Modern medicine is threatened by the global rise of antibiotic resistance, especially among Gram-negative bacteria. Metallo-β-lactamase (MBL) enzymes are a particular concern and are increasingly disseminated worldwide, though particularly in Asia. Many MBL producers have multiple further drug resistances, leaving few obvious treatment options. Nonetheless, and more encouragingly, MBLs may be less effective agents of carbapenem resistance in vivo, under zinc limitation, than in vitro.
KEYWORDS: metallo-β-lactamase, treatment, pharmacology, drug development, metalloenzymes
ABSTRACT
Modern medicine is threatened by the global rise of antibiotic resistance, especially among Gram-negative bacteria. Metallo-β-lactamase (MBL) enzymes are a particular concern and are increasingly disseminated worldwide, though particularly in Asia. Many MBL producers have multiple further drug resistances, leaving few obvious treatment options. Nonetheless, and more encouragingly, MBLs may be less effective agents of carbapenem resistance in vivo, under zinc limitation, than in vitro. Owing to their unique structure and function and their diversity, MBLs pose a particular challenge for drug development. They evade all recently licensed β-lactam–β-lactamase inhibitor combinations, although several stable agents and inhibitor combinations are at various stages in the development pipeline. These potential therapies, along with the epidemiology of producers and current treatment options, are the focus of this review.
INTRODUCTION
Antimicrobial therapy is threatened by the global rise of resistance, especially in Gram-negative bacteria (1), where resistance to β-lactams is largely mediated by β-lactamases (2). Carbapenems evade most β-lactamases but are hydrolyzed by metallo-β-lactamases (MBLs) as well as by a few active-site serine β-lactamases (SBLs), notably members of the KPC and OXA-48-like groups. MBLs are chromosomal and ubiquitous in some nonfermenters, including Stenotrophomonas maltophilia, Aeromonas spp., and Chryseobacterium spp., which are of modest clinical concern. A minority of Bacteroides fragilis strains have a chromosomally encoded MBL, CfiA or CcrA, but this is uncommon and is expressed strongly only if an upstream insertion sequence provides an efficient promoter (3). More important are the acquired MBLs that are spreading among members of the Enterobacterales and Pseudomonas aeruginosa (4); these are associated with extremely drug-resistant (XDR) phenotypes, with the producers generally also being resistant to multiple aminoglycosides, fluoroquinolones, and other agents as well as to β-lactams.
CLASSIFICATION AND DIVERSITY OF METALLO-β-LACTAMASES
β-Lactamases are classified by two major systems. The first is based on substrate profiles and vulnerability to inhibitors (5) and places MBLs in its group 3, whereas groups 1 and 2 comprise SBLs. The second classifies β-lactamases according to their amino acid sequences, recognizing four enzyme classes (6). MBLs form class B, while SBLs are divided among classes A, C, and D (7). The MBLs are structurally and mechanistically dissimilar from SBLs, suggesting a separate evolutionary origin.
Class B enzymes are further divided into three subclasses—B1, B2, and B3—based on differences in amino acid sequence at the active site, zinc ligands, zinc stoichiometry, loop architecture, and substrate profiles (8). The important acquired MBLs, comprising the IMP, NDM, and VIM types, fall into subclass B1. They hydrolyze all currently available β-lactam antibiotics except monobactams (e.g., aztreonam) (9), as do most or all other subclass B1 or B3 enzymes. In contrast, the CphA (subclass B2) MBLs of Aeromonas spp. have narrow-spectrum activity directed exclusively against carbapenems. Irrespective of subclass, MBLs are not inhibited by clavulanic acid, sulbactam, tazobactam, or avibactam or by developmental penicillanic acid sulfones and diazabicyclooctanes.
The important acquired subgroup B1 MBLs (Table 1) are mostly named based on where they were first described; thus, for example, Verona integron-encoded metallo-β-lactamase (VIM) and New Delhi metallo-β-lactamase (NDM). The first acquired MBL (imipenemase; IMP-1), was reported from clinical isolates of P. aeruginosa and Serratia marcescens in Japan in the 1990s (10), and its family now includes over 85 sequence variants (11). The first VIM enzyme was found in P. aeruginosa in 1997 (12), with over 69 variants since described (11). NDM—now the most prevalent MBL in Enterobacterales and Acinetobacter baumannii—was first identified in 2008 in Klebsiella pneumoniae and Escherichia coli isolates from a patient who had travelled to Sweden from New Delhi, India (13). Twenty-nine NDM variants have since been described, (11).
TABLE 1.
Examples of chromosomal and plasmid-associated MBLs (11)
| MBL type | Species | Enzyme(s) | Subclass |
|---|---|---|---|
| Chromosomal | Bacillus cereus | BcII | B1 |
| Chryseobacterium indologenes | IND | B1 | |
| Elizabethkingia meningoseptica | BlaB | B1 | |
| Myroides odoratimimus | MUS and MYO | B1 | |
| Bacteroides fragilisa | CfiA/CcrA | B1 | |
| Aeromonas spp. | CphA | B2 | |
| Stenotrophomonas maltophilia | L1 | B3 | |
| Elizabethkingia meningoseptica | GOB | B3 | |
| Plasmid associated | Verona integron-encoded metallo-β-lactamase (VIM) | B1 | |
| New Delhi metallo-β-lactamase (NDM) | B1 | ||
| Imipenemase (IMP) | B1 | ||
| Sao Paulo metallo-β-lactamase (SPM) | B1 | ||
| German imipenemase (GIM) | B1 | ||
| KHM | B1 | ||
| Dutch imipenemase (DIM) | B1 | ||
| Serratia metallo-β-lactamase (SMB) | B3 | ||
| Adelaide imipenemase (AIM) | B3 | ||
Unlike most other chromosomal MBLs, the Bacteroides fragilis enzyme is rare in the species.
It is easy to be dismissive of the chromosomal subclass B2 and B3 MBLs, but recent reports highlight Stenotrophomonas maltophilia as a multidrug-resistant pathogen in immunocompromised hosts (14). S. maltophilia carries a subclass B3 MBL (L1 enzyme), which is unique among MBLs in having four identical subunits (15), in addition to a chromosomally mediated SBL (L2 enzyme). This combination confers resistance to almost all β-lactams, although MICs vary with methodology, because media affect the expression and/or function of these enzymes (16). Elizabethkingia meningoseptica has two chromosomal MBLs, a B1 enzyme (BlaB) and a B3 type (GOB), with the former predominantly contributing to resistance (17).
GENETIC SUPPORT OF ACQUIRED MBLS
Acquired IMP and VIM enzymes generally are encoded by gene cassettes within class 1 or class 3 integrons. These may be embedded within transposons, allowing insertion into the bacterial chromosome or plasmids (18). In contrast, the blaNDM gene is not integron associated and has been observed on narrow-host-range plasmids belonging to incompatibility group IncF, in addition to wide-host-range plasmids belonging to IncA/C, IncL/M, IncH, and IncN (19–22). K. pneumoniae and E. coli are the frequent hosts of these plasmids, and there are particular associations with K. pneumoniae sequence type 11 (ST11), ST14, ST15, and ST147 and E. coli ST167, ST410, or ST617 (23). These should not, however, be seen as global epidemic strains along the lines of K. pneumoniae ST258 variants with KPC carbapenemases, for many are common STs without carbapenemases. In A. baumannii, the blaNDM-1 gene is generally located within the composite transposon Tn125 and embedded between two copies of a strong promoter gene ISAba125 (24, 25); it is much less prevalent in this genus than are OXA carbapenemases (class D).
B2 and B3 MBLs are generally chromosomally encoded, ubiquitous in their host species, and not transmissible. However, exceptions exist, with horizontal transfer having been observed. Thus, the AIM-1 MBL (B3) was initially reported, in 2012, to be encoded by a gene inserted in (and atypical of) the chromosome of a P. aeruginosa isolate; subsequently, in 2019, it was reported from K. pneumoniae (26). The blaLMB-1 gene, encoding another subclass B3 enzyme, was reported to be located on a plasmid in Rheinheimera pacifica, where it was flanked by ISCR mobilization sequences, implying transfer from some other (unknown) source organism. (27). Mobilization of blaSMB-1, encoding a third subclass B3 enzyme, has occurred similarly (28).
STRUCTURE AND CATALYTIC FUNCTION OF MBLS
Irrespective of subgroup, MBLs contain the αβ/βα fold typical of the metallo-hydrolase/oxidoreductase superfamily (29). The S. maltophilia enzyme has four identical subunits (15), whereas other MBLs are monomeric.
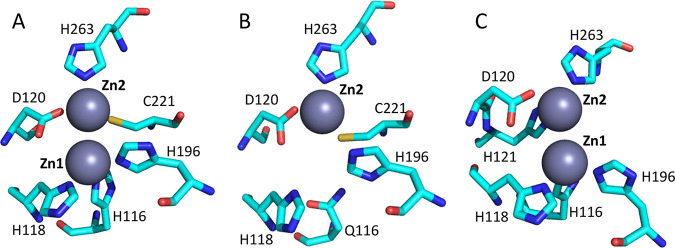
B1 and B3 MBLs have a shallow active-site groove containing 1 or 2 catalytically functional divalent zinc ions, flanked by flexible loops (29). In contrast, the B2 enzymes have an active site that is less accessible and flanked by a helix (30). Except for these consistencies, MBLs are highly divergent even within subclasses and have as little as 20% sequence identity between subclasses (7). Figure 1 illustrates the amino acid residues that bind zinc at the active sites of B1, B2, and B3 MBLs (8).
FIG 1.
Amino acid residues that bind zinc at the active sites of B1, B2, and B3 MBLs. (A) Crystal structures of B1 enzymes, including IMP, VIM, NDM, and B. fragilis CcrA, reveal two zinc-binding sites (Zn1 and Zn2). The Zn1 site contains three histidine residues (His116, His118, and His196), whereas the ligands for the Zn2 site are aspartic acid (Asp120), cysteine (Cys221), and histidine (His263). (B and C) There is only one zinc ion in the active site of the Aeromonas hydrophila enzyme (subclass B2) (B) and two in the active site S. maltophilia enzyme (subclass B3) (C). (Republished with permission from reference 8.)
Mechanistically, the zinc ion(s) activates a water molecule, which acts to open the β-lactam ring (31). There is no covalent intermediate, as with SBL-mediated catalysis. Anionic intermediates have been characterized when MBLs hydrolyze carbapenems (32), but not when NDM-1 enzymes hydrolyze penicillins or cephalosporins (33). In general, imipenem and meropenem are similarly good substrates for MBLs: for example, NDM-1 displays similar catalytic activity, reflected in values of the kcat/Km ratio, for imipenem (0.09 μM−1 s−1) and meropenem (0.06 μM−1 s−1) (34); biapenem is a weaker substrate, owing to high Km values, but seems unsuitable for high-dose development (35).
Differences in assay methodology between workers make it difficult to compare hydrolytic efficiencies for different MBLs. Variation within, e.g., the VIM, IMP, Sao Paulo metallo-β-lactamase (SPM), and German imipenemase (GIM) family appears largely inconsequential (36). Nevertheless, subtle but important evolution may be ongoing, as illustrated in the NDM family. Here, experimental data do not define major differences in the catalytic efficiencies among NDM-1, -3, -4, -5, -6, -7, and -8 enzymes (37) under standard conditions, but differences are seen under zinc deprivation. Thus, studies comparing NDM-1, NDM-4 (Met154Leu), and NDM-12 (Met154Leu, Gly222Asp) demonstrate that the Met154Leu substitution, present in 50% of clinical NDM variants in some locales, enhances the ability to confer resistance at low Zn2+ concentrations (38, 39). This is potentially important because, as discussed below, zinc is restricted in infection (40) and its scarcity may impede the ability of classical NDM-1 enzyme to confer clinical resistance. NDM variants that have increased affinity for zinc (up to ∼10-fold-decreased dissociation constant for zinc [Kd, Zn2]) display selective advantages in experiments that mimic zinc scarcity imposed by the host immune system (41). Perhaps driven by similar pressures, the NDM-15 variant has evolved to function efficiently as a mono- rather than a bi-zinc enzyme (41). In addition, there are suggestions that NDM enzymes are evolving to develop greater thermodynamic stability (37).
EPIDEMIOLOGY AND DISTRIBUTION OF ACQUIRED MBLs
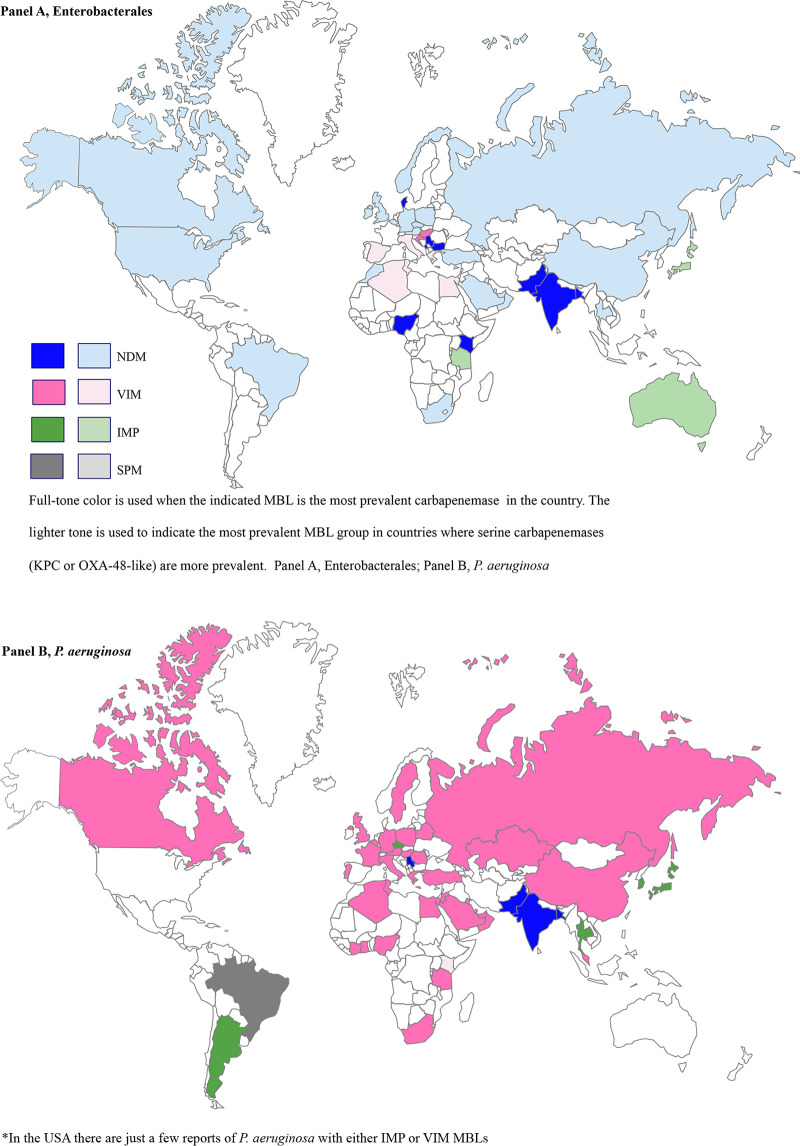
Bacteria with IMP, VIM, and NDM enzymes have been identified in a range of community, hospital, and environmental settings (42). Their prevalence and their importance relative to serine carbapenemases vary greatly by country. Figure 2 illustrates the global distribution of acquired MBLs.
