Ceftriaxone is widely used for respiratory and urinary infections in elderly and frail patients, but there are few pharmacokinetic studies. A prospective population pharmacokinetic study of ceftriaxone in adults over 65 years old was undertaken. Dried blood spots collected at baseline (predose) and 0.5, 1, 4, 8, and 24 h after administration of 1 g of ceftriaxone were assayed using a validated liquid chromatography-mass spectroscopy analytical method. Frailty was classified using the Edmonton frailty scale and grip strength via a hand dynamometer.
KEYWORDS: ceftriaxone, frailty, population pharmacokinetics
ABSTRACT
Ceftriaxone is widely used for respiratory and urinary infections in elderly and frail patients, but there are few pharmacokinetic studies. A prospective population pharmacokinetic study of ceftriaxone in adults over 65 years old was undertaken. Dried blood spots collected at baseline (predose) and 0.5, 1, 4, 8, and 24 h after administration of 1 g of ceftriaxone were assayed using a validated liquid chromatography-mass spectroscopy analytical method. Frailty was classified using the Edmonton frailty scale and grip strength via a hand dynamometer. Estimates of glomerular filtration rate were determined using creatinine-based and cystatin C-based equations. Of 26 patients recruited, 23 (88%) were vulnerable or very frail. Estimates of drug clearance improved significantly with a cystatin C-based estimate of renal function that accounted for frailty. Simulations indicate that the combined effects of ranges of size and renal function resulted in a 6-fold range in peak ceftriaxone concentrations and 9-fold range in total exposure (area under the concentration-time curve [AUC]). For elderly patients with moderate or severe renal impairment, 48-h dosing results in greater trough concentrations and total exposure than the trough concentrations and total exposure in patients with normal renal function receiving 24-h dosing. Cystatin C-based measures of renal function improved predictions of ceftriaxone clearance in elderly patients.
TEXT
Ceftriaxone is a broad-spectrum, third-generation cephalosporin antibiotic used widely for common severe infections like community-acquired pneumonia (1), urosepsis (2), and gonococcal infections (3, 4). As with many older, extensively used antibiotics, there are few quality pharmacokinetic (PK)/pharmacodynamic (PD) data. This knowledge gap is considered a research priority (5). This has particular relevance in elderly, frail populations that, in resource-rich settings, carry the large burden of the infections for which ceftriaxone is recommended therapy. There are few published PK studies in this population, with current dosing regimens extrapolated from studies conducted in healthy older adults or younger individuals (6, 7).
Aging is associated with a number of physiological changes that may influence the PK of antimicrobial and other drugs (8, 9). However, the large interindividual variability in PK parameters in elderly patients suggests that chronological age alone should not determine drug-dosing regimens (10). Frailty is a state of heightened vulnerability to functional dependence or death in response to a stressor (11). The “frailty phenotype” is an independent risk factor and better predictor of hospitalization and mortality than chronological age (12). Frailty also has PK implications, given its association with reduced lean body mass, sarcopenia, increased adiposity, and altered hepatic and renal drug clearance (CL) (13, 14). These changes in body composition may mean that estimates of renal function derived from plasma creatinine (Cr) concentrations may not reflect renal clearance of drugs in elderly populations. This is important for antibiotics with a narrow therapeutic index, such as gentamicin, with frail patients exhibiting reduced drug clearance even after adjusting for renal function using established formulae (8). Cystatin C (CysC), a lower-molecular-weight protein that is produced by all nucleated cells at a constant rate, is freely filtered by the glomerulus and is a measure of kidney function that is less dependent on muscle mass (15). Studies have shown that cystatin C is associated with increased frailty status and that a cystatin C-based equation is a better marker than a creatinine-based measure in detecting mild to moderate kidney function reductions in older adults (15, 16).
Given the lack of available PK data for ceftriaxone in elderly patients, we performed a population PK study in this patient group with frailty as a candidate covariate in the PK model. We then explored whether estimation of renal function through plasma CysC might have more utility in a PK model than Cr-based equations. Because ceftriaxone is typically initiated in an emergency department (ED) setting, we used validated measurement of ceftriaxone concentrations in capillary dried blood spots (DBS) that allowed repeated, nondisruptive, small-volume sampling (17, 18).
RESULTS
Subject characteristics.
Twenty-six patients were enrolled, but 24 were included in the analysis due to 2 participants withdrawing consent. Most of the participants (92%) were Caucasian, and 12 (50%) were female. Six (25%) received ceftriaxone for urinary tract infections and the remainder for lower respiratory tract infections. The median (interquartile range [IQR]) age was 81 (74 to 86) years. The median (IQR) body mass index (BMI), Cr clearance with body weight (CLCr) (Cockcroft-Gault), albumin, and estimated glomerular filtration rate using the Cr-cystatin C method (eGFRCr-Cys) were 27.5 (22 to 33) kg/m2, 48 (26 to 63) ml/min, 38 (35 to 41.5) g/liter, and 36 (23 to 49) ml/min, respectively. The median (IQR) grip strength and Edmonton frailty scale (EFS) score were 13.5 (7 to 21) kg and 10 (7.5 to 11.5), respectively. Only three participants (12%) were considered to be not frail, while 15 (63%) and 6 (25%) patients were considered to be vulnerable or very frail, respectively. There were strong correlations between CysC and age (0.60; P = 0.002), EFS and eGFR using the modification of diet in renal disease formula (eGFRMDRD) (−0.41; P = 0.041), grip strength and EFS (−0.51; P = 0.012), EFS and CysC (0.57; P = 0.003), and grip strength and serum albumin (0.43; P = 0.036). There was also a significant correlation between the two measures of renal function (−0.50; P = 0.014).
