Fosmanogepix is a novel prodrug in a new class of antifungal agents. Manogepix is the active moiety. We evaluated the CLSI and EUCAST MICs of manogepix and eight comparators against Candida auris. CLSI M27-A3 susceptibility testing of manogepix was performed for 122 C. auris isolates and compared to CLSI and EUCAST MICs for manogepix and eight comparators. Differences and agreement were calculated for each compound. Wild-type upper limits (WT-ULs; the upper MIC where the wild-type distribution ends) for manogepix and correlations with other drugs’ MICs were determined.
KEYWORDS: APX001A, manogepix, fosmanogepix, APX001, candidemia, antifungal susceptibility, EUCAST, CLSI, fluconazole, amphotericin B, echinocandins
ABSTRACT
Fosmanogepix is a novel prodrug in a new class of antifungal agents. Manogepix is the active moiety. We evaluated the CLSI and EUCAST MICs of manogepix and eight comparators against Candida auris. CLSI M27-A3 susceptibility testing of manogepix was performed for 122 C. auris isolates and compared to CLSI and EUCAST MICs for manogepix and eight comparators. Differences and agreement were calculated for each compound. Wild-type upper limits (WT-ULs; the upper MIC where the wild-type distribution ends) for manogepix and correlations with other drugs’ MICs were determined. Manogepix MICs (CLSI/EUCAST [mg/liter]) and WT-ULs were as follows: MIC50s, 0.008/0.016; MIC90s, 0.03/0.03; ranges, 0.001 to 0.25/0.001 to 0.125; 97.5% and 99% WT-ULs, 0.03/0.125 and 0.06/0.125, respectively. The manogepix CLSI/EUCAST MIC distributions spanned 9/8 dilutions, respectively. Significant correlation was found for all azoles, particularly fluconazole (r = 0.22 to 0.74, P < 0.05). Isolates with EUCAST manogepix MICs of ≤0.004 had 7.6-/10.2-fold-lower fluconazole CLSI/EUCAST MICs than the remaining isolates that had higher manogepix MICs. The highest essential agreement between CLSI and EUCAST results was observed for manogepix and fluconazole, with a median difference of −1 to 0 2-fold dilutions, 90th percentile absolute difference of 1, and 90 to 92% and 98 to 100% agreement within ±1 and ±2 dilutions. The lowest agreements within ±1 and ±2 dilutions were found for isavuconazole and anidulafungin (44 to 50% and 69 to 76%). The correlation between CLSI and EUCAST manogepix MICs against C. auris was excellent. Differential MICs were found, and these correlated with fluconazole MICs, suggesting that the C. auris population is a mix of wild-type isolates and non-wild-type isolates with low-grade manogepix MIC elevation, probably involving efflux pump expression. However, manogepix was the most potent agent against C. auris in this in vitro study.
INTRODUCTION
Manogepix (APX001A) is the active moiety of the novel drug candidate fosmanogepix (APX001), currently in clinical trials for the treatment of invasive fungal infections. Manogepix has demonstrated activity against a wide range of human-pathogenic fungi, including multidrug-resistant Candida auris (1–4). Fosmanogepix has been granted fast track designations by the U.S. Food and Drug Administration (FDA) for seven indications, including invasive candidiasis. It is currently in phase 2 clinical trials (ClinicalTrials.gov identifier) for C. auris infections (NCT04148287), invasive aspergillosis (NCT04148287), and invasive candidiasis/candidemia (NCT03604705).
Fosmanogepix has been evaluated in vivo and proven efficacious against C. auris in immunosuppressed mouse models when therapy is initiated early, as well as when delayed for 24 h (4–6). The 24-h free-drug area under the concentration-time curve (AUC)/MIC ratio has been identified as the pharmacokinetics (PK)/pharmacodynamics (PD) index that best correlated with outcome and targets for stasis and is established as follows: 14.67 ± 8.30 (mean ± standard deviation [SD]) for C. auris, compared to 20.60 ± 6.50 for Candida albicans and 1.31 ± 0.27 for Candida glabrata (5).
