Abstract
Purpose
To assess SARS-CoV-2 virus in conjunctival tears and secretions of positive confirmed COVID-19 patients.
Methods
A case series study that included 28 positive COVID-19 patients confirmed with nasopharyngeal swab in the period 18–28 May 2020 at Sohag Tropical Medicine Hospital. Tears and conjunctival secretions of these confirmed positive cases were collected with disposable sampling swabs at interval of 3 days after admission due to respiratory symptoms. They were examined for the presence of SARS-CoV-2 by reverse transcription-polymerase chain reaction (RT‐PCR) assay.
Results
Thirteen (46.43%) patients were stable, 4 (14.28%) patients suffered from dyspnea, 3 (10.72%) patients suffered from high fever, 5 (17.85%) patients suffered from cough, and 3 (10.72%) patients were on mechanical ventilation. Ten (35.71%) patients suffered from conjunctivitis. Tear and conjunctival swabs were positive in 8 (28.57%) patients, while other patients’ swabs were negative (71.43%). Out of 10 patients with conjunctival manifestations, 3 patients had SARS-CoV-2 in their conjunctiva using (RT‐PCR) test. Out of the 18 patients with no conjunctival manifestations, 5 patients had positive SARS-CoV-2 in their conjunctiva using (RT‐PCR) test.
Conclusion
The SARS-CoV-2 virus could be found in tears and conjunctival secretions in SARS-CoV-2 patients with or without conjunctivitis.
Keywords: SARS-CoV-2, conjunctiva, conjunctival secretions, tears, swab, RT‐PCR
Introduction
An outbreak of SARS-CoV-2 occurred in Wuhan, China in December 2019. It is a highly infectious disease with severe respiratory symptoms in many cases that may lead to death. The World Health Organization (WHO) considered it a pandemic and published guide for all countries for protection and treatment with a changeable protocol according to the situation.1,2
The presence of the virus in body secretions such as saliva, nasal secretions and tears (eyes) is confirmed; however, its transmission through the conjunctiva is still under investigation, though its presence in the conjunctiva enforces the possibility of its transmission through the eye, especially to ophthalmologists.3
Although great concern has been declared to COVID-19 infection acquired through ocular transmission, the underlying mechanism of this transmission has not currently been clarified.4
Few previous studies have evaluated ophthalmological signs and symptoms in patients infected with SARS-CoV-1 and SARS-CoV-2. A few studies have evaluated the presence of SARS-CoV-2 in tear fluid.5
Many ophthalmologists worldwide are infected during routine or emergency practice, as recorded by their health authorities. The infection may be due to eye route, droplets, breath or others.6 The proximity between the ophthalmologist and the patient raises the chance of infection. The presence of SARS-CoV-2 virus in patients’ tears with or without ocular symptoms suggests contamination through the conjunctiva in the maneuvers needing intimate conjunctival evaluation or direct contact with the conjunctiva; however, the possibility of transmission by other routes is still present.7,8
Recommendations for ophthalmologists to have protective measures to avoid infections, emergency practice only, telemedicine for suitable cases, and strict personal protective equipment are recommended by the WHO and the American Academy of Ophthalmology AAO; otherwise, many ophthalmologists in the world could be infected with deficiency in ophthalmology health-care services affecting the population besides the fatal pandemic.9,10
The aim of this study was to assess the presence of SARS-CoV-2 virus in the conjunctival tears and secretions of positive confirmed patients.
Patients and Methods
A case series study that included 28 positive COVID-19 patients confirmed with nasopharyngeal swab in the period 18–28 May 2020 at Sohag Tropical Medicine Hospital.
Hospitalized patients with positive SARS-CoV-2 were examined using a portable slit lamp or by-side examination (more used) for ocular manifestations and then were recorded. Conjunctival swabs were taken and examined by (RT‐PCR) for the presence of SARS-CoV-2.
Ethical Consideration
Full ethical considerations were followed and comprehensive written informed consent was taken from all involved patients in this study. The present study adhered to the tenants of Helsinki Declaration and ethical board committee. The approval of our institution (Sohag Faculty of Medicine) was obtained with ethical committee number: IBR# S20-129.
This study did not include children but only adult patients. The patients did not bear any expenses for the conjunctival swabs.
