Antibiotic failure not only is due to the development of resistance by pathogens but can also often be explained by persistence and tolerance. Persistence and tolerance can be included in the “persistent phenotype,” with high relevance for clinics. Two of the most important molecular mechanisms involved in tolerance and persistence are toxin-antitoxin (TA) modules and signaling via guanosine pentaphosphate/tetraphosphate [(p)ppGpp], also known as “magic spot.” (p)ppGpp is a very important stress alarmone which orchestrates the stringent response in bacteria; hence, (p)ppGpp is produced during amino acid or fatty acid starvation by proteins belonging to the RelA/SpoT homolog family (RSH).
KEYWORDS: (p)ppGpp, TA systems, persistence, slow growth
ABSTRACT
Antibiotic failure not only is due to the development of resistance by pathogens but can also often be explained by persistence and tolerance. Persistence and tolerance can be included in the “persistent phenotype,” with high relevance for clinics. Two of the most important molecular mechanisms involved in tolerance and persistence are toxin-antitoxin (TA) modules and signaling via guanosine pentaphosphate/tetraphosphate [(p)ppGpp], also known as “magic spot.” (p)ppGpp is a very important stress alarmone which orchestrates the stringent response in bacteria; hence, (p)ppGpp is produced during amino acid or fatty acid starvation by proteins belonging to the RelA/SpoT homolog family (RSH). However, (p)ppGpp levels can also accumulate in response to a wide range of signals, including oxygen variation, pH downshift, osmotic shock, temperature shift, or even exposure to darkness. Furthermore, the stringent response is not only involved in responses to environmental stresses (starvation for carbon sources, fatty acids, and phosphates or heat shock), but it is also used in bacterial pathogenesis, host invasion, and antibiotic tolerance and persistence. Given the exhaustive and contradictory literature surrounding the role of (p)ppGpp in bacterial persistence, and with the aim of summarizing what is known so far about the magic spot in this bacterial stage, this review provides new insights into the link between the stringent response and persistence. Moreover, we review some of the innovative treatments that have (p)ppGpp as a target, which are in the spotlight of the scientific community as candidates for effective antipersistence agents.
INTRODUCTION
Antimicrobial resistance crisis is a serious health problem worldwide. During the past 50 years, very few new anti-infective molecules have been discovered (1). Hence, microbial pathogens have been able to accumulate molecular mechanisms enabling them to counteract antibiotics.
Nonetheless, there are more antibiotic evasion strategies other than resistance that are of great interest, such as persistence and tolerance. Persisters are a subpopulation of cells that are nongrowing, nonreplicative, dormant bacteria that exhibit transient high levels of tolerance to antibiotics without affecting their MICs (2–4). Once the drug pressure is removed, these persisters can rapidly regrow, thus returning to an antibiotic-sensitive state. Moreover, the persistent state can be maintained for hours up to days before persisters revert to an antibiotic-sensitive cell type, resuming growth under drug-free conditions (5).
The term “triggered persistence” was recently coined to indicate a form of persistence that is induced by particular signals, such as starvation and nutrient transitions, acid and oxidant stresses, DNA damage, subinhibitory concentrations of antibiotics, and intracellular infections (6).
Similar to persisters, tolerant cells are populations of bacteria that can also overcome antibiotic therapy. Tolerance allows cells to temporarily counteract the lethal consequences of high doses of antibiotics, maintaining their vital processes slowed (4, 7, 8). Also, tolerant bacteria arise when the whole population slows its growth (e.g., stationary phase), whereas persister bacteria are a small subpopulation of the population (4).
Both tolerant and persistent bacteria can be included in the “persistent phenotype,” which has high relevance in clinics because (i) there is evidence that persistent cells are responsible for relapses of infections, which is common in tuberculosis, cystic fibrosis, and Lyme disease (9, 10); (ii) antibiotic therapy does not effectively work against these types of infections; (iii) persisters are responsible for the majority of biofilm-associated infections (11, 12); and (iv) they are associated with better survival of bacteria inside macrophages (13). Furthermore, persister cells can also survive in immunocompromised patients and in patients in whom antibiotics do not effectively kill pathogenic bacteria, as they might deploy immune-evasion strategies (14).
Differences between resistance, persistence, and tolerance have been established; nonetheless, there are also relationships among these bacterial populations which are worthy of consideration. Despite evidence showing that tolerance and persistence to antibiotics promote the evolution of resistance in bacteria (8, 14, 15), both mechanisms are currently underestimated by the scientific and medical communities.
There are several molecular mechanisms involved in bacterial persistence and tolerance to antibiotics, reviewed by Trastoy and colleagues (7), which include the (p)ppGpp network, toxin-antitoxin (TA) system, the quorum sensing (QS) system, drug efflux pumps, reactive oxygen species (ROS), the SOS response, and RpoS (sigma factor of stationary phase) (7). (p)ppGpp orchestrates the stringent response (SR) in bacteria; thus, it is produced during nutrient stress (such as amino acid or fatty acid starvation) by proteins belonging to the RelA/SpoT homolog family (RSH) (16). In Escherichia coli, RelA is the (p)ppGpp synthetase I or GTP-pyrophosphokinase that synthesizes (p)ppGpp from GTP/GDP and ATP, whereas SpoT is a bifunctional (p)ppGpp synthetase II or pyrophosphohydrolase (17). However, (p)ppGpp levels in bacteria do not depend exclusively on nutrient availability (18), since it can also accumulate in response to a wide range of signals, including oxygen variation (19), pH downshift (20), osmotic shock, temperature shift (21), or even exposure to darkness (22). Furthermore, the SR is not only involved in responses to environmental stresses (starvation for carbon sources, fatty acids, and phosphates or heat shock) but also in bacterial pathogenesis (23), host invasion (24), antibiotic tolerance, and persister cell formation (25).
Given the exhaustive and contradictory literature surrounding the role of (p)ppGpp in bacterial persistence, and with the aim of summarizing what is known so far about the “magic spot” in this bacterial stage, this review provides new insights into the link between the SR and persisters. Finally, we have reviewed and discussed some of the innovative treatments that have (p)ppGpp as a target, which are in the spotlight of the scientific community as candidates of effective antipersistence agents.
(p)ppGpp AS A KEY REGULATOR
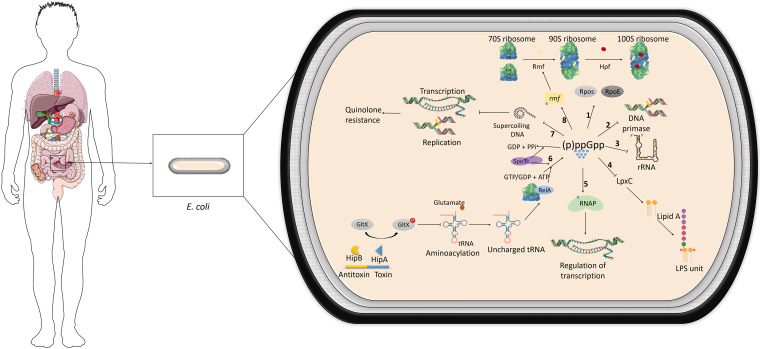
It was in 1969 that Cashel and Gallant described for the first time guanosine penta/tetraphosphate (26). During these 50 years, many functions have been attributed to this alarmone as it plays a key role in the physiology of bacteria, controlling mainly energetic metabolism (26, 27) but also their virulence and immune evasion (26). Despite being widely studied in the model organism E. coli, (p)ppGpp behaves differently in other species, and its regulation changes among phylogenetically related bacterial groups (27). In E. coli, the accumulation of (p)ppGpp causes the differential expression of approximately 500 genes, as it activates RpoS and RpoE (the stress response sigma factor for misfolded proteins in the periplasm) (28). Also, in E. coli, (p)ppGpp directly inhibits DNA primase (29) and is thought to inhibit the synthesis of rRNA, which also affects translation globally, by regulating the transcription of the ribosomal modulation factor (Rmf) (30). More specifically, in response to stresses such as amino acid starvation, in Gram-negative bacteria, (p)ppGpp binds to the RNA polymerase, inducing an allosteric signal to the catalytic Mg2+ site, which severely decreases transcription and causes a global rewiring of the gene expression profile. Taken together, all these changes lead to dormancy or slow growth for most cells (Fig. 1) (31, 32).
FIG 1.
Physiological pathways regulated by (p)ppGpp and the stringent response in E. coli. 1, increased levels of (p)ppGpp induce transcription of RpoS, sigma factor for the stationary phase, and RpoE, the sigma factor that regulates the expression of genes related to misfolded proteins; 2, (p)ppGpp inhibits DNA primase and thus the replication of the chromosome; 3, (p)ppGpp also inhibits transcription of rRNA, affecting the general translation; 4, (p)ppGpp also deregulates LpxC, an enzyme catalyzing the first step of LPS formation; 5, (p)ppGpp binds to RNA polymerase (RNAP), regulating the transcription of many genes; 6, according to certain models, HipA toxin would phosphorylate glutamyl-tRNA-synthetase (GltX), inactivating it and therefore impairing aminoacylation. Empty tRNAs then trigger the stringent response: RelA associates with ribosome and synthesizes (p)ppGpp from GTP/GDP plus ATP; 7, (p)ppGpp can directly inhibit negative supercoiling of DNA in E. coli, associated with resistance to quinolones; 8, (p)ppGpp induces the transcription of the ribosomal modulation factor (Rmf) and hibernation-promoting factor (Hpf), which play a role in ribosome dimerization, typical of persister cells. (p)ppGpp is also involved in immune evasion, virulence, and human pathogenesis.
