Abstract
Aim: To examine the association between carotid plaque and variants in genes involved in inflammation and endothelial function.
Methods: This was a multicenter, cross-sectional survey in southwestern China. The residents aged ≥ 40 years volunteered to participate in the face-to-face survey in eight communities. A total of 2,377 subjects with high stroke risk were enrolled. Carotid plaque and plaque phenotype were assessed by carotid ultrasound. Genotypes of 19 variants in 10 genes related to inflammation and endothelial function were examined. Gene-gene interaction was analyzed by generalized multifactor dimensionality reduction (GMDR).
Results: Carotid plaques were found in 852 (35.8%) subjects, and 454 (53.3%) had stable plaques, whereas 398 (46.7%) had vulnerable plaques. PPARA rs4253655, HABP2 rs7923349, and IL1A rs1609682 were associated with the presence of carotid plaque, and NOS2A rs2297518 and PPARA rs4253655 were associated with vulnerable plaque in univariate analysis. The GMDR analysis revealed that there was a significant gene–gene interaction among HABP2 rs7923349, ITGA2 rs1991013, IL1A rs1609682, and NOS2A rs8081248, and the high-risk interactive genotype among the four variants was independently associated with a higher risk of carotid vulnerable plaque after adjusting the covariates (OR, 2.86, 95% CI: 1.32–7.13, P = 0.003).
Conclusion: The prevalence of carotid plaque was very high in the high-risk stroke population in southwestern China. Variants in genes involved in the endothelial function and inflammation were associated with the carotid plaque. The high-risk interactive genotype among rs7923349, rs1991013, rs1609682, and rs8081248 was independently associated with a higher risk of vulnerable plaque.
Keywords: High-risk stroke population, Inflammation, Carotid plaque, Plaque vulnerability, Genetic polymorphism
Introduction
Stroke is the leading cause of mortality and adult disability in the world, especially in China1). Carotid atherosclerosis is associated with an increased risk of stroke, coronary events, and cardiovascular mortality as a result of both plaque rupture and luminal stenosis2–4). Carotid atherosclerotic plaque is an important subclinical precursor of stroke and other vascular diseases5). Therefore, the identification of etiology for carotid plaque, including genetic etiology, is very important for the prevention of atherosclerosis and stroke5). However, until now, such a genetic influence on carotid atherosclerotic plaque is not fully understood.
Atherosclerosis is a chronic inflammatory disease6). Several different mechanisms play key roles in the pathogenesis of atherosclerosis, including endothelial injury, recruitment and activation of immuneinflammatory cells, influx of lipoproteins through the vessel injury space, and smooth muscle cell proliferation6, 7). In addition, substantial heritability of subclinical carotid atherosclerosis has been reported8, 9). A different risk of atherosclerosis in the population may reflect variants in genes that modulate the inflammatory response to oxidized lipids in the arterial walls7). Therefore, genetic variants in inflammation and endothelial function relevant genes may influence metabolism of lipids and affect atherogenesis. Several studies have investigated the association between carotid atherosclerosis and inflammation- and endothelial function-related genetic variants10–13). However, information about specific genetic determinants of carotid plaque is lacking, particularly among Chinese populations.
It is known that atherosclerosis as a common complex trait does not follow the Mendelian pattern of inheritance14). Gene–gene and gene–environment interactions may be responsible for the complex trait12, 13). It has been emphasized that assessment of multiple genes is necessary to identify the genetic mechanisms for carotid plaque using the generalized multifactor dimensionality reduction (GMDR) approach15). However, few studies used the GMDR method to investigate complex genetic risk factors for carotid plaque. Therefore, the aim of this study was to investigate (1) the prevalence of carotid plaque in high-risk stroke population in southwestern China and (2) the association of 19 single nucleotide polymorphisms (SNPs) in genes involved in inflammation and endothelial function with carotid plaque among individuals in southwestern China.
Materials and Methods
Study Design and Participants
This population-based cross-sectional survey was part of the China National Stroke Screening Survey (CNSSS) program of the National Health and Family Planning Commission of China (grant No. 2011BAI08B01), which was supervised by the Chinese National Centre for Stroke Care Quality Control and Management1, 16, 17). The survey protocol was reviewed and approved by the ethics committee of the participating hospitals (People's Hospital of Deyang City, Suining Central Hospital, the Third Affiliated Hospital of Wenzhou Medical University, and the Affiliated Hospital of Southwest Medical University), and informed consent was obtained from all participants during recruitment.
This study was conducted in eight communities of Sichuan Province in southwestern China and Wenzhou City from May 2015 to September 2015. The eight communities were randomly selected, and a cluster survey method was used. Details on the organization and implementation of the CNSSS can be found at the official website17). Briefly, we screened permanent residents aged 40 years or older who had lived in the area for more than 6 months in each community. All participants were initially screened using a structured face-to-face questionnaire by investigators. The questionnaire included demographic characteristics, behavioral factors, personal and family medical history of stroke and chronic diseases, and physical examination. More detailed information of laboratory examinations (such as fasting blood glucose [FBG], lipid, electrocardiogram [ECG], and carotid ultrasonography) was obtained from the individuals who were identified to be at a high risk for stroke. The investigators were physicians or neurologists from community hospitals who had at least 5 years of education in medicine.
Definitions of Stroke and Evaluation of Risk Factors
In this survey, stroke history and stroke types were established by a combination of self-reporting and the judgment of a physician or neurologist according to neuroimaging (brain computed tomography scan and magnetic resonance imaging). By definition, patients with a history of transient ischemic attack only were excluded.
The eight stroke-related risk factors were assessed, including overweight/obesity (body mass index ≥ 26 kg/m2), smoking, physical inactivity (physical exercise less than three times a week for less than 30 min each time), family history of stroke, and hypertension, diabetes mellitus, dyslipidemia, and atrial fibrillation. Hypertension was defined as using antihypertensive drugs or the average of two resting systolic blood pressure readings of ≥ 140 mm Hg and/or diastolic blood pressure ≥ 90 mm Hg in the field survey. Diabetes mellitus was defined as using insulin and/or oral hypoglycemic medications or fasting blood glucose ≥ 7.0 mmol/L in the field survey. Dyslipidemia was defined as using a lipid-lowering medication or having one or more of the following in the field survey: triglycerides ≥ 1.70 mmol/L, cholesterol ≥ 5.18 mmol/L, and low-density lipoprotein cholesterol ≥ 3.37 mmol/L. Atrial fibrillation was defined as reported by the respondent or diagnosed by electrocardiogram in the field survey. Subjects with at least three of the aforementioned eight stroke-related risk factors or a history of stroke were classified as the high-risk population for stroke17, 18).
Data Cleaning Procedures and Quality Control
The detailed data cleaning procedure and quality control according to the CNSSS is presented in Fig. 1. Briefly, 18595 participants volunteered to participate in the face-to-face survey, and questionnaires were obtained in 17213 participants. The response rate was 93.6% (17413/18595). Five hundred twenty-one participants with incomplete questionnaires on stroke history or risk factor records were excluded. Finally, 16892 valid individual records were completed, including 2893 high-risk stroke population. Among 2893 high-risk stroke population, carotid ultrasonography was examined, and DNA from peripheral blood was obtained in 2377 participants (516 were unwilling to accept examination for carotid ultrasonography and offer DNA sample). The response rate of examination for carotid ultrasonography and DNA sample was 82.2% (2377/2893).
Fig. 1.
