Abstract
Since its outbreak in the last December, coronavirus disease 2019 (COVID-19) caused by SARS-CoV-2 has rapidly spread worldwide at a pandemic proportion and thus is regarded as a global public health emergency. The existing therapeutic options for COVID-19 beyond the intensive supportive care are limited, with an undefined or modest efficacy reported so far. Drug repurposing represents an enthusiastic mechanism to use approved drugs outside the scope of their original indication and accelerate the discovery of new therapeutic options. With the emergence of COVID-19, drug repurposing has been largely applied for early clinical testing. In this review, we discuss some repurposed anticancer drugs for the treatment of COVID-19, which are under investigation in clinical trials or proposed for the clinical testing.
Keywords: COVID-19, SARS-CoV-2, Anticancer drugs, Repurposing
Graphical abstract
1. Introduction
Novel coronavirus disease 2019 (COVID-19) has recently emerged as a potential life-threatening disease of public health interest, worldwide. Few pharmacotherapeutic options are available at the present time, and patients are experiencing adverse outcomes in the most severe presentations. The treatments used in the clinical care are mainly based on supportive measures and intensive care management for complicated cases [138]. Antiviral, antibiotic and antimalarial molecules have also been used to avoid severe outcomes as well as plasma therapy from recovered patients [12,19,75]. Despite the lack of strong evidence from clinical trials, several pharmacological treatments have been used in clinical practice and included in possible protocols based on preliminary findings of promising efficacy and a rationale against COVID-19, as suggested by recent systematic reviews [37,217]. However, if available, clinical trials remain the option of choice for patients with COVID-19, to enhance the data and progress in medical research. In this context, drug repurposing can be an attractive and promising approach for patients with COVID-19 in order to benefit from the safety and effectiveness of the molecules with proposed antiviral and/or immunomodulating properties [80,142]. Repurposed pipelines may have a significant impact, particularly in this critical time [44,78,161]. In this review article, we discuss the potential of a number of compounds licensed for cancer conditions that seem to have a rationale to target some pathogenic key mechanisms of COVID-19, under clinical investigation or proposed for clinical trials.
2. Drug repurposing as a substitute solution for emerging viral diseases of public health interest
The development of novel antiviral drugs requires long-term investigations in clinical trials. The advantage to repurpose drugs to validate off-label use is related to the known safety profile, though it may vary across the diseases, and consolidated data of pharmacodynamics, pharmacokinetics and efficacy in phase I–IV trials. Of interest, some host cellular targets interfering with the viral growth cycle, such as kinases, are broadly shared in the mechanisms of several viral infections and other conditions such as cancer, suggesting the possibility to translate knowledge across medical disciplines and disease models [56,156]. Therefore, pharmacologic approaches for targeting key pathogenic mechanisms, such as signalling pathways, constitute a promising tactic during outbreaks of emerging pathogens. A cost-effective drug repurposing has been previously used to combat intracellular pathogens as an urgent alternative during the occurrence of rapidly spreading and deadly infectious diseases: in fact, repurposed medicines may help reduce the investments in the earlier phases of drug development, with an immediate use in the specific setting of unmet need [66,118]. For instance, an accumulated experience on repurposed therapeutics during previous outbreaks caused by Ebola virus, dengue virus, severe acute respiratory syndrome coronavirus (SARS-CoV), Zika virus and Middle Eastern respiratory syndrome coronavirus (MERS-CoV) has led to the establishment of a rationale for synergistic drug combinations [9,20,32,47,213]. For example, the use of sofosbuvir plus ribavirin treatment, which is a combination of a nucleotide polymerase inhibitor and a synthetic antiviral nucleoside analogue approved for hepatitis C infection by the Food and Drug Administration (FDA) [187], has been repurposed for treating Zika virus infection. The two antiviral drugs were all tested in vivo for Zika disease and showed promising efficacy [22,57,160], thus prompting the clinical testing [16]. The antiprotozoal nitazoxanide developed by Romark Laboratories® is another important example [1,6]. Nitazoxanide is a thiazolide that triggers the host immune response by potentiating interferon release, interfering with post-translational stage of viral haemagglutinin and intracellular trafficking [157,191]. This drug showed potential properties with its broad-spectrum effects on other microbes encompassing Mycobacterium leprae [10], human norovirus [40] and Ebola virus [95]. Additionally, it has also been repurposed for influenza and is being tested in late-phase clinical trials ([79] (for details, see: NCT03336619, NCT02612922, NCT01227421 and NCT01610245). Meanwhile, nitazoxanide is presently under investigation in two randomised clinical trials (NCT04343248 and NCT04341493) for COVID-19. Romark® is a phase III, randomised, double-blind, placebo-controlled trial evaluating the efficacy and safety of nitazoxanide (twice daily for 6 weeks) for post-exposure prophylaxis of COVID-19 in 300 patients (NCT04343248). A phase IV trial is already currently enroling patients with COVID-19 and will compare nitazoxanide-hydroxychloroquine to hydroxychloroquine alone (NCT04341493). Hydroxychloroquine is an antimalarial drug that has been shown to interfere with the late-stage entry process of viruses based on cellular endocytic pathways. It is currently being investigated worldwide in more than 70 urgent clinical trials for COVID-19 (https://www.clinicaltrials.gov; accessed 14-04-2020). However, emerging evidence from a randomised and controlled phase III trial comparing hydroxychloroquine alone or with azithromycin versus standard care treatments for hospitalised patients with mild to moderate disease did not show clinically significant improvements after 15 d of treatment [25]. In the real world, similar results were obtained in an observational study with a larger cohort [68]. Notably, the latest meta-analysis with Bayesian examination of 29 studies including three randomised trials on this topic found evidence against the use of hydroxychloroquine alone or in combination with azithromycin in this setting [58].
3. Druggable targets in COVID-19-host signalling pathways
COVID-19 exhibits mild to moderate symptoms, which may progress into severe pneumonia, with lymphopenia, and an exhausted cytokine release syndrome [24]. The disease is caused by a novel strain of coronaviruses (SARS-CoV-2) that emerged in November–December 2019, probably naturally selected in an animal host before zoonotic transfer. However, SARS-CoV-2 has then crossed the animal species barriers and acquired the capacity of human-to-human infection, resulting in a rapid spread of COVID-19 at a pandemic proportion [83].
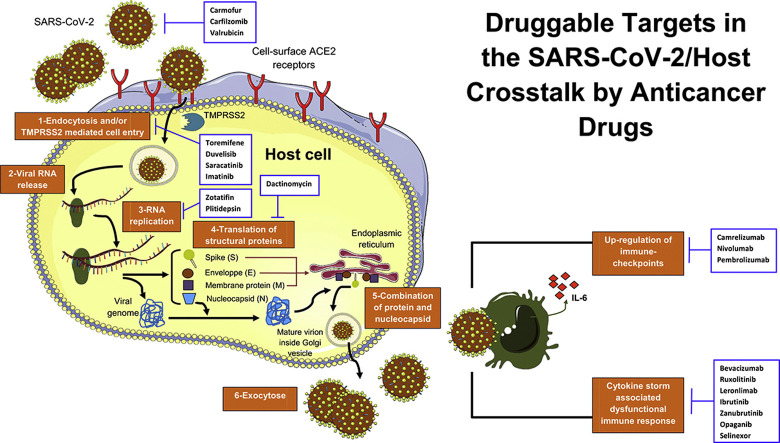
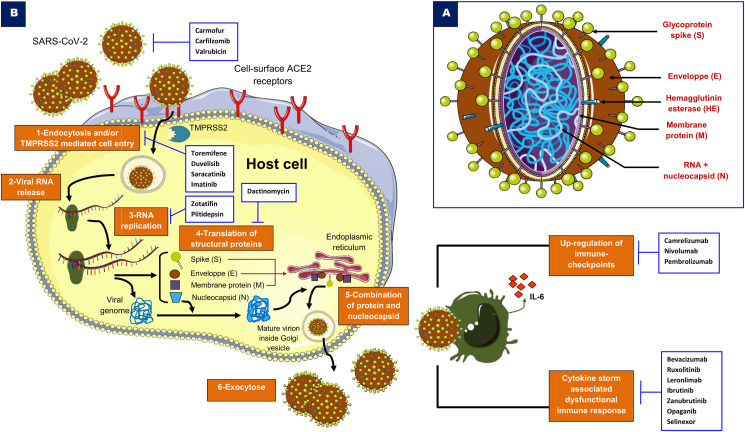
The SARS-CoV-2 is closely related to the previous strain SARS-CoV-1 that has caused the SARS epidemic in China during the period 2002–2004 [216]. SARS-CoV-2 is an enveloped, non-segmented and single-stranded RNA virus and belongs to the sarbecovirus subgenus of the Coronaviridae family [226]. The ultrastructure of SARS-CoV-2 is characterised by four key proteins, namely surface spike S glycoprotein, envelope protein E, membrane protein (M) and nucleocapsid protein (N) (Fig. 1 A).
Fig. 1.
A: SARS-CoV-2 structure. B: A simplified illustration of the targetable pathways by anticancer drugs in COVID-19. For details, see text. Abbreviations: ACE2: angiotensin-converting enzyme 2, IL-6: interleukin 6, RNA: ribonucleic acid, TMPRSS2: transmembrane protease serine 2.
The mechanisms of infectivity and pathogenesis of this coronavirus are poorly known and seem to be similar to those of SARS-CoV and MERS-CoV [106] (Fig. 1B). The host cell entry of SARS-CoV-2 depends on angiotensin-converting enzyme 2 (ACE2) and transmembrane protease serine 2 (TMPRSS2) [86,205,207]. Human cells with rich cell-surface receptor ACE2, an important modulator involved in the blood pressure regulation, are susceptible to SARS-CoV-2 infection. According to the recently emerged evidence, the elucidated crystal structure of SARS-CoV-2 showed that its receptor-binding domain (RBD) has an enhanced affinity to bind to the human ACE2 [167]. The receptor-binding motif of the RBD mediates the contact with ACE2. Two virus-binding hotspots at the RBD–ACE interface are stabilised by several residue changes of amino-acids in SARS-CoV-2 as compared to the previous SARS-CoV-1 [167]. The entry into cells requires the protein S of the viral spike that binds to the receptor ACE2 facilitated by some host cell proteases, such as TMPRSS2 [71,173]. This phenomenon, based on protein S priming, enables fusion of viral and cellular membranes and escapes from antiviral humoral immune response [71,173]. This interaction seems to be targetable by inhibiting host proteases [86,169].
The marketed orally bioavailable camostat mesylate approved in Japan for the treatment of chronic pancreatitis and reflux oesophagitis after gastrectomy [101,123] demonstrated a significant inhibition of TMPRSS2 [86], suggesting a potential role to treat COVID-19. These effects were also observed in prostate cancer in which TMPRSS2 inhibition by camostat was associated with suppression of metastasis in vivo [109]. In addition, the use of soluble human ACE2 can inhibit early stage SARS-CoV-2 infections as revealed by a recent study including human organoids, by using the recombinant ACE2 as a decoy receptor [121]. Fig. 1B shows an overview about the potential targets for COVID-19 therapy.
After the fusion of viral and host cell membranes, the virus accesses by the endosomal pathway, facilitated by cathepsins (cysteine pH-dependent proteases) [18,144]. The virus dissembles and releases its nucleocapsid and RNA material into the host cell cytoplasm via caveolae- and clathrin-mediated endocytosis [215]. This pathway involves phosphatidylinositol binding clathrin assembly protein (PICALM), which is a cargo-selecting clathrin adaptor that drives membrane curvature during endocytosis [90,119]. The PICALM is a target of chloroquine that suppresses its expression [90,209], inhibiting in this way the clathrin-mediated endocytosis process. The genomic RNA of SARS-CoV-2 contains open reading frames, which are translated into two replicase polyproteins (pp1a and pp1b) [55]. Therefore, the replicase transcriptase complex (RTC) is generated by processing pp1a and pp1b to produce non-structural proteins (16 putative NSPs), which form RNA polymerase and helicase that have a central role in the transcription and replication of coronaviruses [26,195]. The RTC attaches to the endoplasmic reticulum (ER) membranes and produces double-membrane vesicles of the coronavirus. A part of the RTC undergoes other molecular changes for mRNA synthesis and splicing to generate the structural and accessory proteins of the virus. The glycoproteins of the envelope will reach the ER and Golgi apparatus in which they form with the nucleocapsid protein and the viral RNA the vesicle containing the virion [26]. After assembly via the ER–Golgi intermediate compartment, the release of the new virion-containing vesicles from the infected host cells is achieved by exocytosis [26,31]. An alternative cell surface non-endosomal route for coronavirus entry involves TMPRSS2 that cleaves the spike glycoprotein into S1 and S2 subunits [26]. This enables a fusion of the membranes of the coronavirus and target cell. Of note, TMPRSS2 is druggable by camostat that demonstrated efficacy as a single treatment in lung cell entry blockade of MERS-CoV [172].
The SARS-CoV-2 action on host immunity encompasses innate and adaptive immune response activation. Unfortunately, local and systemic uncontrolled inflammatory response as well as altered adaptive immune reactions can damage organ tissues [24]. Leukopenia and lymphopenia with reduced natural killer (NK) cells in addition to increased C-reactive protein can be seen in patients with severe COVID-19 [24,27]. A marked decrease of total CD8+ T cells and NK translates the early failure of antiviral immunity during SARS-CoV-2 infection [223]. Upregulation of the expression of CD94/NK group 2 member A (NKG2A) by these immune cells suggested a functional exhaustion of cytotoxic lymphocytes in the development of COVID-19 [223]. These features can result in high mortality and more severity of the disease [27,91,92,146].
Elevated serum levels of pro-inflammatory cytokines (known as cytokine release syndrome or cytokine storm), such as tumour-necrosis factor alpha (TNF-α) and interleukin 6 (IL-6), seem to cause multiple organ failure by damaging tissues, contributing to the poor outcomes in COVID-19 patients [14,24,70,117,159,210,218,220]. Notably, immunosuppression obtained with drugs targeting IL-6 axis such as tocilizumab (Actemra®, Genentech) or siltuximab (Sylvant®, Janssen) has been proposed [61,117,134,221]. Based on these assumptions, several phase III trials are currently ongoing (NCT04320615, NCT04345445 and NCT04330638). However, at least for tocilizumab, the clinical use in hospitalised patients with severe COVID-19 has not met the expectations in randomised clinical trials (COVACTA/NCT04320615), and areas of implementation are now explored to identify possible predictive biomarkers of benefit for a subset of selected patients [124]. Finally, the pro-inflammatory microenvironment in COVID-19-associated lung failure induces massive infiltration of immune cells and damages the alveolar tissues. This mechanism increases vascular permeability and alveolar fluids accumulation, which is a hallmark of the deadly acute respiratory distress syndrome (ARDS) [115]. Both the mechanisms of immune-escape in COVID-19 might be potentially targetable and should be urgently delineated.
