Abstract
Purpose
Many studies have shown that the enhanced recovery after surgery (ERAS) protocols improve postoperative surgical outcomes. The purpose of this study was to observe the effects on postoperative inflammatory markers and to explore the effects of a high degree of compliance and the use of epidural anesthesia on inflammation and surgical outcomes.
Methods
Four hundred patients underwent colorectal cancer surgery at 2 hospitals during 2 different periods, namely, from January 2006 to December 2009 and from January 2017 to July 2017. Data related to the patient’s clinicopathological features, inflammatory markers, percentage of compliance with elements of the ERAS protocol, and use of epidural anesthesia were collected from a prospectively maintained database.
Results
The complication rate and the length of hospital stay (LOS) were less in the ERAS group than in the conventional group (P = 0.005 and P ≤ 0.001, respectively). The postoperative white blood cell count and the duration required for leukocytes to normalize were reduced in patients following the ERAS protocol (P ≤ 0.001). Other inflammatory markers, such as lymphocyte count (P = 0.008), neutrophil/lymphocyte ratio (P = 0.032), and C-reactive protein level (P ≤ 0.001), were lower in the ERAS protocol group. High compliance ( ≥ 70%) was strongly associated with the complication rate and the LOS (P = 0.008 and P ≤ 0.001, respectively).
Conclusion
ERAS protocols decrease early postoperative inflammation and improves short-term postoperative recovery outcomes such as complication rate and the LOS. High compliance ( ≥ 70%) with the ERAS protocol elements accelerates the positive effects of ERAS on surgical outcomes; however, the effect on inflammation was very small.
Keywords: Enhanced recovery after surgery; Colorectal neoplasms; Laparoscopy; Inflammation, Length of stay
INTRODUCTION
The link between inflammation and cancer has been thoroughly deliberated since 1863, when Virchow noted that tumors arise at sites of chronic inflammation [1]. It has been proven that inflammation plays a role in tumor growth and aggressiveness; the preoperative and early postoperative inflammatory response and the inflammatory period induce a micrometastatic environment and negatively impact cancer prognosis [1-4]. Colorectal cancer is the third most common cancer in the world and the fourth leading cause of death. Minimally invasive surgery (MIS) is the preferred modality of treatment, and many systematic reviews and meta-analyses have demonstrated its safety and oncological efficacy, in addition to its ability to reduce the postoperative inflammatory response [5-7]. Currently, the goal is to perform surgery in combination with an enhanced recovery after surgery (ERAS) protocol (EP), as many studies have shown favorable effects of EP on short-term postoperative recovery outcomes, such as the length of hospital stays (LOS), morbidity, and readmission rate [8-13]. Many studies have revealed that the postoperative surgical stress response reflected by the levels of markers such as C-reactive protein (CRP) and interleukins has been improved when surgery is combined with EP [14, 15]. Our objective in the present study was to address the direct and independent effects of EP on postoperative inflammation, regardless of the procedure type and to determine if the degree of inflammation would decrease with increased compliance with the multimodal EP elements.
METHODS
Patients
Data were collected for this study from 400 patients who underwent colorectal cancer surgery at 2 different hospitals. One hundred thirty-seven patients underwent the surgery and followed the EP at Seoul St. Mary’s Hospital, The Catholic University of Korea from January 2017 to July 2017. Two hundred sixty-three patients underwent surgery and followed the conventional protocol (CP) at Yeouido St. Mary’s Hospital, The Catholic University of Korea from January 2006 to December 2009. All procedures were performed by the same surgeon at both hospitals, but one additional surgeon at Seoul St. Mary’s Hospital shared in performing the procedures. Patients with either missing laboratory values or an emergency presentation were excluded, as shown in Fig. 1. The final number of included patients was 296, with 82 patients (27.7%) who followed the EP and 214 patients (72.3%) who followed the CP. This study was approved by the Institutional Review Board (IRB) of Seoul and Yeouido St. Mary’s Hospital, College of Medicine, The Catholic University of Korea (SC11TISI0080, KC17TESI0796) and informed consent was waived by IRB.
Fig. 1.
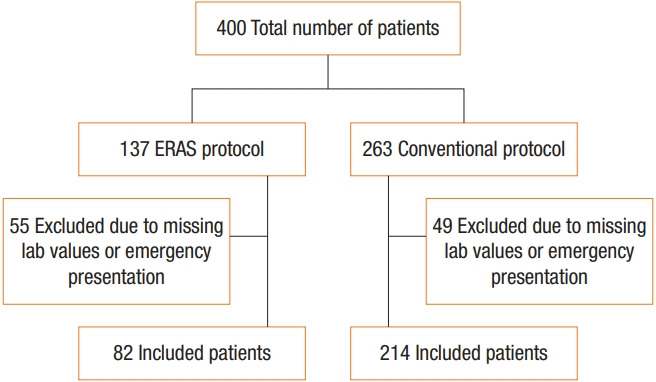
Patient flow chart. ERAS, early recovery after surgery.