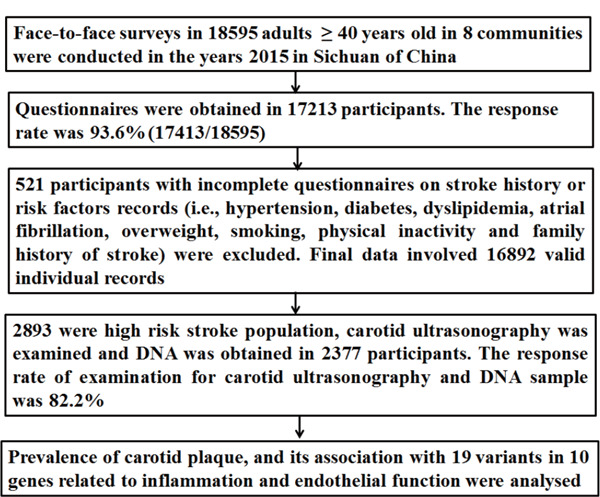
Data preparing and cleaning process in this survey
Carotid Ultrasonography
Bilateral common and internal carotid arteries, as well as bifurcations, were assessed using a diagnostic ultrasound (type 512, ACUSON Sequoia Apparatus, 7.5-MHz probe, Berlin, Germany) in 2377 high-risk stroke population according to standard scanning and reading protocols5). Carotid characteristics, including intima–media thickness (IMT), plaque morphology, and degree of carotid stenosis, were evaluated. The detailed procedures for evaluating plaques, types of plaques, degree of carotid stenosis, intraobserver, and interobserver coefficients were described in our previous study12, 13, 19). Briefly, bilateral internal and common carotid arteries, as well as bifurcations, were examined for the presence of atherosclerotic plaque. Atherosclerotic plaque was defined as an endoluminal protrusion of at least 1.5 mm or a focal thickening > 50% of the IMT relative to the adjacent wall segment5). Thereafter, the participants were divided into two groups: carotid plaque and non-carotid plaque groups. Furthermore, according to plaque echogenicity and surface structure, carotid plaque was further graded from class I to class IV as echolucent, predominantly echolucent, predominantly echogenic, and echogenic, respectively20). Plaque of class I or class II was defined as vulnerable plaque, and plaque of class III or class IV was defined as stable plaque12, 13). Carotid characteristics were graded independently by one ultrasound imaging doctor blinded to clinical status. The main aim of this study was to investigate the prevalence of carotid plaque in high-risk stroke population and the association of 19 single nucleotide polymorphisms (SNPs) in genes involved in inflammation and endothelial function with carotid plaque. Thus, carotid stenosis and IMT in common carotid artery were not involved in this analysis.
Polymorphism Selection and Genotyping
SNPs in 10 genes involved in inflammation and endothelial function were selected from the NCBI database (http://www.ncbi.nlm.nih.gov/SNP), which met the following criteria: (1) these SNPs have been evaluated in previous studies10, 21); (2) SNPs with minor allele frequency > 0.05; (3) tagging SNPs across different human populations (http://pga.gs.washington.edu); and (4) the SNPs may lead to amino acid changes. According to the criteria, 19 SNPs from 10 genes implicated in inflammation and endothelial function were evaluated, including PPARA (rs4253655, rs4253778), NOS2A (rs2297518, rs8081248), TNF rs3093662, IL6R (rs1386821, rs4845625), TNFSF4 (rs1234313, rs11811788), TLR4 (rs752998, rs1927911), IL1A (rs1800587, rs1609682), VCAM1 (rs3783615, rs2392221), ITGA2 (rs1991013, rs4865756), and HABP2 (rs932650, rs7923349).
DNA from peripheral blood was extracted using a modified phenol/chloroform method and purified using the UNIQ-10 kit (Sangon Biotech Co., Ltd. Shanghai, China). The genotyping of the 19 SNPs was performed by investigators blinded to the clinical data of participants, using the matrix-assisted laser desorption/ionization time of flight mass spectrometry method, as previously described12, 13, 19).
Statistical Analysis
The data were analyzed using SPSS 17.0 (SPSS Inc. New York, New York, USA). The results are expressed as percentages for categorical variables, and continuous variables are expressed as mean ± standard deviation. Baseline clinical characteristics and genotype distributions of the 19 variants were compared using χ2 test (categorical variables) and Student's t-test (continuous variables) between subjects with and without carotid plaque.
The allele frequencies for Hardy–Weinberg equilibrium were assessed using χ2 test. The GMDR software (β version 0.7, www.healthsystem.virginia.edu/internet/addiction-genomics/Software) was used to assess gene-gene interaction among the 19 variants under various scenarios as previously reported15, 19). The prevalence of carotid plaque between subjects with and without high-risk interactive genotype was compared by χ2 test. Furthermore, we evaluated the risk of carotid vulnerable plaque conferred by the high-risk interactive genotype using multivariate logistic regression analysis, and reported as the hazard ratio (HR) with the 95% confidence interval (CI). The other variables that were statistically significant at P value < 0.05 in the univariate analysis were entered into the multivariate logistic regression analyses to adjust. All tests were two-sided, and P value < 0.05 was considered statistically significant.
Results
The Baseline Characteristics of Subjects and Prevalence of Carotid Plaque
Among 2377 participants in the high-risk stroke population, carotid plaque was present in 852 (35.8%) participants, and 454 (53.3%) participants had stable plaque, whereas 398 (46.7%) had vulnerable plaque. As presented in Table 1, compared with individuals with no plaque, older age, male, hypertension and intake of antihypertensive drugs, and current smoker were significantly more frequent, and the levels of total cholesterol, fasting blood glucose, and homocysteine were higher in subjects with plaque.
Table 1. Demographic characteristics of the study population.
| Carotid plaque | Non-carotid plaque | ||
|---|---|---|---|
| (n = 852) | (n = 1,525) | P value | |
| Age (years) | 67.1 ± 16.8 | 61.9 ± 21.7 | < 0.001 |
| Male (n, %) | 422 (49.5) | 643 (42.2) | < 0.001 |
| Weight (kg) | 62.9 ± 15.4 | 63.5 ± 18.9 | 0.413 |
| Height (cm) | 157.3 ± 16.7 | 156.8 ± 17.9 | 0.462 |
| Body mass index (kg/m2) | 26.4 ± 5.2 | 26.2 ± 5.6 | 0.411 |
| Hypertension (n, %) | 692 (81.2) | 1111 (72.9) | < 0.001 |
| Diabetes mellitus (n, %) | 231 (27.1) | 422 (27.7) | 0.386 |
| Current smoking (n, %) | 349 (41.0) | 467 (30.6) | < 0.001 |
| History of stroke (n, %) | 143 (16.8) | 301 (19.7) | 0.072 |
| Stroke subtype | |||
| Hemorrhagic | 26 (3.1) | 71 (4.7) | 0.071 |
| Ischemic stroke | 117 (13.7) | 230 (15.1) | 0.574 |
| Atherothromobosis | 73 (8.6) | 136 (8.9) | - |
| Cardioembolism | 16 (1.9) | 29 (1.9) | - |
| Lacunar | 28 (3.3) | 65 (4.3) | - |
| Coronary artery disease (n, %) | 77 (9.0) | 118 (7.7) | 0.289 |
| Atrial fibrillation (n, %) | 15 (1.8) | 35 (2.3) | 0.367 |
| Dyslipidemia (n, %) | 291 (34.2) | 527 (34.6) | 0.972 |
| Total cholesterol (mM) | 5.4 ± 1.6 | 5.2 ± 1.8 | 0.006 |
| LDL (mM) | 3.1 ± 1.2 | 3.0 ± 1.3 | 0.072 |
| HDL (mM) | 1.6 ± 0.7 | 1.6 ± 0.8 | 0.965 |
| Triglycerides (mM) | 1.8 ± 0.9 | 3.5 ± 0.9 | < 0.001 |
| Fasting blood glucose (mM) | 6.6 ± 1.8 | 6.3 ± 2.1 | < 0.001 |
| Homocysteine (mM) | 13.9 ± 5.5 | 13.2 ± 5.7 | < 0.001 |
| Antihypertensive drugs | 387 (45.4) | 563 (36.9) | < 0.001 |
| Antithrombotic medications | 140 (16.4) | 271 (17.8) | 0.435 |
| Statin use | 64 (7.5) | 110 (7.2) | 0.773 |
| Plaque characteristics (n, %) | |||
| Stable plaque | 454 (53.3) | - | |
| Vulnerable plaque | 398 (46.7) | - |
LDL, low-density lipoprotein; HDL, high-density lipoprotein;
Genotype Distributions in Subjects with and without Carotid Plaque
The genotype distributions of the 19 SNPs assessed in this study were consistent with the Hardy–Weinberg equilibrium (all P value > 0.05). Three genes involved in endothelial function and inflammation had SNPs significantly associated with the presence of carotid plaque (PPARA rs4253655, HABP2 rs7923349, and IL1A rs1609682, Table 2), and NOS2A rs2297518 and PPARA rs4253655 were significantly associated with vulnerable plaque in the single SNP analysis (Table 3). However, there were no significant differences in the genotype distributions in the 19 SNPs between individuals with stable plaque and vulnerable plaque (Supplemental Table 1), or in individuals with stable plaque and individuals without plaque (Supplemental Table 2).
