Abstract
Background
Data on mepolizumab in patients with severe eosinophilic asthma (EA) and comorbidities are needed to assess whether randomized controlled trial results are applicable in the real world.
Objective
To evaluate real-life effectiveness and the presence/absence of predictors of treatment response in patients with one or more comorbidities (nasal polyps, allergic rhinitis, gastro-esophageal reflux disease, nonallergic rhinitis with eosinophilia syndrome, obesity, bronchiectasis) who received mepolizumab (MEPO) for the treatment of severe EA.
Methods
We performed a single-center retrospective study in patients with severe asthma and presence of comorbidities treated with mepolizumab at the respiratory outpatient clinic, Policlinico-Vittorio Emanuele, Catania, Italy. Health records of 31 severe asthmatic patients were retrieved and analyzed. Asthma control test (ACT) score, blood eosinophil count, forced expiratory volume in 1 s (FEV1), FEV1% of predicted and FEV1/FVC (Forced Vital Capacity) ratio, oral corticosteroid (OCS) dosage, and exacerbations were recorded at baseline (T0), after 3 (T1), 6 (T3), 9 (T6), and 12 months (T12). Clinical response was defined when 3 of these 4 criteria were fulfilled: i) 30% exacerbation decrease; ii) 80% blood eosinophilia reduction; iii) 3 point ACT increase; iv) FEV1 increase ≥200 mL.
Results
83.87% of patients were classified as responsive to MEPO treatment. Substantial depletion of the blood eosinophils (>80%) was found in 87.1% of patients, FEV1 > 200 mL was seen in 54.84% of patients, a 3-point ACT improvement from baseline was recorded in 80.65% 25 of patients and a 30% reduction of exacerbations rates was seen in 96.77% of patients. Moreover, the majority 38.71% of patients met 3/4 parameters after 12 months. Neither the comorbidities nor other characteristics (sex, BMI, age, smoking) influenced treatment response.
Conclusions
MEPO in patients with severe EA is effective regardless of the presence of comorbidities.
Keywords: Mepolizumab, Severe eosinophilic asthma, Multiple comorbidities
Abbreviations: ACT, Asthma Control Test; BMI, Body Mass Index; DREAM, Dose Ranging Efficacy And safety with Mepolizumab; EA, Eosinophilic Asthma; ERS/ATS, European Respiratory Society/American Thoracic Society; ECRS, Eosinophilic Chronic Rhinosinusitis; FEV1, Forced Expiratory Volume in 1 s; FVC, Forced Vital Capacity; FEV1/FVC, Forced Expiratory Volume in 1 s/Forced Vital Capacity ratio; GERD, Gastro-Esophageal Reflux Disease; GINA, Global INitiative for Asthma; IgG, Immunoglobulin G; IL-5, Interleukin-5; IQR, Interquartile Range; MEPO, Mepolizumab; NARES, Non Allergic Rhinitis with Eosinophilia Syndrome; OCS, Oral Corticosteroid; RCTs, Randomized Controlled Trials; RV, Residual Volume; SD, Standard Deviation; SEM, Standard Error Mean; T0, baseline; T1, 3 months after baseline; T3, 6 months after baseline; T6, 9 months after baseline; T12, 12 months after baseline
Introduction
Severe eosinophilic asthma (EA) is a subtype of asthma characterized by persistent eosinophilic airway inflammation and recurrent exacerbations despite treatment with high doses of glucocorticoids.1
In the last decade, several biological molecules with a steroid-sparing effect have been introduced in the field of severe asthma. Mepolizumab (MEPO) is an IgG1/k class humanized monoclonal antibody approved in patients ≥12 years of age for the treatment of moderate-to-severe eosinophilic asthma, owing to its ability to block circulating interleukin-5 (IL-5) responsible for eosinophil development, maturation, and survival.2
In large placebo-controlled trials, treatment with MEPO was well tolerated, resulting in a substantial fall in blood eosinophils and a significant reduction of intake/dosage of oral corticosteroids (OCS), reduction of exacerbations, and an overall improvement of lung function.3, 4, 5, 6
In practice, MEPO was shown to change the course of severe eosinophilic asthma thanks to its ability to reduce asthma exacerbation rates and improve quality of life in these patients, as clearly outlined in a meta-analysis of 7 randomized controlled trials (RCTs).7 Moreover, severe eosinophilic asthma, just like asthma, can be associated with several comorbidities (eg, nasal polyposis, gastro-esophageal reflux disease (GERD), bronchiectasis, allergic and nonallergic rhinitis, obesity) which have a consistent impact on treatment outcome, asthma symptoms, risk of exacerbations, and patient's quality of life.8, 9, 10, 11
Recently, researchers have been trying to identify, based on the presence of comorbidities or lifestyle habits (ie, smoking), specific asthma phenotypes with the ultimate goal of personalizing the therapeutic approach. However, at present, the characteristics of these phenotypes and the impact of treatment on each of them are still not fully answered questions.12
Thus, the monitoring of new biological agent effectiveness in real-life practice may provide, in a heterogeneous disease like asthma, relevant data complementary to those of randomized control trials.13 Moreover, a detailed assessment of comorbidities in patients with severe eosinophilic asthma is important for clinical practice and, to the best of our knowledge, has not been outlined yet.
Under this perspective, we retrospectively examined a group of patients all presenting one or more comorbidity who received MEPO for the treatment of severe eosinophilic asthma in order to evaluate its real-life effectiveness, determine whether the presence of the comorbidities modifies the treatment response, and explore the presence/absence of potential predictors for treatment response.