Method of Conjunctival Swab
Conjunctival swabs were taken after a time interval of 3 days after admission due to respiratory symptoms to ensure the presence of higher viral load levels to be detected by (RT‐PCR). [11–12] Personal protective equipment (PPE) used for tear film swabs was the same as personal protective equipment, which was used for handling Kit reagents, PPE included gloves, eye protection and lab coats. Conjunctival swabs were taken from samples of the inferior conjunctival sac13 and collected by Virus specimen transport For Molecular and Culture Techniques (Sigma Virocult®) (Figure 1A) it was done through eversion of the lower eyelids by sterile cotton tips to explore the lower fornices of the eye. Topical anesthesia was not used, and the tips of the sterile sticks were placed into liquid Virocult ® medium. Sterile gloves were used and changed between patients to avoid contamination risk either for the samples or patients. Samples were put on ice and rapidly transmitted to the PCR laboratory. Virus specimen transport for molecular and culture techniques.
Figure 1.
(A) Virus specimen transport For Molecular and Culture Techniques (Sigma Virocult®) (B) Applied Biosystem® 7500 Real-Time PCR System. (C) QIAamp DSP Virus spin kit, cat. No. 61704, QIagen Inc.
Qualitative (RT‐PCR) for the detection of SARS-CoV-2 viral RNA14,15
RNA Extraction
SARS-CoV-2 viral RNA was extracted using a fully automated QIAcube instrument and using specific kits; Qiagen columns (QIAamp DSP Virus spin kit, cat. No. 61704, QIagen Inc.) (Figure 1C) according to the manufacturer’s instructions.
II-(RT‐PCR).
SARS-CoV-2 viral RNA was quantified using specific TaqMan® probe-based technology (The Genesig® (RT‐PCR) Coronavirus (COVID-19) kit cat. No. Z-Path-COVID-19-CE, Primerdesign Ltd)) and StepOne (RT‐PCR) system (Applied Biosystem® 7500 (RT‐PCR) System, CA, USA) (Figure 1B) according to the manufacturer’s instructions.
The reaction mixture was used in a total volume of 20 µ, including 8 µ of the sample extract, negative extraction control (NEC) or positive control template (PCT) and 12 µ of the Master Mix, prepared by: 10 µ oasig qPCR OneStep Master Mix and 2 µ Coronavirus (COVID-19) CE IVD Primer/Probe.
The real-time cycler conditions were performed according to the following conditions: reverse transcription at 55°C for 10 min and then at 95°C for 2 min to activate the Taq enzyme (initial denaturation) followed by 45 amplification cycles. The cycle consisted of denaturation at 95°C for 10 seconds, primer annealing and extension at 60°C for 60 seconds.
The oligonucleotide primers and probe for the detection of SARS-CoV-2 were selected from the orf1ab genome region. The supplied primer/probe mix was designed for the specific detection of SARS-CoV-2 RNA (probe labeled with FAM fluorophore) and the supplied genesig® Easy RNA internal Extraction control (ICE-specific probe labeled with HEX fluorophore).
The sensitivity of the Coronavirus test CE IVD genesig® kit included detection of 0.58 copies/µL of COVID-19 viral RNA with a confidence ≥95%. This concentration therefore serves as the limit of detection of the kit. As regards the test specificity; this was assessed with in silico sequence comparison analyses and in vitro specimen testing. In silico analysis of the genesig® (RT‐PCR) Coronavirus (COVID-19) CE IVD design was found to detect all COVID-19 virus strains and exhibited no cross-reactivity with non-COVID-19 species.
Data were collected and recorded.
Results
A total of 28 COVID-19 patients were included in this study. Out of them, there were 15 (53.57%) men and 13 (46.43%) women with a mean age (Mean±SD) is (51.79±11.81). Thirteen (46.42%) patients were stable, 4 (14.28%) patients suffered from dyspnea, 3 (10.72%) patients suffered from high fever, 5 (17.86%) patients suffered from cough and 3 (10.72%) patients were on mechanical ventilation. Ten (35.71%) suffered from conjunctivitis. Tear and conjunctival swabs were positive in 8 (28.57%) patients, while other patients’ swabs were negative (71.43%).
