Abstract
Objective
To analyze the cardiovascular disease (CVD) burden in hospitalized patients with a diagnosis of coronavirus from the pre–coronavirus disease 2019 era in the United States.
Patients and Methods
We identified hospitalized adults with a diagnosis of coronavirus in a large US administrative database, the National (Nationwide) Inpatient Sample, from January 1, 2016, to December 3, 2017, to study patient demographic characteristics, clinical comorbidities, and outcomes (in-hospital mortality and health care resource utilization) based on the presence or absence of CVD.
Results
A total of 21,300 hospitalized adults with a diagnosis of coronavirus in 2016 and 2017 from all across the United States were included in the final analysis; the mean age was 63.6 years, 11,033 (51.8%) were female, and 15,911 (74.7%) had public insurers. Among these hospitalized patients, 11,930 (56.0%) had a diagnosis of CVD. Compared with those without CVD, the patients with CVD were older (70.1 vs 55.4 years) and had higher Charlson comorbidity index scores (2.5 vs 1.6) and Elixhauser comorbidity index scores (4.3 vs 2.4) (all P<.001). After multivariable risk adjustment, patients with CVD had higher mortality than those without CVD (5.3% [632 of 11,930] vs 1.5% [140 of 9370]; adjusted odds ratio, 2.0 [95% CI, 1.2 to 3.4]; P=.008). The mean length of hospital stay (6.9 vs 6.1 days; P=.003), hospital charges ($78,377 vs $66,538; P=.002), and discharge to nursing home (24.6% [2945 of 11,930] vs 12.9% [1208 of 9370]; P<.001) were higher in those with CVD compared with the patients without CVD.
Conclusion
Cardiovascular disease was present in a notable proportion of hospitalized patients with coronavirus in the pre–coronavirus disease 2019 era in United States and was associated with higher risk of in-hospital mortality and health care resource utilization.
Abbreviations and Acronyms: AOR, adjusted odds ratio; COVID-19, coronavirus disease 2019; CV, cardiovascular; CVD, CV disease; HCoV, human coronavirus; HF, heart failure; IL, interleukin; LOS, length of stay; MI, myocardial infarction; NIS, National (Nationwide) Inpatient Sample; SARS, severe acute respiratory syndrome
Coronaviruses are the largest group of viruses, and since their discovery in 1960, several different strains have been associated with respiratory tract illnesses, such as human coronavirus (HCoV) 229E (alpha), NL63 (alpha), OC43 (beta), and HKU1 (beta), severe acute respiratory syndrome (SARS) coronavirus in 2003, Middle East respiratory syndrome coronavirus in 2012, and most recently, SARS coronavirus 2 in 2019 (later designated as coronavirus disease 2019 [COVID-19]).1 In the pre–COVID-19 era, strains such as HCoV-NL63, HCoV-229E, HCoV-OC43, and HCoV-HKU1 have been associated with upper respiratory tract diseases in immunocompetent hosts, infants, young children, and elderly individuals.2 The first multiplex polymerase chain reaction panel for a large number of respiratory pathogens was approved by the US Food and Drug Administration in 2008. The incidence of coronavirus in respiratory tract specimens using such tests has been reported as approximately 8%.3 To the best of our knowledge, this is the first report to describe the characteristics and impact of cardiovascular disease (CVD) on hospitalized patients with a diagnosis of coronavirus from the pre–COVID-19 era in United States.4, 5, 6, 7 We searched a large administrative national database, the National (Nationwide) Inpatient Sample (NIS), for patients hospitalized between January 1, 2016, and December 3, 2017, to identify those with a diagnosis of coronavirus and describe in this article their demographic characteristics, hospitalization characteristics, clinical comorbidities, outcomes, and health care resource utilization based on the presence and absence of CVD.
Patients and Methods
Study Population and Design
The study records utilized were derived from the NIS, the largest publicly available all-payer inpatient care database in the United States. The NIS is a subset of the Healthcare Cost and Utilization Project sponsored by the Agency for Healthcare Research and Quality. The details regarding the NIS data have been published and used previously.8, 9, 10 Because coronavirus-related International Classification of Diseases diagnostic codes were introduced as part of tenth revision, our study sample spans 2016 through 2017 (2017 is the latest available data set in the NIS). The International Classification of Diseases, Tenth Revision, Clinical Modification codes B34.2, J12.81, B97.21, and B97.29 were used to identify our coronavirus study population. We followed the recommendations from the Agency for Healthcare Research and Quality for analysis using survey data such as survey-specific statements and patient-specific and hospital-specific discharge weights, as done previously.9 , 11 Estimates were weighted, unless otherwise noted, to allow for nationally representative interpretations. We accounted for hospital-level clustering of patients and the sampling design. Given that the NIS is a publicly available, deidentified database, we obtained an institutional review board exemption from the University of California, Los Angeles.
