This study evaluated the safety, tolerability, and pharmacokinetics of a novel oral amphotericin B (AmB) formulation (iCo-019) following single doses to healthy humans. The data from this study suggest that iCo-019 has a long circulation time and systemic exposure without the associated gastrointestinal, liver, and kidney toxicity associated with AmB. This novel oral AmB formulation can serve as a new treatment strategy to overcome the limitations of the use of parenterally administered AmB products.
KEYWORDS: phase I human clinical trials, oral amphotericin B, safety and tolerability, pharmacokinetics, healthy human subjects, antimicrobial safety
ABSTRACT
This study evaluated the safety, tolerability, and pharmacokinetics of a novel oral amphotericin B (AmB) formulation (iCo-019) following single doses to healthy humans. The data from this study suggest that iCo-019 has a long circulation time and systemic exposure without the associated gastrointestinal, liver, and kidney toxicity associated with AmB. This novel oral AmB formulation can serve as a new treatment strategy to overcome the limitations of the use of parenterally administered AmB products.
TEXT
Amphotericin B, which is administered parenterally, has been considered a first-line therapy in the treatment of systemic fungal and parasitic infections, with a broad spectrum of activity and limited drug resistance (1, 2); however, its use has been limited by dose-dependent nephrotoxicity and the requirement for parenteral administration. The latter creates barriers to access, including its expense, the need for supplies and trained personnel for intravenous administration, and the necessity of cold-chain shipping and storage (1, 2). Until very recently, it has been particularly challenging to develop an oral formulation of amphotericin B (3–32) due to its physical and chemical properties, its limited water and lipid solubility, and its very poor oral absorption.
To overcome these challenges, the development of an oral formulation of amphotericin B that is cost effective, easy to administer, stable at ambient temperatures, and nontoxic yet retains excellent pharmacological activity is the ideal and represents the formulation used in the present study.
The primary objective of this study was to evaluate the safety and tolerability of iCo-019 following oral administration of single ascending doses (4 dose levels) in healthy subjects. The secondary objectives of this study were to assess the pharmacokinetic (PK) profile of iCo-019 after single-dose oral administration in healthy subjects and to identify the maximum tolerated dose of iCo-019 and its systemic exposure after a single oral dose in healthy subjects.
Based on the no-observed-adverse-effect level (NOAEL) determined for iCo-019 in dogs (58.8 to 93.75 mg/kg body weight/day for male and female dogs, respectively) in the good laboratory practice (GLP) 14-day toxicology studies (K. M. Wasan, E. K. Wasan, and P. Hnik, unpublished data), the human equivalent dose (HED) was calculated to be 32.7 mg/kg body weight/day (calculations based on 2005 U.S. FDA guidance). Applying a conservative safety factor of 10 to the HED, the maximum safe starting dose in the first-in-human trial is estimated to be 3.3 mg/kg body weight/day (198 mg/day for a 60-kg individual). A phase I clinical trial was conducted with iCo-019 as a single capsule dose of 100 mg (1.67 mg for a 60-kg subject) which is far below the NOAEL in dogs. Dose escalation proceeded in a doubling approach, i.e., the next cohort received 2 capsules for a 200 mg dose, the next cohort received 4 capsules for a 400 mg dose, and the fourth cohort received 8 capsules for 800 mg and dosing was adjusted based on safety evaluation committee (SEC) review after each cohort.
