A total of 191 soil samples from Hangzhou, China, were submitted to detect non-wild-type (non-WT) Aspergillus fumigatus and its associated mechanisms. There were 2 (4.7%), 13 (12.4%), and 31 (23.1%) isolates identified as non-WT in 2014, 2016, and 2018, respectively. The resistant mutations of TR34/L98H, TR46/Y121F/T289A, and TR34/L98H/S297T/F495I were found in 3, 5, and 5 non-WT isolates. The G448S mutation, previously only found in clinical settings, was detected in A. fumigatus from soil samples.
KEYWORDS: Aspergillus fumigatus, environmental resistance, microsatellite typing, G448S
ABSTRACT
A total of 191 soil samples from Hangzhou, China, were submitted to detect non-wild-type (non-WT) Aspergillus fumigatus and its associated mechanisms. There were 2 (4.7%), 13 (12.4%), and 31 (23.1%) isolates identified as non-WT in 2014, 2016, and 2018, respectively. The resistant mutations of TR34/L98H, TR46/Y121F/T289A, and TR34/L98H/S297T/F495I were found in 3, 5, and 5 non-WT isolates. The G448S mutation, previously only found in clinical settings, was detected in A. fumigatus from soil samples.
INTRODUCTION
Aspergillus fumigatus, a globally distributed opportunistic pathogen, is the main cause of invasive aspergillosis (IA), which causes 40% to 90% mortality in immunocompromised patients (1). Triazole drugs (itraconazole, voriconazole, posaconazole, and isavuconazole) are the only oral drugs approved for clinical treatment and prevention of IA. Unfortunately, azole-resistant A. fumigatus (ARAF) has been increasingly found in patients who had received long-term antifungal treatment, azole-naive patients, and the environment (2–7). The emergence of ARAF is the primary cause of the increased numbers of treatment failures.
The mechanism of azole resistance is primarily characterized by alterations in cyp51A, the gene coding for sterol 14α-demethylase, which plays an important role in sterol synthesis. G54, G138, G434, M220, H147, Y121, G448, and P216 are common hot spot mutations that have been detected in ARAF from the patients under long-term antifungal treatment (8, 9). TR34/L98H and TR46/Y121F/T289A are the two most frequently identified resistance mechanisms found in environmental A. fumigatus strains and azole-naive patients and may result from the agricultural use of azole fungicides (9–11). Upregulation in intracellular sterol 14α-demethylase concentration because of overexpression of cyp51A is involved in resistance to triazole drugs. In addition, a decrease in intracellular drug concentration because of the overexpression of efflux pump genes can also result in resistance (9).
Immunocompromised patients may be infected by inhalation of airborne ARAF spores. Therefore, understanding the developmental processes and evolution of ARAF in the environment is significant for the treatment of patients with aspergillosis. Two resistant A. fumigatus soil isolates harboring mutations of TR34/L98H/S297T/F495I were identified in Beijing and Fuzhou, respectively (12). Ren et al. (6) reported the emergence of ARAF with the mutations TR46/Y121F/T289A and TR34/L98H/S297T/F495I in agricultural fields. We report here a 5-year survey concerning resistance in environmental A. fumigatus from Hangzhou in China.
A total of 191 soil samples were collected at a depth of 0 to 5 cm from the garden belt of eight hospitals during the 2014 to 2018 period from Hangzhou, Zhejiang, China (data not shown). A. fumigatus isolates were identified according to their microscopic and macroscopic morphology, by their capability to grow at 48°C, and by the internal transcribed spacer ribosomal DNA and β-tubulin gene (6, 13). Susceptibility testing of A. fumigatus against triazole medicines (voriconazole [VRC], itraconazole [ITZ], and posaconazole [POC]) was conducted according to the CLSI M38-A2 method (14). Isolates with a MIC of >1 mg/liter for ITZ and VRC and MIC of >0.25 mg/liter for POC were regarded as non-wild-type (non-WT) (15). To explore the underlying mutations of isolated non-WT A. fumigatus, the cyp51A and promoter regions were amplified and sequenced according to the method proposed by Ren et al. (6). For the non-WT strain without mutation, the mRNA expression levels of cyp51 (cyp51A and cyp51B) and two transporters (AfuMDR3 and AfuMDR4) were assessed according to the methods described by Cao et al. (16). The results were analyzed according to the 2−△△CT method (17). Additionally, genotyping of non-WT A. fumigatus isolates was realized by microsatellite typing using a panel of nine short tandem repeats (STR) as described previously (18). To determine the genotypic relationship between environmental non-WT A. fumigatus and the clinical isolates, a total of 29 non-WT A. fumigatus isolates were used. These data originated from China (environmental, n = 2; clinical, n = 15) (12, 19–21), Netherlands (environmental, n = 1; clinical, n = 6) (19, 22), Colombia (environmental, n = 1) (10), India (environmental, n = 3) (19), and Tanzania (environmental, n = 1) (19).