Table 2. Genotype distribution in individuals with and without carotid plaque (%).
| Carotid plaque | Non-carotid plaque | Wald χ2 value | P value | |
|---|---|---|---|---|
| (n = 852) | (n = 1,525) | |||
| IL6R (rs4845625) | 3.427 | 0.180 | ||
| TT | 250 (29.4) | 405 (26.6) | ||
| CC | 182 (21.2) | 368 (24.1) | ||
| CT | 420 (49.4) | 752 (49.3) | ||
| HABP2 (rs932650) | 2.477 | 0.289 | ||
| CT | 372 (43.7) | 658 (43.1) | ||
| CC | 72 (8.5) | 159 (10.4) | ||
| TT | 408 (47.9) | 708 (46.4) | ||
| TLR4 (rs1927911) | 1.252 | 0.535 | ||
| AG | 424 (49.8) | 740 (48.5) | ||
| AA | 135 (15.8) | 226 (14.8) | ||
| GG | 293 (34.4) | 559 (36.7) | ||
| VCAM1 (rs3783615) | ||||
| AA | 852 (100.0) | 1525 (100.0) | - | - |
| PPARA (rs4253778) | 0.016 | 0.898 | ||
| CG | 2 (0.2) | 4 (0.3) | ||
| GG | 850 (99.8) | 1521 (99.7) | ||
| PPARA (rs4253655) | 7.172 | 0.007 | ||
| AG | 4 (0.5) | 0 (0.0) | ||
| GG | 848 (99.5) | 1525 (100) | ||
| VCAM1 (rs2392221) | 2.560 | 0.278 | ||
| CT | 205 (24.1) | 340 (22.3) | ||
| CC | 632 (74.2) | 1145 (75.1) | ||
| TT | 15 (1.8) | 40 (2.6) | ||
| IL1A (rs1800587) | 1.645 | 0.439 | ||
| AG | 121 (14.2) | 193 (12.7) | ||
| GG | 727 (85.3) | 1321 (86.6) | ||
| AA | 4 (0.5) | 11 (0.7) | ||
| TNFSF4 (rs1234313) | 2.229 | 0.328 | ||
| AG | 399 (46.8) | 667 (43.7) | ||
| GG | 94 (11.0) | 185 (12.1) | ||
| AA | 359 (42.1) | 673 (44.1) | ||
| HABP2 (rs7923349) | 6.745 | 0.034 | ||
| TT | 64 (7.5) | 53 (3.5) | ||
| GT | 325 (38.1) | 563 (36.9) | ||
| GG | 463 (54.3) | 909 (59.6) | ||
| TNFSF4 (rs11811788) | 0.446 | 0.800 | ||
| CG | 136 (16.0) | 237 (15.5) | ||
| GG | 10 (1.2) | 14 (0.9) | ||
| CC | 706 (82.9) | 1274 (83.5) | ||
| TLR4 (rs752998) | 1.875 | 0.392 | ||
| TT | 16 (0.019) | 39 (2.6) | ||
| GG | 605 (71.0) | 1059 (69.4) | ||
| GT | 231 (27.1) | 427 (28.0) | ||
| IL1A (rs1609682) | 7.068 | 0.029 | ||
| GG | 392 (46.0) | 641 (42.0) | ||
| GT | 378 (44.4) | 791 (51.9) | ||
| TT | 82 (9.6) | 93 (6.1) | ||
| NOS2A (rs8081248) | 1.265 | 0.531 | ||
| AG | 384 (45.1) | 666 (43.7) | ||
| AA | 84 (9.9) | 172 (11.3) | ||
| GG | 384 (45.1) | 687 (45.0) | ||
| TNF (rs3093662) | 0.536 | 0.464 | ||
| AG | 44 (5.2) | 68 (4.5) | ||
| AA | 808 (94.8) | 1457 (95.5) | ||
| ITGA2 (rs1991013) | 4.407 | 0.110 | ||
| GG | 71 (8.3) | 160 (10.5) | ||
| AA | 391 (45.9) | 716 (47.0) | ||
| AG | 390 (45.8) | 648 (42.5) | ||
| ITGA2 (rs4865756) | 1.620 | 0.445 | ||
| AG | 333 (39.1) | 556 (36.5) | ||
| GG | 464 (54.5) | 868 (56.9) | ||
| AA | 55 (6.5) | 101 (6.6) | ||
| IL6R (rs1386821) | 1.739 | 0.419 | ||
| GT | 58 (6.8) | 123 (8.1) | ||
| GG | 3 (0.4) | 4 (0.3) | ||
| TT | 791 (92.8) | 1398 (91.7) | ||
| NOS2A (rs2297518) | 1.070 | 0.586 | ||
| AG | 241 (28.3) | 409 (26.8) | ||
| AA | 20 (2.3) | 30 (2.0) | ||
| GG | 591 (69.4) | 1086 (71.2) |
Table 3. Genotype distribution between vulnerable plaque group and non-plaque group (%).