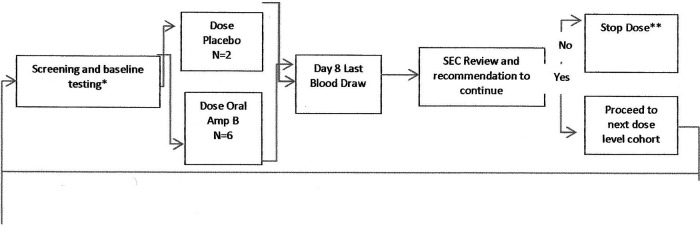
This was a randomized, double-blind, placebo-controlled, single-dose ascending study to assess the safety, tolerability, and pharmacokinetics of iCo-019 in healthy male and female subjects between 18 and 55 years of age. Subjects were randomized into 4 cohorts in a 3:1 ratio, each representing an ascending single dose of treatment (Fig. 1) or placebo. Cohorts were dosed sequentially in ascending fashion. Each cohort consisted of eight subjects, where six subjects were randomized to receive the investigational product (IP) and two subjects were randomized to receive placebo. A sentinel group consisting of two subjects (one subject receiving the IP and one subject receiving the placebo) was dosed before the other subjects in each cohort. When the sentinel group completed a 24-h follow-up after dosing in the study, the safety profile of these two subjects was reviewed by the investigator (or delegate) to determine whether it was safe to dose the remaining subjects. All subjects were followed for 7 days after dosing.
FIG 1.
Study diagram. *, each cohort will be screened up to 21 days prior to dosing; therefore, it is possible that screening for the next cohort will begin when the current cohort is being dosed. **, if severe AEs are noted during the first two cohorts, the SEC may stop the study. If severe AEs are noted during the final two cohorts, the SEC may proceed to the next cohort with a reduced dose level.
Subjects were dosed on day 1 and remained fasted for 4 h after the study drug administration. No other food or water restrictions were applied during the study. Blood samples for pharmacokinetic testing were taken at 0 h (prior to dosing) and at 0.5, 1, 2, 4, 6, 8, 10, 12, 24, 36, 48, 60, and 72 h after dosing. Subjects were required to report to the clinic at least 12 h before dosing (day −1) and required to stay overnight. Dosing was performed on day 1 of the study, and subjects were required to stay in the clinic until the last blood draw at 72 h on day 4 for a total confinement period of 3.5 days. Subjects were evaluated for safety by measuring vital signs, electrocardiography (ECG), clinical laboratory parameters, and physical exam, and subjects were monitored for adverse events (AEs) throughout the study. Subjects returned to the clinic on day 6 for clinical laboratory testing, safety evaluation, and review of adverse events. Vital signs (blood pressure and heart rate) were measured, and end of study procedures were performed on day 8 postdose. After the last subject in the cohort completed the day-8 visit, the safety profile for each subject treated in that cohort was reviewed by the safety evaluation committee (SEC). The SEC met to discuss safety findings and determined the next step in the dose escalation schedule using guidelines prespecified in the protocol. Adverse events were graded according to the Common Toxicity Criteria for Adverse Events version 4.0 (NIH). Adverse events considered associated with oral Amp B and of a high severity would have resulted in immediate cessation of treatment at that dose level.
Thirty-two volunteers (men, 43.8%; women, 56.2%) participated in the study. Their mean age was 26.6 years (18 to 52 years), mean body weight was 68.1 kg (48.4 to 87.7 kg), mean height was 170.5 cm (152 to 188 cm), and mean body mass index (BMI) was 23.26 kg/m2 (18.1 to 29.6 kg/m2). No volunteers had clinically significant renal, liver, cardiac, pulmonary, gastrointestinal, or hematological diseases. The study was performed in Australia based on ethics committee approval and under the supervision of an independent safety evaluation committee (SEC). The primary endpoints of safety and tolerability of iCo-019 following oral administration of all single ascending doses were met, showing no serious adverse events and no drug-related adverse events, including no signs of kidney, liver, or gastrointestinal (GI) toxicity of note. A safety summary dose escalation review form assessing any clinically relevant abnormal vital signs, acute ECG changes observed in P wave, PR interval, QRS complexes, ST segments, QTc, and T waves, relevant changes in laboratory safety data, and clinically relevant changes in the physical examination was completed at each dose and reviewed by the safety evaluation committee (SEC). No clinically relevant abnormal vital signs, acute ECG changes observed in P wave, PR interval, QRS complexes, ST segments, QTc, and T waves, relevant changes in laboratory safety data, and clinically relevant changes in the physical examination were observed at all doses administered. The adverse events reported within each dosing group were classified as mild and not related to the administration of iCo-019.