All patients older than 17 years who had a diagnosis of coronavirus (in primary or secondary diagnosis field) were included in the study. The NIS variables were used to identify patients’ demographic characteristics, clinical comorbidities, hospital length of stay (LOS), total charges, and discharge disposition (home, nursing home or similar ancillary services, morgue).11 The International Classification of Diseases, Tenth Revision, Clinical Modification or Clinical Classifications Software codes were used to define comorbidities (Supplemental Table 1, available online at http://www.mayoclinicproceedings.org). The hospitalization was classified as a cardiovascular (CV) admission if a CV etiology–related International Classification of Diseases diagnosis code was present in the primary diagnostic field, as done previously.10 The severity of comorbid conditions was studied using the Deyo modification of the Charlson comorbidity index, which contains 17 comorbid conditions with differential weights, and the Elixhauser comorbidity index, which is a sum of the 29 Elixhauser comorbidity variables.12 , 13
We defined CVD as the presence of one of the following: coronary artery disease, myocardial infarction (MI), heart failure (HF), sudden cardiac arrest, conduction disorders, cardiac dysrhythmias, cardiomyopathy, pulmonary heart disease, venous thromboembolic disorders, pericardial diseases, heart valve disorders, and peripheral arterial disease (Supplemental Table 1).
We initially studied the characteristics of age, female sex, race, insurance, median socioeconomic status, Charlson comorbidity index score, Elixhauser comorbidity index score, clinical comorbidities (hypertension, hyperlipidemia, diabetes, obesity, chronic obstructive pulmonary disease, renal disease, liver disease, cancer, dementia, pneumonia, acute respiratory failure, shock, sepsis, HIV status, history of organ transplant, invasive mechanical ventilation) based on the presence and absence of CVD (Table 1 and Supplemental Table 2, available online at http://www.mayoclinicproceedings.org). Then we studied the outcomes of in-hospital mortality and health care resource utilization (LOS days, total charges, discharge to nursing home) (Table 2 ). We performed additional analysis for the outcome of in-hospital mortality (1) by comparing patients with a primary CV diagnosis vs those with a non-CV primary diagnosis and (2) by comparing patients with systolic HF vs those without systolic HF (Supplemental Table 3, available online at http://www.mayoclinicproceedings.org). For the outcomes of LOS and total charges, additional analysis was performed after excluding those who died before discharge (Supplemental Table 4, available online at http://www.mayoclinicproceedings.org).
Table 1.
Baseline Patient Demographic and Hospitalization Characteristics, Overall and by Cardiovascular Diseasea
| Variable | Overall (N=21,300) | Cardiovascular disease |
P value | |
|---|---|---|---|---|
| No (n=9370) | Yes (n=11,930) | |||
| Age (y), mean (95% CI) | 63.6 (62.9-64.3) | 55.4 (55.5-6.3) | 70.1 (69.3-70.7) | <.001 |
| Age groups (y) | <.001 | |||
| 18-40 | 2513 (11.8) | 2015 (21.5) | 498 (4.2) | |
| 41-64 | 7562 (35.5) | 4207 (44.9) | 3335 (28.1) | |
| ≥65 | 11225 (52.7) | 3148 (33.6) | 8077 (67.7) | |
| Female | 11040 (51.8) | 5360 (57.2) | 5680 (47.6) | <.001 |
| Race | <.001 | |||
| White | 66.9 | 61.4 | 71.3 | |
| Black | 11.3 | 12.8 | 9.8 | |
| Other | 21.8 | 25.8 | 18.9 | |
| Payer status | <.001 | |||
| Public | 74.7 | 63.8 | 83.3 | |
| Private | 20.5 | 29.4 | 13.5 | |
| Other | 4.8 | 6.8 | 3.2 | |
| Median socioeconomic status by national quartiles | .14 | |||
| 0-25 | 26.2 | 27.6 | 25.1 | |
| 25-50 | 26.1 | 25.7 | 26.4 | |
| 50-75 | 24.5 | 24.0 | 25.0 | |
| 75-100 | 23.2 | 22.7 | 23.5 | |
| Charlson comorbidity index score, mean | 2.1 | 1.6 | 2.5 | <.001 |
| Elixhauser comorbidity index score, mean | 3.5 | 2.4 | 4.3 | <.001 |
| Comorbidities | ||||
| Hypertension | 64.4 | 49.2 | 76.4 | <.001 |
| Hyperlipidemia | 36.0 | 22.9 | 46.4 | <.001 |
| Diabetes | 31.4 | 23.7 | 37.4 | <.001 |
| Obesity | 15.6 | 14.1 | 16.8 | .01 |
| Dementia | 8.3 | 5.1 | 10.9 | <.001 |
| Chronic pulmonary disease | 50.5 | 47.7 | 52.7 | .002 |
| Chronic renal disease | 32.4 | 19.6 | 42.3 | <.001 |
| History of organ transplant | 4.9 | 6.0 | 4.1 | .008 |
| HIV infection | 1.5 | 2.4 | 0.7 | <.001 |
| Chronic liver disease | 3.6 | 3.5 | 3.7 | .79 |
| Cancer | 19.4 | 23.3 | 16.2 | <.001 |
| Pneumonia | 17.7 | 14.8 | 20.2 | <.001 |
| Sepsis | 21.9 | 19.7 | 23.7 | .001 |
| Shock | 1.3 | 0.5 | 1.9 | <.001 |
| Acute respiratory failure | 39.7 | 32.2 | 45.6 | <.001 |
| Invasive mechanical ventilation | 14.2 | 10.4 | 17.1 | <.001 |
Data are presented as No. (percentage) of patients unless indicated otherwise.