| Vulnerable plaque | Non- plaque | Wald χ2 value | P value | |
|---|---|---|---|---|
| (n = 398) | (n = 1,525) | |||
| IL6R (rs4845625) | 1.7719 | 0.4123 | ||
| TT | 113 (28.4) | 405 (26.6) | ||
| CC | 85 (21.4) | 368 (24.1) | ||
| CT | 200 (50.3) | 752 (49.3) | ||
| HABP2 (rs932650) | 0.1649 | 0.9209 | ||
| CT | 170 (42.5) | 658 (43.1) | ||
| CC | 40 (10.0) | 159 (10.4) | ||
| TT | 188 (147.5) | 708 (46.4) | ||
| TLR4 (rs1927911) | 1.3796 | 0.5017 | ||
| AG | 202 (50.7) | 740 (48.5) | ||
| AA | 63 (15.8) | 226 (14.8) | ||
| GG | 133 (33.5) | 559 (36.7) | ||
| VCAM1 (rs3783615) | ||||
| AA | 398 (100.0) | 1525 (100.0) | ||
| PPARA (rs4253778) | 0.0018 | 0.9657 | ||
| CG | 1 (0.2) | 4 (0.3) | ||
| GG | 397 (99.8) | 1521 (99.7) | ||
| PPARA (rs4253655) | 11.4554 | 0.007 | ||
| AG | 3 (0.8) | 0 (0.0) | ||
| GG | 395 (99.2) | 1525 (100) | ||
| VCAM1 (rs2392221 | 1.1946 | 0.5503 | ||
| CT | 98 (24.6) | 340 (22.3) | ||
| CC | 291 (73.1) | 1145 (75.1) | ||
| TT | 9 (2.3) | 40 (2.6) | ||
| IL1A (rs1800587) | 2.6445 | 0.2665 | ||
| AG | 62 (15.6) | 193 (12.7) | ||
| GG | 333 (83.7) | 1321 (86.6) | ||
| AA | 3 (0.8) | 11 (0.7) | ||
| TNFSF4 (rs1234313) | 1.526 | 0.4663 | ||
| AG | 188 (47.2) | 667 (43.7) | ||
| GG | 43 (10.8) | 185 (12.1) | ||
| AA | 167 (42.0) | 673 (44.1) | ||
| HABP2 (rs7923349) | 4.0035 | 0.1351 | ||
| TT | 33 (8.3) | 53 (3.5) | ||
| GT | 154 (38.7) | 563 (36.9) | ||
| GG | 211 (53.0) | 909 (59.6) | ||
| TNFSF4 (rs11811788) | 2.2025 | 0.3325 | ||
| CG | 65 (16.3) | 237 (15.5) | ||
| GG | 7 (1.8) | 7 (1.8) | ||
| CC | 326 (81.9) | 326 (81.9) | ||
| TLR4 (rs752998) | 2.5483 | 0.2797 | ||
| TT | 6 (1.5) | 6 (1.5) | ||
| GG | 276 (69.3) | 276 (69.3) | ||
| GT | 116 (29.1) | 116 (29.1) | ||
| IL1A (rs1609682) | 0.4909 | 0.7824 | ||
| GG | 183 (46.0) | 183 (46.0) | ||
| GT | 174 (43.7) | 174 (43.7) | ||
| TT | 41 (10.3) | 41 (10.3) | ||
| NOS2A (rs8081248) | 1.6235 | 0.4441 | ||
| AG | 170 (42.7) | 170 (42.7) | ||
| AA | 38 (9.5) | 38 (9.5) | ||
| GG | 190 (48.7) | 190 (48.7) | ||
| TNF (rs3093662) | 0.0068 | 0.9345 | ||
| AG | 19 (4.8) | 19 (4.8) | ||
| AA | 379 (95.2) | 379 (95.2) | ||
| ITGA2 (rs1991013) | 1.1302 | 0.5683 | ||
| GG | 36 (9.0) | 36 (9.0) | ||
| AA | 183 (46.0) | 183 (46.0) | ||
| AG | 179 (45.0) | 179 (45.0) | ||
| ITGA2 (rs4865756) | 0.9263 | 0.6293 | ||
| AG | 153 (38.4) | 153 (38.4) | ||
| GG | 223 (56.0) | 223 (56.0) | ||
| AA | 22 (5.5) | 22 (5.5) | ||
| IL6R (rs1386821) | 2.1854 | 0.3353 | ||
| GT | 26 (6.5) | 26 (6.5) | ||
| GG | 2 (0.5) | 2 (0.5) | ||
| TT | 370 (93.0) | 370 (93.0) | ||
| NOS2A (rs2297518) | 7.3901 | 0.0375 | ||
| AG | 119 (29.9) | 119 (29.9) | ||
| AA | 8 (2.0) | 8 (2.0) | ||
| GG | 271 (68.1) | 271 (68.1) |
Supplemental Table 1. Genotype distribution comparison between stable plaque and vulnerable plaque (%).
| Stable plaque (454) | vulnerable plaque (398) | Wald χ2 value | P value | |
|---|---|---|---|---|
| rs4845625 | 0.424 | 0.809 | ||
| TT | 137 (30.2) | 113 (28.4) | ||
| CT | 220 (48.5) | 200 (50.3) | ||
| CC | 97 (21.4) | 85 (21.4) | ||
| rs932650 | 2.1474 | 0.3418 | ||
| TT | 220 (48.2) | 188 (147.5) | ||
| CC | 32 (7.3) | 40 (10.0) | ||
| CT | 202 (44.5) | 170 (42.5) | ||
| rs1927911 | 0.3481 | 0.8403 | ||
| AA | 72 (15.9) | 63 (15.8) | ||
| GG | 160 (35.2) | 133 (33.5) | ||
| AG | 222 (48.9) | 202 (50.7) | ||
| rs3783615 | ||||
| AA | 454 (100.0) | 398 (100.0) | ||
| rs4253778 | 0.0089 | 0.9248 | ||
| CG | 1 (0.2) | 1 (0.2) | ||
| GG | 453 (99.8) | 397 (99.8) | ||
| rs4253655 | 1.2955 | 0.255 | ||
| AG | 1 (0.2) | 3 (0.8) | ||
| GG | 453 (99.8) | 395 (99.2) | ||
| rs2392221 | 341(75.1) | 291(73.1) | 1.302 | 0.5215 |
| CC | 107 (23.6) | 98 (24.6) | ||
| CT | 6 (1.3) | 9 (2.3) | ||
| TT | ||||
| rs1800587 | 2.5591 | 0.2782 | ||
| AG | 59 (13.0) | 62 (15.6) | ||
| GG | 394 (86.8) | 333 (83.7) | ||
| AA | 1 (0.2) | 3 (0.8) | ||
| rs1234313 | ||||
| AG | 211 (46.5) | 188 (47.2) | 0.0931 | 0.9545 |
| AA | 192 (42.3) | 167 (42.0) | ||
| GG | 51 (11.2) | 43 (10.8) | ||
| rs7923349 | 0.9447 | 0.6235 | ||
| GT | 171 (37.7) | 154 (38.7) | ||
| TT | 31 (6.8) | 33 (8.3) | ||
| GG | 252 (55.5) | 211 (53.0) | ||
| rs11811788 | 2.2837 | 0.3192 | ||
| GG | 3 (0.7) | 7 (1.8) | ||
| CG | 71 (15.6) | 65 (16.3) | ||
| CC | 380 (83.7) | 326 (81.9) | ||
| rs752998 | 2.5488 | 0.2796 | ||
| TT | 10 (0.022) | 6 (1.5) | ||
| GG | 329 (0.724) | 276 (69.3) | ||
| GT | 115 (0.254) | 116 (29.1) | ||
| rs1609682 | 0.6008 | 0.7405 | ||
| GT | 204 (44.9) | 174 (43.7) | ||
| TT | 41 (9.0) | 41 (10.3) | ||
| GG | 209 (46.0) | 183 (46.0) | ||
| rs8081248 | 2.4557 | 0.2929 | ||
| AG | 214 (47.1) | 170 (42.7) | ||
| AA | 46 (10.1) | 38 (9.5) | ||
| GG | 194 (42.7) | 190 (48.7) | ||
| rs3093662 | 0.450 | 60.502 | ||
| AG | 25 (5.5) | 19 (4.8) | ||
| AA | 429 (94.5) | 379 (95.2) | ||
| rs1991013 | 0.5878 | 0.7454 | ||
| AG | 211 (46.5) | 179 (45.0) | ||
| GG | 35 (7.7) | 36 (9.0) | ||
| AA | 208 (45.8) | 183 (46.0) | ||
| rs4865756 | 1.4549 | 0.4831 | ||
| AA | 33 (0.072) | 22 (5.5) | ||
| GG | 241 (0.532) | 223 (56.0) | ||
| AG | 180 (0.396) | 153 (38.4) | ||
| rs1386821 | 0.5624 | 0.7549 | ||
| GT | 32 (7.0) | 26 (6.5) | ||
| TT | 421(92.7) | 370 (93.0) | ||
| GG | 1 (0.2) | 2 (0.5) | ||
| rs2297518 | 1.2464 | 0.5362 | ||
| GG | 320 (70.5) | 271 (68.1) | ||
| AA | 12 (2.6) | 8 (2.0) | ||
| AG | 122 (26.9) | 119 (29.9) |
Supplemental Table 2. Genotype distribution between stable plaque group and non-plaque group (%).