From 2014 to 2018, a total of 282 A. fumigatus isolates were recovered from 191 environment samples collected from gardens around eight hospitals in Hangzhou, China (Table 1 and data not shown). The prevalence rates of A. fumigatus were 45.8%, 64.7%, and 62.5% in environmental samples collected in 2014, 2016, and 2018, respectively. This was consistent with previous reports showing a range of 35 to 77.8% (6, 23, 24). Out of the obtained 282 isolates, 46 strains were determined as non-WT A. fumigatus (Table 2). The isolate rates of non-WT A. fumigatus were 4.7%, 12.4%, and 23.1% in 2014, 2016, and 2018, respectively. These results show that the proportion of non-WT A. fumigatus in soils around hospitals in Hangzhou seemly increased during the 5 years.
TABLE 1.
The frequency of isolation of total and non-wild-type A. fumigatus during 2014 to 2018a
| PS | TS | SCDAF | Rate of SCDAF/TS (%) | NIAF | NINAF | Rate of NINAF/NIAF (%) |
|---|---|---|---|---|---|---|
| Jun–Sep 2014 | 59 | 27 | 45.8 | 43 | 2 | 4.7 |
| Mar–May 2016 | 68 | 44 | 64.7 | 105 | 13 | 12.4 |
| Mar–May 2018 | 64 | 40 | 62.5 | 134 | 31 | 23.1 |
PS, period of sampling; TS, total samples; SCDAF, samples containing detectable A. fumigatus; NIAF, numbers of isolated A. fumigatus; NINAF, numbers of isolated non-wild-type A. fumigatus.
TABLE 2.
The MICs and mutations of non-wild-type A. fumigatus isolates from Chinaa
| Strain no. | Date of sample collection (day/mo/yr) | Location of sampling sites | MIC (mg/liter) |
cyp51A mutations | ||
|---|---|---|---|---|---|---|
| VRC | ITZ | POC | ||||
| H-13 | 18/06/2014 | ZPPH | 1 | 16 | 0.5 | TR34/L98H/S297T/F495I |
| H-20 | 12/07/2014 | HCH | 1 | 8 | 0.125 | TR34/L98H/S297T/F495I |
| H-58 | 02/03/2016 | ZPPH | 16 | 4 | 1 | TR46/Y121F/T289A |
| H-90 | 02/03/2016 | ZPPH | 2 | 0.5 | 0.125 | None |
| H-102 | 02/03/2016 | ZPPH | 2 | 0.5 | 0.25 | None |
| H-197 | 02/03/2016 | ZPPH | 2 | 0.125 | 0.0625 | None |
| H-85 | 02/03/2016 | AHFPH | 8 | 4 | 0.5 | G448S |
| H-96 | 02/03/2016 | AHFPH | 4 | 0.5 | 0.125 | G448S |
| H-98 | 02/03/2016 | AHFPH | 8 | 2 | 1 | G448S |
| H-103 | 02/03/2016 | AHFPH | 8 | 2 | 0.5 | G448S |
| H-107 | 02/03/2016 | AHFPH | 8 | 2 | 0.5 | G448S |
| H-111 | 02/03/2016 | AHFPH | 8 | 2 | 0.5 | G448S |
| H-114 | 27/03/2016 | ZPTCM | 8 | 2 | 0.5 | G448S |