4. Potential anticancer drugs for COVID-19
4.1. Ruxolitinib
Ruxolitinib is a potent orally bioavailable Janus kinase (JAK) 1 and JAK2 inhibitor indicated for various myeloproliferative malignancies including myelofibrosis and polycythemia vera [3,198,200]. These neoplasms, alongside with the acute myeloid leukaemia, are known by their aberrant activation of the JAK-STAT signalling [199]. This pathway plays a pivotal role in cell proliferation and survival and seems to be targetable for a spectrum of conditions beyond malignancies.
Regarding COVID-19, ruxolitinib was recently repurposed to quell the immune-hyperactivation, thus dampening the cytokine storm and improving ARDS. An artificial intelligence-driven study has identified ruxolitinib as a promising drug for COVID-19 along with other molecules capable to disrupt the JAK-STAT signalling, used for myelofibrosis and rheumatoid arthritis, such as baricitinib and fedratinib [181]. The clinical trials using ruxolitinib for COVID-19 are summarised in Table 1 . In particular, ruxolitinib is being investigated against placebo for COVID-19 patients with ARDS in two randomised phase III trials (NCT04362137 and NCT04377620). The RUXCOVID study (n = 402, NCT04362137) is a phase III, multicentred, randomised (2:1) and placebo-controlled trial, which evaluates the safety and efficacy of ruxolitinib added to best supportive care in patients with cytokine storm. The RUXCOVID-DEVENT (NCT04377620) is a phase III, placebo-controlled trial, which is currently evaluating ruxolitinib for the treatment of COVID-19 patients with ARDS requiring mechanical ventilation. Both the phase III trials are designed for clinically critical patients, aiming to tackle the pathological immune response and control the hyper-inflammatory syndrome in more severe patients. In addition, another ongoing phase III trial (NCT04424056) combines multiple immune suppressors including anakinra (an anti-IL-1 inhibitor), tocilizumab, in association with ruxolitinib for more severe COVID-19 cases, including ARDS, associated with possible cytokine storm and other immune dysregulations.
Table 1.
Summary of potential anticancer drugs for repurposing in COVID-19.
| Anticancer drug | Mechanism of action | Viral–host targets | Tested in clinical trials for COVID-19? (country) | NCT identifier | Eligible population | Primary end-point | Estimated completion date |
|---|---|---|---|---|---|---|---|
| Ruxolitinib | Reduction of hyperinflammation during cytokine storm | JAK-STAT pathway | Yes (USA, Germany, France, Mexico, Canada, Italy and Spain) | NCT04348071 | Hospitalised patients | Cumulative incidence of Grade 3 and 4 AE; eight-point ordinal scale | October 2021 |
| NCT04359290 | Severe lung disease | Overall survival | July 2021 | ||||
| NCT04355793 | Hospitalised patients | Description of the disease course | Not provided | ||||
| NCT04354714 | Critical disease | Overall survival | Withdrawn | ||||
| NCT04362137 | Hospitalised patients | Proportion of patients who die, develop respiratory failure or require ICU care | November 2020 | ||||
| NCT04377620 | COVID-19-associated ARDS | Overall survival | October 2020 | ||||
| NCT04366232 | Hypoxic pneumonia, ARDS or end-stage organ failure | Biological criteria of response, based on biomarkers change∗ | September 2020 | ||||
| NCT04334044 | Pneumonia with dyspnea | Recovery of pneumonia | June 2020 | ||||
| NCT04374149 | Critical disease | Overall response rate | April 2021 | ||||
| NCT04338958 | Severe lung disease | Overall response rate | August 2021 | ||||
| NCT04337359 | Critical disease | Description of the disease course | Not provided | ||||
| NCT04331665 | Disease requiring O2-support | Rate of clinical worsening | February 2021 | ||||
| NCT04361903 | Disease requiring O2-support | Number of patients who avoid mechanical-assisted ventilation | June 2020 | ||||
| NCT04348695 | Hospitalised patients | Rate of clinical worsening | May 2020 | ||||
| NCT04424056 |
|
Ventilation-free days at day 28 | November 2022 | ||||
| Bevacizumab | Vascular permeability inhibition | VEGF | Yes (France and China) | NCT04275414 | Severe lung disease or critical disease | Change of PaO2 to FiO2 ratio | May 2020 |
| NCT04344782 | Severe lung disease | Number of patients who avoid mechanical-assisted ventilation | December 2020 | ||||
| NCT04305106 | Disease requiring O2-support | Time to clinical improvement | August 2020 | ||||
| Carmofur | Blockade of viral replication | SARS-CoV-2 main protease | No | – | – | – | – |
| Carfilzomib | Blockade of viral replication | SARS-CoV-2 main protease | No | – | – | – | – |
| Toremifene | Inhibition of viral membranes fusion with host cell endosomes | Interaction with coronavirus proteins | No | – | – | – | – |
| Zotatifin | Inhibition of protein biogenesis | Blockade of eIF4A | No | – | – | – | – |
| Plitidepsin | Interference with the viral cycle | Blockade of eEF1A | Yes (Spain) | NCT04382066 | Hospitalised patients | Frequency of occurrence of Grade 3 or higher AEs | November 2020 |
| Dactinomycin | Inhibition of viral cellular transcription | Inhibition of DNA topoisomerase | No | – | – | – | – |
| Valrubicin | Blockade of viral replication | SARS-CoV-2 main protease | No | – | – | – | – |
| Leronlimab | Immune homeostasis restoration | Disruption of the CCL5/RANTES-CCR5 pathway | Yes (USA) | NCT04343651 | Mild/moderate disease | Clinical improvement | September 2020 |
| NCT04347239 | Severe lung disease or critical disease | Overall survival | April 2021 | ||||
| Camrelizumab | Immune homeostasis restoration | PD-1/PD-L1 pathway blockade | Yes (China) | ChiCTR2000029806 | Severe lung disease | Proportion of patients with a lung injury score reduction | February 2021 |
| Nivolumab | Immune homeostasis restoration | PD-1/PD-L1 pathway blockade | Yes (France and China) | NCT04343144 | Disease requiring O2-support | Time to clinical improvement | October 2020 |
| NCT04333914 | Disease requiring O2-support | 28-day survival rate | Suspended | ||||
| NCT04356508 | Clinically stable patients with mild or moderate disease and asymptomatic patients | Viral clearance kinetics | September 2021 | ||||
| Pembrolizumab | Immune homeostasis restoration | PD-1/PD-L1 pathway blockade | Yes (Spain) | NCT04335305 | Severe lung disease or critical disease | Percentage of patients with normalisation of SpO2 ≥96% on room air | September 2020 |
| Imatinib | Blockade of cell entry and endosomal trafficking | BCR/ABL kinase inhibition | Yes (France, Spain and USA) | NCT04357613 | Hospitalised patients | Rate of prevented severe disease worsening | December 2021 |
| NCT04346147 | Disease diagnosed <7 d | Time to clinical improvement | September 2020 | ||||
| NCT04394416 | Hospitalised patients diagnosed <7 d | All-cause mortality | June 2023 | ||||
| NCT04356495 | Outpatients, diagnosed <5 d | Hospitalisation rate, death rate | February 2021 | ||||
| Duvelisib | Immune homeostasis restoration and viral replication inhibition | PI3K inhibition | Yes (USA) | NCT04372602 | Critical disease | Overall survival | April 2022 |
| Zanubrutinib | Protection against immune, lethal and sepsis-induced pulmonary injuries | Inhibition of the Bruton tyrosine kinase | Yes (USA) | NCT04382586 | Disease requiring O2-support | Respiratory failure-free survival rate | April 2021 |
| Ibrutinib | Protection against immune-induced lung injury | Inhibition of the Bruton tyrosine kinase | Yes (USA) | NCT04375397 | Hospitalised patients with severe pneumonia | Respiratory failure-free survival rate, overall survival | April 2021 |
| Opaganib | Anti-inflammatory and antiviral properties | Inhibition of sphingosine kinase-2 | Yes (Israel) | NCT04414618 | Disease requiring O2-support | Measurement of the daily O2 requirements | January 2021 |
| Saracatinib | Antiviral properties | Possible blockade of endocytic pathways and nucleocapsid protein | No | – | – | – | – |
| Selinexor | Antiviral and anti-inflammatory properties | Blockade of nucleocytoplasmic transport | Yes (USA, France, Austria, Spain and United Kingdom) | NCT04355676 | Hospitalised patients with moderate or severe disease | Percentage of participants with at least a two-point improvement in the ordinal scale | September 2020 |
| NCT04349098 | Hospitalised patients with severe disease | Idem | Idem |
Abbreviations: AE: adverse event, FiO2: fraction of inspiration O2, ICU: intensive care unit, O2: oxygen, PaO2: partial arterial oxygen pressure, VEGF: vascular endothelial growth factor. ∗ C-reactive protein, ferritinemia, creatininemia, transaminases, eosinophil count and lymphocyte count.
4.2. Carmofur
Carmofur (1-hexylcarbamoyl-5-fluorouracil) is an old pyrimidine analogue and a lipophilic masked compound of fluorouracil. Its mechanism of action is independent of 5-fluorouracil-antitumour activity, based on the inhibition of the lysosomal acid ceramidase [43,148]. The upregulation of acid ceramidase is linked to survival and growth of cancer cells as well as drug resistance [23]. This enzyme is an emerging target for several malignancies [74], such as prostate cancer [108] and glioblastoma [130] as well as paediatric brain tumours [45]. In addition, carmofur exerts its antiproliferative and antimetastatic effects through the blockade of the Wnt/β-catenin signalling pathway [107]. This molecule has been previously tested in clinical trials for the treatment of colon cancer as single or in combination with other chemotherapeutics [162] and in early breast cancer [122]. Recently, carmofur has been found to block SARS-CoV-2 replication by inhibiting the viral protease through the covalent binding between the carbonyl reactive group and the catalytic Cys145. In another molecular modelling report, Gao et al. indicated that carmofur strongly bound to SARS-CoV-2 Mpro [63]. This suggests that repurposing low-molecular weight drugs such as carmofur against the main protease of SARS-CoV-2 based on in silico studies may show a therapeutic potential. Importantly, the identification of the specific mechanisms of viral blockade provided the paradigm to design analogues of carmofur, with improved efficacy for COVID-19 and less neurotoxicity. This last aspect is particularly relevant because of its association with drug-related leukoencephalopathy.
4.3. Bevacizumab
Bevacizumab is a monoclonal antibody with antiangiogenic properties, capable to target the vascular endothelial growth factor [64]. This biological agent has been successfully implemented in the clinic management of various cancer types, including advanced ovarian cancer, renal cell carcinoma as well as colorectal cancer in combination with chemotherapy [49,64]. The key mechanism of bevacizumab is related to the disruption of the malignant neo-angiogenesis, ultimately reducing the abnormal vascular permeability and nutrients supply to cancer cells. Based on this mechanism [15,208], the use of bevacizumab has been proposed for the control of the abnormal angiogenesis and the related vascular permeability undermining the accumulation of intra-alveolar fibrin deposition, resulting in ARDS and occurring during COVID-19 disease. A recent study found that new vessel growth induced by intussusceptive angiogenesis in the lungs of COVID-19 patients is approximately three times higher than in the lungs of patients with influenza [2]. Therefore, bevacizumab is being investigated also in this field (NCT04344782, NCT04305106 and NCT04275414). More in detail, the BEST-CP clinical trial (NCT04275414) (n = 20) is a Chinese pilot study that is currently recruiting patients with COVID-19 to be treated with intravenous bevacizumab (500 mg flat dose) with the aim to control the pulmonary oedema. Another multicentred and randomised trial in China is investigating the safety and efficacy of bevacizumab (7.5 mg/kg) in COVID-19 patients with severe pneumonia (NCT04305106). Additionally, the French CORIMMUNO-BEVA phase II trial (NCT04344782) is randomising COVID-19 patients to receive bevacizumab (7.5 mg/kg) versus best of the standard supportive care (Table 1). Based on the proposed mechanism of action, the use of bevacizumab is now included in clinical trials for more severe patients, presenting with the clinical features of ARDS.
4.4. Carfilzomib
Carfilzomib is an approved antineoplastic drug for the treatment of relapsed or refractory multiple myeloma patients who have received ≤3 previous lines of therapies [170,183,214]. It has been investigated in several clinical trials for other malignancies such as Hodgkin lymphoma (NCT02867618) and non-Hodgkin lymphoma like in diffuse large B-cell lymphoma (NCT02073097) and mantle cell lymphoma (NCT03891355). In detail, carfilzomib is a second-generation antiproteasome with higher maximal percentage of proteasome inhibition at the maximum tolerated dose than bortezomib [103]. This mechanism leads to the irreversible inhibition of the 26S proteasome, which plays a key role in the hallmarks of myeloma, by binding to its chymotrypsin-like site of the proteolytic core [62,227]. The multiple targets of proteasome targeting encompass the NF-κB pathway, ER stress, cell-cycle arrest, downregulation of growth factor receptors and adhesion molecules expression, angiogenesis inhibition as well as immunogenic cell death [103]. Carfilzomib was recently repurposed for COVID-19 based on virtual docking screening, a structure-based virtual screening method for molecules capable of binding specific proteins in key binding sites or domains [206]. Among several other promising drugs, including eravacycline, elbasvir, lopinavir and valrubicin, carfilzomib was excellent in terms of binding free energy, which is an important parameter for a rational drug design targeting the SARS-CoV-2 main protease, anticipating a potent antiviral activity in silico [206].
4.5. Toremifene
Toremifene is a selective modulator of oestrogen receptors used in the endocrine therapy of breast cancer since 1997 [201]. Toremifene represents an alternative drug to tamoxifen, showing a similar efficacy in patients with advanced breast cancer [113]. Additionally, this antioestrogen drug was shown to destabilise the envelope glycoprotein of Ebola virus [222], leading to the inhibition of the fusion between viral and endosome membranes during intracellular trafficking [222]. The subsequent in vivo studies confirmed the anti-Ebola activity of toremifene regardless of its oestrogen-modulating activity, by inhibiting virus entry and internalisation [97].
Finally, a recent in silico study identified toremifene as a potential therapeutic approach against SARS-CoV-2, particularly when combined to emodin [225]. Emodin is a natural-derived compound with anticoronavirus pharmacological properties, belonging to the traditional Chinese medicine Lianhua Qingwen used for the prevention and treatment of viral influenza [84,165]. The authors suggested that toremifene interacts with several key host coronavirus proteins including ribosomal proteins like RPL19, HNRNPA1, NPM1, EIF3I, EIF3F and EIF3E [225]. However, the exact mechanism of its activity is still unclear. Based on the action on viral entry, the use of this agent can be proposed for prophylaxis of the infection and in patients with milder disease, in the earlier stages of infection.