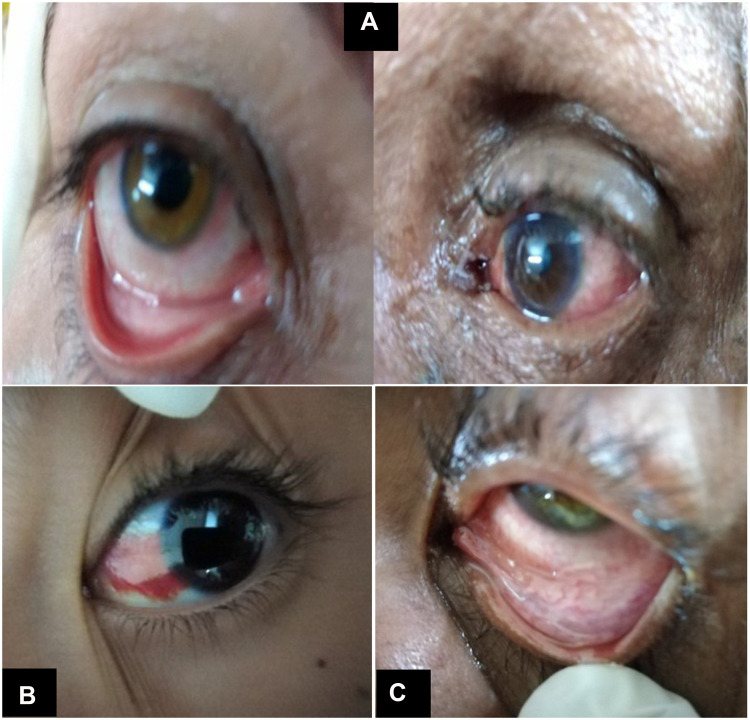
Ten (35.71%) patients had conjunctival manifestations in the form of follicular conjunctivitis (Figure 2A), subconjunctival hemorrhage (Figure 2B) and conjunctival hyperemia (Figure 2C). General and chest manifestations, ranging from mild to severe manifestations (examined by intensive care and chest specialists) along with the presence of conjunctival manifestations and (RT‐PCR) results are shown in (Table 1).
Figure 2.
(A) Follicular conjunctivitis. (B) Subconjunctival hemorrhage. (C) Conjunctival hyperemia.
Table 1.
Characteristics of the Examined Patients
| Patient (ID) | Age (Years) | Sex | General Condition* | Patient’s Onset Time ** | Conjunctival Manifestations *** | Conjunctival Swab PCR Result | Threshold Cycle Value (Cycle)**** |
|---|---|---|---|---|---|---|---|
| 1 | 66 | Male | Dyspnea | Third day | Present | Positive | 22.43 |
| 2 | 25 | Male | Stable | Third day | Free | Negative | ——— |
| 3 | 55 | Female | High grade fever | Third day | Present | Positive | 28.72 |
| 4 | 58 | Male | On ventilator | Third day | Free | Negative | ——— |
| 5 | 68 | Male | On ventilator | Third day | Present | Positive | 17.35 |
| 6 | 36 | Female | Dyspnea | Third day | Present | Negative | ——— |
| 7 | 74 | Female | Stable | Third day | Free | Negative | ——— |
| 8 | 44 | Male | Stable | Third day | Free | Positive | 34.91 |
| 9 | 25 | Female | Dyspnea | Third day | Free | Negative | ———– |
| 10 | 54 | Male | Stable | Third day | Present | Negative | ———– |
| 11 | 62 | Female | Dyspnea | Third day | Free | Positive | 20.65 |
| 12 | 54 | Female | Stable | Third day | Free | Negative | ——— |
| 13 | 38 | Male | High grade fever | Third day | Present | Negative | ——— |
| 14 | 45 | Female | Severe cough | Third day | Free | Positive | 25.85 |
| 15 | 47 | Female | On ventilator | Third day | Free | Negative | ——— |
| 16 | 49 | Male | Stable | Third day | Present | Negative | ——— |
| 17 | 45 | Male | Stable | Third day | Free | Negative | ——— |
| 18 | 58 | Female | High grade fever | Third day | Free | Positive | 29.38 |
| 19 | 45 | Male | Stable | Third day | Free | Negative | ——— |
| 20 | 70 | Female | Cough | Third day | Present | Positive | 31.57 |
| 21 | 48 | Male | Cough | Third day | Free | Negative | ——— |
| 22 | 51 | Female | Stable | Third day | Free | Negative | ——— |
| 23 | 52 | Male | Stable | Third day | Present | Negative | ———– |
| 24 | 53 | Male | Cough | Third day | Free | Negative | ——— |
| 25 | 49 | Male | Stable | Third day | Free | Negative | ——— |
| 26 | 61 | Female | Cough | Third day | Present | Negative | ——— |
| 27 | 66 | Male | Stable | Third day | Free | Negative | ——— |
| 28 | 52 | Female | Stable | Third day | Free | Negative | ——— |
Notes: *General condition: respiratory manifestations (cough, dyspnea); High grade fever (temperature> 38.5 degree Celsius °C); stable (moderate respiratory manifestations without respiratory failure). **Patient’s onset time: Onset time interval of Patient’s manifestations at the time of sampling. *** Conjunctival manifestations: Follicular conjunctivitis; Subconjunctival hemorrhage; Conjunctival hyperemia. **** Threshold cycle value for patients with positive conjunctival swabs.