Methods
Study design and subjects
This was a single-center, retrospective study based on health records of patients who consulted a specialist from January 2018 to June 2019 at respiratory outpatient clinic, Azienda Ospedaliera Policlinico-Vittorio Emanuele di Catania. All outpatients ≥12 years of age prescribed with MEPO were included in the study. Severity at baseline was defined according to the GINA guidelines.14
All patients met the criteria for severe uncontrolled asthma according to the ATS/ERS guidelines1 and received MEPO 100 mg subcutaneously every 4 weeks from T0 for at least 12 months (T12). All patients had >150 eosinophils/μl and a history of at least 300 eosinophils/μl in the previous 12 months. Treatment compliance was strictly assessed at each clinical visit. Socio-demographic characteristics (age, sex, body mass index, smoking status, age at onset of asthma, sensitization to perennial aeroallergens) were included in the database as well as the presence of any comorbidities (nasal polyps, allergic rhinitis, GERD, nonallergic rhinitis with eosinophilia syndrome - NARES, obesity, bronchiectasis), which were objectively assessed according to standardized definitions and eventually confirmed by additional tests which are described in the online supplement (Supplementary 1). According to ERS/ATS guidelines, patients with other respiratory diseases that may share common clinical manifestations of severe asthma (ie, bronchopulmonary aspergillosis, vasculitis, chronic cough) were excluded.1 This study used anonymous retrospective claims data; as such, it did not require institutional review board review and approval or informed consent. Moreover, as it refers to outpatients treated with drugs already approved by regulatory agencies, it does not need approval by the Ethics Committee.
Measurements
The health records for each patient were recorded at baseline (T0), after 3 (T1), 6 (T3), 9 (T6), and 12 months (T12) of treatment with MEPO. The following parameters were assessed: asthma control test (ACT) score,15 blood eosinophil count, forced expiratory volume in 1 s (FEV1), FEV1% of predicted, and FEV1/FVC (forced vital capacity) ratio. Spirometry was performed according to the ATS/ERS guidelines.16 FEV1 and FVC were measured using a spirometer (Sensormedics, Milan, Italy). The best value of 3 consecutive maneuvers was expressed as the percentage of the normal value. After the baseline assessment, spirometry was repeated 15 min after administration of salbutamol (400 μg). Reversibility of airway obstruction was expressed in terms of percentage change from baseline FEV1. Monthly intake (mg) of prednisone and exacerbations (per period of time, corrected per year and calculated as episodes requiring systemic corticosteroid treatment for at least 3 days, and/or emergency visit or hospitalization for acute asthma) were also included in the database for all time points.
Evaluation of the response to mepolizumab
We selected 5 parameters which are crucial in the treatment of severe eosinophilic asthma, and accordingly, patients were divided into 2 groups: responders and non-responders.
Clinical relevant response was defined as: i) a 30% decrease in the exacerbations rate;17 ii) an improvement in pulmonary function (FEV1 ≥ 200 mL) by analogy to the cut-offs used by the Global Lung Initiative;14 iii) an 80% reduction of eosinophils in peripheral blood from baseline by analogy to the approval studies of mepolizumab;3, 4, 5, 6 and iv) a change in ACT from baseline, whereby minimal clinically relevant difference was defined as an ACT score of 3 points.18
We did not include OCS reduction as a clinical response parameter, as not every single patient was on continuous OCS at T0. Fulfilling at least 3 of the 4 components of the primary outcome was considered a treatment success.
Statistical analysis
Statistical analysis was performed using GraphPad Prism v8.0 (Graphpad Software, Inc., La Jolla, CA). Categorical variables are stated as numbers (n) and percentages (%).
Results are reported as mean ± standard deviation (SD) if normally distributed and median and interquartile range (IQR) if non-normally distributed unless indicated otherwise. Comparisons were performed using Chi-squared or Fisher's exact test for categorical data and Student's t-test or Wilcoxon matched-pairs signed-rank test or Mann-Whitney-U-test for continuous data.
The normality of data distribution was checked using the Shapiro-Wilk test. A logistic regression model was created to determine the effects of comorbidities, eosinophil count, body mass index, smoking, age, and gender on the outcomes. A p-value <0.05 was considered as statistically significant.
Results
Assessment of all patients
We analyzed the data from 31 patients (mean age 52.35 years; 58% females) with severe eosinophilic asthma and on treatment with MEPO. Patients’ socio-demographic characteristics, including smoking status and comorbid conditions, are displayed in Table 1. The pre-treatment IgE value of all patients involved in the study was 181 UI/mL (interquartile from 88 UI/mL to 355 UI/mL) (data not shown).
Table 1.
Demographic and baseline characteristics.
