Candida auris has been shown to have a high risk of skin colonization in hospitalized patients, possibly contributing to nosocomial spread. In a guinea pig skin model, animals were evaluated for clinical appearance, tissue fungal burden, histology, and pharmacokinetics. Oral dosing with 10 mg/kg ibrexafungerp (IBX) reduced the severity of lesions and significantly reduced the C. auris fungal burden in infected animals compared with untreated controls.
KEYWORDS: Candida auris, cutaneous, ibrexafungerp
ABSTRACT
Candida auris has been shown to have a high risk of skin colonization in hospitalized patients, possibly contributing to nosocomial spread. In a guinea pig skin model, animals were evaluated for clinical appearance, tissue fungal burden, histology, and pharmacokinetics. Oral dosing with 10 mg/kg ibrexafungerp (IBX) reduced the severity of lesions and significantly reduced the C. auris fungal burden in infected animals compared with untreated controls. This indicates promise for use of IBX in controlling skin infection and colonization of hospitalized patients.
TEXT
A report from a major hospital outbreak in London in 2015 to 2016 concluded that Candida auris demonstrates high survival rates on clinical environmental surfaces and the ability to rapidly colonize the skin of patients. The report also identified that, although most of the patients had skin C. auris colonization only, a significant percentage (44%, 22/50) required the use of a systemic antifungal treatment because of clinical manifestations of infection, including candidemia in eight patients (1). Other supporting evidence has led to current guidelines recommending that infection control measures should be the same for infection and colonization with C. auris (2).
We examined the ability of ibrexafungerp (IBX; formerly SCY-078), administered orally, to prevent the establishment of skin infection in an in vivo guinea pig model as a means to evaluate its potential utility in treatment and decolonization strategies. IBX belongs to a novel class of glucan synthase inhibitors (triterpenoids). It shares the same mechanism of action as the echinocandins but with both oral and intravenous formulations. It is well known that some Candida species, such as Candida albicans, can cause infection in immunocompetent animal models; however, C. auris isolates are less virulent and therefore required localized immunosuppression by subcutaneous administration of prednisolone 30 mg/kg 1 day before infection and 1 and 3 days postinfection. C. auris strain MRL 35368, with known micafungin (MICA) and IBX MIC values of 1.0 and 0.5 μg/ml, respectively, was taken from the culture collection at the Center for Medical Mycology. Animals were infected cutaneously with a concentration of 1 × 109 CFU/ml C. auris and divided into the following treatment groups (n = 5): oral IBX 10, 20, or 30 mg/kg; intraperitoneal MICA 5 mg/kg; and untreated control.
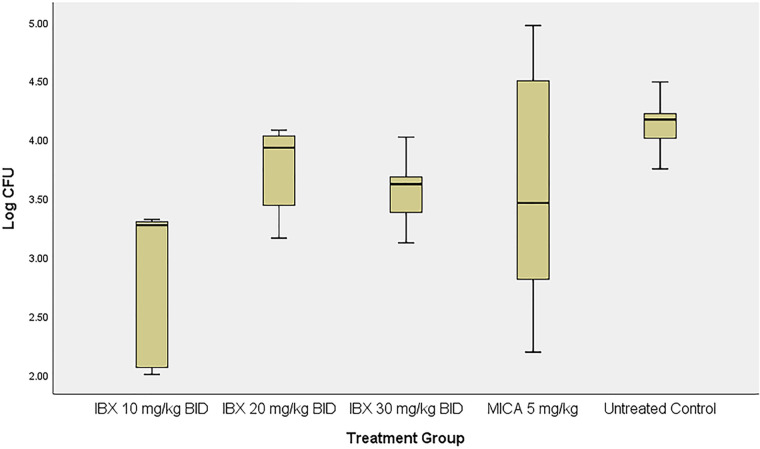
Figure 1 shows the tissue fungal burden of guinea pig skin on day 7 postinoculation. A full-thickness skin sample from the infected area was harvested using an 8-mm biopsy punch, weighed, and homogenized in sterile normal saline. Serial dilutions were plated for colony counting, with tissue fungal burden expressed as CFU per gram of tissue. Skin samples taken from guinea pigs treated with IBX 10 mg/kg showed significantly lower fungal burden (average log CFU/g ± SD, 2.8 ± 0.7) than untreated controls (P = 0.039), whereas animals treated with IBX 20 or 30 mg/kg or with MICA 5 mg/kg demonstrated a decrease in tissue fungal burden (log CFU/g ± SD, 3.7 ± 0.4, 3.6 ± 0.3, and 3.6 ± 1.2, respectively) versus the vehicle control, albeit not statistically significantly (P > 0.05 for all). Fungal infections of the skin tend not to be homogenous across the affected area, attributing to the variation in CFU between samples, which may account for the lack of significance in the IBX 20- and 30-mg/kg-treated groups.
FIG 1.
Boxplot of the tissue fungal burden of infected skin after treatment with ibrexafungerp (IBX) or micafungin (MICA).
Data were analyzed using one-way analysis of variance with a post hoc Bonferroni test; a P value of <0.05 was considered statistically significant.