The in vitro susceptibility of C. auris to manogepix has been previously investigated using the CLSI reference methodology in four studies, three of which included 16 or fewer isolates. Presented as the MIC50, MIC90 (MIC range), Berkow and Lockhart reported 0.002 mg/liter, 0.008 mg/liter (<0.0005 to 0.015 mg/liter) in a study including 100 isolates from four clades (1); Hager et al. reported 0.004 mg/liter, 0.03 mg/liter (0.002 to 0.06 mg/liter) in a study including 16 isolates (4); Wiederhold et al. reported 0.03 mg/liter, 0.03 mg/liter (≤0.002 to 0.03 mg/liter) in a study including 13 isolates (6), and finally, Pfaller et al. included a single isolate with a MIC of 0.06 mg/liter (3). In contrast, only a single study investigated the susceptibility by the EUCAST E.Def 7.3.1 method, reporting the following MIC50, MIC90 (MIC range): 0.008 mg/liter, 0.03 mg/liter (0.001 to 0.125 mg/liter) (2). MIC50 and modal MIC values in general reflect the typical MIC for a given species as long as the majority of isolates are wild type (WT) to the compound in question. Consequently, differences in these values normally reflect differences in susceptibility testing method or interlaboratory variation associated with technical issues. For C. auris specifically, clonal outbreaks have occurred and been associated with four different major C. auris clusters of South American, South Asian, African, and East Asian origins (7). A potential fifth clade of Iranian origin has been described recently (8). Isolates from four clusters have been associated with differential susceptibilities to amphotericin B and, most notably, to fluconazole, and different mutations in the erg11 azole target gene have been associated with different clades (7, 8). Here, we investigated the method-specific agreement between MICs obtained using CLSI and EUCAST reference methods when testing manogepix and eight comparators against a collection of 122 C. auris isolates.
RESULTS
Manogepix MICs.
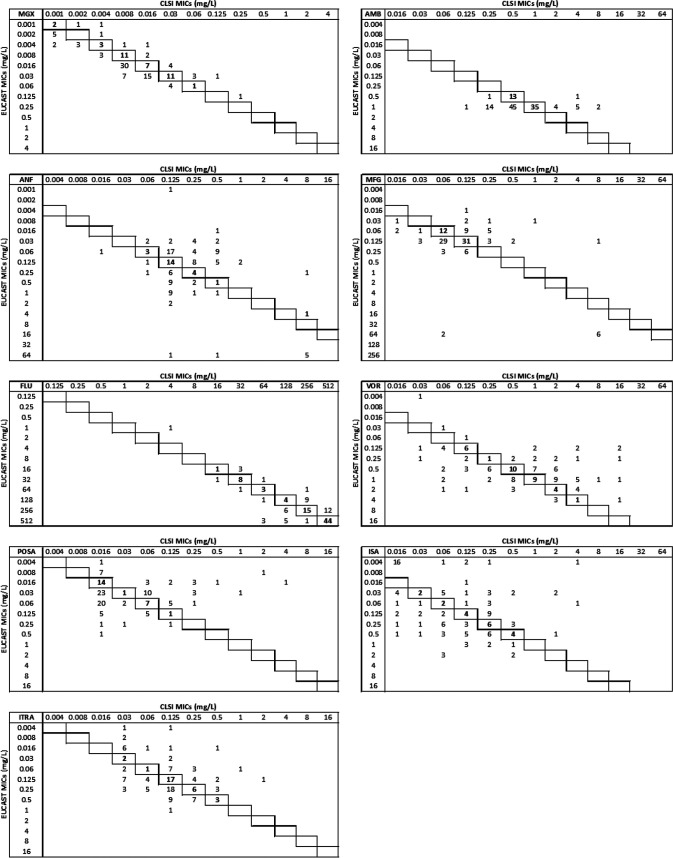
Manogepix MICs against C. auris were low and, in general, 1 2-fold dilution lower using the CLSI versus the EUCAST method, with the following key parameters, indicated as CLSI/EUCAST (mg/liter): MIC50s, 0.008/0.016; MIC90s, 0.03/0.03; ranges, 0.001 to 0.25/0.001 to 0.125; statistical wild-type upper limits (WT-ULs) encompassing 97.5% and 99% of the fitted population, 0.03/0.125 and 0.06/0.125, respectively (Tables 1 and 2). At the individual isolate level, the agreements between CLSI and EUCAST MICs were excellent, with MICs within ±1 dilutions in 90% of the cases and within ±2 dilutions for 100%. The manogepix MIC distributions spanned 9 and 8 dilutions