Abstract
Mycoplasma bovis (M. bovis) is an important bovine mycoplasma implicated in economically important clinical diseases, such as respiratory diseases, otitis media, and mastitis. The prevalence of M. bovis-associated mastitis in both cattle and buffaloes has been increasingly recognized as a global problem. High morbidity rates and consequential economic losses have been devastating to the affected cattle and buffalo farms, especially those in developing countries. Therefore, a rapid and accurate method is urgently needed to detect M. bovis. In this study, a rapid and simple lateral flow strip for detecting antibodies against M. bovis was established that used carbon nanoparticles (CNPs) as the labelled materials. The results from the test strip were highly consistent with those from ELISA. The test showed high specificity (100%) and no cross-reaction with other bovine pathogens. The detection sensitivity of the test was also relatively high (97.67%). All the results indicated that the colloidal carbon test strip could serve as a simple, rapid, sensitive, and specific diagnostic method for detecting antibodies against M. bovis at cattle farms.
Keywords: Colloidal carbon, ELISA, Lateral flow, Mycoplasma bovis, Visual detection
Introduction
Mycoplasma bovis, a prokaryotic microorganism that lacks a cell wall, causes severe pneumonia, mastitis, arthritis, otitis media, and reproductive disorders in cattle. It also causes exudative pleuritic tuberculous in humans (Fox et al. 2005; Kay et al. 2015). M. bovis is mainly transmitted by droplets from the respiratory tract (Foster et al. 2009). M. bovis mainly spreads by droplets ejected from the respiratory tract, and secondly by contacts. It often causes acute and chronic diseases in cattle in a direct or indirect way (Ball and Nicholas 2010). The development of disease severely affects the cattle industry by increasing the cost of feeding and the costs associated with the diagnosis and treatment of disease, including permanent lung injury (Ayling et al. 2014).
Traditionally, the identification and diagnosis of M. bovis has been performed via microbial culture. (Hazelton et al. 2018; Parker et al. 2018; Zhao et al. 2018). More recently, the use of polymerase chain reaction (PCR) to detect M. bovis species from various bovine samples has increased. PCR has a higher efficiency, specificity, and sensitivity for laboratory diagnosis when compared with conventional culture-based methods (Andersson et al. 2019). Serological diagnosis can detect anti-mycoplasma antibodies in serum and milk which includes indirect haemagglutination, immunohistochemistry, agar diffusion, growth inhibition, complement binding, and indirect ELISA (I-ELISA) (Caswell and Archambault 2007; Nielsen et al. 2015; Parker et al. 2017). While each testing method has its strengths and limitations, these methods require professional technicians and test instruments and are not suitable for testing in pastures. Therefore, it is vital to develop a rapid and easy-to-perform method that might be used to effectively eradicate M. bovis from cattle flocks.
Lateral flow testing (LFT) is one of the most commonly used transversal flow immunoassay techniques and is considered an ideal method for detecting and measuring objects during the analysis of samples (Huang et al. 2016; Jiang et al. 2019; Kim et al. 2019). CNPs are relatively inexpensive labels compared with other materials, such as gold and polymers. Additionally, the intense black colour of CNPs provides good contrast for visual detection, which has been demonstrated in many sensitive diagnostic tests (Noguera et al. 2011a, b; Suárez-Pantaleón et al. 2013).
In the present study, we compared and identified the M. bovis p81 membrane protein, p48 membrane protein, M. bovis whole protein and M. bovis outer membrane protein and screened for specific antigens. A double-antigen sandwich immunochromatography assay utilizing CNP label materials with specifically screened antigens as coating antigens was developed for the detection of antibodies against M. bovis in whole blood. On the lateral flow test line and control line, the specific antigens and polyclonal antibody (pAb) against the specific antigens were coated. Then, samples were added onto the sample pad, and a characteristic black band was subsequently observed in the test zone, indicating the accumulation of CNPs. A black band was also observed in the control zone, which indicated the usability of the test strip. The colour intensity of the test line represented the level of target antibody in the sample and could be observed visually. Furthermore, the quantitative detection of antibodies against M. bovis was also achieved by analysing the colour intensities using commercially available optical readers. We anticipate that this CNP-based test strip could be utilized as a novel, direct, and effective immunological method for the detection of antibodies against M. bovis.
Materials and methods
Safety
The care and use of the animals were conducted in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. Procedures involving animals were approved by the Shihezi University Institutional Animal Care and Use Committee.