PK modeling.
There were 101 concentrations from 24 patients available for PK analysis, none of which were below the limit of quantification. A two-compartment model performed better than a single compartment model in characterizing the data. Adding a third compartment did not result in further improvement. It was possible to estimate interindividual variability for central volume of distribution (VC) and CL.
Of the size measures tested, all improved the fit of the model except ideal body weight. The use of normal fat weight, which allowed the estimation of the contribution of fat, resulted in the lowest objective function value (OFV) (−16.00 compared to no allometric scaling). However, compared to body weight, there was no significant improvement given the need for four additional parameters (ΔOFV = −3.70, P = 0.45, χ2 df = 4). There were also large standard errors for the FFAT terms (see Materials and Methods; FFAT represents the relative contribution of fat mass compared to the contribution of fat-free mass to the size covariate for each parameter). Therefore, allometric scaling with body weight was used for further modeling.
Assessment of which measure of renal function was most appropriate for the PK population model is summarized in Table 1. A model with CLCr performed better than eGFRMDRD and resulted in a significant fall in the OFV. Plots for the various renal function estimates against individual clearance estimates obtained from the base model demonstrated that eGFRCr-Cys correlated best, followed by eGFR using the cystatin C method (eGFRCys), then Cr clearance with adjusted body weight (CLCr-Adj), eGFR using the Cr method (eGFRCr), Cr clearance with body weight (CLCr), and finally, eGFRMDRD (Fig. S1 in the supplemental material). As a result, eGFRCr-Cys was included in the model and was associated with a 45% reduction in the population variability estimate for CL. After accounting for allometry based on size and renal function, no other tested covariates were suitable for inclusion in the final model. Of these, serum albumin values were available but did not improve model fit.
TABLE 1.
Changes in OFV with different measures of renal function and the addition of grip strength as a covariate for clearance
| Modela | OFVb | ΔOFV | P value | Result with addition of grip strength to CL |
||
|---|---|---|---|---|---|---|
| OFV | ΔOFV | P value | ||||
| Base model (allometric scaling only) | −195.018 | |||||
| eGFRMDRD | −207.875 | −12.857 | <0.001 | −216.815 | −8.94 | 0.003 |
| CLCr | −209.856 | −14.838 | <0.001 | −214.573 | −4.717 | 0.030 |
| eGFRCr | −210.851 | −15.833 | <0.001 | −213.563 | −2.712 | 0.100 |
| CLCr-Adj | −211.568 | −16.55 | <0.001 | −216.239 | −4.671 | 0.031 |
| eGFRCys | −213.673 | −18.655 | <0.001 | −213.952 | −0.279 | 0.597 |
| eGFRCr-Cys | −217.224 | −22.206 | <0.001 | −218.401 | −1.177 | 0.278 |
eGFRMDRD, estimated glomerular filtration rate using the modification of diet in renal disease formula; CLCr, creatinine clearance; CLCr-Adj, creatinine clearance adjusted for body weight; eGFRCys, estimated glomerular filtration rate with cystatin C; eGFRCr-Cys, estimated glomerular filtration rate with cystatin C and Cr.
OFV, objective function value.
In the assessment of the relationship between frailty and clearance, grip strength correlated with clearance when CLCr, CLCr-Adj, or eGFRMDRD was used as the renal function measure (Table 1). In these cases, the inclusion of grip strength resulted in a significant reduction in the OFV, as well as reduction in the population variability estimate for CL. This relationship suggested that a lower CL was associated with weaker grip strength, a correlate of increased frailty. This is also consistent with a significant bias with lower grip strength for these renal function estimates compared to estimates using eGFRCr-Cys (Table 1). There was a nonsignificant trend for a positive bias with eGFRCr, while there was an opposite trend for eGFRCys. The addition of grip strength did not improve the model when eGFRCr-Cys, eGFRCys, or eGFRCr was used. The EFS did not have any significant effect when tested with any of the renal function measures.
