The mobile colistin resistance gene mcr-3 has globally disseminated since it was first reported in 2017 in Escherichia coli. In vitro mobilization assays in this study demonstrate the functionality of the composite transposon structure ISKpn40-mcr-3.11-dgkA-ISKpn40 in wild-type and recA− E. coli strains. These transpositions generated 4-bp duplications at the target sites.
KEYWORDS: transposition, mcr-3.11, ISKpn40, colistin resistance, transposition mechanism
ABSTRACT
The mobile colistin resistance gene mcr-3 has globally disseminated since it was first reported in 2017 in Escherichia coli. In vitro mobilization assays in this study demonstrate the functionality of the composite transposon structure ISKpn40-mcr-3.11-dgkA-ISKpn40 in wild-type and recA− E. coli strains. These transpositions generated 4-bp duplications at the target sites. This is the first report demonstrating the mobility of the mcr-3.11 gene by transposition.
TEXT
Colistin is one of the last lines of defense against multidrug-resistant (MDR) Gram-negative bacteria. The first plasmid-mediated colistin resistant mcr-1 gene was identified in November 2015, indicating the capacity of horizontal transfer of colistin resistance (1). Since then, 9 additional mcr genes have been identified (mcr-2 to mcr-10) (2–9).
The mcr-3 gene was first identified in Escherichia coli from a healthy pig fecal sample in Shandong Province, China (3). This gene shares 45 and 47% nucleotide sequence homology with mcr-1 and mcr-2, respectively (3). The mcr-3.11 gene, first identified in an E. coli isolate from a swine feedlot, had an upstream ISKpn40 insertion (3). ISKpn40 is an IS3, insertion sequence element family member of 1,213 bp and is flanked by 12-bp inverted repeats (IR) (5′-TGTAATGACCCA-3′). Other IS3 family members, such as IS911 and IS150, can transpose via circular intermediates (10, 11), and therefore transposition of mcr-3 via a circular form mediated by ISKpn40 has been postulated.
Previous studies have detected a 3,535-bp circle of mcr-3.1-dgkA-ISKpn40 using inverse PCR (12), suggesting that ISKpn40 might be involved in the mobilization of this resistance gene. Moreover, direct repeats (DRs) (5′-CACC-3′) were identified both immediately upstream and downstream of the ISKpn40-mcr-3.1-dgkA-ISKpn40 segment in two plasmids, pZR5_mcr-3 and pZR10_mcr3 (13). These previous studies suggested the ability of ISKpn40 to mobilize mcr-3.11; however, its putative role in the mobilization of the mcr-3.11 gene remains to be determined. In this current work, we aimed to close this gap in knowledge and determine experimentally if ISKpn40 can mobilize the mcr-3.11 gene.
A plasmid pYH01-TraJ, carrying an R6K ori which can only replicate in a pir+ host, was constructed by cloning the structure ISKpn40-mcr-3.11-dgkA-ISKpn40 along with its flanking sequence into plasmid pJS05. The pJS05 was constructed as follows. First, the RP4oriT conjugation transfer fragment was amplified using primers traj-1 and traj-2 (Table S2 in the supplemental material) using the plasmid pCVD442 (14) as a template. Then the RP4oriT conjugation transfer fragment and pJS01 (15) were both digested with speI and SalI and ligated to give rise to the recombinant plasmid pJS05. Next, the ISKpn40-mcr-3.11-dgkA-ISKpn40 element was amplified by PCR using primers pYH-AflII and pYH-BglII, and using E. coli 22FS3-1 genomic DNA as the template. E. coli 22FS3-1 is a clinical isolate from Foshan City, China carrying ISKpn40-mcr-3.11-dgkA-ISKpn40 confirmed by primers MCR3-F and MCR3-R. (Table S2). PCR primers pJS05-AflII and pJS05-BglII were used to amplify the backbone of pJS05 using pJS05 as the template that contained a conditional replication origin R6K, which relies on π protein encoded by the pir gene, CmR (chloramphenicol resistance), and an RP4oriT fragment. This suicide plasmid only survives in a bacterial host with the pir gene (e.g., E. coli WM 3064) and is unable to replicate in other hosts. Ligation of these fragments after digestion with the restriction enzymes noted in the primer names resulted in the recombinant plasmid pYH01-TraJ (Table 1, Fig. S1).