Definitions
The clinicopathological data were collected from a prospectively maintained database and analyzed. Routine laboratory measurements, including white blood cell (WBC) count, neutrophil count, lymphocyte count, monocyte count, eosinophil count, platelet count, CRP level, and albumin level were performed preoperatively and then daily until postoperative day 4. For the comparative analysis, the inflammatory marker variables were grouped using the standard threshold of the hospital laboratory. The WBC count was categorized as either < 10,000 mm3/L or ≥ 10,000 mm3/L. The time required after surgery for the leukocyte count to drop below 10,000/mm3 (in days) was grouped into 3 categories, namely, < 1 day, 1 to 3 days, and ≥ 4 days. The neutrophil/lymphocyte ratio (N/L ratio) and Glasgow prognostic score (GPS) were calculated pre- and postoperatively. Age was classified as either < 65 years or ≥ 65 years. Tumor location was categorized as either colon or rectum; the colon category included all tumors in the right colon, left colon, and rectosigmoid colon above the peritoneal reflection. The colorectal procedures were performed through either conventional surgery or MIS (laparoscopic and robotic surgery). Staging evaluation was carried out according to the guidelines of the American Joint Committee on Cancer sixth edition for the CP group, and eighth edition for the EP group. A complication was defined as the presence of any deviation from the normal postoperative course (e.g., fever, diarrhea, voiding difficulties, ileus, and anastomosis leak); the absence or presence of complications was indicated as either yes or no, and the severity of complications was graded according to the classification proposed by Clavien-Dindo. The LOS was defined as the duration from the first postoperative day until discharge. The compliance response cutoff level was set as 70%, and EP patients were divided into 2 groups according to their compliance as follows: ≥ 70%, high compliance response (HCR) group; and < 70%, low compliance response (LCR) group. For the analysis of epidural anesthesia (EPA), the EP patients were divided into the EPA group and the patient-controlled analgesia (PCA) group.
EP elements
In the EP group, the effects of compliance with the EP elements on postoperative inflammation and surgical outcomes were assessed. The compliance with 18 elements was checked during the preoperative (5 elements), intraoperative (4 elements), and postoperative (9 elements) phases as follows: preoperative phase (preadmission patient education, preoperative oral carbohydrate treatment, preoperative formula intake, thrombosis prophylaxis, and antibiotics prophylaxis), intraoperative phase (EPA, prevention of hypothermia, restrictive fluid strategy, and postoperative nausea and vomiting prophylaxis), and postoperative phase (postoperative epidural analgesia, effective pain control, balanced fluids, stimulation of gut motility, early surgical drain removal, early urinary catheter removal, termination of intravenous fluid infusion, early mobilization, and early intake) (Table 1).
Table 1.
Early recovery after surgery protocol of our institution
| Period | Component | Content |
|---|---|---|
| Preoperative | Preadmission patient education | |
| Preoperative oral carbohydrate treatment | By 2 hours before surgery | |
| Preoperative formula intake | Parenteral nutrient solution after mechanical bowel preparation | |
| Thrombosis prophylaxis | Preoperative vascular surgery team consultation, application of pneumatic compression | |
| Antibiotics prophylaxis | Administered at 30 minutes before incision | |
| Intraoperative | Epidural or spinal anesthesia | |
| Body temperature preservation | Use of air warmer and transesophageal monitoring device | |
| Restrictive fluid strategy | Crystalloid 2–4 mL/hr | |
| PONV prophylaxis | Administered before the end of surgery | |
| Postoperative | Postoperative epidural analgesia | Using at least one day of PCA through epidural route |
| Effective pain control | Well controlled pain with NSAID only | |
| Balanced fluids | Daily total fluid level 500 mL or less | |
| Stimulation of gut motility I | Laxatives used | |
| Stimulation of gut motility II | Chewing gum used | |
| Termination of urinary drainage | Withdrawal of Foley catheter before the third postoperative day | |
| Drainage remove | Until the third postoperative day | |
| Termination of intravenous fluid infusion | Until the third postoperative day | |
| Mobilization on day of surgery | Postoperative movement outside the bed | |
| Mobilization on postoperative day 1 | Over 4 hours | |
| Mobilization on postoperative day 2 | Over 6 hours | |
| Mobilization on postoperative day 3 | Over 6 hours | |
| Energy intake on day of surgery, postoperatively | Intake 200 kcal or more | |
| Energy intake on postoperative day 1 | Intake 500 kcal or more |
PONV, postoperative nausea and vomiting; PCA, patient-controlled analgesia; NSAID, nonsteroidal anti-inflammatory drug.
Statistical analysis
We used descriptive statistics with numbers (percentage) for the categorical data and medians (range) or means± standard deviation for the continuous data, according to their distributions. Comparisons between the EP group and the CP group were made using the chi-square test or Fisher exact test for discrete outcomes, and t-tests or the Mann-Whitney U-test were used for continuous outcomes. HCR and LCR were compared using the same analytical methods. Different regression models were used to perform univariate and multivariate analyses to determine the factors associated with postoperative inflammatory markers and LOS. LOS data was skewed, and therefore the data was log transformed. Binary logistic regression, ordinal logistic regression, and log-linear regression were used as appropriate according to the response variables. All statistical tests were 2-tailed, and the significance level was set at P≤ 0.05. All statistical analyses were performed using IBM SPSS Statistics ver. 21.0 (IBM Corp., Armonk, NY, USA) and SAS ver. 9.4 (SAS Institute, Cary, NC, USA).
RESULTS
Patient clinicopathological characteristics and short-term postoperative surgical outcomes
Clinical and surgical features were compared between EP and CP patients (Table 2). The patient male to female ratio was 1.4:1. In the EP group, 77 patients (93.9%) underwent MIS for colorectal cancer, while only 5 patients (6.1%) underwent conventional surgery. In the CP group, 181 patients (84.6%) underwent laparoscopic surgery, and 33 patients (15.4%) underwent conventional surgery. There was a significant difference between the frequency with which each type of surgical procedure was performed (P= 0.032).
Table 2.