Table 2.
| Outcome | Overall (N=21,300) | Cardiovascular disease |
P value | |
|---|---|---|---|---|
| No (n=9370) | Yes (n=11,930) | |||
| Length of stay (d) | 6.6 (3.0-8.0) | 6.1 (5.7-6.6) | 6.9 (6.6-7.2) | .003 |
| Adjusted length of stay (d) | NA | 5.8 (5.5-6.2) | 6.3 (6.0-6.6) | .03 |
| Total chargesc | $73,137 ($66,443-$79,831) | $66,538 ($59,033-$74,043) | $78,377 ($70,611-$86,145) | .002 |
| Adjusted total charges | NA | $54,477 ($50,073-$58,881) | $67,536 ($62,210-$72,861) | <.001 |
| Discharge to nursing home or similar facility, No. (%; 95% CI) | 4153 (19.5; 18.1-20.8) | 1208 (12.9; 11.4-14.5) | 2945 (24.6; 22.9-26.5) | <.001 |
| Adjusted odds ratio (95% CI) | NA | Reference | 1.07 (0.88-1.33) | .475 |
| In-hospital mortality, No. (%; 95% CI) | 775 (3.6; 3.1-4.3) | 141 (1.5; 1.0-2.1) | 634 (5.3; 4.5-6.4) | <.001 |
| Adjusted odds ratio (95% CI) | NA | Reference | 2.0 (1.2-3.4) | .008 |
NA, not applicable.
Data are presented as mean (95% CI) unless indicated otherwise.
Sum of total charges: overall cohort, $1.55 billion; CVD group, $927 million.
Statistical Analyses
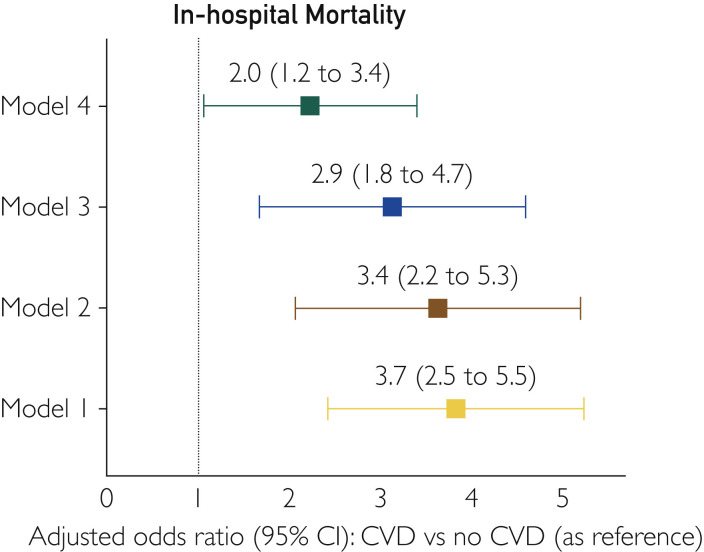
Descriptive analyses were performed using mean (interquartile range) and proportions (interquartile range) as appropriate. The Pearson χ2 test was used to describe the patient demographic and hospitalization characteristics in those with and without CVD (Table 1). Comparisons among continuous variables were made with the Student t test. Significance testing was performed with multivariable unconditional logistic regression with CVD as a categorical variable for the outcomes of in-hospital mortality and discharge to nursing home or similar facility. The influence of potential confounders was analyzed by incremental adjustments (Figure 1 ). Model 1 was unadjusted. Model 2 adjusted for age, sex, race, and insurance. Model 3 adjusted for covariates included in model 2 plus Charlson comorbidity index score, Elixhauser comorbidity index score, and clinical comorbidities (hypertension, hyperlipidemia, diabetes, obesity, chronic pulmonary disease, renal disease, cancer, liver disease, and dementia). Model 4 (Supplemental Table 5, available online at http://www.mayoclinicproceedings.org) adjusted for all covariates in model 3 plus HIV status, history of organ transplant, pneumonia, shock, acute respiratory failure, sepsis, and invasive mechanical ventilation. We used multivariable Poisson regression analyses with robust SEs (using model 4) to compare LOS and total charges per hospitalization (Table 2). We also performed a subgroup analysis to study the association of specific CVDs with in-hospital mortality using events reported recently in the coronavirus literature by the presence of one of the following: acute MI, HF, pericardial diseases, myocarditis, acute pulmonary embolism, and sudden cardiac arrest4 , 6 , 14 , 15 (Supplemental Tables 6, 7, and 8, Supplemental Figure, available online at http://www.mayoclinicproceedings.org). Adjusted odds ratios (AORs) and 95% CIs were used to report the results of logistic regression. All analyses were conducted with Stata/MP statistical software, version 16.1 (StataCorp).