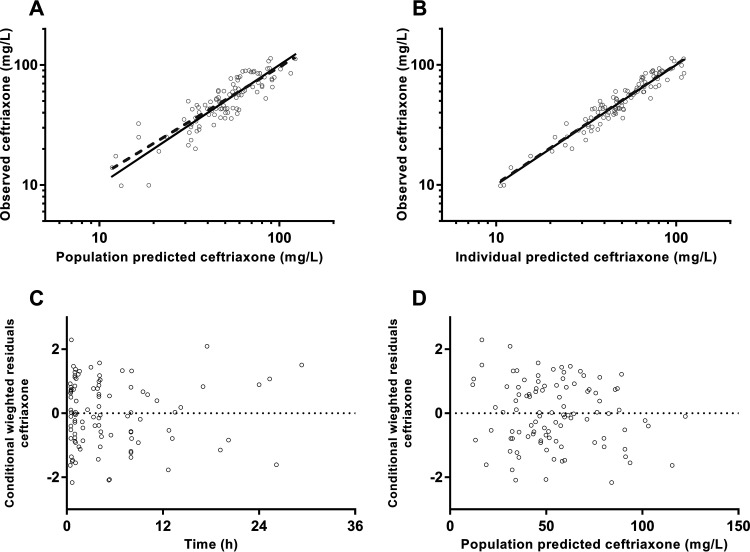
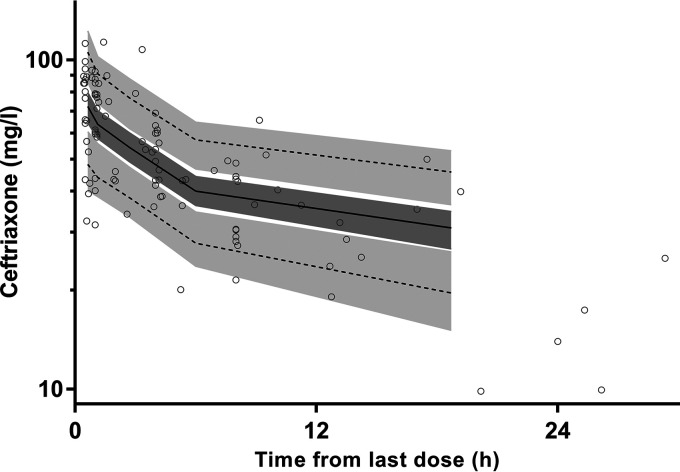
Final parameter estimates and bootstrap results are summarized in Table 2. Bias was <9% for all structural and random model parameters. Goodness-of-fit plots (Fig. 1) and prediction-corrected visual predictive checks (pcVPC) (Fig. 2) are presented with all observed percentiles falling within their respective 95% simulated confidence intervals.
TABLE 2.
Base and final-model population pharmacokinetic estimates and final-model bootstrap results for ceftriaxone in elderly patients
| Parametera | Value for: |
|||
|---|---|---|---|---|
| Mean base | Final model |
|||
| Mean | RSE (%)b | Median bootstrap (95% CI) | ||
| Objective function value | −195.018 | −217.224 | −226.335 (−255.871 to −191.59) | |
| Structural-model parameters | ||||
| CLR (liters/h per 60 ml/min) | 2.125 | 11 | 2.044 (0.98 to 2.66) | |
| CLNR (liters/h per 70 kg) | 0.225 | 33 | 0.248 (0.028 to 0.667) | |
| CL (liters/h per 70 kg) | 0.279 | |||
| VC (liters per 70 kg) | 13.2 | 13.3 | 8 | 13.06 (9.27 to 16.2) |
| Q (liters/h per 70 kg) | 4.99 | 4.90 | 34 | 5.16 (0.95 to 16.0) |
| VP (liters per 70 kg) | 4.87 | 4.73 | 17 | 5.06 (2.87 to 7.86) |
| Variable-model parameters (% shrinkage) | ||||
| IIV in CL | 42 (8) | 22 (22) | 21 | 21 (10 to 30) |
| IIV in VC | 25 (13) | 25 (13) | 15 | 24 (16 to 34) |
| r(CL, VC) | 0.085 | −0.177 | 85 | −0.248 (−0.839 to 0.452) |
| RV (%) | 16 (8) | 16 (16) | 15 | 16 (13 to 18) |
CLR, renal clearance—final model; CLNR, nonrenal clearance—final model; CL, clearance—base model; VC, central volume of distribution; Q, intercompartmental clearance; VP, peripheral volume of distribution; IIV, interindividual variability; RV, residual variability. IIV and RV are presented as .
RSE, residual standard error.
FIG 1.
Goodness-of-fit plots for ceftriaxone, including observed concentration against population (A) and individual (B) predicted concentrations and conditional weighted residuals against time from dose (C) and population predicted concentrations (D).
FIG 2.
Prediction-corrected visual predictive checks for ceftriaxone in elderly patients with observed 50th (solid line) and 5th and 95th (dotted lines) percentiles within their simulated 95% CIs (gray shaded areas) are shown with overlying data points (○).
Simulation results.