Clinicopathological characteristics of the EP and CP groups (n=296)
| Variable | EP | CP | P-value |
|---|---|---|---|
| Patient | 82 (27.7) | 214 (72.3) | |
| Age (yr) | 0.804 | ||
| < 65 | 37 (45.1) | 100 (46.7) | |
| ≥ 65 | 45 (54.9) | 114 (53.3) | |
| Sex | 0.375 | ||
| Male | 44 (53.7) | 127 (59.3) | |
| Female | 38 (46.3) | 87 (40.7) | |
| Tumor location | 0.417 | ||
| Colon | 65 (79.3) | 160 (74.8) | |
| Rectum | 17 (20.7) | 54 (25.2) | |
| Procedure | 0.032 | ||
| Open | 5 (6.1) | 33 (15.4) | |
| Minimally invasive surgery | 77 (93.9) | 181 (84.6) | |
| TNM classificationa | 0.100 | ||
| Stage 0 | 3 (3.7) | 16 (7.5) | |
| Stage I | 21 (25.6) | 53 (24.8) | |
| Stage II | 20 (24.4) | 63 (29.4) | |
| Stage III | 34 (41.5) | 59 (27.6) | |
| Stage IV | 4 (4.9) | 23 (10.7) | |
| Radical resection | 0.758 | ||
| R0 | 76 (92.7) | 196 (91.6) | |
| ≥ R1 | 6 (7.3) | 18 (8.4) | |
| Neoadjuvant therapy | 0.616 | ||
| Yes | 8 (9.8) | 17 (7.9) | |
| No | 74 (90.2) | 197 (92.1) | |
| Complication | 0.005 | ||
| Yes | 10 (12.2) | 59 (27.6) | |
| No | 72 (87.8) | 155 (72.4) | |
| Clavien-Dindo classification | 0.002 | ||
| Grade I | 1 (1.2) | 19 (8.9) | |
| Grade II | 4 (4.9) | 32 (15) | |
| Grade IIIa | 4 (4.9) | 3 (1.4) | |
| Grade IIIb | 0 (0.0) | 5 (2.3) | |
| Grade IVa | 1 (1.2) | 0 (0.0) | |
| Surgical site infection | 0.974 | ||
| Yes | 3 (3.7) | 8 (3.7) | |
| No | 79 (96.3) | 206 (96.3) | |
| Length of hospital stay (day) | 5 (4–34) | 9 (6–60) | < 0.001b |
Values are presented as number (%) or median (range).
EP, enhanced recovery after surgery protocol; CP, conventional protocol.
American Joint Committee on Cancer TNM classification, sixth and eighth editions.
Mann-Whitney U-test.
There were no significant differences between the 2 groups regarding age, sex, tumor location, TNM stage, extent of radical resection, neoadjuvant chemoradiotherapy, or surgical site infection. Complications occurred in 10 patients (12.2%) in the EP group and in 59 patients (27.6%) in the CP group; there was a significant difference in the frequency of complications between the 2 groups (P= 0.005). There was a significant difference between EP and CP group with regard to the number of patients with Clavien-Dindo classification IIIa or greater (P= 0.027) (Table 2). A univariate analysis of EP and other predictive factors was also performed to investigate their impact on the complication rate. The analysis showed (data not shown) that the EP significantly reduced the complication rate (P= 0.005), while other predictive factors, such as age (P = 0.380), sex (P = 0.751), tumor location (P = 0.430), procedure type (P = 0.379), TNM stage (P = 0.164), radical resection (P= 0.838), and neoadjuvant chemoradiotherapy (P= 0.682), did not affect the complication rate. The median LOS was 5 (4 to 34) days in the EP group and 9 (6 to 60) days in the CP group, and the difference between the 2 groups was significantly different (P≤ 0.001) (Table 2).
Comparison of the postoperative inflammatory markers between EP and CP groups after controlling for the preoperative inflammatory markers
The levels of inflammatory markers were compared between the EP and CP groups (Table 3). Starting with normal preoperative WBC counts, increased postoperative WBC count was found in significantly fewer patients in the EP group (42.7%) than in the CP group (72.9%) (P≤ 0.001). The time required for the postoperative WBC count to normalize was significantly shorter in the EP group than in the CP group (P≤ 0.001). Both the postoperative lymphocyte count and the postoperative N/L ratio were significantly different between the 2 groups (P= 0.008 and P= 0.032, respectively), while the other postoperative WBC differentials, such as neutrophil count, monocyte count, eosinophil count and platelet count, did not show any significant differences (P= 0.582, P= 0.097, P= 0.624, and P= 0.959, respectively [data not shown]).
Table 3.
Comparison of the postoperative inflammatory markers between the EP and CP groups after controlling for the preoperative inflammatory markers
| Inflammatory marker |
EP | CP | P-value | |
|---|---|---|---|---|
| Preoperative | Postoperative | |||
| WBC count (× 109/L) | ||||
| < 10,000 | < 10,000 | 43 (57.3) | 55 (27.1) | < 0.001 |
| ≥ 10,000 | 32 (42.7) | 148 (72.9) | ||
| ≥ 10,000 | < 10,000 | 0 (0) | 2 (18.2) | 0.497 |
| ≥ 10,000 | 7 (100) | 9 (81.8) | ||
| C-reactive protein level (mg/L) | ||||
| ≤ 0.5 | ≤ 0.5 | 50 (80.6) | 13 (24.1) | < 0.001 |
| > 0.5 | 12 (19.4) | 41 (75.9) | ||
| > 0.5 | ≤ 0.5 | 7 (35.0) | 2 (1.4) | < 0.001 |
| > 0.5 | 13 (65.0) | 144 (98.6) | ||
| Albumin level (g/dL) | ||||
| ≥ 3.5 | ≥ 3.5 | 9 (100) | 133 (100) | - |
| < 3.5 | 0 (0) | 0 (0) | ||
| < 3.5 | ≥ 3.5 | 33 (45.2) | 7 (8.6) | < 0.001 |
| < 3.5 | 40 (54.8) | 74 (91.4) | ||
| Glasgow prognostic score | ||||
| 0 | 0 | 28 (50.0) | 15 (62.5) | 0.489 |
| 1 | 25 (44.6) | 7 (29.2) | ||
| 2 | 3 (5.4) | 2 (8.3) | ||
| 1 | 0 | 6 (27.3) | 6 (7.0) | 0.032 |
| 1 | 10 (45.4) | 55 (63.9) | ||
| 2 | 6 (27.3) | 25 (29.1) | ||
| 2 | 0 | 0 (0) | 0 (0) | 0.013 |
| 1 | 2 (50.0) | 3 (3.3) | ||
| 2 | 2 (50.0) | 87 (96.7) | ||
Values are presented as number (%).
