Abstract
Background and study aims Pancreatic cancer represents the fourth most common cause of cancer-related deaths in Western countries and the need of a low-risk investigation to obtain an accurate histopathological diagnosis has become increasingly pressing. Endoscopic ultrasonography (EUS) with fine-needle aspiration (FNA) is the standard method for obtaining samples from pancreatic masses. In recent years, there has been an increasing need to obtain histological specimens during EUS procedures, rather than cytological ones, to guide oncological treatment options, leading to the so-call “FNB concept.” Different needles have been developed for fine-needle biopsy (FNB) in recent years, enabling acquisition of larger specimens on which to perform histological and molecular analyses. The aim of this narrative review was to assess the role of EUS-guided FNA and FNB in patients with pancreatic masses, and to identify which needle and which acquisition technique should be used to improve tissue acquisition.
Evolution from FNA to FNB
Pancreatic cancer represents the fourth most common cause of cancer-related deaths in Western countries, with a 5-year survival rate of 6 % 1 2 3 .To improve treatment for these patients and to increase their survival, the need for a low-risk investigation has become essential to obtain an accurate histopathological diagnosis 4 . The standard method for sampling pancreatic masses is fine-needle aspiration (FNA) performed under endoscopic ultrasonography (EUS) guidance. The sensitivity, specificity and diagnostic accuracy of FNA for malignant cytological diagnosis have been reported to range between 85 % to 95 %, 95 % to 98 % and 78 % to 95 %, respectively 5 6 . Several studies published in recent years aimed to identify factors related to non-diagnostic or false-negative EUS-FNA sampling; moreover, to improve its diagnostic yield, different sizes of needles and tissue acquisition (TA) techniques, such as fanning technique, slow-pull stylet extraction or suction technique, have been implemented.
Despite data reporting a high sensitivity and specificity, more accurate diagnostic results with EUS-FNA have been linked to the availability of rapid on-site evaluation (ROSE). However, studies evaluating the diagnostic efficacy of EUS-FNA plus ROSE showed a sensitivity and specificity of 83 % (95 %CI, 64 % to 93 %) and 98 % (95 %CI, 80 % to 100 %), compared with 65 % (95 %CI, 57 % to 73 %) and 94 % (95 %CI, 31 % to 100 %) when ROSE was not available 7 8 . Thus, no significant difference regarding diagnostic accuracy was found between the two groups, with no clear benefit from the use of ROSE. Recently, a systematic review with meta-analysis evaluated the pooled diagnostic accuracy of a repeat EUS-FNA (rEUS-FNA) following a previous non-diagnostic result, demonstrating that rEUS-FNA had an optimal pooled specificity (97 %) with high pooled sensitivity (78 %) for diagnosis of pancreatic malignancies. In particular, the pooled sensitivity increased from 65 % (95 %CI 57 – 73) without ROSE to 83 % (95 %CI 64 – 93) when ROSE was available, confirming the beneficial role of ROSE in this setting to increase the amount of definitive diagnoses 9 .
Another limit of EUS-FNA is that it does not allow to obtain a specimen adequate for histological evaluation; therefore, for some pancreatic masses, such as in the presence of autoimmune pancreatitis, a cytological examination obtained with FNA is of limited value 10 .
During recent years, there has been an increasing need to obtain histological specimens during EUS procedures, rather than cytological samples, to guide oncological treatment options. Therefore, the need for TA needles with different shapes and new configurations has led to the concept of fine-needle biopsy (FNB). Several needles for FNB are available with different design and technical features, such as needles with reverse bevel, fork tip or Franseen tip. A histological specimen could avoid the need of a cytopathologist in the endoscopic room, reducing procedures time, number of passes and additional costs of a possible repeated EUS-FNA, in case of an inconclusive diagnosis.
Is FNB better than FNA?
Different articles comparing FNA vs FNB have been published in recent years. Recently, in a prospective comparison study, conducted in a consecutive cohort of 36 patients affected by pancreatic cancer who underwent EUS-TA with 22 G FNA and 22 G FNB, Tian et al. showed that the number of passes needed to obtain a diagnosis was significantly lower for patients who underwent EUS-FNB (1.11 vs 1.83); in particular, the proportion of diagnoses of malignancies obtained with just one needle pass were significantly higher in the FNB group (80 % vs 66.67 %) 11 .
