Fluconazole is frequently used for the treatment of invasive Candida infections in critically ill patients. However, alterations in renal functions might influence fluconazole clearance. Therefore, our objective was to study the impact of renal function on the population pharmacokinetics of fluconazole in critically ill patients with various degrees of renal function or undergoing continuous renal replacement therapy (CRRT). This was an open-label, multicenter observational study.
KEYWORDS: azoles, fungal disease, intensive care unit, pharmacometrics, renal clearance, renal replacement therapy
ABSTRACT
Fluconazole is frequently used for the treatment of invasive Candida infections in critically ill patients. However, alterations in renal functions might influence fluconazole clearance. Therefore, our objective was to study the impact of renal function on the population pharmacokinetics of fluconazole in critically ill patients with various degrees of renal function or undergoing continuous renal replacement therapy (CRRT). This was an open-label, multicenter observational study. Critically ill patients receiving fluconazole were included. Baseline and clinical data were collected. At days 3 and 7 of enrollment, blood samples were drawn for pharmacokinetic curves. Additionally, daily trough samples were taken. A nonlinear mixed-effects model was built, followed by Monte Carlo simulations for assessment of exposure to various dosages of fluconazole. Nineteen patients were included with a median age of 64.4 (range, 23 to 81) years and median weight of 82.0 (range, 44.0 to 119.5) kg. A linear two-compartment model best described fluconazole pharmacokinetics and demonstrated higher clearance than expected in critically ill patients. Simulations showed that daily dosages of 600 mg and 800 mg are needed for intensive care unit (ICU) patients with normal renal function and patients on CRRT, respectively, to achieve the EUCAST-recommended target fAUC (area under the concentration-time curve for the free, unbound fraction of the drug)/MIC ratio of 100. In conclusion, fluconazole clearance is highly variable in ICU patients and is strongly dependent on renal function and CRRT. Trough concentrations correlated well with the AUC, opening up opportunities for tailored dosing using therapeutic drug monitoring. We recommend doses of 400 mg for patients with poor to moderate renal function, 600 mg for patients with adequate renal function, and 800 mg for patients treated with CRRT. (This study has been registered at ClinicalTrials.gov under identifier NCT02666716.)
TEXT
While echinocandins are recommended for the initial treatment of invasive Candida infections, the azole fluconazole is still frequently used. Despite years of use, it can be questioned whether fluconazole is dosed adequately in the intensive care unit (ICU) setting, given the variation in renal function in ICU patients and the frequent use of renal replacement therapies. Alterations in renal function might influence fluconazole pharmacokinetics, since 89% of fluconazole is excreted, followed by extensive tubular reabsorption. Solid pharmacokinetic data about the influence of renal function on fluconazole concentrations are lacking. Critically ill patients, especially those with moderate to severe renal dysfunction or those who receive continuous renal replacement therapy (CRRT), are at risk of suboptimal dosing of fluconazole and may not attain the target fAUC (area under the concentration-time curve for the free, unbound fraction of the drug)/MIC ratio of 100 which has been set for invasive candidiasis by the European Committee on Antimicrobial Susceptibility Testing (EUCAST) (1).
In this study, we investigated the implications of renal function and CRRT for the pharmacokinetics of fluconazole in order to develop appropriate dosing strategies for ICU patients.
(This work was presented in part at ECCMID [European Congress of Clinical Microbiology and Infectious Diseases] 2019, Amsterdam, The Netherlands.)
RESULTS
Patient characteristics.
Twenty-three patients enrolled in this study, resulting in 19 evaluable patients. Four patients were not evaluable due to death (n = 1), switching of antifungal therapy (n = 2), or removal of a central venous or arterial catheter (n = 1) before the first pharmacokinetic assessment. Patient and clinical characteristics are shown in Table 1. Two consecutive pharmacokinetic curves were available for 11 patients. No drugs interacting with fluconazole were identified. Fluconazole doses ranged from 150 mg once a day (QD) to 800 mg QD; 11 patients received a loading dose on day 1 according to the registration text.
TABLE 1.