EP, enhanced recovery after surgery protocol; CP, conventional protocol; WBC, white blood cells.
Other inflammatory markers, such as CRP and GPS, were evaluated in both the preoperative and postoperative phases. Significantly more patients maintained a normal preoperative or improved a high preoperative CRP level during the postoperative phase in the EP group (80.6% and 35%, respectively) than in the CP group (24.1% and 1.4%, respectively) (P≤ 0.001) (Table 3). No difference was found between the 2 groups in terms of patients with a preoperative GPS of 0 (P= 0.489). Among the patients with preoperative scores of 1, 72.7% of patients in the EP group and 70.9% of patients in the CP group postoperatively maintained a score of 1 or improved their score to 0. In contrast, significantly fewer patients progressed to score 2 in the EP group (27.3%) than in the CP group (29.1%) (P= 0.032). Significantly more patients with poor preoperative GPS (score 2) in the EP group (50.0%) than in the CP group (3.3%) experienced postoperative improvement to score 1 (P= 0.013).
Factors associated with postoperative inflammatory markers and LOS in the multiple regression model
The multivariate regression analysis revealed that EP was significantly independently associated with LOS (P≤ 0.01) and inflammatory markers, namely, postoperative WBC count (P ≤ 0.01), time required for WBC count to normalize (P≤ 0.01), postoperative CRP value (P≤ 0.01), and postoperative GPS (P≤ 0.01). MIS was associated with postoperative WBC count (P = 0.03), GPS (P≤ 0.02), and LOS (P= 0.01). Radical resection was significantly associated with postoperative GPS (P= 0.04), and TNM staging, neoadjuvant chemoradiotherapy, and the complication rate had significant effects on LOS (P≤ 0.01 for all factors). Tumor location did not affect inflammatory markers or LOS (Table 4).
Table 4.
Factors associated with postoperative inflammatory markers and LOS in the multiple regression model
| Predictive factor | Outcome variablea |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Postoperative WBC count |
Time for WBC count to decrease <10,000/mm3 |
Postoperative CRP |
Postoperative GPS |
LOS |
||||||||||||
| OR | 95% CI | P-valueb | OR | 95% CI | P-valuec | OR | 95% CI | P-valueb | OR | 95% CI | P-valuec | OR | 95% CI | P-valued | ||
| Protocol | 3.84 | 2.15–6.84 | < 0.01 | 2.08 | 1.24–3.47 | < 0.01 | 17.92 | 7.63–42.10 | < 0.01 | 8.22 | 4.72–14.34 | < 0.01 | 0.67 | 0.61–0.73 | < 0.01 | |
| ERAS | ||||||||||||||||
| Conventionale | ||||||||||||||||
| Tumor location | 0.63 | 0.32–1.27 | 0.20 | 0.76 | 0.41–1.40 | 0.39 | 0.73 | 0.25–2.07 | 0.55 | 0.89 | 0.47–1.67 | 0.72 | 0.85 | 0.76–0.95 | 0.07 | |
| Colon | ||||||||||||||||
| Rectume | ||||||||||||||||
| Procedure | 0.43 | 0.20–0.92 | 0.03 | 0.72 | 0.37–1.42 | 0.34 | 1.99 | 0.38–10.55 | 0.42 | 2.47 | 1.13–5.40 | 0.02 | 0.78 | 0.69–0.88 | < 0.01 | |
| MIS | ||||||||||||||||
| Opene | ||||||||||||||||
| TNM classificationf | 1.24 | 0.71–2.15 | 0.45 | 1.33 | 0.82–2.15 | 0.24 | 1.79 | 0.75–4.25 | 0.19 | 1.16 | 0.71–1.90 | 0.55 | 0.99 | 0.91–1.08 | < 0.01 | |
| Advanced | ||||||||||||||||
| Earlye | ||||||||||||||||
| Radical resection | 0.97 | 0.37–2.57 | 0.95 | 0.63 | 0.27–1.49 | 0.29 | 0.27 | 0.04–1.74 | 0.17 | 0.36 | 0.13–0.95 | 0.04 | 1.31 | 1.12–1.53 | 0.89 | |
| ≥ R1 | ||||||||||||||||
| R0e | ||||||||||||||||
| Neoadjuvant CRT | 1.72 | 0.62–4.74 | 0.30 | 2.00 | 0.78–5.17 | 0.15 | 0.88 | 0.19–4.05 | 0.87 | 0.89 | 0.34–2.33 | 0.82 | 0.88 | 0.74–1.04 | < 0.01 | |
| Yes | ||||||||||||||||
| Noe | ||||||||||||||||
| Complication | 1.26 | 0.68–2.34 | 0.45 | 1.11 | 0.65–1.90 | 0.70 | 0.44 | 0.14–1.37 | 0.16 | 0.83 | 0.47–1.46 | 0.52 | 1.16 | 1.05–1.27 | < 0.01 | |
| Yes | ||||||||||||||||
| Noe | ||||||||||||||||
Adjusted by age, sex, group (ERAS protocol and conventional protocol group) (relevant preoperative inflammatory markers) and above clinicopathological characteristics.