Recently, promising results have been reported by a randomized controlled trial, analyzing tissue and molecular diagnostic yield of FNA and FNB. Comparing the two techniques, median total tissue area for each specimen was significantly higher for FNB (1.9 vs 5.2 mm 2 ), with a consequent higher on-site diagnostic rate. One of the most important novelties arising from this study was that quantification of nucleic acid obtained from the samples was higher in patients who underwent FNB compared to FNA, regarding both DNA and RNA extraction. Indeed, EUS-FNB can obtain more tissue with higher content of nucleic acid allowing downstream genomics applications such as nucleotide genomic sequence assay (NGS). Because it is a convenient and safe method for obtaining tumor samples for genetic analysis, EUS-FNB could play a pivotal role in guiding new genomics therapies 12 .
Other comparative studies showed similar results, in terms of fewer needle passes required to achieve adequate samples and more core TA with a single pass in favor of FNB. A recent retrospective study from Varadarajulu et al. , which included more than 3000 patients, showed that significantly fewer passes were needed to obtain a correct diagnosis using FNB compared to FNA (1 vs 2; P < 0.001), with an increased diagnostic yield on cell-block using FNB (92.3 % vs 71.1 %; P < 0.001) and an overall superior performance for pancreatic lesions ( P < 0.001) 13 .
On the basis of the available literature, FNB has shown a diagnostic yield of more than 90 % 14 . Moreover, the diagnostic performance of FNB sampling of pancreatic masses was significantly better than FNA sampling, and was also associated with ease of diagnosis and shorter viewing times by the pathologists 15 . As a result, presence of an onsite cytopathologist is not required during EUS-FNB sampling, reducing the overall duration of the procedures.
Similar results in terms of diagnostic yield and specimen adequacy for a correct diagnosis were also reported with use of 25 G needles compared to larger ones; however, despite the high diagnostic yield of 25 G needles, the smaller samples obtained with these needles could represent a limit considering advances in new oncological and molecular discoveries related to pancreatic cancer 16 .
In the near future, FNA needles will probably be used only for selected cases, such as patients with complex anatomy or with a high risk of bleeding (portal hypertension, coagulopathies, and deep arterial or venous thrombosis). FNA needles could also be used for therapeutic EUS procedures, such as contrast injection into the pancreatic or bile ducts, release of a guide-wire, or injection therapies 17 .
Which size needle should be used to sample pancreatic masses?
The two most important variables influencing the diagnostic accuracy of EUS-TA of pancreatic masses are the type of needle (FNA and FNB), as previously discussed, and the size (19G, 20G, 22 G or 25 G). Several types and designs of FNB needles are currently available 18 . The first FNB needle was launched in 2011 (ProCore – Wilson-Cook Medical Inc., United States); its main characteristic was the distal reversed bevel design and different sizes of needles were available for sampling: 19 G, 22 G and 25 G. A second-generation 20 G needle with a proximal forward bevel design and “Menghini” tip type was launched in 2015: the EchoTip ProCore HD Ultrasound Biopsy Needle (Wilson-Cook Medical Inc., United States).
Recently, two different types of FNB needles have been introduced in clinical endoscopic practice: one with fork-tip design (SharkCore, Medtronic, Minneapolis, Minnesota, United States), and another with Franseen tip design (Acquire, Boston Scientific, Natick, Massachusetts, United States). A large network meta-analysis from Facciorusso et al. compared different sizes and types of needles for EUS tissue-acquisition, evaluating a total of 2711 patients. The meta-analysis showed that no EUS-guided tissue sampling technique was superior to another, regardless of needle type (FNA vs FNB) or size (19 G vs 22 G vs 25 G). Moreover, no difference was found between 25G FNA and 22G FNA needles (relative risk [RR], 1.03; 95 % confidence interval [CI], 0.91 – 1.17) and between 22 G FNB and 22 G FNA needles (RR, 1.03; 95 % CI, 0.89 – 1.18) in terms of diagnostic accuracy, sample adequacy and histologic core procurement 19 .