Baseline demographic and clinical characteristics
| Parameter | Valuea for evaluable patients (n = 19) |
|---|---|
| Demographics | |
| Sex | |
| Male | 11 |
| Female | 8 |
| Age (yr) | 64 (23–81) |
| Race | |
| Caucasian | 18 |
| Afro-Surinamese | 1 |
| Wt (kg) | 82.0 (44.0–119.5) |
| Ht (m) | 1.75 (1.55–1.90) |
| BMI (kg/m2) | 26.8 (16.4–38.6) |
| Clinical characteristics | |
| APACHE II score | |
| ≤20 | 9 |
| >20 | 10 |
| SOFA score | 9 (2–18) |
| ICU indication | |
| Respiratory failure | 9 |
| Post-abdominal/cardiac surgery | 6 |
| Abdominal sepsis | 3 |
| Cardiac arrest | 1 |
| Kidney function (eGFR [ml/min/m2])b | |
| >120 | 1 |
| 91–120 | 5 |
| 50–90 | 7 |
| 31–50 | 1 |
| 10–31 | 1 |
| CRRT | 4 |
| Fluconazole indication | |
| Therapy | 17 |
| Empirical therapy/prophylaxis | 2 |
| Days of fluconazole therapy before start of study | 1 (0–17) |
| Fluconazole daily dose (mg)c | |
| Day 3 | |
| 150 | 1 |
| 200 | 7 |
| 400 | 9 |
| 800 | 1 |
| 1,200 | 1 |
| Day 7 | |
| 150 | 0 |
| 200 | 4 |
| 400 | 4 |
| 800 | 2 |
| 1,200 | 1 |
| Site of infection | |
| Blood | 1 |
| Normally sterile location | 16 |
| Mediastinal fluid | 6 |
| Catheter tip | 5 |
| Sputum | 3 |
| Ascites | 2 |
| Species | |
| Candida spp. | 17 |
| Candida albicans | 7 |
| Unspecified | 3 |
| Mixed | 7 |
| C. albicans + unspecified | 4 |
| C. albicans + C. glabrata | 1 |
| C. albicans + C. dubliniensis + unspecified | 1 |
| C. albicans + C. guilliermondii + unspecified | 1 |
| Cryptococcus spp. | 1 |
Values are the number of patients for categorical variables and the median (range) for continuous variables.
eGFR, estimated glomerular filtration rate, calculated using the CKD-EPI formula.
Fluconazole daily dose data are given for 19 patients on day 3 and 11 patients on day 7.
Fluconazole pharmacokinetics.
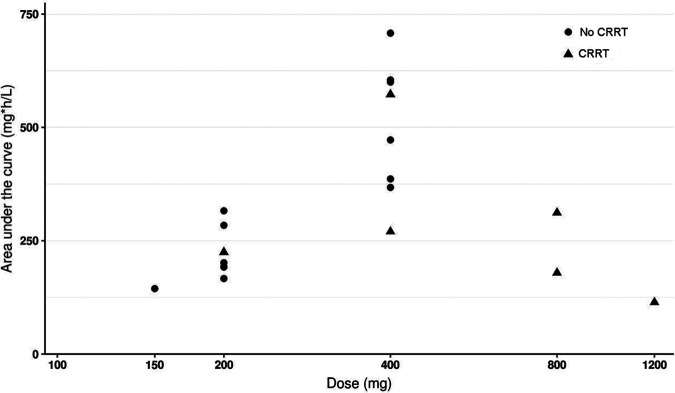
The population pharmacokinetics of fluconazole are best described by a linear two-compartment model. Further details and final model parameters are listed in Table S1 and Fig. S1 in the supplemental material. Figure 1 depicts the relation between the dose and the area under the concentration-time curve (AUC). Linear regression of dose and AUC showed R2 values of 0.67 and 0.58 (for patients without CRRT and with CRRT, respectively).
FIG 1.
Daily dose of fluconazole in steady state plotted against fluconazole exposure in patients without dialysis and patients undergoing CRRT.