4.6. Zotatifin
Zotatifin is a potent and selective small molecule inhibiting the eukaryotic initiation factor 4A (eIF4A) [190]. eIF4A is an RNA helicase activated by the B-cell receptor to stimulate several oncogenes linked to tumour growth, cell survival and metastasis [82,190]. The inhibition of this target by zotatifin and other synthetic molecules demonstrated promising antineoplastic preclinical efficacy [28,125,190]. For example, zotatifin is being studied in a phase I/II dose-escalation and cohort expansion clinical trial (n = 45) as monotherapy for pancreatic cancer and other malignancies (NCT04092673). Promisingly, a recent study published in Nature suggests a potential of zotatifin repurposing for COVID-19 [73]. Based on affinity-purification mass spectrometry, the authors revealed 332 SARS-CoV-2-human protein–protein interactions after cloning and expressing the key SARS-CoV-2 proteins in human cells [73]. Importantly, 66 targetable human proteins were identified to be pharmacologically druggable by 69 compounds including 29 FDA-approved drugs [73]. Several of these compounds exhibited antiviral activities against mRNA translation and Sigma1 and Sigma2 virus receptors expressed on host cells [73]. In this study, zotatifin emerged as an effective drug in reducing viral infectivity by inhibiting protein biogenesis through eIF4A blockade along with other compounds such as ternatin-4 [73,135]. The proposed disruption of the translation of the Sigma proteins coupled to the direct inhibitory activity against them seems a promising strategy of drug targeting against SARS-CoV-2 infection and propagation, possibly indicated in the earlier stages of the infection.
4.7. Plitidepsin
Plitidepsin (also known as dehydrodidemnin B) is a natural compound discovered in a Caribbean marine organism (Aplidium albicans) [48]. Its antitumour effects encompass several cancer targets such as angiogenesis and cell survival [5,72]. Additionally, plitidepsin may also enhance radiotherapy response by a bystander effect [155]. The activation of c-Jun N-terminal kinase is a key mediator of plitidepsin sensitivity along with the eukaryotic elongation factor 1 alpha 2 that were recently suggested as potential predictive biomarkers of drug response [50,126]. So far, the drug has been investigated in several clinical trials for multiple myeloma, lymphoma, liposarcoma and prostate cancer [48]. Only one phase III randomised and controlled trial has evaluated the clinical activity of plitidepsin combined to dexamethasone in relapsed/refractory multiple myeloma (NCT01102426/ADMYRE), showing mild improvements in survival outcomes [180]. The development of this drug has been halted or terminated because of the arrival of competitive targeted agents as well as the poor enrolment in clinical trials.
On the other hand, plitidepsin has also antiviral properties, showing promising findings in the COVID-19 treatment on in vitro models (Press release 1, [136,137]). According to these models, plitidepsin blocks the eEF1A protein in human cells that is required for SARS-CoV-2 infection (Press release 2, [136,137]). Therefore, the drug is being tested in a phase I Spanish trial (NCT04382066/APLICOV) with the aim to assess the safety and early efficacy of three doses of plitidepsin (1.5, 2.0 and 2.5 mg/day) for three consecutive days in patients requiring hospitalisation for COVID-19 (n = 27). Based on the activity on a key mechanism of the viral pathogenesis, the use of this drug can be considered either in the earlier stages of infection, in a multidrug regimen and for patients with more advanced disease, together with proper immunomodulators.
4.8. Dactinomycin
Historically, the anticancer drug dactinomycin was discovered in Streptomyces parvulus bacterium by Selman Waksman and colleagues in the 1940s [203] and launched after its initial FDA-approval in 1964. This intravenously administered cytotoxic antibiotic acts by intercalation to DNA and interruption of RNA transcription [38,87]. Recently, dactinomycin was shown to induce immunogenic cell death, suggesting a direct antineoplastic activity enhanced by immunomodulatory properties [93]. In fact, this drug is used in combination with other cytotoxic drugs in the management of a broad array of solid malignancies, such as gestational trophoblastic disease, Wilms’ tumour and Ewing sarcoma [4,67,105,196].
The use of dactinomycin against coronaviruses was firstly described in 1979 by Kennedy et al. in the context of alpha-coronavirus 229E, involved in a spectrum of respiratory diseases from upper to lower airways infections [98]. Later on, Lewis et al. showed a potent inhibition of the feline enteric coronavirus strain 79-1683 in whole feline embryo cells [104]. Based on these and subsequent evidence, dactinomycin was widely used as an in vitro inhibitor of viral cellular transcription in infected cells [197]. Recently, a combination of dactinomycin and sirolimus showed a synergistic action against SARS-CoV-2-host proteins [225]. In fact, the association may inhibit DNA topoisomerase required for RNA synthesis as well as mammalian target of rapamycin (mTOR) signalling in human coronavirus infected cells [225]. Notably, the mTOR pathway is a target of sirolimus that has also shown a promising activity against MERS-CoV in addition to its known anticancer, immunosuppressive and antifungal properties [100]. However, these results for SARS-CoV-2 are limited by their in silico nature and warrant more clinical experiences beyond speculations. In addition, the use of this antibiotic is associated with an adverse safety profile when used at therapeutic doses as an antineoplastic agent that may be less suitable for severely ill patients with an acute infectious disease. In particular, the emetogenesis and myelosuppression can deserve a special consideration, and antiviral doses may be acceptable only if lower, as expecting a better safety profile.
4.9. Valrubicin
Valrubicin is a semisynthetic derivative of the anthracycline doxorubicin, used as intravesical topic treatment for the management of refractory non-muscle invasive bladder cancer [168]. The mechanism of action of valrubicin affects DNA synthesis and metabolism by inhibiting the incorporation of nucleosides and topoisomerase II [13,168]. Based on computational hierarchical virtual screening, recently Wang showed that valrubicin alongside with other drugs, such as lopinavir and carfilzomib inhibits the main protease of SARS-CoV-2 [206]. Moreover, another report based on a machine-learning simulation has recently shown that valrubicin is a potential COVID-19 protease inhibitor [99]. However, no considerations on the effective doses have been modelled: the clinical use as an agent for systemic administration is not validated, being approved only as a topic solution, and any speculation for the design of clinical trials should account for the adverse safety profile of this chemotherapeutic.
4.10. Leronlimab
Leronlimab (PRO 140) is a humanised monoclonal antibody targeting the C–C chemokine receptor type 5 (CCR5) on T lymphocytes [96]. The role of CCR5 in the immunopathology of disease has been extensively studied since its discovery as a co-receptor for human immunodeficiency virus entry in 1996 [21] (NCT03902522 and NCT02859961). In addition, CCR5 is also expressed on a broad array of cancers and it is linked to various cancer hallmarks, particularly “avoiding immune destruction” [96]. It is under investigation in trials for few cancers, such as metastatic colon and breast cancers (NCT03838367), for which several other inhibitors of CCR5 are being developed (reviewed elsewhere: [96]. Previously, Chen et al. demonstrated that mRNA transcripts of CCR5 and other chemokine receptors were found upregulated in animals infected by SARS-CoV [30]. Additionally, enhanced production of these mediators was associated with recruitment of inflammatory cells and T lymphocytes influx into the lung parenchyma [30].
PRO 140 has shown a promising antiviral potency with a high barrier to resistance and encouraging short-term safety and tolerability [189]. A recently published Preprint suggested that leronlimab disrupts the CCL5/RANTES-CCR5 axis and restores immune homeostasis in COVID-19 patients [133]. The authors observed a marked CCR5 receptor occupancy on macrophages and T lymphocytes, rapid drop of circulating IL-6, CD4/CD8 ratio restoration and a significant decrease of patients’ viremia [133]. These encouraging findings boosted the rapid entrance of leronlimab into two clinical trials for mild to moderate and severe COVID-19 infection (NCT04343651 and NCT04347239). The largest trial using this drug is a randomised, double-blind and placebo-controlled, two-arm phase IIb/III trial investigating weekly leronlimab at a dose of 700 mg given subcutaneously (NCT04347239). A multicentred enrolment will include 390 patients with severe or critical disease with a mortality rate after four weeks of treatment as a primary end-point. Likewise, the other study is a phase II (n = 75) with a similar design that will enrol patients with mild to moderate symptoms (NCT04343651). Patients will be randomised to receive leronlimab (700 mg) or placebo weekly in a 2:1 ratio with changes in total symptom score based on fever, myalgia, dyspnea and cough at day 14 as a primary end-point. Notably, the other outcome measures in this clinical trial include serum changes of cytokines and chemokines from baseline, CCR5 occupancy levels on macrophages and Treg cells and CD3+, CD4+ and CD8+ T cell count from baseline. Based on the specific action in-between the viral pathogenesis and the immune-dysregulation, the use of this agent has been proposed for patients with mild clinical COVID-19 to prevent severe outcomes and reduce the related mortality.
4.11. Antineoplastic immune-checkpoint inhibitors
Based on the hypothesis suggesting the upregulation of immune-checkpoints such as PD-1/PD-L1, cytotoxic T lymphocyte antigen-4 (CTLA-4), lymphocyte activation-gene-3 (LAG-3) and T cell membrane protein-3 (TIM-3) pathways in excessive inflammatory response during sepsis [132], these types of drugs were repurposed beyond their use for the treatment of cancers [132,147]. Importantly, a recent meta-analysis including four studies showed PD-1 blockade improves patient outcome in sepsis [219].
Camrelizumab is a humanised IgG4-kappa antiprogrammed cell death 1 (PD-1) monoclonal antibody used in cancer immunotherapy [114]. The blockade of PD-1 binding to its ligand PD-L1 enables reactivation of cell-mediated immune response against cancer cells [114]. Camrelizumab was tested in phases II/III clinical trials for advanced or metastatic oesophageal squamous cell carcinoma [91,92], osteosarcoma [212], relapsed or refractory classical Hodgkin lymphoma [177,178] and advanced hepatocellular carcinoma [145]. It received its first approval in China for treating RRHCL patients who received at least two lines of chemotherapy [114]. Additionally, it is currently being studied in two ongoing phase III trials for local advanced nasopharyngeal carcinoma (NCT03427827) and gastric and gastroesophageal junction adenocarcinoma (NCT04208347). The use of anti-PD-1 inhibitor camrelizumab in COVID-19 patients with pneumonia is being evaluated in a Chinese clinical trial (n = 120; ChiCTR2000029806) in comparison with the standard supportive care and the immunomodulatory agent thymosin.
Nivolumab and pembrolizumab are other immune-checkpoint inhibitors (ICIs) that were successfully introduced into the management of various solid cancers, particularly for melanoma [110,204]. These ICIs are both humanised and fully human anti-PD-1 IgG4 monoclonal antibodies. Their marked clinical activity depends on PD-L1 expression in some tumours, as well as on emerging predictive biomarkers, such as tumour-mutational burden and microbiota that seem to mediate drug response [51,81,158].
Nivolumab is currently used in a multicentred phase II trial comparing its efficacy against tocilizumab, GNS561 (a chloroquine analogue), and standard supportive care in advanced or metastatic cancer patients with COVID-19 (NCT04333914). A total of 273 participants will be randomised to receive one of three treatments based on two cohorts. Patients that are asymptomatic or with mild disease will be prospectively assigned to the group comparing nivolumab versus GNS561 versus standard of care in a 1:1:1 ratio. Similarly, in patients with moderate to severe symptoms, nivolumab will be compared to GNS561 and tocilizumab. Survival after four weeks of randomised treatment is the primary end-point in this trial. Moreover, another ongoing French phase II trial (n = 92) is investigating the safety and efficacy of nivolumab in COVID-19 patients requiring hospitalisation (NCT04343144). Patients will be randomly allocated to receive nivolumab or standard of care (1:1 ratio). One pilot trial is also testing nivolumab in the earlier setting of COVID-19, in patients presenting with no or mild symptoms and not hospitalised – to examine the role of this ICI as an immune-enhancer, to accelerate the clearance of SARS-CoV-2 (NCT04356508).
Pembrolizumab was also repurposed for COVID-19. The COPERNICO is a multicentred two-arm phase II trial (NCT04335305) that is currently enroling patients with COVID-19 associated with pneumonia in Spain and who are non-responsive to frontline therapy. Twenty-four participants will be prospectively randomised to receive intravenous tocilizumab plus pembrolizumab or standard treatments (at physician's choice). The use of ICIs for COVID-19 is essentially intended as an immunomodulator, based on the proposed role of PD-L1 axis in the pathogenesis of ARDS and the expedited clearance of SARS-CoV-2 with anti-PD-L1 therapies [163]. Accordingly, the studies ongoing are testing these ICIs both for more severe cases, intending to attenuate the exuberant cytokine production [166], and for mild presentations, to prevent the onset of the hyper-immune pathological response and then dampen the viral replication [163]. Moreover, a recent report showed that PD-1 checkpoint inhibition in lung cancer patients who were diagnosed with COVID-19 does not appear to affect the prognostic outcomes [111]. Therefore, this provides a promising rationale for immunotherapy, which seems to have no unfavorable effects in this setting.
4.12. Kinase inhibitors
The blockade of kinase cascade is one of the successful targeted strategies in treating cancer in the modern era of oncology. Several kinase inhibitors including palbociclib, crizotinib, dabrafenib and imatinib, were introduced in practice for various cancers [69]. The rationale for using kinase inhibitors in COVID-19 is principally based on their ability to inhibit host intracellular-viral events particularly during trafficking induced during the viral life cycle [140]. Viruses commonly use a number of host kinases during their invasion and thus, they are considered potential targets for kinase inhibitors repurposing as broad-spectrum antivirals [66,164]. Several previous reports demonstrated potent antiviral properties for kinase inhibitors in various virus-induced diseases, including SARS-CoV infections [8,17,35,46,100,128,129].
Imatinib is a marketed tyrosine kinase inhibitor for chronic myeloid leukaemia and gastrointestinal stromal tumours in the first-line setting [54,85]. The BCR-ABL1 oncoprotein with kinase activity was found to mediate virus entry in several reports encompassing hepatitis C and Ebola viruses [65,120]. In coronaviruses, recent studies indicated that imatinib blocks cell–cell and cell–virus membrane fusions [36,175]. Additionally, in in vivo and ex-vivo mouse models, the use of imatinib protected the lungs from ischemia/reperfusion injury [112,187], attenuated inflammation and vascular leak [154] and prevented lung injury and death [182].
Clinically, the IMAGE-19 trial is a single-arm phase II study in France that will enrol 90 elderly COVID-19 patients (NCT04357613). Early imatinib therapy at a dose of 800 mg daily for two weeks will be evaluated to prevent the progression to severe disease in patients >70 years with COVID-19. The efficacy of imatinib at a dose of 100 mg is also being examined in combination with hydroxychloroquine in the Spanish Covid-19HUF phase II trial (n = 165) (NCT04346147). Participants will be randomised to receive either oral lopinavir/ritonavir (protease inhibitors), imatinib or baricitinib (JAK inhibitor), all combined to hydroxychloroquine. The trial completion date is estimated in September 2020.
The COVERAGE study is a large French multiarm phase III trial (NCT04356495) that will investigate the efficacy of imatinib and several other experimental treatments. A total of 1057 symptomatic COVID-19 patients will be randomly allocated to receive imatinib, or telmisartan, or favipiravir, or hydroxychloroquine as compared to a control group with the objective to reduce the risk of hospitalisation or death in elderly patients. Another phase III trial using imatinib versus placebo for hospitalised COVID-19 patients is currently recruiting in the United States (n = 204, NCT04394416). Given the acceptable safety profile of imatinib and the several available generics, this seems to be an effective strategy for clinical repurposing. The drug repurposing of imatinib in COVID-19 has been embedded for the early phases of the disease, commonly referred to as the “viral response” phase [174]. Imatinib seems to tackle the mechanisms of viral entry and cell–cell spread, functioning as an antiviral agent. In this initial stage, the drug development is intended to reduce the duration of the symptoms, minimise the contagiousness among people and prevent the progression to severe COVID-19.
