Institutes of Health (NIMH/NIEHS/NICHD) as well as Allergan, Neuronetics and Otsuka. He has received material support from and provided consultation to Myriad Genetics. He also is a consultant to the US Food and Drug Administration and to Intra-cellular Therapeutics and receives royalties from the publication of two texts (Springer). Dr. Strawn serves as an author for UpToDate and an Associate Editor for Current Psychiatry. Dr. Strawn also receive research support from the Yung Family Foundation, the National Institute of Environmental Health and Safety and the National Institute of Mental Health. Both Drs. Levine and Strawn receive research support from the Eunice Kennedy Shriver National Institutes of Child Health and Development (NICHD).
References
- Amitai M, Taler M, Carmel M, Michaelovsky E, Eilat T, Yablonski M, Orpaz N, Chen A, Apter A, Weizman A, Fennig S, 2016. The relationship between plasma cytokine levels and response to selective serotonin reuptake inhibitor treatment in children and adolescents with depression and/or anxiety disorders. J. Child Adolesc. Psychopharmacol doi: 10.1089/cap.2015.0147 [DOI] [PubMed] [Google Scholar]
- Ball S, Marangell LB, Lipsius S, Russell JM, 2013. Brain-derived neurotrophic factor in generalized anxiety disorder: Results from a duloxetine clinical trial. Prog. Neuro-Psychopharmacology Biol. Psychiatry doi: 10.1016/j.pnpbp.2013.01.002 [DOI] [PubMed] [Google Scholar]
- Bankier B, Barajas J, Martinez-Rumayor A, Januzzi JL, 2009. Association between anxiety and C-reactive protein levels in stable coronary heart disease patients. Psychosomatics 50, 347–353. doi: 10.1176/appi.psy.50.4.347 [DOI] [PubMed] [Google Scholar]
- Barros PO, Ferreira TB, Vieira MMM, Almeida CRM, Araújo-Lima CF, Silva-Filho RG, Hygino J, Andrade RM, Andrade AF, Bento CA, 2011. Substance P enhances Th17 phenotype in individuals with generalized anxiety disorder: An event resistant to glucocorticoid inhibition. J. Clin. Immunol 31, 51–59. doi: 10.1007/s10875-010-9466-6 [DOI] [PubMed] [Google Scholar]
- Baxter AJ, Vos T, Scott KM, Ferrari AJ, Whiteford HA, 2014. The global burden of anxiety disorders in 2010. Psychol. Med 44, 2363–2374. doi: 10.1017/S0033291713003243 [DOI] [PubMed] [Google Scholar]
- Beckman D, Santos LE, Americo TA, Ledo JH, De Mello FG, Linden R, 2015. Prion protein modulates monoaminergic systems and depressive-like behavior in mice. J. Biol. Chem 290, 20488–20498. doi: 10.1074/jbc.M115.666156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beesdo-Baum K, Knappe S, 2012. Developmental epidemiology of anxiety disorders. Child Adolesc. Psychiatr. Clin. N. Am doi: 10.1016/j.chc.2012.05.001 [DOI] [PubMed] [Google Scholar]
- Beesdo K, Pine DS, Lieb R, Wittchen H-U, 2010. Incidence and risk patterns of anxiety and depressive disorders and categorization of generalized anxiety disorder. Arch. Gen. Psychiatry 67, 47–57. doi: 10.1001/archgenpsychiatry.2009.177 [DOI] [PubMed] [Google Scholar]
- Beninson LA, Brown PN, Loughridge AB, Saludes JP, Maslanik T, Hills AK, Woodworth T, Craig W, Yin H, Fleshner M, 2014. Acute stressor exposure modifies plasma exosome-associated heat shock protein 72 (Hsp72) and microRNA (miR-142–5p and miR-203). PLoS One 9. doi: 10.1371/journal.pone.0108748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousman CA, Dunlop BW, 2018. Genotype, phenotype, and medication recommendation agreement among commercial pharmacogenetic-based decision support tools. Pharmacogenomics J. doi: 10.1038/s41397-018-0027-3 [DOI] [PubMed] [Google Scholar]
- Bradley P, Shiekh M, Mehra V, Vrbicky K, Layle S, Olson MC, Maciel A, Cullors A, Garces JA, Lukowiak AA, 2018. Improved efficacy with targeted pharmacogenetic-guided treatment of patients with depression and anxiety: A randomized clinical trial demonstrating clinical utility. J. Psychiatr. Res. 96, 100–107. doi: 10.1016/j.jpsychires.2017.09.024 [DOI] [PubMed] [Google Scholar]
- Brennan BP, Admon R, Perriello C, LaFlamme EM, Athey AJ, Pizzagalli DA, Hudson JI, Pope HG, Jensen JE, 2017. Acute change in anterior cingulate cortex GABA, but not glutamine/glutamate, mediates antidepressant response to citalopram. Psychiatry Res. - Neuroimaging doi: 10.1016/j.pscychresns.2017.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhouse KL, Kujawa A, Hosseini B, Klumpp H, Fitzgerald KD, Langenecker SA, Monk CS, Phan KL, 2018. Anterior cingulate activation to implicit threat before and after treatment for pediatric anxiety disorders. Prog. Neuro-Psychopharmacology Biol. Psychiatry doi: 10.1016/j.pnpbp.2018.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuron L, Gumnick JF, Musselman DL, Lawson DH, Reemsnyder A, Nemeroff CB, Miller AH, 2002. Neurobehavioral effects of interferon-α in cancer patients: Phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology. doi: 10.1016/S0893-133X(01)00407-9 [DOI] [PubMed] [Google Scholar]
- Charney DS, Heninger GR, Breier A, 1984. Noradrenergic function in panic anxiety: effects of yohimbine in healthy subjects and patients with agoraphobia and panic disorder. Arch. Gen. Psychiatry doi: 10.1001/archpsyc.1984.01790190025003 [DOI] [PubMed] [Google Scholar]
- Charney DS, Woods SW, Krystal JH, Nagy LM, Heninger GR, 1992. Noradrenergic neuronal dysregulation in panic disorder: the effects of intravenous yohimbine and clonidine in panic disorder patients. Acta Psychiatr. Scand doi: 10.1111/j.1600-0447.1992.tb03266.x [DOI] [PubMed] [Google Scholar]
- Chojnacka-Wójcik E, Klodzinska A, Pilc A, 2001. Glutamate receptor ligands as anxiolytics. Curr. Opin. Investig. Drugs [PubMed] [Google Scholar]
- Chugani DC, Muzik O, Juhász C, Janisse JJ, Ager J, Chugani HT, 2001. Postnatal maturation of human GABAA receptors measured with positron emission tomography. Ann. Neurol doi: 10.1002/ana.1003 [DOI] [PubMed] [Google Scholar]