| H-120 | 27/03/2016 | ZPTCM | 8 | 1 | 0.5 | G448S |
| H-126 | 27/03/2016 | ZPTCM | 8 | 2 | 0.5 | G448S |
| H-149 | 04/03/2018 | ZPPH | 2 | 0.5 | 0.0625 | None |
| H-152 | 04/03/2018 | ZPPH | 2 | 0.5 | 0.0625 | None |
| H-161 | 04/03/2018 | ZPPH | 2 | 0.5 | 0.0625 | None |
| H-190 | 04/03/2018 | ZPPH | >16 | 16 | 0.25 | TR46/Y121F/T289A |
| H-202 | 04/03/2018 | ZPPH | 8 | >16 | 1 | TR34/L98H |
| H-247 | 04/03/2018 | ZPPH | 8 | 4 | 0.125 | F46Y/G89G/M172V/N248T/D255E/L358L/E427K/C454C |
| H-234 | 26/03/2018 | HCH | >16 | 1 | 1 | TR46/Y121F/T289A |
| H-237 | 26/03/2018 | HCH | 4 | 0.25 | 0.0625 | None |
| H-264 | 26/03/2018 | HCH | 4 | >16 | 1 | TR34/L98H/S297T/F495I |
| H-176 | 04/03/2018 | TFHZP | 2 | 0.25 | 0.0625 | None |
| H-184 | 04/03/2018 | TFHZP | 2 | 0.25 | 0.0625 | None |
| H-186 | 04/03/2018 | TFHZP | 2 | 0.125 | 0.0625 | None |
| H-191 | 04/03/2018 | TFHZP | 2 | 0.25 | 0.0625 | None |
| H-197 | 04/03/2018 | TFHZP | 2 | 0.125 | 0.0625 | None |
| H-206 | 04/03/2018 | TFHZP | 2 | 0.125 | 0.0625 | None |
| H-208 | 04/03/2018 | TFHZP | 4 | 0.5 | 0.0625 | G170G |
| H-248 | 04/03/2018 | AHFPH | 2 | 0.5 | 0.0625 | None |
| H-252 | 04/03/2018 | AHFPH | 2 | 0.25 | 0.0625 | None |
| H-256 | 04/03/2018 | AHFPH | 2 | 0.5 | 0.25 | None |
| H-258 | 04/03/2018 | AHFPH | 4 | 0.25 | 0.125 | None |
| H-218 | 04/03/2018 | AHFPH | 2 | 0.5 | 0.0625 | None |
| H-242 | 26/03/2018 | ZPTCM | >16 | 0.5 | 0.5 | TR46/Y121F/T289A |
| H-244 | 26/03/2018 | ZPTCM | >16 | 16 | 0.25 | TR46/Y121F/T289A |
| H-260 | 26/03/2018 | ZPTCM | 2 | 0.25 | 0.0625 | None |
| H-261 | 26/03/2018 | ZPTCM | 2 | 0.125 | 0.0625 | None |
| H-266 | 26/03/2018 | ZPTCM | 2 | 0.125 | 0.0625 | None |
| H-259 | 26/03/2018 | HTH | 4 | >16 | 0.5 | TR34/L98H |
| H-263 | 26/03/2018 | HTH | 4 | >16 | 1 | TR34/L98H |
| H-272 | 01/05/2018 | THZP | 2 | >16 | 1 | TR34/L98H/S297T/F495I |
| H-277 | 01/05/2018 | THZP | 2 | 0.5 | 0.0625 | None |
| H-280 | 01/05/2018 | THZP | 2 | >16 | 1 | TR34/L98H/S297T/F495I |
ZPPH, Zhejiang Provincial People’s Hospital; HCH, Hangzhou Children’s Hospital; AHFPH, Affiliated Hangzhou First People’s Hospital, Zhejiang University School of Medicine; ZPTCM, Zhejiang Provincial Hospital of TCM; HTH, Hangzhou Third Hospital; THZP, Tongde Hospital of Zhejiang Province; TFHZP, The First Hospital of Zhejiang Province.
