Abstract
Aim: Coronary artery disease (CAD) and cognitive impairment are common in the elderly, with evidence for shared risk factors and pathophysiological processes. The coronary artery calcium (CAC) score is a marker of subclinical CAD, which may allow early detection of individuals prone to cognitive decline. Prior studies on associations of CAC and clinical CAD with cognitive impairment had discrepant results. This systematic review aims to evaluate the association of (sub)clinical CAD with cognitive function, cognitive decline, and diagnosis of mild cognitive impairment (MCI) or dementia.
Methods: A systematic search was conducted in MEDLINE, Embase, and Web of Science until February 2019, supplemented with citations tracking. Two reviewers independently screened studies and extracted information including odds ratios (ORs) and hazard ratios (HRs).
Results: Forty-six studies, 10 on CAC and 36 on clinical CAD, comprising 1,248,908 participants were included in the systematic review. Studies about associations of (sub)clinical CAD with cognitive function and cognitive decline had heterogeneous methodology and inconsistent findings. Two population-based studies investigated the association between CAC and risk of dementia over 6–12.2 years using different CAC scoring methods. Both found a tendency toward higher risk of dementia as CAC severity increased. Meta-analysis in 15 studies (663,250 individuals) showed an association between CAD and MCI/dementia (pooled OR 1.32, 95%CI 1.17–1.48) with substantial heterogeneity (I2 = 87.0%, p < 0.001). Pooled HR of CAD for incident MCI/dementia over 3.2–25.5 years in six longitudinal studies (70,060 individuals) was 1.51 (95%CI 1.24–1.85), with low heterogeneity (I2 = 14.1%, p = 0.32). Sensitivity analysis did not detect any study that was of particular influence on the pooled OR or HR.
Conclusions: : Limited evidence suggests the CAC score is associated with risk of dementia. In clinical CAD, risk of MCI and dementia is increased by 50%, as supported by stronger evidence.
Keywords: Coronary artery disease, Dementia, Coronary artery calcium, Mild cognitive impairment, Atherosclerosis
Introduction
Coronary artery disease (CAD) and dementia are common in the elderly. The prevalence of CAD and dementia is estimated to be 14.9% and 5.2%, respectively, among adults over 60 years of age1, 2). Mortality of CAD has declined during the past decades because of improvement in disease management, resulting in an increasing number of CAD patients with a higher life expectancy1). These patients, although they survive CAD, may develop other age-related diseases such as dementia in their late life. Mild cognitive impairment (MCI) is an intermediate stage between age-related cognitive decline and clinically diagnosed dementia and may be a prodromal stage of Alzheimer's disease (AD) or other neurodegenerative disorders3). Potentially, early detection of MCI/dementia combined with preventive intervention could delay the progression to dementia. It is important to research the relationship between CAD and MCI/dementia in view of the possibility of measures to prevent dementia in CAD patients.
Epidemiological studies have shown that vascular risk factors are associated with cognitive decline and with incidence of MCI and dementia including AD4, 5). There is evidence for shared pathophysiological mechanisms between cardiovascular disease and dementia: vascular risk factors and heart diseases might contribute to MCI and dementia through pathways including neurodegeneration, cerebral atherosclerosis, and cerebral hypoperfusion and hypoxia6). Vascular pathology such as intracranial atherosclerosis can convert low-grade AD to overt dementia7). Reviews and meta-analysis have found cardiovascular diseases such as atrial fibrillation and heart failure to be associated with increased risk of dementia8, 9). However, so far, results on associations between clinical CAD and the risk of cognitive decline are inconsistent10–13). Caution is needed when summarizing evidence from longitudinal studies linking CAD to dementia; particularly, estimated effects may be distorted by study populations with prior invasive intervention14).
There is increasing interest to use coronary artery calcium (CAC) scoring as imaging biomarker for subclinical CAD to estimate cardiovascular disease risk15, 16). Also, in population-based studies on calcium scoring, discrepant results on the relationship of CAC with cognitive function decline were found17–19). So far, there has been no systematic review of associations between CAC and cognitive impairment.
The aim of the current study was to systematically review the literature on the association of (sub) clinical CAD with cognitive function. To meet this aim, we addressed the following question: What is the association of CAC and clinical CAD with (1) cognitive function, (2) cognitive decline, and (3) risk of MCI or dementia?