- Connolly SD, Bernstein GA, 2007. Practice parameter for the assessment and treatment of children and adolescents with anxiety disorders. J. Am. Acad. Child Adolesc. Psychiatry 46, 267–83. doi: 10.1097/01.chi.0000246070.23695.06 [DOI] [PubMed] [Google Scholar]
- Cotton S, Luberto CM, Sears RW, Strawn JR, Stahl L, Wasson RS, Blom TJ, Delbello MP, 2016. Mindfulness-based cognitive therapy for youth with anxiety disorders at risk for bipolar disorder: a pilot trial. Early Interv. Psychiatry 10, 426–434. doi: 10.1111/eip.12216 [DOI] [PubMed] [Google Scholar]
- Curtis GC, Abelson JL, Gold PW, 1997. Adrenocorticotropic hormone and cortisol responses to corticotropin-releasing hormone: Changes in panic disorder and effects of alprazolam treatment. Biol. Psychiatry doi: 10.1016/S0006-3223(95)00578-1 [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Geracioti TD, Altemus M, Kling MA, 1993a. Cerebrospinal fluid monoamine metabolites in fluoxetine-treated patients with major depression and in healthy volunteers. Biol. Psychiatry doi: 10.1016/0006-3223(93)90103-K [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Gold PW, Geracioti TD, Listwak SJ, Kling MA, 1993b. Association of fluoxetine treatment with reductions in CSF concentrations of corticotropin-releasing hormone and arginine vasopressin in patients with major depression. Am. J. Psychiatry doi: 10.1176/ajp.150.4.656 [DOI] [PubMed] [Google Scholar]
- Doehrmann O, Ghosh SS, Polli FE, Reynolds GO, Horn F, Keshavan A, Triantafyllou C, Saygin ZM, Whitfield-Gabrieli S, Hofmann SG, Pollack M, Gabrieli JD, 2013. Predicting treatment response in social anxiety disorder from functional magnetic resonance imaging. Arch. Gen. Psychiatry doi: 10.1001/2013.jamapsychiatry.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, 2017. BDNF, 5-HT, and anxiety: Identification of a critical periadolescent developmental period. Am. J. Psychiatry doi: 10.1176/appi.ajp.2017.17101084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egami M, Imamura Y, Nabeta H, Mizoguchi Y, Yamada S, 2013. Saliva levels of 3-methoxy-4-hydroxyphenylglycol and clinical efficacy of mirtazapine or selective serotonin reuptake inhibitors in patients with major depression. Hum. Psychopharmacol doi: 10.1002/hup.2273 [DOI] [PubMed] [Google Scholar]
- Eichelbaum M, Ingelman-Sundberg M, Evans WE, 2006. Pharmacogenomics and Individualized Drug Therapy. Annu. Rev. Med 57, 119–137. doi: 10.1146/annurev.med.56.082103.104724 [DOI] [PubMed] [Google Scholar]
- Endo T, Minami M, Hirafuji M, Ogawa T, Akita K, Nemoto M, Saito H, Yoshioka M, Parvez SH, 2000. Neurochemistry and neuropharmacology of emesis - The role of serotonin. Toxicology. doi: 10.1016/S0300-483X(00)00314-0 [DOI] [PubMed] [Google Scholar]
- Fischer S, Cleare AJ, 2017. Cortisol as a predictor of psychological therapy response in anxiety disorders—Systematic review and meta-analysis. J. Anxiety Disord doi: 10.1016/j.janxdis.2017.02.007 [DOI] [PubMed] [Google Scholar]
- Fluvoxamine for the treatment of anxiety disorders in children and adolescents. The Research Unit on Pediatric Psychopharmacology Anxiety Study Group, 2001. . N Engl J Med 344, 1279–1285. doi: 10.1056/nejm200104263441703 [DOI] [PubMed] [Google Scholar]
- Frick A, Engman J, Wahlstedt K, Gingnell M, Fredrikson M, Furmark T, 2018. Anterior cingulate cortex activity as a candidate biomarker for treatment selection in social anxiety disorder. BJPsych Open. doi: 10.1192/bjo.2018.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ, 2001. Validation of clinical classification schemes for predicting stroke. JAMA 285, 2864. doi: 10.1001/jama.285.22.2864 [DOI] [PubMed] [Google Scholar]
- Geracioti TD, Loosen PT, Orth DN, 1997. Low cerebrospinal fluid corticotropin-releasing hormone concentrations in eucortisolemic depression. Biol. Psychiatry doi: 10.1016/s0006-3223(96)00312-5 [DOI] [PubMed] [Google Scholar]
- Geracioti TD, Schmidt D, Ekhator NN, Shelton R, Parris W, Loosen PT, Ebert MH, 1993. Cerebrospinal fluid norepinephrine concentrations and dynamics in depressed patients and normal volunteers. Depression. doi: 10.1002/depr.3050010306 [DOI] [Google Scholar]
- Goddard AW, Mason GF, Almai A, Rothman DL, Behar KL, Petroff OAC, Charney DS, Krystal JH, 2001. Reductions in occipital cortex GABA levels in panic disorder detected with 1H-magnetic resonance spectroscopy. Arch. Gen. Psychiatry doi: 10.1001/archpsyc.58.6.556 [DOI] [PubMed] [Google Scholar]
- Granger DA, Kivlighan KT, El-Sheikh M, Gordis EB, Stroud LR, 2007. Salivy α-amylase in biobehavioral research: Recent developments and applications, in: Annals of the New York Academy of Sciences. doi: 10.1196/annals.1384.008 [DOI] [PubMed] [Google Scholar]
- Gratch I, Choo TH, Galfalvy H, Keilp JG, Itzhaky L, Mann JJ, Oquendo MA, Stanley B, 2020. Detecting suicidal thoughts: The power of ecological momentary assessment. Depress. Anxiety. doi: 10.1002/da.23043 [DOI] [PubMed] [Google Scholar]
- Gyorkos A, Baker MH, Miutz LN, Lown DA, Jones MA, Houghton-Rahrig LD, 2019. Carbohydrate-restricted diet and exercise increase brain-derived neurotrophic factor and cognitive function: a randomized crossover trial. Cureus. doi: 10.7759/cureus.5604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler G, van der Veen JW, Geraci M, Shen J, Pine D, Drevets WC, 2009. Prefrontal cortical gamma-aminobutyric acid levels in panic disorder determined by proton magnetic resonance spectroscopy. Biol. Psychiatry doi: 10.1016/j.biopsych.2008.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hek K, Direk N, Newson RS, Hofman A, Hoogendijk WJG, Mulder CL, Tiemeier H, 2013. Anxiety disorders and salivary cortisol levels in older adults: A population-based study. Psychoneuroendocrinology. doi: 10.1016/j.psyneuen.2012.06.006 [DOI] [PubMed] [Google Scholar]