for the CLSI and EUCAST methods, respectively. Both MIC distributions were slightly asymmetrical due to a tail to the left of the modal MIC (Fig. 1). Moreover, both MIC distributions, but particularly the one for CLSI, were truncated at the lowest concentration tested (0.001 mg/liter), suggesting that the true MIC distributions might have been wider if lower concentrations had been tested. Twenty isolates had low EUCAST manogepix MICs of ≤0.004 mg/liter (Table 2). By CLSI/EUCAST, these isolates were also 7.6-/10.2-fold more susceptible to fluconazole than the remaining isolates, with geometric mean (range) fluconazole MICs of 40.8 (4 to 512)/32.0 (1 to 512) mg/liter versus 310.8 (64 to 512)/326.3 (64 to 512) mg/liter, respectively (Fig. 2). Significant correlations between manogepix MIC and the MICs of the other drugs were found using the CLSI method with fluconazole (r = 0.53, P < 0.001), voriconazole (r = 0.22, P = 0.017), isavuconazole (r = 0.22, P = 0.017), and itraconazole (r = 0.22, P = 0.016) and for EUCAST with fluconazole (r = 0.74, P < 0.001), voriconazole (r = 0.58, P < 0.001), isavuconazole (r = 0.64, P < 0.001), itraconazole (r = 0.66, P < 0.001), and posaconazole (r = 0.44, P < 0.001) (Fig. 3).
TABLE 1.
Comparative CLSI and EUCAST MICs of nine antifungal drugs against C. auris
| Druga | MIC50/geometric mean (range), MIC90 (mg/liter) |
Dilution differences [median (range), 90th percentile of absolute differences]b | % agreement within indicated no. of dilutions |
|||
|---|---|---|---|---|---|---|
| CLSI | EUCAST | Zero | ±1 | ±2 | ||
| MGX | 0.008/0.01 (0.001 to 0.25), 0.03 | 0.016/0.014 (0.001 to 0.125), 0.03 | −1 (−2 to 2), 1 | 29 | 90 | 100 |
| AMB | 0.5/0.674 (0.125 to 8), 1 | 1/0.918 (0.5 to 1), 1 | −1 (−3 to 3), 2 | 40 | 81 | 97 |
| ANI | 0.125/0.222 (0.016 to 8), 0.5 | 0.125/0.196 (0.001 to 64), 1 | 0 (−9 to 7), 3 | 18 | 50 | 69 |
| MFG | 0.125/0.12 (0.016 to 8), 0.25 | 0.125/0.155 (0.016 to 64), 0.25 | 0 (−10 to 6), 2 | 36 | 76 | 90 |
| FLU | 256/220.6 (4 to 512), 512 | 256/221.6 (1 to >256), 512 | 0 (−3 to 2), 1 | 63 | 92 | 98 |
| VOR | 1/0.698 (0.03 to 16), 4 | 0.5/0.554 (0.004 to 4), 2 | 0 (−5 to 7), 3 | 26 | 64 | 83 |
| POSA | 0.016/0.035 (0.016 to 8), 0.125 | 0.03/0.036 (0.004 to 0.5), 0.125 | 0 (−5 to 9), 3 | 19 | 63 | 83 |
| ISA | 0.125/0.102 (0.016 to 4), 0.5 | 0.125/0.091 (0.004 to 2), 0.5 | 0 (−5 to 10), 4 | 15 | 44 | 76 |
| ITRA | 0.125/0.115 (0.03 to 2), 0.25 | 0.125/0.131 (0.004 to 1), 0.5 | 0 (−3 to 5), 2 | 24 | 66 | 92 |
MGX, manogepix; AMB, amphotericin B; ANI, anidulafungin; MFG, micafungin; FLU, fluconazole; VOR, voriconazole; POSA, posaconazole; ISA, isavuconazole; ITRA, itraconazole.