FIG 2.
Global distribution of acquired MBLs.
Indian subcontinent, Asia, and Russia.
The greatest burden of acquired, plasmid-mediated MBLs lies in South and Southeast Asia (43), where NDM types are prevalent. As already noted, blaNDM-1 was first identified in bacteria isolated in 2008 from a patient who had travelled to Sweden from India (44). NDM variants have subsequently been spread worldwide via patient transfers and travel (45). Epidemiological surveillance has confirmed that NDM-1 and its variants are widely disseminated throughout India, Pakistan, and Bangladesh (46, 47); moreover, a review of 39 carbapenem-resistant Enterobacterales (CRE) collected in India in 2006-2007 by the SENTRY Antimicrobial Surveillance Program found that 15 harbored blaNDM-1 (48), indicating that it was circulating prior to its “discovery” in 2008. Enterobacterales with blaNDM were isolated from public tap water in India (49) and in river systems around pilgrimage sites (42), demonstrating that the gene has become established beyond health care environments.
In India, there is frequent cocarriage with other carbapenemases in Enterobacterales (50); thus, in 2012, of 113 nonclonal CRE isolates at a Mumbai hospital, 106 produced NDM enzymes, and 21 of these also had a second carbapenemase, most often an OXA-48-like (n = 17) or VIM-type (n = 4) enzyme. Surprisingly, given that most international reports of NDM enzymes relate to Enterobacterales, P. aeruginosa was the most common MBL host (24%) among 3,414 carbapenem-resistant Gram-negative bacteria collected from community and hospital settings in North India (51), with blaNDM-1 (36%) being the most prevalent carbapenemase gene, followed by blaVIM (18.4%).
Although KPC is the principal carbapenemase among Enterobacterales (CPE) in China, a survey across 25 provinces showed that 32% of phenotypic carbapenem resistance in Enterobacterales was linked to blaNDM-1 (52), while a study (2012 to 2016) of clinical Enterobacter cloacae across three tertiary hospitals found blaNDM-1 to be the most common carbapenemase gene (80%), followed by blaIMP-26 (8%) and blaIMP-4 (6%) (53). The importance of IMP MBLs, particularly IMP-4, in China has been underscored by others; thus, multiple Enterobacterales species carrying a plasmid encoding IMP-4 enzyme were identified from patients with epidemiological links to China (54), and surveillance at a Beijing hospital highlighted both IMP-4 and NDM-1 in K. pneumoniae (55). Colocalization of blaNDM-9 and the plasmid-mediated colistin resistance gene mcr-1 was seen in an E. coli strain recovered from retail chicken meat in Guangzhou, China (56). Having been recognized 30 years ago in Japan, IMP-type enzymes are now endemic there, though not highly prevalent (57).
NDM MBLs are the second most prevalent carbapenemases after OXA-48 in the Middle East, excepting Israel (58, 59). This probably reflects extensive interactions with the Indian subcontinent. As in India, there is significant penetration of blaNDM into P. aeruginosa, for which a much greater proportion of carbapenem resistance appears to be carbapenemase mediated in the Middle East than in Europe or the United States. Thus, in the Gulf Cooperation Council countries, blaVIM was found in 39% of carbapenem-resistant P. aeruginosa isolates (60), with most hosts belonging to internationally disseminated high risk clones, including ST235, ST111, ST233, ST654, and ST357 (60). These lineages seem unusually adept at acquiring extrinsic resistance genes. In Dubai, 32% of resistant P. aeruginosa isolates produced VIM-type MBLs (61), though a larger proportion had outer membrane impermeability.
The proportion of carbapenem-resistant P. aeruginosa harboring MBLs in Russia rose from 4.5% between 2002 and 2004 to 28.7% between 2008 and 2010 (62), largely reflecting the spread of an XDR blaVIM-2-positive ST235 high-risk clone, also present in Belarus and Kazakhstan (62). NDM is reported as the predominant carbapenemase among Enterobacterales in St. Petersburg (63, 64), whereas OXA-48 is predominant in Moscow (65).
Europe.
Although Italy had earlier reported both IMP and VIM enzymes (66), Greece was the first European country to report extensive dissemination of Enterobacterales with MBLs. Specifically, K. pneumoniae with VIM carbapenemases were reported from multiple hospitals in 2003 to 2007, and multilocus sequence typing identified three major clonal complexes (CCs)—CC147, CC18, and CC14—among the producers (67). By 2006, 20% of K. pneumoniae isolates collected from hospital wards and 50% of those from intensive care units (ICUs) monitored by the Greek System for the Surveillance of Antimicrobial Resistance were carbapenem resistant, largely owing to the spread of the blaVIM-1 cassette (68). By 2010, KPC had displaced VIM to become the dominant carbapenemase in Greece, largely through the spread of a K. pneumoniae ST258 variant (69). Nonetheless, VIM types remained scattered, and they may now be reemerging due to suppression of the KPC carbapenemases via the use of ceftazidime-avibactam (70).
Elsewhere in Europe, concern about carbapenemases grew following a flurry of press interest in NDM enzymes from 2008 to 2010 and with the spread of K. pneumoniae ST258/512 lineages with KPC carbapenemases in Italy from 2010. The United Kingdom, taken as an example, recorded a few P. aeruginosa and Enterobacterales strains with IMP and VIM MBLs before 2008. Thereafter, the number of Enterobacterales with NDM enzymes increased (46). Most early cases were imports via patients who had travelled to (and often been hospitalized in) the Indian subcontinent. Multiple NDM variants have subsequently been reported in the United Kingdom, with NDM-1 being the most frequent among Enterobacterales, followed by NDM-5 and NDM-7 (71). In contrast, VIM variants account for 91% of the (uncommon) MBLs in P. aeruginosa, again associated with international high-risk clones, including ST235, ST111, ST233, and ST357 (72).
While referral of CPE isolates to the national reference laboratory has increased 100-fold since 2008, many producers are from screening rather than clinical samples. OXA-48 is now the fastest-spreading carbapenemase, but isolates with NDM enzymes account for 20 to 25% of CPE submitted. A growing minority of these, particularly E. coli, have both NDM- and OXA-48-like enzymes (71, 73).
In 2012, the European Centre for Disease Prevention and Control launched its European Survey of Carbapenemase-Producing Enterobacteriaceae (EuSCAPE) project. The geographic distribution of enzyme types was estimated by national experts across 38 European countries in 2015 (74). A random sample of carbapenem-susceptible and -nonsusceptible K. pneumoniae and E. coli strains subsequently were collected prospectively to determine the occurrence of carbapenemases (75). The results, published in 2017, revealed that SBLs (KPC or OXA-48 enzymes) were more prevalent than MBLs in most countries but that MBLs were widely scattered and were the most prevalent carbapenemases among Enterobacterales in a few countries. Thus, VIM enzymes were the dominant carbapenemases in Hungary, and NDM enzymes were dominant in Serbia and Montenegro. The prevalence of NDM enzymes in the latter countries tallies with early descriptions of producers linked to these Balkan states. It is unclear whether these originated as imports from India or as independent local gene escapes from the unknown source organism (76).
North America.
Infections due to Enterobacterales carrying blaVIM-2, blaVIM-7, blaIMP-4, and blaIMP-18 genes were recorded in the United States prior to 2005 but, in general, MBLs remained extremely rare (1, 77). In 2010, Enterobacterales harboring NDM-1 were isolated from three patients in different states (78) and, as with many contemporaneous cases in the United Kingdom and elsewhere, the source patients had all recently been in India or Pakistan (21). Subsequent expansion of NDM enzymes in the United States has been less marked than in the United Kingdom, with KPC carbapenemases becoming considerably more prevalent. Nevertheless, until December 2017, 379 CPE with NDM carbapenemases were reported to the CDC from 34 states, with just under a third (i.e., 109) from Illinois (79), where an outbreak was associated with contaminated endoscopes.
Enterobacterales with NDM enzymes have been increasing in Canada since 2008, and these MBLs are now the second most common carbapenemases in the country, with a higher prevalence in the western provinces (80). Surveillance conducted between 2007 and 2015 in Toronto revealed that, among 291 clinical CPE, 51% had NDM enzymes, and 24% of the patients had never received health care abroad or travelled to high-risk areas (81), suggesting that the enzymes are established locally. In 2019, a novel MBL, blaCAM-1, was identified from isolates that were collected in 2007 (82). No subsequent isolates harboring this gene have been reported.
Africa.
Because of the paucity of data, the prevalence of CPE carrying MBLs in Africa is difficult to estimate. Apparent infrequency may reflect true rarity, limited sampling, or a lack of infrastructure for accurate detection. CPE with VIM MBLs nonetheless have been identified in Nigeria, Morocco, Algeria, Tunisia, Tanzania, and South Africa, and CPE with NDM enzymes have been found in Kenya, Nigeria, Morocco, Algeria, Tunisia, Tanzania, and South Africa (83, 84). Infections caused by Enterobacterales producing MBLs are reported from both imported and local cases, raising concerns regarding emerging endemicity (85). Those with IMP-type enzymes have been identified in small numbers in Morocco, Tunisia, and Tanzania and appear genuinely uncommon (84). An outbreak caused by Klebsiella spp. carrying blaNDM-5 was reported from a neonatal unit in Nigeria (86). A concern is that African patients are strongly represented in medical tourism to India, which is a risk factor for colonization with Enterobacterales producing MBLs (87).
Rest of the world.
KPC enzymes dominate among carbapenemases from Enterobacterales in Latin America, with (unusually) some penetration also into P. aeruginosa. Nonetheless, Enterobacterales with NDM enzymes are endemic in Brazil, with several outbreaks reported (88). Early case reports of MBL-producing Enterobacterales in Latin America often concerned members of the Proteeae, including Providencia spp. and Morganella (89, 90), which are infrequent hosts of blaNDM elsewhere. This creates a treatment issue, since these genera are inherently resistant to polymyxins and newer tetracyclines, which remain options against other MBL-producing Enterobacterales (below).
Unique to South America is the wide distribution in Brazil of P. aeruginosa with SPM-1 MBL (91), principally associated with an ST277 clone. Outcomes of severe infections with this clone are often poor, reflecting a lack of good treatment options (92).
Carbapenemases are rare in Australasia, but there is spread of blaIMP-4 among Enterobacterales (93), as in parts of China. E. cloacae is a major host, with dissemination mediated by an IncHI2 plasmid (94). Production of IMP-4 has also been recorded in Salmonella spp. from domestic pets (95) and seagulls (96), but the significance of this is uncertain.
MBL FUNCTION IN RESISTANCE, IN VITRO AND IN VIVO
For many years MBLs were perceived as clinically unimportant chromosomally encoded enzymes from nonpathogenic organisms, notably Bacillus cereus (97, 98). This perception changed with the recognition that MBLs confer much of the resistance seen in Chryseobacterium spp. and E. meningoseptica (99) and with heightened awareness of the morbidity and mortality associated with S. maltophilia bacteremia (100, 101). Interest then escalated with the discovery and proliferation of acquired MBLs, especially NDM-1, which drew extensive press coverage in 2010.
Many MBL producers are broadly resistant in vitro and, on this basis, real concern exists about lack of treatments. On the other hand, there is evidence that in vivo resistance to carbapenems may be less than it appears in vitro, because susceptibility tests are conventionally done in media (e.g., cation-adjusted Mueller-Hinton broth) with high zinc concentrations (102), whereas the host immune system imposes a state of zinc deprivation in infection (40, 103). This lack of zinc may not only impede the catalytic function of MBLs but may also interfere with their protein folding (102) and may promote degradation of the enzyme in the periplasm (104).
Several preclinical studies suggest a disconnect between high-level in vitro resistance to carbapenems associated with NDM-1 enzymes and a weak ability to protect against carbapenems in standard murine infection models (105). Moreover, NDM enzymes appear to be less effective than other carbapenemases in causing resistance to carbapenems in patients (106, 107). Thus, mortality in severe infections due to Enterobacterales with blaNDM appears to be relatively low, ranging from 13% (108) to 55% (109), compared to that seen with bacteria expressing other MBLs (18% to 67%) (13) or KPC carbapenemases (41% to 65%) (110, 111). Good clinical outcomes have been reported despite treatment with agents to which NDM enzymes confer resistance in vitro (106, 107, 112). As yet, there are no studies that confirm or refute whether the higher-numbered NDM alleles, encoding variants with greater affinity for zinc (above), are better able to cause clinical resistance than NDM-1 (39, 41).
Finally, it should be underscored that while these indications that NDM MBLs are less potent in vivo are intriguing, they should be approached with caution. Double-blinded randomized controlled trials have not been conducted, and existing outcome data are subject to various biases (113, 114). For VIM MBLs, clinical outcomes correlate with carbapenem MICs, implying little or no such in vitro-in vivo discordance (115).
CURRENT TREATMENT OPTIONS
Limited data exist to inform clinicians on the optimal treatment for infections caused by MBL-producing Gram-negative bacteria (106). Cotrimoxazole remains the standard of care for infections due to S. maltophilia, but most Enterobacterales with acquired MBLs also have sul and dfr genes, conferring resistance. Genes encoding resistance to fluoroquinolones and aminoglycosides are often present alongside genes encoding acquired MBLs. In particular, blaNDM genes are often linked to the genes encoding ArmA or RmtB methyltransferases, which modify ribosomes to block binding of aminoglycosides, including plazomicin; blaIMP and blaVIM generally occur within integrons that often also carry aac(6′), encoding an acetyltransferase that compromises amikacin and tobramycin, though not gentamicin or plazomicin (116). A thorough review of treatment options for MDR and XDR Enterobacterales is available (117). This highlights observational studies comparing monotherapy to combination therapy for bloodstream infection (BSI) involving CRE, although few of these were specifically identified as having MBLs (118, 119).
Colistin.
Colistin is the current mainstay of treatment for infections due to MBL producers. A multinational survey of MBL-producing Enterobacterales and P. aeruginosa conducted from 2012 to 2014 found >97% susceptibility among MBL-producing P. aeruginosa strains (variously with IMP, VIM, and NDM enzymes) and >85% for MBL-producing Enterobacterales (>86.1% NDM type and >88.9% IMP type) (83). Exceptions are Proteeae and Serratia spp., which have intrinsic polymyxin resistance.
For bacteria harboring KPC and OXA-48 carbapenemases, colistin has recently been shown to be less effective than microbiologically active β-lactamase inhibitor combinations (120), making it plausible that an active β-lactam likewise would be more efficacious than colistin against MBL producers. Of note, the emergence of colistin resistance during treatment, with secondary transmission of resistant variants, is a concern (121, 122).
Tigecycline, omadacycline, and eravacycline.