Alterations in the cyp51A gene are usually the main causes of triazole resistance in A. fumigatus (9). There were six different mutations (Table 2 and data not shown) found in the isolated strains of non-WT A. fumigatus. The multiazole resistance mutations of TR34/L98H and TR46/Y121F/T289A were detected in 3 and 5 isolates, respectively. TR34/L98H and TR46/Y121F/T289A were first reported in Dutch patients and have been frequently found in azole-naive patients, clinics, and environmental samples worldwide (25). There is increasing evidence linking the occurrence and spread of these two resistance mechanisms to the use of triazole fungicides in agriculture (6, 26, 27). TR34/L98H/S297T/F495I, a widely reported mutation in China (12, 28), was found in 5 strains. The point mutations G448S and G170G and the mutations of F46Y/G89G/M172V/N248T/D255E/L358L/E427K/C454C were observed in 9, 1, and 1 strains, respectively.
In non-WT strains without mutations in cyp51A, the resistance may result from the decrease in intracellular toxin accumulation or an increase of target enzyme content. The apparent upregulation of AfuMDR3 and AfuMDR4 genes in the non-mutant, non-WT strains induced by ITZ was reported by Nascimento et al. (29). Recently, Cui et al. (30) reported the overexpression of AtrF, AfuMDR1, cyp51A, and cyp51B genes in non-WT strains HI-30 and HI-36 treated with tebuconazole. The expression of cyp51A, cyp51B, AfuMDR3, and AfuMDR4 in strains H-237 and H-258 was assessed by quantitative reverse transcription PCR, and the results are listed in Table 3. The expression of cyp51A and AfuMDR4 genes in H-237 were 4.12- and 3.12-fold greater, respectively, than those in the sensitive strains. Compared to the controls, 5.55- and 2.51- fold expression of cyp51A and AfuMDR3, respectively, was detected in strain H-258. These results imply that the overexpression of cyp51A and efflux pumps are responsible for the resistance in H-237 and H-258 to triazole drugs.
TABLE 3.
The expression levels of cyp51 and efflux transporter genes of nonmutant strains (H-237 and H-258) and susceptible strain (HT)
| Strain | Relative mRNA gene expression levela |
|||
|---|---|---|---|---|
| cyp51A | cyp51B | AfuMDR3 | AfuMDR4 | |
| HT | 1.00 (0.73–1.19) | 1.00 (0.81–1.22) | 1.00 (0.68–1.22) | 1.00 (0.80–1.16) |
| H-237 | 4.12 (3.53–5.12) | 1.53 (1.27–1.70) | 0.81 (0.66–1.10) | 3.12 (2.45–3.32) |
| H-258 | 5.55 (4.81–6.61) | 1.10 (1.05–1.33) | 2.51 (2.07–3.12) | 0.63 (0.61–0.77) |
HT values are averages from 5 randomly selected wild-type A. fumigatus isolates.
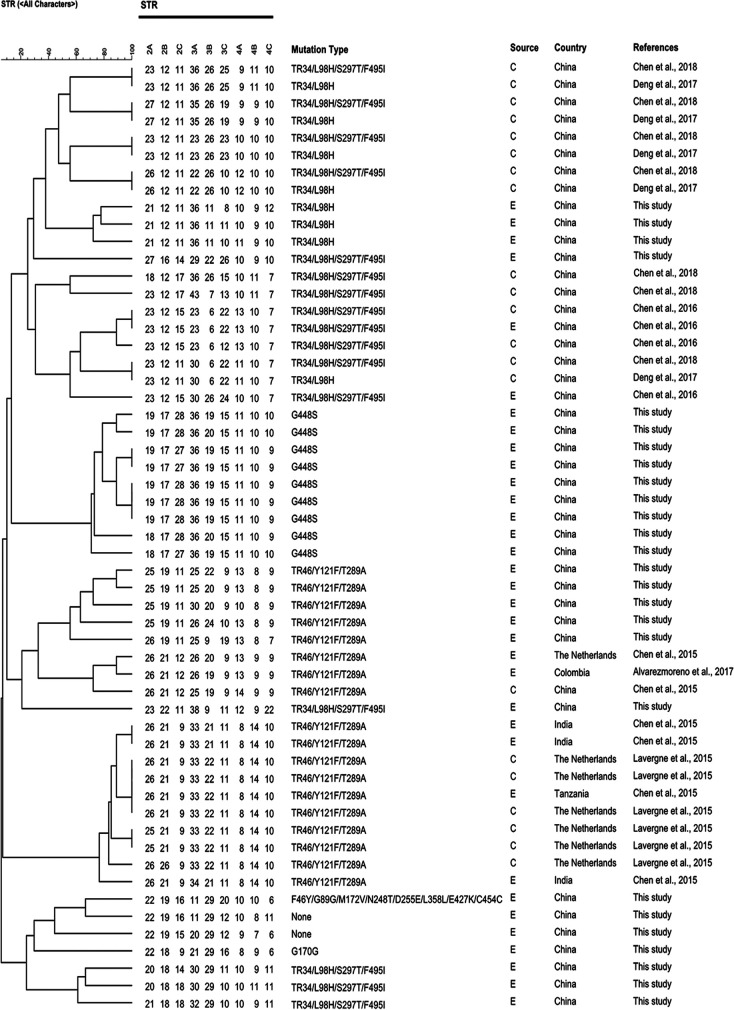
STR genotyping has been widely used to study the genetic similarity of A. fumigatus strains due to its reproducibility and high resolution (12, 18). The genotyping data showed a large genotypic diversity in TR34/L98H (S297T/F495I), TR46/Y121F/T289A, and no-mutation genotypes, whereas most of the G448S mutations were closely clustered with similarity observed at 6 to 9 of the nine loci studied (Fig. 1). Analysis of the genetic similarity between the present environmental isolates and