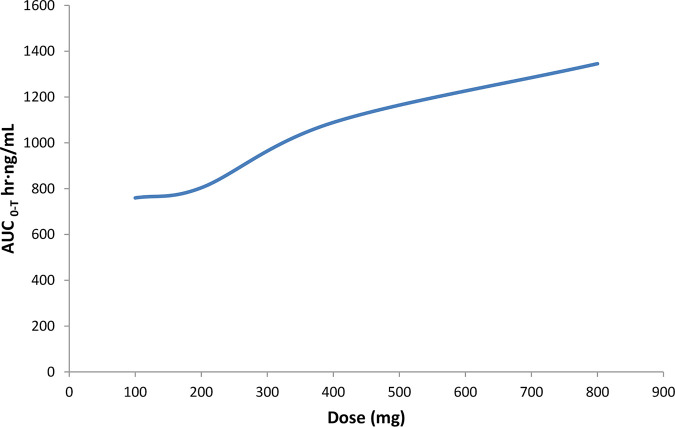
iCo-019 achieved a median maximum concentration in plasma (Cmax) of 28 ng AmB/ml and an area under the concentration-time curve from 0 h to infinity (AUC0–∞) of 1,030 h·ng/ml at the lowest dose of iCo-019 of 100 mg (Table 1). At the 400-mg dose of iCo-019, a median AUC0–∞ of 2,029 h·ng/ml was achieved, representing an approximate doubling of the AUC measure at an increased dose. On further analysis median AUC from 0 to the last measurable concentration (AUC0–Tlast) increased from 759.7 h·ng/ml at the 100-mg dose to 1,345.5 h·ng/ml at the 800-mg dose (Table 1 and Fig. 2).
TABLE 1.
Summary of pharmacokinetic parameters for amphotericin B in human plasma in subjects that were administered a single oral dose of iCo-019 at 100 mg, 200 mg, 400 mg, and 800 mg of amphotericin B
| Dose (mg) | Median (range) valuesa |
|||
|---|---|---|---|---|
| Tmax (h) | Cmax (ng/ml) | AUC0–Tlast (h·ng/ml) | t1/2 (h) | |
| 100 | 6.0 (6–6) | 28.0 (22.7–43.6) | 759.7 (635.8–1606.4) | 27.3 (14.4–55.1) |
| 200 | 6.0 (6–8) | 28.6 (18.8–42.5) | 804.0 (596.1–1344.9) | 24.6 (15.3–68.8) |
| 400 | 6.0 (6–10) | 28.4 (20.2–41.1) | 1,089.2 (461.8–1,856.8) | 39.0 (13.7–142.1) |
| 800 | 7.0 (6–10) | 32.1 (29.9–42.8) | 1,345.5 (915.1–1,854.7) | 25.6 (23.1–32.7) |
n = 6 subjects per dosing cohort. Tmax, time to reach the maximum observed concentration; Cmax, maximum observed amphotericin B plasma concentration; AUC0–Tlast, area under the concentration-time curve from hour 0 to the last measurable concentration, estimated by the linear trapezoidal rule; t1/2, elimination half-life.
FIG 2.
Increase in iCo-019 dose is associated with an increase in AUC0–Tlast.
Unlike other drugs, AmB, due to its lack of water and lipid solubility, has a very unusual pharmacokinetic and pharmacodynamic profile. The absolute bioavailability of AmB from iCo-019 administration can be approximated as 2% to 3%. However, it is the accumulation of drug within infected tissues as a function of time (i.e., depot effect) and the systemic exposure of the drug as a function of time (AUC) and not blood levels (Cmax) that is correlated with its pharmacological activity (1–8). The clearance of the drug from systemic circulation is faster than the clearance of the drug from the tissues, leading to an increased tissue accumulation and enhanced pharmacological effect. This type of pharmacokinetic profile is fundamentally different from parenteral liposomal amphotericin B, the most used commercially available form of the drug; the liposomal form is long circulating, which results in a large AUC. However, iCo-019 exhibits prolonged tissue levels that are the most important factor for efficacy against leishmaniasis and systemic fungal infections. In this study, we report a prolonged AmB half-life and sustained systemic drug exposure as measured by AUC. Furthermore, the AUC observed in this trial is superior to that of the cochleate AmB formulation that recently completed human clinical trials (30, 31), suggesting pharmacological activity can be expected at the doses tested in this study (1–8).