Reagents and materials
The p48 proteins and candidate proteins used in this study were listed in Table 1. The bovine serum samples containing M. bovis, Pasteurella multocida (P. multocida), Bovine viral diarrhea virus (BVDV), Bovine Brucella, Mycobacterium bovis, Escherichia coli (E.coli), Mycoplasma bovigenitalium (M. bovigenitalium), Mannheimia haemolytica (M. haemolytica), Histophilus somni (H. somni) and Mycoplasma ovipneumoniae (M. ovipneumoniae) were preserved in a preventative veterinary science research laboratory at Shihezi University (Xinjiang, China). All serum samples used in this study were identified by commercial ELISA kits and PCR. The nitrocellulose (NC) membranes, absorbent pads, sample pads, conjugate pads, and polyvinyl chloride (PVC) sheets were purchased from Millipore corporation (Shanghai, China). Bovine serum albumin (BSA), 3,3′,5,5′-tetramethylbenzidine (TMB) and rabbit anti-bovine IgG antibodies were purchased from Sigma (St. Louis, MO, USA). A TRM-502 Biovet antibody test kit for M. bovis was purchased from Canadian Biovet (Saint-Hyacinthe, QC, Canada). CNPs (Special Black SB4) were purchased from Evonik Degussa Frankfurt. The XYZ-3030 dispenser and CT 200 cutting system were purchased from Kinbio Tech. Co., Ltd. (Shanghai, China; kinbio.bioon.com.cn). Rabbit anti-bovine IgG horseradish peroxidase (HRP-Rabbit anti-bovine IgG, 1:5000) was from Tiangen (Beijing, China). UV–visible absorption spectra were recorded on a TU-1810 ultraviolet and visible spectrophotometer (Beijing Persee Co., Ltd., China; www.pgeneral.com).
Table 1.
Various proteins used in this study
| Protein | Source |
|---|---|
| Whole protein | M. bovis(PG45)a |
| Outer membrane protein | M. bovis(PG46)b |
| p81 protein | M. bovis(PG47)c |
| p48 protein | M. bovis(PG48)c |
aProteins were obtained by inactivation, ultrasound and filtration of M. bovis, stored at − 20 °C
bProteins were obtained according to the operation of the Mycoplasma Extracorporeal Membrane Protein Extraction Kit (BestBio, Shanghai), stored at − 20 °C
cThe protein was obtained after optimizing the codon, inducing expression and purifying, stored at − 20 °C
Western blot analysis
According to previously described procedures with slight modifications (Li et al. 2016), the purified recombinant p48 protein and p81 protein produced in the laboratory were diluted to 5 mg/mL and subjected to SDS-PAGE. The separated proteins were transferred to an NC membrane, which was blocked with 5% skim milk. M. bovis-positive serum (1:1000) was used as the primary antibody, and HRP-rabbit anti-cattle IgG was used as the secondary antibody.
I-ELISA of p81, p48, whole protein, and outer membrane protein from M. bovis
I-ELISA was used to screen proteins with good affinity and specificity. Different concentrations of whole protein, outer membrane protein, p81 protein and p48 protein were coated on independent microplates, each protein was set with blank pore, negative serum pore and positive serum pore, and each sample pore was made with three biological replicates.
Microplates coated with 100μL of protein of different concentrations (0.5 μg/mL, 2 μg/mL, 5 μg/mL, 10 μg/mL, 20 μg/mL) per well were blocked with 3% BSA for 2 h at 37 °C. After washing three times with PBST, 100 μL of negative and positive serum (1:40) was added to each well and the microplates were incubated for 1 h at 37 °C. After washing three times with PBST, A volume of 100 μL of HRP-Rabbit anti-bovine IgG (1:5000) was added to each well and incubated for 30 min at 37 °C. The plates were washed four times with PBST, and then 100 μL of TMB was added to each well. After incubating for 10 min at 37 °C, 2 M sulfuric acid was added to stop the reaction. The optical densities were read at 450 nm on a microplate reader.
Preparation of pAbs against the p48 protein
Purified p48 protein was mixed with an equal volume of Freund’s complete adjuvant to obtain a final concentration of 300 μg/mL. Subsequently, approximately 500 μg of antigen-adjuvant mixture was injected subcutaneously into New Zealand white rabbits. Immunizations using the antigen-adjuvant mixture were performed at 10-day intervals. An enzyme-linked immunosorbent assay (ELISA) was used to test the rabbit antisera for the presence of antibodies against p48 and was repeated daily until the threshold was reached (1:100,000). Protein A was used for the isolation of IgG antibodies using column chromatography according to the manufacturer’s recommendations.