Most Gram-positive bacteria possess one long RSH, named Rel, which has both (p)ppGpp synthetase and hydrolase activities (33, 34), together with a short RSH, named small alarmone synthetase (SAS) or small alarmone hydrolase (SAH) (35). Nonetheless, the existence of a diverse population of (p)ppGpp synthetases and hydrolases in bacteria has been demonstrated (35–38).
There is evidence that (p)ppGpp is related to antibiotic tolerance and persister cell formation (25, 39–42). For example, increased levels of (p)ppGpp inhibit negative supercoiling of DNA in E. coli, thus preventing DNA replication and transcription, resulting in tolerance toward ofloxacin and quinolones (Fig. 1) (43, 44). Persister cells tend to form in biofilms, in which the bacterial cells are embedded in a three-dimensional matrix that provides protection during pathogenesis and other conditions. Thus, it is logical that bacteria in biofilms encounter limited access to nutrients and therefore display the SR (45). Consistently, different groups have shown that multidrug tolerance of Pseudomonas aeruginosa and E. coli grown in biofilms depends on (p)ppGpp (45–47). Also, in 2014, Helaine and colleagues performed an experiment where they studied the invasion of mouse macrophages by Salmonella enterica, and they observed that (p)ppGpp production by bacteria residing in acidified vacuoles of macrophages was required for persistence (13).
(p)ppGpp AS A MEDIATOR OF BACTERIAL GROWTH
Accumulation of (p)ppGpp promotes transcriptional alterations in the bacterial cell, such as general repression of rapid growth genes and activation of genes involved in amino acid biosynthesis and survival to stress (29). Therefore, loss of (p)ppGpp under some conditions (for example, in minimal medium) leads to amino acid auxotrophy of the whole population and an increased survival due to high tolerance. Similarly, many other conditions or mutations that decrease the bacterial growth rate have been shown to induce the same tolerant phenotype (48). Several authors suggested that, at least in Salmonella, there was no specific molecular pathway underlying bacterial persistence but that slow growth was the main factor to induce persistence (49, 50). Regarding the involvement of (p)ppGpp in bacterial growth, it has been shown that relA spoT double null mutants of E. coli, completely depleted of (p)ppGpp, are more elongated than wild-type cells (51). LpxC, a key enzyme that catalyzes one of the first steps in the synthesis of lipopolysaccharide (LPS) in E. coli, is degraded during slow growth but stabilized when cells grow faster (Fig. 1) (52). Hence, in 2013, it was reported that in relA spot double null mutants, there was a deregulation of LpxC degradation, resulting in rapid proteolysis in fast-growing cells and stabilization during slow growth in opposition to the normal state where (p)ppGpp is present (52). In 2015, Yamaguchi and colleagues found that elevated levels of (p)ppGpp led to inhibition of bacterial growth by interfering with the FtsZ protein assembly in Salmonella enterica serovar Paratyphi A (53). FtsZ is a protein essential for the prokaryotic cell division that needs to form linear structures and has a GTP binding site; however, increased levels of (p)ppGpp (20-fold higher than the required GTP levels) causes FtsZ to form helical structures unable to form the Z-ring at the division site (53), which impairs the division of the bacterial cell and, therefore, population growth. In short, high levels of (p)ppGpp can promote persistence by halting growth in a subpopulation (even in a nutrient-rich medium), while absence of this alarmone can contribute to tolerance (by preventing the whole population from appropriately handling a nutritional stress).
ASSOCIATION BETWEEN OTHER MOLECULAR MECHANISMS AND (p)ppGpp IN PERSISTENCE
According to Trastoy and colleagues (7), some of the molecular mechanisms that have been related to bacterial persistence are TA systems, QS and secretion systems (SS), efflux pumps, the SOS system, and ROS response (Fig. 2A). We focus only on those where a link between (p)ppGpp or SR and persistence has been reported: TA systems, efflux pumps, and ROS systems (Fig. 2B).
FIG 2.
(A) Molecular mechanisms underlying bacterial persistence. (B) Different models explaining the involvement of (p)ppGpp in persistence and representative publications.
TA systems.
The persistent state actually describes many different growth-arrested physiologies, and it has been associated, throughout the years, with the activity of TA systems, at least partially (54, 55). TA systems are a module of two genes encoding a stable toxin and a usually unstable antitoxin which is degraded under stress conditions; however, bound antitoxin to toxin is not likely the source of free toxin, since the two proteins are bound tightly (56) (Fig. 2A and B). Once unbound toxin is produced, the harmful toxin slows growth, maintains plasmids (57), inhibits phage (58), and induces biofilm formation (59, 60). TA systems are widely distributed in pathogenic bacteria and found in the bacterial chromosome, plasmids, and bacteriophages (61, 62).
In 1983, Moyed and Bertrand identified the first persistence gene related to increased survival in the presence of ampicillin in E. coli (63). They discovered a high-persistence gain-of-function mutation, named hipA7; hipA encodes the toxin of the HipA/HipB TA system. Similarly, the second link between TA systems and persistence was reported by Aizenman and colleagues in 1996, when they observed that (p)ppGpp was required to activate MazF toxicity, the toxin of the MazF/MazE TA system (64). In 2003, Korch and colleagues studied the role of the HipAB TA system in persistence, showing that the ability of E. coli to survive to a prolonged exposure to penicillin was due to two mutations in the nontoxic hipA7 allele (65). Both mutations, G22S and D291A, were required for the full range of phenotypes associated with high persistence, increasing 1,000- to 10,000-fold the frequency of persisters. Moreover, in this study, they also demonstrated that relA knockouts diminished the high-persistence phenotype in hipA7 mutants and that relA spoT knockouts completely eliminated this high persistence, suggesting that hipA7 facilitates the establishment of the persistent state by inducing (p)ppGpp synthesis (65). This result was confirmed in 2011 by Nguyen and colleagues in P. aeruginosa, where a relA spot double mutant led to a decrease of 68-fold in persistence (45). Interestingly, Correia and colleagues demonstrated that HipA exhibits a eukaryotic serine-threonine kinase activity, required for both the inhibition of cell growth and the stimulation of persister cells (66). In 2009, Schumacher and colleagues suggested that HipA inhibited cell growth by the phosphorylation of the essential elongation factor Tu (EF-Tu), involved in translation (67). Notwithstanding, in 2013, Germain and colleagues challenged the previous model in which HipA inactivated translation by the phosphorylation of the EF-Tu and proposed a novel paradigm in which HipA inactivates the glutamyl-tRNA-synthetase (GltX) by phosphorylation of the conserved Ser239 near the active center (68). In addition, the authors claimed that this phosphorylation inhibited aminoacylation, which halts translation and induces the SR by the generation of “hungry” codons at the ribosomal A site. Thus, RelA binds to the ribosome, is activated, and increases (p)ppGpp levels that inhibit translation, transcription, replication, and cell wall synthesis, thereby leading to slow growth, multidrug tolerance, and persistence (Fig. 1) (47, 68–70).
In this context, further studies have been conducted to unravel the association of TA systems and (p)ppGpp in persistence. In 2012, Gerdes and Maisonneuve reported that the removal of 10 mRNAse-encoding TA loci of E. coli led to a dramatic decrease of persistence in the presence of the antibiotic (71). A similar phenomenon was observed with a mutant lacking Lon protease, which indicated that TA systems and Lon protease were somehow correlated and both implicated in the persistence of E. coli (72). Since many antitoxins of E. coli were degraded by Lon protease, Gerdes and Maisonneuve hypothesized that HipB was one of the targets. In E. coli, Lon can be activated by polyphosphate (polyP), synthesized by polyphosphate kinase (PPK), and degraded by an exopolyphosphatase (PPX). PPX is inhibited by (p)ppGpp, which leads to an increase in polyP. Hence, these authors claimed that high levels of (p)ppGpp associated with persistence inhibited PPX, thus allowing polyP to activate Lon protease, which would degrade the HipB antitoxin. Furthermore, they suggested that polyP functions as an intracellular signaling molecule controlled by the SR, and (p)ppGpp reprograms the cells to survive starvation. (71). In 2014, these authors completed this model by adding one additional step, which is a speculative positive-feedback loop that ensures even more synthesis of (p)ppGpp (42). Thus, the model predicts that the degradation of HipB enables free HipA to phosphorylate GltX and inhibits charging of tRNA-Glu. When uncharged tRNAs enter the ribosomal A site, RelA-dependent synthesis of (p)ppGpp is triggered (Table 1 and Fig. 1) (42). Even though this model became very influential, the authors retracted the key articles in 2018, claiming that the apparent inhibition of persistence in the multiple-deletion strain was due to an inadvertent lysogenization with the bacteriophage Φ80, a contaminant that caused artifacts in their experiments (73). In addition, there were conflicting reports for the (p)ppGpp/polyP/Lon persistence model, such as that Lon protease is not activated but deactivated by polyP (74) or not required for persistence (Table 1 and Fig. 2B) (75).