Diagnostic performance of the 20G Procore FNB needle was evaluated in a prospective, randomized, multicenter clinical trial compared to the 25 G FNB needles for sampling of solid pancreatic lesions. This study included 88 patients and showed a significantly higher procurement rate of core biopsy specimens among the 20G FNB needle group (41/45, 91.1 %) compared to the 25G FNB needle group (32/43, 74.4 %, P = 0.037). No significant differences were observed in the overall diagnostic accuracy between the 20 G FNB needle (40/45, 88.9 %) and the 25 G FNB (34 /43, 79.1 %, P = 0.208). Therefore, although both FNB needles provided high overall diagnostic accuracy, the reliability of the 20 G FNB needle was superior to the 25 G FNB when retrieving samples for histological analysis 19 , and should be preferred when sampling pancreatic masses.
A recent observational study comparing the 20G Procore vs the 22 G Acquire needles for EUS-FNB of solid pancreatic masses, conducted on 68 patients, showed that histological diagnosis on core biopsy specimens was obtained in 28 of 34 patients (82 %) in the 20 G group and in 33 of 34 patients (97 %) in the 22G group ( P = 0.1). Mean cumulative length of tissue core biopsies per needle pass was significantly higher with the 22G needle with 8.2 ± 4.2 mm versus 4.2 ± 3.8 mm for the 20 G needle ( P < .01), in the absence of intraobserver and inter-observer variability 21 . In our opinion, thinner needles (22 G vs. 20 G) may be easier to maneuver, especially during EUS-FNB of pancreatic masses in the head or the uncinate process, and the EUS scope is bent into the duodenum. Several new TA needles currently exist and none of them has shown a significant superiority in prospective controlled trials. The aim of EUS sampling should be to obtain an adequate sample for diagnosis in more than 90 % of cases, and allow to performance of immunohistochemistry and evaluation of tumor markers, if necessary, to ensure the most appropriate oncological treatment for patients ( Fig. 1 ).
Fig. 1.
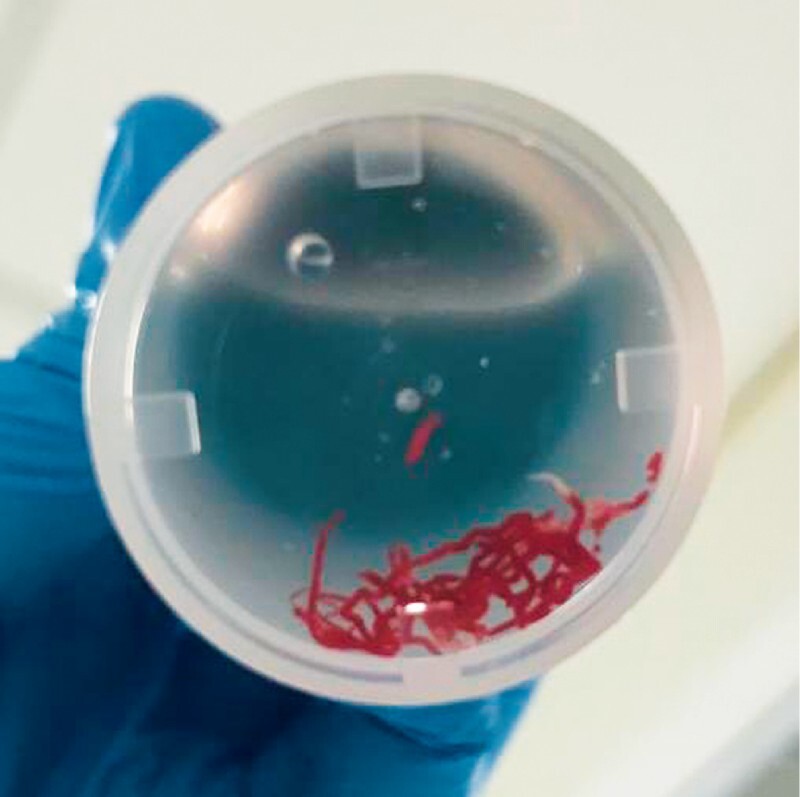
Specimen of a solid pancreatic mass obtained after two passes with a 22G FNB needle.
Is on-site evaluation necessary during FNB procedures?