Preparation of p48 protein-labelled CNPs
An approximately 1% (w/v) suspension of carbon was prepared in demineralized water by sonication. Then, 100 mL sodium borate buffer containing 5 mg p48 was added dropwise to 500 mL of a fivefold dilution of carbon (0.2%, w/v) in sodium borate buffer. After overnight incubation at 4 °C with gentle stirring, the solution was washed four times with washing solution by centrifugation (13600 g; 15 min). Then, the p48-conjugate was reconstituted in 100 mM sodium borate buffer, pH 8.8, with 1% (w/v) BSA and 0.02% (w/v) NaN3 at a final carbon concentration of 0.2% and stored at 4 °C. Before performing the experiments, the working dilution of the p48-carbon conjugate was sonicated for 10 s.
Assembly, testing, and assessment of the test paper strip
The p48-carbon conjugate was dried onto a conjugate pad, which overlapped the NC membrane containing the two capture lines that were visible within the results window. An absorbent pad was laid on top of the membrane to ensure rapid flow up the membrane. The top line was the control line and comprised a polyclonal anti-p48 antibody that was immobilized on the NC membrane to capture the p48-carbon conjugate. This test line confirmed the presence of p48 antibody. Approximately 3–5 drops were placed onto the sample pad, and the results were observed after 10 min. Negative results were observed when only black strips appeared in the quality control line.
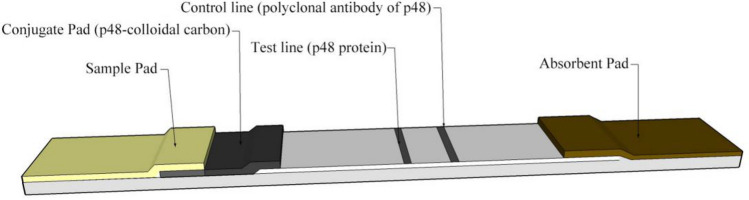
The test strip included a sample pad, a conjugate pad, an absorbent pad, an NC membrane. The conjugate pad contained the dried carbon-labelled p48 protein, which produced an easily visible black colour. There were two lines on the NC membrane: the control line and the test line. The test line was coated with p48 protein. The control line was coated with the pAb against p48 (Fig. 1).
Fig. 1.
Schematic of the colloidal carbon test strip
Cross-reactivity of the CNP test strip
To evaluate the cross reactivity of the CNP test strip, positive serum samples of M. bovis, P. multocida, BVDV, Bovine Brucella, Mycobacterium bovis, E.coli, M.bovigenitalium, M. haemolytica, H. somni and M.ovipneumoniae were added to the sample pad. After 10 min, the results were observed.
Detection limit of the CNP strip
The standard positive serum of M. bovis was diluted 10-, 50-, 100-, 200-, 400-, 800-fold with 1 × PBS (pH 7.4). A 100 μL droplet was added to the sample pad under various conditions, and the results were observed after 10 min.
Comparison of LFA strips to commercial ELISA kits
A total of 197 blood samples collected from seven dairy cow farms in Xinjiang Province, China, were screened for antibodies against M. bovis using the developed CNP immunochromatography strips in parallel to an M. bovis Ab ELISA test kit, which was used according to the manufacturer’s instructions, to compare their specificity, sensitivity, and accuracy.
Results
Western blot analysis
To identify the composition characteristics of p81 and p48 proteins obtained by gene recombination technology in vitro, Western blot analysis was used. M. bovis-negative and -positive serum samples were subjected to Western blot analysis. Figure 2 shows that the p81 protein reacted with the M. bovis-positive and M. bovis-negative sera, whereas the p48 protein reacted with the M. bovis-positive serum but not with the M. bovis-negative sera. The recombinant proteins p81 and p48 showed good reactivity, but the specificity of the p48 protein was higher than that of p81.
Fig. 2.
