Staphylococcus argenteus infection was initially described in Aboriginal patients in the Northern Territories of Australia as a predominant cause of skin infections and is rare outside Southeast Asia. A first well-characterized case of S. argenteus infection has now been described in the United States, involving a recurrent hemodialysis catheter infection, in which unstable daptomycin resistance evolved during daptomycin therapy. The unique colonial pigmentation of S. argenteus isolates in strains otherwise identified as Staphylococcus aureus is noteworthy.
KEYWORDS: Staphylococcus argenteus, daptomycin resistance
ABSTRACT
Staphylococcus argenteus infection was initially described in Aboriginal patients in the Northern Territories of Australia as a predominant cause of skin infections and is rare outside Southeast Asia. A first well-characterized case of S. argenteus infection has now been described in the United States, involving a recurrent hemodialysis catheter infection, in which unstable daptomycin resistance evolved during daptomycin therapy. The unique colonial pigmentation of S. argenteus isolates in strains otherwise identified as Staphylococcus aureus is noteworthy.
INTRODUCTION
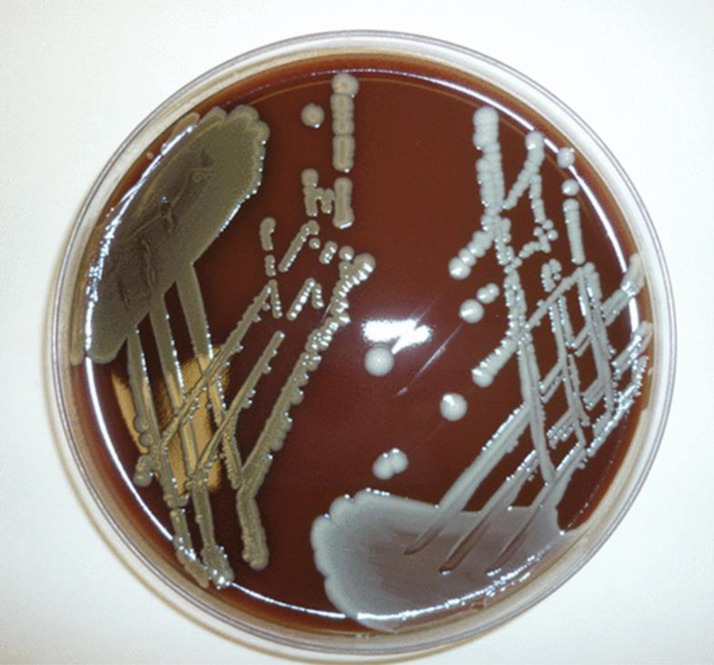
Among recent changes in microbiological taxonomy, Staphylococcus aureus is now recognized as an S. aureus-related complex, comprising S. aureus, Staphylococcus argenteus, and Staphylococcus schweitzeri (1). Key questions for clinicians relate to the degree with which the epidemiology, clinical manifestations, and response to therapy are similar or distinct between these species and, thus, whether respective infections should be managed differently. First observed as a divergent S. aureus genotype usually associated with skin infections in northern Australian Aboriginal populations (2), S. argenteus isolates have now been identified globally. As suggested by its name, S. argenteus does not demonstrate the typical golden colony appearance on laboratory culture because it lacks the gene operon for the carotenoid pigment staphyloxanthin (Fig. 1), giving it the characteristic white colonial phenotype (3). In retrospective analyses, the proportion of isolates previously identified as S. aureus that were in fact S. argenteus ranged from 0.16% (3/1,903) in Belgium (4) to 12% (47/394) among blood culture isolates in Taiwan (5) and 19% (58/311) of sepsis-related isolates in Thailand (6). S. argenteus infection is typically methicillin susceptible but is capable of acquiring genes mediating methicillin and other antibiotic resistances (5, 6). In fact, in the Australian Aboriginal populations (where antibiotic use is high), methicillin-resistant S. argenteus outnumbered methicillin-resistant S. aureus (MRSA) infections; moreover, S. argenteus isolates were more likely than MRSA isolates to be resistant to multiple antibiotic classes (7).
FIG 1.
Comparison of colony pigmentation on chocolate agar for Staphylococcus aureus (left) and Staphylococcus argenteus (right). (Republished from reference 3 with permission of the publisher.)