As previously reported, in some settings, such as during repeat EUS-FNA, ROSE can increase diagnostic accuracy. To evaluate its role during FNB sampling, a multicenter, randomized, non-inferiority trial was conducted in 16 international centers, including a large prospective cohort of patients. The authors randomized 800 patients with solid pancreatic masses to sampling with EUS-FNB plus ROSE or EUS-FNB alone, demonstrating the non-inferiority of FNB alone 22 .
With the advent of FNB, macroscopic on-site evaluation (MOSE) of the specimen by the endosonographer has been proposed as an alternative to ROSE 23 24 . A recent retrospective study including a cohort of 54 patients who underwent TA with a 22G Franseen-tip needle, showed an overall diagnostic accuracy of 94 % adding MOSE to the FNB sampling. Moreover, the reported sensitivity, specificity, positive predictive value, and negative predictive value for malignancy were 92 %, 100 %, 100 %, and 81 %, respectively, with no adverse events 25 .
Which acquisition technique should be used for sampling pancreatic masses?
Techniques for specimen acquisition
There is no a standard technique for TA both for FNA and FNB. Different features influence the acquisition technique, such as the size of the pancreatic lesion, its location, the size and type of needle, and the availability of the ROSE 26 27 28 .
Three main techniques have been described, and endosonographers usually choose which technique to use based on their personal experience. The first technique to have been described was the standard suction technique (SST). Briefly, after the needle tip is placed inside the target lesion, the stylet is pulled-back completely and a syringe, with high negative pressure, is mounted on the handle of the needle. The suction induced by the negative pressure facilitates entry of tissue into the needle. This technique is generally used for FNA but may damage the cellular structure of the tissue and cause considerable blood contamination of the specimen 29 .
The second technique is the stylet slow-pull (SSP) technique. While the endosonographer performs to-and-fro movements with the needle inside the lesion, the stylet is slowly removed to create negative pressure, which allows the tissue to enter the needle 30 31 . The low negative pressure induced by slowly removing the stylet avoids damaging the specimen 32 and reduces blood contamination of the sample 33 34 35 .
The third is the non-suction technique (NST) after stylet removal. After puncturing the targeted lesions, the stylet is completely removed and the needle is moved to-and-fro within the lesion, without a negative pressure syringe mounted onto the needle handle 36 .
Recently, the three TA techniques were compared in a cohort of 50 patients, without using ROSE. The study showed that the rate of a good or excellent proportion of cellularity in the sample was highest when the SSP technique was used compared to the standard suction and non-suction techniques (SSP 72 % vs SST 60 % vs NST 50 %, P = .049). A > 25 % rate of blood contamination was more prevalent in patients in which TA was performed with the standard suction technique (SSP 30 % vs SST 42 % vs NST 10 %, P = .009). The rate of adequate core-TA was not significantly different among the three groups (SSP 52 % vs SST 34 % vs NST 50 %, P = .140).
Use of the SSP technique and tumor size > 40 mm were favorable factors for diagnostic adequacy 37 .
Another TA technique has also recently been proposed, the wet-suction (WS) technique. This technique, rarely used, consists on irrigating the needle with saline solution to replace the air. The saline solution, being liquid, is less compressible than air, allowing better transmission of the negative pressure to the tip of the needle 38 . A RCT compared the WS to the standard suction technique during EUS-FNA in patients with solid masses and showed that WS improved both sample adequacy and quality 39 .
Techniques for targeting lesions
Different techniques have been proposed for targeting lesions during TA. The standard technique (ST) consists of placing the tip of the needle within the lesion, moving the needle to-and-fro on the same axis, regardless of the TA technique applied (standard suction, slow-pull, wet or non-suction technique).
Two other techniques, the fanning technique (FT) and the torque technique (TT), have been proposed to establish accurate diagnosis with fewer needle passes, resulting in shorter procedure duration and lower sedation requirement to carry out the procedure. FT consists of placing the needle in four different areas of the lesion by using the “up-down” wheel of the scope, applying to-and-fro movements four times in each area to procure tissue (4 × 4). This technique was initially proposed by Bang et al. who conducted a RCT comparing the two techniques. No significant differences were found regarding diagnostic accuracy (76.9 % vs. 96.4 %; P = 0.05), technical failure, or complication rates; however, there was a significant difference between the ST and FT in both the number of passes needed to establish a diagnosis and the percentage of patients in whom a diagnosis was achieved with the first needle pass (57.7 % vs. 85.7 %; P = 0.02) 40 .