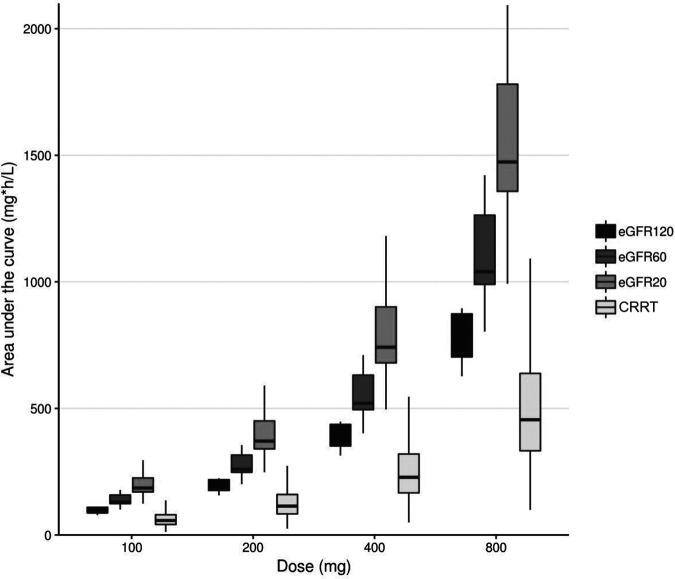
Simulated median (interquartile range [IQR]) values for the AUC from 0 to 24 h (AUC0-24) at steady state for the four dose regimens (100 mg QD, 200 mg QD, 400 mg QD, and 800 mg QD) for patients with a glomerular filtration rate (GFR) of 120 ml/min, 60 ml/min, or 20 ml/min and for patients on CRRT are shown in Fig. 2. Substantial differences in exposure are seen, depending on renal function. To achieve an AUC of 400 mg·h/liter, we showed that 600- and 800-mg daily doses, respectively, are needed for critically ill patients with a creatinine clearance of 120 ml/min and for patients on CRRT; 400-mg daily doses lead to adequate exposure in patients with reduced renal function (creatinine clearance of 60 or 20 ml/min).
FIG 2.
Simulation of fluconazole dose (100 mg – 200 mg – 400 mg – 800 mg QD) in patients with varying degrees in renal function plotted against fluconazole exposure.
DISCUSSION
Our study demonstrates that 100 mg or 200 mg fluconazole QD is not sufficient to achieve the desired target exposure of 400 mg·h/liter, regardless of renal function. These dosages should not be applied for critically ill patients. In patients with poor renal function (estimated GFR [eGFR], <60 ml/min), 400 mg QD is an effective dose, but in patients with adequate renal function (eGFR, >90 ml/min), and especially in patients with augmented renal clearance or patients on CRRT, dosages above 600 to 800 mg per day are necessary to achieve optimal therapy.
The clearance we found for patients with adequate renal function corresponds with earlier findings (2–5). Dose adjustments are often advised for patients with renal dysfunction. For compounds that are filtered and reabsorbed, the net effect of impaired renal function or the use of CRRT may be difficult to predict, since fixed dose reductions by estimated creatinine clearance (CrCl) may not be sufficient to prevent the accumulation of fluconazole in the setting of attenuated filtration or could potentially result in underdosing if tubular reabsorption is compromised.
Our study shows that exposure is higher in patients with an eGFR of <60 ml/min, while there is lower exposure in patients undergoing CRRT than in patients with normal renal function, supporting earlier findings for patients undergoing continuous venovenous hemodiafiltration (CVVHDF) (6). Our cohort received CVVHDF, which is more efficacious in clearing fluconazole than continuous venovenous hemodialysis (CVVH). This study shows that doses of >600 to 800 mg QD are needed to achieve the target fAUC/MIC ratio in patients on CVVHDF. The renal drug handbook recommends maintaining standard dosing in the setting of CVVH. We believe that also for this cohort, a higher dose, but not one as high as that needed during CVVHDF, is needed.
Total fluconazole clearance could not be sufficiently predicted by renal clearance alone; adding an apparent nonrenal clearance component led to improvement of the model-predicted clearance (6, 7).
Some limitations of this study need to be addressed. First, measuring renal function based on plasma creatinine is notoriously unreliable. Yet many ICUs still guide dosing by creatinine-based estimations. Alternatively, the use of other biomarkers, such as cystatin C, might be more suitable for dose adjustments of renally cleared drugs such as fluconazole in the future, but this has to be demonstrated. Second, the number of patients on CRRT was limited (n = 4). Considering this small number, confirmation of our results in a similar population is needed. Third, we did not measure fluconazole concentrations in urine or dialysate, which would enable clearance calculations. This, nevertheless, does not influence the clinical relevance of our finding that patients with normal renal function or on CRRT are underdosed.