In conclusion, we have reported that all single doses of iCo-019 were well tolerated with no serious adverse events and no drug-related adverse events, including no signs of gastrointestinal, kidney, and liver toxicities. iCo-019 achieved favorable AmB pharmacokinetics with a prolonged AmB half-life and increasing area under the concentration-time curve as the dose increased. These data suggest that iCo-19 has a long circulation time which may result in the ability of the formulation to increase and sustain amphotericin B tissue concentrations within infected tissues without the associated GI, liver, or kidney toxicity typically associated with this drug. This novel oral formulation may also serve as a new treatment strategy to overcome the limitations of and barriers to the use of parenterally administered amphotericin B products.
ACKNOWLEDGMENTS
Funding for this project was provided by iCo Therapeutics Inc.
P.H. is the chief medical officer of iCo Therapeutics Inc., and K.M.W. is the director of research at iCo Therapeutics Inc. E.K.W. declares no conflict of interest.
REFERENCES
- 1.Wasan K. 2020. Development of an oral amphotericin B formulation as an alternative approach to parenteral amphotericin B administration in the treatment of blood-borne fungal infections. Curr Pharm Des 26:1521–1523. doi: 10.2174/1381612826666200311130812. [DOI] [PubMed] [Google Scholar]
- 2.Cuddihy G, Wasan EK, Di Y, Wasan KM. 2019. The development of oral amphotericin B to treat systemic fungal and parasitic infections: has the myth been finally realized? Pharmaceutics 11:99. doi: 10.3390/pharmaceutics11030099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kleinberg M. 2006. What is the current and future status of conventional amphotericin B? Int J Antimicrob Agents 27 (Suppl 1):12–16. doi: 10.1016/j.ijantimicag.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 4.Sachs-Barrable K, Lee SD, Wasan EK, Thornton SJ, Wasan KM. 2008. Enhancing drug absorption using lipids: a case study presenting the development and pharmacological evaluation of a novel lipid-based oral amphotericin B formulation for the treatment of systemic fungal infections. Adv Drug Deliv Rev 60:692–701. doi: 10.1016/j.addr.2007.08.042. [DOI] [PubMed] [Google Scholar]
- 5.Ibrahim F, Gershkovich P, Sivak O, Wasan EK, Wasan KM. 2012. Assessment of novel oral lipid-based formulations of amphotericin B using an in vitro lipolysis model. Eur J Pharm Sci 46:323–328. doi: 10.1016/j.ejps.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 6.Gershkovich P, Wasan EK, Lin M, Sivak O, Leon CG, Clement JG, Wasan KM. 2009. Pharmacokinetics and biodistribution of amphotericin B in rats following oral administration in a novel lipid-based formulation. J Antimicrob Chemother 64:101–108. doi: 10.1093/jac/dkp140. [DOI] [PubMed] [Google Scholar]
- 7.Wasan EK, Gershkovich P, Zhao J, Zhu X, Werbovetz K, Tidwell RR, Clement JG, Thornton SJ, Wasan KM. 2010. A novel tropically stable oral amphotericin B formulation (iCo-010) exhibits efficacy against visceral leishmaniasis in a murine model. PLoS Negl Trop Dis 4:e913. doi: 10.1371/journal.pntd.0000913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wasan KM, Wasan EK, Gershkovich P, Zhu X, Tidwell RR, Werbovetz KA, Clement JG, Thornton SJ. 2009. Highly effective oral amphotericin B formulation against murine visceral leishmaniasis. J Infect Dis 200:357–360. doi: 10.1086/600105. [DOI] [PubMed] [Google Scholar]