- Heuser I, Bissette G, Dettling M, Schweiger U, Gotthardt U, Schmider J, Lammers C‐H, Nemeroff CB, Holsboer F, 1998. Cerebrospinal fluid concentrations of corticotropin‐releasing hormone, vasopressin, and somatostatin in depressed patients and healthy controls: Response to amitriptyline treatment. Depress. Anxiety doi: [DOI] [PubMed] [Google Scholar]
- Heuser IJE, Schweiger U, Gotthardt U, Schmider J, Lammers CH, Dettling M, Yassouridis A, Holsboer F, 1996. Pituitary-adrenal-system regulation and psychopathology during amitriptyline treatment in elderly depressed patients and normal comparison subjects. Am. J. Psychiatry doi: 10.1176/ajp.153.1.93 [DOI] [PubMed] [Google Scholar]
- Hicks JK, Bishop JR, Sangkuhl K, Muller DJ, Ji Y, Leckband SG, Leeder JS, Graham RL, Chiulli DL, LLerena A, Skaar TC, Scott SA, Stingl JC, Klein TE, Caudle KE, Gaedigk A, 2015. Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline for CYP2D6 and CYP2C19 genotypes and dosing of selective serotonin reuptake inhibitors. Clin. Pharmacol. Ther 98, 127–134. doi: 10.1002/cpt.147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou R, Baldwin DS, 2012. A neuroimmunological perspective on anxiety disorders. Hum. Psychopharmacol doi: 10.1002/hup.1259 [DOI] [PubMed] [Google Scholar]
- Howells FM, Hattingh CJ, Syal S, Breet E, Stein DJ, Lochner C, 2015. 1H-magnetic resonance spectroscopy in social anxiety disorder. Prog. Neuro-Psychopharmacology Biol. Psychiatry doi: 10.1016/j.pnpbp.2014.12.008 [DOI] [PubMed] [Google Scholar]
- Jakubovski E, Johnson JA, Nasir M, Müller-Vahl K, Bloch MH, 2019. Systematic review and meta-analysis: Dose–response curve of SSRIs and SNRIs in anxiety disorders. Depress. Anxiety doi: 10.1002/da.22854 [DOI] [PubMed] [Google Scholar]
- James SL, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, Abbastabar H, Abd-Allah F, Abdela J, Abdelalim A, Abdollahpour I, Abdulkader RS, Abebe Z, Abera SF, Abil OZ, Abraha HN, Abu-Raddad LJ, Abu-Rmeileh NME, Accrombessi MMK, Acharya D, Acharya P, Ackerman IN, Adamu AA, Adebayo OM, Adekanmbi V, Adetokunboh OO, Adib MG, Adsuar JC, Afanvi KA, Afarideh M, Afshin A, Agarwal G, Agesa KM, Aggarwal R, Aghayan SA, Agrawal S, Ahmadi A, Ahmadi M, Ahmadieh H, Ahmed MB, Aichour AN, Aichour I, Aichour MTE, Akinyemiju T, Akseer N, Al-Aly Z, Al-Eyadhy A, Al-Mekhlafi HM, Al-Raddadi RM, Alahdab F, Alam K, Alam T, Alashi A, Alavian SM, Alene KA, Alijanzadeh M, Alizadeh-Navaei R, Aljunid SM, Alkerwi A, Alla F, Allebeck P, Alouani MML, Altirkawi K, Alvis-Guzman N, Amare AT, Aminde LN, Ammar W, Amoako YA, Anber NH, Andrei CL, Androudi S, Animut MD, Anjomshoa M, Ansha MG, Antonio CAT, Anwari P, Arabloo J, Arauz A, Aremu O, Ariani F, Armoon B, Ärnlöv J, Arora A, Artaman A, Aryal KK, Asayesh H, Asghar RJ, Ataro Z, Atre SR, Ausloos M, Avila-Burgos L, Avokpaho EFGA, Awasthi A, Ayala Quintanilla BP, Ayer R, Azzopardi PS, Babazadeh A, Badali H, Badawi A, Bali AG, Ballesteros KE, Ballew SH, Banach M, Banoub JAM, Banstola A, Barac A, Barboza MA, Barker-Collo SL, Bärnighausen TW, Barrero LH, Baune BT, Bazargan-Hejazi S, Bedi N, Beghi E, Behzadifar Masoud, Behzadifar Meysam, Béjot Y, Belachew AB, Belay YA, Bell ML, Bello AK, Bensenor IM, Bernabe E, Bernstein RS, Beuran M, Beyranvand T, Bhala N, Bhattarai S, Bhaumik S, Bhutta ZA, Biadgo B, Bijani A, Bikbov B, Bilano V, Bililign N, Bin Sayeed MS, Bisanzio D, Blacker BF, Blyth FM, Bou-Orm IR, Boufous S, Bourne R, Brady OJ, Brainin M, Brant LC, Brazinova A, Breitborde NJK, Brenner H, Briant PS, Briggs AM, Briko AN, Britton G, Brugha T, Buchbinder R, Busse R, Butt ZA, Cahuana-Hurtado L, Cano J, Cárdenas R, Carrero JJ, Carter A, Carvalho F, Castañeda-Orjuela CA, Castillo Rivas J, Castro F, Catalá-López F, Cercy KM, Cerin E, Chaiah Y, Chang AR, Chang HY, Chang JC, Charlson FJ, Chattopadhyay A, Chattu VK, Chaturvedi P, Chiang PPC, Chin KL, Chitheer A, Choi JYJ, Chowdhury R, Christensen H, Christopher DJ, Cicuttini FM, Ciobanu LG, Cirillo M, Claro RM, Collado-Mateo D, Cooper C, Coresh J, Cortesi PA, Cortinovis M, Costa M, Cousin E, Criqui MH, Cromwell EA, Cross M, Crump JA, Dadi AF, Dandona L, Dandona R, Dargan PI, Daryani A, Das Gupta Rajat, Das Neves J, Dasa TT, Davey G, Davis AC, Davitoiu DV, De Courten B, De La Hoz FP, De Leo D, De Neve JW, Degefa MG, Degenhardt L, Deiparine S, Dellavalle RP, Demoz GT, Deribe K, Dervenis N, Des Jarlais DC, Dessie GA, Dey S, Dharmaratne SD, Dinberu MT, Dirac MA, Djalalinia S, Doan L, Dokova K, Doku DT, Dorsey ER, Doyle KE, Driscoll TR, Dubey M, Dubljanin E, Duken EE, Duncan BB, Duraes AR, Ebrahimi H, Ebrahimpour S, Echko MM, Edvardsson D, Effiong A, Ehrlich JR, El Bcheraoui C, El Sayed Zaki M, El-Khatib Z, Elkout H, Elyazar IRF, Enayati A, Endries AY, Er B, Erskine HE, Eshrati B, Eskandarieh S, Esteghamati A, Esteghamati S, Fakhim H, Fallah Omrani V, Faramarzi M, Fareed M, Farhadi F, Farid TA, Farinha C.S.E. s., Farioli A, Faro A, Farvid MS, Farzadfar F, Feigin VL, Fentahun N, Fereshtehnejad SM, Fernandes E, Fernandes JC, Ferrari AJ, Feyissa GT, Filip I, Fischer F, Fitzmaurice C, Foigt NA, Foreman KJ, Fox J, Frank TD, Fukumoto T, Fullman N, Fürst T, Furtado JM, Futran ND, Gall S, Ganji M, Gankpe FG, Garcia-Basteiro AL, Gardner WM, Gebre AK, Gebremedhin AT, Gebremichael TG, Gelano TF, Geleijnse JM, Genova-Maleras R, Geramo YCD, Gething PW, Gezae KE, Ghadiri K, Ghasemi Falavarjani K, Ghasemi-Kasman M, Ghimire M, Ghosh R, Ghoshal AG, Giampaoli S, Gill PS, Gill TK, Ginawi IA, Giussani G, Gnedovskaya EV, Goldberg EM, Goli S, Gómez-Dantés H, Gona PN, Gopalani SV, Gorman TM, Goulart AC, Goulart BNG, Grada A, Grams ME, Grosso G, Gugnani HC, Guo Y, Gupta PC, Gupta Rahul, Gupta Rajeev, Gupta T, Gyawali B, Haagsma JA, Hachinski V, Hafezi-Nejad N, Haghparast Bidgoli H, Hagos TB, Hailu