The tetracyclines tigecycline, omadacycline, and eravacycline have strong in vitro activity against many MBL-producing Enterobacterales, except Proteeae, although not against P. aeruginosa. During November 2018, 275 unique Enterobacterales isolates carrying blaNDM collected by the U.S. Centers for Disease Control and Prevention were tested with tigecycline (86.5% susceptible, based on a ≤2-μg/ml FDA breakpoint), eravacycline (66.2% susceptible, based on a ≤0.5-μg/ml FDA breakpoint), and omadacycline (59.6% susceptible, based on a ≤4-μg/ml breakpoint) (123). The higher susceptibility rate for tigecycline than eravacycline reflects the higher FDA breakpoint for Enterobacterales; in Europe, both agents have an identical 0.5-μg/ml breakpoint, and eravacycline is the more active on a simple gravimetric basis, though it is unclear whether this confers a clinical advantage (124). Merits of omadacycline are its minimal known drug interactions and its ability to be administered orally (125); however, it has the least relevant license (for community-acquired bacterial pneumonia and acute bacterial skin and skin structure infections) in relation to the clinical burden of MBL producers.
While the in vitro activity of these tetracyclines is encouraging, there are multiple caveats. First, tigecycline carries an FDA “black box” warning of increased mortality when the drug was used as monotherapy (126); second, both tigecycline and eravacycline have failed to achieve noninferiority to comparators in one or more clinical trials (ventilator-associated pneumonia and diabetic foot infection for tigecycline, complicated urinary tract infection [cUTI] for eravacycline); third, there is little provenance for tetracyclines as monotherapy in the severely ill patients who commonly develop infections due to MBL-producing opportunists; fourth, particularly for tigecycline, the disparity between EUCAST (≤0.5 μg/ml) and FDA (≤2 μg/ml) susceptibility breakpoints creates categorization uncertainty; last, the lack of anti-Proteeae activity is important in Latin America, where Providencia spp. are frequent hosts of blaNDM (127). Given these uncertainties, the best advice is to consider using these tetracyclines in combination against MBL producers, not as monotherapy.
Aztreonam.
Aztreonam is stable to MBLs, though activity is lost against organisms that coproduce extended-spectrum β-lactamases (ESBLs) or AmpC enzymes (128), which are common in MBL-producing Enterobacterales. Clinical experience with aztreonam as monotherapy is lacking for MBL producers, although some success was recorded when aztreonam was used in combination with ceftazidime-avibactam (129, 130), with avibactam serving to inhibit ESBLs. Six of 10 patients survived following treatment with this combination during an outbreak of K. pneumoniae with NDM-1, OXA-48, and CTX-15 β-lactamases in Barcelona (129). Although no adverse events were reported, the safety is unclear, and it is difficult to match the regimen of aztreonam-avibactam (1.5 g + 0.5 g every six hours [q6h]) that is presently being developed (see below).
Fosfomycin.
Fosfomycin commonly retains full in vitro activity against MBL-producing Enterobacterales and has been successful in trials, very recently, as an i.v. agent in cUTI (131). It may be an option against MBL producers—particularly E. coli, which is more susceptible than other Enterobacterales—but it is mainly advocated for use in combination due to concerns about emergence of resistance, particularly in Klebsiella spp. (132). Fosfomycin has little direct antipseudomonal activity, with typical MICs above breakpoints. However, in vitro synergy is seen when fosfomycin is combined with meropenem against MBL-producing P. aeruginosa strains (133), suggesting a need for in vivo exploration.
DEVELOPMENT PIPELINE
The development pipeline represents four main strategies against MBL producers: (i) protection of MBL-stable-monobactams from other coproduced β-lactamases, as, e.g., with aztreonam-avibactam; (ii) development of β-lactams stable to MBLs as well as SBLs, as with, e.g., cefiderocol and BOS-228; (iii) combinations of cephalosporins and carbapenems with triple-action diazabicyclooctanes (DBOs); and (iv) direct inhibition of MBLs with cyclic boronates, thiols, chelators, dicarboxylic acids, and other agents.
Aztreonam-avibactam.
Aztreonam-avibactam is the first antibiotic to be developed under a public-private partnership agreement (134, 135), with partial financing from the European Union’s Innovative Medicine’s Initiative and, latterly, also the U.S. Biomedical Advanced Research and Developmental Authority (BARDA). A prospective randomized phase 3 study (NCT03580044) is scheduled to begin in 2020 to determine efficacy, safety, and tolerability versus best available therapy (BAT) for hospitalized adults with complicated intra-abdominal infections (cIAI), nosocomial pneumonia (NP), cUTI, or BSI due to MBL-producing Gram-negative bacteria (135).
Aztreonam evades hydrolysis by MBLs (128) but is compromised by the ESBL and AmpC enzymes that are coproduced by many MBL-positive CPE. These SBLs are inhibited by avibactam, a diazabicyclooctane (DBO) (136, 137), and consequently, MBL-producing Enterobacterales that also carry ESBLs or AmpC are susceptible to aztreonam-avibactam in vitro (138) and in vivo (139). The combination is less reliably active against MBL-producing P. aeruginosa (140), because aztreonam has weak antipseudomonal activity.
Considerable interest in this approach exists, because the safety and efficacy of aztreonam are well established and because avibactam was established to be effective at inactivating ESBLs and AmpC enzymes during trials with ceftazidime. Moreover, case reports suggest success against infections caused by MBL producers when aztreonam was coadministered with ceftazidime-avibactam (see “Aztreonam” above) (129, 130).
MBL-stable β-lactams.
(i) Cefiderocol (S-649266). Cefiderocol (S-649266) is a novel parenteral siderophore cephalosporin designed by Shionogi & Co., Ltd., with a catechol linked to its 3-position side chain. It is licensed in the United States for cUTI and in the European Union and United Kingdom for “treatment of infections due to aerobic Gram-negative organisms in adults with limited treatment options” (141). It is retained among developmental agents here, rather than being included in the established treatments, because there is little published experience with MBL producers to date (142, 143).
Critically, the catechol moiety forms a chelation complex with ferric iron, and this complex is actively accumulated by Gram-negative bacteria, which are forced to scavenge this essential element (144). Cefiderocol has good activity in vitro under iron starvation against Gram-negative bacteria, including CPE, P. aeruginosa, and A. baumannii (145). It is relatively stable to both SBLs and MBLs (144); however, the MICs for Enterobacterales and nonfermenters with NDM carbapenemases tend to be slightly higher than those for isolates of the same species with other carbapenemase types (146). Cefiderocol proved effective against carbapenem-resistant P. aeruginosa (expressing IMP-1 enzymes), A. baumannii (expressing OXA-51-like enzymes), and K. pneumoniae (expressing NDM-1 enzymes) in immunocompetent rat respiratory tract infection models, achieving a ≥3-log reduction in the number of viable bacteria in the lungs when given over 4 days so as to recreate the human exposures of an infusion regimen involving 2 g q8h given i.v. for 3 h (147). Efficacy decreased when the infusion time was reduced to 1 h, owing to a lower percentage of the dosing interval during which free-drug concentrations were above the MIC (Tf >MIC) (147). Interestingly, the mean Tf >MIC required for a 1-log10 reduction was 18 to 24% greater for A. baumannii isolates (expressing OXA-23 or OXA-24) in the murine lung infection model than for Enterobacterales expressing NDM-1, NDM-4, or KPC-2 enzymes and for P. aeruginosa isolates expressing IMP-1 or VIM-10 MBLs (148).
In humans, the infusion regimen of 2 g q8h i.v. over 3 h provided >90% probability of target attainment (PTA) with 75% Tf >MIC for MICs of 4 μg/ml for patients with normal renal function (149). A phase 3 trial (NCT03032380) has shown noninferiority to meropenem in nosocomial pneumonia (150). Less encouragingly, another trial (NCT02714595) found excessive deaths in the cefiderocol arm, compared with best available therapy, for patients with severe infections caused by carbapenem-resistant Gram-negative pathogens (151). Full analysis is awaited but, notably, deaths were mostly associated with Acinetobacter infections (152), not Enterobacterales.
(ii) BOS-228 (formerly LYS228). BOS-228 is a monobactam and, like aztreonam, is stable to MBLs (153). Unlike aztreonam, it is also stable to many potent SBLs, including carbapenemases, ESBLs, and AmpC types (154); BOS-228 binds to penicillin-binding protein 3 (PBP3) similarly to aztreonam, in addition to weak binding to PBP1a and PBP1b of Enterobacterales (155). BOS-228 had an MIC90 of 2 μg/ml for a clinical panel of 88 Enterobacterales isolates expressing ESBLs, KPCs, and MBLs (153), and no single-step mutants were selected from 12 β-lactamase-expressing Enterobacterales exposed to the drug at 8× MIC, though mutants were selected from 2/12 strains, neither of which expressed MBLs, at 4× MIC (155).
A randomized evaluator-blinded multicenter phase 2 trial (NCT03354754) to evaluate pharmacokinetics, clinical responses, safety, and tolerability of BOS-228 in cIAI commenced in 2018. The drug is being administered as i.v. monotherapy (without metronidazole) q6h for at least 5 days and compared to standard of care, with outcomes evaluated at day 28. A randomized controlled evaluator-blinded multicenter trial (NCT03377426) in cUTI has also been initiated.
Cephalosporins or carbapenems combined with triple-action DBOs—zidebactam and nacubactam.
Unlike with cyclic boronates (see below), it has not been possible to discover DBOs that directly inhibit MBLs. However, nacubactam and zidebactam are DBO analogs that combine inhibition of SBLs with direct antibacterial activity by inhibiting PBP2 (156). When combined with PBP3-targetted β-lactams, this attack on PBP2 leads to an “enhancer” effect, with further β-lactamase inhibition-independent synergy observed (156, 157). Consequently, cefepime-zidebactam and cefepime- or meropenem-nacubactam combinations are active in vitro against >75% of MBL-producing Enterobacterales and, in cefepime-zidebactam’s case, also against many MBL-producing P. aeruginosa strains (158).
Although the direct antibacterial activity of nacubactam and zidebactam is readily lost via mutations compensating for inhibition of PBP2 (159), the enhancer effect is retained, with many of the mutants consequently remaining susceptible to, e.g., cefepime-zidebactam or meropenem-nacubactam at low concentrations (156, 157). Cefepime-zidebactam is currently the most advanced of these combinations, with a phase 3 trial due to commence (160).
Direct inhibitors of MBLs.
(i) Cyclic boronates—VNRX-5133 (taniborbactam) and QPX7228. Inhibitors that target both SBLs and MBLs are of great interest but have proved difficult to obtain owing to structural and functional differences between and among these enzymes. This combination of inhibitory activities nonetheless has recently been achieved with several cyclic boronates, notably taniborbactam and QPX7228. These mimic the tetrahedral anionic intermediate common to SBL and MBL catalysis (161) and additionally inhibit some penicillin-binding proteins (e.g., PBP 5, which is nonessential) by the same mechanism (162). They represent a considerable expansion in spectrum over vaborbactam, their progenitor, which inhibits only few class A β-lactamases, notably KPC types (163).
Taniborbactam (VenatoRx) is the more advanced of these two boronates and is in phase III trials combined with cefepime (164). It irreversibly inhibits class A, C, and D SBLs and is a reversible competitive inhibitor of VIM and NDM MBLs, though not of IMP types (165). Safety has been established in healthy volunteers (NCT02955459), and the FDA has allowed cefepime-taniborbactam to proceed via a fast-track pathway for the clinical indications of cUTI and cIAI. QPX7728 (QPEX) likewise inhibits both SBLs and MBLs: 50% inhibitory concentrations (IC50) for KPC enzymes are around 2.9 ± 0.4 nM, compared with 22 ± 8 nM for the class C cephalosporinase of E. cloacae P99, 55 ± 25 nM for the NDM-1 MBL, and 14 ± 4 nM for VIM-1. As with taniborbactam, the IC50 for IMP-1 is considerably higher, at 610 ± 70 nM (166). An i.v. combination of QPX7728 with meropenem is being explored. This significantly lowered bacterial counts in murine thigh and lung infection models with carbapenem-resistant K. pneumoniae, P. aeruginosa, and A. baumannii compared to meropenem alone, although strain genotypes were not reported. Unlike taniborbactam, QPX7228 is orally bioavailable, and combinations with ceftibuten and tebipenem were evaluated in vitro against CPE, including those with MBLs (167).
(ii) Thiol-containing MBL inhibitors and chelating agents. Small molecules that bind and/or chelate zinc ions include thiols, dicarboxylates, hydroxamates, and tetrazoles; these are widely reported to inhibit MBLs, but human metalloproteases are vulnerable too, so toxicity may preclude clinical development.
Thiol-containing compounds inhibit all MBL subtypes (B1, B2, and B3) (168), with strong competitive inhibition of IMP-1 by thioester derivatives first being reported in 1999 (169). The dipeptide l-captopril deserves mention in this context. It is used as an ACE (angiotensin-converting enzyme) inhibitor in the treatment of hypertension and is reported also to inhibit MBLs by chelating the active-site zinc ions via its thiol group (170); the corresponding d-stereoisomer is a more potent inhibitor and can potentiate meropenem against strains with VIM-2 MBLs (170). Both captopril isomers act via zinc chelation, and repurposing is attractive, given the known safety of the l-isomer at its licensed dose; however, the economic model for development is yet to be established, and safety issues for the d-isomer need exploration. Other thio-carbonyl compounds, such as thiomandelic acid, exhibit synergy with meropenem against Enterobacterales with VIM, NDM, and IMP enzymes (171).
Bisthiazolidines are carboxylate-containing bicyclic compounds, considered penicillin analogs that inhibit MBLs through a zinc-bridging thiol group and a carboxylate that interacts with K224 (172). The orientations of the carboxylate and thiol moieties create diverse binding that is observed on X-ray crystal structures and has been shown to inhibit all MBL types (173). The bisthiazolidine scaffold inhibits NDM-1 enzymes in vitro, with Ki values in the low micromolar range (from 7 ± 1 to 19 ± 3 μM); they restore imipenem activity against E. coli strains producing NDM-1 (172).
ANT2681 (Antabio) is the lead compound from a series of thiazole carboxylate derivatives which inhibit NDM and VIM through interaction with the dinuclear zinc ion cluster present at the active site (189, 190). It binds in a noncovalent competitive manner with respect to the substrate and is in late-stage preclinical development.
The divalent cation chelator EDTA has raised interest, too, both as an inhibitor of MBLs and because it disrupts the Gram-negative outer membrane and neutralizes various bacterial enzymes and toxins (174, 175). It is widely used in identification tests for MBLs. Sodium calcium EDTA, which is licensed for use for treatment of lead poisoning, reportedly restored imipenem’s activity in vivo against P. aeruginosa strains producing IMP and VIM enzymes and against E. coli strains producing NDM-1 enzyme (176, 177), raising the issue of whether it might be used to potentiate carbapenems in human infections. Elores, which is marketed in India, combines ceftriaxone, sulbactam, and EDTA (178, 179) and reportedly achieved cures of infections due to MBL producers in multiple patients, with no serious adverse events (178). However, prospective and controlled studies are lacking, the dose of EDTA is low, and there remains uncertainty (see above) about the function of NDM-1 in vivo. More negatively, the FDA has placed strict limits on the amount of EDTA permissible even in food (180), and sodium calcium EDTA is capable of producing toxic effects that can be fatal (181). High concentrations of EDTA are likely to strip divalent cations from human metalloenzymes, including matrix metalloproteinases, carbonic anhydrase, and carboxypeptidases, thus limiting clinical applicability.