Methods
This systematic review was performed in line with the Meta-analysis of Observational Studies in Epidemiology (MOOSE) statement20) and Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA) statement21).
Search Strategy
MEDLINE, Embase, and Web of Science were searched from inception to February 2019 without limits on publication dates. The search strategy included terms relevant to coronary atherosclerosis and cognitive impairment (see Supplementary Table 1). Additional appropriate articles were manually added when discovered by tracking citations. An experienced medical information expert checked the search strategy.
Supplementary Table 1. Literature search strategy.
| Search Strings |
|---|
| Pubmed |
| (“Myocardial Ischemia”[Mesh] OR “Atherosclerosis”[Mesh:Noexp] OR coronary atherosclerosis[tiab] OR coronary artery disease [tiab] OR coronary heart disease [tiab] OR coronary calcium[tiab] OR coronary calcification[tiab] OR coronary calcific[tiab] OR coronary calcified[tiab] ) AND (“Dementia”[Mesh] OR “Cognitive Dysfunction”[Mesh] OR dementia [tiab] OR Alzheimer*[tiab] OR cognitive impairment[tiab] OR cognitive decline[tiab] OR cognitive function[tiab] OR cognitive disorder[tiab] OR cognitive performance [tiab] OR cognitive dysfunction[tiab]) NOT (“Animals”[Mesh] NOT “Humans”[Mesh]) |
| EmBase |
| (‘coronary artery disease’/exp OR ‘coronary artery calcium score’/exp OR ‘coronary atherosclerosis’:ab,ti OR ‘coronary artery disease’:ab,ti OR ‘coronary heart disease’:ab,ti OR ‘coronary calcium’:ab,ti OR ‘coronary calcification’:ab,ti OR ‘coronary calcific’:ab,ti OR ‘coronary calcified’:ab,ti) AND (‘mild cognitive impairment’/exp OR ‘dementia’/exp OR ‘dementia’:ab,ti OR ‘alzheimer disease’:ab,ti OR ‘mild cognitive impairment’:ab,ti OR ‘cognitive decline’:ab,ti OR ‘cognitive function’:ab,ti OR ‘cognitive disorder’:ab,ti OR ‘cognitive performance’:ab,ti OR ‘cognitive dysfunction’:ab,ti) NOT (‘animal’/exp NOT ‘human’/exp) |
| Web of Science |
| #1 TS = (coronary artery disease) OR TS = (Coronary atherosclerosis) OR TS = (coronary artery calcium score) OR TS = (coronary calcium) OR TS = (coronary calcified) OR TS = (coronary calcific) OR TS = (coronary calcification) OR TS = (coronary heart disease) #2 TS = (dementia) OR TS = (mild cognitive impairment) OR TS = (alzheimer) OR TS = (cognitive function) OR TS = (cognitive dysfunction) OR TS = (cognitive disorder) OR TS = (cognitive performance) OR TS = (cognitive decline) #3 #1 AND #2 |
Selection Criteria
The following inclusion criteria were used to determine eligibility of a study: (1) all types of studies that examined both coronary atherosclerosis and cognitive function regardless of the study design concerned, that is, cross-sectional, case–control, or longitudinal cohorts (≥ 1 year follow-up); (2) coronary atherosclerosis as defined by clinical CAD events or by subclinical CAD as quantified by CAC scoring; (3) cognitive function based on either validated mental state examinations and neuropsychological testing or clinically diagnosed MCI or dementia; and (4) study sample size larger than 100.
Invasive interventions for CAD may have a negative effect on subsequent cognitive performance14). In addition, the effect of CAD on cognition may be distorted by atrial fibrillation, stroke, or heart failure because of different underlying pathophysiological mechanisms22). Therefore, we excluded studies (1) that clearly mentioned that participants had undergone invasive cardiac procedures prior to cognitive function testing; (2) that did not consider invasive intervention as a confounder for statistical analysis; and (3) that solely focused on patients with atrial fibrillation, stroke, or heart failure. We also excluded case reports, reviews, conference abstracts, editorials, or articles not published in English. In case of multiple articles that reported results based on the same cohort, we only included those articles that reported the largest sample size or that best addressed our research question.