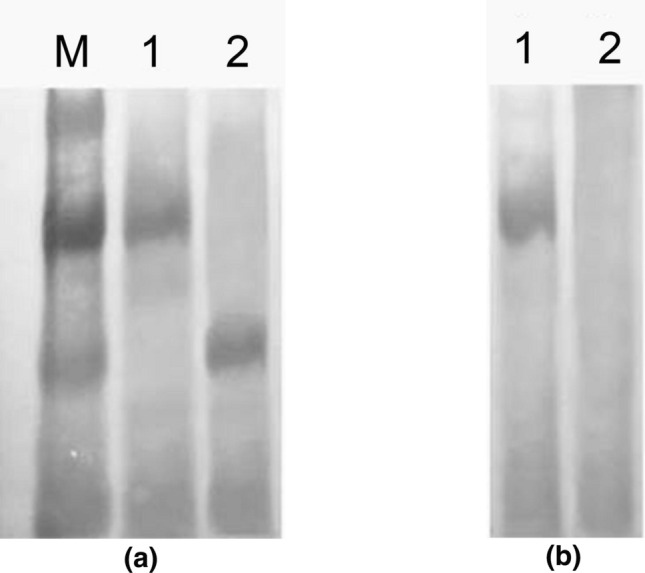
Western blot results for p81 and p48 protein. a Western blot results for p81 and p48 protein and M. bovis-positive serum; lane M, low MW protein marker; lane 1, positive bands for p81 protein and M. bovis-positive serum; lane 2, positive bands for p48 protein and M. bovis-positive serum. b Western blot results for p81 and p48 protein and M. bovis-negative serum; lane 1, positive bands for p81 protein and M. bovis-negative serum; lane 2, p48 protein does not react with M. bovis-negative serum
Comparison of specificity and affinity between the four proteins
I-ELISA was used to screen proteins with good affinity and specificity. A series of proteins with dilution concentrations ranging from 0.5 to 20.0 µg were prepared and the results are shown in Table 2. Under the same conditions, p48 protein showed a good positive detection rate, the optimal concentration was 5.0 µg/mL. Interestingly, the whole protein showed a good positive detection rate because it contains p48 protein, but its specificity is not as good as p48 protein, so we chose p48 protein as the specific protein to establish the detection method.
Table 2.
Comparison of specificity and affinity between the four proteins
| p48 protein concentrations (μg) | Whole protein concentrations (μg) |
Outer membrane protein concentrations (μg) | p81 protein concentrations (μg) |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.5 | 2.0 | 5.0 | 10.0 | 20.0 | 0.5 | 2.0 | 5.0 | 10.0 | 20.0 | 0.5 | 2.0 | 5.0 | 10.0 | 20.0 | 0.5 | 2.0 | 5.0 | 10.0 | 20.0 | |
| M. bovis positive serum | + | + | + + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| M. bovis negative serum | − | − | − | − | − | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
+ positive, − negative
Verification of pAbs against p48 protein
To identify the composition and concentration of the pAbs against p48 protein, SDS-PAGE was used. After the rabbits were immunized five times with the purified p48 protein, the pAb against p48 was purified using a protein A chromatography column. The pAb was observed with SDS-PAGE, and the subunit dissociation resulted in bands corresponding to approximately 55 kDa for the heavy chain and approximately 25 kDa for the light chain; no non-specific bands were observed, indicating that the pAb was pure (Fig. 3a). The titer of the pAb according to ELISA was 1:204,800. These results revealed that the pAbs could recognize the recombinant p48 protein, which is indicative of high specificity (Fig. 3b).
Fig. 3.