High off-scale MICs were converted to the next-higher 2-fold dilution. The 90th percentile was calculated from the absolute differences.
TABLE 2.
Comparative MIC distributions of nine antifungal drugs obtained with CLSI and EUCAST methods against C. aurisa
Framed cells represent the identity lines where the MICs are the same between the two methods. MGX, manogepix; AMB, amphotericin B; ANI, anidulafungin; MFG, micafungin; FLU, fluconazole; VOR, voriconazole; POSA, posaconazole; ISA, isavuconazole; ITRA, itraconazole.
FIG 1.
Manogepix MICs (mg/liter) against 122 C. auris isolates determined by the CLSI (a) and EUCAST (b) reference methods. The ECOFFs (mg/liter) determined using ECOFFinder XL 2.0, including various proportions of the fitted population, are indicated for both methods in the embedded tables.
FIG 2.
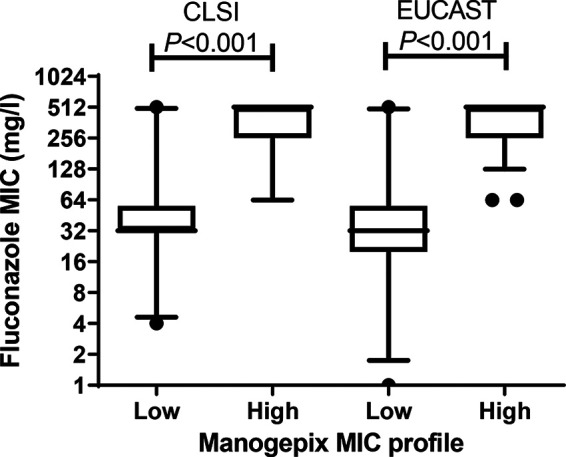
Comparison of fluconazole CLSI and EUCAST MICs for isolates with low (≤0.004 mg/liter) and high (0.008 to 0.125 mg/liter) EUCAST MIC profiles. Whiskers show 5th to 95th percentiles.
FIG 3.
Correlations between manogepix MICs and MICs of the other drugs with the EUCAST methodology. Correlation coefficients are shown.
Agreement between CLSI and EUCAST MICs for manogepix and comparators.
Comparing CLSI and EUCAST MICs pairwise for each compound, statistically significant correlations were found for all drugs (r > 0.40, P < 0.001) except posaconazole (r = −0.03, P = 0.67) and amphotericin B (r = 0.10, P = 0.27). No significant differences were found between CLSI and EUCAST MICs for each compound. The highest agreement across CLSI and EUCAST methods was observed for manogepix and fluconazole, with a median difference of −1 to 0 2-fold dilutions, a 90th percentile absolute difference of 1, and in both cases, ≥90% agreement within ±1 dilution (Table 1). The agreement was also high for amphotericin B and micafungin, ranging from 76 to 81% and 90 to 97% within ±1 and ±2 dilutions, respectively. The agreement was less optimal for itraconazole, voriconazole, and posaconazole (ranging from 63 to 66% and 83 to 92% within ±1 and ±2 dilutions, respectively), and low for isavuconazole and anidulafungin (ranging from 44 to 50% and 69 to 76% within ±1 and ±2 dilutions, respectively, Table 1).
DISCUSSION
We found an excellent essential agreement between manogepix MICs determined by the CLSI and EUCAST reference methodologies when a collection of 122 C. auris isolates was tested in two different laboratories. Compared to the results for the eight other antifungal compounds included in our study, the essential agreement was highest for manogepix and fluconazole and lowest for anidulafungin and isavuconazole.