TABLE 1.
Summary of the main ppGpp models
| Yr | Journal | Authors | Model | Reference(s) |
|---|---|---|---|---|
| 2014 | Cell | E. Maisonneuve and K. Gerdes | (p)ppGpp induces persistence by activating TA loci via polyP and Lon protease in E. coli K-12 strain MG1655 | 42, 71–73a |
| 3, 65, 107 | ||||
| 2016 | Scientific Reports | N. Chowdhury, B. W. Kwan, and T. K. Wood | Formation of persister cells in E. coli K-12 strain MG1655 is attributed to production of any toxic protein (e.g., MazF, RelB, and YafO), and (p)ppGpp is not essential but increases persistence 1000× | 75 |
| 2019 | Science Signaling | M. H. Pontes and E. A. Groisman | Low cytoplasmatic Mg2+ induces S. enterica serovar Typhimurium tolerance to antibiotic independently of (p)ppGpp and TA modules; however, (p)ppGpp reduces antibiotic tolerance under certain conditions | 49 |
| 2020 | Biochemical and Biophysical Research Communications | S. Song and T. K. Wood | (p)ppGpp generates persister cells directly by inactivation of ribosomes via Rmf and Hpf.b | 82 |
Some of these publications have been retracted due to the contamination with the Φ80 bacteriophage causing artifacts in the results.
Rmf, ribosomal modulation factor; Hpf, hibernation-promoting factor.
In 2019, Pontes and Groisman studied the implication of TA modules and (p)ppGpp in the persistence of Salmonella, and they revealed that low cytoplasmic Mg2+ induced tolerance to antibiotics independently of (p)ppGpp and TA modules (Table 1) (49). In fact, a relA spoT double mutant of Salmonella, unable to produce (p)ppGpp, exhibited similar tolerance to antibiotics after growing in low Mg2+ as the wild-type strain. The same phenomenon occurred with the mutant strain lacking 12 TA systems (Δ12TA). However, when the antibiotic treatment was added at neutral pH, they saw 5- to 8-fold fewer persisters than for the wild type (WT) both in the Δ12TA strain and in double mutant relA spoT (49). Nonetheless, when they deleted one single TA module, they found that this mutation had no effect on persistence (49) (Fig. 2B).
Recently, Song and Wood claimed that TA systems are not involved in the formation of persistent bacteria (56). The many contradictory observations in diverse experimental setups lead to no clear conclusive understanding of the implications of TA systems in persistence, and further work is needed.
Efflux pumps.
Efflux pumps are proteic complexes that allow bacteria to draw out intracellular toxins or antibiotic molecules. Some genes encoding efflux pumps are upregulated in cells that constitute biofilms, which are composed mainly by nongrowing persistent cells (76). This upregulation in persister cells can be triggered by different signals, such as the ROS response, QS, and (p)ppGpp (77).
The first data that linked SR with efflux pumps were reported by Chuang and colleagues (78), who observed that a ppx2 (encoding the exopolyphosphatase that degrades polyP) knockout mutant of Mycobacterium tuberculosis [where polyP and (p)ppGpp accumulate] exhibited increased levels of several efflux genes, including iniA, iniB, mmpL10, and Rv2459 (Fig. 2B). Notwithstanding, the authors concluded that the element which contributed the most to isoniazid tolerance in this mutant was the change in the cell wall thickness, which limited the diffusion of polar molecules such as isoniazid (78).
Some years later, in 2018, Ge and colleagues described that a glucose/galactose transporter of Helicobacter pylori, Hp1174, functions as an efflux pump and is highly expressed in biofilm-forming and multidrug-resistant (MDR) H. pylori strains. This transporter, encoded by the gluP gene, is upregulated by SpoT (77). An H. pylori mutant lacking gluP and its product Hp1174 constituted an unstructured biofilm whose matrix was damaged. As this study revealed that the SpoT enzyme upregulates Hp1174 in persistent biofilm-forming cells, and provided that the transcription of this gene is controlled by an alternative sigma factor (σ54), they hypothesized that SpoT may upregulate the expression of gluP by σ54-dependent transcription.
Finally, Pu and colleagues demonstrated that β-lactam-induced E. coli persisters exhibited less cytoplasmic drug accumulation because of an enhanced efflux activity compared to that of nonpersister cells. Combining time-lapse imaging and mutagenesis techniques, scientists determined a positive correlation between tolC expression and the emergence of E. coli persisters (79).
ROS response.
Reactive oxygen species (ROS) are produced as a natural response to the normal metabolism of oxygen and perform important functions in cell signaling and homeostasis. However, when cells are exposed to environmental pressure, such as antibiotics, UV, or heat pressure, ROS levels can increase; this increase can cause damage to the DNA, lipids, and proteins, which subsequently leads to cell death. Like all molecular mechanisms, ROS are subject to regulation (Fig. 2B). Among the molecules capable of eliminating ROS, we find enzymes, such as superoxide dismutase (SOD) and catalase, as well as antioxidant agents, such as glutathione and vitamin C. However, when an increase in ROS levels occurs due to an imbalance between production and elimination mechanisms, cells are said to be subject to oxidative stress (7).
The relationship between the ROS response and persistent bacteria is widely described in the literature. Nguyen and colleagues, in 2011, showed that the survival of multidrug-tolerant persisters in biofilms of P. aeruginosa was largely dependent on catalase or SOD enzymes, which are under the control of (p)ppGpp signaling (45). Along the same lines, the study by Khakimova and colleagues demonstrated that the SR regulates catalases, likely through a complex interplay of regulators (80) (Fig. 2A and B). Furthermore, they also demonstrated that H2O2 and antibiotic tolerance were the result of a balance between prooxidant stress and antioxidant stress (80). Similarly, the work of Molina-Quiroz and colleagues (81) gave more evidence of the relationship between oxidative stress and bacterial survival to antibiotics. In this study, the authors demonstrated the impact of ROS on the generation of persister cells, exposing the cultures of a WT strain and its corresponding mutant lacking the cAMP synthase adenylate cyclase (ΔcyaA) under the antibiotic pressure of ampicillin in the presence and absence of oxygen. For both strains, they observed a 100-fold increase of ampicillin survival in the absence of oxygen compared to that of the strain under aerobic conditions. This study concluded that the damage that ROS caused to DNA was regulated by cAMP, a negative regulator of persistence in uropathogenic E. coli (81).
PRDP: (p)ppGpp RIBOSOME DIMERIZATION PERSISTER MODEL
Song and Wood proposed a novel model in which the alarmone (p)ppGpp would generate persister cells by inactivating ribosomes via the Rmf and the hibernation-promoting factor (Hpf) (Fig. 1 and 2B; Table 1) (82). Among their findings, the following should be highlighted: (i) E. coli persisters contain a large fraction of inactivated 100S ribosomes; (ii) Rmf and Hpf induced persistence, and the inactivation of these proteins increased single-cell persister resuscitation, and (iii) (p)ppGpp did not affect the single-cell persister resuscitation. In another work, it was reported that (p)ppGpp induced Hpf, converting the 90S ribosomes into 100S ribosomes, and that overproduction of Rmf and Hpf increased persistence as well as reduced single-cell resuscitation (83). Furthermore, the authors based their theory on the fact that (p)ppGpp inhibits the ribosome-associated GTPase Era, essential in assembling ribosomal 30S subunits in Staphylococcus aureus (84). Hence, a connection between (p)ppGpp and persistence via ribosome dimerization was demonstrated.
In 2019, Libby and colleagues showed that there was an enormous variability in sasA expression (the gene encoding SasA in Bacillus subtilis) among bacterial cells, linking a higher expression of sasA with an increase in antibiotic survival (85). (p)ppGpp synthetases in B. subtilis, such as SasA, are important in ribosome dimerization, as YwaC induces the transcription of the yvyD gene, whose product, YvyD protein, is essential for the dimerization of 70S ribosomes (86). 70S dimers are similar to the above-mentioned 100S ribosomes in E. coli and therefore translationally inactive. These results agree with the ppGpp ribosome dimerization persister (PRDP) model, where (p)ppGpp would induce bacterial persistence by promoting ribosome dimerization and compromise translation inside the cells (82).
ANTIPERSISTER TREATMENTS WITH (p)ppGpp AS A TARGET
Most antibiotics used in clinics target active metabolic processes. Therefore, bacteria that exhibit a reduction in metabolism and growth rate, such as tolerant or persistent cells, are not a target for the classic antibiotics. In the literature, a few reviews summarizing some of the most useful strategies to combat persistent infectious diseases can be found (2, 87, 88).