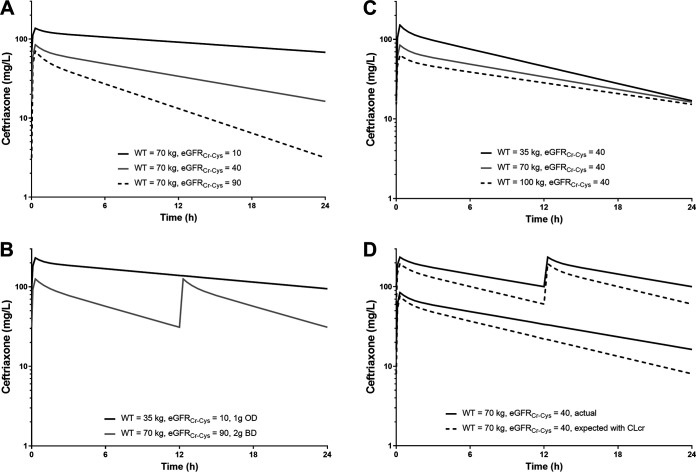
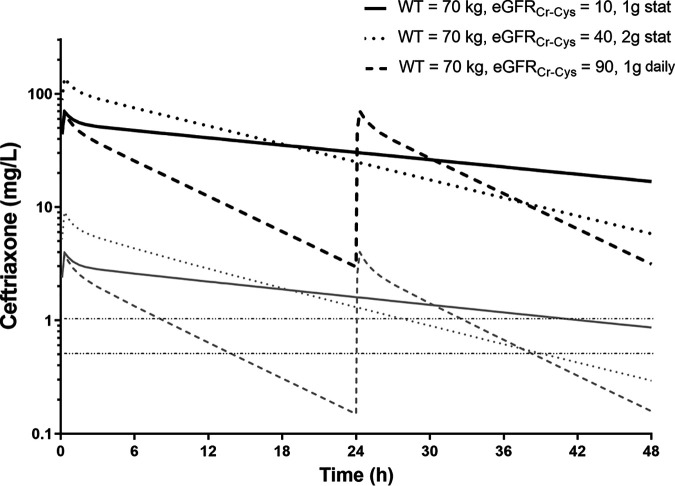
Simulations demonstrated the significant effect of impaired renal function (eGFRCr-Cys = 10 ml/min/1.73m2) on exposure (area under the curve [AUC]), with up to 5-fold greater exposure and close to three times higher peak concentrations than with normal renal function (eGFRCr-Cys = 90 ml/min/1.73m2) (Fig. 3A). Size also affected drug exposure, with an approximate doubling of peak concentrations and AUC (Fig. 3C). The combined effects of these two covariates resulted in a 6-fold range in peak concentrations and 9-fold range in AUC in the simulations. To highlight these important differences, comparisons of simulations between a light patient with impaired renal function receiving 1 g daily and a heavier patient with normal renal function receiving 2 g twice daily are shown (Fig. 3B). The exposure profiles indicate that peak and trough concentrations are both higher in the former. The differences in concentrations derived using CLCr compared with eGFRCr-Cys for dosing are presented in Fig. 3D. AUC and peak concentrations were 40% and 10% higher than expected, respectively, when using traditional measures of renal function. Simulations for ceftriaxone dosing given every 48 h for patients with moderate (2 g) or severe (1 g) renal impairment are shown in Fig. 4. Following these doses, AUC and trough concentrations were higher than for standard dosing with 1 g every 24 h for patients with normal renal function. Additional simulations for estimated free ceftriaxone concentrations over time indicated that the times above the breakpoints of 1 mg/liter (Escherichia coli) and 0.5 mg/liter (Streptococcus pneumoniae) were also prolonged compared with the times above the breakpoints with standard dosing in patients with normal renal function (Fig. 4).
FIG 3.
Results of simulations at steady state demonstrating the effects of changes in renal function (A), changes in body weight (B), the greatest difference in simulated individuals (C), and the difference in expected (from CLCr) and actual concentrations in frail individuals (D).
FIG 4.
Results of simulations at steady state demonstrating time-concentration profiles for ceftriaxone given every 48 h in patients with severely impaired renal function (black solid line; 1-g dose) and moderate renal impairment (black dotted line; 2-g dose) compared with normal renal function (black dashed line; 1 g every 24 h). The corresponding gray lines represent simulated free concentrations over time and are presented in relation to target breakpoints for Escherichia coli (1 mg/liter) and Streptococcus pneumoniae (0.5 mg/liter). stat, single dose only.
DISCUSSION
Many elderly patients presenting to hospital with infections are frail. As demonstrated with ceftriaxone in the present study, traditional Cr-based measures of renal function do not always adequately predict the renal clearance of antibiotics and other drugs in this patient group. When estimates of renal function are based on measurement of CysC, the effects of frailty can be better accounted for and estimates of drug clearance significantly improved.
Although estimates based on serum Cr are recommended for drug dose adjustment, we found that eGFRCr-Cys performed best when intravenous (i.v.) ceftriaxone was administered to a group of elderly patients. Renal clearance estimated with Cr-based calculations had an increasing positive bias with decreasing grip strength (up to 50% in severely frail patients) compared with eGFRCr-Cys. In these patients, exposure to ceftriaxone (AUC) was underestimated by up to 40%. Although ceftriaxone has a wide therapeutic index, the consequences of underestimated drug concentrations are likely to be greater for medications with a narrow therapeutic index, such as gentamicin, where concentration-dependent toxicity is a major challenge.
There are few data on the optimal dose of ceftriaxone in the elderly, and current guidelines do not recommend alterations to the dosing regimen in elderly patients. Our data accord with a small study of six elderly patients with a mean eGFR of 55 ml/min/1.73m2 that identified a large difference between observed drug clearance in the group of elderly patients compared with that in younger healthy volunteers (19). In contrast, another study did not show as large a difference, but the recruited elderly volunteers had a higher mean eGFR (6). If CysC-based estimates of GFR were routinely available, this could have implications for ceftriaxone dosing in elderly and frail adults. Our data show that, among elderly patients with moderately to severely impaired renal function based on eGFRCr-Cys, there may be the potential to decrease the frequency of dosing from daily to every second day. If this is confirmed, a decreased dose frequency would have favorable resourcing implications, including earlier hospital discharge and simpler ceftriaxone administration in domiciliary-based patients requiring parenteral antibiotic therapy.