The CLSI and EUCAST MIC distributions for manogepix against C. auris were quite broad, spanning 9 and 8 dilutions, respectively, and potentially more due to an apparent truncation at the low end. Wide distributions normally reflect either suboptimal intralaboratory reproducibility of the MIC testing, which appears contradictory to the excellent agreement found between the MICs, or differential susceptibilities among the isolates tested. Manogepix targets an essential fungal acyltransferase, Gwt1 (9), blocking inositol acylation of glycosylphosphatidylinositol (GPI) anchors and trafficking of GPI-anchored proteins from the endoplasmic reticulum (ER) (10). It is not yet in clinical use, and therefore, differential susceptibility was unexpected, as these clinical isolates have no prior exposure to manogepix. However, recent studies have demonstrated a correlation between fluconazole and manogepix MICs for other Candida species (2). Moreover, two mutants selected in vitro for elevated manogepix MICs also demonstrated a 4-fold and a 2-fold increase in fluconazole MICs, which was mediated by increased expression of multidrug efflux pumps. Specifically, one Candida parapsilosis and one C. albicans mutant showed activated expression of the major facilitator superfamily transporter gene MDR1 and of the ATP-binding cassette transporter genes CDR11 and SNQ2, respectively (11, 12). This is in line with the significant correlation between manogepix MICs and all azole MICs, particularly with EUCAST methodology. The majority of the Indian C. auris isolates included here were fluconazole resistant, and when the fluconazole MICs were compared for isolates with low and high manogepix MICs, a significant correlation was observed between manogepix and fluconazole MICs. Fluconazole resistance in C. auris was first related to Y132F (a change of Y to F at position 132), K143R, and F126T alterations in the Erg11 target protein (7, 13), but involvement of efflux pumps and target gene upregulation have subsequently been demonstrated in aging cells (14). Taken together, we therefore hypothesize that the differential susceptibilities to fluconazole may explain the wide manogepix MIC distribution observed for C. auris and that both CLSI and EUCAST testing of manogepix against C. auris are robust and reproducible. This also implies that our isolates include both a minor true wild-type population (with very low MICs) and a larger non-wild-type population (with slightly elevated manogepix MICs) and thus, that in principle, future epidemiological cutoff (ECOFF) setting for this species may be difficult. Importantly, however, the manogepix MIC variation among these populations is low compared to the variation among wild-type and non-wild-type isolates for the azoles, which span up to at least 11 dilutions. Therefore, the highest manogepix MICs among the isolates are much less elevated than the fluconazole MICs for the most azole-resistant isolates (0.25 mg/liter compared to 4 to 512 mg/liter). The clinical implications for the elevation of manogepix MIC values are unknown and will be informed by the patient outcomes in the clinical development program.
We observed a discrete but systematic difference between CLSI and EUCAST endpoints, with CLSI MICs being 1 2-fold dilution lower than EUCAST MICs. In comparison to published data, Berkow and Lockhart reported 2 2-fold-dilution-lower CLSI MICs than were found with CLSI in this study (MIC50s of 0.002 versus 0.008 mg/liter) for 100 isolates from four clades (1). Those isolates were selected to represent different C. auris clades and patterns of susceptibility to other antifungal agents, and the manogepix MIC distribution was multimodal, with a fifth of the isolates at <0.0005 mg/liter, a peak at 0.001 mg/liter, and another peak spanning 0.004 to 0.008 mg/liter (20 isolates at 0.004 and 19 at 0.008 mg/liter). This may suggest that the overall lower MICs reported in the Berkow and Lockhart study may be explained by more isolates belonging to clades with lower MICs rather than by interlaboratory variation. However, a multicenter study testing a shared strain collection is warranted to clarify whether interlaboratory variability also plays a role.
Our study has limitations. First, we have not characterized the mechanism behind the azole resistance in our isolates. Thus, it is unclear whether efflux pumps, which have been associated with MIC elevation for both manogepix and fluconazole in other Candida species, were highly expressed in our isolates. Second, the MIC determinations were performed in two separate laboratories, and thus, the 1 2-fold-dilution difference observed between the CLSI and EUCAST data set may be related to factors other than method differences, such as differential potencies of the stock solution, binding to plasticware, etc. On the other hand, it is also a strength that an excellent essential agreement was found despite tests being performed in separate laboratories. Third, our lowest concentration tested for manogepix was 0.001 mg/liter, and 10 and 4 isolates had MICs at or lower than this value with CLSI and EUCAST, respectively. However, as this is a small fraction of the isolates, it is not likely that this alters the conclusion drawn in this study.