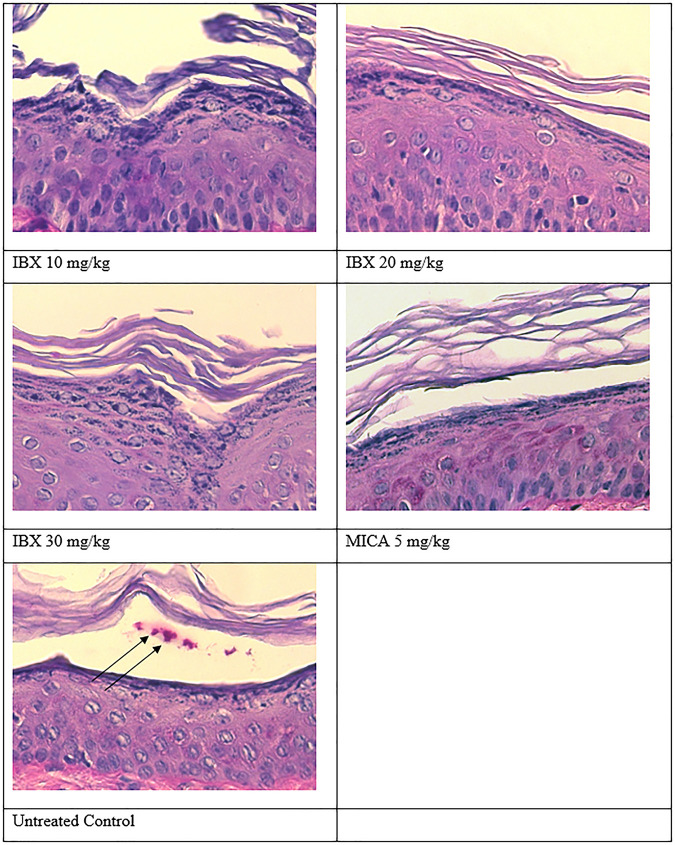
Figure 2 shows the results for the periodic acid-Schiff (PAS)-stained skin samples on day 7 postinoculation (end of treatment). No fungal elements were found in the histopathology of the animals treated with IBX at any of the dose levels tested or with MICA, indicating that the C. auris infection was cleared. In contrast, fungal elements were evident in the skin samples of untreated control animals, as expected. There was no significant difference in clinical scores between the treatment groups.
FIG 2.
PAS stain of infected skin after treatment with ibrexafungerp (IBX) or micafungin (MICA). Arrows, fungal cells.
Pharmacokinetic analysis showed that animals dosed with 10, 20, or 30 mg/kg twice daily of IBX had exposures (area under the concentration-time curve from 0 to 24 h [AUC0–24]) of 2.8, 5.6, and 15 μg · h/ml, respectively. Our results are in line with those of previous studies using radioactive [14C]SCY-078, which showed that IBX achieves high concentrations in the skin, with skin AUC ratios 12 to 18 times higher than those of blood in rats after oral administration (3). In comparison, studies of tissue concentrations in humans have indicated that peak concentrations of MICA in the skin are from below the level of detection to ≤0.5 times the plasma concentration (4). Our data showing the effectiveness of IBX in reducing the C. auris load in an in vivo cutaneous model may indicate promise for its use in controlling skin infection and colonization of hospitalized patients. Therefore, development of a C. auris colonization model, in addition to this skin infection model, is warranted.
Candida auris is emerging as a more frequent cause of nosocomial infections, and skin colonization of health care workers and patients is suspected to be a major contributing factor. In the study by Schelenz et al. (1), no health care workers had C. auris colonization of the hands, but eight patients with candidemia did demonstrate skin colonization. Other studies have shown that hands play an important part in the transmission of Candida species by direct contact with either infected patients or contaminated surfaces (5). The ability to clear C. auris isolates from the skin, not only from colonized patients but also from those who may have established skin wounds, is extremely important for the disruption of hospital transmission.
In the effort to prevent nosocomial spread of C. auris infection from patients being treated for multiple serious conditions, the oral bioavailability of IBX presents a distinct advantage over MICA and other echinocandins that must be administered intravenously; thus, a colonization model will add important data supporting the use of this novel antifungal.
ACKNOWLEDGMENTS
This work is supported by a grant from the National Institutes of Health (grant 5R21AI143305-02) and by a contract from Scynexis, Inc., to M.A.G.
REFERENCES
- 1.Schelenz S, Hagen F, Rhodes JL, Abdolrasouli A, Chowdhary A, Hall A, Ryan L, Shackleton J, Trimlett R, Meis JF, Armstrong-James D, Fisher MC. 2016. First hospital outbreak of the globally emerging Candida auris in a European hospital. Antimicrob Resist Infect Control 5:35. doi: 10.1186/s13756-016-0132-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Candida auris: healthcare professionals FAQ. https://www.cdc.gov/fungal/candida-auris/c-auris-health-qa.html.
- 3.Wring S, Borroto-Esoda K, Solon E, Angulo D. 2018. SCY-078, a novel fungicidal agent, demonstrates distribution to tissues associated with fungal infections during mass balance studies with intravenous and oral [14C]SCY-078 in albino and pigmented rats. Antimicrob Agents Chemother 63:e02119-18. doi: 10.1128/AAC.02119-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Felton F, Troke PF, Hope WW. 2014. Tissue penetration of antifungal agents. Clin Microbiol Rev 27:68–88. doi: 10.1128/CMR.00046-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vazquez JA, Dembry LM, Sanchez V, Vazquez MA, Sobel JD, Dmuchowski C, Zervos MJ. 1998. Nosocomial Candida glabrata colonization: an epidemiologic study. J Clin Microbiol 36:421–426. doi: 10.1128/JCM.36.2.421-426.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]