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Description | Reference |
|---|---|---|
| Strains | ||
| E. coli MG1655 (wild type) | K-12 strain F− λ− ilvG rfb-50 rph-1 | (19) |
| E. coli MG1655(recA∷Km) | K-12 strain F− λ− ilvG rfb-50 rph-1 recA- | (19) |
| E. coli WM3064 | RP4(tra) in chromosome, DAP- | (20) |
| E. coli 22FS3-1 | Clinical isolate carrying ISKpn40-mcr-3.11-dgkA-ISKpn40 | This study |
| Plasmids | ||
| pJS01 | Suicide plasmid (R6K replication origin) contains oriTRP4 fragment | (15) |
| pJS05 | Suicide plasmid (R6K replication origin) contains and chloramphenicol resistance gene (CmR) | This study |
| pYH01-traJ | Suicide plasmid (R6K replication origin) contains and ISKpn40-mcr-3.11-dgkA-ISKpn40 | This study |
The pYH01-TraJ plasmid was electroporated into E. coli WM3064 (16) and transformants were selected through plating on LB agar supplemented with 25 μg/ml chloramphenicol. The integrity of both ISKpn40 elements and mcr-3.11 were confirmed by DNA sequencing. E. coli WM3064 is a pir+ diaminopimelic acid auxotroph (DAP) and contains the RP4 transfer machinery necessary for conjugation. Next, the suicide plasmid was conjugated into two recipient strains, E. coli MG1655 (wild type) and E. coli MG1655 (recA∷Km). The survival of the transconjugants was contingent upon transposition of the selectable marker into the host genome. The transposition frequencies of pYH01-TraJ into the two E. coli strains were 2.85 × 10−6 and 2.53 × 10−6 per transformed cell, respectively.
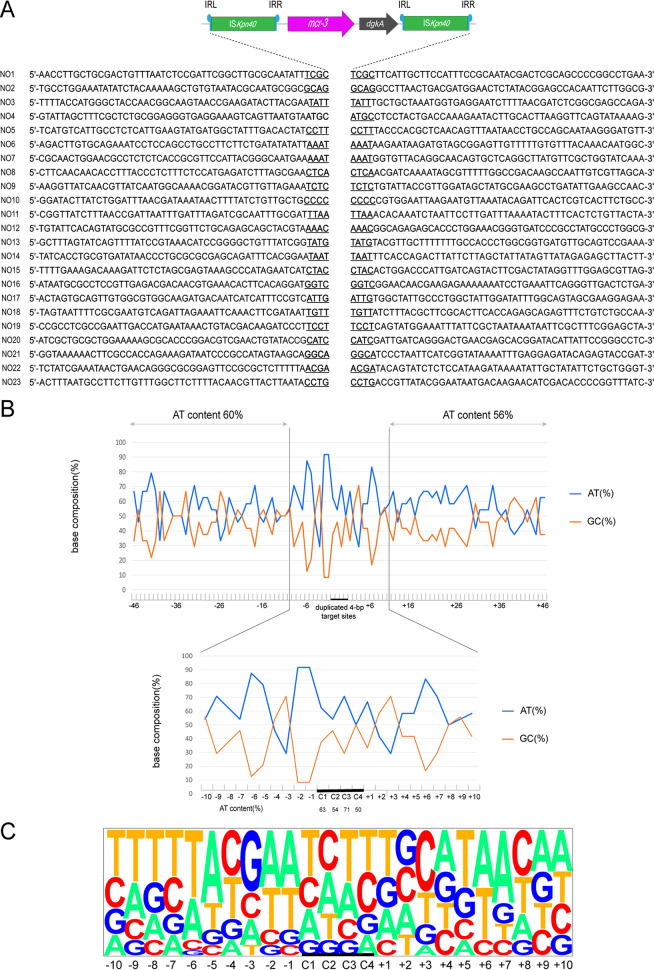
Arbitrary primed PCR-based analyses (17) revealed 23 integration sites of transposon ISKpn40-mcr-3.11-dgkA-ISKpn40. The insertion locations of the mcr-3.11 gene were further confirmed by PCR and Sanger sequencing. All primers are listed in Table S1. The results showed that all transposition events were separated by 4-bp direct repeats (DRs) at the insertion sites (Fig. 1). The mean AT content extending in each direction from the 4-bp target sites (−46 to −1 bp and +1 to +46 bp) were 60 and 56%, respectively (Fig. 1A). In addition, the AT content was higher in the sequences closer to the target site, and was 92% at positions −2 and −1 and 85% at positions −6 and +6. At the duplicated target site positions (C1, C2, C3, and C4), the AT content was 63%, 54%, 71%, and 50%, respectively (Fig. 1B).