| Characteristic | All (n = 31) |
|---|---|
| Age (years), mean (SD) | 52.35 (9.714) |
| Female sex, n (%) | 18 (58) |
| Male sex, n (%) | 13 (42) |
| Body Mass Index, mean (SD) | 26.68 (5.237) |
| Diagnosis of asthma, years, median (IQR) | 15 (10–23) |
| Blood eosinophils, mean (range) median (SEM) |
1219 (293–7180) 791 (273.8) |
| FEV1 (l), mean (SD) | 2.15 (0.81) |
| OCS therapy dependent, n (%) | 21 (67.7) |
| OCS mg/30days, median (IQR) | 56.25 (0 -112-.5) |
| Number of exacerbations/year, median (IQR) | 6 (4–12) |
| Smoking status, n (%) | |
| Active smoker | 3 (10) |
| Ex-smoker | 7 (22) |
| Non-smoker | 21 (68) |
| Comorbid conditions prevalence, n (%) | |
| Nasal polyps | 24 (77.4) |
| GERD | 10 (32.2) |
| NARES | 12 (38.7) |
| Obesity | 11 (35.5) |
| Allergy | 22 (71) |
| Bronchiectasis | 17 (54.8) |
SD, standard deviation; IQR, interquartile range; SEM, standard error mean; GERD, Gastro-esophageal reflux disease; NARES, Nonallergic rhinitis with eosinophilia syndrome
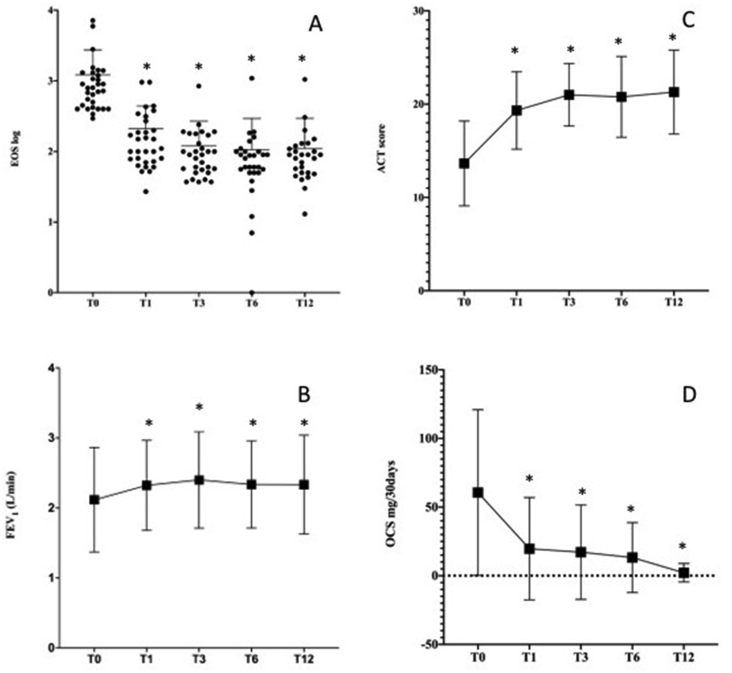
The variables at baseline and 12 months (T12) are shown in Table 2. The overall median blood eosinophil count decreased from 791 cells/ul (IQR 420–1300) at baseline to 80 cells/ul (IQR 43–109) at T12 (p > 0.0001). As shown in Fig. 1A, the median decrease was already significant (p < 0.0001) at the first time point (T1, 3 months) and was sustained at each consecutive time point.
Table 2.
Summary of effectiveness outcomes.
| Before treatment | After treatment | p value | |
|---|---|---|---|
| FEV1% of predicted, mean (SD) | 73.68 (21.43) | 82.94 (21) | 0.0069 |
| FEV1 (L), mean (SD) | 2.11 (0.748) | 2.33 (0.70) | 0.0224 |
| FEV1/FVC, mean (SD) | 69.48 (15.52) | 69.31 (11.23) | Ns |
| ACT, mean (SD) | 13.65 (4.54) | 21.29 (4.49) | <0.0001 |
| Blood eosinophils, median (IQR) | 791 (420–1300) | 80 (43–109) | <0.0001 |
| OCS therapy dependent, n (%) | 21 (67.7) | 5 (16.1) | <0.0001 |
| OCS mg/30days, median (IQR) | 56.25 (0–112.5) | 0 (0–0) | 0.0012 |
| Number of exacerbations/year, median (IQR) | 6 (4–12) | 0 (0–1) | <0.0001 |
SD, standard deviation; IQR, interquartile range; OCS, oral corticosteroid; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; ACT, asthma control test
Fig. 1.
Comparison of clinical parameters from baseline (T0) to 12 months (T12). (A) Median blood eosinophil count; (B) Mean of FEV1; (C) Mean of ACT score; (D) Median of OCS mg/30days. ∗indicates p < 0.05. EOS, Eosinophil count; FEV1, forced expiratory volume in 1 s; ACT, asthma control test; OCS, oral corticosteroids
A significant change in mean predicted FEV1 and FEV1% compared to baseline was observed at T12 (2.12 ± 0.75 versus 2.33 ± 0.7; p = 0.0224) and (73.68 ± 21.43 versus 82.94 ± 21; p = 0.0069), respectively. As shown in Fig. 1B, mean FEV1 increase was already significant (p = 0.0455) at T1 and was sustained at each consecutive time point. FEV1/FVC was significantly different from baseline only at T1 (79.19 ± 16.38; p = 0.0036) and at T3 (82.71 ± 16.24; p = 0.0001), but not at T6 and T12 (data not shown).
There was a significant improvement in ACT after treatment with MEPO, with a mean of 13.65 ± 4.54 points at baseline and 21.29 ± 4.49 points at T12 (p > 0.0001). As shown in Fig. 1C, also for this outcome, the mean increase was already significant (p = 0.0455) at T1 and was sustained at each consecutive time point.
At baseline, 67.7% of patients were on continuous OCS therapy with a median 30-days dose of 59.25 mg (IQR 0–112.5) of prednisone. Both OCS rates and dosage were significantly reduced at T12. Only 16.1% were still on OCS (p < 0.0001), with a lower median 30-day dose of 0 mg (IQR 0-0).
After 1 year of MEPO treatment, we observed a significant difference in the number of exacerbations/year (6, IQR 4–12 vs. 0, IQR 0–1; p < 0.00001). All patients except one (96.77%) reduced their number of exacerbations by at least 70%. In particular, of the 17 patients (54.93%) who had more than 5 exacerbations in the year before therapy, 100% had no exacerbations at T12.
Finally, no adverse effects were observed in our cohort.
Assessment of patients based on comorbidities
All patients had at least 1 comorbidity with a median (IQR) number of comorbidities of 3.2, 3, 4
In order to evaluate the potential impact of comorbidities on treatment effect, a comparison of blood eosinophils count, ACT score, FEV1 values, and OCS median dose among the 6 groups of patients with/without nasal polyps, GERD, NARES, obesity, bronchiectasis, and allergy was performed. Table 3 shows that there was no significant difference at baseline between groups with/without comorbidities, with the only exception of ACT score between patients without and with GERD (12.52 ± 5.47 versus 16 ± 4.807, respectively; p = 0.0443).
Table 3.