Figure 1.
Forest plot showing the adjusted odds ratio for association of cardiovascular disease (CVD) with in-hospital mortality. Model 1, unadjusted; model 2, adjusted for age, sex, race, and insurance; model 3, adjusted for model 2 plus hypertension, hyperlipidemia, diabetes, obesity, chronic pulmonary disease, renal disease, liver disease, cancer, and dementia; model 4, adjusted for model 3 plus HIV status, history of organ transplant, pneumonia, sepsis, acute respiratory failure, shock, and mechanical ventilation.
Results
Baseline Demographic Characteristics and Clinical Comorbidities
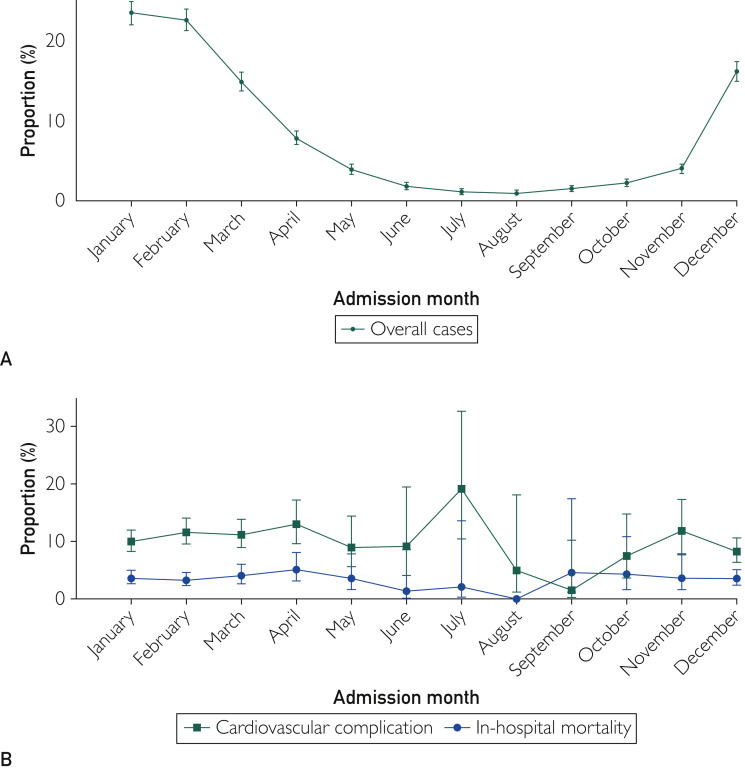
There were 60,618,339 adult hospitalizations during 2016 (n=30,195,772) and 2017 (n=30,422,617) across the United States. Among these hospitalizations, 21,300 patients (8250 in 2016 and 13,050 in 2017) had a diagnosis of coronavirus and formed our final analytic cohort. The major reasons documented for hospitalizations were sepsis in 3855 (18.1%), chronic obstructive pulmonary disease in 3046 (14.3%), acute respiratory failure in 1981 (9.3%), viral infection in 1640 (7.7%), pneumonia in 980 (4.6%), and upper respiratory tract infection in 746 (3.5%). The proportion of patients with a primary CV diagnosis was 20.0% (4258 patients). The mean age was 63.6 years, 11,033 (51.8%) were female, 14,250 (66.9%) were White, and 15,911 (74.7%) had public insurers. As shown in Table 1, the mean Charlson comorbidity index score was 2.1, and the mean Elixhauser comorbidity index score was 3.5. Most admissions (13,185 [61.9%]) occurred during the winter season (December through February) (Figure 2 A).
Figure 2.
A, Trends of hospitalizations with a diagnosis of coronavirus by admission month. Overall (%) = total number of hospitalizations with a diagnosis of coronavirus during a particular month divided by the total number of hospitalizations with a diagnosis of coronavirus. B, Trends of in-hospital mortality and hospitalizations with a diagnosis of cardiovascular disease by admission month. In-hospital mortality (%) = (total number of hospitalizations with death as an outcome before hospital discharge during a particular month divided by the total number of hospitalizations with a diagnosis of coronavirus during that month) × 100. Cardiovascular disease (%) = (total number of hospitalizations with cardiovascular disease during a particular month divided by the total number of hospitalizations with a diagnosis of coronavirus during that month) × 100.