FIG 1.
Target site analyses of 23 transposition events. (A) Molecular characterization of 23 transposition events of transposons in E. coli MG1655 (recA∷Km). The duplicated 4-bp target sites are underlined in the context of the surrounding 46 nucleotides upstream and downstream of the target sites. (B) Statistical analyses of the 23 transposition sites. The percentage of AT and GC at each position from 46 nucleotides upstream to 46 nucleotides downstream of the target sites are shown. The 4-bp duplicated target sites (C1, C2, C3, and C4) are indicated by black bars. The AT and GC percentages of regions spanning positions –46 to –1 bp and positions +1 to +46 bp and that of the region spanning positions –10 to +10 bp are indicated in the upper and lower graphs, respectively. (C) Pictogram showing the relative frequencies of each A, T, C, and G at the target site deduced from the 23 experimental transposition events.
To further characterize the distribution of ISKpn40-mediated mcr-3.11 transposition, we determined the insertion sites for 23 transposon events in E. coli MG1655. We found that 18 ISKpn40-mcr-3.11-dgkA-ISKpn40 sites were randomly located into nonessential genes and 5 were inserted between two nonessential genes in the bacterial chromosome (Table S5). That this study only found insertions into nonessential genes may be because insertions into essential genes are deleterious and/or may negatively impact growth, and therefore cannot be selected on plates to be identified. Finally, we verified those sites containing the whole ISKpn40-mcr-3.11-dgkA-ISKpn40 genetic structure in the genome of E. coli MG1655 by PCR with primers (Table S3) binding the upstream and downstream sequences, respectively.
In this study, we demonstrated the functionality of ISKpn40-mcr-3.11-dgkA-ISKpn40 transposition from plasmids where cell survival was dependent on transposition of the mcr-3.11 selective marker. This ISKpn40-mcr-3.11-dgkA-ISKpn40 structure from plasmid pYH01-TraJ can transpose efficiently and randomly into the E. coli chromosome. Notably, several transposases and IS elements, including IS4321, IS26, ΔTnAs2, or the ISKpn40 have been identified in the flanking regions of mcr-3.1 (13). ISKpn40 is present in E. coli, Salmonella enterica, Aeromonas caviae, A. veronii, and Klebsiella pneumoniae (Table S4). The ISKpn40-mediated translocation of mcr-3.11 may accelerate transmission of mcr-3.11 among these species, as ISKpn40 can efficiently mobilize mcr-3.11 (this study). Conversely, the transposition of mcr-1 has always been associated with ISApl1, and no other insertion sites were identified in flanking regions (15, 18).
The association of multiple insertion elements in mcr-3.11 also suggests the possibility that the new mobile colistin resistance gene mcr-3.11 may utilize different transposons to mobilize. In this study, we confirmed the functionality of ISKpn40; however, whether and how other IS elements and transposons contribute to the mobility of mcr-3 in different species or genera has yet to be determined. This work demonstrates the effective mobilization of the mcr-3.11 gene into the E. coli chromosome mediated by ISKpn40. Interestingly, the ISKpn40-mcr-3.11-dgkA-ISKpn40 structure was incorporated randomly in or near nonessential genes with no obvious preference for GC- or AT-rich DNA domains.