During the TT, the needle is first advanced into the lateral margin of the targeted lesion. Then, the operator performs repetitive to-and-fro movements while twisting the endoscope without using the left/right wheel. This technique was recently compared to the ST, showing significant differences between the groups regarding the procurement rate for the histologic cores and optimal quality cores (ST vs TT: 87.1 % vs 98.4 %, P = 0.038 and 79.0 % vs 93.5 %, P = 0.037). Sensitivity, specificity, positive predictive value, and negative predictive values for EUS-FNB were 85.5 %, 100 %, 100 %, and 46.7 %, respectively, using the ST, and 96.5 %, 100 %, 100 %, and 71.4 % for the TT. Diagnostic accuracy of the ST and TT was 87.1 % and 96.8 %, respectively. In conclusion, the TT for EUS-TA offered acceptable technical feasibility and superior diagnostic performance, including optimal histologic core procurement, compared with the ST 41 . No study comparing the FT vs TT has yet been published. Nevertheless, most endosonographers combine these three techniques based on their personal experience and on the characteristic of the lesion being targeted.
Conclusions
On the basis of the published data, FNB has proven to be superior to FNA for EUS-TA of pancreatic masses, as it makes it possible to obtain histological specimens that do not require ROSE, reducing the number of needle passes and procedure duration. FNB 22G needles seem to be the best option for sampling pancreatic masses, however, 25G needles can be used for challenging targets, such as small or hypervascular lesions. The fanning technique should be used to obtain specimens from different areas of the target lesion, and the stylet slow-pull technique also should be used to apply a low negative pressure, avoiding fragmentation of the specimen. Finally, FNB-acquired specimens should be evaluated by MOSE to limit needle passes ( Fig. 2 ).
Fig. 2.
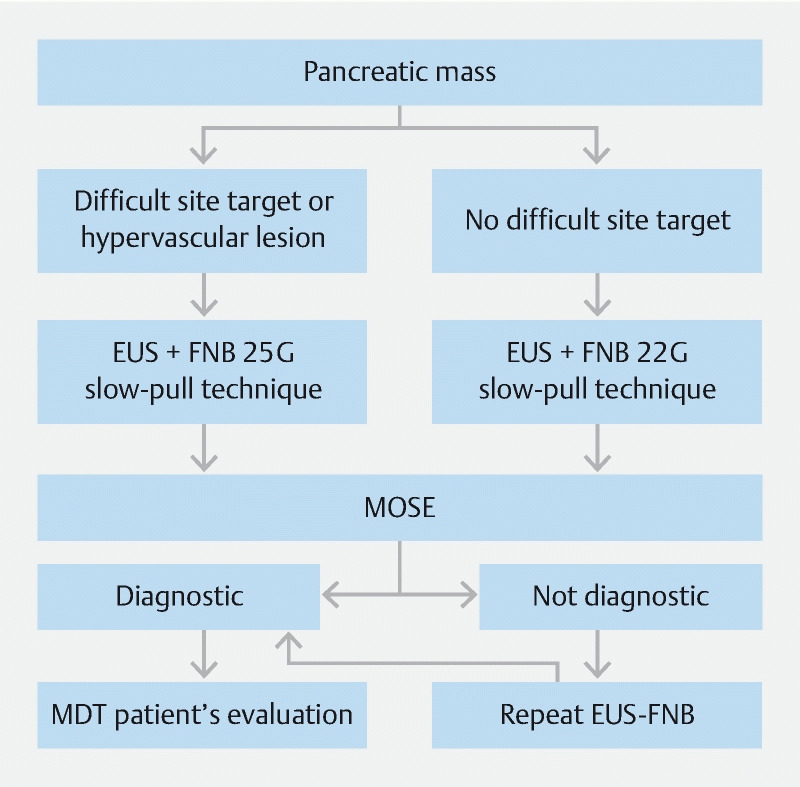
Algorithm for diagnosis of pancreatic masses.
Acknowledgments
Dr. Mangiavillano thank Dr. Shyam Varadarajulu, who changed the panorama of endoscopic ultrasonography with his innovations and with his research in this field; Prof. Laurent Palazzo, who taught him diagnostic and therapeutic EUS; and Prof. Todd H. Baron, who spent time with him me in Paris at the time when heneeded to learn EUS.