Duvelisib is an orally bioavailable phosphatidylinositol 3-kinase (PI3K) selective inhibitor that is being studied for haematologic malignancies [94]. The PI3K–AKT–mTOR signalling is frequently dysregulated in cancer as well as during hyperinflammation and its therapeutic targeting is highly represented in ongoing clinical trials. Duvelisib was investigated in the DUO phase III trial and compared to ofatumumab for refractory and relapsed chronic lymphocytic leukaemia and small lymphocytic lymphoma [59]. It was approved by the FDA in 2018 for treating adult patients with relapsed or refractory chronic lymphocytic leukaemia or small lymphocytic leukaemia after at least two prior therapies [88]. There is currently one pilot phase II trial examining the efficacy of duvelisib twice daily at a dose of 25 mg for 10 d in COVID-19 patients as monotherapy (NCT04372602). This single-arm study will test the hypothesis that PI3K blockade could hamper the immune system hyperactivation and therefore reduce lung inflammation and interfere with the viral cycle. It is estimated to enrol 25 participants, with overall survival as a primary end-point. Notably, the PI3K inhibitors including the drug duvelisib can cause lung inflammation and increase the risk for infections, as consistently demonstrated for multiple compounds of this pharmacological class, and a special caution is warranted during the clinical trials using this class of molecules.
The aberrant activation of the Bruton tyrosine kinase has a key role in the tumourigenesis of B-cell lymphoma [77]. Its abrogation seems to protect lungs from acute immune-induced or lethal influenza-induced injuries [60,102] as well as sepsis-induced injury [224]. Zanubrutinib is an orally administered irreversible inhibitor of the Bruton tyrosine kinase [186,218,220]. This drug showed a durable and high overall response rate in patients with relapsed/refractory Mantle cell lymphoma [179]. Recently, zanubrutinib received accelerated FDA approval for treating adult patients with this rare and aggressive cancer who have received at least one line of therapy [186]. For COVID-19, a phase II trial in the United States is ongoing, aiming to dampen the disease-related immune dysregulation and hyper-inflammation (NCT04382586). This study is expected to randomise 52 participants to receive zanubrutinib combined to supportive care versus placebo and to test the hypothesis of increasing respiratory failure-free survival rates in patients with pulmonary distress. Ibrutinib is another inhibitor of Bruton kinase inhibitor, approved to treat indolent B-cell malignancies and chronic graft-versus-host disease, now being repurposed for COVID-19 [193]. An earlier experience with this drug in COVID-19 patients with cancer showed marked improvements and resolution or near resolution of COVID-19 symptoms during the follow-up [193]. In the same way, the iNSPIRE (NCT04375397) is a phase II that will enrol 46 COVID-19 patients to randomly receive ibrutinib versus placebo along with standard supportive care. The trialists expect that ibrutinib will reduce respiratory failure and their findings are expected to be released in September 2020.
Opaganib (ABC294640) is a first-in-class selective inhibitor of sphingosine kinase-2 (sk2). This key enzyme in lipid signalling promotes tumour growth, cell proliferation and cytokine production during inflammation [139,143,[176], [177], [178]]. It was previously noticed that sk2 is the host factor that impacts the viral replication process of the chikungunya virus [149] and maintains viral latency and survival in Kaposi's sarcoma-associated herpes virus [39]. This drug has an orphan drug designation for cholangiocarcinoma [89] and is being tested in clinical trials for patients with metastatic castration-resistant prostate cancer (NCT04207255) and advanced cholangiocarcinoma (NCT03377179). It is also under development for COVID-19-associated pneumonia in a phase II study (NCT04414618). This is a randomised, double-blind, placebo-controlled trial in 40 adult patients with positive pneumonia, using daily opaganib for two weeks.
Saracatinib (AZD0530) is a tyrosine kinase inhibitor of the c-SRC family of proto-oncogenes. This pathway mediates signal transduction for several cell functions such as proliferation, cell-to-cell transmission, epithelial-to-mesenchymal transition, vascular permeability, invasion and metastasis [33,131]. Moreover, it interferes with additional cell signalling such as PI3K/mTOR and MAPK [151]. Saracatinib selectively abrogates this enzyme and was investigated in several clinical trials for recurrent osteosarcoma [11], platinum-resistant ovarian cancer [116], unresectable thymic malignancy [76] and cancer-induced bone pain [41]. SRC kinases have also a role in endocytic trafficking [150] and there is a growing body of evidence indicating the inhibitory properties of viral replication of dengue viruses and MERS-CoV by the anticancer agent saracatinib [42,171,228]. This makes this drug a possible candidate for further investigations on other members of the Coronaviridae family. In a recently published Preprint, molecular modelling showed that saracatinib binds to the nucleocapsid protein sites of SARS-CoV-2 along with other previously discussed drugs such as imatinib and camostat [188]. To the best of our knowledge, saracatinib has not entered into any COVID-19 clinical trial. The clinical use of molecular blockers of PI3K–AKT–mTOR, Bruton tyrosine kinase, sk2 and c-SRC is anticipated to exert immune-modulating rather than antiviral effect. To date, the drug development of this group of tyrosine kinase inhibitors is included in the protocols for hospitalised patients with severe COVID-19, to rescue the immune-dysregulation and improve the outcome.
4.13. Selinexor
Selinexor is a first-in-class orally bioavailable inhibitor of Exportin-1 (XPO1) [185]. XPO1 is an export receptor for various proteins and RNA molecules with oncogenic properties and is frequently altered in cancer [7]. Moreover, it is implicated in several cancer pathways including cell growth and survival, differentiation and drug resistance [127]. XPO1 suppression by selinexor in human clinical trials demonstrated promising results for patients with heavily treated relapsed or refractory multiple myeloma [29,152,202]. The drug received first an accelerated approval by the FDA in the United States of America (USA) in 2019 in combination with dexamethasone [152]. The antiviral activity of selinexor is based on its potential to interfere with host targets including XPO1 that interacts with SARS-CoV-2 proteins [73,194]. In fact, blockade of this nucleocytoplasmic transporter can sequester viral materials in the host cell nucleus and therefore reduce its replication mechanisms and viral load [194]. Moreover, selinexor has also selective and potent anti-inflammatory effects on sepsis pathophysiology [211]. Based on these concepts, two phase II randomised and controlled trials of low dose selinexor were initiated for COVID-19 patients with moderate to severe disease (NCT04355676 and NCT04349098). The largest trial that is currently recruiting patients is single-blind and placebo-controlled and is expected to enrol 230 hospitalised participants with severe COVID-19 (NCT04349098).
5. Perspectives
The COVID-19 pandemic continues to spread globally with 24,484,968 cases and 832,533 deaths registered worldwide, including 6,016,069 cases only in the USA (as of 27th August 2020). New effective therapeutics are urgently needed, as the results from the first clinical trials or experiences with drugs for COVID-19 are mostly disappointing. There is a massive demand for drug repurposing in this rapidly evolving setting, relying on the benefit of a shorter drug development and the capability to turn preclinical intuitions in clinical experiences. In fact, it is a potential successful drug discovery process, more efficient and resource sparing than the classic drug development from early phases for new molecules, taking generally a longer time (typically 10–15 years). While drug repurposing is not an excuse to design low-quality clinical trials or emphasise biased results from preliminary findings and non-controlled cohorts, the possibility to use molecules with an established safety profile certainly represents an advantage. The US ClinicalTrials.gov database shows that ≥3100 clinical studies for COVID-19, including more than 1700 interventional trials (accessed as of 27-08-2020), are ongoing, portraying the commitment of the global medical community to accelerate the COVID-19 research agenda – in a successful interplay between laboratory researchers and clinical trialists, ultimately to serve clinicians and improve patients’ care. However, in the urgent need to provide a response to a burning unmet need, many isolated smaller clinical trials or off-label use of medicines outside the investigations have been observed, perhaps fragmenting the research agenda in uncoordinated silos – thus diluting the research efficiency. The large proportions of ongoing clinical trials are single-centred observational studies with high risk of bias and therefore providing low level of evidence when their findings are reported. The capacity to inform the decision-makers is inevitably weak when the clinical studies generate doubts over certainties. This was recently outlined in a critical appraisal of registered COVID-19 trials on ClinicalTrials.gov database [141]. In fact, only 13.6% of these clinical trials are prospective cohorts that may provide level 2 evidence [141]. Therefore, the lack of effective international cooperation is suggested, along with the negligence of other key aspects to tackle the pandemic, including the disease prevention in high-risk populations. Another emerged critical issue is the tremendous heterogeneity in the rates of COVID-19 new cases across different geographic areas with a significant decline in some countries that may halt and decrease expected enrolment in ongoing clinical trials [192]. The important number of launched monocentric studies and the observed decline of incident COVID-19 cases in some countries should promote considering adaptive study designs to fulfil enrolment [192]. For this perspective, trialists are encouraged to follow master protocols when testing their hypotheses, as suggested by the World Health Organization, in the design of the international adaptive trial SOLIDARITY (e.g. NCT04330690). A significant experience with master protocols in oncology and haematology was achieved, which may accelerate drug development and reduce ineffectiveness in urgent clinical trials [184].
Among the dozens of coronavirus drugs that are being repurposed or in development, a number of anticancer compounds are also being studied as potential future drugs for COVID-19. At this time, the best option for patients is the enrolment in clinical trials. In the current scenario where no drug has provided a “parachute” effect on the prognosis of patients with COVID-19, especially with more severe disease, the use of off-label medicines based on over-emphasised interpretations of the preliminary data from small cohorts or anecdotes is strongly discouraged. With nearly one thousand clinical trials ongoing, there is virtually room to allocate much more patients in truly promising clinical trials, tailoring specific populations across the continuum of care – from the prevention to the treatments and palliative care. The notion is clear: treatment options in settings of high medical unmet need can be improved only in controlled clinical trials, both offering the most innovative therapeutic approaches and prompting the dis-engagement from established clinical interventions with narrow added value, a common contingency observed when no treatment is available for a particular disease. Having an impact on health requires quality behind.
In response to the “high clinical unmet need” and to support clinicians, who are facing the daily challenges with the aim to manage severe health conditions associated with dismal prognosis, the implementation of clinical trials may serve to boost the access to treatments, making treasure of such precious information. In fact, the off-label use of some medicines, most strikingly hydroxychloroquine almost everywhere, outside a clinical trial or a registry-based accountability is the primordial error and the enhancer of confusions – generated by the disconnect between the anecdotal clinical perception of benefits and the objective data collected. In this regard, the regulatory agencies for medicines in many regions and countries have reformulated simplified procedures and supported more flexibility in the design, evaluation and adoption of clinical trials, to meet the patients' needs. For example, the European Medicines Agency (EMA) has delineated regulatory flexibilities to help pharmaceutical companies cope with the consequences of the pandemic, emphasising the need to keep high-level of quality, safety and efficacy for medicinal products developed for COVID-19. The message is clear: patients’ safety and quality of science in clinical trial conduction is non-negotiable [52] (accessed 30-05-2020). EMA has established rapid procedures through accelerated scientific advice, rolling review and expedited treatment of the applications for marketing authorisations [53] (accessed 30-05-2020).
The harmonisation of regulatory processes and enhanced drug development must necessarily proceed in parallel, committing to accelerate the drug repurposing, ensuring safety thus prompting early interruptions of trials when new safety signals emerge, or major results of activity are collected. However, while endorsing the acceleration in the revisions, decisions, approval and authorisation, the regulators should commit to ensure the design of quality clinical trials, pushing for the definition of the benefits based on patients’ relevant end-points and dissecting the reasonability and opportunity to favour surrogate end-points in selected instances – when appropriate for the setting and highly predictive or correlated with the mortality outcomes – the truly important ones.
The high-throughput screening of approved molecules capable to dock to the key protein domains of SARS-CoV-2 pathogenesis or related to the immune-dysregulation will identify thousands of possible compounds, from in silico models. An important success for this approach was recently achieved based on a library profiling of approximately 12,000 clinical-stage or FDA-approved compounds [153]. The authors of this paper published in Nature identified various molecules such as the kinase inhibitor apilimod that antagonise SARS-CoV-2 replication in vitro and in primary human lung explant model [153]. While it is essential to boost the drug-discovery based on approved medicines, to accelerate the drug development overall, the construct of preclinical models should be in vitro and ex vivo strictly implemented in collaborating research facilities, committed for the research on COVID-19. This is an effort of everyone. In the fight against COVID-19, the entire chain of medical researches has rapidly become multidisciplinary, truly intersected with specialists of several branches, transversally positioned. Offering technology and knowledge to develop the best science at the service of a public health global emergency recalls the humanitarian mandate of medical science – to improve population health and wellbeing for all.
The hope is that drug repurposing to boost the discovery of new treatments for human diseases will find the right place and that this innovative and transversal operational model of scientific research will get common, to result in timely impact on populations at the most effective and efficient utilisation of resources and knowledge. Time for wasteful duplications based on competitive conflicts should be over; the COVID-19 will enlighten the solidarity in science, sublimating narcissism for the ultimate goals of serving people, especially when facing the hardest moments of their lives, for deadly diseases.
6. Conclusion
Controlling the SARS-CoV-2 pandemic warrants multidisciplinary collaboration to find relevant treatment options. From the evidence reviewed here, a number of anticancer drugs seem to retain a promising activity to treat patients with COVID-19. The high level of evidence should still be the primary objective of the clinical research, despite the critical circumstances during a pandemic. Hence, testing a battery of known drugs in clinical cohorts will certainly provide an additional value to these molecules (for more information, see Box 1, Box 2 ). Although the ideas of using repurposed anticancer drugs might be criticised because of the toxicity profile of some of them, we believe that this review article may provide inputs and guide the future directions for clinical research – envisioning an approach beyond the definition of separate medical subjects by vaporising the common barriers between medical disciplines. This might help address innovative operational models and regulatory flexibilities in this unique momentum of the international research, united to control a global pandemic, committed to serve the people in all over the world.