As the activation of the SR leads to the shutdown of nearly all metabolic processes and entrance into a state of dormancy, an interesting therapeutic approach to combat persistent infections is the inhibition of the SR network. Hobbs and Boraston reviewed the recent attempts that have been made to design and discover inhibitors of the SR, and they concluded that there are currently two approaches: (i) inhibition of (p)ppGpp synthetases by using (p)ppGpp analogs and (ii) inhibition of (p)ppGpp accumulation by using protein inhibitors (89).
Inhibition of (p)ppGpp synthetases by using (p)ppGpp analogs.
Several in vitro studies using double relA spoT null mutants in E. coli have shown that this bacterium lacks the (p)ppGpp and therefore has significantly reduced persistence to antibiotics. In this context, some compounds that inhibit the (p)ppGpp production (thus SR) had been developed to abolish persistence. Relacin, one of these compounds, was first designed in 2012 by Wexselblatt and colleagues and was shown to inhibit Rel-mediated (p)ppGpp synthesis, leading to the death of B. subtilis with an estimated 50% inhibitory concentration (IC50) of 200 μM (Table 2) (90). Importantly, relacin also prevented the formation of spores and biofilm in this species. Before bacterial death, relacin also induced a prolonged exponential phase. In 2017, Syal and colleagues performed a slight modification of relacin and found that cells of Mycobacterium smegmatis treated with this molecule were not able to establish any biofilm and were elongated, showing exactly the same phenotype as a rel mutant (91). Interestingly, this relacin-derived compound lacked toxicity with human red blood cells and has good permeability into human lung epithelial cells. One of the most persistent pathogens, M. tuberculosis, could be potentially targeted with this (p)ppGpp inhibitor if its evaluation in humans turns out to be effective and safe (Table 2).
TABLE 2.
Summary of the main treatments having ppGpp as a target
| Yr | Authors | Strategy | Mechanism of action | Reference |
|---|---|---|---|---|
| 2012 | Wexselblatt and colleagues | (p)ppGpp analogs inhibit Rel protein: relacin | Relacin produced death of B. subtilis with an IC50 of 200 μM, prevented sporulation and biofilm formation, and induced a prolonged exponential phase | 90 |
| 2012 | de la Fuente-Núñez and colleagues | Inhibition of (p)ppGpp accumulation: 1037 peptide | 1037 reduced expression of flagellum-associated genes that favor biofilm establishment in P. aeruginosa and B. cenocepacia; also reduced swarming motility | 94 |
| 2014 | de la Fuente-Núñez and colleagues | Inhibition of (p)ppGpp accumulation: 1018 peptide | 1018 marked (p)ppGpp for degradationa: broad antibiofilm activity against Gram-positive and -negative bacteria and lack of effect for planktonic cultures | 93 |
| 2017 | Syal and colleagues | (p)ppGpp analog to inhibit Rel protein: modification of relacin | Impaired biofilm formation by M. smegmatis and emergence of elongated cells. Lack of toxicity, good permeability into human lung epithelial cells. | 91 |
| 2019 | Dutta and colleagues | (p)ppGpp analog to inhibit Rel protein: compound X9 | Highest inhibitory activity against Rel protein: IC50 of ∼15 μM against purified Rel of M. tuberculosis. Enhancement of susceptibility against isoniazid. | 92 |
Andresen and colleagues rejected this hypothesis 2 years later, questioning its specificity for (p)ppGpp and for biofilm-forming cells (95).
In order to find other inhibitors of Rel protein, Dutta and colleagues performed a high-throughput screening approach, using Rel from M. tuberculosis and a novel (p)ppGpp synthetase assay, based on detection of AMP released after Rel catalyzes the transfer of pyrophosphate groups from ATP to GTP/GDP (92). This screening led to the identification of the most potent Rel inhibitor to date, the compound X9, which exhibited an IC50 of ∼15 μM against purified Rel. At 4 μM, when M. tuberculosis was nutrient starved, it enhanced its susceptibility against isoniazid (Table 2). Even if the molecular mechanism by which X9 inhibits Rel is not yet fully understood, this compound displays the most potent activity of any Rel inhibitor to date.
Inhibition of (p)ppGpp accumulation.
A second approach to design antipersistence strategies that target (p)ppGpp would be the inhibition of the accumulation of this alarmone. Biofilms are very important in the establishment and maintenance of many infections caused by pathogenic bacteria; therefore, some cationic peptides with antibiofilm abilities have been tested and proposed to act via disruption of the SR (11, 89).
The 1018 peptide (VRLIVAVRIWRR-NH2) is a small, synthetic l-amino acid peptide derived from a bovine host defense peptide. de la Fuente-Núñez and colleagues first described that 1018 marks (p)ppGpp for degradation, exhibiting potent activity against biofilms produced by Gram-positive (S. aureus) and Gram-negative (E. coli, P. aeruginosa, Klebsiella pneumoniae, or Acinetobacter baumannii) bacteria but not in planktonic cultures (93). They also observed that the 1018 peptide prevented biofilm formation and degraded the preformed biofilm (as old as 2 days). As an overproduction of (p)ppGpp leads to resistance to the 1018 peptide, the authors suggested that this peptide specifically targeted SR (Table 2). The same research group generated another small peptide, called 1037, and observed its effects on biofilm formation and swarming motility in P. aeruginosa, Burkholderia cenocepacia, and Gram-positive Listeria monocytogenes (Table 2) (94). They observed that 1037 reduced flagellum-dependent swimming in P. aeruginosa PA14, P. aeruginosa PAO1, and B. cenocepacia; consistently, transcriptomic analysis revealed that, in the presence of 1037, several genes related to flagella were downregulated by 2- to 3-fold. This is interesting because flagella are involved in biofilm formation and swarming motility, both of which were significantly inhibited by the action of 1037 in the tested species (94).
Notwithstanding, the study of Andresen and colleagues in 2016 rejected the idea that 1018 peptide specifically targets the SR alarmone (p)ppGpp (95). Furthermore, they observed that in P. aeruginosa, this peptide showed antibacterial activity both in planktonic and in biofilm-derived cells.
Interestingly, Allison and colleagues published an innovative article in 2011 describing how they killed E. coli and S. aureus persisters by combining different metabolites with aminoglycosides (96). They reported that glucose, pyruvate, mannitol, or fructose significantly increased the proton motive force (PMF); this leads to a higher uptake of the aminoglycoside and the consequent killing of the persisters, either in vitro or in a mouse model carrying a catheter colonized by uropathogenic E. coli. In conclusion, these findings mean that some of these PMF-stimulating metabolites might be a good adjuvant to aminoglycoside to treat persistent chronic infections (96).
DISCUSSION
We have summarized the functions of (p)ppGpp regarding its role as a global transcription and translation regulator of metabolism, slow growth, dormancy, nutrient starvation, different kinds of stress, virulence, tolerance to antibiotics, persister cell formation, and even persistence inside macrophages. However, an accurate role of this alarmone in persistence has not been determined yet. Clearly, evidence relates this molecule to the persistent phenotype based on its dominant role in the stress response of bacteria.
The diversity among the conclusions obtained by laboratories around the globe raised the question of whether persisters in phylogenetically close organisms are produced through different pathways (49, 97). Nevertheless, this seems unlikely, as the SR is a universal highly conserved network in many phyla, and all microbes use it to protect themselves against different types of stress.
The relevance of the role of the ATP in the antibiotic persistence is revealed as tolerant cells slow down their metabolism and persistent cells are quiescent. Pontes and Groisman showed that Salmonella preexposed to chloramphenicol resisted killing by bactericidal antibiotics (49). However, contradictory results have been obtained from different groups, indicating that ATP does not control persistence (49, 98, 99) or even that persister cell formation is based on the reduction of ATP (100–103).
The results of Pontes and Groisman (49) agree with the findings of both Hobby et al. (104) and Bigger (105) and with many other researchers who have shown that deliberate induction of bacteriostasis promotes antibiotic tolerance (15). Whereas deliberate induction of bacteriostasis overrides bacterial control of growth, it remains to be explored what mechanisms promote growth arrest in individual cells. They showed that Salmonella persisters emerged as a result of slow growth alone and with transitory disturbances to core activities, regardless of the underlying physiological process. They also performed studies with Salmonella mutants lacking 12 TA modules and observed their implication in persistence under some conditions (49). Kaldalu and Tenson claimed that there was no specific molecular mechanism involved in persistence but that it was simply produced by slow growth of bacteria (50).
It remains unclear if efflux pumps and the SOS system have a real link with (p)ppGpp. Despite being important molecular mechanisms widely studied in pathogenic bacteria, there are still few studies linking (p)ppGpp metabolism with these mechanisms. It is even trickier to associate these with persistence, the proof for which is in the article of Ge and colleagues, where they claim that the bifunctional SpoT enzyme upregulates the Hp1174 efflux pump of H. pylori, contributing to biofilm formation (77). Regarding the role of the ROS system in persistence, different research groups have demonstrated that (p)ppGpp and the SR regulate the expression of antioxidant enzymes, e.g., SOD or catalases, in order to avoid the intracellular accumulation of ROS (81). Even if the intermediate regulators involved in this pathway need further research, this opens the door to antipersister therapies targeting SR (45, 80).