LOS, length of hospital stay; WBC, white blood cells; CRP, C-reactive protein; GPS, Glasgow prognostic score; OR, odds ratio; CI, confidence interval; ERAS, enhanced recovery after surgery; MIS, minimally invasive surgery; CRT, chemoradiotherapy.
The references for the outcome variables: postoperative WBC count, >10,000 mm3/L; time for WBC count to decrease <10,000/mm3, >day 4; postoperative CRP, ≤0.5; postoperative GPS, score 2.
Logistic regression.
Ordinal logistic regression.
Log-linear regression.
Reference group for predictive factors.
American Joint Committee on Cancer TNM classification, sixth and eighth editions.
Postoperative inflammation and short-term surgical
outcomes according to compliance with EP elements The effects of compliance with the EP on inflammation, the complication rate, and LOS were assessed. Eleven EP patients were excluded due to unknown compliance; in total, 59 patients (72.0%) were in the HCR group, and 12 patients (14.6%) were in the LCR group. Postoperative monocyte count (P= 0.015) was the only inflammatory marker that was significantly different between the 2 groups (Table 5). The HCR group had significantly fewer complications (6.8%) than the LCR group (33.3%) (P= 0.008). ClavienDindo classification revealed 2 patients with > grade II severity in the HCR group, while there was only one patient with > grade II severity in the LCR group, which was significant (P= 0.018). LOS, in contrast, was significantly shorter in the HCR group (5.4± 1.6 days) than in the LCR group (9.9± 6 days) (P≤ 0.001) (Table 5).
Table 5.
The influence of high degree of protocol compliance on inflammation and postoperative surgical outcomes
| Variable | HCR (n = 59, 72%) | LCR (n = 12, 14.6%) | P-value |
|---|---|---|---|
| Inflammatory marker | |||
| Postoperative WBC count (× 109/L) | 0.976 | ||
| < 10,000 | 31 (52.5) | 6 (50.0) | |
| ≥ 10,000 | 28 (47.5) | 6 (50.0) | |
| Time for WBC count to decrease < 10,000/mm3 (day) | 0.998 | ||
| < 1 | 31 (52.5) | 6 (50.0) | |
| 1–3 | 17 (28.8) | 4 (33.3) | |
| ≥ 4 | 11 (18.6) | 2 (16.7) | |
| Preoperative monocyte count (%) | 0.476 | ||
| ≤9 | 40 (67.8) | 7 (58.3) | |
| >9 | 19 (32.2) | 5 (41.7) | |
| Postoperative monocyte count (%) | 0.015 | ||
| ≤9 | 51 (86.4) | 6 (50.0) | |
| >9 | 8 (13.6) | 6 (50.0) | |
| Postoperative CRP level (mg/L) | 0.657 | ||
| ≤ 0.5 | 42 (71.2) | 7 (58.3) | |
| > 0.5 | 17 (28.8) | 5 (41.7) | |
| Postoperative Glasgow prognostic score | 0.099 | ||
| 0 | 29 (49.2) | 4 (33.3) | |
| 1 | 24 (40.7) | 5 (41.7) | |
| 2 | 6 (10.2) | 3 (25.0) | |
| Postoperative surgical outcome | |||
| Complication | 0.008 | ||
| Yes | 4 (6.8) | 4 (33.3) | |
| No | 55 (93.2) | 8 (66.7) | |
| Clavien-Dindo classification | 0.018 | ||
| Grade I, II | 2 (3.4) | 3 (25.0) | |
| Grade > II | 2 (3.4) | 1 (8.3) | |
| Length of hospital stay (day) | 5.4 ± 1.6 | 9.9 ± 6.0 | < 0.001a |
aValues are presented as number (%) or mean±standard deviation.
HCR, high compliance response; LCR, low compliance response; WBC, white blood cells, CRP, C-reactive protein.
Mann-Whitney U-tests.
One of the main EP elements during the intraoperative period was the use of EPA, and its effect on inflammation and LOS was evaluated (data not shown). Eighty-two patients in the EP group were divided into 76 patients (92.7%) in the EPA group and 6 patients (7.3%) in the PCA group. Two inflammatory markers were associated with the use of EPA. First, the time required for the leukocyte count to normalize was shorter in the EPA group than in the PCA group. The percentage of cases that required less than one day to normalize was significantly greater in the EPA group (55.3%) than in the PCA group (16.7%) (P= 0.010). Significantly more patients in the EPA group (72.4%) than in the PCA group (33.3%) had normal postoperative CRP values (P= 0.046). The other preoperative and postoperative inflammatory markers did not exhibit any significant differences. There was no difference found in between the 2 groups regarding the LOS (P= 0.820).