Chinese clinical strains (n = 14) with TR34/L98H (S297T/F495I) showed that the genotypes of all environmental TR34/L98H (S297T/F495I) isolates were distinct from those of the clinical TR34/L98H (S297T/F495I) strains. The genotypes of TR46/Y121F/T289A from environmental and clinical isolates showed two major clusters. The clade in the upper part of the phylogenetic tree mainly contained TR46/Y121F/T289A genotypes from China (environmental isolates, n = 5; clinical isolates, n = 1) and other Eurasian countries, the Netherlands (environmental isolates, n = 1), and Colombia (environmental isolates, n = 1). The clade in the lower part of the tree reveals a single microsatellite cluster and was mainly composed of TR46/Y121F/T289A genotypes from the Netherlands (clinical isolates, n = 6), Tanzania (environmental isolates, n = 1), and India (environmental isolates, n = 3). No genetic similarity was found in F46Y/G89G/M172V/N248T/D255E/L358L/E427K/C454C and G170G genotypes. Strikingly, non-WT strains containing the resistance-related mutation of G448S isolated from different hospitals in China had a nearly identical genotype. Although G448S has not been isolated from a clinic, its spread is likely to increase the azole resistance risks to immunocompromised patients. Recently, there is increasing evidence the concerning development of resistance in A. fumigatus through its sexual cycle (31–33). The sexual crossing experiments conducted by Camps et al. (32) demonstrated that TR34/L98H strains could outcross with azole-susceptible isolates of diverse genetic backgrounds. Zhang et al. (34) found that the new mutation of TR463/Y121F/T289A could arise via sexual crossing between isolates with TR46/Y121F/T289A. Sexual reproduction leads to new microsatellite genotypes. Considering the diverse STR types among non-WT isolates in this study, sexual reproduction may be involved in the development and spread of resistant A. fumigatus.
FIG 1.
Genotypic relationships of selected non-wild-type A. fumigatus isolates from environmental samples with non-wild-type strains from China (environmental resistant, n = 2; clinical resistant, n = 15), the Netherlands (environmental resistant, n = 1; clinical resistant, n = 6), Colombia (environmental resistant, n = 1), India (environmental resistant, n = 3), and Tanzania (environmental resistant, n = 1). E, environmental; C, clinical. The dendrogram is based on a categorical analysis of STR repeat numbers in combination with unweighted pair group method using average linkages clustering.
In conclusion, this study revealed an increasing incidence of non-WT A. fumigatus in Hangzhou over a 5-year survey. The G448S mutation, previously reported only in clinical settings, was recovered in environmental isolates. The high prevalence (>20%) of non-WT A. fumigatus in hospital environments suggests that susceptible patients will inhale spores, resulting in azole-resistant aspergillosis. These results demonstrate that regular resistance surveillance of A. fumigatus is necessary to obtain sufficient helpful data to avoid clinical failures.