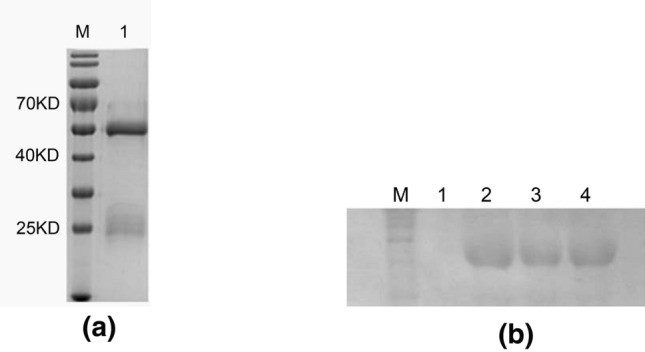
Verification of pAbs against p48 protein. a SDS-PAGE detection of antibody purity. Lane M, molecular weight marker; lane 1, the weight of the heavy chain of the antibody was approximately 55 kDa and that of the light chain of the antibody was approximately 25 kDa. b Lane M, protein molecular standard; lane 1, negative control; lanes 2–4: Positive bands produced by anti-p48 pAb and whole protein
Determination of the optimal ratio of the p48 protein coating concentration and the p48 protein-labelled CNPs
To determine the optimal ratio of p48 protein coating concentration and p48 protein-labeled CNPs, p48 protein-labeled CNPs were diluted at the ratio of 1:4, 1:6, 1:10, and p48 protein was diluted to 1 mg/mL, 2 mg/mL, 4 mg/mL, and 5 mg/mL, respectively. When the concentration of the p48 protein-labelled CNPs was diluted at a ratio of 1:4, the C line was clearly observed; however, the T line was not observed. With increasing concentrations of p48 protein-labelled CNPs, the colour of the T line became increasingly darker until the CNPs were diluted at a ratio of 1:10 (Fig. 4). When the p48 protein was diluted to a concentration of 4 mg/mL, it was coated in NC membrane, and the colour of the T line increased in intensity; furthermore, the negative and positive serum results were clear. When the p48 protein was diluted to a concentration of 2 mg/mL, the T and C lines were of the same colour, indicating that this was the optimal condition (Table 3).
Fig. 4.

p48 protein-labelled CNPs on strips showing different results. Lane 1–3, different ratios (1:4; 1:6; 1:10) of diluted p48 protein-labelled CNPs
Table 3.
Colour rendering of the results obtained with different concentrations of p48 protein and multiple dilutions of p48 protein-labelled CNPs
| p48 protein-labelled CNPs | p48 (mg/mL) | |||
|---|---|---|---|---|
| 1 | 2 | 4 | 5 | |
| 4x | C + + + /T + | C + + + /T + | C + + + + /T + + | C + + + /T + + + |
| 6x | C + + /T + | C + + /T + | C + + /T + | C + + /T + + |
| 10x | C + /T + | C + + /T + + | C + /T + | C + /T + + |
Determination of the optimal dilution ratio for rabbit anti-p48 pAb
To determine the optimal dilution ratio for rabbit anti-p48 pAb, rabbit anti-p48 pAb was diluted at the ratio of 1:50, 1:100, 1:150, 1:200 respectively. When the concentration of the rabbit anti-p48 pAb was high, the colour intensity of the quality control line was markedly higher than that of the detection line. At a 1:100 dilution, the two detection lines were clearly visible, and the colour intensity remained the same. Therefore, the optimal dilution of the rabbit anti-p48 pAb was 1:100. (Table 4).
Table 4.
Colour rendering results for different dilution multiples
| Dilution ratio of rabbit anti-p48 pAb | Chromogenic result |
|---|---|
| 50 | + + + + T < C |
| 100 | + + + T = C |
| 150 | + + T > C |
| 200 | + T > C |
T < C indicates that the T line was lighter than the C line. T = C indicates that the T line showed the same colour intensity as the C line. T > C indicates that the T line was darker than the C line
Cross-reactivity of the CNP test strips
The cross-reactivity of the test strips was evaluated using bovine-susceptible pathogens in serum samples positive for P. multocida, BVDV, Bovine Brucella, M. bovis, E. coli, M. bovigenitalium, M. haemolytica, H. somni and M. ovipneumoniae. While the M. bovis-positive serum sample yielded positive results, all other samples showed negative results (Fig. 5). These data convincingly demonstrated that the test strip could be used to detect antibodies against M. bovis.
Fig. 5.

Cross-reactivity of the CNP strips showing different results for various positive serum samples. Various positive serum samples: Lane 1, P. multocida; Lane 2, BVDV; Lane 3, Bovine Brucella; Lane 4, Mycobacterium bovis; Lane 5, E. coli; Lane 6, M. bovigenitalium; Lane 7, M. haemolytica; Lane 8, H. somni; Lane 9, M. ovipneumoniae; Lane 10, M. bovis
Detection limit of the CNP strips
To evaluate the detection limit of the CNP test strip, the standard positive serum was diluted with normal saline to yield different concentration gradients. When the standard M. bovis-positive sample was diluted 800-fold, the CNP test strip was negative. When the standard M. bovis-positive sample was diluted 400-fold, the CNP test strip was positive, but the test line was indistinct, indicating that the detection limit of the CNP test strip was 1:400; as the dilution ratio was increased, the colour of the T-line deepened gradually (Fig. 6).