Box 1. Recommended reading of particular interest.
| DOI | |
|---|---|
| Larson C et al. COVID-19 and Cancer: A guide with suggested COVID-19 rule-out criteria to support clinical decision-making. Biochimica et Biophysica Acta (BBA) - Reviews on Cancer. 2020; 188412. | https://doi.org/10.1016/j.bbcan.2020.188412 |
| Zeggini E et al. Biomedical Research Goes Viral: Dangers and Opportunities. Cell. 2020; S0092-8674(20)30576-6. | https://doi.org/10.1016/j.cell.2020.05.014 |
| Sanders et al. Pharmacologic Treatments for Coronavirus Disease 2019 (COVID-19): A Review. JAMA. 2020; 10.1001/jama.2020.6019. | https://doi.org/10.1001/jama.2020.6019 |
| Mercorelli B et al. Drug Repurposing for Viral Infectious Diseases: How Far Are We? Trends Microbiol. 2018; 26(10):865–876. | https://doi.org/10.1016/j.tim.2018.04.004 |
| Farha MA, Brown ED. Drug repurposing for antimicrobial discovery. Nat Microbiol. 2019; 4(4):565–577. | https://doi.org/10.1038/s41564-019-0357-1 |
| Boldescu V et al. Broad-spectrum agents for flaviviral infections: dengue, Zika and beyond. Nat Rev Drug Discov. 2017; 16(8):565–586. | https://doi.org/10.1038/nrd.2017.33 |
| Takizawa N, Yamasaki M. Current landscape and future prospects of antiviral drugs derived from microbial products. J Antibiot (Tokyo). 2017; 71(1):45–52. | https://doi.org/10.1038/ja.2017.115 |
| Kaufmann SHE et al. Host-directed therapies for bacterial and viral infections. Nat Rev Drug Discov. 2018; 17(1):35–56. | https://doi.org/10.1038/nrd.2017.162 |
| Pfefferle S et al. The SARS-coronavirus-host interactome: identification of cyclophilins as target for pan-coronavirus inhibitors. PLoSPathog. 2011; 7(10):e1002331. | https://doi.org/10.1371/journal.ppat.1002331 |
| Zumla A et al. Coronaviruses - drug discovery and therapeutic options. Nat Rev Drug Discov. 2016; 15(5):327–347. | https://doi.org/10.1038/nrd.2015.37 |
| de Wit E et al. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol. 2016; 14(8):523–534. | https://doi.org/10.1038/nrmicro.2016.81 |
Alt-text: Box 1
Box 2. Useful resources and database.
| Website | |
|---|---|
| The US Clinical Trials Database | https://www.clinicaltrials.gov/ |
| COVID-19 clinical trials | https://www.clinicaltrials.gov/ct2/results?cond=COVID-19 |
| Coronavirus Disease 2019 (COVID-19) Treatment Guidelines |
https://www.covid19treatmentguidelines.nih.gov/ |
| COVID-19 Studies from the World Health Organization Database | https://www.clinicaltrials.gov/ct2/who_table |
| Elsevier Coronavirus Resource Directory | https://www.elsevier.com/novel-coronavirus-covid-19 |
| COVID-19 Databases and Journals | https://www.cdc.gov/library/researchguides/2019novelcoronavirus/databasesjournals.html |
| EULAR COVID-19 Database | https://www.eular.org/eular_covid19_database.cfm |
| COVID-Evidence Database | https://covid-evidence.org/database |
| Cell Press Coronavirus Resource Hub | https://www.cell.com/COVID-19 |
| COVID-19 SARS-CoV-2 preprints from medRxiv and bioRxiv | https://connect.biorxiv.org/relate/content/181 |
Alt-text: Box 2
Funding
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Conflicts of interest statement
Khalid El Bairi, Dario Trapani, Angelica Petrillo, Cécile Le Page, Hanaa Zbakh, Bruno Daniele, Prof. Rhizlane Belbaraka and Prof. Said Afqir have no COI to declare. Prof. Giuseppe Curigliano has received honoraria from Pfizer, Novartis, Lilly, Roche; fees for expert testimony and medical education from Pfizer and has participated in advisory boards for Pfizer, Roche, Lilly, Novartis, Seattle Genetics, Celltrion.
Acknowledgements
For transparency and scientific publishing ethics, we supported our manuscript by the Turnitin® report of plagiarism (Supplemental material 1). To encourage international collaboration and continuous discussion of this subject, we have created a project on ResearchGate social network and we encourage the readers to ask questions and comment on the topic following this link: https://www.researchgate.net/project/COVID-19-Cancer-and-Biomarkers.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ejca.2020.09.014.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Abubakar I., Aliyu S.H., Arumugam C., Hunter P.R., Usman N.K. Prevention and treatment of cryptosporidiosis in immunocompromised patients. Cochrane Database Syst Rev. 2007;1 doi: 10.1002/14651858.CD004932.pub2. CD004932. Published 2007 Jan 24. [DOI] [PubMed] [Google Scholar]
- 2.Ackermann M., Verleden S.E., Kuehnel M., et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMoa2015432. [published online ahead of print, 2020 may 21] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ajayi S., Becker H., Reinhardt H., et al. Ruxolitinib. Recent Results. Canc Res. 2018;212:119-132. doi: 10.1007/978-3-319-91439-8_6. [DOI] [PubMed] [Google Scholar]
- 4.Alazzam M., Tidy J., Osborne R., Coleman R., Hancock B.W., Lawrie T.A. Chemotherapy for resistant or recurrent gestational trophoblastic neoplasia. Cochrane Database Syst Rev. 2016;2016(1):CD008891. doi: 10.1002/14651858.CD008891.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alonso-Álvarez S., Pardal E., Sánchez-Nieto D., et al. Plitidepsin: design, development, and potential place in therapy. Drug Des Devel Ther. 2017;11:253-264. doi: 10.2147/DDDT.S94165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson V.R., Curran M.P. Nitazoxanide: a review of its use in the treatment of gastrointestinal infections. Drugs. 2007;67(13):1947–1967. doi: 10.2165/00003495-200767130-00015. [DOI] [PubMed] [Google Scholar]
- 7.Azizian N.G., Li Y. XPO1-dependent nuclear export as a target for cancer therapy. J Hematol Oncol. 2020;13(1):61. doi: 10.1186/s13045-020-00903-4. Published 2020 Jun 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Badia R., Angulo G., Riveira-Muñoz E., et al. Inhibition of herpes simplex virus type 1 by the CDK6 inhibitor PD-0332991 (palbociclib) through the control of SAMHD1. J Antimicrob Chemother. 2016;71(2):387–394. doi: 10.1093/jac/dkv363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bai J.P.F., Hsu C.W. Drug repurposing for Ebola virus disease: principles of consideration and the animal rule. J Pharmaceut Sci. 2019;108(2):798–806. doi: 10.1016/j.xphs.2018.09.010. [DOI] [PubMed] [Google Scholar]
- 10.Bailey M.A., Na H., Duthie M.S., Gillis T.P., Lahiri R., Parish T. Nitazoxanide is active against Mycobacterium leprae. PloS One. 2017;12(8) doi: 10.1371/journal.pone.0184107. Published 2017 Aug 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baird K., Glod J., Steinberg S.M., et al. Results of a randomized, double-blinded, placebo-controlled, phase 2.5 study of saracatinib (AZD0530), in patients with recurrent osteosarcoma localized to the lung. Sarcoma. 2020;2020 doi: 10.1155/2020/7935475. Published 2020 Apr 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barlow A., Landolf K.M., Barlow B., et al. Review of emerging pharmacotherapy for the treatment of coronavirus disease 2019. Pharmacotherapy. 2020 doi: 10.1002/phar.2398. [published online ahead of print, 2020 Apr 7] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barlow L.J., Benson M.C. Experience with newer intravesical chemotherapy for high-risk non-muscle-invasive bladder cancer. Curr Urol Rep. 2013;14(2):65-70. doi: 10.1007/s11934-013-0312-2. [DOI] [PubMed] [Google Scholar]
- 14.Barnes B.J., Adrover J.M., Baxter-Stoltzfus A., et al. Targeting potential drivers of COVID-19: neutrophil extracellular traps. J Exp Med. 2020;217(6) doi: 10.1084/jem.20200652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bates D.O. Vascular endothelial growth factors and vascular permeability. Cardiovasc Res. 2010;87(2):262–271. doi: 10.1093/cvr/cvq105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baz M., Boivin G. Antiviral agents in development for Zika virus infections. Pharmaceuticals. 2019;12(3):101. doi: 10.3390/ph12030101. (Basel) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bekerman E., Neveu G., Shulla A., et al. Anticancer kinase inhibitors impair intracellular viral trafficking and exert broad-spectrum antiviral effects. J Clin Invest. 2017;127(4):1338-1352. doi: 10.1172/JCI89857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Belouzard S., Millet J.K., Licitra B.N., Whittaker G.R. Mechanisms of coronavirus cell entry mediated by the viral spike protein. Viruses. 2012;4(6):1011–1033. doi: 10.3390/v4061011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bloch E.M., Shoham S., Casadevall A., et al. Deployment of convalescent plasma for the prevention and treatment of COVID-19. J Clin Invest. 2020:138745. doi: 10.1172/JCI138745. [published online ahead of print, 2020 Apr 7] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Branche E., Tang W.W., Viramontes K.M., et al. Synergism between the tyrosine kinase inhibitor sunitinib and Anti-TNF antibody protects against lethal dengue infection. Antivir Res. 2018;158:1–7. doi: 10.1016/j.antiviral.2018.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brelot A., Chakrabarti L.A. CCR5 revisited: how mechanisms of HIV entry govern AIDS pathogenesis. J Mol Biol. 2018;430(17):2557–2589. doi: 10.1016/j.jmb.2018.06.027. [DOI] [PubMed] [Google Scholar]
- 22.Bullard-Feibelman K.M., Govero J., Zhu Z., et al. The FDA-approved drug sofosbuvir inhibits Zika virus infection. Antivir Res. 2017;137:134–140. doi: 10.1016/j.antiviral.2016.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Canals D., Perry D.M., Jenkins R.W., Hannun Y.A. Drug targeting of sphingolipid metabolism: sphingomyelinases and ceramidases. Br J Pharmacol. 2011;163(4):694-712. doi: 10.1111/j.1476-5381.2011.01279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cao X. COVID-19: immunopathology and its implications for therapy. Nat Rev Immunol. 2020 doi: 10.1038/s41577-020-0308-3. [published online ahead of print, 2020 Apr 9] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cavalcanti A.B., Zampieri F.G., Rosa R.G., et al. Hydroxychloroquine with or without azithromycin in mild-to-moderate covid-19 [published online ahead of print, 2020 jul 23] N Engl J Med. 2020 doi: 10.1056/NEJMoa2019014. NEJMoa2019014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan J.F., Lau S.K., To K.K., Cheng V.C., Woo P.C., Yuen K.Y. Middle East respiratory syndrome coronavirus: another zoonotic betacoronavirus causing SARS-like disease. Clin Microbiol Rev. 2015;28(2):465–522. doi: 10.1128/CMR.00102-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chan J.F., Yuan S., Kok K.H., et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chan K., Robert F., Oertlin C., et al. eIF4A supports an oncogenic translation program in pancreatic ductal adenocarcinoma. Nat Commun. 2019;10(1):5151. doi: 10.1038/s41467-019-13086-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chari A., Vogl D.T., Gavriatopoulou M., et al. Oral selinexor-dexamethasone for triple-class refractory multiple myeloma. N Engl J Med. 2019;381(8):727–738. doi: 10.1056/NEJMoa1903455. [DOI] [PubMed] [Google Scholar]
- 30.Chen J., Lau Y.F., Lamirande E.W., et al. Cellular immune responses to severe acute respiratory syndrome coronavirus (SARS-CoV) infection in senescent BALB/c mice: CD4+ T cells are important in control of SARS-CoV infection. J Virol. 2010;84(3):1289-1301. doi: 10.1128/JVI.01281-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Y., Liu Q., Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J Med Virol. 2020;92(4):418–423. doi: 10.1002/jmv.25681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheng F., Murray J.L., Rubin D.H. Drug repurposing: new treatments for Zika virus infection? Trends Mol Med. 2016;22(11):919–921. doi: 10.1016/j.molmed.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 33.Choi Y.R., Kim J.B., Kang S.J., et al. The dual role of c-src in cell-to-cell transmission of α-synuclein. EMBO Rep. 2020 doi: 10.15252/embr.201948950. [published online ahead of print, 2020 May 5] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clark M.J., Miduturu C., Schmidt A.G., et al. GNF-2 inhibits dengue virus by targeting abl kinases and the viral E protein. Cell Chem Biol. 2016;23(4):443–452. doi: 10.1016/j.chembiol.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coleman C.M., Sisk J.M., Mingo R.M., Nelson E.A., White J.M., Frieman M.B. Abelson kinase inhibitors are potent inhibitors of severe acute respiratory syndrome coronavirus and Middle East respiratory syndrome coronavirus fusion. J Virol. 2016;90(19):8924–8933. doi: 10.1128/JVI.01429-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cortegiani A., Ingoglia G., Ippolito M., Giarratano A., Einav S. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J Crit Care. 2020;S0883-9441(20):30390–30397. doi: 10.1016/j.jcrc.2020.03.005. [published online ahead of print, 2020 Mar 10] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cytotoxic antibiotics . LiverTox: clinical and research information on drug-induced liver injury. National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda (MD): 2012. [Google Scholar]
- 39.Dai L., Plaisance-Bonstaff K., Voelkel-Johnson C., et al. Sphingosine kinase-2 maintains viral latency and survival for KSHV-infected endothelial cells. PloS One. 2014;9(7) doi: 10.1371/journal.pone.0102314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dang W., Xu L., Ma B., et al. Nitazoxanide inhibits human norovirus replication and synergizes with ribavirin by activation of cellular antiviral response. Antimicrob Agents Chemother. 2018;62(11) doi: 10.1128/AAC.00707-18. e00707-18. Published 2018 Oct 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Danson S., Mulvey M.R., Turner L., et al. An exploratory randomized-controlled trial of the efficacy of the Src-kinase inhibitor saracatinib as a novel analgesic for cancer-induced bone pain. J Bone Oncol. 2019;19:100261. doi: 10.1016/j.jbo.2019.100261. Published 2019 Sep 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Wispelaere M., LaCroix A.J., Yang P.L. The small molecules AZD0530 and dasatinib inhibit dengue virus RNA replication via Fyn kinase. J Virol. 2013;87(13):7367–7381. doi: 10.1128/JVI.00632-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dementiev A., Joachimiak A., Nguyen H., et al. Molecular mechanism of inhibition of acid ceramidase by carmofur. J Med Chem. 2019;62(2):987–992. doi: 10.1021/acs.jmedchem.8b01723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Di Cosimo S., Malfetton A., Pérez-García J.M., et al. Immune checkpoint inhibitors: a physiology-driven approach to the treatment of COVID-19. Eur J Canc. 2020;135:62–65. doi: 10.1016/j.ejca.2020.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Doan N.B., Nguyen H.S., Montoure A., et al. Acid ceramidase is a novel drug target for pediatric brain tumors. Oncotarget. 2017;8(15):24753–24761. doi: 10.18632/oncotarget.15800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dyall J., Coleman C.M., Hart B.J., et al. Repurposing of clinically developed drugs for treatment of Middle East respiratory syndrome coronavirus infection. Antimicrob Agents Chemother. 2014;58(8):4885-4893. doi: 10.1128/AAC.03036-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dyall J., Gross R., Kindrachuk J., et al. Middle East respiratory syndrome and severe acute respiratory syndrome: current therapeutic options and potential targets for novel therapies. Drugs. 2017;77(18):1935–1966. doi: 10.1007/s40265-017-0830-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.El Bairi K., Amrani M., Afqir S. Starvation tactics using natural compounds for advanced cancers: pharmacodynamics, clinical efficacy, and predictive biomarkers. Canc Med. 2018;7(6):2221-2246. doi: 10.1002/cam4.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.El Bairi K., Amrani M., Kandhro A.H., Afqir S. Prediction of therapy response in ovarian cancer: where are we now? Crit Rev Clin Lab Sci. 2017;54(4):233–266. doi: 10.1080/10408363.2017.1313190. [DOI] [PubMed] [Google Scholar]