Data availability.
cyp51A sequences were deposited in the NCBI database under GenBank accession numbers MT418853–MT418878.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (no. 21777141, 41977342, and 21477112).
We have no competing interests to declare.
REFERENCES
- 1.Sewell TR, Zhu JN, Rhodes J, Hagen F, Meis JF, Fisher MC, Jombart T. 2019. Nonrandom distribution of azole resistance across the global population of Aspergillus fumigatus. mBio 10:e00392-19. doi: 10.1128/mBio.00392-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Howard SJ, Cerar D, Anderson MJ, Albarrag A, Fisher MC, Pasqualotto AC, Laverdiere M, Arendrup MC, Perlin DS, Denning DW. 2009. Frequency and evolution of azole resistance in Aspergillus fumigatus associated with treatment failure. Emerg Infect Dis 15:1068–1076. doi: 10.3201/eid1507.090043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arendrup MC, Mavridou E, Mortensen KL, Snelders E, Frimodt-Moller N, Khan H, Melchers WJG, Verweij PE. 2010. Development of azole resistance in Aspergillus fumigatus during azole therapy associated with change in virulence. PLoS One 5:e10080. doi: 10.1371/journal.pone.0010080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chowdhary A, Kathuria S, Randhawa HS, Gaur SN, Klaassen CH, Meis JF. 2012. Isolation of multiple-triazole-resistant Aspergillus fumigatus strains carrying the TR/L98H mutations in the cyp51A gene in India. J Antimicrob Chemother 67:362–366. doi: 10.1093/jac/dkr443. [DOI] [PubMed] [Google Scholar]
- 5.Tashiro M, Izumikawa K, Minematsu A, Hirano K, Iwanaga N, Ide S, Mihara T, Hosogaya N, Takazono T, Morinaga Y, Nakamura S, Kurihara S, Imamura Y, Miyazaki T, Nishino T, Tsukamoto M, Kakeya H, Yamamoto Y, Yanagihara K, Yasuoka A, Tashiro T, Kohno S. 2012. Antifungal susceptibilities of Aspergillus fumigatus clinical isolates obtained in Nagasaki, Japan. Antimicrob Agents Chemother 56:584–587. doi: 10.1128/AAC.05394-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ren J, Jin X, Zhang Q, Zheng Y, Lin D, Yu YL. 2017. Fungicides induced triazole-resistance in Aspergillus fumigatus, associated with mutations of TR46/Y121F/T289A and its appearance in agricultural fields. J Hazard Mater 326:54–60. doi: 10.1016/j.jhazmat.2016.12.013. [DOI] [PubMed] [Google Scholar]
- 7.Dabas Y, Xess I, Bakshi S, Mahapatra M, Seth R. 2018. Emergence of azole resistant Aspergillus fumigatus from immunocompromised hosts in India. Antimicrob Agents Chemother 62:e02264-17. doi: 10.1128/AAC.02264-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Snelders E, Karawajczyk A, Schaftenaar G, Verweij PE, Melchers W. 2010. Azole resistance profile of amino acid changes in Aspergillus fumigatus cyp51A based on protein homology modeling. Antimicrob Agents Chemother 54:2425–2430. doi: 10.1128/AAC.01599-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chowdhary A, Sharma C, Kathuria S, Hagen F, Meis JF. 2014. Exploring azole antifungal drug resistance in Aspergillus fumigatus with special reference to resistance mechanisms. Future Microbiol 9:697–711. doi: 10.2217/fmb.14.27. [DOI] [PubMed] [Google Scholar]
- 10.Alvarez-Moreno C, Lavergne RA, Hagen F, Morio F, Meis JF, Le Pape P. 2017. Azole-resistant Aspergillus fumigatus harboring TR34/L98H, TR46/Y121F/T289A and TR53 mutations related to flower fields in Colombia. Sci Rep 7:45631. doi: 10.1038/srep45631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chowdhary A, Sharma C, Meis JF. 2017. Azole-resistant aspergillosis: epidemiology, molecular mechanisms, and treatment. J Infect Dis 216:S436–S444. doi: 10.1093/infdis/jix210. [DOI] [PubMed] [Google Scholar]