Fig. 6.

Limit of detection of LFA strips in detecting antibodies against M. bovis. Lanes 1–6, different concentrations (1:10; 1:50; 1:100; 1:200; 1:400; 1:800) of bovine plasma samples containing antibodies against M. bovis
Comparison of lateral flow strip results to ELISA
To verify the accuracy of the lateral flow strip method and the ELISA method, the test strip was used for the detection of antibodies against M. bovis in 197 clinical samples, including 42 positive samples and 155 negative samples. The results of the comparison of the strip results with those of ELISA are shown in Table 5. The lateral flow strips showed 100% and 97.67% specificity and sensitivity, respectively, compared to ELISA. The lateral flow strips had higher specificity and sensitivity. The results obtained with the lateral flow strips exhibited 99.49% concordance with those of ELISA.
Table 5.
Comparison of the CNP strips with ELISA for the detection of antibodies against M. bovis
| Strip | M. bovis I-ELISA kit results | ||||||
|---|---|---|---|---|---|---|---|
| Positive | Negative | Total | Sensitivity (%) | Specificity (%) | Accuracy (%) | Positive rate (%) | |
| Positive | 42 | 0 | 42 | 97.67 | |||
| Negative | 1 | 154 | 155 | 100 | |||
| Total | 43 | 154 | 197 | 99.49 | 21.82 (43/197) | ||
| Positive rate (%) | 21.32 (42/197) | ||||||
Both positive = 42, both negative = 154, number of samples = 197. The following equations were used: sensitivity (%) = (both positive/positive in ELISA) × 100%; specificity (%) = (both negative/negative in ELISA) × 100%; accuracy (%) = (both positive + both negative)/total) × 100%
Discussion
In recent years, because of the rise in socioeconomic status and the quality of life, there has been a higher demand for lamb and wool products, which has led to increases in cattle farming and husbandry for beef production. However, the incidence of M. bovis targeting cattle has concordantly risen, and epidemic outbreaks were reported recently (Wawegama et al. 2014). Therefore, there is a need to establish a rapid, simple, and reliable detection method for M. bovis. In this study, a fast and efficient lateral flow strip method for detecting antibodies against M. bovis was established that used CNPs as labelled materials. The method is simple, does not require any instruments or equipment, and its results are presented visually.
To the best of our knowledge, this is the first time that CNPs have been selected as labeling materials to establish a method for detecting antibodies against M. bovis in serum and describe the results of conjugated proteins and lateral flow test strips achieved with colloidal carbon labeling. The colloidal carbon label appeared useful for the lateral flow strips that were studied. Considering the desired properties for a new label, colloidal carbon has several advantageous characteristics and properties. First, the label is very cheap, and conjugates with proteins are easy to prepare (O'Keeffe et al. 2003). Second, the colloidal carbon label and the conjugates are very stable. Some conjugates were stored at 4 °C for several months, while others were stored for up to 1 year (Blažková et al. 2009). Finally, the black/grey line on a white nitrocellulose background has a very good contrast ratio, as judged by visual examination and by computer image analysis. Based on these characteristics and properties, colloidal carbon suspensions can be applied as labels in lateral flow strips.
To obtain better lateral flow strip results, we systematically optimized the coating and labeling conditions of the test strip. After optimization, the test strip could show an accurate and clear result that was directly visible to the naked eye within 10 min. We further examined the accuracy of the result, including its specificity, sensitivity, and consistency in comparison with the ELISA results. To examine its practicability, the test strip was used for the detection of 197 clinical samples. Among them, the results obtained from the test strip agreed with the ELISA results 99.49% of the time. There was one sample that was identified as positive by ELISA but was identified as negative by the strip. This sample was retested by PCR, and the final data agreed with the result obtained from the strip. This observation may hint at the possibility of false positive results from ELISA, thereby implying that our test strip may show higher efficacy for detecting antibodies against M. bovis in serum. I-ELISA has been widely used to detect antibodies in serum, but it shows false positives in practical applications (Fu et al. 2014). The reasons for non-specific reactions include the non-specific colour of non-specific antibodies in the sample, the binding of the clad protein, and the non-specific colour reaction caused by the enzyme marker in the solid carrier. The strip uses a sandwich antigen method, which may help reduce the non-specific adsorption of the sandwich antigen. The double-antigen sandwich method has higher specificity than the indirect method. Therefore, the sandwich method can promote the detection of weakly positive samples. Therefore, this study used the double-antigen sandwich method to establish a test strip for antibody detection.