Traditionally, the persistence of bacteria has been widely attributed to TA systems, as Chowdhury and colleagues published in 2016, where they claimed that persister cells can form in the absence of (p)ppGpp (although at much-reduced levels), mainly due to the effect of production of any toxic protein (75). Nevertheless, T. K. Wood reported some years later an essential role of (p)ppGpp in the establishment of persistence via induction of dimerization of ribosomes. In this new model, called PRDP and proposed by Song and Wood in 2020, there is evidence of a direct role of the magic spot in the persistence phenotype (82).
An interesting issue is the individual variability within a population of cells regarding their tolerance to antibiotics. Whether this heterogeneity is regulated or, on the contrary, is an unavoidable consequence of stochastic fluctuations remains unknown. In 2004, Balaban and colleagues showed that spontaneous persisters are rare, suggesting that they were not stochastically produced (3). Similarly, the PRDP model also suggests that both persistence and resuscitation are sophisticatedly regulated by ribosome content and their activation status (82). Finally, another hint of the regulation of SR was proposed by Libby and colleagues (85), based on the fact that SasA in B. subtilis has multiple sites of phosphorylation, which can explain the cell-to-cell variability in sasA expression (85, 86). These differences may be the reason for the physiologically relevant variability in (p)ppGpp levels and shed some light into the heterogeneity within a bacterial population and their phenotypic variability.
There are some arguments in favor of the PRDP model: one is that Hpf, which converts 90S ribosomes into inactivated 100S ribosomes in E. coli, is highly conserved in most bacteria (83); second, other (p)ppGpp synthetases found in B. subtilis are essential for ribosome dimerization in Gram-positive bacteria, generating translationally inactive ribosomes associated with persistence (86).
In this review, we have contrasted different models that have been proposed over many years, all of them aimed at answering the same question: what is the precise role of (p)ppGpp and SR in bacterial persistence? Definitely, after comparing all those models, we can conclude that uncontrolled variables such as contaminants (such as Φ80 phage), particular setups that differ from lab to lab, artifacts that mislead to conclusions, changes in the tested strains, and, in short, different experimental conditions can be some of the underlying reasons to explain the controversy around this question.
The current lack of effective antibiotics against multidrug-resistant, persister, and tolerant pathogens leads the urgency to develop new antibacterial treatments, such as the antipersistence treatments targeting the (p)ppGpp network. The inhibitors of (p)ppGpp synthetases are good candidates as antimicrobial agents because of their high efficacies in avoiding biofilm formation (90), in the loss of the persistence phenotype, and even in the prolongation of exponential phase, when bacteria replicate more actively, being more susceptible to antibiotics. This also opens the door to the possibility of a combination of therapies [inhibitors of (p)ppGpp synthetase with antibiotics] and to the establishment of potential synergies. Even if no effect of the Rel inhibitor relacin was observed in E. coli, the authors suggested that this could be due to the inability of relacin to penetrate Gram-negative cells and reach its target. However, agents such as polymyxins can destabilize the outer membrane in Gram-negative organisms, facilitating the entrance of therapeutic molecules, e.g., relacin, or endolysins (106). The absence of known (p)ppGpp synthetases in mammalian cells and the specificity of these inhibitors for the Rel protein make this protein a good candidate as an antibacterial agent.
The second strategy of inhibition of (p)ppGpp accumulation, supported by the 1018 and 1037 peptides, exhibited promising antipersistence activities, as they specifically targeted biofilm-forming cells and had no effect on planktonic cells (93, 94). According to the few studies that focus on inhibiting the SR as an antipersistence therapeutic approach, we can consider this as an emerging field; therefore, further research and financial investment are needed to efficiently prevent persistent, chronic, and life-threatening infections.
Conclusion.
The emergence of contradictory models about the involvement of the “magic spot” in bacterial persistence highlights the need to deepen the studies in this field. In summary, one potential strategy to fight persistent infectious diseases resides in specifically targeting SR or (p)ppGpp of pathogenic bacteria, but further knowledge is necessary to provide a better understanding of the complexity of bacterial persistence as well as its implications in clinics.
ACKNOWLEDGMENTS
This study was funded by grant PI16/01163 and PI19/00878 awarded to M. Tomás within the State Plan for R+D+I 2013-2016 (National Plan for Scientific Research, Technological Development and Innovation 2008-2011) and cofinanced by the ISCIII-Deputy General Directorate for Evaluation and Promotion of Research–European Regional Development Fund, A way of Making Europe, Instituto de Salud Carlos III FEDER, Spanish Network for the Research in Infectious Diseases (REIPI, RD16/0016/0006 and RD16/0016/0011), and by the Study Group on Mechanisms of Action and Resistance to Antimicrobials, GEMARA (SEIMC, http://www.seimc.org/). M. Tomás was financially supported by the Miguel Servet Research Program (SERGAS and ISCIII). L. Fernández-García was supported by postdoctoral fellowship from the Diputation A Coruña (Xunta Galicia). T.J.K. is supported by a National Health and Medical Research Council Early Career Fellowship (GNT1088448).
O.P., L.B., I.B., L.F.-G., A.A., and M.L. wrote the manuscript; G.B., R.C., R.G.-C., T.K.W., and M.T. supervised and revised the manuscript.
We declare no conflict of interest.
O.P., L.B., I.B., L.F.-G., A.A., M.L., G.B., R.C., and M.T. are members of the Study Group on Mechanisms of Action and Resistance to Antimicrobials (GEMARA) of Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC). G.B., R.C., and M.T. are members of the Spanish Network for the Research in Infectious Diseases (REIPI).
REFERENCES
- 1.Lewis K. 2020. The science of antibiotic discovery. Cell 181:29–45. doi: 10.1016/j.cell.2020.02.056. [DOI] [PubMed] [Google Scholar]
- 2.Dewachter L, Fauvart M, Michiels J. 2019. Bacterial heterogeneity and antibiotic survival: understanding and combatting persistence and heteroresistance. Mol Cell 76:255–267. doi: 10.1016/j.molcel.2019.09.028. [DOI] [PubMed] [Google Scholar]
- 3.Balaban NQ, Merrin J, Chait R, Kowalik L, Leibler S. 2004. Bacterial persistence as a phenotypic switch. Science 305:1622–1625. doi: 10.1126/science.1099390. [DOI] [PubMed] [Google Scholar]
- 4.Brauner A, Fridman O, Gefen O, Balaban NQ. 2016. Distinguishing between resistance, tolerance and persistence to antibiotic treatment. Nat Rev Microbiol 14:320–330. doi: 10.1038/nrmicro.2016.34. [DOI] [PubMed] [Google Scholar]
- 5.Girgis HS, Harris K, Tavazoie S. 2012. Large mutational target size for rapid emergence of bacterial persistence. Proc Natl Acad Sci U S A 109:12740–12745. doi: 10.1073/pnas.1205124109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balaban NQ, Helaine S, Lewis K, Ackermann M, Aldridge B, Andersson DI, Brynildsen MP, Bumann D, Camilli A, Collins JJ, Dehio C, Fortune S, Ghigo JM, Hardt WD, Harms A, Heinemann M, Hung DT, Jenal U, Levin BR, Michiels J, Storz G, Tan MW, Tenson T, Van Melderen L, Zinkernagel A. 2019. Definitions and guidelines for research on antibiotic persistence. Nat Rev Microbiol 17:441–448. doi: 10.1038/s41579-019-0196-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trastoy R, Manso T, Fernández-García L, Blasco L, Ambroa A, Pérez Del Molino ML, Bou G, García-Contreras R, Wood TK, Tomás M. 2018. Mechanisms of bacterial tolerance and persistence in the gastrointestinal and respiratory environments. Clin Microbiol Rev 31:e00023-18. doi: 10.1128/CMR.00023-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Windels EM, Michiels JE, Van den Bergh B, Fauvart M, Michiels J. 2019. Antibiotics: combatting tolerance to stop resistance. mBio 10:e02095-19. doi: 10.1128/mBio.02095-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y. 2014. Persisters, persistent infections and the Yin-Yang model. Emerg Microbes Infect 3:e3. doi: 10.1038/emi.2014.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lewis K. 2007. Persister cells, dormancy and infectious disease. Nat Rev Microbiol 5:48–56. doi: 10.1038/nrmicro1557. [DOI] [PubMed] [Google Scholar]