There are a number of noteworthy microbiological features in the case reported by Hao et al. (8). (i) This is the first well-described clinical case of S. argenteus infection in the United States. (ii) The strain was misidentified as methicillin-susceptible S. aureus by matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS). With the inclusion of S. argenteus in some newer commercial MALDI-TOF MS databases, clinical microbiology laboratories should be able to distinguish S. aureus from S. argenteus isolates. However, the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) has recommended not distinguishing species within the S. aureus-related complex for routine reporting until there is evidence that pathogenicity or clinical outcomes differ markedly between species (9). (iii) In contradistinction to the majority experience in northern Australia concerning S. argenteus infections among Aboriginal patients (in which nonbacteremic skin and soft tissue infections have predominated) (2), the current patient’s clinical course featured health care-associated (hemodialysis catheter) bacteremia. To this point, although relatively uncommon, selected reports have confirmed invasive endovascular infections, including vascular graft syndromes, caused by S. argenteus (10). (iv) The microbiology laboratory in this medical center apparently did not observe the typical pure white colonies of S. argenteus isolates on their blood culture plates (Fig. 1). (v) Until the present report from Hao and colleagues (8), S. argenteus resistance to daptomycin or vancomycin was not documented. (vi) The emergence of a single nucleotide polymorphism mutation in the mprF gene (as delineated by whole-genome sequencing) accompanied the evolution of increased MICs to daptomycin into the nonsusceptible range (4 μg/ml).
The latter event would not be unexpected in this particular patient given the lack of source control in a probable high-inoculum infection (vascular device) plus the repetitive courses of daptomycin. Although the MprF mutation described (S337L) occurs within one of the classic bifunctional domain hot spots of this protein (11, 12), the instability of this mutation was surprising. As pointed out by the authors, in S. aureus (usually MRSA) infection, daptomycin nonsusceptibility is a stable phenotype, most frequently linked to acquisition of mprF mutations (13). This genetic phenomenon has been seen clinically (as in this case), complicating daptomycin therapy of high-inoculum infections occurring at anatomic sites that are notoriously difficult to eradicate (e.g., endocarditis, osteomyelitis) (13). In fact, when S. aureus strains are serially passaged in vitro in daptomycin, mprF mutations are invariably the first ones induced, followed by rpoB,C and yycFG (14). Most investigations have ascribed the mechanism(s) of daptomycin resistance related to mprF polymorphisms to be based in gain-in-function enhancement of lysinylation of phosphatidylglycerol in the cell membrane of staphylococci, as well as its increased translocation of this positively charged phospholipid to the outer cell membrane leaflet (13, 15). This would, in turn, theoretically create a relatively more positively charged surface, with a resultant charge-repulsive milieu that could repel calcium-complexed daptomycin (15). However, recent data from Ernst et al. (11) have implicated a non-surface-charge-associated mechanism for daptomycin resistance in strains with mprF mutations, i.e., a specific cell membrane biophysical perturbation that appears to allow for selective daptomycin efflux. Unfortunately, the instability of the mprF mutation in the current S. argenteus infection precludes further understanding of the mechanism of daptomycin resistance in this case.
An unanswered question remains. Were there any other S. argenteus strains isolated temporally at their medical center in and around the time of this patient’s multiple admissions? The same question may be asked concerning other hemodialysis patients at the inpatient or outpatient facilities utilized by this patient. The community and nosocomial life cycles of S. argenteus isolates are poorly understood; thus, any insights in this regard may be enlightening.
ACKNOWLEDGMENTS
A.S.B. is supported in part by grants from the National Institutes of Allergy and Infectious Diseases (NIAID) (5R01AI130056 and 1R01-AI146078). S.Y.C.T. is an Australian National Health and Medical Research Council Career Development Fellow (1145033).
We have no conflicts of interest to declare.
Footnotes
For the case presentation, see https://doi.org/10.1128/AAC.00961-20.