Although fluconazole still has an important place in the treatment of invasive candidiasis and candidemia in critically ill patients, the data presented in this study could open up a discussion about using fluconazole as primary therapy or earlier “step-down therapy” when optimally dosed. The head-to-head comparison studies did not report fluconazole pharmacokinetics; hence, underdosing of fluconazole leading to therapy failure could be a reason why fluconazole was outperformed by echinocandins. The fact that the trough concentrations correlated well with the AUC opens up opportunities for tailored dosing using therapeutic drug monitoring.
In conclusion, low doses of 100 mg and 200 mg fluconazole QD are insufficient to adequately treat ICU patients with invasive candidiasis and candidemia regardless of renal function. We recommend that 400 mg be given to patients with poor to moderate renal function, 600 mg to patients with good renal function, and 800 mg to patients on CRRT.
MATERIALS AND METHODS
Study design.
A prospective, multicenter, open-label, observational pharmacokinetic trial was performed with patients receiving fluconazole for prophylaxis or for suspected or proven (invasive) fungal infection in three ICUs (ClinicalTrials.gov identifier NCT02666716). The local ethics committees from the Radboudumc (Nijmegen, The Netherlands) and University Medical Center Utrecht (Utrecht, The Netherlands) waived the need for informed consent due to the observational nature of the study and the noninvasive procedures. Patients were treated with fluconazole at the discretion of the treating physician regarding indication, dose. and treatment duration.
Study population.
Patients in ICUs receiving intravenous fluconazole were eligible for participation if they were ≥18 years old and were managed with a central venous or arterial catheter.
Study procedures.
Demographic and clinical data were collected at baseline and on days 3 and 7 of this study. Disease severity scores—the Acute Physiology and Chronic Health Evaluation (APACHE) II score, the Sequential Organ Failure Assessment (SOFA) score, and the Child-Pugh score—were determined on the day of ICU admission (APACHE II), at baseline, and on days 3 and 7 (SOFA and Child-Pugh scores). Renal function (serum creatinine) and CRRT were determined at baseline, on days 3 and 7, and twice weekly thereafter.
The use and administration of fluconazole and concomitant medication were registered in electronic patient records. Rich pharmacokinetic sampling was performed on day 3 (±1) of the study at 0 (predose), 0.5, 1, 2, 4, 6, 8, 12, 18, and 24 h postinfusion and on day 7 (±1) at 0 (predose), 1, 4, 8, 12, and 24 h postinfusion. Furthermore, sampling was performed during therapy trough levels, and samples were taken up to 72 h after drug cessation. Fluconazole was analyzed by a validated assay using liquid chromatography coupled with tandem mass spectrometry (validated range, 0.0302 to 30.21 mg/liter).
Pharmacokinetic analysis.
Pharmacokinetic analysis was performed by nonlinear mixed-effect modeling using NONMEM (v7.41). All flow and volume parameters in the pharmacokinetic model were allometrically scaled to fat-free mass a priori (8). Bootstrapping (n = 1,000) and visual predictive checks were used for the internal validity of the model.
Simulation of various regimens in relation to renal function.
We performed a Monte Carlo simulation study with an existing cohort of 1,706 patients reported previously (9), adding creatinine clearance and clearance during CRRT. Renal clearance (CL) was calculated with the Cockcroft-Gault equation, which was allometrically scaled to fat-free mass, as proposed earlier (10). Four different dose regimens (100, 200, 400, and 800 mg QD) as well as three estimates of renal function (GFR, 120, 60, and 20 ml/min) and CRRT were used as typical profiles to show the exposure of fluconazole in these patient cohorts.
In the setting of no dialysis, clearance was described by a base intercept and the function that incorporated renal clearance performance. In the setting of dialysis, this intercept was used plus an estimate of machine clearance.