- 50.El Bairi K., Atanasov A.G., Amrani M., Afqir S. The arrival of predictive biomarkers for monitoring therapy response to natural compounds in cancer drug discovery. Biomed Pharmacother. 2019;109:2492-2498. doi: 10.1016/j.biopha.2018.11.097. [DOI] [PubMed] [Google Scholar]
- 51.El Bairi K., Jabi R., Trapani D., et al. Can the microbiota predict response to systemic cancer therapy, surgical outcomes, and survival? The answer is in the gut [published online ahead of print, 2020 May 24] Expet Rev Clin Pharmacol. 2020:1–19. doi: 10.1080/17512433.2020.1758063. [DOI] [PubMed] [Google Scholar]
- 52.EMA . 2020. Covid-19: guidance on more regulatory flexibility for medicines.https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/guidance-medicine-developers-companies-covid-19 Available at: [Google Scholar]
- 53.EMA . 2020. EMA initiatives for acceleration of development support and evaluation procedures for COVID-19 treatments and vaccines.https://www.ema.europa.eu/en/documents/other/ema-initiatives-acceleration-development-support-evaluation-procedures-covid-19-treatments-vaccines_en.pdf Available at: [Google Scholar]
- 54.Farag S., Smith M.J., Fotiadis N., Constantinidou A., Jones R.L. Revolutions in treatment options in gastrointestinal stromal tumours (GISTs): the latest updates. Curr Treat Options Oncol. 2020;21(7):55. doi: 10.1007/s11864-020-00754-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fehr A.R., Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Methods Mol Biol. 2015;1282:1–23. doi: 10.1007/978-1-4939-2438-7_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ferguson F.M., Gray N.S. Kinase inhibitors: the road ahead. Nat Rev Drug Discov. 2018;17(5):353–377. doi: 10.1038/nrd.2018.21. [DOI] [PubMed] [Google Scholar]
- 57.Ferreira A.C., Zaverucha-do-Valle C., Reis P.A., et al. Sofosbuvir protects Zika virus-infected mice from mortality, preventing short- and long-term sequelae. Sci Rep. 2017;7(1):9409. doi: 10.1038/s41598-017-09797-8. Published 2017 Aug 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fiolet T., Guihur A., Rebeaud M., Mulot M., Peiffer-Smadja N., Mahamat-SalehY Effect of hydroxychloroquine with or without azithromycin on the mortality of COVID-19 patients:a systematic review and meta-analysis. Clin Microbiol Infect. 2018 doi: 10.1016/j.cmi.2020.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Flinn I.W., Hillmen P., Montillo M., et al. The phase 3 DUO trial: duvelisib vs ofatumumab in relapsed and refractory CLL/SLL. Blood. 2018;132(23):2446–2455. doi: 10.1182/blood-2018-05-850461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Florence J.M., Krupa A., Booshehri L.M., Davis S.A., Matthay M.A., Kurdowska A.K. Inhibiting Bruton's tyrosine kinase rescues mice from lethal influenza-induced acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2018;315(1):L52-L58. doi: 10.1152/ajplung.00047.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fu B., Xu X., Wei H. Why tocilizumab could be an effective treatment for severe COVID-19? J Transl Med. 2020;18(1):164. doi: 10.1186/s12967-020-02339-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gandolfi S., Laubach J.P., Hideshima T., Chauhan D., Anderson K.C., Richardson P.G. The proteasome and proteasome inhibitors in multiple myeloma. Canc Metastasis Rev. 2017;36(4):561-584. doi: 10.1007/s10555-017-9707-8. [DOI] [PubMed] [Google Scholar]
- 63.Gao J., Zhang L., Liu X., et al. Repurposing low-molecular-weight drugs against the main protease of severe acute respiratory syndrome coronavirus 2. J Phys Chem Lett. 2020;11:7267–7272. doi: 10.1021/acs.jpclett.0c01894. [published online ahead of print, 2020 aug 20] [DOI] [PubMed] [Google Scholar]
- 64.Garcia J., Hurwitz H.I., Sandler A.B., et al. Bevacizumab (Avastin®) in cancer treatment: a review of 15 years of clinical experience and future outlook. Canc Treat Rev. 2020;86:102017. doi: 10.1016/j.ctrv.2020.102017. [published online ahead of print, 2020 Mar 26] [DOI] [PubMed] [Google Scholar]
- 65.García M., Cooper A., Shi W., et al. Productive replication of Ebola virus is regulated by the c-Abl1 tyrosine kinase. Sci Transl Med. 2012;4(123) doi: 10.1126/scitranslmed.3003500. 123ra24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.García-Serradilla M., Risco C., Pacheco B. Drug repurposing for new, efficient, broad spectrum antivirals. Virus Res. 2019;264:22–31. doi: 10.1016/j.virusres.2019.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gaspar N., Hawkins D.S., Dirksen U., et al. Ewing sarcoma: current management and future approaches through collaboration. J Clin Oncol. 2015;33(27):3036–3046. doi: 10.1200/JCO.2014.59.5256. [DOI] [PubMed] [Google Scholar]
- 68.Geleris J., Sun Y., Platt J., et al. Observational study of hydroxychloroquine in hospitalized patients with covid-19. N Engl J Med. 2020;382(25):2411–2418. doi: 10.1056/NEJMoa2012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gharwan H., Groninger H. Kinase inhibitors and monoclonal antibodies in oncology: clinical implications. Nat Rev Clin Oncol. 2016;13(4):209–227. doi: 10.1038/nrclinonc.2015.213. [DOI] [PubMed] [Google Scholar]
- 70.Giamarellos-Bourboulis E.J., Netea M.G., Rovina N., et al. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020 doi: 10.1016/j.chom.2020.04.009. [published online ahead of print, 2020 apr 17] S1931-3128(20)30236-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Glowacka I., Bertram S., Müller M.A., et al. Evidence that TMPRSS2 activates the severe acute respiratory syndrome coronavirus spike protein for membrane fusion and reduces viral control by the humoral immune response. J Virol. 2011;85(9):4122–4134. doi: 10.1128/JVI.02232-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gomes N.G.M., Valentão P., Andrade P.B., Pereira R.B. Plitidepsin to treat multiple myeloma. Drugs Today. 2020;56(5):337–347. doi: 10.1358/dot.2020.56.5.3135886. (Barc) [DOI] [PubMed] [Google Scholar]
- 73.Gordon D.E., Jang G.M., Bouhaddou M., et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020;583(7816):459–468. doi: 10.1038/s41586-020-2286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Govindarajah N., Clifford R., Bowden D., Sutton P.A., Parsons J.L., Vimalachandran D. Sphingolipids and acid ceramidase as therapeutic targets in cancer therapy. Crit RevOncolHematol. 2019;138:104-111. doi: 10.1016/j.critrevonc.2019.03.018. [DOI] [PubMed] [Google Scholar]
- 75.Grein J., Ohmagari N., Shin D., et al. Compassionate use of remdesivir for patients with severe covid-19 [published online ahead of print, 2020 apr 10] N Engl J Med. 2020 doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gubens M.A., Burns M., Perkins S.M., et al. A phase II study of saracatinib (AZD0530), a Src inhibitor, administered orally daily to patients with advanced thymic malignancies. Lung Canc. 2015;89(1):57-60. doi: 10.1016/j.lungcan.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 77.Guo Y., Liu Y., Hu N., et al. Discovery of zanubrutinib (BGB-3111), a novel, potent, and selective covalent inhibitor of bruton's tyrosine kinase. J Med Chem. 2019;62(17):7923–7940. doi: 10.1021/acs.jmedchem.9b00687. [DOI] [PubMed] [Google Scholar]
- 78.Guy R.K., DiPaola R.S., Romanelli F., Dutch R.E. Science; 2020. Rapid repurposing of drugs for COVID-19. eabb9332. [DOI] [PubMed] [Google Scholar]
- 79.Haffizulla J., Hartman A., Hoppers M., et al. Effect of nitazoxanide in adults and adolescents with acute uncomplicated influenza: a double-blind, randomised, placebo-controlled, phase 2b/3 trial. Lancet Infect Dis. 2014;14(7):609–618. doi: 10.1016/S1473-3099(14)70717-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Harrison C. Coronavirus puts drug repurposing on the fast track. Nat Biotechnol. 2020;38(4):379–381. doi: 10.1038/d41587-020-00003-1. [DOI] [PubMed] [Google Scholar]
- 81.Havel J.J., Chowell D., Chan T.A. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat Rev Canc. 2019;19(3):133-150. doi: 10.1038/s41568-019-0116-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Heerma van Voss M.R., van Diest P.J., Raman V. Targeting RNA helicases in cancer: the translation trap. Biochim Biophys Acta Rev Canc. 2017;1868(2):510-520. doi: 10.1016/j.bbcan.2017.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Helmy Y.A., Fawzy M., Elaswad A., Sobieh A., Kenney S.P., Shehata A.A. The COVID-19 pandemic: a comprehensive review of taxonomy, Genetics, epidemiology, diagnosis, treatment, and control. J Clin Med. 2020;9(4):1225. doi: 10.3390/jcm9041225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ho T.Y., Wu S.L., Chen J.C., Li C.C., Hsiang C.Y. Emodin blocks the SARS coronavirus spike protein and angiotensin-converting enzyme 2 interaction. Antivir Res. 2007;74(2):92-101. doi: 10.1016/j.antiviral.2006.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hochhaus A., Baccarani M., Silver R.T., et al. European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia. 2020;34(4):966-984. doi: 10.1038/s41375-020-0776-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hoffmann M., Kleine-Weber H., Schroeder S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020 doi: 10.1016/j.cell.2020.02.052. [published online ahead of print, 2020 Mar 4] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hollstein U. Actinomycin. Chemistry and mechanism of action. Chem Rev. 1974:625–652. [Google Scholar]
- 88.https://www.fda.gov/drugs/resources-information-approved-drugs/duvelisib-copiktra-verastem-inc-adult-patients-relapsed-or-refractory-chronic-lymphocytic-leukemia.
- 89.https://www.redhillbio.com/RedHill/Templates/showpage.asp?DBID=1&LNGID=1&TMID=178&FID=1365&PID=0&IID=5106Accessed 06-06-2020.
- 90.Hu T.Y., Frieman M., Wolfram J. Insights from nanomedicine into chloroquine efficacy against COVID-19. Nat Nanotechnol. 2020;15(4):247–249. doi: 10.1038/s41565-020-0674-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [published correction appears in Lancet. 2020 Jan 30;:] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Huang J., Xu J., Chen Y., et al. Camrelizumab versus investigator's choice of chemotherapy as second-line therapy for advanced or metastatic oesophageal squamous cell carcinoma (ESCORT): a multicentre, randomised, open-label, phase 3 study. Lancet Oncol. 2020 doi: 10.1016/S1470-2045(20)30110-8. [published online ahead of print, 2020 May 13] S1470-2045(20)30110-30118. [DOI] [PubMed] [Google Scholar]
- 93.Humeau J., Sauvat A., Cerrato G., et al. Inhibition of transcription by dactinomycin reveals a new characteristic of immunogenic cell stress. EMBO Mol Med. 2020;12(5) doi: 10.15252/emmm.201911622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Janku F., Yap T.A., Meric-Bernstam F. Targeting the PI3K pathway in cancer: are we making headway? Nat Rev Clin Oncol. 2018;15(5):273–291. doi: 10.1038/nrclinonc.2018.28. [DOI] [PubMed] [Google Scholar]
- 95.Jasenosky L.D., Cadena C., Mire C.E., et al. The FDA-approved oral drug nitazoxanide amplifies host antiviral responses and inhibits Ebola virus. iScience. 2019;19:1279–1290. doi: 10.1016/j.isci.2019.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jiao X., Nawab O., Patel T., et al. Recent advances targeting CCR5 for cancer and its role in immuno-oncology. Canc Res. 2019;79(19):4801–4807. doi: 10.1158/0008-5472.CAN-19-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Johansen L.M., Brannan J.M., Delos S.E., et al. FDA-approved selective estrogen receptor modulators inhibit Ebola virus infection. Sci Transl Med. 2013;5(190) doi: 10.1126/scitranslmed.3005471. 190ra79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kennedy D.A., Johnson-Lussenburg C.M. Inhibition of coronavirus 229E replication by actinomycin D. J Virol. 1979;29(1):401-404. doi: 10.1128/jvi.29.1.401-404.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Khandelwal R., Nayarisseri A., Madhavi M., et al. Shape-based machine learning models for the potential novel COVID-19 protease inhibitors assisted by molecular dynamics simulation. Curr Top Med Chem. 2020 doi: 10.2174/1568026620666200704135327. [published online ahead of print, 2020 jul 4] [DOI] [PubMed] [Google Scholar]
- 100.Kindrachuk J., Ork B., Hart B.J., et al. Antiviral potential of ERK/MAPK and PI3K/AKT/mTOR signaling modulation for Middle East respiratory syndrome coronavirus infection as identified by temporal kinome analysis. Antimicrob Agents Chemother. 2015;59(2):1088–1099. doi: 10.1128/AAC.03659-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kono K., Takahashi A., Sugai H., et al. Oral trypsin inhibitor can improve reflux esophagitis after distal gastrectomy concomitant with decreased trypsin activity. Am J Surg. 2005;190:412–417. doi: 10.1016/j.amjsurg.2005.05.044. [DOI] [PubMed] [Google Scholar]
- 102.Krupa A., Fol M., Rahman M., et al. Silencing Bruton's tyrosine kinase in alveolar neutrophils protects mice from LPS/immune complex-induced acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2014;307(6):L435–L448. doi: 10.1152/ajplung.00234.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Landgren O., Sonneveld P., Jakubowiak A., et al. Carfilzomib with immunomodulatory drugs for the treatment of newly diagnosed multiple myeloma. Leukemia. 2019;33(9):2127-2143. doi: 10.1038/s41375-019-0517-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lewis E.L., Harbour D.A., Beringer J.E., Grinsted J. Differential in vitro inhibition of feline enteric coronavirus and feline infectious peritonitis virus by actinomycin D. J Gen Virol. 1992;73(Pt 12):3285-3288. doi: 10.1099/0022-1317-73-12-3285. [DOI] [PubMed] [Google Scholar]
- 105.Li J., Li S., Yu H., Wang J., Xu C., Lu X. The efficacy and safety of first-line single-agent chemotherapy regimens in low-risk gestational trophoblastic neoplasia: a network meta-analysis. Gynecol Oncol. 2018;148(2):247-253. doi: 10.1016/j.ygyno.2017.11.031. [DOI] [PubMed] [Google Scholar]