REFERENCES
- 1.Tong SY, Schaumburg F, Ellington MJ, Corander J, Pichon B, Leendertz F, Bentley SD, Parkhill J, Holt DC, Peters G, Giffard PM. 2015. Novel staphylococcal species that form part of a Staphylococcus aureus-related complex: the non-pigmented Staphylococcus argenteus sp. nov. and the non-human primate-associated Staphylococcus schweitzeri sp. nov. Int J Syst Evol Microbiol 65:15–22. doi: 10.1099/ijs.0.062752-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tong SY, Sharma-Kuinkel BK, Thaden JT, Whitney AR, Yang SJ, Mishra NN, Rude T, Lilliebridge RA, Selim MA, Ahn SH, Holt DC, Giffard PM, Bayer AS, Deleo FR, Fowler VG Jr. 2013. Virulence of endemic nonpigmented northern Australian Staphylococcus aureus clone (clonal complex 75, S. argenteus) is not augmented by staphyloxanthin. J Infect Dis 208:520–527. doi: 10.1093/infdis/jit173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holt DC, Holden MT, Tong SY, Castillo-Ramirez S, Clarke L, Quail MA, Currie BJ, Parkhill J, Bentley SD, Feil EJ, Giffard PM. 2011. A very early-branching Staphylococcus aureus lineage lacking the carotenoid pigment staphyloxanthin. Genome Biol Evol 3:881–895. doi: 10.1093/gbe/evr078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Argudin MA, Dodemont M, Vandendriessche S, Rottiers S, Tribes C, Roisin S, de Mendonca R, Nonhoff C, Deplano A, Denis O. 2016. Low occurrence of the new species Staphylococcus argenteus in a Staphylococcus aureus collection of human isolates from Belgium. Eur J Clin Microbiol Infect Dis 35:1017–1022. doi: 10.1007/s10096-016-2632-x. [DOI] [PubMed] [Google Scholar]
- 5.Chen SY, Lee H, Wang XM, Lee TF, Liao CH, Teng LJ, Hsueh PR. 2018. High mortality impact of Staphylococcus argenteus on patients with community-onset staphylococcal bacteraemia. Int J Antimicrob Agents 52:747–753. doi: 10.1016/j.ijantimicag.2018.08.017. [DOI] [PubMed] [Google Scholar]
- 6.Chantratita N, Wikraiphat C, Tandhavanant S, Wongsuvan G, Ariyaprasert P, Suntornsut P, Thaipadungpanit J, Teerawattanasook N, Jutrakul Y, Srisurat N, Chaimanee P, Anukunananchai J, Phiphitaporn S, Srisamang P, Chetchotisakd P, West TE, Peacock SJ. 2016. Comparison of community-onset Staphylococcus argenteus and Staphylococcus aureus sepsis in Thailand: a prospective multicentre observational study. Clin Microbiol Infect 22:458.e11. doi: 10.1016/j.cmi.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McDonald M, Dougall A, Holt D, Huygens F, Oppedisano F, Giffard PM, Inman-Bamber J, Stephens AJ, Towers R, Carapetis JR, Currie BJ. 2006. Use of a single-nucleotide polymorphism genotyping system to demonstrate the unique epidemiology of methicillin-resistant Staphylococcus aureus in remote Aboriginal communities. J Clin Microbiol 44:3720–3727. doi: 10.1128/JCM.00836-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hao S, Abdelghany M, Lyden A, Sit R, Tan M, Tato CM, DeRisi JL, Miller S, Doernberg SB, Langelier C. 2020. Genomic profiling of evolving daptomycin resistance in a patient with recurrent Staphylococcus argenteus sepsis. Antimicrob Agent Chemother 64:e00961-20. doi: 10.1128/AAC.00961-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Becker K, Schaumburg F, Kearns A, Larsen AR, Lindsay JA, Skov RL, Westh H. 2019. Implications of identifying the recently defined members of the Staphylococcus aureus complex S. argenteus and S. schweitzeri: a position paper of members of the ESCMID Study Group for Staphylococci and Staphylococcal Diseases (ESGS). Clin Microbiol Infect 25:1064–1070. doi: 10.1016/j.cmi.2019.02.028. [DOI] [PubMed] [Google Scholar]
- 10.Chu C, Wong MY, Tseng YH, Lin CL, Tung CW, Kao CC, Huang YK. 2019. Vascular access infection by Staphylococcus aureus from removed dialysis accesses. Microbiologyopen 8:e00800. doi: 10.1002/mbo3.800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ernst CM, Slavetinsky CJ, Kuhn S, Hauser JN, Nega M, Mishra NN, Gekeler C, Bayer AS, Peschel A. 2018. Gain-of-function mutations in the phospholipid flippase MprF confer specific daptomycin resistance. mBio 9:e01659-18. doi: 10.1128/mBio.01659-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ernst CM, Staubitz P, Mishra NN, Yang SJ, Hornig G, Kalbacher H, Bayer AS, Kraus D, Peschel A. 2009. The bacterial defensin resistance protein MprF consists of separable domains for lipid lysinylation and antimicrobial peptide repulsion. PLoS Pathog 5:e1000660. doi: 10.1371/journal.ppat.1000660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bayer AS, Schneider T, Sahl HG. 2013. Mechanisms of daptomycin resistance in Staphylococcus aureus: role of the cell membrane and cell wall. Ann N Y Acad Sci 1277:139–158. doi: 10.1111/j.1749-6632.2012.06819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedman L, Alder JD, Silverman JA. 2006. Genetic changes that correlate with reduced susceptibility to daptomycin in Staphylococcus aureus. Antimicrob Agents Chemother 50:2137–2145. doi: 10.1128/AAC.00039-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones T, Yeaman MR, Sakoulas G, Yang SJ, Proctor RA, Sahl HG, Schrenzel J, Xiong YQ, Bayer AS. 2008. Failures in clinical treatment of Staphylococcus aureus infection with daptomycin are associated with alterations in surface charge, membrane phospholipid asymmetry, and drug binding. AAC 52:269–278. doi: 10.1128/AAC.00719-07. [DOI] [PMC free article] [PubMed] [Google Scholar]