| Stable plaque | Non- plaque | Wald χ2 value | P value | |
|---|---|---|---|---|
| (n = 454) | (n = 1,525) | |||
| IL6R (rs4845625) | 3.1825 | 0.2037 | ||
| TT | 137 (30.2) | 405 (26.6) | ||
| CC | 97 (21.4) | 368 (24.1) | ||
| CT | 220 (48.5) | 752 (49.3) | ||
| HABP2 (rs932650) | 4.1404 | 0.1262 | ||
| CT | 202 (44.5) | 658 (43.1) | ||
| CC | 32 (7.3) | 159 (10.4) | ||
| TT | 220 (48.2) | 708 (46.4) | ||
| TLR4 (rs1927911) | 0.4055 | 0.8165 | ||
| AG | 222 (48.9) | 740 (48.5) | ||
| AA | 72 (15.9) | 226 (14.8) | ||
| GG | 160 (35.2) | 559 (36.7) | ||
| VCAM1 (rs3783615) | ||||
| AA | 454 (100.0) | 1525 (100.0) | ||
| PPARA (rs4253778) | 0.0264 | 0.8709 | ||
| CG | 1 (0.2) | 4 (0.3) | ||
| GG | 453 (99.8) | 1521 (99.7) | ||
| PPARA (rs4253655) | 3.3387 | 0.0677 | ||
| AG | 1 (0.2) | 0 (0.0) | ||
| GG | 453 (99.8) | 1525 (100) | ||
| VCAM1 (rs2392221) | 2.8784 | 0.2371 | ||
| CT | 107 (23.6) | 340 (22.3) | ||
| CC | 341 (75.1) | 1145 (75.1) | ||
| TT | 6 (1.3) | 40 (2.6) | ||
| IL1A (rs1800587) | 1.5282 | 0.4658 | ||
| AG | 59 (13.0) | 193 (12.7) | ||
| GG | 394 (86.8) | 1321 (86.6) | ||
| AA | 1 (0.2) | 11 (0.7) | ||
| TNFSF4 (rs1234313) | ||||
| AG | 211 (46.5) | 667 (43.7) | 1.0144 | 0.6022 |
| GG | 51 (11.2) | 185 (12.1) | ||
| AA | 192 (42.3) | 673 (44.1) | ||
| HABP2 (rs7923349) | 0.6553 | 0.7206 | ||
| TT | 31 (6.8) | 53 (3.5) | ||
| GT | 171 (37.7) | 563 (36.9) | ||
| GG | 252 (55.5) | 909 (59.6) | ||
| TNFSF4 (rs11811788) | 0.2909 | 0.8646 | ||
| CG | 71 (15.6) | 237 (15.5) | ||
| GG | 3 (0.7) | 14 (0.9) | ||
| CC | 380 (83.7) | 1274 (83.5) | ||
| TLR4 (rs752998) | 1.52 | 0.4677 | ||
| TT | 10 (0.022) | 39 (2.6) | ||
| GG | 329 (0.724) | 1059 (69.4) | ||
| GT | 115 (0.254) | 427 (28.0) | ||
| IL1A (rs1609682) | 1.108 | 0.5746 | ||
| GG | 209 (46.0) | 641 (42.0) | ||
| GT | 204 (44.9) | 791 (51.9) | ||
| TT | 41 (9.0) | 93 (6.1) | ||
| NOS2A (rs8081248) | 1.6624 | 0.4355 | ||
| AG | 214 (47.1) | 666 (43.7) | ||
| AA | 46 (10.1) | 172 (11.3) | ||
| GG | 194 (42.7) | 687 (45.0) | ||
| TNF (rs3093662) | 0.9554 | 0.3284 | ||
| AG | 25 (5.5) | 68 (4.5) | ||
| AA | 429 (94.5) | 1457 (95.5) | ||
| ITGA2 (rs1991013) | 4.1734 | 0.1241 | ||
| GG | 35 (7.7) | 160 (10.5) | ||
| AA | 208 (45.8) | 716 (47.0) | ||
| AG | 211 (46.5) | 648 (42.5) | ||
| ITGA2 (rs4865756) | 2.0025 | 0.3674 | ||
| AG | 180 (0.396) | 556 (36.5) | ||
| GG | 241 (0.532) | 868 (56.9) | ||
| AA | 33 (0.072) | 101 (6.6) | ||
| IL6R (rs1386821) | 0.5635 | 0.7545 | ||
| GT | 32 (7.0) | 123 (8.1) | ||
| GG | 1 (0.2) | 4 (0.3) | ||
| TT | 421 (92.7) | 1398 (91.7) | ||
| NOS2A(rs2297518) | 0.7359 | 0.6921 | ||
| AG | 122 (26.9) | 409 (26.8) | ||
| AA | 12 (2.6) | 30 (2.0) | ||
| GG | 320 (70.5) | 1086 (71.2) |
Gene–Gene Interactions of the 19 Variants and Risk of Vulnerable Plaque
We evaluated the relationship between the higher-order interaction for the 19 variants and carotid vulnerable plaque using the GMDR analysis and found that there was a significant gene–gene interaction among genes involved in inflammation and endothelial function. After adjusting for confounding variables, the best interactive model for carotid plaque was interaction among HABP2 rs7923349, ITGA2 rs1991013, IL1A rs1609682, and NOS2A rs8081248, in which the cross-validation consistency was 10/10 and sign test was 9 (P = 0.017, Table 4). Then, the one-locus model was computed for each variant of the four SNPs, and the P value for prediction error was 0.019 using permutation testing, indicating that the interaction among the four variants strongly synergistically contributed to a higher risk of carotid vulnerable plaque compared with did each single variant alone.
Table 4. GMDR analysis of the best models, prediction accuracies, cross-validation consistencies, and P values for carotid vulnerable plaque.
| Best model* | Training balanced accuracy | Testing balanced accuracy | Cross- validation consistency | Sign test (P value) |
|---|---|---|---|---|
| 1 | 0.453 | 0.386 | 6/10 | 5 (0.635) |
| 1,2 | 0.531 | 0.494 | 7/10 | 7 (0.347) |
| 1, 2, 3 | 0.608 | 0.597 | 9/10 | 9 (0.082) |
| 1, 2, 3, 4 | 0.642 | 0.626 | 10/10 | 9 (0.017) |
| 1, 2, 3, 4, 5 | 0.359 | 0.572 | 7/10 | 7 (0.685) |
| 1, 2, 3, 4, 5, 6 | 0.467 | 0.546 | 8/10 | 6 (0.721) |
| 1, 2, 3, 4, 5, 6, 7 | 0.398 | 0.435 | 7/10 | 6 (0.796) |
| 1, 2, 3, 4, 5, 6, 7, 8 | 0.612 | 0.625 | 9/10 | 8 (0.267) |
| 1, 2, 3, 4, 5, 6, 7, 8, 9 | 0.513 | 0.524 | 8/10 | 7 (0.535) |
| 1, 2, 3, 4, 5, 6, 7, 8, 9, 10 | 0.596 | 0.537 | 5/10 | 6 (0.689) |
| 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11 | 0.572 | 0.499 | 8/10 | 8 (0.267) |
| 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12 | 0.515 | 0.488 | 6/10 | 7 (0.496) |
| 1, 2,3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13 | 0.433 | 0.397 | 5/10 | 6 (0.734) |
| 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14 | 0.582 | 0.605 | 8/10 | 6 (0.317) |
| 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15 | 0.631 | 0.538 | 7/10 | 7 (0.296) |
| 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16 | 0.382 | 0.418 | 4/10 | 6 (0.698) |
| 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17 | 0.507 | 0.553 | 5/10 | 5 (0.723) |
| 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18 | 0.468 | 0.486 | 7/10 | 7 (0.467) |
| 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19 | 0.523 | 0.545 | 8/10 | 8 (0.485) |
GMDR, generalized multifactor dimensionality reduction
Numbers 1–19 represent rs7923349, rs1991013, rs1609682, rs8081248, rs4253655, rs4253778, rs2297518, rs3093662, rs1386821, rs4845625, rs1234313, rs11811788, rs752998, rs1927911, rs1800587, rs3783615, rs2392221, rs4865756, and rs932650, respectively.