Our study was designed to assess the impact of the frailty phenotype on ceftriaxone PK. In a study of 38 elderly patients, half were frail (EFS ≥ 8) and their gentamicin clearance was reduced by 12% (20), but CLCr calculated from ideal body weight was applied to estimate renal function. When either CLCr, CLcr-Adj, or eGFRMDRD was applied in the present study, we also demonstrated a statistically significant relationship between decreasing grip strength (as a marker for increasing frailty) and lower clearance. In the case of CLCr, this relationship estimated a 35% lower ceftriaxone clearance in patients with the lowest compared to the highest grip strength. These results suggest that previously reported effects of frailty on drug clearance are likely due to bias in estimates of renal clearance in this population.
CysC-based estimates of renal function could improve the safety and efficacy of antibiotics with a narrow therapeutic index, including for situations other than in the elderly where frailty is common. Compared with gold standard methods for measuring GFR, eGFRCr-Cys outperforms Cr-based estimates across different patient groups with a range of degrees of renal impairment (21). A review found that CysC correlated better with drug clearance and/or trough levels (22), and current Kidney Disease Improving Global Outcomes (KDIGO) guidelines recommend CysC-based measures of renal function when precision is required (23). A case in point is vancomycin, where the use of eGFRCr-Cys improved the proportion of patients achieving trough concentration targets (24). Despite this evidence, the use of CysC-based measures has not been widely adopted. This may be in part due to cost considerations or due to the lack of international standardization of the assay to allow valid comparisons (25).
The present study had limitations. We did not measure unbound (free) ceftriaxone concentrations because we used the DBS sampling method. However, by applying a concentration-dependent correction for free concentrations (26), the estimated times above established breakpoints for the common bacteria causing community-acquired pneumonia and urinary tract infections were longer in patients with impaired CysC-based measurements of renal function than for standard dosing. Serum albumin concentrations were available but were not a significant covariate in our final PK model. Low serum albumin caused by malnutrition, decreased hepatic production, or urinary or gastrointestinal protein loss may increase the unbound fraction. Taken together, these data provide reassurance that theoretical antibiotic efficacy would not be impacted in those elderly patients with increased ceftriaxone exposure profiles. We measured Cr and CysC on only one occasion during treatment. As with Cr-based measurements, large changes in renal clearance of drugs can be observed during severe illness, and it is possible that this applies to CysC with biased estimates of clearance. Nevertheless, clinicians will often determine the drug dosage based on a single baseline Cr measurement in this setting.
We conclude that CysC-based measures of renal function improve the prediction of ceftriaxone clearance in elderly patients and should be considered routine practice for this patient group. Extending this approach to other renally excreted medications with a low therapeutic index also warrants further investigation. Due to variations in body size and renal function in elderly patients, ceftriaxone concentrations and exposure profiles vary widely, raising the possibility for more efficient dosing regimens, such as every 48 h, to facilitate provision of ceftriaxone in domiciliary settings.
MATERIALS AND METHODS
Approvals, patients, and sample collection.
This study was approved by the South Metropolitan Health Service Human Research Ethics Committee (SMHS HREC; 2016-129), Western Australia. Participants were eligible for recruitment if they were >65 years old and required ceftriaxone as part of their infection management plan, as assessed by ED staff. Participants were excluded if they had received ceftriaxone within 72 h of admission or had a history of adverse reaction to cephalosporins or if language barriers prevented provision of informed consent.
It was expected that some participants might not be able to provide informed consent due to acute delirium or background cognitive impairment. To address this issue, we applied a multistage process in assessing relevant capacity. The treating ED clinician first determined whether there was a concern that the prospective participant was incapable of providing informed consent. If no concerns were identified, the researchers (M.C. and S.J.T.) provided a verbal explanation of the study as well as an information sheet before written informed consent was obtained. If the ED clinician or researchers had any concerns regarding capacity, a mini-mental state examination (MMSE) was performed (27). If the participant scored <21, or ≥21 but the treating ED clinician was still concerned about capacity, a relative/guardian provided informed consent on the participant’s behalf.
Because sampling was minimally invasive, a process of delayed consent was followed so that ceftriaxone could be promptly administered and initial DBS samples taken. If the prospective participant did not have capacity and no relative/guardian was contactable, the prospective participant was enrolled in the study while a researcher attempted to contact a relative/guardian. If no contact was made prior to the third DBS sample, the participant was withdrawn from the study. Participants were free to withdraw at any time without prejudice to their ongoing care.
A standard questionnaire, clinical assessment, and blood sample protocol were followed in each case. Data collected included age, gender, ethnicity, height, weight, serum Cr, liver function tests including albumin concentrations, full blood count, and CysC. Frailty was assessed using the Edmonton frailty scale (EFS) (28). Grip strength was measured three times on each hand using a dynamometer (Jamar hydraulic hand dynamometer), with the greatest value recorded. All measurements were taken at the time of recruitment or as soon as the patient was alert enough to cooperate. Absence of frailty, apparent vulnerability, or severe frailty was assumed if the EFS was ≤5, 6 to 11, or ≥12, respectively.
Ceftriaxone 1g (Sandoz Australia, Pyrmont, NSW, Australia) was administered intravenously via a slow push over 3 to 5 min. DBS samples were collected from finger prick samples at baseline (predose) and then at 0.5, 1, 4, 8, and 24 h postadministration of ceftriaxone. At least two DBS samples were collected onto DBS cards at each time point (Whatman 903 protein saver cards; GE Healthcare Australia Pty. Ltd., Parramatta, NSW, Australia). The DBS cards were air dried for ≥1 h before being placed into an airtight foil bag with two desiccant sachets and stored at −80°C until analyzed. Ceftriaxone concentrations from DBS were measured using a validated liquid chromatography-tandem mass spectroscopy (LC-MS/MS) assay with a limit of quantification of 0.1 mg/liter (17).