Clinical parameters by comorbidities at baseline and at ΔT12-T0.
| Baseline | ΔT12-T0 | |||||
|---|---|---|---|---|---|---|
|
Nasal polyps | ||||||
|
Without (7) |
With (24) |
p value |
Without (7) |
With (24) |
p value |
|
| FEV1 (L) | 2.024 (1.002) | 2.146 (0.68) | 0.712 | 0.284 (0.52) | 0.15 (0.508) | 0.7208 |
| ACT | 15.57 (5.028) | 13.08 (4.343) | 0.266 | 5.286 (6.157) | 8.333 (4.556) | 0.2585 |
| OCS mg/30days | 112.5 (0–112.5) | 16.88 (0–112.5) | 0.51 | −112.5 (−112.5–0) | - 11.25 (- 11.25–0) | 0.6997 |
| Exacerbations/year | 12 (4–12) | 6 (3.25–12) | 0.312 | −11 (−12–−4) | −6 (−12–−2.25) | 0.284 |
| Eosinophils |
1200 (500–1400) |
711 (400–1063) |
0.245 |
−995 (−1200–−500) |
−640 (−927 to −347) |
0.0395 |
| GERD | ||||||
|
Without (21) |
With (10) |
p value |
Without (21) |
With (10) |
p value |
|
| FEV1 (L) | 2.033 (0.782) | 2.398 (0.871) | 0.366 | 0.259 (0.424) | 0.02 (0.64) | 0.302 |
| ACT | 12.52 (5.47) | 16 (4.807) | 0.044 | 8.048 (5.47) | 6.8 (4.022) | 0.482 |
| OCS mg/30days | 56.25 (0–112.5) | 67.5 (0–112.5) | 0.95 | −33.75 (−112.5–0) | −56.25 (−112.5–0) | 0.835 |
| Exacerbations/year | 8 (4–12) | 6 (2.75–12) | 0.471 | −7 (−12–−4) | −5.5 (−12–−2) | 0.665 |
| Eosinophils |
800 (460–1135) |
755.5 (400–1764) |
0.811 |
−700 (−987.5–−420) |
−515.5 (−1569–−386.8) |
0.811 |
|
Obesity | ||||||
|
Without (20) |
With (11) |
p value |
Without (20) |
With (11) |
p value |
|
| FEV1 (L) | 2.295 (0.950) | 1.868 (0.515) | 0.134 | 0.142 (0.355) | 0.09 (0.59) | 0.791 |
| ACT | 14.85 (4.082) | 12.09 (5.224) | 0.148 | 7.25 (4.529) | 8 (6.017) | 0.7 |
| OCS mg/30days | 112.5 (2.813–112.5) | 22.5 (0–98.44) | 0.065 | −112.5 (−112.5 – 2.813) | −21.5 (−111.5–0) | 0.061 |
| Exacerbations/year | 6 (4–12) | 8 (4–12) | 0.961 | −6 (−12–−4) | −7 (−12–−3) | 0.88 |
| Eosinophils |
711 (405–1200) |
1040 (550–1418) |
0.42 |
−653.5 (−960.8–−400) |
−743 (−1110–−368.0) |
0.8 |
| Bronchiectasis | ||||||
|
Without (14) |
With (17) |
p value |
Without (14) |
With (17) |
p value |
|
| FEV1 (L) | 2.133 (0.793) | 2165 (0.858) | 0.925 | 0.173 (0.49) | 0.189 (0.534) | 0.932 |
| ACT | 13.5 (4.926) | 13.76 (4.352) | 0.877 | 6.643 (5.583) | 8.471 (4.501) | 0.332 |
| OCS mg/30days | 22.5 (0–112.5) | 112.5 (0–112.5) | 0.334 | −11.25 (−112.5–0) | −112.5 (−112.5–0) | 0.313 |
| Exacerbations/year | 4 (2.75–12) | 12 (5–12) | 0.082 | −4 (-9–−2) | −11 (−12–−5) | 0.066 |
| Eosinophils |
970 (437.5–1449) |
720 (410–985) |
0.32 |
−685 (−1248–−358.5) |
−657 (−919–−370) |
0.604 |
|
NARES | ||||||
|
Without (19) |
With (12) |
p value |
Without (19) |
With (12) |
p value |
|
| FEV1 (L) | 2.144 (0.7882) | 2.078 (0.712) | 0.984 | 0.127 (0.469) | 0.269 (0.570) | 0.479 |
| ACT | 13.79 (4.709) | 13.42 (4.461) | 0.826 | 7.526 (5.621) | 7.833 (4.108) | 0.862 |
| OCS mg/30days | 56.25 (0–112.5) | 22.5 (0–112.5) | 0.553 | −56.25 (−112.5–0) | −22.5 (−112.5–0) | 0.742 |
| Exacerbations/year | 8 (4–12) | 6 (3.25–12) | 0.56 | −7 (-12–−4) | −6 (−11.75–−2.5) | 0.719 |
| Eosinophils |
898 (500–1400) |
691 (400–1235) |
0.482 |
−700 (−1110–−368.0) |
−653.5 (−983.8–−345.8) |
0.726 |
| Allergy | ||||||
|
Without (9) |
With (22) |
p value |
Without (9) |
With (22) |
p value |
|
| FEV1 (L) | 2.421 (0.675) | 1.995 (0.7558) | 0.142 | 0.136 (0.571) | 0.2 (0.49) | 0.772 |
| ACT | 14 (4.5) | 13.5 (4.657) | 0.784 | 4.778 (4.764) | 8.818 (4.727) | 0.048 |
| OCS mg/30days | 56.25 (0–112.5) | −39.38 (−112.5–0) | 0.977 | −33.75 (−112.5–0) | −39.38 (−112.5–0) | 0.681 |
| Exacerbations/year | 12 (2.5–12) | 6 (4–12) | 0.741 | −8 (−11.5–−1.5) | −6 (−12–−4) | 0.640 |
| Eosinophils | 702 (410–1364) | 795.5 (437.5–1225) | 0.888 | −500 (-979-5 to −333.5) | −721.5 (−1024–−398.3) | 0.384 |
ACT values are expressed ad mean (SD), OCS mg/30days as median (IQR), Number of exacerbations/year as median (IQR), Eosinophils as median (IQR), FEV1 as mean (SD). SD, standard deviation; IQR, interquartile range; OCS, oral corticosteroid; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; ACT, asthma control test; ns, not significant
We did not find any significant difference for ΔT12-T0 in any of the analyzed clinical parameters among patients with and without comorbidities, with the only exceptions of patients without nasal polyps, who showed a greater blood eosinophil reduction than those with nasal polyps (−995, IQR −1200 to −500 vs. −640, IQR −927 to −347; p = 0.0395), and patients with allergy, who showed a greater ACT score than those without allergy (8.818 ± 4.727 vs 4.778 ± 4.764; p = 0.048) (Table 3).