Study Selection, Data Collection, and Quality Assessment
Two reviewers (C.X. and M.V.) independently performed the selection process and data extraction of included studies. Articles were first evaluated for eligibility on the basis of the selection criteria. A standardized data extraction form was used to collect the following information for eligible articles: publication details, study population characteristics, study setting, CAC measurements, determination of CAD and cognitive function, and description of results. Studies included for meta-analysis were evaluated for study quality. For observational studies including cohort and case–control studies, the Newcastle–Ottawa Scale (NOS) was used for the quality assessment23), whereas for cross-sectional studies, an adapted NOS version was used24). In case of a disagreement in article selection or data extraction, this was discussed between the two reviewers and consensus was obtained, or a third reviewer (R.V.) was consulted.
Data Analysis
This systematic review evaluates the relationship of CAC score and clinical CAD with cognitive function. For each part, the following three questions were evaluated: the association of CAC score or clinical CAD with (1) cognitive function, (2) changes of cognitive function over time/cognitive decline, and (3) risk of MCI or dementia. The strength of associations between CAC score or clinical CAD and MCI or dementia as dichotomous outcomes was estimated using either odds ratio (OR) or hazard ratio (HR) and 95% confident intervals (CIs). Meta-analysis was conducted if there were at least two studies that reported the same outcome of interest (MCI and/or dementia). If studies reported the results of myocardial infarction (MI) and angina pectoris (AP) separately, then only the results of the MI were used in this systematic review, and the AP results were excluded since MI is a harder endpoint of CAD. OR and HR (derived from a multivariable model in each study if available) were pooled separately using the inverse variance method with DerSimonian–Laird random-effects model despite inter-study heterogeneity. Pooled estimated effect was tested using the Z test. Heterogeneity was assessed using the Q statistic test and I2 statistic. A two-tailed p value for Q statistic < 0.10 and I2 > 50% was considered to indicate heterogeneity. Reporting biases or small-study effects were evaluated by visual evaluation of the funnel plot of each pooling analysis for symmetry. Egger's test for funnel plot asymmetry was performed only if the number of studies for pooling analysis was sufficient (≥ 10). Sensitivity analysis was conducted using the leave-one-out method to detect any study that may be influential in the overall estimated effect. To explore potential source of heterogeneity across the studies, subgroup analysis was performed on the basis of study design. Statistical analysis was conducted using R (Package “meta,” R Foundation, Vienna, Austria). A two-tailed value of p < 0.05 was considered as statistically significant except for the test of heterogeneity.
Results
Study Selection
The results of the search strategy and selection process are shown in Fig. 1. After removal of duplicates, 6,601 studies were screened on the basis of title and abstract. Finally, 46 studies (10 for CAC, 36 for clinical CAD), comprising 1,248,908 participants, were included for the systematic review. The main study characteristics are provided in Supplementary Tables 2 and 3. The main results of the studies are shown in Supplementary Tables 4 and 5, sorted by outcome: cognitive function, changes of cognitive function over time, and risk of MCI/dementia. Quality of studies that investigated the association between clinical CAD and diagnosis of MCI/dementia was variable (Supplementary Tables 6–8). Ten studies on clinical CAD had suboptimal ascertainment of exposure as determination of clinical CAD was self-reported or not described11, 25–33).
Fig. 1.
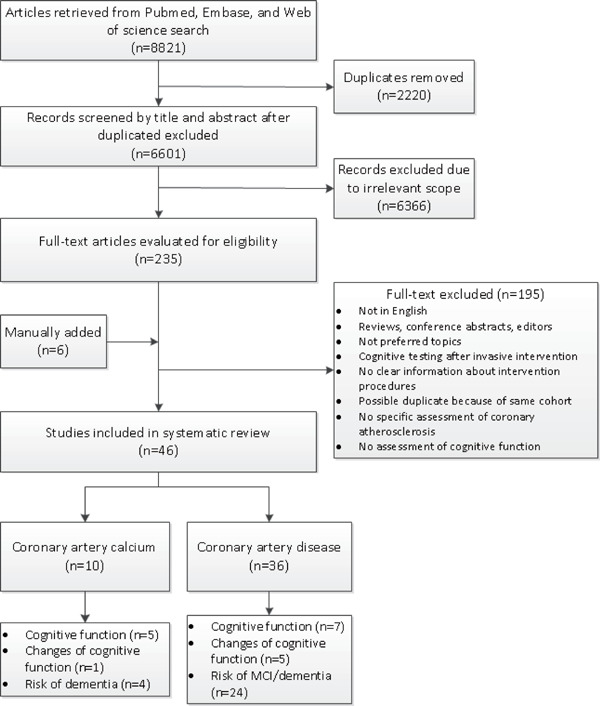
Flowchart of literature search and selection
Supplementary Table 2. Characteristics of included studies on the association between coronary artery calcium and cognitive function.