Footnotes
Competing interests The authors declare that they have no conflict of interest.
References
- 1.Maitra A, Hruban R H. Pancreatic cancer. Annu Rev Pathol. 2008;3:157–188. doi: 10.1146/annurev.pathmechdis.3.121806.154305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cui X, Zhang Y, Yang J. ZIP4 confers resistance to zinc deficiency-induced apoptosis in pancreatic cancer. Cell Cycle. 2014;13:1180–1186. doi: 10.4161/cc.28111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pietryga J A, Morgan D E. Imaging preoperatively for pancreatic adenocarcinoma. J Gastrointest Oncol. 2015;6:343–357. doi: 10.3978/j.issn.2078-6891.2015.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Del Chiaro M, Segersvard R, Lohr M et al. Early detection and prevention of pancreatic cancer: is it really possible today? World J Gastroenterol. 2014;20:12118–12131. doi: 10.3748/wjg.v20.i34.12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ngamruengphong S, Li F, Zhou Y et al. EUS and survival in patients with pancreatic cancer: a population-based study. Gastrointest Endosc. 2010;72:78–83. doi: 10.1016/j.gie.2010.01.072. [DOI] [PubMed] [Google Scholar]
- 6.Othman M O, Wallace M B. The role of endoscopic ultrasonography in the diagnosis and management of pancreatic cancer. Gastroenterol Clin North Am. 2012;41:179–188. doi: 10.1016/j.gtc.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 7.Kappelle W FW, Van Leerdam M E, Schwartz M P et al. Rapid on-site evaluation during endoscopic ultrasound-guided fine-needle aspiration of lymph nodes does not increase diagnostic yield: A randomized, multicenter trial. Am J Gastroenterol. 2018;113:677–685. doi: 10.1038/s41395-018-0025-8. [DOI] [PubMed] [Google Scholar]
- 8.Fabbri C, Fuccio L, Fornelli A et al. The presence of rapid on-site evaluation did not increase the adequacy and diagnostic accuracy of endoscopic ultrasound-guided tissue acquisition of solid pancreatic lesions with core needle. Surg Endosc. 2017;31:225–230. doi: 10.1007/s00464-016-4960-4. [DOI] [PubMed] [Google Scholar]
- 9.Lisotti A, Frazzoni L, Fuccio L. Repeated EUS-FNA of pancreatic masses after nondiagnostic or inconclusive results: systematic review and meta-analysis. Gastrointest Endosc. 2020;91:1234–1.241E7. doi: 10.1016/j.gie.2020.01.034. [DOI] [PubMed] [Google Scholar]
- 10.Mizuno N, Bhatia V, Hosoda W et al. Histological diagnosis of autoimmune pancreatitis using EUS-guided trucut biopsy: a comparison study with EUS-FNA. J Gastroenterol. 2009;44:742–750. doi: 10.1007/s00535-009-0062-6. [DOI] [PubMed] [Google Scholar]
- 11.Tian L, Tang A L, Zhang L et al. Evaluation of 22G fine-needle aspiration (FNA) versus fine-needle biopsy (FNB) for endoscopic ultrasound-guided sampling of pancreatic lesions: a prospective comparison study. Surg Endosc. 2018;32:3533–3539. doi: 10.1007/s00464-018-6075-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Asokkumar R, Yung Ka C, Loh T et al. Comparison of tissue and molecular yield between fine-needle biopsy (FNB) and fine-needle aspiration (FNA): a randomized study. Endosc Int Open. 2019;7:E955–E963. doi: 10.1055/a-0903-2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bang J Y, Kirtane S, Krall K et al. In memoriam: Fine-needle aspiration, birth: Fine-needle biopsy: The changing trend in endoscopic ultrasound-guided tissue acquisition. Dig Endosc. 2019;31:197–202. doi: 10.1111/den.13280. [DOI] [PubMed] [Google Scholar]
- 14.Ishigaki K, Nakai Y, Oyama H.Endoscopic ultrasound-guided tissue acquisition by 22-Gauge Franseen and standard needles for solid pancreatic lesions Gut Liver 2020 10.5009/gnl19171[Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oppong K W, Bekkali N LH, Leeds J S et al. Fork-tip needle biopsy versus fine-needle aspiration in endoscopic ultrasound-guided sampling of solid pancreatic masses: a randomized crossover study. Endoscopy. 2020;52:454–461. doi: 10.1055/a-1114-5903. [DOI] [PubMed] [Google Scholar]