The association between the median number of observed comorbidities and the ΔT12-T0 of ACT mean scores (1–2 comorbidities: 4.625 ± 5.878; 3 comorbidities: 8.615 ± 5.06; ≥4 comorbidities: 8.8 ± 3.46; p = 0.1412), of FEV1 mean values (1–2 comorbidities: 0.22 ± 0.304, 3 comorbidities: 0.1662 ± 0.537, ≥4 comorbidities: 0.173 ± 0.628; p = 0.9719), OCS median dose (1–2 comorbidities: 112.5, IQR-140.6 to −19.69, 3 comorbidities: 112.5, IQR −112.5 to −90, ≥4 comorbidities: 84.38, IQR −112.5 to −30.94; p = 0.7741) and median blood eosinophilia (1–2 comorbidities: 645.5, IQR −109.5 to −380, 3 comorbidities: 657, IQR −1087 to −380, ≥4 comorbidities: 696.5, IQR −1013 to −375; p = 0.9933) was not significant.
Clinical response
According to our clinical response parameters, 83.87%26 of patients were classified as responsive to MEPO treatment. A substantial depletion of the blood eosinophils (less than 80% from baseline) was found in 87.1% of patients, improvement in lung function (FEV1 > 200 mL) was seen in 17 patients (54.84%), 3-point improvement in ACT from baseline was recorded in 25 patients (80.65%) and a 30% reduction of exacerbations rates was seen in 30 patients (96.77%).
Moreover, the majority of patients (38.71%) met 3/4 parameters after 12 months, as shown in Table 4 (Table 4).
Table 4.
Response to treatment with mepolizumab.
| N | % | |
|---|---|---|
| Overall response | 26 | 83.87 |
| Outcome summary (number of fulfilled parameters) | ||
| 4/4 | 12 | 38.71 |
| 3/4 | 14 | 45.16 |
| 2/4 | 4 | 12.9 |
| 1/4 | 1 | 3.22 |
| 0/4 | 0 | 0 |
| With Nasal polyps | 21 | 87.5 |
| With GERD | 8 | 72.72 |
| With NARES | 9 | 75 |
| With Obesity | 9 | 87.5 |
| With Allergy | 18 | 81.82 |
| With Bronchiectasis | 14 | 82.35 |
GERD, Gastro-esophageal reflux disease; NARES, Nonallergic rhinitis with eosinophilia syndrome
The characteristics of the 5 non-responding patients are summarized in Table 5.
Table 5.
Characteristics of non-responder patients.
| Patient | Age | Comorbidity | Missed Outcome |
|---|---|---|---|
| A | 70 | Bronchiectasie | FEV1↑, ACT↑ |
| B | 46 | Nasal polyps, GERD, Obesity | FEV1↑, ACT↑, Blood eosinophils↓ |
| C | 69 | GERD, NARES, Allergy | FEV1↑, ACT↑ |
| D | 55 | Nasal polyps, NARES, Allergy, Bronchiectasie | FEV1↑, ACT↑ |
| E | 41 | Nasal polyps, GERD, NARES, Allergy | FEV1↑, Blood eosinophils↓ |
GERD, Gastro-esophageal reflux disease; NARES, Nonallergic rhinitis with eosinophilia syndrome; FEV1, forced expiratory volume in 1 s; ACT, asthma control test
Predictive factors
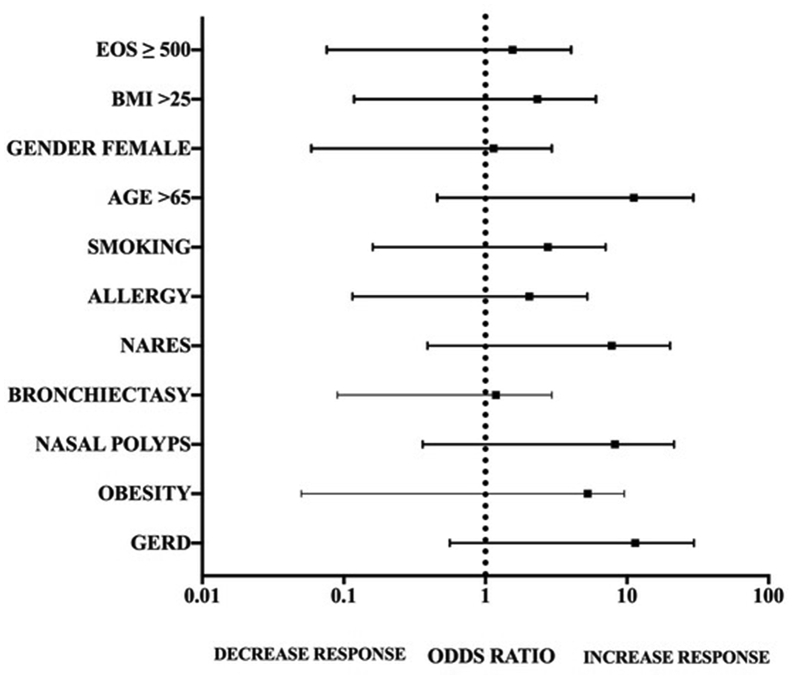
In order to identify potential predictive factors of MEPO response, we analyzed if every single comorbidity, and smoking status, gender (female), age ≥65 years-old, BMI ≥ 25 kg/m2, and blood eosinophil count ≥ 500/mm3 of these 31 patients were associated with allocation to a specific treatment response group (responders or non-responders). As shown in Fig. 2, each of the analyzed variables achieved a significant value of p > 0.05 in the univariate model; thus, none of them influenced allocation to a specific treatment response group.