- 11.Lebeaux D, Chauhan A, Létoffé S, Fischer F, de Reuse H, Beloin C, Ghigo J-M. 2014. pH-mediated potentiation of aminoglycosides kills bacterial persisters and eradicates in vivo biofilms. J Infect Dis 210:1357–1366. doi: 10.1093/infdis/jiu286. [DOI] [PubMed] [Google Scholar]
- 12.Wood TK, Knabel SJ, Kwan BW. 2013. Bacterial persister cell formation and dormancy. Appl Environ Microbiol 79:7116–7121. doi: 10.1128/AEM.02636-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Helaine S, Cheverton AM, Watson KG, Faure LM, Matthews SA, Holden DW. 2014. Internalization of Salmonella by macrophages induces formation of nonreplicating persisters. Science 343:204–208. doi: 10.1126/science.1244705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fisher RA, Gollan B, Helaine S. 2017. Persistent bacterial infections and persister cells. Nat Rev Microbiol 15:453–464. doi: 10.1038/nrmicro.2017.42. [DOI] [PubMed] [Google Scholar]
- 15.Windels EM, Michiels JE, Fauvart M, Wenseleers T, Van den Bergh B, Michiels J. 2019. Bacterial persistence promotes the evolution of antibiotic resistance by increasing survival and mutation rates. ISME J 13:1239–1251. doi: 10.1038/s41396-019-0344-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Potrykus K, Cashel M. 2008. (p)ppGpp: still magical? Annu Rev Microbiol 62:35–51. doi: 10.1146/annurev.micro.62.081307.162903. [DOI] [PubMed] [Google Scholar]
- 17.Ronneau S, Hallez R. 2019. Make and break the alarmone: regulation of (p)ppGpp synthetase/hydrolase enzymes in bacteria. FEMS Microbiol Rev 43:389–400. doi: 10.1093/femsre/fuz009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Irving SE, Corrigan RM. 2018. Triggering the stringent response: signals responsible for activating (p)ppGpp synthesis in bacteria. Microbiology 164:268–276. doi: 10.1099/mic.0.000621. [DOI] [PubMed] [Google Scholar]
- 19.Glass TL, Holmes WM, Hylemon PB, Stellwag EJ. 1979. Synthesis of guanosine tetra- and pentaphosphates by the obligately anaerobic bacterium Bacteroides thetaiotaomicron in response to molecular oxygen. J Bacteriol 137:956–962. doi: 10.1128/JB.137.2.956-962.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wells DH, Gaynor EC. 2006. Helicobacter pylori initiates the stringent response upon nutrient and pH downshift. J Bacteriol 188:3726–3729. doi: 10.1128/JB.188.10.3726-3729.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gallant J, Palmer L, Pao CC. 1977. Anomalous synthesis of ppGpp in growing cells. Cell 11:181–185. doi: 10.1016/0092-8674(77)90329-4. [DOI] [PubMed] [Google Scholar]
- 22.Hood RD, Higgins SA, Flamholz A, Nichols RJ, Savage DF. 2016. The stringent response regulates adaptation to darkness in the cyanobacterium Synechococcus elongatus. Proc Natl Acad Sci U S A 113:E4867–E4876. doi: 10.1073/pnas.1524915113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dalebroux ZD, Svensson SL, Gaynor EC, Swanson MS. 2010. ppGpp conjures bacterial virulence. Microbiol Mol Biol Rev 74:171–199. doi: 10.1128/MMBR.00046-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geiger T, Francois P, Liebeke M, Fraunholz M, Goerke C, Krismer B, Schrenzel J, Lalk M, Wolz C. 2012. The stringent response of Staphylococcus aureus and its impact on survival after phagocytosis through the induction of intracellular PSMs expression. PLoS Pathog 8:e1003016. doi: 10.1371/journal.ppat.1003016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodionov DG, Ishiguro EE. 1995. Direct correlation between overproduction of guanosine 3',5'-bispyrophosphate (ppGpp) and penicillin tolerance in Escherichia coli. J Bacteriol 177:4224–4229. doi: 10.1128/jb.177.15.4224-4229.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cashel M, Gallant J. 1969. Two compounds implicated in the function of the RC gene of Escherichia coli. Nature 221:838–841. doi: 10.1038/221838a0. [DOI] [PubMed] [Google Scholar]
- 27.Hauryliuk V, Atkinson GC, Murakami KS, Tenson T, Gerdes K. 2015. Recent functional insights into the role of (p)ppGpp in bacterial physiology. Nat Rev Microbiol 13:298–309. doi: 10.1038/nrmicro3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Costanzo A, Ades SE. 2006. Growth phase-dependent regulation of the extracytoplasmic stress factor, sigmaE, by guanosine 3',5'-bispyrophosphate (ppGpp). J Bacteriol 188:4627–4634. doi: 10.1128/JB.01981-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gaca AO, Colomer-Winter C, Lemos JA. 2015. Many means to a common end: the intricacies of (p)ppGpp metabolism and its control of bacterial homeostasis. J Bacteriol 197:1146–1156. doi: 10.1128/JB.02577-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shimada T, Yoshida H, Ishihama A. 2013. Involvement of cyclic AMP receptor protein in regulation of the rmf gene encoding the ribosome modulation factor in Escherichia coli. J Bacteriol 195:2212–2219. doi: 10.1128/JB.02279-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Durfee T, Hansen AM, Zhi H, Blattner FR, Jin DJ. 2008. Transcription profiling of the stringent response in Escherichia coli. J Bacteriol 190:1084–1096. doi: 10.1128/JB.01092-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu K, Bittner AN, Wang JD. 2015. Diversity in (p)ppGpp metabolism and effectors. Curr Opin Microbiol 24:72–79. doi: 10.1016/j.mib.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wendrich TM, Marahiel MA. 1997. Cloning and characterization of a relA/spoT homologue from Bacillus subtilis. Mol Microbiol 26:65–79. doi: 10.1046/j.1365-2958.1997.5511919.x. [DOI] [PubMed] [Google Scholar]
- 34.Mittenhuber G. 2001. Comparative genomics of prokaryotic GTP-binding proteins (the Era, Obg, EngA, ThdF (TrmE), YchF and YihA families) and their relationship to eukaryotic GTP-binding proteins (the DRG, ARF, RAB, RAN, RAS and RHO families). J Mol Microbiol Biotechnol 3:21–35. [PubMed] [Google Scholar]
- 35.Atkinson GC, Tenson T, Hauryliuk V. 2011. The RelA/SpoT homolog (RSH) superfamily: distribution and functional evolution of ppGpp synthetases and hydrolases across the tree of life. PLoS One 6:e23479. doi: 10.1371/journal.pone.0023479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nanamiya H, Kasai K, Nozawa A, Yun CS, Narisawa T, Murakami K, Natori Y, Kawamura F, Tozawa Y. 2008. Identification and functional analysis of novel (p)ppGpp synthetase genes in Bacillus subtilis. Mol Microbiol 67:291–304. doi: 10.1111/j.1365-2958.2007.06018.x. [DOI] [PubMed] [Google Scholar]
- 37.Das B, Pal RR, Bag S, Bhadra RK. 2009. Stringent response in Vibrio cholerae: genetic analysis of spoT gene function and identification of a novel (p)ppGpp synthetase gene. Mol Microbiol 72:380–398. doi: 10.1111/j.1365-2958.2009.06653.x. [DOI] [PubMed] [Google Scholar]
- 38.Lemos JA, Lin VK, Nascimento MM, Abranches J, Burne RA. 2007. Three gene products govern (p)ppGpp production by Streptococcus mutans. Mol Microbiol 65:1568–1581. doi: 10.1111/j.1365-2958.2007.05897.x. [DOI] [PubMed] [Google Scholar]
- 39.Goodell W, Tomasz A. 1980. Alteration of Escherichia coli murein during amino acid starvation. J Bacteriol 144:1009–1016. doi: 10.1128/JB.144.3.1009-1016.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kusser W, Ishiguro EE. 1985. Involvement of the relA gene in the autolysis of Escherichia coli induced by inhibitors of peptidoglycan biosynthesis. J Bacteriol 164:861–865. doi: 10.1128/JB.164.2.861-865.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Joseleau-Petit D, Thévenet D, D'Ari R. 1994. ppGpp concentration, growth without PBP2 activity, and growth-rate control in Escherichia coli. Mol Microbiol 13:911–917. doi: 10.1111/j.1365-2958.1994.tb00482.x. [DOI] [PubMed] [Google Scholar]
- 42.Maisonneuve E, Gerdes K. 2014. Molecular mechanisms underlying bacterial persisters. Cell 157:539–548. doi: 10.1016/j.cell.2014.02.050. [DOI] [PubMed] [Google Scholar]