Subsequently, we assessed the association between different genotype combinations of the four variants and risk of carotid vulnerable plaque. Compared with the individuals carrying wild-type genotypes rs7923349 GG, rs1991013 GG, rs1609682 GG, and rs8081248 GG, the relative risk of different genotype combinations among the four variants for vulnerable plaque was investigated. The results revealed that four genotype combinations making larger contributions to carotid vulnerable plaque were those individuals carrying rs7923349 TT, rs1991013 AA, rs1609682 TT, and rs8081248 AA (OR = 2.83, 95% CI: 1.26–5.38, P = 0.005); rs7923349 TT, rs1991013 GA, rs1609682 TT, and rs8081248 GA (OR = 2.02, 95% CI: 1.12–4.33, P = 0.008); rs7923349 GT, rs1991013 AA, rs1609682 GT, and rs8081248AA (OR = 1.93, 95% CI: 1.07–3.84, P = 0.028); and rs7923349 GT, rs1991013 GA, rs1609682 GT, and rs8081248GA (OR = 1.85, 95% CI: 1.03–2.98, P = 0.038). The aforementioned four genotype combinations were defined as the high-risk interactive genotype. The other genotype combinations did not reach statistical significance (P > 0.05) and were defined as the low-risk interactive genotype.
Association between High-Risk Interactive Genotype and Carotid Vulnerable Plaque
There were 401 carriers of the high-risk interactive genotype among 2377 participants. The incidence of carotid vulnerable plaque was significantly higher in carriers of the high-risk interactive genotypes than in noncarriers (32.7% [131/401] vs. 13.5% [267/1976], P < 0.001).
Furthermore, we evaluated the risk of the presence of carotid vulnerable plaque conferred by the high-risk interactive genotype using multivariate logistic regression analysis. The high-risk interactive genotype was assigned as one, and the low-risk interactive genotype was assigned as zero. The other variables that were statistically significant at P value < 0.05 in the univariate analysis were entered into the multivariate logistic regression analyses to adjust. After adjusting the covariates, the high-risk interactive genotype among rs7923349, rs1991013, rs1609682, and rs8081248 was independently associated with a higher risk of carotid vulnerable plaque (OR, 2.86, 95% CI: 1.32–7.13, P = 0.003, Table 5).
Table 5. Multivariate analysis of the major risk factors for vulnerable plaque.
| Risk factor | OR* | 95% CI | P value |
|---|---|---|---|
| Age | 1.75 | 1.07–4.58 | 0.042 |
| Male | 0.89 | 0.76–1.66 | 0.368 |
| Hypertension | 2.01 | 1.08–6.68 | 0.019 |
| Current smoking | 1.87 | 1.16–5.76 | 0.028 |
| Total cholesterol | 1.76 | 1.13–5.14 | 0.033 |
| Triglycerides | 0.89 | 0.83–1.99 | 0.432 |
| Fasting blood glucose | 1.21 | 0.92–2.57 | 0.235 |
| Homocysteine | 1.32 | 0.95–2.89 | 0.132 |
| PPARA rs4253655AG | 0.86 | 0.73–1.64 | 0.553 |
| HABP2 rs7923349TT | 1.13 | 0.99–4.02 | 0.186 |
| IL1A rs1609682TT | 1.08 | 0.95–3.48 | 0.232 |
| High-risk interactive genotype | 2.86 | 1.32–7.13 | 0.003 |
OR, odds ratios; CI, confidence interval.
OR for continuous variables means per 1 - Standard Deviation increase.
Discussion
In this study, we have identified a high prevalence of carotid plaque (35.8%) in the high-risk stroke population in southwestern China and found significant associations of three genetic variants (PPARA rs4253655, IL1A rs1609682, and NOS2A rs2297518) in three genes related to inflammation and one variant (NOS2A rs2297518) in one gene related to endothelial function with carotid plaque phenotypes in the single SNP analysis. In addition, the GMDR analysis revealed that there was a significant gene–gene interaction among HABP2 rs7923349, ITGA2 rs1991013, IL1A rs1609682, and NOS2A rs8081248, and the high-risk interactive genotype among the four variants was independently associated with a higher risk of carotid vulnerable plaque.
Numerous studies have investigated the association of inflammatory genes and endothelial function relevant genes with ischemic stroke22, 23), but few studies have focused on subclinical atherosclerosis. Carotid plaque is an important subclinical precursor of stroke and other vascular diseases5). Certain plaque phenotypes, such as irregular plaques and maximal carotid plaque thickness, may be important markers of vulnerable plaques susceptible to rupture leading to stroke24). To our knowledge, our study is the first to investigate the association between variants in genes related to endothelial function and inflammatory processes and possible markers of vulnerable plaque in Chinese population.
Inflammation plays a key role in increased migration of inflammatory cells and development of atherosclerosis6, 7). The “response to injury” model in atherosclerosis highlights the role of cytokines such as IL-1β and tumor necrosis factor-α in the response of endothelial cells25). Polymorphisms in inflammatory genes may directly or indirectly interact with vascular risk factors to influence the progression and development of atherosclerosis20). Previous studies have demonstrated overrepresentation of IL1A gene in patients with coronary artery disease26). Furthermore, other data and our current results support an association between IL1A and carotid plaque21). The IL1A gene represents a susceptibility factor for the development of carotid atherosclerosis21). The IL1A alleles may affect the inflammatory environment in the vascular endothelium27). NOS2A was associated with the presence of plaque10), and this was in accordance with our current results. NOS2A gene regulates inducible nitric oxide synthase to produce nitric oxide and is involved in vascular tone regulation, immune response, and neurotransmission. In humans, inducible nitric oxide synthase has been observed in the core of carotid plaques, and its inhibitor can slow the development and progression of atherosclerosis in experimental rabbits28). One study from carotid endarterectomy specimens revealed that the non-ruptured plaques had inducible nitric oxide synthase mRNA and protein, whereas the ruptured plaques did not, indicating that inducible nitric oxide synthase might contribute to the carotid plaque instability29). PPARA has been shown to affect lipid metabolism and oxidative stress, and association between PPARA SNPs and myocardial infarction has been reported30). However, few studies to investigate the potential relationship between PPARA and plaque. In a cohort of Finnish men, PPARA polymorphisms were associated with the progression of coronary atherosclerosis31). In this study, PPARA gene was associated with the presence of carotid plaque, implying its important role in different stages of atherosclerotic plaque.
HABP2 gene encodes a protein involved in cell adhesion and regulates vascular integrity. HABP2 is observed to affect vascular smooth muscle cell proliferation and atherosclerotic plaque vulnerability32). A HABP2 SNP was associated with carotid stenosis progression33). A significant association between HABP2 variants and venous thromboembolic disease has also been reported34). ITGA2 regulates cell adhesion and cell surface-mediated signaling. Its polymorphisms have been associated with the risk of ischemic stroke and carotid IMT and plaque in patients with type 2 diabetes35, 36). An ITGA2 SNP (rs1991013) was related with carotid calcified plaque, a surrogate measure of an increased risk of carotid atherosclerosis10).
Besides these inspiring findings, we evaluated the relationship of gene–gene interaction among the 19 variants with carotid vulnerable plaque using the GMDR analysis. The most noteworthy finding in this study was that there was a significant gene–gene interaction among HABP2 rs7923349, ITGA2 rs1991013, IL1A rs1609682, and NOS2A rs8081248, and the high-risk interactive genotype among the four variants was independently associated with a higher risk of carotid vulnerable plaque, indicating that interaction among the four variants synergistically contributes to vulnerable plaque. The GMDR analysis underscores the complex nature of the genetic effects and the potentially synergistic role of variants in conferring an increased risk of carotid vulnerability. Furthermore, previous studies have also examined the role of various genes in plaque etiology12, 19).