Aspergillomarasmine A (AMA) is a fungal natural product discovered in the 1960s (182) and reevaluated in the 1980s as an inhibitor of the human metalloproteinase angiotensin-converting enzyme (ACE). AMA inhibits MBLs via a metal ion sequestration mechanism and displays rapid and potent inhibition of NDM-1 and VIM-2 enzymes in vitro (183). It restored the activity of meropenem against a K. pneumoniae strain expressing NDM-1 enzyme in an intraperitoneal murine infection model (184). Again, the hazard of inhibiting human metalloenzymes requires careful investigation.
CHALLENGES FOR THE DEVELOPMENT OF INHIBITORS OF MBLS
One of the biggest challenges in designing MBL inhibitors is the diversity among these enzymes, which share less than one-third sequence identity at their active sites. Thus, for example, taniborbactam and QPX7728 target NDM and VIM enzymes, but not IMP types (185). Development of inhibitors that bind remotely from the active site might overcome this limitation, but possible target areas also vary within class B1 and seem even better able to tolerate mutations than the active site (29). Another challenge is the shallow binding site in B1 enzymes, meaning that inhibitors can carry out only limited interactions (29). Specificity for bacterial MBLs is a further recurring challenge; interactions with human metalloenzymes and contingent toxicity are major concerns. Molecules that solely inhibit MBLs are limited by the fact that many MBL producers also coproduce SBLs, including carbapenemases, meaning that the partner β-lactam must evade these enzymes, that the inhibitor must inactivate both MBLs and SBLs, or that a second inhibitor is required.
Preclinical development is challenging, too, because it is difficult to establish reliable animal models in which MBL-mediated resistance is expressed, perhaps owing to the already-mentioned lack of essential zinc at infection sites. Moreover, bacteria are prone to lose MBL-encoding plasmids, or fail to reliably express them, in murine models, resulting in pharmacodynamic data that suggest meropenem susceptibility (186, 187). Consequently, it is difficult to establish the efficacy of candidate MBL-stable drugs or inhibitor combinations. It is unclear if the same phenomena occur in patients (188), and this requires further research. Irrespective of this aspect, it is also challenging to find and recruit the required number of patients with MBL-producing pathogens to clinical trials. Rapid diagnostics should help, but their use is complicated by cost and the need to deploy them to all trial sites, including in countries where they are not licensed or are licensed only to inform infection control, not treatment.
CONCLUSION
MBLs are disseminating internationally, particularly in Asia, and often are produced by Gram-negative bacteria with extremely broad spectra of in vitro resistance. Unlike for KPC and OXA-48-like carbapenemases, producers are typically not susceptible to recently licensed β-lactamase inhibitor combinations such as ceftazidime-avibactam, meropenem-vaborbactam, imipenem-relebactam, although cefiderocol may be a potential answer. The ability of MBLs to confer resistance to carbapenems may not be as great in vivo as in vitro, though this is uncertain and may vary by enzyme type even within MBL subclasses.
Inhibitors are known, and the developmental compounds boronates, taniborbactam, and QPX7728 are of particular interest. Nonetheless, the quest for effective inhibitors is complicated by differences in active-site structure and zinc ligand interactions among MBLs and by difficulties in the design of appropriate preclinical and clinical trials. Nonboronate inhibitors face toxicity issues, particularly if they interact with other metalloenzymes or are general chelators. Other approaches to overcoming MBLs include avibactam-protected aztreonam; stable β-lactams, notably BOS-228 as well as cefiderocol; and combinations of β-lactams with triple-action DBOs, notably cefepime-zidebactam and meropenem-nacubactam.
And that is the positive aspect on which to close: there is now a diverse and exciting pipeline of potential agents for the treatment of infections caused by bacteria that produce MBLs. It remains to be seen which will be the most effective of these agents.
ACKNOWLEDGMENTS
S.E.B. is funded by a UKRI Innovation Fellowship through the North West MRC Scheme in Clinical Pharmacology and receives research support from Roche Pharma. D.M.L. serves on advisory boards or has ad-hoc consultancy with Accelerate, Allecra, Antabio, Centauri, Entasis, GlaxoSmithKline, J&J, Meiji, Melinta, Menarini, Mutabilis, Nordic, ParaPharm, Pfizer, QPEX, Roche, Sandoz, Shionogi, T.A.Z., Tetraphase, VenatoRx, Wockhardt, and Zambon; has delivered paid lectures for Astellas, bioMérieux, Beckman Coulter, Cardiome, Cepheid, Merck/MSD, Menarini, Nordic, Pfizer, and Shionogi; and has relevant shareholdings or options in Dechra, GSK, Merck, Perkin Elmer, Pfizer, and T.A.Z., amounting to <10% of portfolio value. D.C.H. is a consultant for Selux Diagnostics, Day Zero Diagnostics, Wockhardt Pharmaceuticals, and Shionogi Pharmaceuticals. W.W.H. holds or has recently held research grants with F2G, Astellas Pharma, Spero Therapeutics, Antabio, Allecra, Bugworks, and NAEJA-RGM; holds awards from the Medical Research Council, National Institute of Health Research, FDA, and the European Commission; and has received personal fees in his capacity as a consultant for F2G, Amplyx, Ausperix, Spero Therapeutics, VenatoRx, Pfizer, and BLC/TAZ.
REFERENCES
- 1.Walsh TR, Toleman MA, Poirel L, Nordmann P. 2005. Metallo-β-lactamases: the quiet before the storm? Clin Microbiol Rev 18:306–325. doi: 10.1128/CMR.18.2.306-325.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. 2015. Global action plan on antimicrobial resistance. World Health Organization, Geneva, Swizterland. [Google Scholar]
- 3.Salonen JH, Eerola E, Meurman O. 1998. Clinical significance and outcome of anaerobic bacteremia. Clin Infect Dis 26:1413–1417. doi: 10.1086/516355. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization, Kahlmeter G, Singh N. 2017. Global priority list of antibiotic-resistant bacteria to guide research, discovery and development of new antibiotics. World Health Organization, Geneva, Swizterland. [Google Scholar]
- 5.Bush K, Jacoby GA. 2010. Updated functional classification of β-lactamases. Antimicrob Agents Chemother 54:969–976. doi: 10.1128/AAC.01009-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hall BG, Barlow M. 2005. Revised Ambler classification of β-lactamases. J Antimicrob Chemother 55:1050–1051. doi: 10.1093/jac/dki130. [DOI] [PubMed] [Google Scholar]
- 7.Garau G, García-Sáez I, Bebrone C, Anne C, Mercuri P, Galleni M, Frère J-M, Dideberg O. 2004. Update of the standard numbering scheme for class B β-lactamases. Antimicrob Agents Chemother 48:2347–2349. doi: 10.1128/AAC.48.7.2347-2349.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palzkill T. 2013. Metallo-β-lactamase structure and function. Ann N Y Acad Sci 1277:91–104. doi: 10.1111/j.1749-6632.2012.06796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marshall S, Hujer AM, Rojas LJ, Papp-Wallace KM, Humphries RM, Spellberg B, Hujer KM, Marshall EK, Rudin SD, Perez F, Wilson BM, Wasserman RB, Chikowski L, Paterson DL, Vila AJ, van Duin D, Kreiswirth BN, Chambers HF, Fowler VG, Jacobs MR, Pulse ME, Weiss WJ, Bonomo RA, Bonomo RA. 2017. Can ceftazidime-avibactam and aztreonam overcome β-lactam resistance conferred by metallo-β-lactamases in Enterobacteriaceae? Antimicrob Agents Chemother 61:e02243-16. doi: 10.1128/AAC.02243-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Watanabe M, Iyobe S, Inoue M, Mitsuhashi S. 1991. Transferable imipenem resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother 35:147–151. doi: 10.1128/aac.35.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naas T, Oueslati S, Bonnin RA, Dabos ML, Zavala A, Dortet L, Retailleau P, Iorga BI. 2017. Beta-lactamase database (BLDB)—structure and function. J Enzyme Inhib Med Chem 32:917–919. doi: 10.1080/14756366.2017.1344235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lauretti L, Riccio ML, Mazzariol A, Cornaglia G, Amicosante G, Fontana R, Rossolini GM. 1999. Cloning and characterization of blaVIM, a new integron-borne metallo-β-lactamase gene from a Pseudomonas aeruginosa clinical isolate. Antimicrob Agents Chemother 43:1584–1590. doi: 10.1128/AAC.43.7.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nordmann P, Naas T, Poirel L. 2011. Global spread of carbapenemase-producing Enterobacteriaceae. Emerg Infect Dis 17:1791–1798. doi: 10.3201/eid1710.110655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brooke JS. 2012. Stenotrophomonas maltophilia: an emerging global opportunistic pathogen. Clin Microbiol Rev 25:2–41. doi: 10.1128/CMR.00019-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Denton M, Kerr KG. 1998. Microbiological and clinical aspects of infection associated with Stenotrophomonas maltophilia. Clin Microbiol Rev 11:57–80. doi: 10.1128/CMR.11.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akova M, Bonfiglio G, Livermore DM. 1991. Susceptibility to β-lactam antibiotics of mutant strains of Xanthomonas maltophilia with high- and low-level constitutive expression of L1 and L2 beta-lactamases. J Med Microbiol 35:208–213. doi: 10.1099/00222615-35-4-208. [DOI] [PubMed] [Google Scholar]
- 17.González LJ, Vila AJ. 2012. Carbapenem resistance in Elizabethkingia meningoseptica is mediated by metallo-β-lactamase BlaB. Antimicrob Agents Chemother 56:1686–1692. doi: 10.1128/AAC.05835-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bennett PM. 2008. Plasmid encoded antibiotic resistance: acquisition and transfer of antibiotic resistance genes in bacteria. Br J Pharmacol 153(Suppl 1):S347–S357. doi: 10.1038/sj.bjp.0707607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bonnin RA, Poirel L, Carattoli A, Nordmann P. 2012. Characterization of an IncFII plasmid encoding NDM-1 from Escherichia coli ST131. PLoS One 7:e34752. doi: 10.1371/journal.pone.0034752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Villa L, García-Fernández A, Fortini D, Carattoli A. 2010. Replicon sequence typing of IncF plasmids carrying virulence and resistance determinants. J Antimicrob Chemother 65:2518–2529. doi: 10.1093/jac/dkq347. [DOI] [PubMed] [Google Scholar]
- 21.Rasheed JK, Kitchel B, Zhu W, Anderson KF, Clark NC, Ferraro MJ, Savard P, Humphries RM, Kallen AJ, Limbago BM. 2013. New Delhi metallo-β-lactamase–producing Enterobacteriaceae, United States. Emerg Infect Dis 19:870–878. doi: 10.3201/eid1906.121515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nithya N, Remitha R, Jayasree PR, Faisal M, Manish Kumar PR. 2017. Analysis of β-lactamases, blaNDM-1 phylogeny and plasmid replicons in multidrug-resistant Klebsiella spp. from a tertiary care centre in south India. Indian J Med Res 146:S38–S45. doi: 10.4103/ijmr.IJMR_31_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu W, Feng Y, Tang G, Qiao F, McNally A, Zong Z. 2019. NDM metallo-β-lactamases and their bacterial producers in health care settings. Clin Microbiol Rev 32:e00115-18. doi: 10.1128/CMR.00115-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poirel L, Bonnin RA, Boulanger A, Schrenzel J, Kaase M, Nordmann P. 2012. Tn125-related acquisition of blaNDM-like genes in Acinetobacter baumannii. Antimicrob Agents Chemother 56:1087–1089. doi: 10.1128/AAC.05620-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joshi PR, Acharya M, Kakshapati T, Leungtongkam U, Thummeepak R, Sitthisak S. 2017. Co-existence of blaOXA-23 and blaNDM-1 genes of Acinetobacter baumannii isolated from Nepal: antimicrobial resistance and clinical significance. Antimicrob Resist Infect Control 6:21. doi: 10.1186/s13756-017-0180-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou H, Guo W, Zhang J, Li Y, Zheng P, Zhang H. 2019. Draft genome sequence of a metallo-β-lactamase (blaAIM-1)-producing Klebsiella pneumoniae ST1916 isolated from a patient with chronic diarrhoea. J Glob Antimicrob Resist 16:165–167. doi: 10.1016/j.jgar.2019.01.010. [DOI] [PubMed] [Google Scholar]
- 27.Dabos L, Rodriguez CH, Nastro M, Dortet L, Bonnin RA, Famiglietti A, Iorga BI, Vay C, Naas T. 2020. LMB-1 producing Citrobacter freundii from Argentina, a novel player in the field of MBLs. Int J Antimicrob Agents 55:105857. doi: 10.1016/j.ijantimicag.2019.11.014. [DOI] [PubMed] [Google Scholar]
- 28.Wachino J, Yoshida H, Yamane K, Suzuki S, Matsui M, Yamagishi T, Tsutsui A, Konda T, Shibayama K, Arakawa Y. 2011. SMB-1, a novel subclass B3 metallo-β-lactamase, associated with ISCR1 and a class 1 integron, from a carbapenem-resistant Serratia marcescens clinical isolate. Antimicrob Agents Chemother 55:5143–5149. doi: 10.1128/AAC.05045-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mojica MF, Bonomo RA, Fast W. 2016. B1-metallo-β-lactamases: where do wesStand? Curr Drug Targets 17:1029–1050. doi: 10.2174/1389450116666151001105622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brem J, Struwe WB, Rydzik AM, Tarhonskaya H, Pfeffer I, Flashman E, van Berkel SS, Spencer J, Claridge TDW, McDonough MA, Benesch JLP, Schofield CJ. 2015. Studying the active-site loop movement of the São Paolo metallo-β-lactamase-1. Chem Sci 6:956–963. doi: 10.1039/c4sc01752h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Page MI, Badarau A. 2008. The mechanisms of catalysis by metallo-β-lactamases. Bioinorg Chem Appl 2008:576297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lisa M-N, Palacios AR, Aitha M, González MM, Moreno DM, Crowder MW, Bonomo RA, Spencer J, Tierney DL, Llarrull LI, Vila AJ. 2017. A general reaction mechanism for carbapenem hydrolysis by mononuclear and binuclear metallo-β-lactamases. Nat Commun 8:538. doi: 10.1038/s41467-017-00601-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feng H, Liu X, Wang S, Fleming J, Wang D-C, Liu W. 2017. The mechanism of NDM-1-catalyzed carbapenem hydrolysis is distinct from that of penicillin or cephalosporin hydrolysis. Nat Commun 8:2242. doi: 10.1038/s41467-017-02339-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shen B, Yu Y, Chen H, Cao X, Lao X, Fang Y, Shi Y, Chen J, Zheng H. 2013. Inhibitor discovery of full-length New Delhi metallo-β-lactamase-1 (NDM-1). PLoS One 8:e62955. doi: 10.1371/journal.pone.0062955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Livermore D, Mushtaq S, Warner M, Woodford N. 2015. Activity of OP0595/β-lactam combinations against Gram-negative bacteria with extended-spectrum, AmpC and carbapenem-hydrolysing β-lactamases. J Antimicrob Chemother 70:3032–3041. doi: 10.1093/jac/dkv239. [DOI] [PubMed] [Google Scholar]
- 36.Walsh TR. 2005. The emergence and implications of metallo-β-lactamases in Gram-negative bacteria. Clin Microbiol Infect 11:2–9. doi: 10.1111/j.1469-0691.2005.01264.x. [DOI] [PubMed] [Google Scholar]