DISCUSSION
EP is a recent development in the treatment of colorectal cancer patients and many surgeons are currently embracing this new strategy. The ability of EP to accelerate short-term postoperative recovery has been proven by many studies [8-14, 16]. This effect has been evaluated in different ways, with some addressing the favorable effects in combination with laparoscopic procedures [9, 11, 12, 16], conventional procedures [13], or both [10, 14]. In the present study, we did not exclude any cases of elective colorectal cancer surgery, and we chose to observe the impact of EP on postoperative recovery outcomes in general and on postoperative inflammation in particular, with a combination of different surgical techniques (open and MIS). Although the strongly favorable effect of MIS on postoperative inflammation has been proven by many studies [5, 6], to eliminate the common confusion regarding which factor, EP or MIS, caused that effect, a regression analysis was conducted. The results demonstrated that the EP independently significantly affected inflammation postoperatively when compared to the CP in colorectal cancer patients, regardless of whether MIS was used. In our regression analysis, MIS reduced the postoperative WBC count and GPS, while EP significantly reduced the postoperative WBC count, the time required for the WBC count to normalize, the postoperative CRP level and the postoperative GPS. A study by Feng et al. [11] supported this positive effect of EP on the acceleration of the reduction of the postoperative WBC count (P= 0.05). At our institution, we additionally reinforced the EP effect on inflammation by using immunenutrition modulation that reduces the inflammatory response and therefore improves postoperative surgical outcomes after major procedures [17-20]. We provided our patients with an enriched oral formula (Encover, JW Pharmaceutical, Seoul, Korea) that contains omega-3 fatty acids and other elements, with 2 packs consumed on the last preoperative day during the bowel preparation procedure and one pack consumed on the first postoperative day.
To evaluate the effect of EP on short-term postoperative recovery outcomes, some randomized controlled trials (RCTs) include only colon cancer patients [9, 10, 14], while others include only rectal cancer patients [11]. It has been shown that patients with rectal cancer, especially those required stoma, have higher complication rates and longer LOS than those of colon cancer patients. A recent multicenter RCT study [16] that included colon and all rectal cancer patients reported a limited effect of EP in rectal cancer patients. In contrast, our study included all cases, including low rectal tumors that required stoma and neoadjuvant chemoradiotherapy, and a further subgroup analysis to check for bias in the heterogenous group of patients was conducted (data not shown). There was no difference between colon cancer and rectal cancer patients regarding the following postoperative inflammatory marker levels: postoperative WBC count (P= 0.084), time required for the WBC to normalize (P= 0.223), and postoperative N/L ratio (P= 0.699). There was also no difference in their postoperative complication rate (P= 0.430). However, there was a significantly longer LOS in the rectal cancer group (mean, 10± 8 days) than in the colon cancer group (mean, 9 ± 5.4 days) (P= 0.033), which agreed with the results of other studies [16]. The present study proved the significant ability of the EP to decrease the complication rate, which has also been supported by several meta-analyses and RCTs [8, 11, 12, 17]. However, based on our analysis of the complication severity according to the classification proposed by Clavien-Dindo, there were still many patients with severe complications (> grade IIIa) among those who followed the EP, indicating that even though the EP reduces the complication rate, it cannot reduce the severity of complications caused by the surgery itself. Some studies showed no difference in the complication rate between the EP and the CP [9, 18].
Improving postoperative inflammation and decreasing the postoperative complication rate might affect the LOS. Most of the published articles [8, 10, 17-23] have demonstrated that the EP facilitates an earlier hospital discharge than that after the CP, without increasing morbidity or mortality rates; our findings were in line with the findings of those studies. In the LAFA study [10], fast track surgery in combination with laparoscopic procedures for colon cancer cases had the shortest LOS, and laparoscopy was the only independent predictive factor found to reduce LOS in their regression analysis. In contrast, in our regression analysis results, the EP was found to be an independent factor in the reduction of the LOS. Other factors, such as MIS, TNM stage, neoadjuvant chemoradiotherapy, and complication rate, were also associated with the LOS.
To accurately determine the effect of the EP, it is important to measure the degree of compliance with the elements of the EP during the pre-, intra-, and postoperative phases. Several authors found that achieving high degrees of compliance (more than 70%) improved postoperative recovery [24, 25]. Our study identified a compliance level ≥ 70% at 72% of EP patients. This result prompted us to assess the influence of a high degree of compliance on postoperative surgical outcomes in general and on postoperative inflammation in particular. With a high degree of compliance with the EP, the postoperative monocyte count was the only marker that was significantly reduced; therefore, a high degree of compliance has an unsatisfactory effect on reducing inflammatory markers, and further investigation is required. In contrast, both the complication rate and LOS were significantly reduced when ≥ 70% compliance was achieved. This result was in line with the observations of other authors [24, 25] and encourages all surgeons to increase their degree of compliance to improve postoperative surgical outcomes after colorectal cancer surgery. A recent systematic review that included 34 studies showed how decreasing compliance increases postoperative morbidity, hospital readmission and LOS, and further studies are needed to determine which specific elements of the EP have the greatest impacts on recovery outcomes after laparoscopic colorectal surgery [26]. Although the EP and a high degree of compliance with the elements of the EP decrease the complication rate, their effect on reducing the severity of complications is limited.
The use of EPA is one of the elements of the EP with the highest degree of compliance (67% to 100%) [27]. The influence of EPA on the attenuation of the postoperative inflammatory response and the acceleration of postoperative recovery has been mentioned by many authors [28, 29]. However, some studies reported no beneficial effect of EPA in comparison to intravenous analgesia on the LOS or other postoperative outcomes, and it is expensive [30]. Our study showed that EPA had some effect on postoperative inflammation, decreasing the time needed for leukocytes to normalize and reducing the postoperative CRP level, but it had no effect on the LOS. It seems that the effect of EPA on postoperative inflammation is still controversial, and more studies are needed.