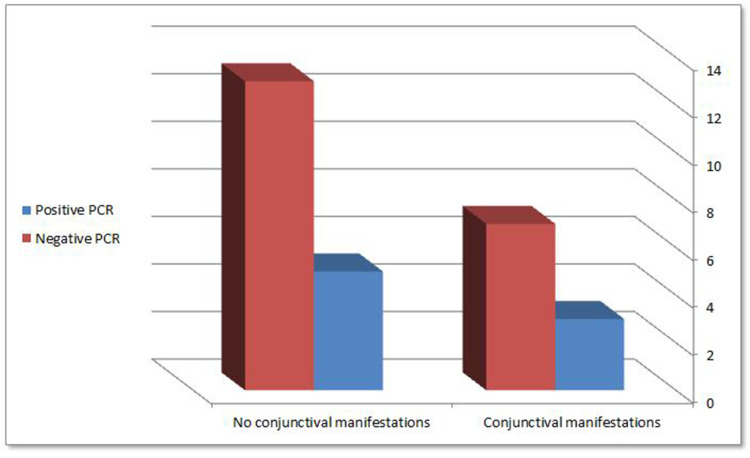
Out of the 10 patients with conjunctival manifestations, 3(30%) patients had SARS-CoV-2 virus in their conjunctiva by (RT‐PCR) test. From the other 18 patients with no conjunctival manifestations, 5 (27.78%) patients had positive SARS-CoV-2 virus in their conjunctiva by (RT‐PCR), with no statistically significant difference between both positive and negative conjunctival swabs patients (P value < 0.9) (RT‐PCR) results are shown in Figure 3.
Figure 3.
(RT‐PCR) results in symptomatic and asymptomatic patients (P value < 0.9).
Discussion
The Coronaviridae virus family consists of enveloped viruses with a large plus-strand RNA genome that is capped and polyadenylated.16 The serology of each type is characterized by a specific host range and genome sequence. The most pathogenic form of the types of coronaviruses is SARS-CoV, which causes life-threatening pneumonia.17
The pandemic of coronavirus disease in 2019 resulted in the suspension or sharp reduction of various ophthalmic activities considered non-urgent. There are currently little-shared and vague recommendations among the various countries on safety in ophthalmic operating rooms due little data about virus transmission through ocular secretions.18
A few previous researches have evaluated the presence of SARS-CoV-2 RNA in tear fluid in patients.19 Our research suggests that COVID-19 patients, who have ocular manifestations or not, can shed RNA in their conjunctival secretions and tears. It is not related to the presence of the symptoms or signs either general or ocular.
The results of the current study showed positive results for SARS-CoV-2 RNA in conjunctival swabs of patients with COVID-19 in 28.5% of patients. Published reports have shown that RNA shedding can occur in asymptomatic patients, which may be a very dangerous source of infection for ophthalmologists and other people.3 A previous study reported that unprotected eyes were associated with a high risk of transmission of SARS-CoV-1.20 This result enforces our current results due to the similarity between the two viruses.