- 37.Makena A, Brem J, Pfeffer I, Geffen REJ, Wilkins SE, Tarhonskaya H, Flashman E, Phee LM, Wareham DW, Schofield CJ. 2015. Biochemical characterization of New Delhi metallo-β-lactamase variants reveals differences in protein stability. J Antimicrob Chemother 70:463–469. doi: 10.1093/jac/dku403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stewart AC, Bethel CR, VanPelt J, Bergstrom A, Cheng Z, Miller CG, Williams C, Poth R, Morris M, Lahey O, Nix JC, Tierney DL, Page RC, Crowder MW, Bonomo RA, Fast W. 2017. Clinical variants of New Delhi metallo-β-lactamase are evolving to overcome zinc scarcity. ACS Infect Dis 3:927–940. doi: 10.1021/acsinfecdis.7b00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bahr G, Vitor-Horen L, Bethel CR, Bonomo RA, González LJ, Vila AJ. 2017. Clinical evolution of New Delhi metallo-β-lactamase (NDM) optimizes resistance under Zn(II) deprivation. Antimicrob Agents Chemother 62:e01849-17. doi: 10.1128/AAC.01849-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Corbin BD, Seeley EH, Raab A, Feldmann J, Miller MR, Torres VJ, Anderson KL, Dattilo BM, Dunman PM, Gerads R, Caprioli RM, Nacken W, Chazin WJ, Skaar EP. 2008. Metal chelation and inhibition of bacterial growth in tissue abscesses. Science 319:962–965. doi: 10.1126/science.1152449. [DOI] [PubMed] [Google Scholar]
- 41.Cheng Z, Thomas PW, Ju L, Bergstrom A, Mason K, Clayton D, Miller C, Bethel CR, VanPelt J, Tierney DL, Page RC, Bonomo RA, Fast W, Crowder MW. 2018. Evolution of New Delhi metallo-β-lactamase (NDM) in the clinic: effects of NDM mutations on stability, zinc affinity, and mono-zinc activity. J Biol Chem 293:12606–12618. doi: 10.1074/jbc.RA118.003835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ahammad ZS, Sreekrishnan TR, Hands CL, Knapp CW, Graham DW. 2014. Increased waterborne blaNDM-1 resistance gene abundances associated with seasonal human pilgrimages to the Upper Ganges River. Environ Sci Technol 48:3014–3020. doi: 10.1021/es405348h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Duin D, Doi Y. 2017. The global epidemiology of carbapenemase-producing Enterobacteriaceae. Virulence 8:460–469. doi: 10.1080/21505594.2016.1222343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yong D, Toleman MA, Giske CG, Cho HS, Sundman K, Lee K, Walsh TR. 2009. Characterization of a new metallo-β-lactamase gene, blaNDM-1, and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob Agents Chemother 53:5046–5054. doi: 10.1128/AAC.00774-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Woodford N, Johnson AP. 2013. Global spread of antibiotic resistance: the example of New Delhi metallo-β-lactamase (NDM)-mediated carbapenem resistance. J Med Microbiol 62:499–513. doi: 10.1099/jmm.0.052555-0. [DOI] [PubMed] [Google Scholar]
- 46.Kumarasamy KK, Toleman MA, Walsh TR, Bagaria J, Butt F, Balakrishnan R, Chaudhary U, Doumith M, Giske CG, Irfan S, Krishnan P, Kumar AV, Maharjan S, Mushtaq S, Noorie T, Paterson DL, Pearson A, Perry C, Pike R, Rao B, Ray U, Sarma JB, Sharma M, Sheridan E, Thirunarayan MA, Turton J, Upadhyay S, Warner M, Welfare W, Livermore DM, Woodford N. 2010. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. Lancet Infect Dis 10:597–602. doi: 10.1016/S1473-3099(10)70143-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heinz E, Ejaz H, Bartholdson Scott J, Wang N, Gujaran S, Pickard D, Wilksch J, Cao H, Haq I-U, Dougan G, Strugnell RA. 2019. Resistance mechanisms and population structure of highly drug resistant Klebsiella in Pakistan during the introduction of the carbapenemase NDM-1. Sci Rep 9:2392. doi: 10.1038/s41598-019-38943-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Castanheira M, Deshpande LM, Mathai D, Bell JM, Jones RN, Mendes RE. 2011. Early dissemination of NDM-1- and OXA-181-producing Enterobacteriaceae in Indian hospitals: report from the SENTRY Antimicrobial Surveillance Program, 2006–2007. Antimicrob Agents Chemother 55:1274–1278. doi: 10.1128/AAC.01497-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Walsh TR, Weeks J, Livermore DM, Toleman MA. 2011. Dissemination of NDM-1 positive bacteria in the New Delhi environment and its implications for human health: an environmental point prevalence study. Lancet Infect Dis 11:355–362. doi: 10.1016/S1473-3099(11)70059-7. [DOI] [PubMed] [Google Scholar]
- 50.Kazi M, Drego L, Nikam C, Ajbani K, Soman R, Shetty A, Rodrigues C. 2015. Molecular characterization of carbapenem-resistant Enterobacteriaceae at a tertiary care laboratory in Mumbai. Eur J Clin Microbiol Infect Dis 34:467–472. doi: 10.1007/s10096-014-2249-x. [DOI] [PubMed] [Google Scholar]
- 51.Garg A, Garg J, Kumar S, Bhattacharya A, Agarwal S, Upadhyay G. 2019. Molecular epidemiology and therapeutic options of carbapenem-resistant Gram-negative bacteria. Indian J Med Res 149:285–289. doi: 10.4103/ijmr.IJMR_36_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang R, Liu L, Zhou H, Chan EW, Li J, Fang Y, Li Y, Liao K, Chen S. 2017. Nationwide surveillance of clinical carbapenem-resistant Enterobacteriaceae (CRE) strains in China. EBioMedicine 19:98–106. doi: 10.1016/j.ebiom.2017.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cai Y, Chen C, Zhao M, Yu X, Lan K, Liao K, Guo P, Zhang W, Ma X, He Y, Zeng J, Chen L, Jia W, Tang Y-W, Huang B. 2019. High prevalence of metallo-β-lactamase-producing Enterobacter cloacae from three tertiary hospitals in China. Front Microbiol 10:1610. doi: 10.3389/fmicb.2019.01610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang Y, Lo W-U, Lai R-M, Tse C-S, Lee RA, Luk W-K, Cheng V-C, Que T-L, Chow K-H, Ho P-L. 2017. IncN ST7 epidemic plasmid carrying blaIMP-4 in Enterobacteriaceae isolates with epidemiological links to multiple geographical areas in China. J Antimicrob Chemother 72:99–103. doi: 10.1093/jac/dkw353. [DOI] [PubMed] [Google Scholar]
- 55.Dong F, Lu J, Wang Y, Shi J, Zhen JH, Chu P, Zhen Y, Han SJ, Guo YL, Song WQ. 2017. A five-year surveillance of carbapenemase-producing Klebsiella pneumoniae in a pediatric hospital in China reveals increased predominance of NDM-1. Biomed Environ Sci 30:562–569. [DOI] [PubMed] [Google Scholar]
- 56.Du H, Chen L, Tang Y-W, Kreiswirth BN. 2016. Carbapenem-resistant and colistin-resistant Escherichia coli co-producing NDM-9 and MCR-1. Lancet Infect Dis 16:288–289. [DOI] [PubMed] [Google Scholar]
- 57.Lutgring JD, Limbago BM. 2016. The problem of carbapenemase-producing-carbapenem-resistant-Enterobacteriaceae detection. J Clin Microbiol 54:529–534. doi: 10.1128/JCM.02771-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Al-Agamy MH, Aljallal A, Radwan HH, Shibl AM. 2018. Characterization of carbapenemases, ESBLs, and plasmid-mediated quinolone determinants in carbapenem-insensitive Escherichia coli and Klebsiella pneumoniae in Riyadh hospitals. J Infect Public Health 11:64–68. doi: 10.1016/j.jiph.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 59.Zowawi HM, Sartor AL, Balkhy HH, Walsh TR, Al Johani SM, AlJindan RY, Alfaresi M, Ibrahim E, Al-Jardani A, Al-Abri S, Al Salman J, Dashti AA, Kutbi AH, Schlebusch S, Sidjabat HE, Paterson DL. 2014. Molecular characterization of carbapenemase-producing Escherichia coli and Klebsiella pneumoniae in the countries of the Gulf Cooperation Council: dominance of OXA-48 and NDM producers. Antimicrob Agents Chemother 58:3085–3090. doi: 10.1128/AAC.02050-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zowawi HM, Syrmis MW, Kidd TJ, Balkhy HH, Walsh TR, Al Johani SM, Al Jindan RY, Alfaresi M, Ibrahim E, Al-Jardani A, Al Salman J, Dashti AA, Sidjabat HE, Baz O, Trembizki E, Whiley DM, Paterson DL. 2018. Identification of carbapenem-resistant Pseudomonas aeruginosa in selected hospitals of the Gulf Cooperation Council States: dominance of high-risk clones in the region. J Med Microbiol 67:846–853. doi: 10.1099/jmm.0.000730. [DOI] [PubMed] [Google Scholar]
- 61.Ayoub Moubareck C, Hammoudi Halat D, Akkawi C, Nabi A, AlSharhan MA, AlDeesi ZO, Peters CC, Celiloglu H, Karam Sarkis D. 2019. Role of outer membrane permeability, efflux mechanism, and carbapenemases in carbapenem-nonsusceptible Pseudomonas aeruginosa from Dubai hospitals: results of the first cross-sectional survey. Int J Infect Dis 84:143–150. doi: 10.1016/j.ijid.2019.04.027. [DOI] [PubMed] [Google Scholar]
- 62.Edelstein MV, Skleenova EN, Shevchenko OV, D'souza JW, Tapalski DV, Azizov IS, Sukhorukova MV, Pavlukov RA, Kozlov RS, Toleman MA, Walsh TR. 2013. Spread of extensively resistant VIM-2-positive ST235 Pseudomonas aeruginosa in Belarus, Kazakhstan, and Russia: a longitudinal epidemiological and clinical study. Lancet Infect Dis 13:867–876. doi: 10.1016/S1473-3099(13)70168-3. [DOI] [PubMed] [Google Scholar]
- 63.Ageevets VA, Partina IV, Lisitsyna ES, Ilina EN, Lobzin YV, Shlyapnikov SA, Sidorenko SV. 2014. Emergence of carbapenemase-producing Gram-negative bacteria in Saint Petersburg, Russia. Int J Antimicrob Agents 44:152–155. doi: 10.1016/j.ijantimicag.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 64.Lazareva IV, Ageevets VA, Ershova TA, Zueva LP, Goncharov AE, Darina MG, Svetlichnaya YS, Uskov AN, Sidorenko SV. 2016. Prevalence and antibiotic resistance of carbapenemase-producing Gram-negative bacteria in Saint Petersburg and some other regions of the Russian Federation. Antibiot Khimioter 61:28–38. [PubMed] [Google Scholar]
- 65.Fursova NK, Astashkin EI, Knyazeva AI, Kartsev NN, Leonova ES, Ershova ON, Alexandrova IA, Kurdyumova NV, Sazikina SY, Volozhantsev NV, Svetoch EA, Dyatlov IA. 2015. The spread of blaOXA-48 and blaOXA-244 carbapenemase genes among Klebsiella pneumoniae, Proteus mirabilis and Enterobacter spp. isolated in Moscow, Russia. Ann Clin Microbiol Antimicrob 14:46. doi: 10.1186/s12941-015-0108-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Luzzaro F, Docquier J-D, Colinon C, Endimiani A, Lombardi G, Amicosante G, Rossolini GM, Toniolo A. 2004. Emergence in Klebsiella pneumoniae and Enterobacter cloacae clinical isolates of the VIM-4 metallo-β-lactamase encoded by a conjugative plasmid. Antimicrob Agents Chemother 48:648–650. doi: 10.1128/aac.48.2.648-650.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hasan CM, Turlej-Rogacka A, Vatopoulos AC, Giakkoupi P, Maâtallah M, Giske CG. 2014. Dissemination of blaVIM in Greece at the peak of the epidemic of 2005–2006: clonal expansion of Klebsiella pneumoniae clonal complex 147. Clin Microbiol Infect 20:34–37. doi: 10.1111/1469-0691.12187. [DOI] [PubMed] [Google Scholar]
- 68.Vatopoulos A. 2008. High rates of metallo-β-lactamase-producing Klebsiella pneumoniae in Greece—a review of the current evidence. Euro Surveill 13:8023. [PubMed] [Google Scholar]
- 69.Giakoupi P, Maltezou H, Polemis M, Pappa O, Saroglou G, Vatopoulos A, Greek System for the Surveillance of Antimicrobial Resistance. 2009. KPC-2-producing Klebsiella pneumoniae infections in Greek hospitals are mainly due to a hyperepidemic clone. Eurosurveillance 14:19218. doi: 10.2807/ese.14.21.19218-en. [DOI] [PubMed] [Google Scholar]
- 70.Papadimitriou-Olivgeris M, Bartzavali C, Lambropoulou A, Solomou A, Tsiata E, Anastassiou ED, Fligou F, Marangos M, Spiliopoulou I, Christofidou M. 2019. Reversal of carbapenemase-producing Klebsiella pneumoniae epidemiology from blaKPC to blaVIM harbouring isolates in a Greek ICU after introduction of ceftazidime/avibactam. J Antimicrob Chemother 74:2051–2054. doi: 10.1093/jac/dkz125. [DOI] [PubMed] [Google Scholar]
- 71.Public Health England. 2017. English surveillance programme for antimicrobial utilisation and resistance (ESPAUR) report 2017. Public Health England, London, United Kingdom. [Google Scholar]
- 72.Wright LL, Turton JF, Livermore DM, Hopkins KL, Woodford N. 2015. Dominance of international “high-risk clones” among metallo-β-lactamase-producing Pseudomonas aeruginosa in the UK. J Antimicrob Chemother 70:103–110. doi: 10.1093/jac/dku339. [DOI] [PubMed] [Google Scholar]
- 73.Public Health England. 2018. English surveillance programme for antimicrobial utilisation and resistance (ESPAUR) report. Public Health England, London, United Kingdom. [Google Scholar]
- 74.Albiger B, Glasner C, Struelens MJ, Grundmann H, Monnet DL, European Survey of Carbapenemase-Producing Enterobacteriaceae (EuSCAPE) Working Group. 2015. Carbapenemase-producing Enterobacteriaceae in Europe: assessment by national experts from 38 countries, May 2015. Euro Surveill 20:30062. doi: 10.2807/1560-7917.ES.2015.20.45.30062. [DOI] [PubMed] [Google Scholar]
- 75.Grundmann H, Glasner C, Albiger B, Aanensen DM, Tomlinson CT, Andrasević AT, Cantón R, Carmeli Y, Friedrich AW, Giske CG, Glupczynski Y, Gniadkowski M, Livermore DM, Nordmann P, Poirel L, Rossolini GM, Seifert H, Vatopoulos A, Walsh T, Woodford N, Monnet DL, Koraqi A, Lacej D, Apfalter P, Hartl R, Glupczynski Y, Huang T-D, Strateva T, Marteva-Proevska Y, Andrasevic AT, Butic I, Pieridou-Bagatzouni D, Maikanti-Charalampous P, Hrabak J, Zemlickova H, Hammerum A, Jakobsen L, Ivanova M, Pavelkovich A, Jalava J, Österblad M, Dortet L, Vaux S, Kaase M, Gatermann SG, Vatopoulos A, Tryfinopoulou K, Tóth Á, Jánvári L, Boo TW, McGrath E, Carmeli Y, Adler A, Pantosti A, Monaco M, Raka L, Kurti A, Balode A, Saule M, Miciuleviciene J, Mierauskaite A, Perrin-Weniger M, Reichert P, Nestorova N, Debattista S, Mijovic G, Lopicic M, Samuelsen Ø, Haldorsen B, Zabicka D, Literacka E, Caniça M, Manageiro V, Kaftandzieva A, Trajkovska-Dokic E, Damian M, Lixandru B, Jelesic Z, Trudic A, Niks M, Schreterova E, Pirs M, Cerar T, Oteo J, Aracil B, Giske C, Sjöström K, Gür D, Cakar A, Woodford N, Hopkins K, Wiuff C, Brown DJ, European Survey of Carbapenemase-Producing Enterobacteriaceae (EuSCAPE) Working Group. 2017. Occurrence of carbapenemase-producing Klebsiella pneumoniae and Escherichia coli in the European survey of carbapenemase-producing Enterobacteriaceae (EuSCAPE): a prospective, multinational study. Lancet Infect Dis 17:153–163. doi: 10.1016/S1473-3099(16)30257-2. [DOI] [PubMed] [Google Scholar]
- 76.Livermore DM, Walsh TR, Toleman M, Woodford N. 2011. Balkan NDM-1: escape or transplant? Lancet Infect Dis 11:164. doi: 10.1016/S1473-3099(11)70048-2. [DOI] [PubMed] [Google Scholar]
- 77.Little ML, Qin X, Zerr DM, Weissman SJ. 2012. Molecular diversity in mechanisms of carbapenem resistance in paediatric Enterobacteriaceae. Int J Antimicrob Agents 39:52–57. doi: 10.1016/j.ijantimicag.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Centers for Disease Control and Prevention. 2010. Detection of Enterobacteriaceae isolates carrying metallo-beta-lactamase—United States, 2010. MMWR Morb Mortal Wkly Rep 59:750. [PubMed] [Google Scholar]
- 79.Centers for Disease Control and Prevention. 2013. Notes from the Field: New Delhi metallo-β-lactamase–producing Escherichia coli associated with endoscopic retrograde cholangiopancreatography—Illinois. MMWR Morb Mortal Wkly Rep 62:1051. [PMC free article] [PubMed] [Google Scholar]
- 80.Canadian Public Health Laboratory Network. 2017. Canadian Public Health Laboratory Network (CPHLN) voluntary reporting of carbapenemase-producing Enterobacteriaeceae.