- 12.Chen Y, Lu Z, Zhao J, Zou Z, Gong Y, Qu F, Bao Z, Qiu G, Song M, Zhang Q, Liu L, Hu M, Han X, Tian S, Zhao J, Chen F, Zhang C, Sun Y, Verweij PE, Huang L, Han L. 2016. Epidemiology and molecular characterizations of azole resistance in clinical and environmental Aspergillus fumigatus isolates from China. Antimicrob Agents Chemother 60:5878–5884. doi: 10.1128/AAC.01005-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahmad S, Khan Z, Hagen F, Meis JF. 2014. Occurrence of triazole-resistant Aspergillus fumigatus with TR34/L98H mutations in outdoor and hospital environment in Kuwait. Environ Res 133:20–26. doi: 10.1016/j.envres.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 14.Clinical Laboratory Standards Institute (CLSI). 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi, approved standard, 2nd ed CLSI document M38-A2 Clinical Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 15.Pfaller MA, Diekema DJ, Ghannoum MA, Rex JH, Alexander BD, Andes D, Brown SD, Chaturvedi V, Espinel-Ingroff A, Fowler CL, Johnson EM, Knapp CC, Motyl MR, Ostrosky-Zeichner L, Sheehan DJ, Walsh TJ, Clinical and Laboratory Standards Institute Antifungal Testing Subcommittee. 2009. Wild-type MIC distribution and epidemiological cutoff values for Aspergillus fumigatus and three triazoles as determined by the Clinical and Laboratory Standards Institute broth microdilution methods. J Clin Microbiol 47:3142–3146. doi: 10.1128/JCM.00940-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cao D, Yao S, Zhang H, Wang S, Jin X, Lin D, Fang H, Yu Y. 2020. Mutation in cyp51A and high expression of efflux pump gene of Aspergillus fumigatus induced by propiconazole in liquid medium and soil. Environ Pollut 256:113385. doi: 10.1016/j.envpol.2019.113385. [DOI] [PubMed] [Google Scholar]
- 17.Schmittgen TD, Livak KJ. 2008. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 18.de Valk HA, Meis J, Curfs IM, Muehlethaler K, Mouton JW, Klaassen C. 2005. Use of a novel panel of nine short tandem repeats for exact and high-resolution fingerprinting of Aspergillus fumigatus isolates. J Clin Microbiol 43:4112–4120. doi: 10.1128/JCM.43.8.4112-4120.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Y, Wang H, Lu Z, Li P, Zhang Q, Jia T, Zhao J, Tian S, Han X, Chen F, Zhang C, Jia X, Huang L, Qu F, Han L. 2015. Emergence of TR46/Y121F/T289A in an Aspergillus fumigatus isolates from a Chinese patient. Antimicrob Agents Chemother 59:7148–7150. doi: 10.1128/AAC.00887-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Y, Li Z, Han X, Tian S, Zhao J, Chen F, Su X, Zhao J, Zou Z, Gong Y, Qu F, Qiu G, Wang S, Jia X, Lu Z, Hu M, Huang L, Verweij P, Han L. 2018. Elevated MIC values of imidazole drugs against Aspergillus fumigatus isolates with TR34/L98H/S297T/F495I mutation. Antimicrob Agents Chemother 62:e01549-17. doi: 10.1128/AAC.01549-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deng S, Zhang L, Ji Y, Verweij PE, Tsui KM, Hagen F, Houbraken J, Meis JF, Abliz P, Wang XD, Zhao JJ, Liao WQ. 2017. Triazole phenotypes and genotypic characterization of clinical Aspergillus fumigatus isolates in China. Emerg Microbes Infect 6:e109. doi: 10.1038/emi.2017.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lavergne RA, Morio F, Favennec L, Dominique S, Meis JF, Gargala G, Verweij PE, Le Pape P. 2015. First description of azole-resistant Aspergillus fumigatus due to TR46/Y121F/T289A mutation in France. Antimicrob Agents Chemother 59:4331–4335. doi: 10.1128/AAC.00127-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chowdhary A, Sharma C, Kathuria S, Hagen F, Meis JF. 2014. Azole-resistant Aspergillus fumigatus with the environmental TR46/Y121F/T289A mutation in India. J Antimicrob Chemother 69:555–557. doi: 10.1093/jac/dkt397. [DOI] [PubMed] [Google Scholar]