To our knowledge, this is the first screening of specific proteins in four candidate proteins of M. bovis by I-ELISA. The results indicate that the assay has high specificity for p48 recombinant protein. We describe a simple, rapid, and specific strip assay using CNPs as labelled materials for the detection of antibodies against M. bovis. The strip results indicate that the specificity was relatively increased, and no cross-reactions with other bovine pathogens were observed; the detection sensitivity was also increased. This technique is suitable for use in areas with a high incidence of M. bovis because of its simple operation, visual results and low costs.
Acknowledgements
This study was supported by a project undertaken by Shihezi University (2017AA003). Project Name: Development of New Brucella Vaccine and Detection Reagent.
Compliance with ethical standards
Conflict of interests
The authors declare that they have no competing interests.
Research involving human and animal participants
This study was conducted in compliance with ethical standards. All applicable international, national, and institutional guidelines for the care and use of animals were followed.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Feng Shi and Yang Zhao have contributed equally to this work.
Contributor Information
Feng Shi, Email: shifeng2314@yeah.net.
Chuangfu Chen, Email: ccf-xb@163.com.
References
- Andersson AM, Aspán A, Wisselink HJ, et al. A European inter-laboratory trial to evaluate the performance of three serological methods for diagnosis of Mycoplasma bovis infection in cattle using latent class analysis. BMC Vet Res. 2019;15:369. doi: 10.1186/s12917-019-2117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayling RD, Rosales RS, Barden G, Gosney FL. Changes in antimicrobial susceptibility of Mycoplasma bovis isolates from Great Britain. Vet Rec. 2014;175:486. doi: 10.1136/vr.102303. [DOI] [PubMed] [Google Scholar]
- Blažková M, Mičková-Holubová B, Rauch P, Fukal L. Immunochromatographic colloidal carbon-based assay for detection of methiocarb in surface water. Biosens Bioelectron. 2009;25:753–758. doi: 10.1016/j.bios.2009.08.023. [DOI] [PubMed] [Google Scholar]
- Ball HJ, Nicholas RAJ. Mycoplasma bovis-associated disease: here, there and everywhere. Vet J. 2010;186:280–281. doi: 10.1016/j.tvjl.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Caswell JL, Archambault M. Mycoplasma bovis pneumonia in cattle. Anim Health Res Rev. 2007;8:161–186. doi: 10.1017/S1466252307001351. [DOI] [PubMed] [Google Scholar]
- Fox LK, Kirk JH, Britten A. Mycoplasma mastitis: a review of transmission and control. J Vet Med B Infect Dis Vet Public Health. 2005;52:153–160. doi: 10.1111/j.1439-0450.2005.00845.x. [DOI] [PubMed] [Google Scholar]
- Foster AP, Naylor RD, Howie NM, Nicholas RA, Ayling RD. Mycoplasma bovis and otitis in dairy calves in the United Kingdom. Vet J. 2009;179:455–457. doi: 10.1016/j.tvjl.2007.10.020. [DOI] [PubMed] [Google Scholar]
- Fu P, Sun Z, Zhang Y, Yu Z, Zhang H, Su D, Jiang F, Wu W. Development of a direct competitive ELISA for the detection of Mycoplasma bovis infection based on a monoclonal antibody of p48 protein. Bmc Vet Res. 2014;10:42. doi: 10.1186/1746-6148-10-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Aguilar ZP, Xu H, Lai W, Xiong Y. Membrane-based lateral flow immunochromatographic strip with nanoparticles as reporters for detection: a review. Biosens Bioelectron. 2016;75:166–180. doi: 10.1016/j.bios.2015.08.032. [DOI] [PubMed] [Google Scholar]
- Hazelton MS, Morton JM, Bosward KL, Sheehy PA, Parker AM, Dwyer CJ, Niven PG, House JK. Isolation of Mycoplasma spp. and serological responses in bulls prior to and following their introduction into Mycoplasma bovis-infected dairy herds. J Dairy Sci. 2018;101:7412–7424. doi: 10.3168/jds.2018-14457. [DOI] [PubMed] [Google Scholar]