In summary, we verified that transposition of mcr-3.11 is mediated by ISKpn40. Our work demonstrates that ISKpn40 can transpose mcr-3.11 in E. coli. This is especially important for other clinically relevant bacterial species in the Enterobacteriaceae family, in which ISKpn40 is present, for possible translocation of mcr-3.11. Future studies will focus on the regulatory mechanisms of ISKpn40-mcr-3.11-dgkA-ISKpn40 transposition. Additional future work will focus on the other routes of mcr-3.11 gene transmission to elucidate these pathways and help to control the spread of mcr-3.11 and colistin resistance.
MATERIALS AND METHODS
Data availability.
The nucleotide sequence of amplicon ISKpn40-mcr-3.11-dgkA-ISKpn40 has been deposited in GenBank under accession number MT561503.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (31972735), the National Key Research and Development Program of China (2016YFD0501300), the Program for Innovative Research Team in the University of Ministry of Education of China (IRT_17R39), and the 111 Project (D20008).
We declare that we have no competing interests.
J.S. designed this project. Y.-Z.H., T.-F.L., C.-P.C., and B.H. performed the experiments. Y.-Z.H., X.-P. Li, L.C., and J.S. analyzed the data. Y.-Z.H. made the figures. Y.-Z.H. wrote the manuscript. X.-P. Liao, and J.S. edited and revised the manuscript. Y.-H.L. coordinated the whole project.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Liu YY, Wang Y, Walsh TR, Yi LX, Zhang R, Spencer J, Doi Y, Tian G, Dong B, Huang X, Yu LF, Gu D, Ren H, Chen X, Lv L, He D, Zhou H, Liang Z, Liu JH, Shen J. 2015. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis 16:161–168. doi: 10.1016/S1473-3099(15)00424-7. [DOI] [PubMed] [Google Scholar]
- 2.Xavier BB, Lammens C, Ruhal R, Kumar-Singh S, Butaye P, Goossens H, Malhotra-Kumar S. 2016. Identification of a novel plasmid-mediated colistin-resistance gene, mcr-2, in Escherichia coli, Belgium, June 2016. Euro Surveill 21. doi: 10.2807/1560-7917.ES.2016.21.27.30280. [DOI] [PubMed] [Google Scholar]
- 3.Yin W, Li H, Shen Y, Liu Z, Wang S, Shen Z, Zhang R, Walsh TR, Shen J, Wang Y. 2017. Novel plasmid-mediated colistin resistance gene mcr-3 in Escherichia coli. mBio 8:e00543-17. doi: 10.1128/mBio.01166-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carattoli A, Villa L, Feudi C, Curcio L, Orsini S, Luppi A, Pezzotti G, Magistrali CF. 2017. Novel plasmid-mediated colistin resistance mcr-4 gene in Salmonella and Escherichia coli, Italy 2013, Spain and Belgium, 2015 to 2016. Euro Surveill 22:18–22. doi: 10.2807/1560-7917.ES.2017.22.31.30589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borowiak M, Fischer J, Hammerl JA, Hendriksen RS, Szabo I, Malorny B. 2017. Identification of a novel transposon-associated phosphoethanolamine transferase gene, mcr-5, conferring colistin resistance in D-tartrate fermenting Salmonella enterica subsp. enterica serovar Paratyphi B. J Antimicrob Chemother 72:3317–3324. doi: 10.1093/jac/dkx327. [DOI] [PubMed] [Google Scholar]
- 6.AbuOun M, Stubberfield EJ, Duggett NA, Kirchner M, Dormer L, Nunez-Garcia J, Randall LP, Lemma F, Crook DW, Teale C, Smith RP, Anjum MF. 2018. mcr-1 and mcr-2 (mcr-6.1) variant genes identified in Moraxella species isolated from pigs in Great Britain from 2014 to 2015. J Antimicrob Chemother 73:2904. doi: 10.1093/jac/dky272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang YQ, Li YX, Lei CW, Zhang AY, Wang HN. 2018. Novel plasmid-mediated colistin resistance gene mcr-7.1 in Klebsiella pneumoniae. J Antimicrob Chemother 73:1791–1795. doi: 10.1093/jac/dky111. [DOI] [PubMed] [Google Scholar]