Data analysis.
Correlations were assessed using Spearman’s rank correlation coefficient (r) (GraphPad Prism version 6.05; GraphPad Software, Inc., La Jolla, CA, USA).
Pharmacokinetic modeling.
NONMEM (version 7.2.0, ICON Development Solutions, Ellicott City, MD, USA) with an Intel Visual FORTRAN 10.0 compiler was utilized for nonlinear mixed-effects modeling of the loge predicted plasma concentration-time data sets for ceftriaxone. Predicted plasma concentrations were determined from measured dried blood spot concentrations using a previously validated method (17). The first-order conditional estimates method with interaction (FOCE INTER) estimation was used, with the minimum value of the objective function value (OFV), goodness-of-fit plots, condition number (<1,000), and predictive checks used to arrive at suitable models during the model-building process. A significance level of P < 0.05 was set for comparison of nested models. An additive residual variability (RV) model, equivalent to proportional RV structures on the normal scale, was tested for the log-transformed data. Base models were parameterized using VC (central volume of distribution), CL (clearance), and peripheral volume(s) of distribution (VP) and its/their respective intercompartmental clearance(s) (Q).
Ceftriaxone concentration profiles were modeled using one-, two- and three-compartment models (ADVAN1, -3, and -11) with first-order elimination from the central compartment. Once the structure of the models was established, interindividual variability (IIV) of parameters was estimated where supported by the data.
The effect of body size on pharmacokinetic parameters was investigated before testing of other covariates. Allometric scaling based on size was applied a priori, with coefficients of 1 and 3/4 for clearance and volume parameters, respectively. Several measures of size were tested in the model building process to identify the most appropriate descriptor. These were body weight, adjusted body weight, ideal body weight, and lean body mass (29). Additionally, models with normal fat weight using an additional parameter, FFAT, were assessed as follows: normal fat weight = fat-free mass + FFAT × (body weight − fat-free mass) (30), where FFAT represents the relative contribution of fat mass compared to the contribution of fat-free mass to the size covariate for each parameter.
Subsequently, the effect of renal function on clearance was assessed. Renal function was estimated using Cr clearance (Cockcroft-Gault) both with body weight (CLCr) (31) and adjusted body weight (CLCr-Adj) (32) and the estimated glomerular filtration rate (eGFR) using the modification of diet in renal disease formula (eGFRMDRD) (33), the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) (2009) Cr method (eGFRCr) (34), the CKD-EPI (2012) cystatin C method (eGFRCys), and the Cr-cystatin C method (eGFRCr-Cys). (35). Estimates of eGFR (in ml/min/1.73 m2) were converted to ml/min using each patient’s body surface area (33).
Clearance was modeled as the sum of renal clearance (CLR) and nonrenal clearance (CLNR) as follows: individual CL = CLNR × allometric scaling + CLR × (individual renal function [ml/min]/60).
The effects of other covariates, including gender, grip strength, EFS, and diagnosis (urinary tract infection or respiratory infection), were identified by inspection of individual parameter versus covariate plots. Identified relationships were then tested within NONMEM using a stepwise forward and backward approach (P < 0.10 for forward steps and P < 0.05 for backward steps). The effect sizes (%) of categorical data were assessed, while both linear and power relationships were evaluated for continuous covariates. To test the effect of frailty on drug clearance and its relationship with renal function, the potential effects of grip strength and frailty score on CL were assessed for all measures of renal function.
Model evaluation comprised goodness-of-fit plots, including observed versus individual and population predicted values, residual plots against time from last dose, and population predicted values. A bootstrap using Perl speaks NONMEM (PSN) with 1,000 samples was performed, and the parameters derived from this analysis summarized as the median and 2.5th and 97.5th percentiles (95% empirical confidence interval [CI]) to facilitate evaluation of final estimates. In addition, prediction-corrected visual predictive checks (pcVPC) were performed, with 1,000 data sets simulated from the final models. The observed 10th, 50th, and 90th percentiles were plotted with their respective simulated 95% CIs to assess the predictive performance of the model and to assess for any major bias.
Simulations.
Simulations of plasma concentrations were performed using the final population pharmacokinetic model. These were at steady state after low (1 g daily) and high (2 g twice a day) dose regimens, the latter dose administered for central nervous system infections or as adjunctive therapy for enterococcal endocarditis. Linear PK was assumed, consistent with previous reports of only small, clinically insignificant changes over a large dose range (36, 37). Median simulated time-concentration profiles with various weights (35 to 100 kg) and levels of renal function (10 to 90 ml/min/1.73m2) according to eGFRCr-Cys were obtained. Simulations also explored the potential effect of using CLCr instead of eGFRCr-Cys as the descriptor of renal function in terms of drug exposures. An additional simulation estimated time-concentration profiles for a dosing regimen of 1 g every 48 h. Simulations of free concentrations of ceftriaxone were subsequently estimated from the total concentrations using a validated correction factor (26). Breakpoints for Streptococcus pneumoniae (0.5 mg/liter) and Escherichia coli (1 mg/liter) were applied to provide context for PK-PD for free ceftriaxone concentrations (38).