In conclusion, our study suggests an excellent correlation between EUCAST and CLSI MIC testing of C. auris. This is in line with what has been reported for other Candida spp. and for moulds (15, 16). It also suggests that the differential MICs found for manogepix reflect true but minor MIC differences. We hypothesize that the differential MICs may be linked to differences in efflux pump expression associated with concomitant fluconazole resistance and, thus, that what is perceived as the wild-type population is likely a mix of true wild-type and non-wild-type isolates with low-grade resistance mechanisms, which may complicate the establishment of epidemiological cutoff values. Further analysis of patient outcomes versus C. auris manogepix MICs is necessary to understand the clinical implications of strains with elevated MICs. Overall, the in vitro activity of manogepix was highly potent on a mg/liter basis. This is particularly promising due to the multidrug resistance potential associated with C. auris.
MATERIALS AND METHODS
Fungal isolates.
A total of 122 clinical isolates of C. auris were collected from individual patients in 6 tertiary care hospitals in India from 2010 to 2015 (17). The isolates were mainly from patients with candidemia (blood, n = 100), and the remaining isolates (n = 22) were from invasive Candida infections, with the types of specimens including tissue and pleural fluid and a single isolate from pus.
Species identification.
The isolates were subjected to sequencing of the internal transcribed spacer (ITS) region of the ribosomal subunit as described previously, followed by GenBank basic local alignment search tool (BLAST) pairwise sequence alignment (https://www.ncbi.nlm.nih.gov/BLAST/Blast.cgi) (7). Further, upon subculture on CHROMagar for 24 h at 37°C, all isolates were also identified using matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) (Bruker Daltonics, Bremen, Germany). The ethanol-formic acid extraction procedure was followed according to the manufacturer’s protocol for the identification of yeast isolates (7, 18). The spectra were analyzed using Flex Control 3.1 software (Bruker Daltonics, Inc., Billerica, MA, USA) and MALDI Biotyper OC version 3.1 (Bruker Daltonics, Bremen, Germany). The isolates were identified as C. auris with a score of >2 against the C. auris database (in-house and Bruker’s) (7, 18).
Susceptibility testing.
CLSI susceptibility testing was performed according to the M27-A3/S4 guidelines (19). EUCAST manogepix MICs have previously been reported for these isolates, as have the EUCAST and CLSI MICs for the comparator compounds (2). Manogepix (APX001A; Amplyx Pharmaceuticals, San Diego, USA) was prepared in dimethyl sulfoxide (DMSO) (5,000 mg/liter; Sigma-Aldrich, Brøndby, Denmark). The final drug concentration ranges studied were 0.001 to 0.5 mg/liter. Drug-free and yeast-free controls were included, and microtiter plates were incubated at 35°C and read visually for the CLSI method (19). The recommended Candida krusei ATCC 6258 and Candida parapsilosis ATCC 22019 were used as quality control strains. The MIC end points for azoles and echinocandins were defined as the lowest drug concentration that caused a prominent decrease in visual growth (CLSI) or ≤50% growth (EUCAST) in relation to the growth og the controls. For amphotericin B, the MIC was defined as the lowest concentration at which there was full inhibition of visual growth (CLSI) or ≤10% growth (EUCAST) compared with the growth in the drug-free control wells. MICs for the licensed compounds were classified as wild type and non-wild type by adopting the EUCAST ECOFFs valid 4 February 2020 (www.eucast.org).
Comparison between CLSI and EUCAST.
MIC ranges, modal MICs (the most common MIC), MIC50s (the MIC value that includes 50% of the isolates), and MIC90s (the MIC value that includes 90% of the isolates) were calculated using GraphPad Prism version 6.00 (GraphPad software). High off-scale EUCAST MIC results were converted to the next-highest 2-fold dilution, and low off-scale MIC results were left unchanged for comparison between the two methods. The median (range) differences and the 90th percentile of absolute differences were calculated. The percentages of absolute (±0 2-fold dilutions) and essential (±1 and ±2 2-fold dilutions) agreement between the EUCAST and the CLSI methods were calculated for each compound. Wild-type upper limits (WT-ULs), defined as the upper MIC where the wild-type distribution ends, were determined for manogepix using a statistical method and 97.5% and 99% endpoints and the EUCAST ECOFFinder program (20). However, as the values reported here are not formally accepted EUCAST manogepix ECOFFs, we used the term “WT-UL” to avoid confusion.