- 8.Wang XM, Wang Y, Zhou Y, Wang Z, Wang Y, Zhang SX, Shen ZQ. 2019. Emergence of colistin resistance gene mcr-8 and its variant in Raoultella ornithinolytica. Front Microbiol 10:228. doi: 10.3389/fmicb.2019.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang C, Feng Y, Liu L, Wei L, Kang M, Zong Z. 2020. Identification of novel mobile colistin resistance gene mcr-10. Emerg Microbes Infect 9:508–516. doi: 10.1080/22221751.2020.1732231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haas M, Rak B. 2002. Escherichia coli insertion sequence IS150: transposition via circular and linear intermediates. J Bacteriol 184:5833–5841. doi: 10.1128/jb.184.21.5833-5841.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chandler M, Fayet O, Rousseau P, Ton Hoang B, Duval-Valentin G. 2015. Copy-out-paste-in Transposition of IS911: a major transposition pathway. Microbiol Spectr 3. doi: 10.1128/microbiolspec.MDNA3-0031-2014. [DOI] [PubMed] [Google Scholar]
- 12.Xiang R, Liu BH, Zhang AY, Lei CW, Ye XL, Yang YX, Chen YP, Wang HN. 2018. Colocation of the polymyxin resistance gene mcr-1 and a variant of mcr-3 on a plasmid in an Escherichia coli isolate from a chicken farm. Antimicrob Agents Chemother 62:e00501-18. doi: 10.1128/AAC.00501-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Z, Fu Y, Schwarz S, Yin W, Walsh TR, Zhou Y, He J, Jiang H, Wang Y, Wang S. 2019. Genetic environment of colistin resistance genes mcr-1 and mcr-3 in Escherichia coli from one pig farm in China. Vet Microbiol 230:56–61. doi: 10.1016/j.vetmic.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 14.Philippe N, Alcaraz JP, Coursange E, Geiselmann J, Schneider D. 2004. Improvement of pCVD442, a suicide plasmid for gene allele exchange in bacteria. Plasmid 51:246–255. doi: 10.1016/j.plasmid.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 15.He Y-Z, Li X-P, Miao Y-Y, Lin J, Sun R-Y, Wang X-P, Guo Y-Y, Liao X-P, Liu Y-H, Feng Y, Sun J. 2019. The ISApl12 dimer circular intermediate participates in mcr-1 transposition. Front Microbiol 10:15. doi: 10.3389/fmicb.2019.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang P, Yu Z, Li B, Cai X, Zeng Z, Chen X, Wang X. 2015. Development of an efficient conjugation-based genetic manipulation system for Pseudoalteromonas. Microb Cell Fact 14:11. doi: 10.1186/s12934-015-0194-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Das S, Noe JC, Paik S, Kitten T. 2005. An improved arbitrary primed PCR method for rapid characterization of transposon insertion sites. J Microbiol Methods 63:89–94. doi: 10.1016/j.mimet.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 18.Poirel L, Kieffer N, Nordmann P. 2017. In vitro study of ISApl1-mediated mobilization of the colistin resistance gene mcr-1. Antimicrob Agents Chemother 61:e00127-17. doi: 10.1128/AAC.00127-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blattner FR, Plunkett G 3rd, Bloch CA, Perna NT, Burland V, Riley M, Collado-Vides J, Glasner JD, Rode CK, Mayhew GF, Gregor J, Davis NW, Kirkpatrick HA, Goeden MA, Rose DJ, Mau B, Shao Y. 1997. The complete genome sequence of Escherichia coli K-12. Science 277:1453–1462. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- 20.Dehio C, Meyer M. 1997. Maintenance of broad-host-range incompatibility group P and group Q plasmids and transposition of Tn5 in Bartonella henselae following conjugal plasmid transfer from Escherichia coli. J Bacteriol 179:538–540. doi: 10.1128/jb.179.2.538-540.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The nucleotide sequence of amplicon ISKpn40-mcr-3.11-dgkA-ISKpn40 has been deposited in GenBank under accession number MT561503.