- 81.Kohler PP, Melano RG, Patel SN, Shafinaz S, Faheem A, Coleman BL, Green K, Armstrong I, Almohri H, Borgia S, Borgundvaag E, Johnstone J, Katz K, Lam F, Muller MP, Powis J, Poutanen SM, Richardson D, Rebbapragada A, Sarabia A, Simor A, McGeer A, Toronto Invasive Bacterial Diseases Network (TIBDN). 2018. Emergence of carbapenemase-producing Enterobacteriaceae, South-Central Ontario, Canada. Emerg Infect Dis 24:1674–1682. doi: 10.3201/eid2409.180164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Boyd DA, Lisboa LF, Rennie R, Zhanel GG, Dingle TC, Mulvey MR. 2019. Identification of a novel metallo-β-lactamase, CAM-1, in clinical Pseudomonas aeruginosa isolates from Canada. J Antimicrob Chemother 74:1563–1567. doi: 10.1093/jac/dkz066. [DOI] [PubMed] [Google Scholar]
- 83.Kazmierczak KM, Rabine S, Hackel M, McLaughlin RE, Biedenbach DJ, Bouchillon SK, Sahm DF, Bradford PA. 2016. Multiyear, multinational survey of the incidence and global distribution of metallo-β-lactamase-producing Enterobacteriaceae and Pseudomonas aeruginosa. Antimicrob Agents Chemother 60:1067–1078. doi: 10.1128/AAC.02379-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Manenzhe RI, Zar HJ, Nicol MP, Kaba M. 2015. The spread of carbapenemase-producing bacteria in Africa: a systematic review. J Antimicrob Chemother 70:23–40. doi: 10.1093/jac/dku356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rubin JE, Peirano G, Peer AK, Govind CN, Pitout J. 2014. NDM-1–producing Enterobacteriaceae from South Africa: moving towards endemicity? Diagn Microbiol Infect Dis 79:378–380. doi: 10.1016/j.diagmicrobio.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 86.Brinkac LM, White R, D’Souza R, Nguyen K, Obaro SK, Fouts DE. 2019. Emergence of New Delhi metallo-β-lactamase (NDM-5) in Klebsiella quasipneumoniae from neonates in a Nigerian hospital. mSphere 4:e00685-18. doi: 10.1128/mSphere.00685-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rao P. 2017. India's medical tourism gets Africans' attention. United Nations Africa Renewal blog. https://www.un.org/africarenewal/magazine/december-2016-march-2017/india%E2%80%99s-medical-tourism-gets-africans%E2%80%99-attention. Accessed 28 June 2020. [Google Scholar]
- 88.da Silva IR, Aires CAM, Conceição-Neto OC, de Oliveira Santos IC, Ferreira Pereira N, Moreno Senna JP, Carvalho-Assef APD, Asensi MD, Rocha-de-Souza CM. 2019. Distribution of clinical NDM-1-producing Gram-negative bacteria in Brazil. Microb Drug Resist 25:394–399. doi: 10.1089/mdr.2018.0240. [DOI] [PubMed] [Google Scholar]
- 89.Zurita J, Parra H, Gestal MC, McDermott J, Barba P. 2015. First case of NDM-1-producing Providencia rettgeri in Ecuador. J Glob Antimicrob Resist 3:302–303. doi: 10.1016/j.jgar.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 90.Saavedra-Rojas S-Y, Duarte-Valderrama C, González-de-Arias M-N, Ovalle-Guerro MV. 2017. Emergence of Providencia rettgeri NDM-1 in two departments of Colombia, 2012–2013. Enferm Infecc Microbiol Clin 35:354–358. doi: 10.1016/j.eimc.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 91.Gales AC, Menezes LC, Silbert S, Sader HS. 2003. Dissemination in distinct Brazilian regions of an epidemic carbapenem-resistant Pseudomonas aeruginosa producing SPM metallo-β-lactamase. J Antimicrob Chemother 52:699–702. doi: 10.1093/jac/dkg416. [DOI] [PubMed] [Google Scholar]
- 92.Zavascki AP, Barth AL, Gonçalves ALS, Moro ALD, Fernandes JF, Martins AF, Ramos F, Goldani LZ. 2006. The influence of metallo-β-lactamase production on mortality in nosocomial Pseudomonas aeruginosa infections. J Antimicrob Chemother 58:387–392. doi: 10.1093/jac/dkl239. [DOI] [PubMed] [Google Scholar]
- 93.Sidjabat HE, Townell N, Nimmo GR, George NM, Robson J, Vohra R, Davis L, Heney C, Paterson DL. 2015. Dominance of IMP-4-producing Enterobacter cloacae among carbapenemase-producing Enterobacteriaceae in Australia. Antimicrob Agents Chemother 59:4059–4066. doi: 10.1128/AAC.04378-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Roberts LW, Catchpoole E, Jennison AV, Bergh H, Hume A, Heney C, George N, Paterson DL, Schembri MA, Beatson SA, Harris P. 2020. Genomic analysis of carbapenemase-producing Enterobacteriaceae in Queensland reveals widespread transmission of blaIMP-4 on an IncHI2 plasmid. Microb Genom 6:e000321. doi: 10.1099/mgen.0.000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Abraham S, O'Dea M, Trott DJ, Abraham RJ, Hughes D, Pang S, McKew G, Cheong EYL, Merlino J, Saputra S, Malik R, Gottlieb T. 2016. Isolation and plasmid characterization of carbapenemase (IMP-4) producing Salmonella enterica Typhimurium from cats. Sci Rep 6:35527. doi: 10.1038/srep35527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dolejska M, Masarikova M, Dobiasova H, Jamborova I, Karpiskova R, Havlicek M, Carlile N, Priddel D, Cizek A, Literak I. 2016. High prevalence of Salmonella and IMP-4-producing Enterobacteriaceae in the silver gull on Five Islands, Australia. J Antimicrob Chemother 71:63–70. doi: 10.1093/jac/dkv306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lim HM, Pène JJ, Shaw RW. 1988. Cloning, nucleotide sequence, and expression of the Bacillus cereus 5/B/6 β-lactamase II structural gene. J Bacteriol 170:2873–2878. doi: 10.1128/jb.170.6.2873-2878.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kuwabara S, Abraham EP. 1967. Some properties of two extracellular β-lactamases from Bacillus cereus 569/H. Biochem J 103:27C–30C. doi: 10.1042/bj1030027c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hawkey PM, Warren RE, Livermore DM, McNulty CAM, Enoch DA, Otter JA, Wilson A. 2018. Treatment of infections caused by multidrug-resistant Gram-negative bacteria: report of the British Society for Antimicrobial Chemotherapy/Healthcare Infection Society/British Infection Association Joint Working Party. J Antimicrob Chemother 73:iii2–iii78. doi: 10.1093/jac/dky027. [DOI] [PubMed] [Google Scholar]
- 100.Jeon YD, Jeong WY, Kim MH, Jung IY, Ahn MY, Ann HW, Ahn JY, Han SH, Choi JY, Song YG, Kim JM, Ku NS. 2016. Risk factors for mortality in patients with Stenotrophomonas maltophilia bacteremia. Medicine (Baltimore) 95:e4375. doi: 10.1097/MD.0000000000004375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Senol E, DesJardin J, Stark PC, Barefoot L, Snydman DR. 2002. Attributable mortality of Stenotrophomonas maltophilia bacteremia. Clin Infect Dis 34:1653–1656. doi: 10.1086/340707. [DOI] [PubMed] [Google Scholar]
- 102.Asempa TE, Abdelraouf K, Nicolau DP. 2020. Metallo-β-lactamase resistance in Enterobacteriaceae is an artefact of currently utilized antimicrobial susceptibility testing methods. J Antimicrob Chemother 75:997–1005. doi: 10.1093/jac/dkz532. [DOI] [PubMed] [Google Scholar]
- 103.Gammoh NZ, Rink L. 2017. Zinc in infection and inflammation. Nutrients 9:624. doi: 10.3390/nu9060624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.González LJ, Bahr G, Nakashige TG, Nolan EM, Bonomo RA, Vila AJ. 2016. Membrane anchoring stabilizes and favors secretion of New Delhi metallo-β-lactamase. Nat Chem Biol 12:516–522. doi: 10.1038/nchembio.2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Monogue ML, Abbo LM, Rosa R, Camargo JF, Martinez O, Bonomo RA, Nicolau DP. 2017. In vitro discordance with in vivo activity: humanized exposures of ceftazidime-avibactam, aztreonam, and tigecycline alone and in combination against New Delhi metallo-β-lactamase-producing Klebsiella pneumoniae in a murine lung infection model. Antimicrob Agents Chemother 61:e00486-17. doi: 10.1128/AAC.00486-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chibabhai V, Nana T, Bosman N, Thomas T, Lowman W. 2018. Were all carbapenemases created equal? Treatment of NDM-producing extensively drug-resistant Enterobacteriaceae: a case report and literature review. Infection 46:1–13. doi: 10.1007/s15010-017-1070-8. [DOI] [PubMed] [Google Scholar]
- 107.Naim H, Rizvi M, Azam M, Gupta R, Taneja N, Shukla I, Khan H. 2017. Alarming emergence, molecular characterization, and outcome of blaNDM-1 in patients infected with multidrug-resistant Gram-negative bacilli in a tertiary care hospital. J Lab Physicians 9:170–176. doi: 10.4103/0974-2727.208264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Datta S, Roy S, Chatterjee S, Saha A, Sen B, Pal T, Som T, Basu S. 2014. A five-year experience of carbapenem resistance in Enterobacteriaceae causing neonatal septicaemia: predominance of NDM-1. PLoS One 9:e112101. doi: 10.1371/journal.pone.0112101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.de Jager P, Chirwa T, Naidoo S, Perovic O, Thomas J. 2015. Nosocomial outbreak of New Delhi metallo-β-lactamase-1-producing Gram-negative bacteria in South Africa: a case-control study. PLoS One 10:e0123337. doi: 10.1371/journal.pone.0123337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Freire MP, Abdala E, Moura ML, de Paula FJ, Spadão F, Caiaffa-Filho HH, David-Neto E, Nahas WC, Pierrotti LC. 2015. Risk factors and outcome of infections with Klebsiella pneumoniae carbapenemase-producing K. pneumoniae in kidney transplant recipients. Infection 43:315–323. doi: 10.1007/s15010-015-0743-4. [DOI] [PubMed] [Google Scholar]
- 111.Fraenkel-Wandel Y, Raveh-Brawer D, Wiener-Well Y, Yinnon AM, Assous MV. 2016. Mortality due to blaKPC Klebsiella pneumoniae bacteraemia. J Antimicrob Chemother 71:1083–1087. doi: 10.1093/jac/dkv414. [DOI] [PubMed] [Google Scholar]
- 112.Mathur P, Sagar S, Kumar S, Sharma V, Gupta D, Lalwani S, Rani R, Muruganantham A. 2016. Does the presence of Klebsiella pneumoniae carbapenemase and New Delhi metallo-β-lactamase-1 genes in pathogens lead to fatal outcome? Indian J Med Microbiol 34:495–499. doi: 10.4103/0255-0857.195367. [DOI] [PubMed] [Google Scholar]
- 113.van Duin D, Kaye KS, Neuner EA, Bonomo RA. 2013. Carbapenem-resistant Enterobacteriaceae: a review of treatment and outcomes. Diagn Microbiol Infect Dis 75:115–120. doi: 10.1016/j.diagmicrobio.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Daikos GL, Petrikkos P, Psichogiou M, Kosmidis C, Vryonis E, Skoutelis A, Georgousi K, Tzouvelekis LS, Tassios PT, Bamia C, Petrikkos G. 2009. Prospective observational study of the impact of VIM-1 metallo-β-lactamase on the outcome of patients with Klebsiella pneumoniae bloodstream infections. Antimicrob Agents Chemother 53:1868–1873. doi: 10.1128/AAC.00782-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Daikos GL, Kosmidis C, Tassios PT, Petrikkos G, Vasilakopoulou A, Psychogiou M, Stefanou I, Avlami A, Katsilambros N. 2007. Enterobacteriaceae bloodstream infections: presence of integrons, risk factors, and outcome. Antimicrob Agents Chemother 51:2366–2372. doi: 10.1128/AAC.00044-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Huang S, Dai W, Sun S, Zhang X, Zhang L. 2012. Prevalence of plasmid-mediated quinolone resistance and aminoglycoside resistance determinants among carbapeneme [sic] non-susceptible Enterobacter cloacae. PLoS One 7:e47636. doi: 10.1371/journal.pone.0047636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rodríguez-Baño J, Gutiérrez-Gutiérrez B, Machuca I, Pascual A. 2018. Treatment of infections caused by extended-spectrum-β-lactamase-, AmpC-, and carbapenemase-producing Enterobacteriaceae. Clin Microbiol Rev 31:e00079-17. doi: 10.1128/CMR.00079-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gutiérrez-Gutiérrez B, Salamanca E, de Cueto M, Hsueh P-R, Viale P, Paño-Pardo JR, Venditti M, Tumbarello M, Daikos G, Cantón R, Doi Y, Tuon FF, Karaiskos I, Pérez-Nadales E, Schwaber MJ, Azap ÖK, Souli M, Roilides E, Pournaras S, Akova M, Pérez F, Bermejo J, Oliver A, Almela M, Lowman W, Almirante B, Bonomo RA, Carmeli Y, Paterson DL, Pascual A, Rodríguez-Baño J, del Toro MD, Gálvez J, Falcone M, Russo A, Giamarellou H, Trecarichi EM, Losito AR, García-Vázquez E, Hernández A, Gómez J, Bou G, Iosifidis E, Prim N, Navarro F, Mirelis B, Skiada A, Origüen J, Juan RS, Fernández-Ruiz M, Larrosa N, PuigAsensio M, Cisneros JM, Molina J, González V, Rucci V, de Gopegui ER, Marinescu CI, Martínez-Martínez L, Fariñas MC, Cano ME, Gozalo M, Mora-Rillo M, Francisco C-S, Peña C, Gómez-Zorrilla S, Tubau F, Tsakris A, Zarkotou O, Antoniadou A, Poulakou G, Pitout J, Virmani D, Torre-Cisneros J, Guzmán-Puche J, Helvaci Ö, Sahin AO, Pintado V, Ruiz P, Bartoletti M, Giannella M, Tacconelli E, Riemenschneider F, Calbo E, Badia C, Xercavins M, Gasch O, Fontanals D, Jové E. 2017. Effect of appropriate combination therapy on mortality of patients with bloodstream infections due to carbapenemase-producing Enterobacteriaceae (INCREMENT): a retrospective cohort study. Lancet Infect Dis 17:726–734. doi: 10.1016/S1473-3099(17)30228-1. [DOI] [PubMed] [Google Scholar]