In a study by Xia J et al,21 they assessed the presence of coronavirus (SARS-CoV-2) in tears and conjunctival secretions of COVID-19-infected patients. Only two samples of tear and conjunctival secretions were obtained from one patient with conjunctivitis and showed positive (RT‐PCR) results, while fifty-eight samples from other patients were all negative.
Moreover, in a study by Seah IYJ et al,22 they assessed the presence of SARS-CoV-2 by viral isolation and quantitative (RT-PCR) analysis in seventeen COVID-19 patients. Interestingly, their results showed that all samples (64 samples) showed negative results for SARS-CoV-2 on viral isolation and (RT‐PCR).
In previous studies,3,20–22 the positive rate of the virus detected in the conjunctival sac did not exceed 10%, while in the present study, the positive rate was close to 30%. This can be interpreted due to several factors, such as differences in race, hygiene practices,23 the weather such as humidity and ultraviolet exposure, or whether there is a possibility of virus mutation. Many studies showed there is a correlation between environmental factors and SARS-CoV-2 transmission, other studies did not support that.24–26 so, further studies are needed in the future to confirm the results of the current study.
In 2 case reports,27,28 bilateral follicular conjunctivitis in a COVID-19 positive patient with positive ocular swabs were documented. These reports suggest that tears can be a potential source of infection early in the course of the disease and that the conjunctiva may sustain viral RNA replication for a longer period of time.
Although RNA was detected in the conjunctival secretions, more researches are still needed to get rid of the logistic problems in specimen taking and RNA study of the conjunctiva of COVID-19 patients.
In conclusion, SARS-CoV-2 could be found through tears and conjunctival secretions in COVID-19 patients with or without conjunctivitis. Further studies are needed to confirm these results.
Abbreviations
(RT‐PCR), reverse transcriptase-polymerase chain reaction (SARS-CoV-2), severe acute respiratory syndrome.
Disclosure
The article has not been presented in a meeting. The authors did not receive any financial support from any public or private sources. The authors have no financial or proprietary interest in the product, method, or material described herein. The authors report no conflicts of interest for this work.
References
- 1.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. doi: 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun P, Qie S, Liu Z, Ren J, Li K, Xi J. Clinical characteristics of 50466 hospitalized patients with 2019‐nCoV infection. J Med Virol. 2020. doi: 10.1002/jmv.25735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loon SC, Teoh SC, Oon LL, et al. The severe acute respiratory syndrome coronavirus in tears. Br J Ophthalmol. 2004;88(7):861–863. doi: 10.1136/bjo.2003.035931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Napoli PE, Nioi M, d’Aloja E, Fossarello M. The ocular surface and the coronavirus disease 2019: does a dual ‘ocular route’ exist? J Clin Med. 2020;9(5):1269. doi: 10.3390/jcm9051269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peiris JSM, Yuen KY, Osterhaus ADME, Stöhr K. The severe acute respiratory syndrome. N Engl J Med. 2003;349(25):2431–2441. doi: 10.1056/NEJMra032498 [DOI] [PubMed] [Google Scholar]
- 6.Jorstad OK, Moe MC, Eriksen K, Petrovski G, Bragadottir R. Coronavirus disease 2019 (COVID-19) outbreak at the Department of Ophthalmology, Oslo University Hospital, Norway. Acta Ophthalmol. 2020. doi: 10.1111/aos.14426 [DOI] [PubMed] [Google Scholar]
- 7.Lu CW, Liu XF, Jia ZF. 2019-nCoV transmission through the ocular surface must not be ignored. Lancet (London, England). 2020;395:e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li JO, Lam DS, Chen Y, Ting DS. Novel Coronavirus disease 2019 (COVID-19): the importance of recognising possible early ocular manifestation and using protective eyewear. Br J Ophthalmol. 2020;104:297–298. doi: 10.1136/bjophthalmol-2020-315994 [DOI] [PubMed] [Google Scholar]