Among the 21,300 overall hospitalizations, 11,930 patients (56.0%) had CVD: coronary artery disease, 5623 (26.4%; 95% CI, 25.0% to 27.9%); acute MI, 703 (3.3%; 95% CI, 2.7% to 3.9%); HF, 5708 (26.8%; 95% CI, 25.3% to 28.3%; systolic HF, 2130 [10.0%; 95% CI, 9.1% to 11.05%]; and diastolic HF, 2663 [12.5%; 95% CI, 11.5% to 13.6%]); sudden cardiac arrest, 213 (1.0%; 95% CI, 0.8% to 1.9%); conduction disease disorders, 1512 (7.1%; 95% CI, 6.3% to 7.9%); cardiac dysrhythmias, overall, 5048 (23.7%; 95% CI, 22.4% to 25.1%); atrial arrhythmias, 4665 (21.9%; 95% CI, 20.6% to 23.3%); and ventricular arrhythmias, 511 (2.4%; 95% CI, 2.0% to 3.0%); venous thromboembolic disorders, 746 (3.5%; 95% CI, 3.0% to 4.2%); myocarditis, 43 (0.2%; 95% CI, 0.1% to 0.2%); pulmonary heart disease, 1704 (8.0%; 95% CI, 7.2% to 8.8%); pericardial diseases, 320 (1.5%; 95% CI, 1.1% to 1.9%); heart valve disorders, 1938 (9.1%; 95% CI, 8.1% to 10.1%); peripheral arterial disease, 1278 (6.0%; 95% CI, 5.3% to 6.9%); cardiomyopathies, 1811 (8.5%; 95% CI, 7.6% to 8.8%); and cerebrovascular disease, 873 (4.1%; 95% CI, 3.5% to 4.7%). In subgroup analysis, CVDs acute MI, HF, myocarditis, pericardial diseases, sudden cardiac arrest, and acute pulmonary embolism were present in 2215 patients (10.4%) (Supplemental Table 6).
Compared with those without CVD, patients with CVD were older (70.1 years [95% CI, 69.3 to 70.7 years] vs 55.4 years [95% CI, 55.5 to 56.3 years]; P<.001) and had higher Charlson comorbidity index scores (2.5 [95% CI, 2.4 to 2.6] vs 1.6 [95% CI, 1.5 to 1.7]; P<.001) and Elixhauser comorbidity index scores (4.3 [95% CI, 4.2 to 4.4] vs 2.4 [95% CI, 2.4 to 2.5]; P<.001). Moreover, comorbidities, including hypertension (9115 [76.4%; 95% CI, 74.5% to 78.2%] vs 4610 [49.2%; 95% CI, 46.9% to 51.5%]), diabetes (4462 [37.4%; 95% CI, 35.5% to 39.4%] vs 2221 [23.7%; 95% CI, 21.9% to 25.7%]), hyperlipidemia (5536 [46.4; 95% CI, 44.1% to 48.6%] vs 2146 [22.9%; 95% CI, 21.0% to 25.0%]), chronic pulmonary disease (6287 [52.7%; 95% CI, 50.4% to 55.0%] vs 4470 [47.7%; 95% CI, 45.0% to 50.4%]), and chronic renal failure (5046 [42.3%; 95% CI, 40.4% to 44.4%] vs 1837 [19.6%; 17.9% vs 21.6%]) were present more often among patients with CVD (Table 1). The proportion of hospitalized patients with CVD did not change substantially when stratified by the admission month (Figure 2B). The diagnosis of pneumonia (2410 [20.2%; 95% CI, 18.4% to 21.7%] vs 1387 [14.8%; 95% CI, 13.2% to 16.5%]), sepsis (2827 [23.7%; 95% CI, 22.0% to 25.5%] vs 1846 [19.7%; 95% CI, 17.9% to 21.6%]), acute respiratory failure (5441 [45.6%; 95% CI, 43.3% to 48.0%] vs 3017 [32.2%; 95% CI, 29.8% to 34.7%]), shock (227 [1.9%; 95% CI, 1.4 to 2.6%] vs 47 [0.5%; 95% CI, 0.3% to 0.9%]), and invasive mechanical ventilation (2040 [17.1%; 95% CI, 15.4% to 19.0%] vs 974 [10.4%; 95% CI, 9.0% to 11.9%]) was more common in patients with CVD (Table 1).
In-hospital Mortality
The overall in-hospital mortality was 3.6% (775 of 21,300), and the presence of CVD was associated with higher risk of in-hospital mortality (5.3% [634 of 11,930] vs 1.5% [141 of 9370]; AOR, 2.0 [95% CI, 1.2 to 3.4]; P=.008) when compared with patients without CVD (Table 2). Also, patients with a primary CV diagnosis had similar in-hospital mortality when compared with those who had a primary non-CV diagnosis (3.4% [145 of 4258] vs 3.7% [644 of 17042]; P=.807).