GB, Haj-Mirzaian Arvin, Haj-Mirzaian Arya, Hamadeh RR, Hamidi S, Handal AJ, Hankey GJ, Hao Y, Harb HL, Harikrishnan S, Haro JM, Hasan M, Hassankhani H, Hassen HY, Havmoeller R, Hawley CN, Hay RJ, Hay SI, Hedayatizadeh-Omran A, Heibati B, Hendrie D, Henok A, Herteliu C, Heydarpour S, Hibstu DT, Hoang HT, Hoek HW, Hoffman HJ, Hole MK, Homaie Rad E, Hoogar P, Hosgood HD, Hosseini SM, Hosseinzadeh M, Hostiuc M, Hostiuc S, Hotez PJ, Hoy DG, Hsairi M, Htet AS, Hu G, Huang JJ, Huynh CK, Iburg KM, Ikeda CT, Ileanu B, Ilesanmi OS, Iqbal U, Irvani SSN, Irvine CMS, Islam SMS, Islami F, Jacobsen KH, Jahangiry L, Jahanmehr N, Jain SK, Jakovljevic M, Javanbakht M, Jayatilleke AU, Jeemon P, Jha RP, Jha V, Ji JS, Johnson CO, Jonas JB, Jozwiak JJ, Jungari SB, Jürisson M, Kabir Z, Kadel R, Kahsay A, Kalani R, Kanchan T, Karami M, Karami Matin B, Karch A, Karema C, Karimi N, Karimi SM, Kasaeian A, Kassa DH, Kassa GM, Kassa TD, Kassebaum NJ, Katikireddi SV, Kawakami N, Karyani AK, Keighobadi MM, Keiyoro PN, Kemmer L, Kemp GR, Kengne AP, Keren A, Khader YS, Khafaei B, Khafaie MA, Khajavi A, Khalil IA, Khan EA, Khan MS, Khan MA, Khang YH, Khazaei M, Khoja AT, Khosravi A, Khosravi MH, Kiadaliri AA, Kiirithio DN, Kim C Il, Kim D, Kim P, Kim YE, Kim YJ, Kimokoti RW, Kinfu Y, Kisa A, Kissimova-Skarbek K, Kivimäki M, Knudsen AKS, Kocarnik JM, Kochhar S, Kokubo Y, Kolola T, Kopec JA, Kosen S, Kotsakis GA, Koul PA, Koyanagi A, Kravchenko MA, Krishan K, Krohn KJ, Kuate Defo B, Kucuk Bicer B, Kumar GA, Kumar M, Kyu HH, Lad DP, Lad SD, Lafranconi A, Lalloo R, Lallukka T, Lami FH, Lansingh VC, Latifi A, Lau KMM, Lazarus JV, Leasher JL, Ledesma JR, Lee PH, Leigh J, Leung J, Levi M, Lewycka S, Li S, Li Y, Liao Y, Liben ML, Lim LL, Lim SS, Liu S, Lodha R, Looker KJ, Lopez AD, Lorkowski S, Lotufo PA, Low N, Lozano R, Lucas TCD, Lucchesi LR, Lunevicius R, Lyons RA, Ma S, Macarayan ERK, Mackay MT, Madotto F, Magdy Abd El Razek H, Magdy Abd El Razek M, Maghavani DP, Mahotra NB, Mai HT, Majdan M, Majdzadeh R, Majeed A, Malekzadeh R, Malta DC, Mamun AA, Manda AL, Manguerra H, Manhertz T, Mansournia MA, Mantovani LG, Mapoma CC, Maravilla JC, Marcenes W, Marks A, Martins-Melo FR, Martopullo I, März W, Marzan MB, Mashamba-Thompson TP, Massenburg BB, Mathur MR, Matsushita K, Maulik PK, Mazidi M, McAlinden C, McGrath JJ, McKee M, Mehndiratta MM, Mehrotra R, Mehta KM, Mehta V, Mejia-Rodriguez F, Mekonen T, Melese A, Melku M, Meltzer M, Memiah PTN, Memish ZA, Mendoza W, Mengistu DT, Mengistu G, Mensah GA, Mereta ST, Meretoja A, Meretoja TJ, Mestrovic T, Mezerji NMG, Miazgowski B, Miazgowski T, Millear AI, Miller TR, Miltz B, Mini GK, Mirarefin M, Mirrakhimov EM, Misganaw AT, Mitchell PB, Mitiku H, Moazen B, Mohajer B, Mohammad KA, Mohammadifard N, Mohammadnia-Afrouzi M, Mohammed MA, Mohammed S, Mohebi F, Moitra M, Mokdad AH, Molokhia M, Monasta L, Moodley Y, Moosazadeh M, Moradi G, Moradi-Lakeh M, Moradinazar M, Moraga P, Morawska L, Moreno Velásquez I, Morgado-Da-Costa J, Morrison SD, Moschos MM, Mousavi SM, Mruts KB, Muche AA, Muchie KF, Mueller UO, Muhammed OS, Mukhopadhyay S, Muller K, Mumford JE, Murhekar M, Musa J, Musa KI, Mustafa G, Nabhan AF, Nagata C, Naghavi M, Naheed A, Nahvijou A, Naik G, Naik N, Najafi F, Naldi L, Nam HS, Nangia V, Nansseu JR, Nascimento BR, Natarajan G, Neamati N, Negoi I, Negoi RI, Neupane S, Newton CRJ, Ngunjiri JW, Nguyen AQ, Nguyen Ha Thu, Nguyen HLT, Nguyen Huong Thanh, Nguyen LH, Nguyen M, Nguyen NB, Nguyen SH, Nichols E, Ningrum DNA, Nixon MR, Nolutshungu N, Nomura S, Norheim OF, Noroozi M, Norrving B, Noubiap JJ, Nouri HR, Nourollahpour Shiadeh M, Nowroozi MR, Nsoesie EO, Nyasulu PS, Odell CM, Ofori-Asenso R, Ogbo FA, Oh IH, Oladimeji O, Olagunju AT, Olagunju TO, Olivares PR, Olsen HE, Olusanya BO, Ong KL, Ong SK, Oren E, Ortiz A, Ota E, Otstavnov SS, Øverland S, Owolabi MO, P A M, Pacella R, Pakpour AH, Pana A, Panda-Jonas S, Parisi A, Park EK, Parry CDH, Patel S, Pati S, Patil ST, Patle A, Patton GC, Paturi VR, Paulson KR, Pearce N, Pereira DM, Perico N, Pesudovs K, Pham HQ, Phillips MR, Pigott DM, Pillay JD, Piradov MA, Pirsaheb M, Pishgar F, Plana-Ripoll O, Plass D, Polinder S, Popova S, Postma MJ, Pourshams A, Poustchi H, Prabhakaran D, Prakash S, Prakash V, Purcell CA, Purwar MB, Qorbani M, Quistberg DA, Radfar A, Rafay A, Rafiei A, Rahim F, Rahimi K, Rahimi-Movaghar A, Rahimi-Movaghar V, Rahman M, Rahman M.H. ur, Rahman MA, Rahman SU, Rai RK, Rajati F, Ram U, Ranjan P, Ranta A, Rao PC, Rawaf DL, Rawaf S, Reddy KS, Reiner RC, Reinig N, Reitsma MB, Remuzzi G, Renzaho AMN, Resnikoff S, Rezaei S, Rezai MS, Ribeiro ALP, Robinson SR, Roever L, Ronfani L, Roshandel G, Rostami A, Roth GA, Roy A, Rubagotti E, Sachdev PS, Sadat N, Saddik B, Sadeghi E, Saeedi Moghaddam S, Safari H, Safari Y, Safari-Faramani R, Safdarian M, Safi S, Safiri S, Sagar R, Sahebkar A, Sahraian MA, Sajadi HS, Salam N, Salama JS, Salamati P, Saleem K, Saleem Z, Salimi Y, Salomon JA, Salvi SS, Salz I, Samy AM, Sanabria J, Sang Y, Santomauro DF, Santos IS, Santos JV, Santric Milicevic MM, Sao Jose BP, Sardana M, Sarker AR, Sarrafzadegan N, Sartorius B, Sarvi S, Sathian B, Satpathy M, Sawant AR, Sawhney M, Saxena S, Saylan M, Schaeffner E, Schmidt MI, Schneider IJC, Schöttker B, Schwebel DC, Schwendicke F, Scott JG, Sekerija M, Sepanlou SG, Serván-Mori E, Seyedmousavi S, Shabaninejad H, Shafieesabet A, Shahbazi M, Shaheen AA, Shaikh MA, Shams-Beyranvand M, Shamsi M, Shamsizadeh M, Sharafi H, Sharafi K, Sharif M, Sharif-Alhoseini M, Sharma M, Sharma R, She J, Sheikh A, Shi P, Shibuya K, Shigematsu M, Shiri R, Shirkoohi R, Shishani K, Shiue I, Shokraneh F, Shoman H, Shrime MG, Si S, Siabani S, Siddiqi TJ, Sigfusdottir ID, Sigurvinsdottir R, Silva JP, Silveira DGA, Singam NSV, Singh JA, Singh NP, Singh V, Sinha DN, Skiadaresi E, Slepak ELN, Sliwa