- 106.Li X., Geng M., Peng Y., Meng L., Lu S. Molecular immune pathogenesis and diagnosis of COVID-19 [published online ahead of print, 2020 Mar 5] J Pharm Anal. 2020 doi: 10.1016/j.jpha.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Liu P., Ma S., Liu H., Han H., Wang S. HCFU inhibits cervical cancer cells growth and metastasis by inactivating Wnt/β-catenin pathway. J Cell Biochem. 2017 doi: 10.1002/jcb.26570. [published online ahead of print, 2017 Dec 12] [DOI] [PubMed] [Google Scholar]
- 108.Liu X., Cheng J.C., Turner L.S., et al. Acid ceramidase upregulation in prostate cancer: role in tumor development and implications for therapy. Expert Opin Ther Targets. 2009;13(12):1449-1458. doi: 10.1517/14728220903357512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lucas J.M., Heinlein C., Kim T., et al. The androgen-regulated protease TMPRSS2 activates a proteolytic cascade involving components of the tumor microenvironment and promotes prostate cancer metastasis. Canc Discov. 2014;4(11):1310–1325. doi: 10.1158/2159-8290.CD-13-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Luke J.J., Flaherty K.T., Ribas A., Long G.V. Targeted agents and immunotherapies: optimizing outcomes in melanoma. Nat Rev Clin Oncol. 2017;14(8):463–482. doi: 10.1038/nrclinonc.2017.43. [DOI] [PubMed] [Google Scholar]
- 111.Luo J., Rizvi H., Egger J.V., Preeshagul I.R., Wolchok J.D., Hellmann M.D. Impact of PD-1 blockade on severity of COVID-19 in patients with lung cancers. Canc Discov. 2020;10(8):1121–1128. doi: 10.1158/2159-8290.CD-20-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Magruder J.T., Grimm J.C., Crawford T.C., et al. Imatinib is protective against ischemia-reperfusion injury in an ex vivo rabbit model of lung injury. Ann Thorac Surg. 2018;105(3):950-956. doi: 10.1016/j.athoracsur.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 113.Mao C., Yang Z.Y., He B.F., et al. Toremifene versus tamoxifen for advanced breast cancer. Cochrane Database Syst Rev. 2012;7 doi: 10.1002/14651858.CD008926.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Markham A., Keam S.J. Camrelizumab: first global approval. Drugs. 2019;79(12):1355–1361. doi: 10.1007/s40265-019-01167-0. [published correction appears in drugs. 2019 Aug 24;:] [DOI] [PubMed] [Google Scholar]
- 115.Matthay M.A., Zemans R.L., Zimmerman G.A., et al. Acute respiratory distress syndrome. Nat Rev Dis Prim. 2019;5(1):18. doi: 10.1038/s41572-019-0069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.McNeish I.A., Ledermann J.A., Webber L., et al. A randomised, placebo-controlled trial of weekly paclitaxel and saracatinib (AZD0530) in platinum-resistant ovarian, fallopian tube or primary peritoneal cancer. Ann Oncol. 2014;25(10):1988–1995. doi: 10.1093/annonc/mdu363. [DOI] [PubMed] [Google Scholar]
- 117.Mehta P., McAuley D.F., Brown M., et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mercorelli B., Palù G., Loregian A. Drug repurposing for viral infectious diseases: how far are we? Trends Microbiol. 2018;26(10):865–876. doi: 10.1016/j.tim.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Miller S.E., Mathiasen S., Bright N.A., et al. CALM regulates clathrin-coated vesicle size and maturation by directly sensing and driving membrane curvature. Dev Cell. 2015;33(2):163–175. doi: 10.1016/j.devcel.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Min S., Lim Y.S., Shin D., et al. Abl tyrosine kinase regulates hepatitis C virus entry. Front Microbiol. 2017;8:1129. doi: 10.3389/fmicb.2017.01129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Monteil V., Kwon H., Prado P., Hagelkrüys A., Wimmer R.A., Stahl M., et al. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell. 2020 doi: 10.1016/j.cell.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Morimoto K., Koh M. Postoperative adjuvant use of carmofur for early breast cancer. Osaka City Med J. 2003;49(2):77-83. [PubMed] [Google Scholar]
- 123.Motoo Y. Antiproteases in the treatment of chronic pancreatitis. JOP. 2007;8(4 suppl):533–537. 24. [PubMed] [Google Scholar]
- 124.Mourad J.J., Azria P. Tocilizumab for severe COVID-19 pneumonia. Lancet Rheumatol. 2020 doi: 10.1016/S2665-9913(20)30282-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Müller D., Shin S., Goullet de Rugy T., et al. eIF4A inhibition circumvents uncontrolled DNA replication mediated by 4E-BP1 loss in pancreatic cancer. JCI Insight. 2019;4(21) doi: 10.1172/jci.insight.121951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Muñoz-Alonso M.J., Álvarez E., Guillén-Navarro M.J., et al. c-Jun N-terminal kinase phosphorylation is a biomarker of plitidepsin activity. Mar Drugs. 2013;11(5):1677–1692. doi: 10.3390/md11051677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nachmias B., Schimmer A.D. Targeting nuclear import and export in hematological malignancies. Leukemia. 2020 doi: 10.1038/s41375-020-0958-y. [published online ahead of print, 2020 Jul 5] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Neveu G., Barouch-Bentov R., Ziv-Av A., Gerber D., Jacob Y., Einav S. Identification and targeting of an interaction between a tyrosine motif within hepatitis C virus core protein and AP2M1 essential for viral assembly. PLoS Pathog. 2012;8(8) doi: 10.1371/journal.ppat.1002845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Neveu G., Ziv-Av A., Barouch-Bentov R., Berkerman E., Mulholland J., Einav S. AP-2-associated protein kinase 1 and cyclin G-associated kinase regulate hepatitis C virus entry and are potential drug targets. J Virol. 2015;89(8):4387-4404. doi: 10.1128/JVI.02705-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Nguyen H.S., Awad A.J., Shabani S., Doan N. Molecular targeting of acid ceramidase in glioblastoma: a review of its role, potential treatment, and challenges. Pharmaceutics. 2018;10(2):45. doi: 10.3390/pharmaceutics10020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Patel A., Sabbineni H., Clarke A., Somanath P.R. Novel roles of Src in cancer cell epithelial-to-mesenchymal transition, vascular permeability, microinvasion and metastasis. Life Sci. 2016;157:52-61. doi: 10.1016/j.lfs.2016.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Patil N.K., Guo Y., Luan L., Sherwood E.R. Targeting immune cell checkpoints during sepsis. Int J Mol Sci. 2017;18(11):2413. doi: 10.3390/ijms18112413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Patterson B., Seetthamraju H., Dhody K., et al. ResearchSquare; 2020. Disruption of the CCL5/RANTES-CCR5 pathway restores immune homeostasis and reduces plasma viral load in critical COVID-19. [DOI] [Google Scholar]
- 134.Perrone F., Piccirillo M.C., Ascierto P.A., et al. Tocilizumab for patients with COVID-19 pneumonia. The TOCIVID-19 phase 2 trial. medRxiv. 2020 doi: 10.1101/2020.06.01.20119149. [DOI] [Google Scholar]
- 135.Pessoa C., Silveira E.R., Lemos T.L., Wetmore L.A., Moraes M.O., Leyva A. Antiproliferative effects of compounds derived from plants of Northeast Brazil. Phytother Res. 2000;14(3):187–191. doi: 10.1002/(sici)1099-1573(200005)14:3<187::aid-ptr572>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 136.PharmaMar has submitted a Phase II clinical trial of Aplidin® (plitidepsin) for the treatment of COVID-19 to the Spanish Medicines Agency. PharmaMar. 2020 http://pharmamar.com/wp-content/uploads/2020/04/PR_clinical_trial_plitidepsin_covid19_DEF.pdf Available at: [Google Scholar]
- 137.PharmaMar reports positive results for Aplidin® against coronavirus HCoV-229E. PharmaMar. 2020 http://pharmamar.com/wp-content/uploads/2020/03/PR_Results_Aplidin_coronavirus.pdf Available at: [Google Scholar]
- 138.Phua J., Weng L., Ling L., et al. Intensive care management of coronavirus disease 2019 (COVID-19): challenges and recommendations. Lancet Respir Med. 2020 doi: 10.1016/S2213-2600(20)30161-2. [published online ahead of print, 2020 Apr 6] S2213-2600(20)30161-30162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Pitman M.R., Costabile M., Pitson S.M. Recent advances in the development of sphingosine kinase inhibitors. Cell Signal. 2016;28(9):1349-1363. doi: 10.1016/j.cellsig.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 140.Pu S.Y., Xiao F., Schor S., et al. Feasibility and biological rationale of repurposing sunitinib and erlotinib for dengue treatment. Antivir Res. 2018;155:67-75. doi: 10.1016/j.antiviral.2018.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pundi K., Perino A.C., Harrington R.A., Krumholz H.M., Turakhia M.P. Characteristics and strength of evidence of COVID-19 studies registered on ClinicalTrials.gov. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.2904. [published online ahead of print, 2020 Jul 27] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Pushpakom S., Iorio F., Eyers P.A., et al. Drug repurposing: progress, challenges and recommendations. Nat Rev Drug Discov. 2019;18(1):41–58. doi: 10.1038/nrd.2018.168. [DOI] [PubMed] [Google Scholar]
- 143.Pyne N.J., Adams D.R., Pyne S. Sphingosine kinase 2 in autoimmune/inflammatory disease and the development of sphingosine kinase 2 inhibitors. Trends Pharmacol Sci. 2017;38(7):581-591. doi: 10.1016/j.tips.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 144.Qian Z., Dominguez S.R., Holmes K.V. Role of the spike glycoprotein of human Middle East respiratory syndrome coronavirus (MERS-CoV) in virus entry and syncytia formation. PloS One. 2013;8(10) doi: 10.1371/journal.pone.0076469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Qin C., Zhou L., Hu Z., et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa248. [published online ahead of print, 2020 Mar 12] ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Qin S., Ren Z., Meng Z., et al. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: a multicentre, open-label, parallel-group, randomised, phase 2 trial. Lancet Oncol. 2020;21(4):571-580. doi: 10.1016/S1470-2045(20)30011-5. [DOI] [PubMed] [Google Scholar]
- 147.Qin W., Hu L., Zhang X., et al. The diverse function of PD-1/PD-L pathway beyond cancer. Front Immunol. 2019;10:2298. doi: 10.3389/fimmu.2019.02298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Realini N., Solorzano C., Pagliuca C., et al. Discovery of highly potent acid ceramidase inhibitors with in vitro tumor chemosensitizing activity. Sci Rep. 2013;3:1035. doi: 10.1038/srep01035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Reid S.P., Tritsch S.R., Kota K., et al. Sphingosine kinase 2 is a chikungunya virus host factor co-localized with the viral replication complex. Emerg Microb Infect. 2015;4(10):e61. doi: 10.1038/emi.2015.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Reinecke J., Caplan S. Endocytosis and the Src family of non-receptor tyrosine kinases. Biomol Concepts. 2014;5(2):143-155. doi: 10.1515/bmc-2014-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rho O., Kim D.J., Kiguchi K., Digiovanni J. Growth factor signaling pathways as targets for prevention of epithelial carcinogenesis. Mol Carcinog. 2011;50(4):264-279. doi: 10.1002/mc.20665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Richter J., Madduri D., Richard S., Chari A. Selinexor in relapsed/refractory multiple myeloma. Ther Adv Hematol. 2020;11 doi: 10.1177/2040620720930629. Published 2020 Jun 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Riva L., Yuan S., Yin X., et al. Discovery of SARS-CoV-2 antiviral drugs through large-scale compound repurposing. Nature. 2020 doi: 10.1038/s41586-020-2577-1. [published online ahead of print, 2020 Jul 24] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Rizzo A.N., Sammani S., Esquinca A.E., et al. Imatinib attenuates inflammation and vascular leak in a clinically relevant two-hit model of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2015;309(11):L1294–L1304. doi: 10.1152/ajplung.00031.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Rockwell S., Liu Y. Aplidin as a potential adjunct to radiation therapy: in vitro studies. Int J Radiat Biol. 2010;86(1):63–70. doi: 10.3109/09553000903264531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Roskoski R., Jr. Cyclin-dependent protein serine/threonine kinase inhibitors as anticancer drugs. Pharmacol Res. 2019;139:471–488. doi: 10.1016/j.phrs.2018.11.035. [DOI] [PubMed] [Google Scholar]
- 157.Rossignol J.F. Nitazoxanide: a first-in-class broad-spectrum antiviral agent. Antivir Res. 2014;110:94–103. doi: 10.1016/j.antiviral.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Roy S., Trinchieri G. Microbiota: a key orchestrator of cancer therapy. Nat Rev Canc. 2017;17(5):271-285. doi: 10.1038/nrc.2017.13. [DOI] [PubMed] [Google Scholar]
- 159.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020:1–3. doi: 10.1007/s00134-020-05991-x. [published online ahead of print, 2020 Mar 3] [published correction appears in Intensive Care Med. 2020 Apr 6;:] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sacramento C.Q., de Melo G.R., de Freitas C.S., et al. The clinically approved antiviral drug sofosbuvir inhibits Zika virus replication. Sci Rep. 2017;7 doi: 10.1038/srep40920. [published correction appears in Sci Rep. 2017 Apr 24;7:46772] Published 2017 Jan 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Saini K.S., Lanza C., Romano M., et al. Repurposing anticancer drugs for COVID-19-induced inflammation, immune dysfunction, and coagulopathy. Br J Canc. 2020:1–4. doi: 10.1038/s41416-020-0948-x. [published online ahead of print, 2020 Jun 22] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sakamoto J., Hamada C., Rahman M., et al. An individual patient data meta-analysis of adjuvant therapy with carmofur in patients with curatively resected colon cancer. Jpn J Clin Oncol. 2005;35(9):536-544. doi: 10.1093/jjco/hyi147. [DOI] [PubMed] [Google Scholar]
- 163.SchönrichG, Raftery M.J. The PD-1/PD-L1 axis and virus infections: a delicate balance. Front Cell Infect Microbiol. 2019;9:207. doi: 10.3389/fcimb.2019.00207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Schor S., Einav S. Repurposing of kinase inhibitors as broad-spectrum antiviral drugs. DNA Cell Biol. 2018;37(2):63-69. doi: 10.1089/dna.2017.4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Schwarz S., Wang K., Yu W., Sun B., Schwarz W. Emodin inhibits current through SARS-associated coronavirus 3a protein. Antivir Res. 2011;90(1):64-69. doi: 10.1016/j.antiviral.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Shah K.P., Song H., Ye F., et al. Demographic factors associated with toxicity in patients treated with anti-programmed cell death-1 therapy. Canc Immun. 2019 doi: 10.1158/2326-6066.CIR-19-0986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Shang J., Ye G., Shi K., et al. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020 doi: 10.1038/s41586-020-2179-y. [published online ahead of print, 2020 Mar 30] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Sharma P., Zargar-Shoshtari K., Sexton W.J. Valrubicin in refractory non-muscle invasive bladder cancer. Expert Rev Anticancer Ther. 2015;15(12):1379-1387. doi: 10.1586/14737140.2015.1115350. [DOI] [PubMed] [Google Scholar]