- 43.Viducic D, Ono T, Murakami K, Susilowati H, Kayama S, Hirota K, Miyake Y. 2006. Functional analysis of spoT, relA and dksA genes on quinolone tolerance in Pseudomonas aeruginosa under nongrowing condition. Microbiol Immunol 50:349–357. doi: 10.1111/j.1348-0421.2006.tb03793.x. [DOI] [PubMed] [Google Scholar]
- 44.Amato SM, Orman MA, Brynildsen MP. 2013. Metabolic control of persister formation in Escherichia coli. Mol Cell 50:475–487. doi: 10.1016/j.molcel.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 45.Nguyen D, Joshi-Datar A, Lepine F, Bauerle E, Olakanmi O, Beer K, McKay G, Siehnel R, Schafhauser J, Wang Y, Britigan BE, Singh PK. 2011. Active starvation responses mediate antibiotic tolerance in biofilms and nutrient-limited bacteria. Science 334:982–986. doi: 10.1126/science.1211037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bernier SP, Surette MG. 2013. Concentration-dependent activity of antibiotics in natural environments. Front Microbiol 4:20. doi: 10.3389/fmicb.2013.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maisonneuve E, Castro-Camargo M, Gerdes K. 2013. (p)ppGpp controls bacterial persistence by stochastic induction of toxin-antitoxin activity. Cell 154:1140–1150. doi: 10.1016/j.cell.2013.07.048. [DOI] [PubMed] [Google Scholar]
- 48.Tuomanen E, Cozens R, Tosch W, Zak O, Tomasz A. 1986. The rate of killing of Escherichia coli by beta-lactam antibiotics is strictly proportional to the rate of bacterial growth. J Gen Microbiol 132:1297–1304. doi: 10.1099/00221287-132-5-1297. [DOI] [PubMed] [Google Scholar]
- 49.Pontes MH, Groisman EA. 2019. Slow growth determines nonheritable antibiotic resistance in Salmonella enterica. Sci Signal 12:eaax3938. doi: 10.1126/scisignal.aax3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaldalu N, Tenson T. 2019. Slow growth causes bacterial persistence. Sci Signal 12:eaay1167. doi: 10.1126/scisignal.aay1167. [DOI] [PubMed] [Google Scholar]
- 51.Xiao H, Kalman M, Ikehara K, Zemel S, Glaser G, Cashel M. 1991. Residual guanosine 3',5'-bispyrophosphate synthetic activity of relA null mutants can be eliminated by spoT null mutations. J Biol Chem 266:5980–5990. [PubMed] [Google Scholar]
- 52.Schäkermann M, Langklotz S, Narberhaus F. 2013. FtsH-mediated coordination of lipopolysaccharide biosynthesis in Escherichia coli correlates with the growth rate and the alarmone (p)ppGpp. J Bacteriol 195:1912–1919. doi: 10.1128/JB.02134-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yamaguchi T, Iida K, Shiota S, Nakayama H, Yoshida S. 2015. Elevated guanosine 5'-diphosphate 3'-diphosphate level inhibits bacterial growth and interferes with FtsZ assembly. FEMS Microbiol Lett 362:fnv187. doi: 10.1093/femsle/fnv187. [DOI] [PubMed] [Google Scholar]
- 54.Bokinsky G, Baidoo EE, Akella S, Burd H, Weaver D, Alonso-Gutierrez J, García-Martín H, Lee TS, Keasling JD. 2013. HipA-triggered growth arrest and β-lactam tolerance in Escherichia coli are mediated by RelA-dependent ppGpp synthesis. J Bacteriol 195:3173–3182. doi: 10.1128/JB.02210-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Page R, Peti W. 2016. Toxin-antitoxin systems in bacterial growth arrest and persistence. Nat Chem Biol 12:208–214. doi: 10.1038/nchembio.2044. [DOI] [PubMed] [Google Scholar]
- 56.Song S, Wood TK. 2020. Toxin/antitoxin system paradigms: toxins bound to antitoxins are not likely activated by preferential antitoxin degradation. Adv Biosyst 4:1900290. doi: 10.1002/adbi.201900290. [DOI] [PubMed] [Google Scholar]
- 57.Ogura T, Hiraga S. 1983. Mini-F plasmid genes that couple host cell division to plasmid proliferation. Proc Natl Acad Sci U S A 80:4784–4788. doi: 10.1073/pnas.80.15.4784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pecota DC, Wood TK. 1996. Exclusion of T4 phage by the hok/sok killer locus from plasmid R1. J Bacteriol 178:2044–2050. doi: 10.1128/jb.178.7.2044-2050.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim Y, Wang X, Ma Q, Zhang X-S, Wood TK. 2009. Toxin-antitoxin systems in Escherichia coli influence biofilm formation through YjgK (TabA) and fimbriae. J Bacteriol 191:1258–1267. doi: 10.1128/JB.01465-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ren D, Bedzyk LA, Thomas SM, Ye RW, Wood TK. 2004. Gene expression in Escherichia coli biofilms. Appl Microbiol Biotechnol 64:515–524. doi: 10.1007/s00253-003-1517-y. [DOI] [PubMed] [Google Scholar]
- 61.Yang QE, Walsh TR. 2017. Toxin-antitoxin systems and their role in disseminating and maintaining antimicrobial resistance. FEMS Microbiol Rev 41:343–353. doi: 10.1093/femsre/fux006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fernández-García L, Blasco L, Lopez M, Bou G, García-Contreras R, Wood T, Tomas M. 2016. Toxin-antitoxin systems in clinical pathogens. Toxins (Basel) 8:227. doi: 10.3390/toxins8070227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Moyed HS, Bertrand KP. 1983. hipA, a newly recognized gene of Escherichia coli K-12 that affects frequency of persistence after inhibition of murein synthesis. J Bacteriol 155:768–775. doi: 10.1128/JB.155.2.768-775.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Aizenman E, Engelberg-Kulka H, Glaser G. 1996. An Escherichia coli chromosomal “addiction module” regulated by guanosine [corrected] 3',5'-bispyrophosphate: a model for programmed bacterial cell death. Proc Natl Acad Sci U S A 93:6059–6063. doi: 10.1073/pnas.93.12.6059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Korch SB, Henderson TA, Hill TM. 2003. Characterization of the hipA7 allele of Escherichia coli and evidence that high persistence is governed by (p)ppGpp synthesis. Mol Microbiol 50:1199–1213. doi: 10.1046/j.1365-2958.2003.03779.x. [DOI] [PubMed] [Google Scholar]
- 66.Correia FF, D'Onofrio A, Rejtar T, Li L, Karger BL, Makarova K, Koonin EV, Lewis K. 2006. Kinase activity of overexpressed HipA is required for growth arrest and multidrug tolerance in Escherichia coli. J Bacteriol 188:8360–8367. doi: 10.1128/JB.01237-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schumacher MA, Piro KM, Xu W, Hansen S, Lewis K, Brennan RG. 2009. Molecular mechanisms of HipA-mediated multidrug tolerance and its neutralization by HipB. Science 323:396–401. doi: 10.1126/science.1163806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Germain E, Castro-Roa D, Zenkin N, Gerdes K. 2013. Molecular mechanism of bacterial persistence by HipA. Mol Cell 52:248–254. doi: 10.1016/j.molcel.2013.08.045. [DOI] [PubMed] [Google Scholar]
- 69.Magnusson LU, Farewell A, Nyström T. 2005. ppGpp: a global regulator in Escherichia coli. Trends Microbiol 13:236–242. doi: 10.1016/j.tim.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 70.Srivatsan A, Wang JD. 2008. Control of bacterial transcription, translation and replication by (p)ppGpp. Curr Opin Microbiol 11:100–105. doi: 10.1016/j.mib.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 71.Gerdes K, Maisonneuve E. 2012. Bacterial persistence and toxin-antitoxin loci. Annu Rev Microbiol 66:103–123. doi: 10.1146/annurev-micro-092611-150159. [DOI] [PubMed] [Google Scholar]
- 72.Maisonneuve E, Shakespeare LJ, Jørgensen MG, Gerdes K. 2011. Bacterial persistence by RNA endonucleases. Proc Natl Acad Sci U S A 108:13206–13211. doi: 10.1073/pnas.1100186108. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 73.Anonymous. 2018. Retraction for Maisonneuve et al., Bacterial persistence by RNA endonucleases. Proc Natl Acad Sci U S A 115:E2901. doi: 10.1073/pnas.1803278115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Osbourne DO, Soo VWC, Konieczny I, Wood TK. 2014. Polyphosphate, cyclic AMP, guanosine tetraphosphate, and c-di-GMP reduce in vitro Lon activity. Bioengineered 5:264–268. doi: 10.4161/bioe.29261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chowdhury N, Kwan BW, Wood TK. 2016. Persistence increases in the absence of the alarmone guanosine tetraphosphate by reducing cell growth. Sci Rep 6:20519. doi: 10.1038/srep20519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Alav I, Sutton JM, Rahman KM. 2018. Role of bacterial efflux pumps in biofilm formation. J Antimicrob Chemother 73:2003–2020. doi: 10.1093/jac/dky042. [DOI] [PubMed] [Google Scholar]