- 9.Jensen GM. 2017. The care and feeding of a commercial liposomal product: liposomal amphotericin B (AmBisome). J Liposome Res 27:173–179. doi: 10.1080/08982104.2017.1380664. [DOI] [PubMed] [Google Scholar]
- 10.Tan JSL, Roberts CJ, Billa N. 2018. Mucoadhesive chitosan-coated nanostructured lipid carriers for oral delivery of amphotericin B. Pharm Dev Technol 24:504–512. doi: 10.1080/10837450.2018.1515225. [DOI] [PubMed] [Google Scholar]
- 11.Radwan MA, AlQuadeib BT, Siller L, Wright MC, Horrocks B. 2017. Oral administration of amphotericin B nanoparticles: antifungal activity, bioavailability and toxicity in rats. Drug Deliv 24:40–50. doi: 10.1080/10717544.2016.1228715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wasan EK, Bartlett K, Gershkovich P, Sivak O, Banno B, Wong Z, Gagnon J, Gates B, Leon CG, Wasan KM. 2009. Development and characterization of oral lipid-based amphotericin B formulations with enhanced drug solubility, stability and antifungal activity in rats infected with Aspergillus fumigatus or Candida albicans. Int J Pharm 372:76–84. doi: 10.1016/j.ijpharm.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Barwicz J, Tancrede P. 1997. The effect of aggregation state of amphotericin-B on its interactions with cholesterol- or ergosterol-containing phosphatidylcholine monolayers. Chem Phys Lipids 85:145–155. doi: 10.1016/s0009-3084(96)02652-7. [DOI] [PubMed] [Google Scholar]
- 14.Espada R, Valdespina S, Alfonso C, Rivas G, Ballesteros MP, Torrado JJ. 2008. Effect of aggregation state on the toxicity of different amphotericin B preparations. Int J Pharm 361:64–69. doi: 10.1016/j.ijpharm.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 15.Chaudhari MB, Desai PP, Patel PA, Patravale VB. 2016. Solid lipid nanoparticles of amphotericin B (AmbiOnp): in vitro and in vivo assessment towards safe and effective oral treatment module. Drug Deliv Transl Res 6:354–364. doi: 10.1007/s13346-015-0267-6. [DOI] [PubMed] [Google Scholar]
- 16.Kumar R, Sahoo GC, Pandey K, Das V, Das P. 2015. Study the effects of PLGA-PEG encapsulated amphotericin B nanoparticle drug delivery system against Leishmania donovani. Drug Deliv 22:383–388. doi: 10.3109/10717544.2014.891271. [DOI] [PubMed] [Google Scholar]
- 17.Chen YC, Su CY, Jhan HJ, Ho HO, Sheu MT. 2015. Physical characterization and in vivo pharmacokinetic study of self-assembling amphotericin B-loaded lecithin-based mixed polymeric micelles. Int J Nanomed 10:7265–7274. doi: 10.2147/IJN.S95194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silva AE, Barratt G, Cheron M, Egito ES. 2013. Development of oil-in-water microemulsions for the oral delivery of amphotericin B. Int J Pharm 454:641–648. doi: 10.1016/j.ijpharm.2013.05.044. [DOI] [PubMed] [Google Scholar]
- 19.Richter AR, Feitosa JPA, Paula HCB, Goycoolea FM, de Paula RCM. 2018. Pickering emulsion stabilized by cashew gum- poly-l-lactide copolymer nanoparticles: synthesis, characterization and amphotericin B encapsulation. Colloids Surf B Biointerfaces 164:201–209. doi: 10.1016/j.colsurfb.2018.01.023. [DOI] [PubMed] [Google Scholar]
- 20.Mohamed HA, Radwan RR, Raafat AI, Ali AE. 2018. Antifungal activity of oral (Tragacanth/acrylic acid) amphotericin B carrier for systemic candidiasis: in vitro and in vivo study. Drug Deliv Transl Res 8:191–203. doi: 10.1007/s13346-017-0452-x. [DOI] [PubMed] [Google Scholar]