- 16.Conti C B, Cereatti F, Grassia R. Endoscopic ultrasound-guided sampling of solid pancreatic masses: the fine needle aspiration or fine needle biopsy dilemma. Is the best needle yet to come? World J Gastrointest Endosc. 2019;11:454–471. doi: 10.4253/wjge.v11.i8.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robles-Medranda C, Oleas R, Valero M. Endoscopic ultrasonography-guided deployment of embolization coils and cyanoacrylate injection in gastric varices versus coiling alone: a randomized trial. Endoscopy. 2020;52:268–275. doi: 10.1055/a-1123-9054. [DOI] [PubMed] [Google Scholar]
- 18.James T W, Baron T H. A comprehensive review of endoscopic ultrasound core biopsy needles. Expert Rev Med Devices. 2018;15:127–135. doi: 10.1080/17434440.2018.1425137. [DOI] [PubMed] [Google Scholar]
- 19.Facciorusso A, Wani S, Triantafyllou K et al. Comparative accuracy of needle sizes and designs for EUS tissue sampling of solid pancreatic masses: a network meta-analysis. Gastrointest Endosc. 2019;90:893–903. doi: 10.1016/j.gie.2019.07.009. [DOI] [PubMed] [Google Scholar]
- 20.Cho E, Park C H, Kim T H et al. A prospective, randomized, multicenter clinical trial comparing 25-gauge and 20-gauge biopsy needles for endoscopic ultrasound-guided sampling of solid pancreatic lesions. Surg Endosc. 2020;34:1310–1317. doi: 10.1007/s00464-019-06903-x. [DOI] [PubMed] [Google Scholar]
- 21.Karsenti D, Tharsis G, Zeitoun J D et al. Comparison of 20-gauge Procore® and 22-gauge Acquire® needles for EUS-FNB of solid pancreatic masses: an observational study. Scand J Gastroenterol. 2019;54:499–505. doi: 10.1080/00365521.2019.1599418. [DOI] [PubMed] [Google Scholar]
- 22.Crinò S F, Manfrin E, Scarpa A et al. EUS-FNB with or without on-site evaluation for the diagnosis of solid pancreatic lesions (FROSENOR): Protocol for a multicenter randomized non-inferiority trial. Dig Liver Dis. 2019;51:901–906. doi: 10.1016/j.dld.2019.03.008. [DOI] [PubMed] [Google Scholar]
- 23.Iwashita T, Yasuda I, Mukai T et al. Macroscopic on-site quality evaluation of biopsy specimens to improve the diagnostic accuracy during EUS-guided FNA using a 19-gauge needle for solid lesions: a single-center prospective pilot study (MOSE study) Gastrointest Endosc. 2015;81:177–185. doi: 10.1016/j.gie.2014.08.040. [DOI] [PubMed] [Google Scholar]
- 24.Han P, Che D, Pallav K et al. Models of the cutting edge geometry of medical needles with applications to needle design. Int J Mech Sci. 2012;65:157–67. [Google Scholar]
- 25.Leung Ki E L, Lemaistre A I, Fumex F et al. Macroscopic onsite evaluation using endoscopic ultrasound fine needle biopsy as an alternative to rapid onsite evaluation. Endosc Int Open. 2019;7:E189–E194. doi: 10.1055/a-0770-2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Varadarajulu S, Fockens P, Hawes R H. Best practices in endoscopic ultrasound-guided fine-needle aspiration. Clin Gastroenterol Hepatol. 2012;10:697–703. doi: 10.1016/j.cgh.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 27.Bang J Y, Hebert-Magee S, Trevino J et al. Randomized trial comparing the 22-gauge aspiration and 22-gauge biopsy needles for EUS-guided sampling of solid pancreatic masses. Gastrointest Endosc. 2012;76:321–327. doi: 10.1016/j.gie.2012.03.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tadic M, Stoos-Veic T, Kusec R. Endoscopic ultrasound guided fine needle aspiration and useful ancillary methods. World J Gastroenterol. 2014;20:14292–14300. doi: 10.3748/wjg.v20.i39.14292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wani S. Basic techniques in endoscopic ultrasound-guided fine-needle aspiration: role of a stylet and suction. Endosc Ultrasound. 