- Jiang N, Ahmed R, Damayantharan M, Ünal B, Butt H, Yetisen AK. Lateral and vertical flow assays for point-of-care diagnostics. Adv Healthcare Mater. 2019;8:2192–2640. doi: 10.1002/adhm.201900244. [DOI] [PubMed] [Google Scholar]
- Kim H, Chung DR, Kang M. A new point-of-care test for the diagnosis of infectious diseases based on multiplex lateral flow immunoassays. Analyst. 2019;144:2460–2466. doi: 10.1039/c8an02295j. [DOI] [PubMed] [Google Scholar]
- Kay AW, Itoh M, Valdez J, Chen SF, Mathew R, Gans HA. Pleural effusion and fever in an immunocompromised patient. J Pediat Inf Dis Soc. 2015;4:E6–E9. doi: 10.1093/jpids/piu018. [DOI] [PubMed] [Google Scholar]
- Li X, Qin L, Zhu H, Sun Y, Cui X, Gao Y, Qi X, Wang Y, Gao H, Gao Y, Wang X. Identification of a linear B-cell epitope on the avian leukosis virus P27 protein using monoclonal antibodies. Arch Virol. 2016;161:2871–2877. doi: 10.1007/s00705-016-2971-z. [DOI] [PubMed] [Google Scholar]
- Nielsen PK, Petersen MB, Nielsen LR, Halasa T, Toft N. Latent class analysis of bulk tank milk PCR and ELISA testing for herd level diagnosis of Mycoplasma bovis. Prev Vet Med. 2015;121:338–342. doi: 10.1016/j.prevetmed.2015.08.009. [DOI] [PubMed] [Google Scholar]
- Noguera PS, Posthuma-Trumpie GA, Tuil MV, Wal FJVD, Boer AD, Moers APHA, Amerongen AV. Carbon nanoparticles as detection labels in antibody microarrays. Detection of genes encoding virulence factors in shiga toxin-producing Escherichia coli. Anal Chem. 2011;83:8531–8536. doi: 10.1021/ac201823v. [DOI] [PubMed] [Google Scholar]
- Noguera P, Posthuma-Trumpie GA, Tuil MV, Wal FJVD, Boer AD, Moers APHA, Amerongen AV. Carbon nanoparticles in lateral flow methods to detect genes encoding virulence factors of Shiga toxin-producing Escherichia coli. Anal Bioanal Chem. 2011;399:831–838. doi: 10.1007/s00216-010-4334-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keeffe M, Crabbe P, Salden M, Wichers J, Peteghem CV, Kohen F, Pieraccini G, Moneti G. Preliminary evaluation of a lateral flow immunoassay device for screening urine samples for the presence of sulphamethazine. J Immunol Methods. 2003;278:117–126. doi: 10.1016/S0022-1759(03)00207-2. [DOI] [PubMed] [Google Scholar]
- Parker AM, House JK, Hazelton MS, Bosward KL, Morton JM, Sheehy PA. Bulk tank milk antibody ELISA as a biosecurity tool for detecting dairy herds with past exposure to Mycoplasma bovis. J Dairy Sci. 2017;100:8296–8309. doi: 10.3168/jds.2016-12468. [DOI] [PubMed] [Google Scholar]
- Parker AM, Sheehy PA, Hazelton MS, Bosward KL, House JK. A review of mycoplasma diagnostics in cattle. J Vet Intern Med. 2018;32:1241–1252. doi: 10.1111/jvim.15135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suárez-Pantaleón C, Wichers J, Abad-Somovilla A, Amerongen AV, Abad-Fuentes A. Development of an immunochromatographic assay based on carbon nanoparticles for the determination of the phytoregulator forchlorfenuron. Biosens Bioelectron. 2013;42:170–176. doi: 10.1016/j.bios.2012.11.001. [DOI] [PubMed] [Google Scholar]
- Wawegama NK, Browning GF, Kanci A, Marenda MS, Markham PF. Development of a recombinant protein-based enzyme-linked immunosorbent assay for diagnosis of Mycoplasma bovis infection in cattle. Clin Vaccine Immunol. 2014;21:196–202. doi: 10.1128/CVI.00670-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao G, Hou P, Huan Y, He C, Wang H, He H. Development of a recombinase polymerase amplification combined with a lateral flow dipstick assay for rapid detection of the Mycoplasma bovis. BMC Vet Res. 2018;14:412. doi: 10.1186/s12917-018-1703-x. [DOI] [PMC free article] [PubMed] [Google Scholar]