This study had some limitations. First, the patient population was heterogeneous, with samples were collected from 2 different hospitals at 2 different periods. Second, the analysis of differences between groups with high versus low compliance with ERAS includes very few patients, in particular in the low compliance group, the risk of a type II error might be considered. Third, the influence of EPA on postoperative inflammation was not clear because of a limited number of patients in the present study. Fourth, although this study conducted a regression analysis to eliminate confounding factors between MIS and open methods, which can affect postoperative inflammation, there can be limitation for different rates of surgical methods between EP and CP groups. Fifth, the short duration of the study meant that the longterm effects of the EP on inflammation and oncological outcomes such as overall survival and disease-free survival could not be investigated; we aim to study these effects in the future.
The EP is the most recent advance in the treatment of colorectal cancer patients, in combination with MIS. The EP decreases early postoperative inflammation and improves short-term postoperative recovery outcomes such as complication rate and the LOS. A high degree of compliance (≥ 70%) with the elements of the EP accelerates its positive effect on postoperative surgical outcomes, but the effect on inflammation was limited.
Acknowledgments
This research was supported by Research Fund of The Catholic University of Korea, Seoul St. Mary’s Hospital.
Footnotes
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–44. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 2.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu H, Ouyang W, Huang C. Inflammation, a key event in cancer development. Mol Cancer Res. 2006;4:221–33. doi: 10.1158/1541-7786.MCR-05-0261. [DOI] [PubMed] [Google Scholar]
- 4.Paik KY, Lee IK, Lee YS, Sung NY, Kwon TS. Clinical implications of systemic inflammatory response markers as independent prognostic factors in colorectal cancer patients. Cancer Res Treat. 2014;46:65–73. doi: 10.4143/crt.2014.46.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ordemann J, Jacobi CA, Schwenk W, Stosslein R, Muller JM. Cellular and humoral inflammatory response after laparoscopic and conventional colorectal resections. Surg Endosc. 2001;15:600–8. doi: 10.1007/s004640090032. [DOI] [PubMed] [Google Scholar]
- 6.Zawadzki M, Krzystek-Korpacka M, Gamian A, Witkiewicz W. Comparison of inflammatory responses following robotic and open colorectal surgery: a prospective study. Int J Colorectal Dis. 2017;32:399–407. doi: 10.1007/s00384-016-2697-0. [DOI] [PubMed] [Google Scholar]
- 7.Arezzo A, Passera R, Salvai A, Arolfo S, Allaix ME, Schwarzer G, et al. Laparoscopy for rectal cancer is oncologically adequate: a systematic review and meta-analysis of the literature. Surg Endosc. 2015;29:334–48. doi: 10.1007/s00464-014-3686-4. [DOI] [PubMed] [Google Scholar]
- 8.Zhuang CL, Ye XZ, Zhang XD, Chen BC, Yu Z. Enhanced recovery after surgery programs versus traditional care for colorectal surgery: a meta-analysis of randomized controlled trials. Dis Colon Rectum. 2013;56:667–78. doi: 10.1097/DCR.0b013e3182812842. [DOI] [PubMed] [Google Scholar]
- 9.Lee TG, Kang SB, Kim DW, Hong S, Heo SC, Park KJ. Comparison of early mobilization and diet rehabilitation program with conventional care after laparoscopic colon surgery: a prospective randomized controlled trial. Dis Colon Rectum. 2011;54:21–8. doi: 10.1007/DCR.0b013e3181fcdb3e. [DOI] [PubMed] [Google Scholar]
- 10.Vlug MS, Wind J, Hollmann MW, Ubbink DT, Cense HA, Engel AF, et al. Laparoscopy in combination with fast track multimodal management is the best perioperative strategy in patients undergoing colonic surgery: a randomized clinical trial (LAFA-study) Ann Surg. 2011;254:868–75. doi: 10.1097/SLA.0b013e31821fd1ce. [DOI] [PubMed] [Google Scholar]
- 11.Feng F, Li XH, Shi H, Wu GS, Zhang HW, Liu XN, et al. Fasttrack surgery combined with laparoscopy could improve postoperative recovery of low-risk rectal cancer patients: a randomized controlled clinical trial. J Dig Dis. 2014;15:306–13. doi: 10.1111/1751-2980.12142. [DOI] [PubMed] [Google Scholar]
- 12.Wang Q, Suo J, Jiang J, Wang C, Zhao YQ, Cao X. Effectiveness of fast-track rehabilitation vs conventional care in laparoscopic colorectal resection for elderly patients: a randomized trial. Colorectal Dis. 2012;14:1009–13. doi: 10.1111/j.1463-1318.2011.02855.x. [DOI] [PubMed] [Google Scholar]
- 13.Spanjersberg WR, Reurings J, Keus F, van Laarhoven CJ. Fast track surgery versus conventional recovery strategies for colorectal surgery. Cochrane Database Syst Rev. 2011;(2):CD007635. doi: 10.1002/14651858.CD007635.pub2. [DOI] [PubMed] [Google Scholar]
- 14.Wang G, Jiang Z, Zhao K, Li G, Liu F, Pan H, et al. Immunologic response after laparoscopic colon cancer operation within an enhanced recovery program. J Gastrointest Surg. 2012;16:1379–88. doi: 10.1007/s11605-012-1880-z. [DOI] [PubMed] [Google Scholar]