K, Smith DL, Smith M, Soares Filho AM, Sobaih BH, Sobhani S, Sobngwi E, Soneji SS, Soofi M, Soosaraei M, Sorensen RJD, Soriano JB, Soyiri IN, Sposato LA, Sreeramareddy CT, Srinivasan V, Stanaway JD, Stein DJ, Steiner C, Steiner TJ, Stokes MA, Stovner LJ, Subart ML, Sudaryanto A, Sufiyan MB, Sunguya BF, Sur PJ, Sutradhar I, Sykes BL, Sylte DO, Tabarés-Seisdedos R, Tadakamadla SK, Tadesse BT, Tandon N, Tassew SG, Tavakkoli M, Taveira N, Taylor HR, Tehrani-Banihashemi A, Tekalign TG, Tekelemedhin SW, Tekle MG, Temesgen H, Temsah MH, Temsah O, Terkawi AS, Teweldemedhin M, Thankappan KR, Thomas N, Tilahun B, To QG, Tonelli M, Topor-Madry R, Topouzis F, Torre AE, Tortajada-Girbés M, Touvier M, Tovani-Palone MR, Towbin JA, Tran BX, Tran KB, Troeger CE, Truelsen TC, Tsilimbaris MK, Tsoi D, Tudor Car L, Tuzcu EM, Ukwaja KN, Ullah I, Undurraga EA, Unutzer J, Updike RL, Usman MS, Uthman OA, Vaduganathan M, Vaezi A, Valdez PR, Varughese S, Vasankari TJ, Venketasubramanian N, Villafaina S, Violante FS, Vladimirov SK, Vlassov V, Vollset SE, Vosoughi K, Vujcic IS, Wagnew FS, Waheed Y, Waller SG, Wang Y, Wang YP, Weiderpass E, Weintraub RG, Weiss DJ, Weldegebreal F, Weldegwergs KG, Werdecker A, West TE, Whiteford HA, Widecka J, Wijeratne T, Wilner LB, Wilson S, Winkler AS, Wiyeh AB, Wiysonge CS, Wolfe CDA, Woolf AD, Wu S, Wu YC, Wyper GMA, Xavier D, Xu G, Yadgir S, Yadollahpour A, Yahyazadeh Jabbari SH, Yamada T, Yan LL, Yano Y, Yaseri M, Yasin YJ, Yeshaneh A, Yimer EM, Yip P, Yisma E, Yonemoto N, Yoon SJ, Yotebieng M, Younis MZ, Yousefifard M, Yu C, Zadnik V, Zaidi Z, Zaman S. Bin, Zamani M, Zare Z, Zeleke AJ, Zenebe ZM, Zhang K, Zhao Z, Zhou M, Zodpey S, Zucker I, Vos T, Murray CJL, 2018. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392, 1789–1858. doi: 10.1016/S0140-6736(18)32279-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeshin BR, Strawn JR, Out D, Granger DA, Putnam FW, 2015. Elevated salivary alpha amylase in adolescent sexual abuse survivors with posttraumatic stress disorder symptoms. J. Child Adolesc. Psychopharmacol 25, 344–350. doi: 10.1089/cap.2014.0034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeshin BR, Strawn JR, Out D, Granger DA, Putnam FW, 2014. Cortisol awakening response in adolescents with acute sexual abuse related posttraumatic stress disorder. Depress. Anxiety 31, 107–114. doi: 10.1002/da.22154 [DOI] [PubMed] [Google Scholar]
- Kircanski K, Williams LM, Gotlib IH, 2019. Heart rate variability as a biomarker of anxious depression response to antidepressant medication. Depress. Anxiety doi: 10.1002/da.22843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumpp H, Fitzgerald DA, Phan KL, 2013. Neural predictors and mechanisms of cognitive behavioral therapy on threat processing in social anxiety disorder. Prog. Neuro-Psychopharmacology Biol. Psychiatry doi: 10.1016/j.pnpbp.2013.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A, Swain JE, Hanna GL, Koschmann E, Simpson D, Connolly S, Fitzgerald KD, Monk CS, Phan KL, 2016. Prefrontal reactivity to social signals of threat as a predictor of treatment response in anxious youth. Neuropsychopharmacology. doi: 10.1038/npp.2015.368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leicht G, Mulert C, Eser D, Sämann PG, Ertl M, Laenger A, Karch S, Pogarell O, Meindl T, Czisch M, Rupprecht R, 2013. Benzodiazepines counteract rostral anterior cingulate cortex activation induced by cholecystokinin-tetrapeptide in humans. Biol. Psychiatry. doi: 10.1016/j.biopsych.2012.09.004 [DOI] [PubMed] [Google Scholar]
- Lener MS, Niciu MJ, Ballard ED, Park M, Park LT, Nugent AC, Zarate CA, 2017. Glutamate and gamma-aminobutyric acid systems in the pathophysiology of major depression and antidepressant response to ketamine. Biol. Psychiatry 81, 886–897. doi: 10.1016/j.biopsych.2016.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenze EJ, Mantella RC, Shi P, Goate AM, Nowotny P, Butters MA, Andreescu C, Thompson PA, Rollman BL, 2011. Elevated cortisol in older adults with generalized anxiety disorder is reduced by treatment: A placebo-controlled evaluation of escitalopram. Am. J. Geriatr. Psychiatry doi: 10.1097/JGP.0b013e3181ec806c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litonjua AA, Gong L, Duan QL, Shin J, Moore MJ, Weiss ST, Johnson JA, Klein TE, Altman RB, 2010. Very important pharmacogene summary ADRB2. Pharmacogenet. Genomics doi: 10.1097/FPC.0b013e328333dae6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locher C, Koechlin H, Zion SR, Werner C, Pine DS, Kirsch I, Kessler RC, Kossowsky J, 2017. Efficacy and safety of selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, and placebo in common psychiatric disorders a meta-analysis in children and adolescents. JAMA Psychiatry 74, 1011–1020. doi: 10.1001/jamapsychiatry.2017.2432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long Z, Medlock C, Dzemidzic M, Shin YW, Goddard AW, Dydak U, 2013. Decreased GABA levels in anterior cingulate cortex/medial prefrontal cortex in panic disorder. Prog. Neuro-Psychopharmacology Biol. Psychiatry doi: 10.1016/j.pnpbp.2013.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotrich FE, Ferrell RE, Rabinovitz M, Pollock BG, 2009. Risk for Depression During Interferon-Alpha Treatment Is Affected by the Serotonin Transporter Polymorphism. Biol. Psychiatry doi: 10.1016/j.biopsych.2008.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jockers-Scherübl MC., 2007. Nerve growth factor serum concentrations rise after successful cognitive-behavioural therapy of generalized anxiety disorder. Prog. Neuro-Psychopharmacology Biol. Psychiatry [DOI] [PubMed] [Google Scholar]