LOS, Total Charges, and Discharge Disposition
The mean overall LOS was 6.6 days, and the mean overall total hospital charges was $73,137 (Table 2). Overall, 19.5% of the survivors (4153 of 21,300) were discharged to a nursing home or similar ancillary care facilities. In patients with CVD vs those without CVD, the mean LOS (6.9 days [95% CI, 6.6 to 7.2 days] vs 6.1 days [95% CI, 5.7 to 6.6 days]; P=.003) and mean total charges ($78,377 [95% CI, $70,611 to $86,145] vs $66,538 [95% CI, $59,033 to $74,043]; P=.002) were higher in those with CVD. Also, the CVD subgroup was more likely to be discharged to nursing home facilities (2945 of 11,930 [24.6%; 95% CI, 22.9% to 26.5%] vs 1208 of 9370 [12.9%; 95% CI, 11.4% to 14.5%]; P<.001). After adjustment for covariates, the adjusted mean LOS (5.8 days [95% CI, 5.5 to 6.2 days] vs 6.3 days [95% CI, 6.0 to 6.6 days]; P=.03) and adjusted mean total charges ($54,477 [95% CI, $50,073 to $58,881] vs $67,536 [95% CI, $62,210 to $72,861]; P<.001) were higher in those with CVD. However, after adjustment for covariates, the presence of CVD no longer had a significant association with higher utilization of nursing home or similar ancillary facilities after discharge (AOR, 1.07; 95% CI, 0.88 to 1.33; P=.475) (Table 2).
Discussion
To the best of our knowledge, this report using a national administrative data set from the nonpandemic pre–COVID-19 era, is the largest and first description of hospitalizations with a diagnosis of coronavirus in United States. Our study found that CVD (present in ∼56% of hospitalizations) was associated with worse outcomes and higher health care resource utilization in patients with a diagnosis of coronavirus in a nonpandemic setting. To account for possible confounding from multiple factors that could influence mortality, we rigorously adjusted our findings for age and other demographic factors as well as comorbidities. The persistence of substantially increased risks of poorer outcomes associated with the presence of CVD, despite such adjustments, strengthens the overall validity of our findings. Although the findings are not unexpected, our report helps to raise awareness about a high-risk group of hospitalized patients with coronavirus and CVD. This finding emphasizes the need for further research, clinical care tracks, and other strategies to mitigate the adverse outcomes and improve health care efficiency.
Previous studies focusing on coronavirus from single-center non-US cohorts have reported CVD in 30% to 40% of cases,15, 16, 17 with CVD complications ranging between 7% and 30%.14 , 15 , 18, 19, 20, 21 A systematic analysis of 637 Middle East respiratory syndrome coronavirus cases from Asia and Europe found that diabetes and hypertension were prevalent in about 50% of the patients and CVD was present in 30%.17 A recent study found that among 99 cases of COVID-19 in China, 40% of patients had CVD.22 A recent meta-analysis of 6 studies reported that approximately 8% of patients with COVID-19 had evidence of acute cardiac injury.15 In a single-center study of 138 hospitalizations, Wang et al14 found acute cardiac injury, shock, and arrhythmia in 7.2%, 8.7%, and 16.7% of patients, respectively, while another study described cardiac injury in 19.7% of their patients.19 A case series of 15 patients with SARS reported the presence of myocardial ischemia and arrhythmia in 10 patients.21 Preliminary reports in patients with COVID-19 suggest that there may be higher rates of myocarditis and arrhythmia that necessitate further study.20 , 23 , 24 These dissimilarities from the present study can be attributed to differences in the various strains of coronavirus, study sample size, pandemic vs nonpandemic timeline, patient characteristics, regional differences, and definitions (biomarker/medical record review vs International Classification of Diseases, Tenth Revision code–based).
Patients with CVD represent a high-risk population for pandemic transmission and worse outcomes in viral infections, as documented previously.15 , 17 , 19 , 20 , 25, 26, 27, 28 In a systematic review of 234 articles and 610,782 participants, the CVD subgroup was 3 times more likely to have development of pandemic infections.25 The association of mortality with CV injury defined by troponin elevation was recently reported by Shi et al28 in a cohort of 416 hospitalized patients with COVID-19. Similar to our study, patients with CVD were older, had a higher burden of comorbidities, and were more likely to have critical conditions such as acute respiratory failure. Older adults have different innate responses to viral infections than younger adults in terms of a cascade of events leading to increased type 2 cytokine production and decreased expression of type 1 interferon-β that eventually leads to a cytokine storm.29 Viral infections with coronavirus have been determined to have higher plasma levels of C-reactive protein, cytokines such as interleukin (IL) 2, IL-7, IL-10, and tumor necrosis factor α.14 , 22 The increased cytokine synthesis from metabolic diseases along with cytokine overload from the helper T cell subtype 1 to helper T cell subtype 2 shift can lead to endothelial damage.30 Hence, as a result of increased metabolic demand in the setting of viral sepsis syndrome, it is possible that the underlying stable CVD decompensates, leading to worse outcomes.20 , 31 Previous studies have also found that coronavirus strains of SARS may down-regulate the myocardial angiotensin-converting enzyme 2 system, which leads to myocardial dysfunction and adverse cardiac outcomes.32 The inflammatory cascade up-regulation might contribute to rupture of coronary atherosclerotic plaques along with endothelial dysfunction, predisposing patients to thromboembolic events. Although we did adjust for demographic characteristics (including age) and comorbidities in regression analysis, we cannot exclude the possibility of residual confounding.