- 15.Muehling BM, Ortlieb L, Oberhuber A, Orend KH. Fast track management reduces the systemic inflammatory response and organ failure following elective infrarenal aortic aneurysm repair. Interact Cardiovasc Thorac Surg. 2011;12:784–8. doi: 10.1510/icvts.2010.262337. [DOI] [PubMed] [Google Scholar]
- 16.Maggiori L, Rullier E, Lefevre JH, Regimbeau JM, Berdah S, Karoui M, et al. Does a combination of laparoscopic approach and full fast track multimodal management decrease postoperative morbidity?: a multicenter randomized controlled trial. Ann Surg. 2017;266:729–37. doi: 10.1097/SLA.0000000000002394. [DOI] [PubMed] [Google Scholar]
- 17.Liu VX, Rosas E, Hwang J, Cain E, Foss-Durant A, Clopp M, et al. Enhanced recovery after surgery program implementation in 2 surgical populations in an integrated health care delivery system. JAMA Surg. 2017;152:e171032. doi: 10.1001/jamasurg.2017.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keane C, Savage S, McFarlane K, Seigne R, Robertson G, Eglinton T. Enhanced recovery after surgery versus conventional care in colonic and rectal surgery. ANZ J Surg. 2012;82:697–703. doi: 10.1111/j.1445-2197.2012.06139.x. [DOI] [PubMed] [Google Scholar]
- 19.Wijk L, Franzen K, Ljungqvist O, Nilsson K. Implementing a structured enhanced recovery after surgery (ERAS) protocol reduces length of stay after abdominal hysterectomy. Acta Obstet Gynecol Scand. 2014;93:749–56. doi: 10.1111/aogs.12423. [DOI] [PubMed] [Google Scholar]
- 20.Vignali A, Elmore U, Cossu A, Lemma M, Cali B, de Nardi P, et al. Enhanced recovery after surgery (ERAS) pathway vs traditional care in laparoscopic rectal resection: a single-center experience. Tech Coloproctol. 2016;20:559–66. doi: 10.1007/s10151-016-1497-4. [DOI] [PubMed] [Google Scholar]
- 21.Teeuwen PH, Bleichrodt RP, Strik C, Groenewoud JJ, Brinkert W, van Laarhoven CJ, et al. Enhanced recovery after surgery (ERAS) versus conventional postoperative care in colorectal surgery. J Gastrointest Surg. 2010;14:88–95. doi: 10.1007/s11605-009-1037-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Forsmo HM, Pfeffer F, Rasdal A, Ostgaard G, Mohn AC, Korner H, et al. Compliance with enhanced recovery after surgery criteria and preoperative and postoperative counselling reduces length of hospital stay in colorectal surgery: results of a randomized controlled trial. Colorectal Dis. 2016;18:603–11. doi: 10.1111/codi.13253. [DOI] [PubMed] [Google Scholar]
- 23.Aarts MA, Okrainec A, Glicksman A, Pearsall E, Victor JC, McLeod RS. Adoption of enhanced recovery after surgery (ERAS) strategies for colorectal surgery at academic teaching hospitals and impact on total length of hospital stay. Surg Endosc. 2012;26:442–50. doi: 10.1007/s00464-011-1897-5. [DOI] [PubMed] [Google Scholar]
- 24.Gustafsson UO, Hausel J, Thorell A, Ljungqvist O, Soop M, Nygren J, et al. Adherence to the enhanced recovery after surgery protocol and outcomes after colorectal cancer surgery. Arch Surg. 2011;146:571–7. doi: 10.1001/archsurg.2010.309. [DOI] [PubMed] [Google Scholar]
- 25.Pedziwiatr M, Kisialeuski M, Wierdak M, Stanek M, Natkaniec M, Matlok M, et al. Early implementation of Enhanced Recovery After Surgery (ERAS(R)) protocol–compliance improves outcomes: a prospective cohort study. Int J Surg. 2015;21:75–81. doi: 10.1016/j.ijsu.2015.06.087. [DOI] [PubMed] [Google Scholar]
- 26.Messenger DE, Curtis NJ, Jones A, Jones EL, Smart NJ, Francis NK. Factors predicting outcome from enhanced recovery programmes in laparoscopic colorectal surgery: a systematic review. Surg Endosc. 2017;31:2050–71. doi: 10.1007/s00464-016-5205-2. [DOI] [PubMed] [Google Scholar]
- 27.Ahmed J, Khan S, Lim M, Chandrasekaran TV, MacFie J. Enhanced recovery after surgery protocols: compliance and variations in practice during routine colorectal surgery. Colorectal Dis. 2012;14:1045–51. doi: 10.1111/j.1463-1318.2011.02856.x. [DOI] [PubMed] [Google Scholar]
- 28.Barr J, Boulind C, Foster JD, Ewings P, Reid J, Jenkins JT, et al. Impact of analgesic modality on stress response following laparoscopic colorectal surgery: a post-hoc analysis of a randomised controlled trial. Tech Coloproctol. 2015;19:231–9. doi: 10.1007/s10151-015-1270-0. [DOI] [PubMed] [Google Scholar]
- 29.Chen WK, Ren L, Wei Y, Zhu DX, Miao CH, Xu JM. General anesthesia combined with epidural anesthesia ameliorates the effect of fast-track surgery by mitigating immunosuppression and facilitating intestinal functional recovery in colon cancer patients. Int J Colorectal Dis. 2015;30:475–81. doi: 10.1007/s00384-014-2098-1. [DOI] [PubMed] [Google Scholar]
- 30.Khan SA, Khokhar HA, Nasr AR, Carton E, El-Masry S. Effect of epidural analgesia on bowel function in laparoscopic colorectal surgery: a systematic review and meta-analysis. Surg Endosc. 2013;27:2581–91. doi: 10.1007/s00464-013-2794-x. [DOI] [PubMed] [Google Scholar]