It has become more evident that many common phenotypes are polygenic in nature. It is necessary to evaluate gene–gene interaction when studying the genetic etiology of plaque phenotypes37). However, the nature of the gene-gene interactions among the four variants assessed in this study is not clear. As is known to all, atherosclerosis is a complex inflammatory disease. Endothelial injury, recruitment and activation of immune-inflammatory cells, influx of lipoproteins through the vessel injury space, and smooth muscle cell proliferation play important roles in the pathogenesis mechanisms of atherosclerosis6, 7). A number of studies have explored variants in HABP2, ITGA2, IL1A, and NOS2A are associated with inflammation and endothelial function10, 29, 31, 33, 36). Thus, one possible explanation for the interaction among four variants is that the four genes encode and regulate for inflammation and endothelial function relevant enzymes that participate in the principal pathogenic mechanisms of atherosclerosis. However, further studies are necessary in future. In the next study, we will plan to use the primary cultured neurons or animal models to explain the molecular mechanisms of interaction among the four variants.
Despite our inspiring findings, this study has several limitations. First, this study only sampled residents aged ≥ 40 years; therefore, our results cannot be generalized to all population groups in southwestern China. Second, this study was a cross-sectional study, and recall bias may exist due to the self-reported questionnaire. Third, carotid plaque and plaque vulnerability were evaluated by ultrasound. Although ultrasound can identify carotid plaques and determine the extent of stenosis, high-resolution magnetic resonance imaging (HR-MRI) may provide more information regarding plaque composition and morphology38). Thus, it is necessary to assess carotid plaque using HR-MRI and confirm our current findings in future. In addition, the main aim of this study was to evaluate the association of 19 SNPs in genes involved in inflammation and endothelial function with carotid plaque. Thus, carotid stenosis and IMT in the common carotid artery were not involved in this analysis. Fourth, although we examined the role of several known important genes involved in endothelial function and inflammation, other known and unknown genes were not captured in this study. Future studies involving a larger set of genetic variants should be conducted to elucidate further the gene–gene interaction effects on plaque phenotypes. Finally, antiinflammatory drugs (i.e., statins) and antiplatelet drugs may affect carotid plaque characteristics. In this study, we did not investigate the effect of statins or antiplatelet drugs on carotid plaque stability. Furthermore, a lack of an independent sample for replication was also a limitation in this study.
In conclusion, the prevalence of carotid plaque was very high in the high-risk stroke population in southwestern China. Variants in genes involved in endothelial function and inflammation were associated with carotid plaque and plaque vulnerability in the single SNP analysis. The GMDR analysis revealed that there was a significant gene-gene interaction among HABP2 rs7923349, ITGA2 rs1991013, IL1A rs1609682, and NOS2A rs8081248. The high-risk interactive genotype among the four variants was independently associated with a higher risk of carotid vulnerable plaque and might be potential markers for plaque vulnerability. The GMDR analysis may provide further insight into the complex genetic etiology of carotid plaque vulnerability. However, further studies are needed to validate our findings.
Sources of Funding
This study was supported in part by grants from the Scientific Research Foundation of Sichuan Provincial Health Department (Grant No. 16ZD046), the Sichuan Science and Technology Agency Research Foundation (Grant No.2018JY0164), and Universal Application Program, Health and Family Planning Commission of Sichuan Province in China (Grant No.17PJ084).
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- 1). Guan T, Ma J, Li M, Xue T, Lan Z, Guo J, Shen Y, Chao B, Tian G, Zhang Q, Wang L, Liu Y: Rapid transitions in the epidemiology of stroke and its risk factors in China from 2002 to 2013. Neurology, 2017; 89: 53-61 [DOI] [PubMed] [Google Scholar]
- 2). Donnan GA, Fisher M, Macleod M, Davis SM: Stroke. Lancet, 2008; 371: 1612-1623 [DOI] [PubMed] [Google Scholar]
- 3). O'Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK, Jr: Cartid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med, 1999; 340: 14-22 [DOI] [PubMed] [Google Scholar]
- 4). Hollander M, Bots ML, Del Sol AI, Koudstaal PJ, Witteman JC, Grobbee DE, Hofman A, Breteler MM: Carotid plaques increase the risk of stroke and subtypes of cerebral infarction in asymptomatic elderly: the Rotterdam study. Circulation, 2002; 105: 2872-2877 [DOI] [PubMed] [Google Scholar]
- 5). Rundek T, Arif H, Boden-Albala B, Elkind MS, Paik MC, Sacco RL: Carotid plaque, a subclinical precursor of vascular events: the Northern Manhattan Study. Neurology, 2008; 70: 1200-1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6). Hansson GK: Immune mechanisms in atherosclerosis. Arterioscler Thromb Vasc Biol, 2001; 21: 1876-1890 [DOI] [PubMed] [Google Scholar]
- 7). Berliner JA, Navab M, Fogelman AM, Frank JS, Demer LL, Edwards PA, Watson AD, Lusis AJ: Atherosclerosis: basic mechanisms. Oxidation, inflammation, and genetics. Circulation, 1995; 91:2488-2496 [DOI] [PubMed] [Google Scholar]
- 8). Zhao J, Cheema FA, Bremner JD, Goldberg J, Su S, Snieder H, Maisano C, Jones L, Javed F, Murrah N, Le NA, Vaccarino V: Heritability of carotid intima-media thickness: a twin study. Atherosclerosis, 2008; 197: 814-820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9). Sacco RL, Blanton SH, Slifer S, Beecheam A, Glover K, Garderner H, Wang L, Sabala E, Juo SH, Rundek T: Heritability and linkage analysis of carotid intima-media thickness: the Family Study of STroke Risk and Carotid Atherosclerosis. Stroke, 2009; 40: 2307-2312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10). Gardener H, Beecham A, Cabral D, Yanuck D, Slifer S, Wang L, Blanton SH, Sacco RL, Juo SH, Rundek T: Carotid plaque and candidate genes related to inflammation and endothelial function in Hispanicsfrom northern Manhattan. Stroke, 2011; 42: 889-896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11). Wang L, Yanuck D, Beecham A, Gardener H, Slifer S, Blanton SH, Sacco RL, Rundek T: A candidate gene study revealed sex-specific association between the OLR1 gene and carotidplaque. Stroke, 2011; 42: 588-592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12). Yi X, Lin J, Luo H, Wang C, Liu Y: Genetic variants of PTGS2, TXA2R and TXAS1 are associated with carotid plaque vulnerability, platelet activation and TXA2 levels in ischemic stroke patients. PLoS One, 2017; 12: e0180704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13). Yi XY, Liao DX, Wang C, Cheng W, Fu XQ, Zhang B: Cytochrome P450 Genetic Variants and Their Metabolite Levels Associated with Plaque Stability in Patients with Ischemic Stroke. J Atheroscler Thromb, 2016; 23: 330-338 [DOI] [PubMed] [Google Scholar]