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge the Emergency Department staff members at Royal Perth Hospital and Fiona Stanley Hospital for their assistance with this study.
Matthew Cockcroft wrote the initial protocol with Laurens Manning, submitted ethics applications, and commenced recruitment. Shu Jin Tan completed participant recruitment, collated the data, and wrote the initial draft of the manuscript.
This study was funded through the National Health and Medical Research Council (NHMRC) project grant (grant number 1047105). B.R.M. was supported by an NHMRC Early Career fellowship (grant number 1036951). T.M.E.D. is supported by a Medical Research Future Fund NHMRC Practitioner fellowship (grant number 1124130). The funders had no role in the study design, data collection and interpretation, or the decision to submit the work for publication.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Woodhead M, Blasi F, Ewig S, Garau J, Huchon G, Ieven M, Ortqvist A, Schaberg T, Torres A, van der Heijden G, Read R, Verheij TJM, Joint Taskforce of the European Respiratory Society and European Society for Clinical Microbiology and Infectious Diseases. 2011. Guidelines for the management of adult lower respiratory tract infections—full version. Clin Microbiol Infect 17(Suppl 6):E1–E59. doi: 10.1111/j.1469-0691.2011.03672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scottish Intercollegiate Guidelines Network. 2012. Management of suspected bacterial urinary tract infection in adults: a national clinical guideline. SIGN publication no. 88 SIGN, Edinburgh, United Kingdom. [Google Scholar]
- 3.World Health Organization. 2015. WHO model list of essential medicines, 19th list (April 2015). WHO, Geneva, Switzerland. [Google Scholar]
- 4.Chisholm SA, Mouton JW, Lewis DA, Nichols T, Ison CA, Livermore DM. 2010. Cephalosporin MIC creep among gonococci: time for a pharmacodynamic rethink? J Antimicrob Chemother 65:2141–2148. doi: 10.1093/jac/dkq289. [DOI] [PubMed] [Google Scholar]
- 5.Mouton JW, Ambrose PG, Canton R, Drusano GL, Harbarth S, MacGowan A, Theuretzbacher U, Turnidge J. 2011. Conserving antibiotics for the future: new ways to use old and new drugs from a pharmacokinetic and pharmacodynamic perspective. Drug Resist Updat 14:107–117. doi: 10.1016/j.drup.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 6.Luderer JR, Patel IH, Durkin J, Schneck DW. 1984. Age and ceftriaxone kinetics. Clin Pharmacol Ther 35:19–25. doi: 10.1038/clpt.1984.3. [DOI] [PubMed] [Google Scholar]
- 7.Patel IH, Sugihara JG, Weinfeld RE, Wong EG, Siemsen AW, Berman SJ. 1984. Ceftriaxone pharmacokinetics in patients with various degrees of renal impairment. Antimicrob Agents Chemother 25:438–442. doi: 10.1128/aac.25.4.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chin PK, Florkowski CM, Begg EJ. 2013. The performances of the Cockcroft-Gault, modification of diet in renal disease study and chronic kidney disease epidemiology collaboration equations in predicting gentamicin clearance. Ann Clin Biochem 50:546–557. doi: 10.1177/0004563213492320. [DOI] [PubMed] [Google Scholar]
- 9.Dowling TC, Wang ES, Ferrucci L, Sorkin JD. 2013. Glomerular filtration rate equations overestimate creatinine clearance in older individuals enrolled in the Baltimore Longitudinal Study on Aging: impact on renal drug dosing. Pharmacotherapy 33:912–921. doi: 10.1002/phar.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duffull SB, Wright DF, Winter HR. 2011. Interpreting population pharmacokinetic-pharmacodynamic analyses—a clinical viewpoint. Br J Clin Pharmacol 71:807–814. doi: 10.1111/j.1365-2125.2010.03891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arendts G, Burkett E, Hullick C, Carpenter CR, Nagaraj G, Visvanathan R. 2017. Frailty, thy name is… Emerg Med Australas 29:712–716. doi: 10.1111/1742-6723.12869. [DOI] [PubMed] [Google Scholar]
- 12.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA, Cardiovascular Health Study Collaborative Research Group. 2001. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 13.Ramsay SE, Whincup PH, Shaper AG, Wannamethee SG. 2006. The relations of body composition and adiposity measures to ill health and physical disability in elderly men. Am J Epidemiol 164:459–469. doi: 10.1093/aje/kwj217. [DOI] [PubMed] [Google Scholar]
- 14.Hubbard RE, Lang IA, Llewellyn DJ, Rockwood K. 2010. Frailty, body mass index, and abdominal obesity in older people. J Gerontol A Biol Sci Med Sci 65:377–381. doi: 10.1093/gerona/glp186. [DOI] [PubMed] [Google Scholar]
- 15.Hart A, Paudel ML, Taylor BC, Ishani A, Orwoll ES, Cawthon PM, Ensrud KE, Osteoporotic Fractures in Men Study Group. 2013. Cystatin C and frailty in older men. J Am Geriatr Soc 61:1530–1536. doi: 10.1111/jgs.12413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fliser D, Ritz E. 2001. Serum cystatin C concentration as a marker of renal dysfunction in the elderly. Am J Kidney Dis 37:79–83. doi: 10.1053/ajkd.2001.20628. [DOI] [PubMed] [Google Scholar]