- Maslowsky J, Mogg K, Bradley BP, McClure-Tone E, Ernst M, Pine DS, Monk CS, 2010. A preliminary investigation of neural correlates of treatment in adolescents with generalized anxiety disorder. J. Child Adolesc. Psychopharmacol doi: 10.1089/cap.2009.0049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, 2008. Glutamate and neurotrophic factors in neuronal plasticity and disease, in: Annals of the New York Academy of Sciences. pp. 97–112. doi: 10.1196/annals.1418.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure EB, Adler A, Monk CS, Cameron J, Smith S, Nelson EE, Leibenluft E, Ernst M, Pine DS, 2007. fMRI predictors of treatment outcome in pediatric anxiety disorders. Psychopharmacology (Berl). doi: 10.1007/s00213-006-0542-9 [DOI] [PubMed] [Google Scholar]
- Meeusen R, 2014. Exercise, nutrition and the brain. Sport. Med doi: 10.1007/s40279-014-0150-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas KR, He J-P, Burstein M, Swanson SA, Avenevoli S, Cui L, Benjet C, Georgiades K, Swendsen J, 2010. Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication--Adolescent Supplement (NCS-A). J. Am. Acad. Child Adolesc. Psychiatry 49, 980–9. doi: 10.1016/j.jaac.2010.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musselman DL, Lawson DH, Gumnick JF, Manatunga AK, Penna S, Goodkin RS, Greiner K, Nemeroff CB, Miller AH, 2001. Paroxetine for the prevention of depression induced by high-dose interferon alfa. N. Engl. J. Med doi: 10.1056/NEJM200103293441303 [DOI] [PubMed] [Google Scholar]
- Mustapic M, Eitan E, Werner JK, Berkowitz ST, Lazaropoulos MP, Tran J, Goetzl EJ, Kapogiannis D, 2017. Plasma extracellular vesicles enriched for neuronal origin: a potential window into brain pathologic processes. Front. Neurosci 11, 278. doi: 10.3389/fnins.2017.00278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasrallah HA, 2019. Biomarkers in neuropsychiatric disorders: Translating research to clinical applications. Biomarkers in Neuropsychiatry 1, 100001. doi: 10.1016/j.bionps.2019.100001 [DOI] [Google Scholar]
- Nitschke JB, Sarinopoulos I, Oathes DJ, Johnstone T, Whalen PJ, Davidson RJ, Kalin NH, 2009. Anticipatory activation in the Amygdala and Anterior Cingulate in generalized anxiety disorder and prediction of reatment response. Am. J. Psychiatry doi: 10.1176/appi.ajp.2008.07101682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuvolone M, Aguzzi A, 2015. Altered monoaminergic systems and depressive-like behavior in congenic prion protein knock-out mice. J. Biol. Chem doi: 10.1074/jbc.L115.689117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Z. P, E. D, R. E, di M. F, B. TC, P. A, P. F, 2009. Changes in CCK-4 induced panic after treatment with the GABA-reuptake inhibitor tiagabine are associated with an increase in 3alpha,5alpha-tetrahydrodeoxycorticosterone concentrations. Psychoneuroendocrinology. [DOI] [PubMed] [Google Scholar]
- Palucha A, Pilc A, 2007. Metabotropic glutamate receptor ligands as possible anxiolytic and antidepressant drugs. Pharmacol. Ther doi: 10.1016/j.pharmthera.2007.04.007 [DOI] [PubMed] [Google Scholar]
- Peskind ER, Raskind MA, Wilkinson CW, Flatness DE, Halter JB, 1986. Peripheral sympathectomy and adrenal medullectomy do not alter cerebrospinal fluid norepinephrine. Brain Res. doi: 10.1016/0006-8993(86)91600-8 [DOI] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Cortese BM, Seraji-Bozorgzad N, Tancer ME, Moore GJ, 2005. Anterior cingulate neurochemistry in social anxiety disorder: 1H-MRS at 4 Tesla. Neuroreport. doi: 10.1097/00001756-200502080-00024 [DOI] [PubMed] [Google Scholar]
- Piro B, Mattana G, Noël V, 2019. Recent advances in skin chemical sensors. Sensors (Switzerland). doi: 10.3390/s19204376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack MH, Jensen JE, Simon NM, Kaufman RE, Renshaw PF, 2008. High-field MRS study of GABA, glutamate and glutamine in social anxiety disorder: Response to treatment with levetiracetam. Prog. Neuro-Psychopharmacology Biol. Psychiatry doi: 10.1016/j.pnpbp.2007.11.023 [DOI] [PubMed] [Google Scholar]
- Poweleit EA, Aldrich SL, Martin LJ, Hahn D, Strawn JR, Ramsey LB, 2019. Pharmacogenetics of Sertraline Tolerability and Response in Pediatric Anxiety and Depressive Disorders. J. Child Adolesc. Psychopharmacol 29, 1–14. doi: 10.1089/cap.2019.0017 [DOI] [PubMed] [Google Scholar]
- Raghavan V, 2017. Role of exosomes in psychiatric disorders. Asian J. Psychiatr doi: 10.1016/j.ajp.2017.03.032 [DOI] [PubMed] [Google Scholar]
- Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, Haroon E, Miller AH, 2013. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: The role of baseline inflammatory biomarkers. Arch. Gen. Psychiatry doi: 10.1001/2013.jamapsychiatry.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, 2010. Neurosteroids. Endogenous role in the human brain and therapeutic potentials, in: Progress in Brain Research. doi: 10.1016/B978-0-444-53630-3.00008-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimón R, Lepola U, Jolkkonen J, Halonen T, Riekkinen P, 1995. Cerebrospinal fluid gamma-aminobutyric acid in patients with panic disorder. Biol. Psychiatry doi: 10.1016/0006-3223(95)00076-3 [DOI] [PubMed] [Google Scholar]
- Robinaugh DJ, Brown ML, Losiewicz OM, Jones PJ, Marques L, Baker AW, 2020. Towards a precision psychiatry approach to anxiety disorders with ecological momentary assessment: The example of panic disorder. Gen. Psychiatry doi: 10.1136/gpsych-2019-100161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy-Byrne P, Craske MG, Sullivan G, Rose RD, Edlund MJ, Lang AJ, Bystritsky A, Welch SS, Chavira DA, Golinelli D, Campbell-Sills L, Sherbourne CD, Stein MB, 2010. Delivery of evidence-based treatment for multiple anxiety disorders in primary care: A randomized controlled trial. JAMA - J. Am. Med. Assoc 303, 1921–1928. doi: 10.1001/jama.2010.608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy-Byrne PP, Cowley DS, Hommer D, Greenblatt DJ, Kramer GL, Petty F, 1992. Effect of acute and chronic benzodiazepines on plasma GABA in anxious patients and controls. Psychopharmacology (Berl). doi: 10.1007/BF02245493 [DOI] [PubMed] [Google Scholar]