Fig. 2.
Analysis of potential predictors of treatment outcome. EOS, eosinophil count; BMI, body mass index; GERD, Gastro-esophageal reflux disease; NARES, Nonallergic rhinitis with eosinophilia syndrome
Discussion
Our study assessed not only the efficacy of mepolizumab in patients with severe eosinophilic asthma complicated by the presence of one or more comorbidities but also whether these affected the treatment outcome or not.
Our first analysis led us to conclude that the treatment with mepolizumab for one year substantially improved all the analyzed clinical parameters. Mepolizumab resulted in a significant reduction in asthma exacerbations, use and dose of OCS, blood eosinophilia and a concomitant improvement in pulmonary function and asthma symptoms control in all patients.
The overall response rate was of 83.87%. In particular, blood eosinophil count decreased by 89.89%, a 3-point improvement in ACT from baseline was recorded in 80.65% of patients and exacerbations rates were virtually zeroed, as 96.77% of patients had a reduction in the number of exacerbations by minimum 30% and at least 70% during the year of treatment with mepolizumab.
Also, a sharp reduction in the use of OCS was recorded in our cohort, as 84% of patients discontinued the OCS at follow-up, a percentage higher than so far reported in other studies.19, 20, 21, 22, 23
In our cohort, FEV1 values increased only by 9% and the FEV1/FVC difference between follow-up and baseline was statistically significant at 3 months but not at 12 months; these data seem to align with those of other studies.21 The FEV1/FVC result could be explained as a concomitant increase of airway caliber and reduction in the residual volume (RV), which usually takes place in response to asthma treatment and improves both FEV1 and FVC.24
Overall, our results are comparable with those attained in both randomized and real-life analyses.3, 4, 5, 6,20, 21, 22, 23,25,26
Also, it is important to underline that our data not only confirm the efficacy of MEPO but also highlight the rapidity of the therapeutic effect. In our study, a significant improvement in FEV1 and blood eosinophil count was already evident after 3 months and was sustained for 12 months. The quick beneficial effect of MEPO was in accordance with the reported patient's ACT score, which also significantly improved within the first 3 months. An equally rapid response, even within the first month of treatment, has been highlighted in other real-life studies.19,20,22,23,25
In our cohort, only 5 patients did not exhibit, according to our criteria, an effective response to treatment. These patients had an average age of 56.2 years old (minimum 50, maximum 70) and had distinctive comorbidities or combinations of comorbidities without a recurrent pattern. The outcome that was mostly not achieved among these 5 patients was the increase of 200 mL in FEV1, followed by the 3-point increase in the ACT score, and in only 2 patients the 80% decrease in the level of eosinophils in the blood. However, our clinical response cut-off was particularly stringent and, in general, the clinical conditions of these patients were ameliorated by MEPO therapy.
Our second analysis questioned whether patients with specific comorbidities achieved different results in treatment outcomes. Among patients with or without a comorbidity, we did not find any statistically significant difference. The only exceptions were patients without nasal polyps, who showed a more significant reduction in blood eosinophilia than patients with nasal polyps (p = 0.0395), and patients with allergy, who showed a more considerable improvement in their ACT score than those without allergy (p = 0.048).
Not even the number of comorbidities influenced treatment with MEPO, as no difference in achieving the therapeutic success was found among patients having 1–2 comorbidities, 3 comorbidities or more than 4 comorbidities.
These data are particularly useful to assess the role of mepolizumab better as we provide an insight into real-life characteristics of all eligible patients. Our 31 patients had a median number of 3 comorbidities, a situation that differed largely from that of RCTs, in which patients do not present any concomitant disease. In this regard, a recent real-life study by Bagnasco and colleagues compared the characteristics at baseline of its cohort with those of patients enrolled in MEPO RCTs.27 Their results underline how real-life patients were characterized by a greater age, a worse lung function, a higher level of eosinophilia, and a higher dosage of OCS compared to RCT patients.27
If we compare the baseline characteristics of our cohort with the cohort of the study of Bagnasco and colleagues, it is possible to observe an even higher level of eosinophilia at baseline (653 ± 381 vs. 1219 ± 1585 respectively; p = 0.0034), a similar baseline level of FEV1%, and a greater annual recurrence of exacerbations (3 ± 1.8 vs. 7.58 ± 4.178; p < 0.0001).
Taken together, these data suggest that mepolizumab is capable of exerting its beneficial action in patients with severe eosinophilic asthma despite the presence of one or more comorbidities.