- 14). Bevan S, Traylor M, Adib-Samii P, Malik R, Paul NL, Jackson C, Farrall M, Rothwell PM, Sudlow C, Dichgans M, Markus HS: Genetic heritability of ischemic stroke and the contribution of previously reported candidate gene and genomewide associations. Stroke, 2012; 43: 3161-3167 [DOI] [PubMed] [Google Scholar]
- 15). Lou X-Y, Chen G-B, Yan L, Ma JZ, Zhu J, Elston RC, Li MD: A generalized combinatorial approach for detecting gene-by-gene and gene-by-environment interactions with application to nicotine dependence. Am J Hum Genet, 2007; 80: 1125-1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16). Sun H, Zou X, Liu L: Epidemiological factors of stroke: a survey of the current status in china. J Stroke, 2013; 15: 109-114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17). Stroke Prevention Project Committee Programme of Stroke Screening and Intervention for High-risk Population. http://cnstroke.com/WebManage/InterveneProject/Index (accessed Nov 30, 2018)
- 18). Wang W, Jiang B, Sun H, Ru X, Sun D, Wang L, Wang L, Jiang Y, Li Y, Wang Y, Chen Z, Wu S, Zhang Y, Wang D, Wang Y, Feigin VL: Prevalence, incidence, and mortality of stroke in China: results from a nationwide population-based survey of 480687 adults. Circulation, 2017; 135: 759-771 [DOI] [PubMed] [Google Scholar]
- 19). Yi X, Liao D, Wu L, Chen H, Li J, Wang C: CYP Genetic Variants, CYP Metabolite Levels, and Symptomatic Carotid Stenosis in Ischemic Stroke Patients. J Atheroscler Thromb, 2016; 23: 621-631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20). Mathiesen EB, Bønaa KH, Joakimsen O: Low levels of high-density lipoprotein cholesterol are associated with echolucent carotid artery plaques: the tromsø study. Stroke, 2001; 32: 1960-1965 [DOI] [PubMed] [Google Scholar]
- 21). Worrall BB, Azhar S, Nyquist PA, Ackerman RH, Hamm TL, DeGraba TJ: Interleukin-1 receptor antagonist gene polymorphisms in carotid atherosclerosis. Stroke, 2003; 34: 790-793 [DOI] [PubMed] [Google Scholar]
- 22). Muiño E, Krupinski J, Carrera C, Gallego-Fabrega C, Montaner J, Fernández-Cadenas I: An Inflammatory Polymorphisms Risk Scoring System for the Differentiation of Ischemic Stroke Subtypes. Mediators Inflamm, 2015; 2015: 569714. 10.1155/2015/569714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23). Cheng YC, Stanne TM, Giese AK, Ho WK, Traylor M, Amouyel P, Holliday EG, Malik R, Xu H, Kittner SJ, Cole JW, O'Connell JR, Danesh J, Rasheed A, Zhao W, Engelter S, Grond-Ginsbach C, Kamatani Y, Lathrop M, Leys D, Thijs V, Metso TM, Tatlisumak T, Pezzini A, Parati EA, Norrving B, Bevan S, Rothwell PM, Sudlow C, Slowik A, Lindgren A, Walters MR, WTCCC-2 Consortium. Jannes J, Shen J, Crosslin D, Doheny K, Laurie CC, Kanse SM, Bis JC, Fornage M, Mosley TH, Hopewell JC, Strauch K, Müller-Nurasyid M, Gieger C, Waldenberger M, Peters A, Meisinger C, Ikram MA, Longstreth WT, Jr, Meschia JF, Seshadri S, Sharma P, Worrall B, Jern C, Levi C, Dichgans M, Boncoraglio GB, Markus HS, Debette S, Rolfs A, Saleheen D, Mitchell BD: Genome-wide association analysis of young onset stroke identifies a locus on chromosome 10q25 near HABP2. Stroke, 2016; 47: 307-316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24). Prabhakaran S, Rundek T, Ramas R, Elkind MS, Paik MC, Boden-Albala B, Sacco RL: Carotid plaque surface irregularity predicts ischemic stroke: the northern Manhattan study. Stroke, 2006; 37: 2696-2701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25). Libby P: Coronary artery injury and the biology of atherosclerosis: inflammation, thrombosis, and stabilization. Am J Cardiol, 2000; 86: 3J-8J [DOI] [PubMed] [Google Scholar]
- 26). Francis SE, Camp NJ, Dewberry RM, Gunn J, Syrris P, Carter ND, Jeffery S, Kaski JC, Cumberland DC, Duff GW, Crossman DC: Interleukin-1 receptor antagonist gene polymorphism and coronary artery disease. Circulation, 1999; 99: 861-866 [DOI] [PubMed] [Google Scholar]
- 27). Santtila S, Savinainen K, Hurme M: Presence of the IL-1RA allele 2 (IL-1RN*2) is associated with enhanced IL- 1beta production in vitro. Scand J Immunol, 1998; 47: 195-198 [DOI] [PubMed] [Google Scholar]
- 28). Hayashi T, Matsui-Hirai H, Fukatsu A, Sumi D, Kano-Hayashi H, Rani PJA, Iguchi A: Selective iNOS inhibitor, ONO1714 successfully retards the development of high-cholesterol diet induced atherosclerosis by novel mechanism. Atherosclerosis, 2006; 187: 316-324 [DOI] [PubMed] [Google Scholar]
- 29). Behr-Roussel D, Rupin A, Sansilvestri-Morel P, Fabiani JN, Verbeuren TJ: Histochemical evidence for inducible nitric oxide synthase in advanced but non-ruptured human atherosclerotic carotid arteries. Histochem J, 2000; 32: 41-51 [DOI] [PubMed] [Google Scholar]
- 30). Reinhard W, Stark K, Sedlacek K, Fischer M, Baessler A, Neureuther K, Weber S, Kaess B, Wiedmann S, Mitsching S, Lieb W, Erdmann J, Meisinger C, Doering A, Tolle R, Jeron A, Riegger G, Hengstenberg C: Association between PPARalpha gene polymorphisms and myocardial infarction. Clin Sci (Lond), 2008; 115: 301-308 [DOI] [PubMed] [Google Scholar]
- 31). Flavell DM, Jamshidi Y, Hawe E, Pineda Torra I, Taskinen MR, Frick MH, Nieminen MS, Kesäniemi YA, Pasternack A, Staels B, Miller G, Humphries SE, Talmud PJ, Syvänne M: Peroxisome proliferator-activated receptor alpha gene variants influence progression of coronary atherosclerosis and risk of coronary artery disease. Circulation, 2002; 105: 1440-1445 [DOI] [PubMed] [Google Scholar]
- 32). Kanse SM, Parahuleva M, Muhl L, Kemkes-Matthes B, Sedding D, Preissner KT: Factor VII-activating protease (FSAP): vascular functions and role in atherosclerosis. Thromb Haemost, 2008; 99: 286-289 [DOI] [PubMed] [Google Scholar]
- 33). Willeit J, Kiechl S, Weimer T, Mair A, Santer P, Wiedermann CJ, Roemisch J: Marburg I polymorphism of factor VII-activating protease: a prominent risk predictor of carotid stenosis. Circulation, 2003; 107: 667-670 [DOI] [PubMed] [Google Scholar]
- 34). Reiner AP, Lange LA, Smith NL, Zakai NA, Cushman M, Folsom AR: Common hemostasis and inflammation gene variants and venous thrombosis in older adults from the Cardiovascular Health Study. J Thromb Haemost, 2009; 7: 1499-1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35). Matarin M, Brown WM, Hardy JA, Rich SS, Singleton AB, Brown RD, Jr, Brott TG, Worrall BB, Meschia JF: Association of integrin alpha2 gene variants with ischemic stroke. J Cereb Blood Flow Metab, 2008; 28: 81-89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36). Maeno T, Koyama H, Tahara H, Komatsu M, Emoto M, Shoji T, Inaba M, Miki T, Okuno Y, Nishizawa Y: The 807T allele in alpha2 integrin is protective against atherosclerotic arterial wall thickening and the occurrence of plaque in patients with type 2 diabetes. Diabetes, 2002; 51: 1523-1528 [DOI] [PubMed] [Google Scholar]
- 37). Yamasaki Y, Katakami N, Sakamoto K, Kaneto H, Matsuhisa M, Sato H, Hori M, Haneda M, Kashiwagi A, Tanaka Y, Kawamori R, Kuno S: Combination of multiple genetic risk factors is synergistically associated with carotid atherosclerosis in Japanese subjects with type 2 diabetes. Diabetes Care, 2006; 29: 2445-2451 [DOI] [PubMed] [Google Scholar]
- 38). Fitzpatrick LA, Berkovitz N, Dos Santos MP, Majeed N, Glikstein R, Chakraborty S, Veinot JP, Stotts G, Berthiaume A, Chatelain R: Vulnerable carotid plaque imaging and histopathology without a dedicated MRI receiver coil. Neuroradiol J, 2017; 30: 120-128 [DOI] [PMC free article] [PubMed] [Google Scholar]