- Sakolsky DJ, Nurmi EL, Birmaher B, March JS, Walkup JT, Piacentini JC, Kendall PC, Albano AM, Compton SN, Sherill J, Rynn MA, Ginsburg GS, 2010. Association of GRIK4 with treatment response in the Child/Adolescent Anxiety Multimodal Study (CAMS), Annual Meeting of the American Academy of Child Adolescent Psychiatry. 4.3. [Google Scholar]
- Schulte HM, Chrousos GP, Avgerinos P, Oldfield EH, Gold PW, Cutler GB, Loriaux DL, 1984. The corticotropin-releasing hormone stimulation test: A possible aid in the evaluation of patients with adrenal insufficiency. J. Clin. Endocrinol. Metab doi: 10.1210/jcem-58-6-1064 [DOI] [PubMed] [Google Scholar]
- Sheline YI, Bardgett ME, Csernansky JG, 1997. Correlated reductions in cerebrospinal fluid 5-HIAA and MHPG concentrations after treatment with selective serotonin reuptake inhibitors. J. Clin. Psychopharmacol doi: 10.1097/00004714-199702000-00003 [DOI] [PubMed] [Google Scholar]
- Shen Z, Zhu J, Yuan Y, Ren L, Qian M, Lin M, Cai M, Zhang Z, Shen X, 2019. The roles of brain-derived neurotrophic factor (BDNF) and glial cell line-derived neurotrophic factor (GDNF) in predicting treatment remission in a Chinese Han population with generalized anxiety disorder. Psychiatry Res. doi: 10.1016/j.psychres.2018.08.111 [DOI] [PubMed] [Google Scholar]
- Stein MB, Fyer AJ, Davidson JRT, Pollack MH, Wiita B, 1999. Fluvoxamine treatment of social phobia (social anxiety disorder): A double-blind, placebo-controlled study. Am. J. Psychiatry doi: 10.1176/ajp.156.5.756 [DOI] [PubMed] [Google Scholar]
- Stein MB, Sareen J, 2015. Clinical Practice. Generalized Anxiety Disorder. N. Engl. J. Med 373, 2059–68. doi: 10.1056/NEJMcp1502514 [DOI] [PubMed] [Google Scholar]
- Stranahan AM, Lee K, Martin B, Maudsley S, Golden E, Cutler RG, Mattson MP, 2009. Voluntary exercise and caloric restriction enhance hippocampal dendritic spine density and BDNF levels in diabetic mice. Hippocampus. doi: 10.1002/hipo.20577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strawn JR, Chu WJ, Whitsel RM, Weber WA, Norris MM, Adler CM, Eliassen JC, Phan KL, Strakowski SM, Delbello MP, 2013. A pilot study of anterior cingulate cortex neurochemistry in adolescents with generalized anxiety disorder. Neuropsychobiology. doi: 10.1159/000347090 [DOI] [PubMed] [Google Scholar]
- Strawn JR, Compton SN, Robertson B, Albano AM, Hamdani M, Rynn MA, 2017. Extended release guanfacine in pediatric anxiety disorders: a pilot, randomized, placebo-controlled trial. J. Child Adolesc. Psychopharmacol 27, 29–37. doi: 10.1089/cap.2016.0132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strawn JR, Ekhator NN, Anthenelli RM, Baker DG, Maxwell RA, Hill KK, Geracioti TD, 2002. Intra- and inter-individual relationships between central and peripheral serotonergic activity in humans: A serial cerebrospinal fluid sampling study. Life Sci. 71, 1219–1225. doi: 10.1016/S0024-3205(02)01828-3 [DOI] [PubMed] [Google Scholar]
- Strawn JR, Ekhator NN, Horn PS, Baker DG, Geracioti TD, 2004. Blood pressure and cerebrospinal fluid norepinephrine in combat-related posttraumatic stress disorder. Psychosom. Med. 66, 757–759. doi: 10.1097/01.psy.0000138133.72365.45 [DOI] [PubMed] [Google Scholar]
- Strawn JR, Geracioti L, Rajdev N, Clemenza K, Levine A, 2018a. Pharmacotherapy for generalized anxiety disorder in adult and pediatric patients: an evidence-based treatment review. Expert Opin. Pharmacother 19, 1057–1070. doi: 10.1080/14656566.2018.1491966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strawn JR, Geracioti TD, 2008. Noradrenergic dysfunction and the psychopharmacology of posttraumatic stress disorder. Depress. Anxiety 25, 260–271. doi: 10.1002/da.20292 [DOI] [PubMed] [Google Scholar]
- Strawn JR, Lu L, Peris TS, Levine A, Walkup JT, 2020. Research Review: Peadiatric Anxiety Disorders: What have we learnt in the last 10 years? J. Child Psychol. Psychiatry doi: 10.1111/JCPP.13262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strawn JR, Mills JA, Croarkin PE, 2019a. Switching Selective Serotonin Reuptake Inhibitors in Adolescents with Selective Serotonin Reuptake Inhibitor-Resistant Major Depressive Disorder: Balancing Tolerability and Efficacy. J. Child Adolesc. Psychopharmacol doi: 10.1089/cap.2018.0145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strawn JR, Mills JA, Sauley BA, Welge JA, 2018. The Impact of Antidepressant Dose and Class on Treatment Response in Pediatric Anxiety Disorders: A Meta-Analysis. J. Am. Acad. Child Adolesc. Psychiatry 57. doi: 10.1016/j.jaac.2018.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strawn JR, Mills JA, Schroeder HK, Mossman SA, Varney ST, Ramsey LB, Cecil KM, Delbello MP, 2019b. A randomized, placebo-controlled study of escitalopram in adolescents with generalized anxiety disorder. J. Am. Acad. Child Adolesc. Psychiatry 58, S254. doi: 10.1016/j.jaac.2019.08.341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strawn JR, Prakash A, Zhang Q, Pangallo BA, Stroud CE, Cai N, Findling RL, 2015. A randomized, placebo-controlled study of duloxetine for the treatment of children and adolescents with generalized anxiety disorder. J. Am. Acad. Child Adolesc. Psychiatry 54, 283–93. doi: 10.1016/j.jaac.2015.01.008 [DOI] [PubMed] [Google Scholar]
- Strawn JR, Vollmer LL, McMurray KMJ, Mills JA, Mossman SA, Varney ST, Schroeder HK, Sah R, 2018b. Acid-sensing T cell death associated gene-8 receptor expression in panic disorder. Brain. Behav. Immun 67, 36–41. doi: 10.1016/j.bbi.2017.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strawn JR, Wehry AM, Delbello MP, Rynn MA, Strakowski S, 2012. Establishing the neurobiologic basis of treatment in children and adolescents with generalized anxiety disorder. Depress. Anxiety 29, 328–339. doi: 10.1002/da.21913 [DOI] [PubMed] [Google Scholar]