To date, only 2 studies have assessed the effectiveness of MEPO in patients with comorbidities, and both corroborate our data.28,29 The first study has evaluated MEPO outcomes after 12 months of treatment in 4 severe uncontrolled asthmatic patients with bronchiectasis.28 Results revealed a significant increment in ACT and lung function, a reduction in the number of exacerbations/year, and a reduction of blood eosinophilia.28 The second study has found a correlation between the presence of eosinophilic chronic rhinosinusitis (ECRS) and therapeutic response in patients with severe eosinophilic asthma.29 In particular, Numata and colleagues identified in 28 patients that ECRS was a predictive factor of the response to mepolizumab as patients with eosinophilic chronic rhinosinusitis showed significantly improved systemic corticosteroid-sparing effects, lung function and symptoms compared to patients without the comorbidity.29
In order to extend our analysis, we probed if single comorbidities influenced allocation to the responder or non-responder group. Neither the comorbidities nor other characteristics of patients at baseline (ie, sex, BMI, age, smoking habits, baseline eosinophil count) affected the success or failure of MEPO therapy. Other studies evaluated some socio-demographic factors (ie, allergy, BMI, eosinophils, and lung function at baseline, age, sex, and smoking habits) and 2 of them identified potential predictive factors of MEPO response.25,30,31 A supervised cluster analysis with a recursive partitioning approach applied to the Dose Ranging Efficacy And safety with Mepolizumab (DREAM) data identified BMI as a predictor.30 However, the data on this topic are rather controversial, and there is no general agreement as to the role of BMI as a predictive factor of outcome.25,30
Finally, like other real-life studies, our data do not indicate the number of eosinophils as a predictor of clinical efficacy, suggested by some other authors as a useful biomarker for the selection of patients who are more likely to benefit from treatment with MEPO.21,25,30,32, 33, 34
There are several limitations to the present study. One limitation is that it is a single-center, retrospective study. However, the alignment of our results with those from the literature makes the data more robust. Secondly, the conclusions about predictive factors could be limited due to the small number of patients included in the study. Thirdly, no established criteria for treatment response have been validated yet; therefore, our criteria could be classified as subjective. Finally, we acknowledge that the small sample size, the retrospective design, and methods of the study might limit addressing whether comorbidities modify the treatment effect of mepolizumab on asthma outcomes.
Conclusions
Asthma is a heterogeneous disease with different clinical manifestations; therefore, the systematic investigation of flawless biomarkers or composite indexes which could help clinicians identify patients predisposed to specific therapeutic strategies is still an unmet need.
These findings, while preliminary, suggest that treatment with MEPO is effective in clinical practice in patients with severe eosinophilic asthma complicated by 1 or more comorbidities. However, as we were not able to establish a predictive outcome factor, further larger studies, which take these variables into account, will need to be undertaken.
Funding
Not applicable.
Ethical approval
This study used anonymous retrospective claims data, and as such, it did not require institutional review board and approval or informed consent. Moreover, as it refers to outpatients treated with drugs already approved by regulatory agencies, does not need approval by an Ethics Committee.
Submission declaration
The work has not been published or submitted to another scientific journal and is not being considered for publication elsewhere, though it was uploaded to a preprint server in an earlier version (https://www.medrxiv.org/content/10.1101/2020.05.26.20112052v1). This submission represents original work and is approved by all authors.
Consent for publication
All authors approved publication of the work.
Availability of data and material
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
NC, CC and RC designed the study. GC, SN, RI, MP, CP, contributed to the clinical and laboratory work for the study. GC, SN, RI and MP contributed to data collection. CC contributed to data analysis. RC, CC, and NC drafted the article and revised it critically for important intellectual content. RC, CC, and NC contributed to final approval of the version to be published. All authors contributed to drafting, revising and editing the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Declaration of competing interest
All the authors declare no competing interests.
Acknowledgements
Editorial assistance for the manuscript was provided by sciencED medical communication.
Footnotes
Full list of author information is available at the end of the article
Supplementary data to this article can be found online at https://doi.org/10.1016/j.waojou.2020.100462.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Chung K.F., Wenzel S.E., Brozek J.L. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43(2):343–373. doi: 10.1183/09031936.00202013. [DOI] [PubMed] [Google Scholar]
- 2.European Medicine Agency (Ema). https://www.ema.europa.eu/en/documents/overview/nucala-epar-medicine-overview_en.pdf Last accessed 25 november 2019.
- 3.Chupp G.L., Bradford E.S., Albers F.C. Efficacy of mepolizumab add-on therapy on health-related quality of life and markers of asthma control in severe eosinophilic asthma (MUSCA): a randomised, double-blind, placebo-controlled, parallel-group, multicentre, phase 3b trial. Lancet Respir Med. 2017;5(5):390–400. doi: 10.1016/S2213-2600(17)30125-X. [DOI] [PubMed] [Google Scholar]
- 4.Pavord I.D., Korn S., Howarth P. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012;380(9842):651–659. doi: 10.1016/S0140-6736(12)60988-X. [DOI] [PubMed] [Google Scholar]
- 5.Ortega H.G., Liu M.C., Pavord I.D. MENSA Investigators. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371(13):1198–1207. doi: 10.1056/NEJMoa1403290. [DOI] [PubMed] [Google Scholar]
- 6.Bel E.H., Wenzel S.E., Thompson P.J. SIRIUS Investigators. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371(13):1189–1197. doi: 10.1056/NEJMoa1403291. [DOI] [PubMed] [Google Scholar]