- 21.Kumar V, Sharma K, Nagpal K, Bera T. 2014. Significance of algal polymer in designing amphotericin B nanoparticles. Sci World J 2014:564573. doi: 10.1155/2014/564573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh K, Tiwary A, Rana V. 2013. Spray dried chitosan–EDTA superior microparticles as solid substrate for the oral delivery of amphotericin B. Int J Biol Macromol 58:310–319. doi: 10.1016/j.ijbiomac.2013.04.053. [DOI] [PubMed] [Google Scholar]
- 23.Prajapati VK, Awasthi K, Yadav TP, Rai M, Srivastava ON, Sundar S. 2012. An oral formulation of amphotericin B attached to functionalized carbon nanotubes is an effective treatment for experimental visceral leishmaniasis. J Infect Dis 205:333–336. doi: 10.1093/infdis/jir735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prajapati VK, Awasthi K, Gautam S, Yadav TP, Rai M, Srivastava ON, Sundar S. 2011. Targeted killing of Leishmania donovani in vivo and in vitro with amphotericin B attached to functionalized carbon nanotubes. J Antimicrob Chemother 66:874–879. doi: 10.1093/jac/dkr002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang Z, Tan Y, Chen M, Dian L, Shan Z, Peng X, Wu C. 2012. Development of amphotericin B-loaded cubosomes through the SolEmuls technology for enhancing the oral bioavailability. AAPS PharmSciTech 13:1483–1491. doi: 10.1208/s12249-012-9876-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Serrano DR, Lalatsa A, Dea-Ayuela MA, Bilbao-Ramos PE, Garrett NL, Moger J, Guarro J, Capilla J, Ballesteros MP, Schätzlein AG, Bolás F, Torrado JJ, Uchegbu IF. 2015. Oral particle uptake and organ targeting drives the activity of amphotericin B nanoparticles. Mol Pharm 12:420–431. doi: 10.1021/mp500527x. [DOI] [PubMed] [Google Scholar]
- 27.Zarif L, Graybill JR, Perlin D, Mannino RJ. 2000. Cochleates: new lipid-based drug delivery system. J Liposome Res 10:523–538. doi: 10.3109/08982100009031116. [DOI] [Google Scholar]
- 28.Delmas G, Park S, Chen ZW, Tan F, Kashiwazaki R, Zarif L, Perlin DS. 2002. Efficacy of orally delivered cochleates containing amphotericin B in a murine model of aspergillosis. Antimicrob Agents Chemother 46:2704–2707. doi: 10.1128/AAC.46.8.2704-2707.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalbag S, Lu R, Ngoje J, Mannino RJ. 1992. Oral administration of amphotericin B: toxicokinetic studies in animal models. https://d1io3yog0oux5.cloudfront.net/_5302c050e2e7c359ceb9446db98d9937/matinasbiopharma/db/284/2327/pdf/CAmB-Focus-Tox-Poster.pdf.
- 30.Matinas Biopharma. 2018. MAT2203: LNC formulation of amphotericin B. https://www.matinasbiopharma.com/pipeline/mat2203-lnc-formulation-of-amphotericin-b. Accessed on 15 September 2018.
- 31.Mannino R, De B, Teae A. 2019. Oral administration of amphotericin B (CAmB) in humans: a phase I study of tolerability and pharmacokinetics preliminary pharmacokinetics. https://content.equisolve.net/_db6027646f523d19fe795801a0b7aff1/matinasbiopharma/db/128/510/pdf/Oral_Dosing_of_Encochleated_Amphotericin_B_%28CAmB%29__Rapid_Drug_Targeting_to_Infected_Tissues_in_Mice_with_Invasive_Candidiasis.pdf. Accessed on 21 February 2019.
- 32.Sivak O, Gershkovich P, Lin M, Wasan EK, Zhao J, Owen D, Clement JG, Wasan KM. 2011. Tropically stable novel oral lipid formulation of amphotericin B (iCo-010): biodistribution and toxicity in a mouse model. Lipids Health Dis 10:135. doi: 10.1186/1476-511X-10-135. [DOI] [PMC free article] [PubMed] [Google Scholar]