The AUC target was set at 400 mg·h/liter, since this is necessary to achieve an fAUC/MIC ratio of 100 for species with a MIC of ≤4 (1), taking into account the protein binding of fluconazole of about 11 to 12% (7).
Supplementary Material
ACKNOWLEDGMENTS
We thank all patients who participated in this trial and all the research nurses and analytical pharmacy staff for their contributions to this study.
Paul E. Verweij reports grants from Gilead Sciences, MSD, Pfizer, and F2G and nonfinancial support from OLM and IMMY, outside the submitted work. Roger J. Brüggemann has served as a consultant to Astellas Pharma, F2G, Amplyx, Gilead Sciences, MSD, and Pfizer and has received unrestricted and research grants from Astellas Pharma, Gilead Sciences, MSD, and Pfizer. All contracts were through Radboudumc, and all payments were invoiced by Radboudumc. The remaining authors have disclosed that they have no potential conflicts of interest in relation to this study.
This study was supported by the Department of Pharmacy of the Radboud University Medical Center Nijmegen.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.EUCAST. 2013. Fluconazole. Rationale for the EUCAST clinical breakpoints, version 2.0. http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Rationale_documents/Fluconazole_rationale_2_0_20130223.pdf. Accessed 12 December 2017.
- 2.Aoyama T, Hirata K, Hirata R, Yamazaki H, Yamamoto Y, Hayashi H, Matsumoto Y. 2012. Population pharmacokinetics of fluconazole after administration of fosfluconazole and fluconazole in critically ill patients. J Clin Pharm Ther 37:356–363. doi: 10.1111/j.1365-2710.2011.01297.x. [DOI] [PubMed] [Google Scholar]
- 3.Csajka C, Decosterd LA, Buclin T, Pagani JL, Fattinger K, Bille J, Biollaz J. 2001. Population pharmacokinetics of fluconazole given for secondary prevention of oropharyngeal candidiasis in HIV-positive patients. Eur J Clin Pharmacol 57:723–727. doi: 10.1007/s00228-001-0377-6. [DOI] [PubMed] [Google Scholar]
- 4.McLachlan AJ, Tett SE. 1996. Pharmacokinetics of fluconazole in people with HIV infection: a population analysis. Br J Clin Pharmacol 41:291–298. doi: 10.1046/j.1365-2125.1996.03085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roos JF, Kirkpatrick CM, Tett SE, McLachlan AJ, Duffull SB. 2008. Development of a sufficient design for estimation of fluconazole pharmacokinetics in people with HIV infection. Br J Clin Pharmacol 66:455–466. doi: 10.1111/j.1365-2125.2008.03247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel K, Roberts JA, Lipman J, Tett SE, Deldot ME, Kirkpatrick CM. 2011. Population pharmacokinetics of fluconazole in critically ill patients receiving continuous venovenous hemodiafiltration: using Monte Carlo simulations to predict doses for specified pharmacodynamic targets. Antimicrob Agents Chemother 55:5868–5873. doi: 10.1128/AAC.00424-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.CBG-MEB. 2003. Diflucan: summary of product characteristics. https://db.cbg-meb.nl/ords/f?p=111:3::SEARCH:NO::P0_DOMAIN,P0_LANG,P3_RVG1:H,NL,14769.
- 8.Holford NHG, Anderson BJ. 2017. Allometric size: the scientific theory and extension to normal fat mass. Eur J Pharm Sci 109(Suppl):S59–S64. doi: 10.1016/j.ejps.2017.05.056. [DOI] [PubMed] [Google Scholar]
- 9.Brüggemann RJM, Van Der Velden WJFM, Knibbe CAJ, Colbers A, Hol S, Burger DM, Donnelly JP, Blijlevens NMA. 2015. A rationale for reduced-frequency dosing of anidulafungin for antifungal prophylaxis in immunocompromised patients. J Antimicrob Chemother 70:1166–1174. doi: 10.1093/jac/dku477. [DOI] [PubMed] [Google Scholar]
- 10.Mould DR, Upton RN. 2013. Basic concepts in population modeling, simulation, and model-based drug development—part 2: introduction to pharmacokinetic modeling methods. CPT Pharmacometrics Syst Pharmacol 2:e38. doi: 10.1038/psp.2013.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.