- 7.Liu Y., Zhang S., Li D.W., Jiang S.J. Efficacy of anti-interleukin-5 therapy with mepolizumab in patients with asthma: a meta-analysis of randomized placebo-controlled trials. PloS One. 2013;8(6) doi: 10.1371/journal.pone.0059872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bachert C., Zhang N. Chronic rhinosinusitis and asthma: novel understanding of the role of IgE 'above atopy'. J Intern Med. 2012;272(2):133–143. doi: 10.1111/j.1365-2796.2012.02559.x. [DOI] [PubMed] [Google Scholar]
- 9.Scott H.A., Gibson P.G., Garg M.L. Relationship between body composition, inflammation and lung function in overweight and obese asthma. Respir Res. 2012;13(1):10. doi: 10.1186/1465-9921-13-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crimi C., Ferri S., Crimi N. Bronchiectasis and asthma: a dangerous liaison? Curr Opin Allergy Clin Immunol. 2019;19(1):46–52. doi: 10.1097/ACI.0000000000000492. [DOI] [PubMed] [Google Scholar]
- 11.Boulet L.P., Boulay M.È. Asthma-related comorbidities. Expet Rev Respir Med. 2011;5(3):377–393. doi: 10.1586/ers.11.34. [DOI] [PubMed] [Google Scholar]
- 12.Wenzel S.E. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med. 2012;18(5):716–725. doi: 10.1038/nm.2678. [DOI] [PubMed] [Google Scholar]
- 13.Saturni S., Bellini F., Braido F. Randomized Controlled Trials and real life studies. Approaches and methodologies: a clinical point of view. Pulm Pharmacol Therapeut. 2014;27(2):129–138. doi: 10.1016/j.pupt.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 14.InitiativeforAsthma G . 2016. Global Strategy for Asthma Management and Prevention.www.ginasthma.org Available at: [Google Scholar]
- 15.Korn S., Both J., Jung M. Prospective evaluation of current asthma control using ACQ and ACT compared with GINA criteria. Ann Allergy Asthma Immunol. 2011;107(6):474–479. doi: 10.1016/j.anai.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Miller M.R., Hankinson J., Brusasco V. ATS/ERS task force. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 17.Ortega H.G., Yancey S.W., Mayer B. Severe eosinophilic asthma treated with mepolizumab stratified by baseline eosinophil thresholds: a secondary analysis of the DREAM and MENSA studies. Lancet Respir Med. 2016;4(7):549–556. doi: 10.1016/S2213-2600(16)30031-5. [DOI] [PubMed] [Google Scholar]
- 18.Schatz M., Kosinski M., Yarlas A.S. The minimally important difference of the Asthma Control Test. J Allergy Clin Immunol. 2009;124(4):719–723.e1. doi: 10.1016/j.jaci.2009.06.053. [DOI] [PubMed] [Google Scholar]
- 19.Pelaia C., Busceti M.T., Solinas S. Real-life evaluation of the clinical, functional, and hematological effects of mepolizumab in patients with severe eosinophilic asthma: results of a single-centre observational study. Pulm Pharmacol Therapeut. 2018;53:1–5. doi: 10.1016/j.pupt.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 20.Pertzov B., Unterman A., Shtraichman O. Efficacy and safety of mepolizumab in a real-world cohort of patients with severe eosinophilic asthma. J Asthma. 2019:1–6. doi: 10.1080/02770903.2019.1658208. [DOI] [PubMed] [Google Scholar]
- 21.Bagnasco D., Caminati M., Menzella F. One year of mepolizumab. Efficacy and safety in real-life in Italy. Pulm Pharmacol Therapeut. 2019;58:101836. doi: 10.1016/j.pupt.2019.101836. [DOI] [PubMed] [Google Scholar]
- 22.Caminati M., Cegolon L., Vianello A. Mepolizumab for severe eosinophilic asthma: a real-world snapshot on clinical markers and timing of response. Expet Rev Respir Med. 2019;13(12):1205–1212. doi: 10.1080/17476348.2019.1676734. [DOI] [PubMed] [Google Scholar]
- 23.Montero-Pérez O., Contreras-Rey M.B., Sánchez-Gómez E. Effectiveness and safety of mepolizumab in severe refractory eosinophilic asthma: results in clinical practice. Drugs Context. 2019;8:212584. doi: 10.7573/dic.212584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tepper R.S., Wise R.S., Covar R. Asthma outcomes: pulmonary physiology. J Allergy Clin Immunol. 2012;129(3 Suppl):S65–S87. doi: 10.1016/j.jaci.2011.12.986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drick N., Seeliger B., Welte T. Anti-IL-5 therapy in patients with severe eosinophilic asthma - clinical efficacy and possible criteria for treatment response. BMC Pulm Med. 2018;18(1):119. doi: 10.1186/s12890-018-0689-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quanjer P.H., Stanojevic S., Cole T.J., ERS Global Lung Function Initiative Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40(6):1324–1343. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bagnasco D., Milanese M., Rolla G. The North-Western Italian experience with anti IL-5 therapy and comparison with regulatory trials. World Allergy Organ J. 2018;11(1):34. doi: 10.1186/s40413-018-0210-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carpagnano G.E., Scioscia G., Lacedonia D. Severe uncontrolled asthma with bronchiectasis: a pilot study of an emerging phenotype that responds to mepolizumab. J Asthma Allergy. 2019;12:83–90. doi: 10.2147/JAA.S196200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Numata T., Nakayama K., Utsumi H. Efficacy of mepolizumab for patients with severe asthma and eosinophilic chronic rhinosinusitis. BMC Pulm Med. 2019;19(1):176. doi: 10.1186/s12890-019-0952-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ortega H., Li H., Suruki R. Cluster analysis and characterization of response to mepolizumab. A step closer to personalized medicine for patients with severe asthma. Ann Am Thorac Soc. 2014;11(7):1011–1017. doi: 10.1513/AnnalsATS.201312-454OC. [DOI] [PubMed] [Google Scholar]
- 31.Albers F.C., Papi A., Taillé C. Mepolizumab reduces exacerbations in patients with severe eosinophilic asthma, irrespective of body weight/body mass index: meta-analysis of MENSA and MUSCA. Respir Res. 2019;20(1):169. doi: 10.1186/s12931-019-1134-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Albers F.C., Licskai C., Chanez P. Baseline blood eosinophil count as a predictor of treatment response to the licensed dose of mepolizumab in severe eosinophilic asthma. Respir Med. 2019;159:105806. doi: 10.1016/j.rmed.2019.105806. [DOI] [PubMed] [Google Scholar]
- 33.Walsh G.M. Severe eosinophilic asthma and mepolizumab. Lancet Respir Med. 2016;4(7):528–529. doi: 10.1016/S2213-2600(16)30103-5. [DOI] [PubMed] [Google Scholar]
- 34.Pelaia C., Crimi C., Pelaia G. Real-life evaluation of mepolizumab efficacy in patients with severe eosinophilic asthma, according to atopic trait and allergic phenotype. Clin Exp Allergy. 2020;50(7):780–788. doi: 10.1111/cea.13613. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.