Patients with CVD may have less ability to overcome the stress of infections, leading to worsening of respiratory status and related complications that might have contributed to longer LOS and higher hospital charges. The mean total charges for the overall cohort were $1.55 billion, and those for the CVD subgroup were $927 million. Additionally, postdischarge utilization of nursing home facilities was seen in a notable proportion of patients. Although our analysis is limited to a nonpandemic setting and spans hospitalizations over 2 years, it is reasonable to hypothesize that such charges will be several-fold higher if analyzed during a pandemic setting and after inclusion of the cost of postdischarge ancillary care services. Also, the seasonal variability of viral infections, including the coronavirus family as seen in our study, has been reported previously,33 , 34 but interestingly, the monthly proportion of patients with CVD and mortality remained high and did not change substantially with admission months in our study.
Several limitations merit consideration when interpreting our findings. We conducted a retrospective observational study from an administrative database with unavailability of detailed clinical, laboratory, echocardiographic, and pharmacotherapeutic information. Details about specific strain of coronavirus were not available. Given that the diagnosis of coronavirus depends on the respiratory viral panels that might not be readily available across all health care systems, the cases captured in the data set might reflect only a proportion of cases, from medical centers with accessibility and technical expertise.3 Our analysis is predisposed to selection bias because sicker patients plausibly are more likely to be hospitalized and undergo respiratory viral panel testing. It is not possible to differentiate the complications from preexisting comorbidities in the NIS data set. Plausibly, older patients with CVD are more likely to be tested than younger patients with fewer comorbid conditions. However, because of the nature of our data set, we were not able to determine the testing rates and make such comparisons. The information related to initial clinical presentation, postdischarge events, and long-term outcomes are not available in data set. Because details about cause of death are not available, death might have occurred without identification of CVD events, and hence, our results should be interpreted with caution. We were not able to study the outcomes of patients with CVD at high risk for infection-related hospitalizations such as those with left ventricular assist devices.35 Nevertheless, our findings provide a description of nation-wide US hospitalizations with a diagnosis of coronavirus from the pre–COVID-19 era and document the association of CVD with worse outcomes and greater health care resource utilization in patients with coronavirus.
Conclusion
We found that in the pre–COVID-19 era, CVD in hospitalized patients with a diagnosis of coronavirus was associated with higher in-hospital mortality and a substantially higher health care resource utilization. Further studies are needed to validate our findings in the COVID-19 era and to determine the causes of the increased mortality and associated expenditures that we observed in this high-risk cohort of patients with CVD. Efforts can then be directed toward decreasing these events, reducing costs, and optimizing health-related outcomes.
Footnotes
Potential Competing Interests: Dr Fonarow has received consulting fees from Abbott, Amgen Inc, AstraZeneca, Bayer AG, CHF Solutions, Inc, Janssen Pharmaceuticals, Inc, Medtronic, Merck & Co, Inc, and Novartis AG. The other authors report no competing interests.
Supplemental material can be found online at http://www.mayoclinicproceedings.org. Supplemental material attached to journal articles has not been edited, and the authors take responsibility for the accuracy of all data.
Supplemental Online Material
References
- 1.Fehr A.R., Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Methods Mol Biol. 2015;1282:1–23. doi: 10.1007/978-1-4939-2438-7_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cui J., Li F., Shi Z.-L. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. 2019;17(3):181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schreckenberger P.C., McAdam A.J. Point-counterpoint: large multiplex PCR panels should be first-line tests for detection of respiratory and intestinal pathogens. J Clin Microbiol. 2015;53(10):3110–3115. doi: 10.1128/JCM.00382-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clerkin K.J., Fried J.A., Raikhelkar J. COVID-19 and cardiovascular disease. Circulation. 2020;141(20):1648–1655. doi: 10.1161/CIRCULATIONAHA.120.046941. [DOI] [PubMed] [Google Scholar]
- 5.Li S.S., Cheng C., Fu C. Left ventricular performance in patients with severe acute respiratory syndrome: a 30-day echocardiographic follow-up study. Circulation. 2003;108(15):1798–1803. doi: 10.1161/01.CIR.0000094737.21775.32. [DOI] [PubMed] [Google Scholar]
- 6.Driggin E., Madhavan M.V., Bikdeli B. Cardiovascular considerations for patients, health care workers, and health systems during the COVID-19 pandemic. J Am Coll Cardiol. 2020;75(18):2352–2371. doi: 10.1016/j.jacc.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aggarwal G., Cheruiyot I., Aggarwal S. Association of cardiovascular disease with coronavirus disease 2019 (COVID-19) severity: a meta-analysis. Curr Probl Cardiol. 2020;45(8):100617. doi: 10.1016/j.cpcardiol.2020.100617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agarwal M.A., Shah M., Garg L., Lavie C.J. Relationship between obesity and survival in patients hospitalized for hypertensive emergency [letter] Mayo Clin Proc. 2018;93(2):263–265. doi: 10.1016/j.mayocp.2017.07.015. [DOI] [PubMed] [Google Scholar]
- 9.Agarwal M.A., Jain N., Podila P.S.B. Association of history of heart failure with hospital outcomes of hyperglycemic crises: analysis from a university hospital and national cohort. J Diabetes Complications. 2020;34(1):107466. doi: 10.1016/j.jdiacomp.2019.107466. [DOI] [PubMed] [Google Scholar]
- 10.Agarwal M.A., Aggarwal A., Rastogi S., Ventura H.O., Lavie C.J. Cardiovascular disease burden in cancer patients from 2003 to 2014 [letter] Eur Heart J Qual Care Clin Outcomes. 2018;4(1):69–70. doi: 10.1093/ehjqcco/qcx033. [DOI] [PubMed] [Google Scholar]
- 11.Agency for Healthcare Research and Quality NIS description of data elements. https://www.hcup-us.ahrq.gov/db/nation/nis/nisdde.jsp Updated August 13, 2018.