- 119.Daikos GL, Tsaousi S, Tzouvelekis LS, Anyfantis I, Psichogiou M, Argyropoulou A, Stefanou I, Sypsa V, Miriagou V, Nepka M, Georgiadou S, Markogiannakis A, Goukos D, Skoutelis A. 2014. Carbapenemase-producing Klebsiella pneumoniae bloodstream infections: lowering mortality by antibiotic combination schemes and the role of carbapenems. Antimicrob Agents Chemother 58:2322–2328. doi: 10.1128/AAC.02166-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.van Duin D, Lok JJ, Earley M, Cober E, Richter SS, Perez F, Salata RA, Kalayjian RC, Watkins RR, Doi Y, Kaye KS, Fowler VG, Paterson DL, Bonomo RA, Evans S, Antibacterial Resistance Leadership Group. 2018. Colistin versus ceftazidime-avibactam in the treatment of infections due to carbapenem-resistant Enterobacteriaceae. Clin Infect Dis 66:163–171. doi: 10.1093/cid/cix783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Otter JA, Doumith M, Davies F, Mookerjee S, Dyakova E, Gilchrist M, Brannigan ET, Bamford K, Galletly T, Donaldson H, Aanensen DM, Ellington MJ, Hill R, Turton JF, Hopkins KL, Woodford N, Holmes A. 2017. Emergence and clonal spread of colistin resistance due to multiple mutational mechanisms in carbapenemase-producing Klebsiella pneumoniae in London. Sci Rep 7:12711. doi: 10.1038/s41598-017-12637-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kanwar A, Marshall SH, Perez F, Tomas M, Jacobs MR, Hujer AM, Domitrovic TN, Rudin SD, Rojas LJ, Kreiswirth BN, Chen L, Quinones-Mateu M, van Duin D, Bonomo RA, Antibacterial Resistance Leadership Group. 2018. Emergence of resistance to colistin during the treatment of bloodstream infection caused by Klebsiella pneumoniae carbapenemase–producing Klebsiella pneumoniae. Open Forum Infect Dis 5:ofy054. doi: 10.1093/ofid/ofy054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lutgring JD, Balbuena R, Reese N, Gilbert SE, Ansari U, Bhatnagar A, Boyd S, Campbell D, Cochran J, Haynie J, Ilutsik J, Longo C, Swint S, Rasheed JK, Brown AC, Karlsson M. 2020. Antibiotic susceptibility of NDM-producing Enterobacterales collected in the United States, 2017–2018. Antimicrob Agents Chemother. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Livermore DM, Mushtaq S, Warner M, Woodford N. June 2016. In vitro activity of eravacycline against carbapenem-resistant Enterobacteriaceae and Acinetobacter baumannii. Antimicrob Agents Chemother 60:3840–3844. doi: 10.1128/AAC.00436-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gallagher JC. 2019. Omadacycline: a modernized tetracycline. Clin Infect Dis 69:S1–S5. doi: 10.1093/cid/ciz394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.U.S. Food and Drug Administration. 2010. FDA Drug Safety Communication: Increased risk of death with Tygacil (tigecycline) compared to other antibiotics used to treat similar infections. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-increased-risk-death-tygacil-tigecycline-compared-other-antibiotics. Accessed 17 February 2020.
- 127.Marquez-Ortiz RA, Haggerty L, Olarte N, Duarte C, Garza-Ramos U, Silva-Sanchez J, Castro BE, Sim EM, Beltran M, Moncada MV, Valderrama A, Castellanos JE, Charles IG, Vanegas N, Escobar-Perez J, Petty NK. 2017. Genomic epidemiology of NDM-1-encoding plasmids in Latin American clinical isolates reveals insights into the evolution of multidrug resistance. Genome Biol Evol 9:1725–1741. doi: 10.1093/gbe/evx115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Crandon JL, Nicolau DP. 2013. Human simulated studies of aztreonam and aztreonam-avibactam to evaluate activity against challenging gram-negative organisms, including metallo-β-lactamase producers. Antimicrob Agents Chemother 57:3299–3306. doi: 10.1128/AAC.01989-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shaw E, Rombauts A, Tubau F, Padullés A, Càmara J, Lozano T, Cobo-Sacristán S, Sabe N, Grau I, Rigo-Bonnin R, Dominguez MA, Carratalà J. 2018. Clinical outcomes after combination treatment with ceftazidime/avibactam and aztreonam for NDM-1/OXA-48/CTX-M-15-producing Klebsiella pneumoniae infection. J Antimicrob Chemother 73:1104–1106. doi: 10.1093/jac/dkx496. [DOI] [PubMed] [Google Scholar]
- 130.Davido B, Fellous L, Lawrence C, Maxime V, Rottman M, Dinh A. 2017. Ceftazidime-avibactam and aztreonam, an interesting strategy to overcome β-lactam resistance conferred by metallo-β-lactamases in Enterobacteriaceae and Pseudomonas aeruginosa. Antimicrob Agents Chemother 61:e01008-17. doi: 10.1128/AAC.01008-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kaye KS, Rice LB, Dane AL, Stus V, Sagan O, Fedosiuk E, Das AF, Skarinsky D, Eckburg PB, Ellis-Grosse EJ. 2019. Fosfomycin for injection (ZTI-01) versus piperacillin-tazobactam for the treatment of complicated urinary tract infection including acute pyelonephritis: ZEUS, a phase 2/3 randomized trial. Clin Infect Dis 69:2045–2056. doi: 10.1093/cid/ciz181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Demir T, Buyukguclu T. 2017. Fosfomycin: in vitro efficacy against multidrug-resistant isolates beyond urinary isolates. J Glob Antimicrob Resist 8:164–168. doi: 10.1016/j.jgar.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 133.Albiero J, Mazucheli J, Barros JP, dos R, Szczerepa MM, dos A, Nishiyama SAB, Carrara-Marroni FE, Sy S, Fidler M, Sy SKB, Tognim M. 2019. Pharmacodynamic attainment of the synergism of meropenem and fosfomycin combination against Pseudomonas aeruginosa producing metallo-β-lactamase. Antimicrob Agents Chemother 63:e00126-19. doi: 10.1128/AAC.00126-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Pharmaceutical Technology. 2016. Allergan and AstraZeneca to develop ATM-AVI for antibiotic-resistant gram-negative infections. Pharm Technol. https://www.pharmaceutical-technology.com/news/newsallergan-astrazeneca-develop-atm-avi-antibiotic-resistant-gram-negative-infections-4797880/. Accessed 20 February 2020.
- 135.U.S. National Library of Medicine. Efficacy, safety, and tolerability of ATM-AVI in the treatment of serious infection due to MBL-producing Gram-negative bacteria. https://clinicaltrials.gov/ct2/show/NCT03580044. Accessed 20 June 2020.
- 136.Ehmann DE, Jahić H, Ross PL, Gu R-F, Hu J, Kern G, Walkup GK, Fisher SL. 2012. Avibactam is a covalent, reversible, non-β-lactam β-lactamase inhibitor. Proc Natl Acad Sci U S A 109:11663–11668. doi: 10.1073/pnas.1205073109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Livermore DM, Mushtaq S, Warner M, Zhang J, Maharjan S, Doumith M, Woodford N. 2011. Activity of MK-7655 combined with imipenem against Enterobacteriaceae and Pseudomonas aeruginosa. Antimicrob Agents Chemother 55:390–394. doi: 10.1128/AAC.00756-10. [DOI] [PubMed] [Google Scholar]
- 138.Sader HS, Mendes RE, Pfaller MA, Shortridge D, Flamm RK, Castanheira M. 2017. Antimicrobial activities of aztreonam-avibactam and comparator agents against contemporary (2016) clinical Enterobacteriaceae isolates. Antimicrob Agents Chemother 62:e01856-17. doi: 10.1128/AAC.01856-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gregory S, Paul N, Leanne G, Helen B, Angela W, Katrina Y, Chen Z, Jie S, Chow J. 2018. Clinical activity of ceftazidime/avibactam against MDR Enterobacteriaceae and Pseudomonas Aeruginosa: pooled data from the ceftazidime/avibactam Phase III clinical trial programme. J Antimicrob Chemother 73:2519–2523. doi: 10.1093/jac/dky204. [DOI] [PubMed] [Google Scholar]
- 140.Wenzler E, Deraedt MF, Harrington AT, Danizger LH. 2017. Synergistic activity of ceftazidime-avibactam and aztreonam against serine and metallo-β-lactamase-producing gram-negative pathogens. Diagn Microbiol Infect Dis 88:352–354. doi: 10.1016/j.diagmicrobio.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 141.NHS Specialist Pharmacy Service. Cefiderocol. https://www.sps.nhs.uk/medicines/cefiderocol/. Accessed 20 June 2020.
- 142.Avery LM, Nicolau DP. 2018. Investigational drugs for the treatment of infections caused by multidrug-resistant Gram-negative bacteria. Expert Opin Invest Drugs 27:325–338. doi: 10.1080/13543784.2018.1460354. [DOI] [PubMed] [Google Scholar]
- 143.Portsmouth S, van Veenhuyzen D, Echols R, Machida M, Ferreira JCA, Ariyasu M, Tenke P, Nagata TD. 2018. Cefiderocol versus imipenem-cilastatin for the treatment of complicated urinary tract infections caused by Gram-negative uropathogens: a phase 2, randomised, double-blind, non-inferiority trial. Lancet Infect Dis 18:1319–1328. doi: 10.1016/S1473-3099(18)30554-1. [DOI] [PubMed] [Google Scholar]
- 144.Kohira N, West J, Ito A, Ito-Horiyama T, Nakamura R, Sato T, Rittenhouse S, Tsuji M, Yamano Y. 2016. In vitro antimicrobial activity of a siderophore cephalosporin, S-649266, against Enterobacteriaceae clinical isolates, including carbapenem-resistant strains. Antimicrob Agents Chemother 60:729–734. doi: 10.1128/AAC.01695-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Saisho Y, Katsube T, White S, Fukase H, Shimada J. 2018. Pharmacokinetics, safety, and tolerability of cefiderocol, a novel siderophore cephalosporin for Gram-negative bacteria, in healthy subjects. Antimicrob Agents Chemother 62:e02163-17. doi: 10.1128/AAC.02163-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mushtaq S, Sadouki Z, Vickers A, Livermore D, Woodford N. 2020. In-vitro activity of cefiderocol against multidrug-resistant Enterobacterales, Pseudomonas aeruginosa and Acinetobacter baumannii Isolates from the UK Access Microbiol 2:50. [Google Scholar]
- 147.Matsumoto S, Singley CM, Hoover J, Nakamura R, Echols R, Rittenhouse S, Tsuji M, Yamano Y. 2017. Efficacy of cefiderocol against carbapenem-resistant Gram-negative bacilli in immunocompetent-rat respiratory tract infection models recreating human plasma pharmacokinetics. Antimicrob Agents Chemother 61:e00700-17. doi: 10.1128/AAC.00700-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Nakamura R, Ito-Horiyama T, Takemura M, Toba S, Matsumoto S, Ikehara T, Tsuji M, Sato T, Yamano Y. 2019. In vivo pharmacodynamic study of cefiderocol, a novel parenteral siderophore cephalosporin, in murine thigh and lung infection models. Antimicrob Agents Chemother 63:e02031-18. doi: 10.1128/AAC.02031-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Katsube T, Wajima T, Ishibashi T, Arjona Ferreira JC, Echols R. 2017. Pharmacokinetic/pharmacodynamic modeling and simulation of cefiderocol, a parenteral siderophore cephalosporin, for dose adjustment based on renal function. Antimicrob Agents Chemother 61:e01381-16. doi: 10.1128/AAC.01381-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.U.S. National Library of Medicine. Clinical study of S-649266 for the treatment of nosocomial pneumonia caused by Gram-negative pathogens (APEKS-NP). https://clinicaltrials.gov/ct2/show/NCT03032380/. Accessed 13 February 2020.