- 77.Ge X, Cai Y, Chen Z, Gao S, Geng X, Li Y, Li Y, Jia J, Sun Y. 2018. Bifunctional enzyme SpoT is involved in biofilm formation of Helicobacter pylori with multidrug resistance by upregulating efflux pump Hp1174 (gluP). (Antimicrob Agents Chemother 62:e00957-18. doi: 10.1128/AAC.00957-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chuang YM, Bandyopadhyay N, Rifat D, Rubin H, Bader JS, Karakousis PC. 2015. Deficiency of the novel exopolyphosphatase Rv1026/PPX2 leads to metabolic downshift and altered cell wall permeability in Mycobacterium tuberculosis. mBio 6:e02428. doi: 10.1128/mBio.02428-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pu Y, Zhao Z, Li Y, Zou J, Ma Q, Zhao Y, Ke Y, Zhu Y, Chen H, Baker MAB, Ge H, Sun Y, Xie XS, Bai F. 2016. Enhanced efflux activity facilitates drug tolerance in dormant bacterial cells. Mol Cell 62:284–294. doi: 10.1016/j.molcel.2016.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Khakimova M, Ahlgren HG, Harrison JJ, English AM, Nguyen D. 2013. The stringent response controls catalases in Pseudomonas aeruginosa and is required for hydrogen peroxide and antibiotic tolerance. J Bacteriol 195:2011–2020. doi: 10.1128/JB.02061-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Molina-Quiroz RC, Silva-Valenzuela C, Brewster J, Castro-Nallar E, Levy SB, Camilli A. 2018. Cyclic AMP regulates bacterial persistence through repression of the oxidative stress response and SOS-dependent DNA repair in uropathogenic Escherichia coli. mBio 9:e02144-17. doi: 10.1128/mBio.02144-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Song S, Wood TK. 2020. ppGpp ribosome dimerization model for bacterial persister formation and resuscitation. Biochem Biophys Res Commun 523:281–286. doi: 10.1016/j.bbrc.2020.01.102. [DOI] [PubMed] [Google Scholar]
- 83.Prossliner T, Skovbo Winther K, Sørensen MA, Gerdes K. 2018. Ribosome hibernation. Annu Rev Genet 52:321–348. doi: 10.1146/annurev-genet-120215-035130. [DOI] [PubMed] [Google Scholar]
- 84.Wood A, Irving SE, Bennison DJ, Corrigan RM. 2019. The (p)ppGpp-binding GTPase Era promotes rRNA processing and cold adaptation in Staphylococcus aureus. PLoS Genet 15:e1008346. doi: 10.1371/journal.pgen.1008346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Libby EA, Reuveni S, Dworkin J. 2019. Multisite phosphorylation drives phenotypic variation in (p)ppGpp synthetase-dependent antibiotic tolerance. Nat Commun 10:5133. doi: 10.1038/s41467-019-13127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tagami K, Nanamiya H, Kazo Y, Maehashi M, Suzuki S, Namba E, Hoshiya M, Hanai R, Tozawa Y, Morimoto T, Ogasawara N, Kageyama Y, Ara K, Ozaki K, Yoshida M, Kuroiwa H, Kuroiwa T, Ohashi Y, Kawamura F. 2012. Expression of a small (p)ppGpp synthetase, YwaC, in the (p)ppGpp(0) mutant of Bacillus subtilis triggers YvyD-dependent dimerization of ribosome. Microbiologyopen 1:115–134. doi: 10.1002/mbo3.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yan J, Bassler BL. 2019. Surviving as a community: antibiotic tolerance and persistence in bacterial biofilms. Cell Host Microbe 26:15–21. doi: 10.1016/j.chom.2019.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pacios O, Blasco L, Bleriot I, Fernandez-Garcia L, González Bardanca M, Ambroa A, López M, Bou G, Tomás M. 2020. Strategies to combat multidrug-resistant and persistent infectious diseases. Antibiotics (Basel) 9:65. doi: 10.3390/antibiotics9020065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hobbs JK, Boraston AB. 2019. (p)ppGpp and the stringent response: an emerging threat to antibiotic therapy. ACS Infect Dis 5:1505–1517. doi: 10.1021/acsinfecdis.9b00204. [DOI] [PubMed] [Google Scholar]
- 90.Wexselblatt E, Oppenheimer-Shaanan Y, Kaspy I, London N, Schueler-Furman O, Yavin E, Glaser G, Katzhendler J, Ben-Yehuda S. 2012. Relacin, a novel antibacterial agent targeting the stringent response. PLoS Pathog 8:e1002925. doi: 10.1371/journal.ppat.1002925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Syal K, Flentie K, Bhardwaj N, Maiti K, Jayaraman N, Stallings CL, Chatterji D. 2017. Synthetic (p)ppGpp analogue is an inhibitor of stringent response in mycobacteria. Antimicrob Agents Chemother 61:e00443-17. doi: 10.1128/AAC.00443-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dutta N, Klinkenberg L, Vázquez M-J, Segura-Carro D, Colmenarejo G, Ramón F, Rodriguez-Miquel B, Mata-Cantero L, Porras-De Francisco E, Chuang Y-M, Rubin H, Lee J, Eoh H, Bader J, Perez-Herran E, Mendoza-Losana A, Karakousis P. 2019. Inhibiting the stringent response blocks Mycobacterium tuberculosis entry into quiescence and reduces persistence. Sci Adv 5:eaav2104. doi: 10.1126/sciadv.aav2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.de la Fuente-Núñez C, Reffuveille F, Haney EF, Straus SK, Hancock RE. 2014. Broad-spectrum anti-biofilm peptide that targets a cellular stress response. PLoS Pathog 10:e1004152. doi: 10.1371/journal.ppat.1004152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.de la Fuente-Núñez C, Korolik V, Bains M, Nguyen U, Breidenstein EB, Horsman S, Lewenza S, Burrows L, Hancock RE. 2012. Inhibition of bacterial biofilm formation and swarming motility by a small synthetic cationic peptide. Antimicrob Agents Chemother 56:2696–2704. doi: 10.1128/AAC.00064-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Andresen L, Tenson T, Hauryliuk V. 2016. Cationic bactericidal peptide 1018 does not specifically target the stringent response alarmone (p)ppGpp. Sci Rep 6:36549. doi: 10.1038/srep36549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Allison KR, Brynildsen MP, Collins JJ. 2011. Metabolite-enabled eradication of bacterial persisters by aminoglycosides. Nature 473:216–220. doi: 10.1038/nature10069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bhaskar A, De Piano C, Gelman E, McKinney JD, Dhar N. 2018. Elucidating the role of (p)ppGpp in mycobacterial persistence against antibiotics. IUBMB Life 70:836–844. doi: 10.1002/iub.1888. [DOI] [PubMed] [Google Scholar]
- 98.Cameron DR, Shan Y, Zalis EA, Isabella V, Lewis K. 2018. A genetic determinant of persister cell formation in bacterial pathogens. J Bacteriol 200:e00303-18. doi: 10.1128/JB.00303-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Svenningsen MS, Veress A, Harms A, Mitarai N, Semsey S. 2019. Birth and resuscitation of (p)ppGpp induced antibiotic tolerant persister cells. Sci Rep 9:6056. doi: 10.1038/s41598-019-42403-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dörr T, Vulić M, Lewis K. 2010. Ciprofloxacin causes persister formation by inducing the TisB toxin in Escherichia coli. PLoS Biol 8:e1000317. doi: 10.1371/journal.pbio.1000317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Conlon BP, Rowe SE, Gandt AB, Nuxoll AS, Donegan NP, Zalis EA, Clair G, Adkins JN, Cheung AL, Lewis K. 2016. Persister formation in Staphylococcus aureus is associated with ATP depletion. Nat Microbiol 1:16051. doi: 10.1038/nmicrobiol.2016.51. [DOI] [PubMed] [Google Scholar]
- 102.Shan Y, Brown Gandt A, Rowe SE, Deisinger JP, Conlon BP, Lewis K. 2017. ATP-dependent persister formation in Escherichia coli. mBio 8:e02267-16. doi: 10.1128/mBio.02267-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cheng HY, Soo VW, Islam S, McAnulty MJ, Benedik MJ, Wood TK. 2014. Toxin GhoT of the GhoT/GhoS toxin/antitoxin system damages the cell membrane to reduce adenosine triphosphate and to reduce growth under stress. Environ Microbiol 16:1741–1754. doi: 10.1111/1462-2920.12373. [DOI] [PubMed] [Google Scholar]
- 104.Hobby GL, Meyer K, Chaffee E. 1942. Observations on the mechanism of action of penicillin. Exp Biol Med 50:281–285. doi: 10.3181/00379727-50-13773. [DOI] [Google Scholar]
- 105.Bigger JW. 1944. Treatment of staphylococcal infections with penicillin by intermittent sterilisation. Lancet 244:497–500. doi: 10.1016/S0140-6736(00)74210-3. [DOI] [Google Scholar]
- 106.Blasco L, Ambroa A, Trastoy R, Bleriot I, Moscoso M, Fernández-Garcia L, Perez-Nadales E, Fernández-Cuenca F, Torre-Cisneros J, Oteo-Iglesias J, Oliver A, Canton R, Kidd T, Navarro F, Miró E, Pascual A, Bou G, Martínez-Martínez L, Tomas M. 2020. In vitro and in vivo efficacy of combinations of colistin and different endolysins against clinical strains of multi-drug resistant pathogens. Sci Rep 10:7163. doi: 10.1038/s41598-020-64145-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Schumacher MA, Balani P, Min J, Chinnam NB, Hansen S, Vulic M, Lewis K, Brennan RG. 2015. HipBA-promoter structures reveal the basis of heritable multidrug tolerance. Nature 524:59–64. doi: 10.1038/nature14662. [DOI] [PMC free article] [PubMed] [Google Scholar]