- Strawn JR, Welge JA, Wehry AM, Keeshin B, Rynn MA, 2015. Efficacy and tolerability of antidepressants in pediatric anxiety disorders: A systematic review and meta-analysis. Depress. Anxiety 32. doi: 10.1002/da.22329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suire CN, Eitan E, Shaffer NC, Tian Q, Studenski S, Mattson MP, Kapogiannis D, 2017. Walking speed decline in older adults is associated with elevated pro-BDNF in plasma extracellular vesicles. Exp. Gerontol doi: 10.1016/j.exger.2017.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JH, Landeros-Weisenberger A, Coughlin C, Mulqueen J, Johnson JA, Gabriel D, Reed MO, Jakubovski E, Bloch MH, 2018. Ketamine for social anxiety disorder: A randomized, placebo-controlled crossover trial. Neuropsychopharmacology. doi: 10.1038/npp.2017.194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AG, Gray E, Heman-Ackah SM, Mäger I, Talbot K, Andaloussi S. El, Wood MJ, Turner MR, 2016. Extracellular vesicles in neurodegenerative disease — pathogenesis to biomarkers. Nat. Rev. Neurol 12, 346–357. doi: 10.1038/nrneurol.2016.68 [DOI] [PubMed] [Google Scholar]
- Tsilioni I, Panagiotidou S, Theoharides TC, 2014. Exosomes in neurologic and psychiatric disorders. Clin. Ther doi: 10.1016/j.clinthera.2014.05.005 [DOI] [PubMed] [Google Scholar]
- Van Ameringen MA, Lane RM, Walker JR, Bowen RC, Chokka PR, Goldner EM, Johnston DG, Lavallee YJ, Nandy S, Pecknold JC, Hadrava V, Swinson RP, 2001. Sertraline treatment of generalized social phobia: A 20-week, double-blind, placebo-controlled study. Am. J. Psychiatry doi: 10.1176/appi.ajp.158.2.275 [DOI] [PubMed] [Google Scholar]
- van der Pol E, Boing AN, Harrison P, Sturk A, Nieuwland R, 2012. Classification, Functions, and Clinical Relevance of Extracellular Vesicles. Pharmacol. Rev 64, 676–705. doi: 10.1124/pr.112.005983 [DOI] [PubMed] [Google Scholar]
- Vieira MMM, Ferreira TB, Pacheco PAF, Barros PO, Almeida CRM, Araújo-Lima CF, Silva-Filho RG, Hygino J, Andrade RM, Linhares UC, Andrade AFB, Bento CAM, 2010. Enhanced Th17 phenotype in individuals with generalized anxiety disorder. J. Neuroimmunol 229, 212–218. doi: 10.1016/j.jneuroim.2010.07.018 [DOI] [PubMed] [Google Scholar]
- Vogelzangs N, de Jonge P, Smit JH, Bahn S, Penninx BW, 2016. Cytokine production capacity in depression and anxiety. Transl. Psychiatry doi: 10.1038/tp.2016.92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmer LL, Strawn JR, Sah R, 2015. Acid-base dysregulation and chemosensory mechanisms in panic disorder: A translational update. Transl. Psychiatry 5, e572–11. doi: 10.1038/tp.2015.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walkup JT, Albano AM, Piacentini J, Birmaher B, Compton SN, Sherrill JT, Ginsburg GS, Rynn MA, McCracken J, Waslick B, Iyengar S, March JS, Kendall PC, 2008. Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N. Engl. J. Med 359, 2753–2766. doi: 10.1056/NEJMoa0804633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehry AM, Beesdo-Baum K, Hennelly MM, Connolly SD, & Strawn JR, 2015. Assessment and Treatment of Anxiety Disorders in Children and Adolescents. Curr. Psychiatry Rep 17, 99–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen PJ, Johnstone T, Somerville LH, Nitschke JB, Polis S, Alexander AL, Davidson RJ, Kalin NH, 2008. A Functional Magnetic Resonance Imaging Predictor of Treatment Response to Venlafaxine in Generalized Anxiety Disorder. Biol. Psychiatry doi: 10.1016/j.biopsych.2007.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong ML, Kling MA, Munson PJ, Listwak S, Licinio J, Prolo P, Karp B, McCutcheon IE, Geracioti TD, DeBellis MD, Rice KC, Goldstein DS, Veldhuis JD, Chrousos GP, Oldfield EH, McCann SM, Gold PW, 2000. Pronounced and sustained central hypernoradrenergic function in major depression with melancholic features: Relation to hypercortisolism and corticotropin-releasing hormone. Proc. Natl. Acad. Sci. U. S. A doi: 10.1073/pnas.97.1.325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yáñez-Mó M, Siljander PRM, Andreu Z, Zavec AB, Borràs FE, Buzas EI, Buzas K, Casal E, Cappello F, Carvalho J, Colás E, Cordeiro-Da Silva A, Fais S, Falcon-Perez JM, Ghobrial IM, Giebel B, Gimona M, Graner M, Gursel I, Gursel M, Heegaard NHH, Hendrix A, Kierulf P, Kokubun K, Kosanovic M, Kralj-Iglic V, Krämer-Albers EM, Laitinen S, Lässer C, Lener T, Ligeti E, Line A, Lipps G, Llorente A, Lötvall J, Manček-Keber M, Marcilla A, Mittelbrunn M, Nazarenko I, Nolte-’t Hoen ENM, Nyman TA, O’Driscoll L, Olivan M, Oliveira C, Pállinger É, Del Portillo HA, Reventós J, Rigau M, Rohde E, Sammar M, Sánchez-Madrid F, Santarém N, Schallmoser K, Ostenfeld MS, Stoorvogel W, Stukelj R, Van Der Grein SG, Helena Vasconcelos M, Wauben MHM, De Wever O, 2015. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles doi: 10.3402/jev.v4.27066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokus MA, Songkakul T, Pozdin VA, Bozkurt A, Daniele MA, 2020. Wearable multiplexed biosensor system toward continuous monitoring of metabolites. Biosens. Bioelectron doi: 10.1016/j.bios.2020.112038 [DOI] [PubMed] [Google Scholar]
- Ypenburg C, Bax JJ, van der Wall EE, Schalij MJ, van Erven L, 2007. Intrathoracic Impedance Monitoring to Predict Decompensated Heart Failure. Am. J. Cardiol doi: 10.1016/j.amjcard.2006.08.066 [DOI] [PubMed] [Google Scholar]
- Zorumski CF, Paul SM, Covey DF, Mennerick S, 2019. Neurosteroids as novel antidepressants and anxiolytics: GABA-A receptors and beyond. Neurobiol. Stress doi: 10.1016/j.ynstr.2019.100196 [DOI] [PMC free article] [PubMed] [Google Scholar]