- 24.Mortensen KL, Mellado E, Lass-Florl C, Rodriguez-Tudela JL, Johansen HK, Arendrup MC. 2010. Environmental study of azole-resistant Aspergillus fumigatus and other aspergilli in Austria, Denmark, and Spain. Antimicrob Agents Chemother 54:4545–4549. doi: 10.1128/AAC.00692-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Verweij PE, Chowdhary A, Melchers WJG, Meis JF. 2016. Azole resistance in Aspergillus fumigatus: can we retain the clinical use of mold-active antifungal azoles? Clin Infect Dis 62:362–368. doi: 10.1093/cid/civ885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Snelders E, Veld R, Rijs A, Kema GHJ, Melchers WJG, Verweij PE. 2009. Possible environmental origin of resistance of Aspergillus fumigatus to medical triazoles. Appl Environ Microbiol 75:4053–4057. doi: 10.1128/AEM.00231-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Snelders E, Camps SMT, Karawajczyk A, Schaftenaar G, Kema GHJ, van der Lee HA, Klaassen CH, Melchers WJG, Verweij PE. 2012. Triazole fungicides can induce cross-resistance to medical triazoles in Aspergillus fumigatus. PLoS One 7:e31801. doi: 10.1371/journal.pone.0031801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu MS, Zeng R, Zhang LL, Li DM, Lv GX, Shen YN, Zheng HL, Zhang QQ, Zhao JJ, Zheng N, Liu WD. 2015. Multiple cyp51A-based mechanisms identified in azole-resistant isolates of Aspergillus fumigatus from China. Antimicrob Agents Chemother 59:4321–4325. doi: 10.1128/AAC.00003-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nascimento AM, Goldman GH, Park S, Marras SAE, Delmas G, Oza U, Lolans K, Dudley MN, Mann PA, Perlin DS. 2003. Multiple resistance mechanisms among Aspergillus fumigatus mutants with high-level resistance to itraconazole. Antimicrob Agents Chemother 47:1719–1726. doi: 10.1128/aac.47.5.1719-1726.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cui N, He Y, Yao S, Zhang H, Ren J, Fang H, Yu Y. 2019. Tebuconazole induces triazole-resistance in Aspergillus fumigatus in liquid medium and soil. Sci Total Environ 648:1237–1243. doi: 10.1016/j.scitotenv.2018.08.247. [DOI] [PubMed] [Google Scholar]
- 31.van der Linden JWM, Arendrup MC, Warris A, Lagrou K, Pelloux H, Hauser PM, Chryssanthou E, Mellado E, Kidd SE, Tortorano AM, Dannaoui E, Gaustad P, Baddley JW, Uekotter A, Lass-Florl C, Klimko N, Moore CB, Denning DW, Pasqualotto AC, Kibbler C, Arikan-Akdagli S, Andes D, Meletiadis J, Naumiuk L, Nucci M, Melchers WJG, Verweij PE. 2015. Prospective multicenter international surveillance of azole resistance in Aspergillus fumigatus. Emerg Infect Dis 21:1041–1044. doi: 10.3201/eid2106.140717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Camps SMT, Rijs A, Klaassen CHW, Meis JF, O'Gorman CM, Dyer PS, Melchers WJG, Verweij PE. 2012. Molecular epidemiology of Aspergillus fumigatus isolates harboring the TR34/L98H azole resistance mechanism. J Clin Microbiol 50:2674–2680. doi: 10.1128/JCM.00335-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heitman J, Carter DA, Dyer PS, Soll DR. 2014. Sexual reproduction of human fungal pathogens. Cold Spring Harb Lab Perspect Med 4:a019281. doi: 10.1101/cshperspect.a019281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang JH, Snelders E, Zwaan BJ, Schoustra SE, Meis JF, van Dijk K, Hagen F, van der Beek MT, Kampinga GA, Zoll J, Melchers WJG, Verweij PE, Debets A. 2017. A novel environmental azole resistance mutation in Aspergillus fumigatus and a possible role of sexual reproduction in its emergence. mBio 8:e00791-17. doi: 10.1128/mBio.00791-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
cyp51A sequences were deposited in the NCBI database under GenBank accession numbers MT418853–MT418878.