- 12.Deyo R.A., Cherkin D.C., Ciol M.A. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 13.Elixhauser A., Steiner C., Harris D.R., Coffey R.M. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li B., Yang J., Zhao F. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020;109(5):531–538. doi: 10.1007/s00392-020-01626-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Badawi A., Ryoo S.G. Prevalence of comorbidities in the Middle East respiratory syndrome coronavirus (MERS-CoV): a systematic review and meta-analysis. Int J Infect Dis. 2016;49:129–133. doi: 10.1016/j.ijid.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi K.W., Chau T.N., Tsang O., Princess Margaret Hospital SARS Study Group Outcomes and prognostic factors in 267 patients with severe acute respiratory syndrome in Hong Kong. Ann Intern Med. 2003;139(9):715–723. doi: 10.7326/0003-4819-139-9-200311040-00005. [DOI] [PubMed] [Google Scholar]
- 19.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study [published corrections appear in Lancet. 2020;395(10229):1038] Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiong T.-Y., Redwood S., Prendergast B., Chen M. Coronaviruses and the cardiovascular system: acute and long-term implications. Eur Heart J. 2020;41:1798–1800. doi: 10.1093/eurheartj/ehaa231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pan S., Zhang H., Li C., Wang C. Cardiac arrest in severe acute respiratory syndrome: analysis of 15 cases [in Chinese] Zhonghua Jie He He Hu Xi Za Zhi. 2003;26(10):602–605. [PubMed] [Google Scholar]
- 22.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China [published correction appears in Lancet. 2020;395(10223):496] Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alhogbani T. Acute myocarditis associated with novel Middle east respiratory syndrome coronavirus. Ann Saudi Med. 2016;36(1):78–80. doi: 10.5144/0256-4947.2016.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China [published correction appears in Intensive Care Med. 2020;46(6):1294-1297] Intensive Care Med. 2020;46(5):846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mertz D., Kim T.H., Johnstone J. Populations at risk for severe or complicated influenza illness: systematic review and meta-analysis. BMJ. 2013;347:f5061. doi: 10.1136/bmj.f5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hong K.-H., Choi J.-P., Hong S.-H. Predictors of mortality in Middle East respiratory syndrome (MERS) Thorax. 2018;73(3):286–289. doi: 10.1136/thoraxjnl-2016-209313. [DOI] [PubMed] [Google Scholar]
- 27.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 28.Shi S., Qin M., Shen B. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smits S.L., de Lang A., van den Brand J.M.A. Exacerbated innate host response to SARS-CoV in aged non-human primates. PLoS Pathog. 2010;6(2):e1000756. doi: 10.1371/journal.ppat.1000756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berger A. Th1 and Th2 responses: what are they? BMJ. 2000;321(7258):424. doi: 10.1136/bmj.321.7258.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin G.-L., McGinley J.P., Drysdale S.B., Pollard A.J. Epidemiology and immune pathogenesis of viral sepsis. Front Immunol. 2018;9:2147. doi: 10.3389/fimmu.2018.02147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oudit G.Y., Kassiri Z., Jiang C. SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur J Clin Invest. 2009;39(7):618–625. doi: 10.1111/j.1365-2362.2009.02153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morikawa S., Kohdera U., Hosaka T. Seasonal variations of respiratory viruses and etiology of human rhinovirus infection in children. J Clin Virol. 2015;73:14–19. doi: 10.1016/j.jcv.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fares A. Factors influencing the seasonal patterns of infectious diseases. Int J Prev Med. 2013;4(2):128–132. [PMC free article] [PubMed] [Google Scholar]
- 35.Agrawal S., Garg L., Shah M. Thirty-day readmissions after left ventricular assist device implantation in the United States: insights from the Nationwide Readmissions Database. Circ Heart Fail. 2018;11(3):e004628. doi: 10.1161/CIRCHEARTFAILURE.117.004628. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.