- 9.Seittzman GD, Doan T. No time for tears. Ophthalmology. 2020;127:7. doi: 10.1016/j.ophtha.2020.03.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Radonovich LJ Jr., Simberkoff MS, Bessesen MT. N95 respirators vs medical masks for preventing influenza among health care personnel: a randomized clinical trial. JAMA. 2019;322(9):824–833. doi: 10.1001/jama.2019.11645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pan Y, Zhang D, Yang P, Poon LLM, Wang Q. Viral load of SARS-CoV-2 in clinical samples. Lancet Infect Dis. 2020;20(4):411–412. doi: 10.1016/S1473-3099(20)30113-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang Y, Chen S, Yang Z, et al. SARS-CoV-2 Viral Load in Clinical Samples from Critically Ill Patients. Am J Respir Crit Care Med. 2020;201(11):1435–1438. doi: 10.1164/rccm.202003-0572LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lamagna B, Pasolini MP, Nizza S, et al. Conjunctival cytological examination, bacteriological culture and antimicrobial resistance profiles of healthy Mediterranean buffaloes (Bubalus bubalis) from Southern Italy. Asian Pac J Trop Biomed. 2015;5(11):889–895. [Google Scholar]
- 14.Aziz H, Fatima S, Iqbal H, Faheem M. Recent advances in molecular diagnosis curbing the COVID-19. Int J Infect Dis. 2020;97:322–325. doi: 10.1016/j.ijid.2020.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poljak M, Korva M, Knap Gašper N, et al. Clinical Evaluation of the cobas SARS-CoV-2 test and a diagnostic platform switch during 48 hours in the Midst of the COVID-19 Pandemic. J Clin Microbiol. 2020;58(6):e00599–20. doi: 10.1128/JCM.00599-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lai MM. SARS virus: the beginning of the unraveling of a new coronavirus. J Biomed Sci. 2003;10:664–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drosten C, Gu¨nther S, Preiser W, et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med. 2003;348:1967–1976. [DOI] [PubMed] [Google Scholar]
- 18.Napoli PE, Nioi M, d’Aloja E, Fossarello M. Safety recommendations and medical liability in ocular surgery during the COVID-19 Pandemic: an unsolved dilemma. J Clin Med. 2020;9(5):1403. doi: 10.3390/jcm9051403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoehl S, Berger A, Kortenbusch M, et al. Evidence of SARS-CoV-2 infection in returning travelers from Wuhan, China. N Engl J Med. 2020. doi: 10.1056/NEJMc2001899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raboud J, Shigayeva A, McGeer A, et al. Risk factors for SARS transmission from patients requiring intubation: a multicentre investigation in Toronto, Canada. PLoS One. 2010;5(5):e10717. doi: 10.1371/journal.pone.0010717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xia J, Tong J, Liu M, Shen Y, Guo D. Evaluation of coronavirus in tears and conjunctival secretions of patients with SARS-CoV-2 infection. J Med Virol. 2020;92(6):589–594. doi: 10.1002/jmv.25725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seah IYJ, Anderson DE, Kang AEZ, et al. Assessing viral shedding and infectivity of tears in Coronavirus Disease 2019 (COVID-19) patients. Ophthalmology. 2020;127(7):977–979. doi: 10.1016/j.ophtha.2020.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lodder W, de Roda Husman AM. SARS-CoV-2 in wastewater: potential health risk, but also data source. Lancet Gastroenterol Hepatol. 2020;5(6):533–534. doi: 10.1016/S2468-1253(20)30087-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang J. High temperature and high humidity reduce the transmission of COVID-19. SSRN Elec J. 2020. doi: 10.2139/ssrn.3551767 [DOI] [Google Scholar]
- 25.Qi H, Xiao S, Shi R, Ward MP, Chen Y, Tu W. COVID-19 transmission in Mainland China is associated with temperature and humidity: a time-series analysis. Sci Total Environ. 2020;728. doi: 10.1016/j.scitotenv.2020.138778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang M, Jiang A, Gong L, et al. Temperature significantly change COVID-19 transmission in 429 cities. medRxiv. 2020. doi: 10.1101/2020.02.22.20025791 [DOI] [Google Scholar]
- 27.Chen L, Liu M, Zhang Z, et al. Ocular manifestations of a hospitalized patient with confirmed 2019 novel coronavirus disease. Br J Ophthalmol. 2020:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Colavita F, Lapa D, Carletti F, et al. SARS-CoV-2 isolation from ocular secretions of a patient with COVID-19 in Italy with prolonged viral RNA detection. Ann Intern Med. 2020;173(3):242–243. doi: 10.7326/M20-1176 [DOI] [PMC free article] [PubMed] [Google Scholar]