Any statistical differences between CLSI and EUCAST MICs were investigated using repeated-measures analysis of variance (ANOVA) on log2 MICs followed by Bonferroni’s multiple-comparison test (significance was set at P < 0.05). Correlations between the CLSI and EUCAST log2 MICs of each drug and between manogepix MICs and the MICs of the other drugs using the CLSI and EUCAST methodologies were determined with Pearson analysis for each drug after log2 transformation.
ACKNOWLEDGMENTS
We thank research technician Birgit Brandt for excellent technical assistance.
This study was supported by an unrestricted grant from Amplyx Pharmaceuticals. The funder had no influence on the study design nor on the analysis of the results.
Outside this work, the authors have the following potential conflicts to declare. M.C.A. has, over the past 5 years, received research grants/contract work (paid to the SSI) from Amplyx, Basilea, Cidara, F2G, Gilead, Novabiotics, Scynexis, and T2Biosystems and speaker honoraria (personal fee) from Astellas, Gilead, Novartis, MSD, and Seges. She is the current chairman of the EUCAST-AFST. A.C. has no conflicts to declare. K.M.J. has received travel grants from Amplyx Pharmaceuticals and F2G and a meeting grant from MSD. J.M. has, over the past 5 years, received research grants/contract work (paid to the NKUA) from F2G, Gilead, Astellas, Gilead, Pfizer, MSD, and VenatoRx. He is the current clinical data coordinator of the EUCAST-AFST.
REFERENCES
- 1.Berkow EL, Lockhart SR. 2018. Activity of novel antifungal compound APX001A against a large collection of Candida auris. J Antimicrob Chemother 73:3060–3062. doi: 10.1093/jac/dky302. [DOI] [PubMed] [Google Scholar]
- 2.Arendrup MC, Chowdhary A, Astvad KMT, Jørgensen KM. 2018. APX001A in vitro activity against contemporary blood isolates and Candida auris determined by the EUCAST reference method. Antimicrob Agents Chemother 62:e01225-18. doi: 10.1128/AAC.01225-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pfaller MA, Huband MD, Flamm RK, Bien PA, Castanheira M. 2019. In vitro activity of APX001A (Manogepix) and comparator agents against 1,706 fungal isolates collected during an international surveillance program in 2017. Antimicrob Agents Chemother 63:e00840-19. doi: 10.1128/AAC.00840-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hager CL, Larkin EL, Long L, Zohra Abidi F, Shaw KJ, Ghannoum MA. 2018. In vitro and in vivo evaluation of the antifungal activity of APX001A/APX001 against Candida auris. Antimicrob Agents Chemother 62:e02319-17. doi: 10.1128/AAC.02319-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao M, Lepak AJ, VanScoy B, Bader JC, Marchillo K, Vanhecker J, Ambrose PG, Andes DR. 2018. In vivo pharmacokinetics and pharmacodynamics of APX001 against Candida spp. in a neutropenic disseminated candidiasis mouse model. Antimicrob Agents Chemother 62:e02542-17. doi: 10.1128/AAC.02542-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wiederhold NP, Najvar LK, Shaw KJ, Jaramillo R, Patterson H, Olivo M, Catano G, Patterson TF. 2019. Efficacy of delayed therapy with fosmanogepix (APX001) in a murine model of Candida auris invasive candidiasis. Antimicrob Agents Chemother 63:e01120-19. doi: 10.1128/AAC.01120-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lockhart SR, Etienne KA, Vallabhaneni S, Farooqi J, Chowdhary A, Govender NP, Colombo AL, Calvo B, Cuomo CA, Desjardins CA, Berkow EL, Castanheira M, Magobo RE, Jabeen K, Asghar RJ, Meis JF, Jackson B, Chiller T, Litvintseva AP. 2017. Simultaneous emergence of multidrug-resistant Candida auris on 3 continents confirmed by whole-genome sequencing and epidemiological analyses. Clin Infect Dis 64:134–140. doi: 10.1093/cid/ciw691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Groot T, Puts Y, Berrio I, Chowdhary A, Meis JF. 2020. Development of Candida auris short tandem repeat typing and its application to a global collection of isolates. mBio 11:e02971-19. doi: 10.1128/mBio.02971-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Umemura M, Okamoto M, Nakayama K, Sagane K, Tsukahara K, Hata K, Jigami Y. 2003. GWT1 gene is required for inositol acylation of glycosylphosphatidylinositol anchors in yeast. J Biol Chem 278:23639–23647. doi: 10.1074/jbc.M301044200. [DOI] [PubMed] [Google Scholar]