- 17.Page-Sharp M, Nunn T, Salman S, Moore BR, Batty KT, Davis TM, Manning L. 2016. Validation and application of a dried blood spot ceftriaxone assay. Antimicrob Agents Chemother 60:14–23. doi: 10.1128/AAC.01740-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mukap M, Sprod C, Tefuarani N, Laman M, Page-Sharp M, Salman S, Moore BR, Batty KT, Davis TME, Manning L. 2018. Validation of a dried blood spot ceftriaxone assay in Papua New Guinean children with severe bacterial infections. Antimicrob Agents Chemother 62:e00940-18. doi: 10.1128/AAC.00940-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deeter RG, Weinstein MP, Swanson KA, Gross JS, Bailey LC. 1990. Crossover assessment of serum bactericidal activity and pharmacokinetics of five broad-spectrum cephalosporins in the elderly. Antimicrob Agents Chemother 34:1007–1013. doi: 10.1128/aac.34.6.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnston C, Hilmer SN, McLachlan AJ, Matthews ST, Carroll PR, Kirkpatrick CM. 2014. The impact of frailty on pharmacokinetics in older people: using gentamicin population pharmacokinetic modeling to investigate changes in renal drug clearance by glomerular filtration. Eur J Clin Pharmacol 70:549–555. doi: 10.1007/s00228-014-1652-7. [DOI] [PubMed] [Google Scholar]
- 21.Meeusen JW, Rule AD, Voskoboev N, Baumann NA, Lieske JC. 2015. Performance of cystatin C- and creatinine-based estimated glomerular filtration rate equations depends on patient characteristics. Clin Chem 61:1265–1272. doi: 10.1373/clinchem.2015.243030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brou NA, Jacqz-Aigrain E, Zhao W. 2015. Cystatin C as a potential biomarker for dosing of renally excreted drugs. Br J Clin Pharmacol 80:20–27. doi: 10.1111/bcp.12602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anonymous. 2013. Chapter 4: other complications of CKD: CVD, medication dosage, patient safety, infections, hospitalizations, and caveats for investigating complications of CKD. Kidney Int Suppl 3:91–111. doi: 10.1038/kisup.2012.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frazee EN, Rule AD, Herrmann SM, Kashani KB, Leung N, Virk A, Voskoboev N, Lieske JC. 2014. Serum cystatin C predicts vancomycin trough levels better than serum creatinine in hospitalized patients: a cohort study. Crit Care 18:R110. doi: 10.1186/cc13899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chew JS, Saleem M, Florkowski CM, George PM. 2008. Cystatin C—a paradigm of evidence based laboratory medicine. Clin Biochem Rev 29:47–62. [PMC free article] [PubMed] [Google Scholar]
- 26.McNamara PJ, Trueb V, Stoeckel K. 1988. Protein binding of ceftriaxone in extravascular fluids. J Pharm Sci 77:401–404. doi: 10.1002/jps.2600770509. [DOI] [PubMed] [Google Scholar]
- 27.Folstein MF, Folstein SE, McHugh PR. 1975. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 28.Rolfson DB, Majumdar SR, Tsuyuki RT, Tahir A, Rockwood K. 2006. Validity and reliability of the Edmonton Frail Scale. Age Ageing 35:526–529. doi: 10.1093/ageing/afl041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Green B, Duffull SB. 2004. What is the best size descriptor to use for pharmacokinetic studies in the obese? Br J Clin Pharmacol 58:119–133. doi: 10.1111/j.1365-2125.2004.02157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anderson BJ, Holford NH. 2008. Mechanism-based concepts of size and maturity in pharmacokinetics. Annu Rev Pharmacol Toxicol 48:303–332. doi: 10.1146/annurev.pharmtox.48.113006.094708. [DOI] [PubMed] [Google Scholar]
- 31.Cockcroft DW, Gault H. 1976. Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 32.Winter MA, Guhr KN, Berg GM. 2012. Impact of various body weights and serum creatinine concentrations on the bias and accuracy of the Cockcroft‐Gault equation. Pharmacotherapy 32:604–612. doi: 10.1002/j.1875-9114.2012.01098.x. [DOI] [PubMed] [Google Scholar]
- 33.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. 1999. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med 130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 34.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). 2009. A new equation to estimate glomerular filtration rate. Ann Intern Med 150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J, Levey AS, CKD-EPI Investigators. 2012. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 367:20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patel IH, Chen S, Parsonnet M, Hackman MR, Brooks MA, Konikoff J, Kaplan SA. 1981. Pharmacokinetics of ceftriaxone in humans. Antimicrob Agents Chemother 20:634–641. doi: 10.1128/aac.20.5.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pollock AA, Tee PE, Patel IH, Spicehandler J, Simberkoff MS, Rahal JJ Jr. 1982. Pharmacokinetic characteristics of intravenous ceftriaxone in normal adults. Antimicrob Agents Chemother 22:816–823. doi: 10.1128/aac.22.5.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.The European Committee on Antimicrobial Susceptibility Testing. 2020. Breakpoint tables for interpretation of MICs and zone diameters. http://www.eucast.org/clinical_breakpoints/. Accessed 1 May 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.