2014;3:17–21. doi: 10.4103/2303-9027.123008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kudo T, Kawakami H, Hayashi T et al. High and low negative pressure suction techniques in EUS-guided fine-needle tissue acquisition by using 25-gauge needles: a multicenter, prospective, randomized, controlled trial. Gastrointest Endosc. 2014;80:1030–1037. doi: 10.1016/j.gie.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 31.Nakai Y, Isayama H, Chang K J et al. Slow pull versus suction in endoscopic ultrasound-guided fine-needle aspiration of pancreatic solid masses. Dig Dis Sci. 2014;59:1578–1585. doi: 10.1007/s10620-013-3019-9. [DOI] [PubMed] [Google Scholar]
- 32.Matsubayashi H, Matsui T, Yabuuchi Y et al. Endoscopic ultrasonography guided-fine needle aspiration for the diagnosis of solid pancreaticobiliary lesions: Clinical aspects to improve the diagnosis. World J Gastroenterol. 2016;22:628–640. doi: 10.3748/wjg.v22.i2.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen J Y, Ding Q Y, Lv Y et al. Slow-pull and different conventional suction techniques in endoscopic ultrasound- guided fine-needle aspiration of pancreatic solid lesions using 22-gauge needles. World J Gastroenterol. 2016;22:8790–8797. doi: 10.3748/wjg.v22.i39.8790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang J, Wu X, Yin P et al. Comparing endoscopic ultrasound (EUS)-guided fine needle aspiration (FNA) versus fine needle biopsy (FNB) in the diagnosis of solid lesions: study protocol for a randomized controlled trial. Trials. 2016;17:198. doi: 10.1186/s13063-016-1316-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kin T, Katanuma A, Yane K et al. Diagnostic ability of EUS-FNA for pancreatic solid lesions with conventional 22-gauge needle using the slow pull technique: a prospective study. Scand J Gastroenterol. 2015;50:900–907. doi: 10.3109/00365521.2014.983155. [DOI] [PubMed] [Google Scholar]
- 36.Sahai A V, Paquin S C, Gariepy G. A prospective comparison of endoscopic ultrasound-guided fine needle aspiration results obtained in the same lesion, with and without the needle stylet. Endoscopy. 2010;42:900–903. doi: 10.1055/s-0030-1255676. [DOI] [PubMed] [Google Scholar]
- 37.Lee K Y, Cho H D, Hwangbo Y et al. Efficacy of 3 fine-needle biopsy techniques for suspected pancreatic malignancies in the absence of an on-site cytopathologist. Gastrointest Endosc. 2019;89:825–831. doi: 10.1016/j.gie.2018.10.042. [DOI] [PubMed] [Google Scholar]
- 38.Villa N A, Berzosa M, Wallace M B et al. Endoscopic ultrasound-guided fine needle aspiration: The wet suction technique. Endosc Ultrasound. 2016;5:17–20. doi: 10.4103/2303-9027.175877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Attam R, Arain M A, Bloechl S J et al. “Wet suction technique (WEST)”: a novel way to enhance the quality of EUS-FNA aspirate. Results of a prospective, single-blind, randomized, controlled trial using a 22-gauge needle for EUS-FNA of solid lesions. Gastrointest Endosc. 2015;81:1401–1407. doi: 10.1016/j.gie.2014.11.023. [DOI] [PubMed] [Google Scholar]
- 40.Bang J Y, Magee S H, Ramesh J et al. Randomized trial comparing fanning with standard technique for endoscopic ultrasound-guided fine-needle aspiration of solid pancreatic mass lesions. Endoscopy. 2013;45:445–450. doi: 10.1055/s-0032-1326268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park S W, Lee S S, Song T J et al. The diagnostic performance of novel torque technique for endoscopic ultrasound-guided tissue acquisition in solid pancreatic lesions: A prospective randomized controlled trial. Gastroenterol Hepatol. 2020;35:508–515. doi: 10.1111/jgh.14840. [DOI] [PubMed] [Google Scholar]