- 10.Hata K, Horii T, Miyazaki M, Watanabe N-A, Okubo M, Sonoda J, Nakamoto K, Tanaka K, Shirotori S, Murai N, Inoue S, Matsukura M, Abe S, Yoshimatsu K, Asada M. 2011. Efficacy of oral E1210, a new broad-spectrum antifungal with a novel mechanism of action, in murine models of candidiasis, aspergillosis, and fusariosis. Antimicrob Agents Chemother 55:4543–4551. doi: 10.1128/AAC.00366-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kapoor M, Moloney M, Soltow QA, Pillar CM, Shaw KJ. 2019. Evaluation of resistance development to the Gwt1 inhibitor manogepix (APX001A) in Candida species. Antimicrob Agents Chemother 64:e01387-19. doi: 10.1128/AAC.01387-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liston SD, Whitesell L, Kapoor M, Shaw KJ, Cowen LE. 2020. Enhanced efflux pump expression in Candida mutants results in decreased manogepix susceptibility. Antimicrob Agents Chemother 64:e00261-20. doi: 10.1128/AAC.00261-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Healey KR, Kordalewska M, Jiménez Ortigosa C, Singh A, Berrío I, Chowdhary A, Perlin DS. 2018. Limited ERG11 mutations identified in isolates of Candida auris directly contribute to reduced azole susceptibility. Antimicrob Agents Chemother 62:e01427-18. doi: 10.1128/AAC.01427-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhattacharya S, Holowka T, Orner EP, Fries BC. 2019. Gene duplication associated with increased fluconazole tolerance in Candida auris cells of advanced generational age. Sci Rep 9:5052. doi: 10.1038/s41598-019-41513-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pfaller MA, Watanabe N, Castanheira M, Messer S, Jones RN. 2011. Pre-clinical development of antifungal susceptibility test methods for the testing of the novel antifungal agent E1210 versus Candida: comparison of CLSI and European Committee on Antimicrobial Susceptibility Testing methods. J Antimicrob Chemother 66:2581–2584. doi: 10.1093/jac/dkr342. [DOI] [PubMed] [Google Scholar]
- 16.Jørgensen KM, Astvad KMT, Arendrup MC. 8 June 2020. In vitro activity of manogepix (APX001A) and comparators against contemporary moulds; MEC comparison and preliminary experience with colorimetric MIC determination. Antimicrob Agents Chemother doi: 10.1128/AAC.00730-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arendrup MC, Prakash A, Meletiadis J, Sharma C, Chowdhary A. 2017. Comparison of EUCAST and CLSI reference microdilution MICs of eight antifungal compounds for Candida auris and associated tentative epidemiological cutoff values. Antimicrob Agents Chemother 61:e00485-17. doi: 10.1128/AAC.00485-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Magobo RE, Corcoran C, Seetharam S, Govender NP. 2014. Candida auris-associated candidemia, South Africa. Emerg Infect Dis 20:1250–1251. doi: 10.3201/eid2007.131765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clinical and Laboratory Standards Institute. 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts; third informational supplement. CLSI document M27-S3 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 20.Turnidge J, Kahlmeter G, Kronvall G. 2006. Statistical characterisation of bacterial wild-type MIC value distributions and the determination of epidemiological cut-off values. Clin Microbiol Infect 12:418–425. doi: 10.1111/j.1469-0691.2006.01377.x. [DOI] [PubMed] [Google Scholar]