- 151.U.S. National Library of Medicine. Study of S-649266 or best available therapy for the treatment of severe infections caused by carbapenem-resistant Gram-negative pathogens. https://clinicaltrials.gov/ct2/show/NCT02714595.
- 152.FDA. 2019. FDA Briefing Document: cefiderocol injection. https://www.fda.gov/media/131703/download. Accessed 13 February 2020.
- 153.Reck F, Bermingham A, Blais J, Capka V, Cariaga T, Casarez A, Colvin R, Dean CR, Fekete A, Gong W, Growcott E, Guo H, Jones AK, Li C, Li F, Lin X, Lindvall M, Lopez S, McKenney D, Metzger L, Moser HE, Prathapam R, Rasper D, Rudewicz P, Sethuraman V, Shen X, Shaul J, Simmons RL, Tashiro K, Tang D, Tjandra M, Turner N, Uehara T, Vitt C, Whitebread S, Yifru A, Zang X, Zhu Q. 2018. Optimization of novel monobactams with activity against carbapenem-resistant Enterobacteriaceae—identification of LYS228. Bioorg Med Chem Lett 28:748–755. doi: 10.1016/j.bmcl.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 154.Blais J, Lopez S, Li C, Ruzin A, Ranjitkar S, Dean CR, Leeds JA, Casarez A, Simmons RL, Reck F. 2018. In vitro activity of LYS228, a novel monobactam antibiotic, against multidrug-resistant Enterobacteriaceae. Antmicrob Agents Chemother 62:e00552-18. doi: 10.1138/AAC.00552-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Dean CR, Barkan DT, Bermingham A, Blais J, Casey F, Casarez A, Colvin R, Fuller J, Jones AK, Li C, Lopez S, Metzger LE, Mostafavi M, Prathapam R, Rasper D, Reck F, Ruzin A, Shaul J, Shen X, Simmons RL, Skewes-Cox P, Takeoka KT, Tamrakar P, Uehara T, Wei J-R. 2018. Mode of action of the monobactam LYS228 and mechanisms decreasing in vitro susceptibility in Escherichia coli and Klebsiella pneumoniae. Antimicrob Agents Chemother 62:e01200-18. doi: 10.1128/AAC.01200-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Livermore DM, Mushtaq S, Warner M, Vickers A, Woodford N. 2017. In vitro activity of cefepime/zidebactam (WCK 5222) against Gram-negative bacteria. J Antimicrob Chemother 72:1373–1385. doi: 10.1093/jac/dkw593. [DOI] [PubMed] [Google Scholar]
- 157.Mushtaq S, Vickers A, Woodford N, Haldimann A, Livermore DM. 2019. Activity of nacubactam (RG6080/OP0595) combinations against MBL-producing Enterobacteriaceae. J Antimicrob Chemother 74:953–960. doi: 10.1093/jac/dky522. [DOI] [PubMed] [Google Scholar]
- 158.Moya B, Barcelo IM, Bhagwat S, Patel M, Bou G, Papp-Wallace KM, Bonomo RA, Oliver A. 2017. WCK 5107 (Zidebactam) and WCK 5153 are novel inhibitors of PBP2 showing potent “β-lactam enhancer” activity against Pseudomonas aeruginosa, including multidrug-resistant metallo-β-lactamase-producing high-risk clones. Antimicrob Agents Chemother 61:e02529-16. doi: 10.1128/AAC.02529-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Doumith M, Mushtaq S, Livermore DM, Woodford N. 2016. New insights into the regulatory pathways associated with the activation of the stringent response in bacterial resistance to the PBP2-targeted antibiotics, mecillinam and OP0595/RG6080. J Antimicrob Chemother 71:2810–2814. doi: 10.1093/jac/dkw230. [DOI] [PubMed] [Google Scholar]
- 160.Wockhardt Ltd. 2019. Wockhardt Annual Report 2018–2019. Wockhardt Ltd., Mumbai, India. [Google Scholar]
- 161.Cahill ST, Cain R, Wang DY, Lohans CT, Wareham DW, Oswin HP, Mohammed J, Spencer J, Fishwick CWG, Mcdonough MA, Schofield CJ, Brem J. 2017. Cyclic boronates inhibit all classes of β-lactamases. Antimicrob Agents Chemother 61:e02260-16. doi: 10.1128/AAC.02260-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Brem J, Cain R, Cahill S, McDonough MA, Clifton IJ, Jiménez-Castellanos J-C, Avison MB, Spencer J, Fishwick CWG, Schofield CJ. 2016. Structural basis of metallo-β-lactamase, serine-β-lactamase and penicillin-binding protein inhibition by cyclic boronates. Nat Commun 7:12406. doi: 10.1038/ncomms12406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lomovskaya O, Dongxu S, Rubio-Aparicio D, Nelson K, Tsivkovski R, Griffith DC, Dudley MN. 2017. Vaborbactam: spectrum of β-lactamase inhibition and impact of resistance mechanisms on activity in Enterobacteriaceae. Antimicrob Agents Chemother 61:e01443-17. doi: 10.1128/AAC.01443-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Geibel B, Dowell J, Dickerson D, Henkel T. 2018. A randomized, double-blind, placebo-controlled study of the safety and pharmacokinetics of single and repeat doses of VNRX-5133 in healthy subjects. Open Forum Infect Dis 5:S431–S431. doi: 10.1093/ofid/ofy210.1232. [DOI] [Google Scholar]
- 165.Daigle D, Hamrick J, Chatwin C, Kurepina N, Kreiswirth BN, Shields RK, Oliver A, Clancy CJ, Nguyen M-H, Pevear D, Xerri L. 2018. 1370. Cefepime/VNRX-5133 broad-spectrum activity is maintained against emerging KPC- and PDC-variants in multidrug-resistant K. pneumoniae and P. aeruginosa. Open Forum Infect Dis 5:S419–S420. doi: 10.1093/ofid/ofy210.1201. [DOI] [Google Scholar]
- 166.Tsivkovski R, Totrov M, Lomovskaya O. 2020. Biochemical characterization of QPX7728, a new ultrabroad-spectrum β-lactamase inhibitor of serine and metallo-β-lactamases. Antimicrob Agents Chemother 64:e00130-20. doi: 10.1128/AAC.00130-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Rubio-Aparicio D, Nelson K, Griffith D, Dudley MN, Lomovskaya O. 2019. QPX7228 in vitro activity in combination with oral β-lactam antibiotics against Enterobacteriaceae (ENT), abstr AAR-710 ASM Microbe, 20 to 24 June 2019, San Francisco, CA. [Google Scholar]
- 168.Büttner D, Kramer JS, Klingler F-M, Wittmann SK, Hartmann MR, Kurz CG, Kohnhäuser D, Weizel L, Brüggerhoff A, Frank D, Steinhilber D, Wichelhaus TA, Pogoryelov D, Proschak E. 2018. Challenges in the development of a thiol-based broad-spectrum inhibitor for metallo-β-lactamases. ACS Infect Dis 4:360–372. doi: 10.1021/acsinfecdis.7b00129. [DOI] [PubMed] [Google Scholar]
- 169.Hammond GG, Huber JL, Greenlee ML, Laub JB, Young K, Silver LL, Balkovec JM, Pryor KD, Wu JK, Leiting B, Pompliano DL, Toney JH. 1999. Inhibition of IMP-1 metallo-β-lactamases and sensitization of IMP-1-producing bacteria by thioester derivatives. FEMS Microbiol Lett 179:289–296. doi: 10.1111/j.1574-6968.1999.tb08740.x. [DOI] [PubMed] [Google Scholar]
- 170.Brem J, van Berkel SS, Zollman D, Lee SY, Gileadi O, McHugh PJ, Walsh TR, McDonough MA, Schofield CJ. 2016. Structural basis of metallo-β-lactamase inhibition by captopril stereoisomers. Antimicrob Agents Chemother 60:142–150. doi: 10.1128/AAC.01335-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Tehrani K, Martin NI. 2017. Thiol-containing metallo-β-lactamase inhibitors resensitize resistant Gram-negative bacteria to meropenem. ACS Infect Dis 3:711–717. doi: 10.1021/acsinfecdis.7b00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.González MM, Kosmopoulou M, Mojica MF, Castillo V, Hinchliffe P, Pettinati I, Brem J, Schofield CJ, Mahler G, Bonomo RA, Llarrull LI, Spencer J, Vila AJ. 2015. Bisthiazolidines: a substrate-mimicking scaffold as an inhibitor of the NDM-1 carbapenemase. ACS Infect Dis 1:544–554. doi: 10.1021/acsinfecdis.5b00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hinchliffe P, González MM, Mojica MF, González JM, Castillo V, Saiz C, Kosmopoulou M, Tooke CL, Llarrull LI, Mahler G, Bonomo RA, Vila AJ, Spencer J. 2016. Cross-class metallo-β-lactamase inhibition by bisthiazolidines reveals multiple binding modes. Proc Natl Acad Sci U S A 113:E3745–E3754. doi: 10.1073/pnas.1601368113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lambert RJW, Hanlon GW, Denyer SP. 2004. The synergistic effect of EDTA/antimicrobial combinations on Pseudomonas aeruginosa. J Appl Microbiol 96:244–253. doi: 10.1046/j.1365-2672.2004.02135.x. [DOI] [PubMed] [Google Scholar]
- 175.Vaara M. 1992. Agents that increase the permeability of the outer membrane. Microbiol Rev 56:395–411. doi: 10.1128/MMBR.56.3.395-411.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Aoki N, Ishii Y, Tateda K, Saga T, Kimura S, Kikuchi Y, Kobayashi T, Tanabe Y, Tsukada H, Gejyo F, Yamaguchi K. 2010. Efficacy of calcium-EDTA as an inhibitor for metallo-β-lactamase in a mouse model of Pseudomonas aeruginosa pneumonia. Antimicrob Agents Chemother 54:4582–4588. doi: 10.1128/AAC.00511-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Yoshizumi A, Ishii Y, Kimura S, Saga T, Harada S, Yamaguchi K, Tateda K, Livermore DM, Woodford N, Livermore DM. 2013. Efficacies of calcium–EDTA in combination with imipenem in a murine model of sepsis caused by Escherichia coli with NDM-1 β-lactamase. J Infect Chemother 19:992–995. doi: 10.1007/s10156-012-0528-y. [DOI] [PubMed] [Google Scholar]
- 178.Patil UN, Jambulingappa KL. 2015. A combination strategy of ceftriaxone, sulbactam and disodium edetate for the treatment of multi-drug resistant (MDR) septicaemia: a retrospective, observational study in Indian tertiary care hospital. J Clin Diagn Res 9:FC29–FC32. doi: 10.7860/JCDR/2015/14129.6840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Chaudhary M, Mir MA, Ayub S. 2017. Safety and efficacy of a novel drug elores (ceftriaxone + sulbactam + disodium edetate) in the management of multi-drug resistant bacterial infections in tertiary care centers: a post-marketing surveillance study. Braz J Infect Dis 21:408–417. doi: 10.1016/j.bjid.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.U.S. Department of Health & Human Sciences. 2019. Code of Federal Regulations Title 21. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=73.2120. Accessed 24 June 2020.
- 181.Graceway Pharmaceuticals. 2009. Calcium disodium versenate: NDA 8–922/S-016. https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/008922s016lbl.pdf. Accessed 24 June 2020.
- 182.Haenni AL, Robert M, Vetter W, Roux L, Barbier M, Lederer E. 1965. Structure chimique des aspergillomarasmines A et B. Helv Chim Acta 48:729–750. doi: 10.1002/hlca.19650480409. [DOI] [PubMed] [Google Scholar]
- 183.Bergstrom A, Katko A, Adkins Z, Hill J, Cheng Z, Burnett M, Yang H, Aitha M, Mehaffey MR, Brodbelt JS, Tehrani K, Martin NI, Bonomo RA, Page RC, Tierney DL, Fast W, Wright GD, Crowder MW. 2018. Probing the interaction of aspergillomarasmine A (AMA) with metallo-β-lactamases NDM-1, VIM-2, and IMP-7. ACS Infect Dis 4:135–145. doi: 10.1021/acsinfecdis.7b00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.King AM, Reid-Yu SA, Wang W, King DT, De Pascale G, Strynadka NC, Walsh TR, Coombes BK, Wright GD. 2014. Aspergillomarasmine A overcomes metallo-β-lactamase antibiotic resistance. Nature 510:503–506. doi: 10.1038/nature13445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.VenatoRx Pharmaceuticals. 2019. Venatorx Pharmaceuticals to present data for its injectable and orally bioavailable beta-lactamase inhibitor drug candidates at ECCMID 2019. https://www.venatorx.com/press-releases/venatorx-pharmaceuticals-to-present-data-for-its-injectable-and-orally-bioavailable-beta-lactamase-inhibitor-drug-candidates-at-eccmid-2019. Accessed 9 February 2020.
- 186.Ghazi IM, Crandon JL, Lesho EP, McGann P, Nicolau DP. 2015. Efficacy of humanized high-dose meropenem, cefepime, and levofloxacin against Enterobacteriaceae isolates producing Verona integron-encoded metallo-β-lactamase (VIM) in a murine thigh infection model. Antimicrob Agents Chemother 59:7145–7147. doi: 10.1128/AAC.00794-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Wiskirchen DE, Nordmann P, Crandon JL, Nicolau DP. 2014. In vivo efficacy of human simulated regimens of carbapenems and comparator agents against NDM-1-producing Enterobacteriaceae. Antimicrob Agents Chemother 58:1671–1677. doi: 10.1128/AAC.01946-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.MacVane SH, Crandon JL, Nichols WW, Nicolau DP. 2014. Unexpected in vivo activity of ceftazidime alone and in combination with avibactam against New Delhi metallo-β-lactamase-producing Enterobacteriaceae in a murine thigh infection model. Antimicrob Agents Chemother 58:7007–7009. doi: 10.1128/AAC.02662-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Leiris S, Coelho A, Castandet J, Bayet M, Lozano C, Bougnon J, Bousquet J, Everett M, Lemonnier M, Sprynski N, Zalacain M, Pallin TD, Cramp MC, Jennings N, Raphy G, Jones MW, Pattipati R, Shankar B, Sivasubrahmanyam R, Soodhagani AK, Juventhala RR, Pottabathini N, Pothukanuri S, Benvenuti M, Pozzi C, Mangani S, De Luca F, Cerboni G, Docquier J-D, Davies DT. 2019. SAR studies leading to the identification of a novel series of metallo-β-lactamase inhibitors for the treatment of carbapenem-resistant Enterobacteriaceae infections that display efficacy in an animal infection model. ACS Infect Dis 5:131–140. doi: 10.1021/acsinfecdis.8b00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Everett M, Sprynski N, Coelho A, Castandet J, Bayet M, Bougnon J, Lozano C, Davies DT, Leiris S, Zalacain M, Morrissey I, Magnet S, Holden K, Warn P, De Luca F, Docquier J-D, Lemonnier M. 2018. Discovery of a novel metallo-β-lactamase inhibitor that potentiates meropenem activity against carbapenem-resistant Enterobacteriaceae. Antimicrob Agents Chemother 62:e00074-18. doi: 10.1128/AAC.00074-18. [DOI] [PMC free article] [PubMed] [Google Scholar]