- 169.Shen L.W., Mao H.J., Wu Y.L., Tanaka Y., Zhang W. TMPRSS2: a potential target for treatment of influenza virus and coronavirus infections. Biochimie. 2017;142:1–10. doi: 10.1016/j.biochi.2017.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sheng Z., Li G., Li B., Liu Y., Wang L. Carfilzomib-containing combinations as frontline therapy for multiple myeloma: a meta-analysis of 13 trials. Eur J Haematol. 2017;98(6):601-607. doi: 10.1111/ejh.12877. [DOI] [PubMed] [Google Scholar]
- 171.Shin J.S., Jung E., Kim M., Baric R.S., Go Y.Y. Saracatinib inhibits Middle East respiratory syndrome-coronavirus replication in vitro. Viruses. 2018;10(6):283. doi: 10.3390/v10060283. Published 2018 May 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Shirato K., Kawase M., Matsuyama S. Middle East respiratory syndrome coronavirus infection mediated by the transmembrane serine protease TMPRSS2. J Virol. 2013;87(23):12552–12561. doi: 10.1128/JVI.01890-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Shulla A., Heald-Sargent T., Subramanya G., Zhao J., Perlman S., Gallagher T. A transmembrane serine protease is linked to the severe acute respiratory syndrome coronavirus receptor and activates virus entry. J Virol. 2011;85(2):873–882. doi: 10.1128/JVI.02062-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Siddiqi H.K., Mehra M.R. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J Heart Lung Transplant. 2020;39(5):405–407. doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sisk J.M., Frieman M.B., Machamer C.E. Coronavirus S protein-induced fusion is blocked prior to hemifusion by Abl kinase inhibitors. J Gen Virol. 2018;99(5):619-630. doi: 10.1099/jgv.0.001047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Song D.D., Zhou J.H., Sheng R. Regulation and function of sphingosine kinase 2 in diseases. Histol Histopathol. 2018;33(5):433-445. doi: 10.14670/HH-11-939. [DOI] [PubMed] [Google Scholar]
- 177.Song K., Dai L., Long X., Cui X., Liu Y., Di W. Sphingosine kinase 2 inhibitor ABC294640 displays anti-epithelial ovarian cancer activities in vitro and in vivo. OncoTargets Ther. 2019;12:4437-4449. doi: 10.2147/OTT.S208519. Published 2019 Jun 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Song Y., Wu J., Chen X., et al. A single-arm, multicenter, phase II study of camrelizumab in relapsed or refractory classical Hodgkin lymphoma. Clin Canc Res. 2019;25(24):7363–7369. doi: 10.1158/1078-0432.CCR-19-1680. [DOI] [PubMed] [Google Scholar]
- 179.Song Y., Zhou K.S., Zou D., et al. Treatment of patients with relapsed or refractory mantle cell lymphoma with zanubrutinib, a selective inhibitor of bruton's tyrosine kinase. Clin Canc Res. 2020 doi: 10.1158/1078-0432.CCR-19-3703. [published online ahead of print, 2020 May 27] clincanres.3703.2019. [DOI] [PubMed] [Google Scholar]
- 180.Spicka I., Ocio E.M., Oakervee H.E., et al. Randomized phase III study (ADMYRE) of plitidepsin in combination with dexamethasone vs. dexamethasone alone in patients with relapsed/refractory multiple myeloma. Ann Hematol. 2019;98(9):2139-2150. doi: 10.1007/s00277-019-03739-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Stebbing J., Phelan A., Griffin I., et al. COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect Dis. 2020;20(4):400-402. doi: 10.1016/S1473-3099(20)30132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Stephens R.S., Johnston L., Servinsky L., Kim B.S., Damarla M. The tyrosine kinase inhibitor imatinib prevents lung injury and death after intravenous LPS in mice. Phys Rep. 2015;3(11) doi: 10.14814/phy2.12589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Stewart A.K., Rajkumar S.V., Dimopoulos M.A., et al. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N Engl J Med. 2015;372(2):142-152. doi: 10.1056/NEJMoa1411321. [DOI] [PubMed] [Google Scholar]
- 184.Sudhop T., Brun N.C., Riedel C., Rosso A., Broich K., Senderovitz T. Master protocols in clinical trials: a universal Swiss Army knife? Lancet Oncol. 2019;20(6):e336–e342. doi: 10.1016/S1470-2045(19)30271-2. [DOI] [PubMed] [Google Scholar]
- 185.Syed Y.Y. Selinexor: first global approval. Drugs. 2019;79(13):1485–1494. doi: 10.1007/s40265-019-01188-9. [DOI] [PubMed] [Google Scholar]
- 186.Syed Y.Y. Zanubrutinib: first approval. Drugs. 2020;80(1):91-97. doi: 10.1007/s40265-019-01252-4. [DOI] [PubMed] [Google Scholar]
- 187.Tanaka S., Chen-Yoshikawa T.F., Kajiwara M., et al. Protective effects of imatinib on ischemia/reperfusion injury in rat lung. Ann Thorac Surg. 2016;102(5):1717-1724. doi: 10.1016/j.athoracsur.2016.05.037. [DOI] [PubMed] [Google Scholar]
- 188.Tatar G., Turhan K. Investigation of N Terminal domain of SARS CoV 2 nucleocapsid protein with antiviral compounds based on molecular modeling approach. Sci Open Preprint. 2020 doi: 10.14293/S2199-1006.1.SOR-.PPPT99I.v1. [DOI] [Google Scholar]
- 189.Thompson M.A. The return of PRO 140, a CCR5-directed mAb. Curr Opin HIV AIDS. 2018;13(4):346-353. doi: 10.1097/COH.0000000000000479. [DOI] [PubMed] [Google Scholar]
- 190.Thompson P.A., et al. Preclinical evaluation of eFT226, a novel, potent and selective eIF4A inhibitor with anti-tumor activity in B-cell malignancies. Blood. 2017;130(Suppl 1):1530. [Google Scholar]
- 191.Tilmanis D., van Baalen C., Oh D.Y., Rossignol J.F., Hurt A.C. The susceptibility of circulating human influenza viruses to tizoxanide, the active metabolite of nitazoxanide. Antivir Res. 2017;147:142–148. doi: 10.1016/j.antiviral.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 192.Tini G., Duso B.A., Bellerba F., et al. Semantic and geographical analysis of COVID-19 trials reveals a fragmented clinical research landscape likely to impair informativeness. Front Med. 2020;7:367. doi: 10.3389/fmed.2020.00367. (Lausanne) Published 2020 Jun 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Treon S.P., Castillo J.J., Skarbnik A.P., et al. The BTK inhibitor ibrutinib may protect against pulmonary injury in COVID-19-infected patients. Blood. 2020;135(21):1912-1915. doi: 10.1182/blood.2020006288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Uddin M.H., Zonder J.A., Azmi A.S. Exportin 1 inhibition as antiviral therapy [published online ahead of print, 2020 Jun 20] Drug Discov Today. 2020 doi: 10.1016/j.drudis.2020.06.014. S1359-6446(20)30239-30247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.van Boheemen S., de Graaf M., Lauber C., et al. Genomic characterization of a newly discovered coronavirus associated with acute respiratory distress syndrome in humans. mBio. 2012;3(6) doi: 10.1128/mBio.00473-12. e00473-12. Published 2012 Nov 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.van den Heuvel-Eibrink M.M., Hol J.A., Pritchard-Jones K., et al. Position paper: rationale for the treatment of Wilms tumour in the UMBRELLA SIOP-RTSG 2016 protocol. Nat Rev Urol. 2017;14(12):743–752. doi: 10.1038/nrurol.2017.163. [DOI] [PubMed] [Google Scholar]
- 197.van Hemert M.J., van den Worm S.H., Knoops K., Mommaas A.M., Gorbalenya A.E., Snijder E.J. SARS-coronavirus replication/transcription complexes are membrane-protected and need a host factor for activity in vitro. PLoS Pathog. 2008;4(5) doi: 10.1371/journal.ppat.1000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Vannucchi A.M., Kiladjian J.J., Griesshammer M., et al. Ruxolitinib versus standard therapy for the treatment of polycythemia vera. N Engl J Med. 2015;372(5):426–435. doi: 10.1056/NEJMoa1409002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Venugopal S., Bar-Natan M., Mascarenhas J.O. JAKs to STATs: a tantalizing therapeutic target in acute myeloid leukemia. Blood Rev. 2020;40:100634. doi: 10.1016/j.blre.2019.100634. [DOI] [PubMed] [Google Scholar]
- 200.Verstovsek S., Mesa R.A., Gotlib J., et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012;366(9):799-807. doi: 10.1056/NEJMoa1110557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Vogel C.L., Johnston M.A., Capers C., Braccia D. Toremifene for breast cancer: a review of 20 years of data. Clin Breast Canc. 2014;14(1):1-9. doi: 10.1016/j.clbc.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 202.Vogl D.T., Dingli D., Cornell R.F., et al. Selective inhibition of nuclear export with oral selinexor for treatment of relapsed or refractory multiple myeloma. J Clin Oncol. 2018;36(9):859–866. doi: 10.1200/JCO.2017.75.5207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Waksman S.A., Woodruff H.B. Bacteriostatic and bactericidal substances produced by a soil actinomyces. Proc Soc Exp Biol Med. 1940;45:609. [Google Scholar]
- 204.Waldman A.D., Fritz J.M., Lenardo M.J. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol. 2020:1-18. doi: 10.1038/s41577-020-0306-5. [published online ahead of print, 2020 May 20] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181(2):281–292. doi: 10.1016/j.cell.2020.02.058. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Wang J. Fast identification of possible drug treatment of coronavirus disease-19 (COVID-19) through computational drug repurposing study [published online ahead of print, 2020 may 4] J Chem Inf Model. 2020 doi: 10.1021/acs.jcim.0c00179. acs.jcim. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Wang Q., Zhang Y., Wu L., et al. Structural and functional basis of SARS-CoV-2 entry by using human ACE2. Cell. 2020 doi: 10.1016/j.cell.2020.03.045. [published online ahead of print, 2020 Apr 7] S0092-8674(20)30338-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Weis S.M., Cheresh D.A. Pathophysiological consequences of VEGF-induced vascular permeability. Nature. 2005;437(7058):497–504. doi: 10.1038/nature03987. [DOI] [PubMed] [Google Scholar]
- 209.Wolfram J., Nizzero S., Liu H., et al. A chloroquine-induced macrophage-preconditioning strategy for improved nanodelivery. Sci Rep. 2017;7(1):13738. doi: 10.1038/s41598-017-14221-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Wu C., Chen X., Cai Y., et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in wuhan, China. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.0994. [published online ahead of print, 2020 mar 13] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Wu M., Gui H., Feng Z., et al. KPT-330, a potent and selective CRM1 inhibitor, exhibits anti-inflammation effects and protection against sepsis. Biochem Biophys Res Commun. 2018;503(3):1773–1779. doi: 10.1016/j.bbrc.2018.07.112. [DOI] [PubMed] [Google Scholar]
- 212.Xie L., Xu J., Sun X., et al. Apatinib plus camrelizumab (anti-PD1 therapy, SHR-1210) for advanced osteosarcoma (APFAO) progressing after chemotherapy: a single-arm, open-label, phase 2 trial. J Immunother Canc. 2020;8(1) doi: 10.1136/jitc-2020-000798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Xu M., Lee E.M., Wen Z., et al. Identification of small-molecule inhibitors of Zika virus infection and induced neural cell death via a drug repurposing screen. Nat Med. 2016;22(10):1101–1107. doi: 10.1038/nm.4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Xu W., Sun X., Wang B., Guo H. Pooled analysis of the reports of carfilzomib/ixazomib combinations for relapsed/refractory multiple myeloma. Ann Hematol. 2018;97(2):299-307. doi: 10.1007/s00277-017-3173-9. [DOI] [PubMed] [Google Scholar]
- 215.Yang N., Shen H.M. Targeting the endocytic pathway and autophagy process as a novel therapeutic strategy in COVID-19. Int J Biol Sci. 2020;16(10):1724–1731. doi: 10.7150/ijbs.45498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Yang Y., Peng F., Wang R., et al. The deadly coronaviruses: the 2003 SARS pandemic and the 2020 novel coronavirus epidemic in China. J Autoimmun. 2020;109:102434. doi: 10.1016/j.jaut.2020.102434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Yao T.T., Qian J.D., Zhu W.Y., Wang Y., Wang G.Q. A systematic review of lopinavir therapy for SARS coronavirus and MERS coronavirus-A possible reference for coronavirus disease-19 treatment option. J Med Virol. 2020 doi: 10.1002/jmv.25729. [published online ahead of print, 2020 Feb 27] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Zhang C., Tian B. Nonclinical safety assessment of zanubrutinib: a novel irreversible BTK inhibitor. Int J Toxicol. 2020;39(3):232-240. doi: 10.1177/1091581820918511. [DOI] [PubMed] [Google Scholar]
- 219.Zhang Q., Qi Z., Bo-Liu, Li C.S. Programmed cell death-1/programmed death-ligand 1 blockade improves survival of animals with sepsis: a systematic review and meta-analysis. BioMed Res Int. 2018;2018:1969474. doi: 10.1155/2018/1969474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Zhang X., Tan Y., Ling Y., et al. Viral and host factors related to the clinical outcome of COVID-19. Nature. 2020 doi: 10.1038/s41586-020-2355-0. [published online ahead of print, 2020 May 20] [DOI] [PubMed] [Google Scholar]
- 221.Zhao M. Cytokine storm and immunomodulatory therapy in COVID-19: role of chloroquine and anti-IL-6 monoclonal antibodies. Int J Antimicrob Agents. 2020:105982. doi: 10.1016/j.ijantimicag.2020.105982. [published online ahead of print, 2020 Apr 16] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Zhao Y., Ren J., Harlos K., et al. Toremifene interacts with and destabilizes the Ebola virus glycoprotein. Nature. 2016;535(7610):169-172. doi: 10.1038/nature18615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Zheng M., Gao Y., Wang G., et al. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol Immunol. 2020 doi: 10.1038/s41423-020-0402-2. [published online ahead of print, 2020 Mar 19] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Zhou P., Ma B., Xu S., et al. Knockdown of Burton's tyrosine kinase confers potent protection against sepsis-induced acute lung injury. Cell Biochem Biophys. 2014;70(2):1265-1275. doi: 10.1007/s12013-014-0050-1. [DOI] [PubMed] [Google Scholar]
- 225.Zhou Y., Hou Y., Shen J., Huang Y., Martin W., Cheng F. Network-based drug repurposing for novel coronavirus 2019-nCoV/SARS-CoV-2. Cell Discov. 2020;6:14. doi: 10.1038/s41421-020-0153-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Zhu N., Zhang D., Wang W., et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Ziogas D.C., Terpos E., Kastritis E., Dimopoulos M.A. An overview of the role of carfilzomib in the treatment of multiple myeloma. Expert Opin Pharmacother. 2017;18(17):1883–1897. doi: 10.1080/14656566.2017.1404575. [DOI] [PubMed] [Google Scholar]
- 228.Nabavi S.F., Habtemariam S., Clementi E., et al. Lessons learned from SARS-CoV and MERS-CoV: FDA-approved Abelson tyrosine-protein kinase 2 inhibitors may help us combat SARS-CoV-2. Arch Med Sci. 2020;16(3):519–521. doi: 10.5114/aoms.2020.94504. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.