| Study, year | Study setting | Study population | No. of Participants | Age (Mean, SD), years | Female, % | Follow-up (Mean, SD), years |
|---|---|---|---|---|---|---|
| Topic 1: Association with cognitive function Cross-sectional | ||||||
| Reis, 2013 35) | Cross-sectional analysis of coronary artery risk development in young adults (CARDIA) study | Multi-center, community based including black and white | 2,510 | Range 43–55 years | 54.9 | NA |
| Vidal, 2010 16) | Cross-sectional analysis of the age, gene, environment susceptibility (AGES) - Reykjavik study | Residents in Reykjavik, Iceland | 4,250 | Range 74.5–78.0 | 75.0 | NA |
| Suemoto, 2017 34) | Cross-sectional analysis of Brazilian Longitudinal Study of Adult Health (ELSD-Brasil) study | Residents in São Paulo Center, Brazil | 4,104 | 50.9 ± 8.8 | 54.0 | NA |
| Longitudinal | ||||||
| Hugenschmidt, 2013 36) | Cross-sectional analysis of Diabetes Heart Study (DHS) –Mind | T2DM affected and unaffected siblings (European American, African American) | 514 (T2DM affected n = 422, T2DM unaffected n = 92) | T2DM affected 67.8 ± 8.6, T2DM unaffected 67.0 ± 10.1 | T2DM affected 46.2, T2DM unaffected 36.2 | 6.7 ± 1.6 |
| Rossetti, 2015 37) | Cross-sectional analysis of Dallas heart study (DHS) | African American, white, Hispanic | 1,154 | 50.9 ± 10.4 | 58.0 | 6 |
| Topic 2: Association with changes of cognitive function over time (longitudinal) | ||||||
| Jacobson, 2011 38) | Prospective cohort of Diabetes control and complications trial (DCCT)/Epidemiology of diabetes interventions and complications (EDIC) study | Type 1 diabetes patients | 1,144 | 45.7 ± 6.8 | 47.0 | 18.5 |
| Topic 3: Association with MCI/dementia Longitudinal (risk of MCI/dementia) | ||||||
| Bos, 2015 17) | Prospective Rotterdam cohort | Residents in Rotterdam, The Netherlands | 2,364 (2,212 censored for stroke) | 69.4 ± 6.7 | 52.3 | 6 |
| Fujiyoshi, 2017 18) | Prospective cohort of Multi-Ethnic Study of Atherosclerosis (MESA) | 12.2% Chinese, 26.1% black, 22.5% Hispanic, and 39.2% white | 6,293 (6,120 excluded interim stroke) | 68.4 ± 5.9 | 52.5 | 12.2 (Median) |
| Kuller, 2016 40) | Prospective cohort of Cardiovascular Health Study | Predominantly 80+ years (white, African-American, others) | 311 | ≥ 80 | 65.0 | 10+ |
| Guo, 2019 39) | Pittsburgh epidemiology of diabetes complications (EDC) study | Diagnosed with childhood-onset type 1 diabetes | 148 | 37.2 ± 7.0 | 51.0 | 14.0 ± 3.5 |
SD, Standard Deviation; T2DM, Type 2 Diabetes Mellitus; NA, Not applicable.
Supplementary Table 3. Characteristics of included studies on the association between coronary artery disease and cognitive function.
| Study, year | Study setting | Study population | No. of Participants | Age (Mean, SD), years | Female, % | Follow-up (Mean, SD), years |
|---|---|---|---|---|---|---|
| Topic 1: Association with cognitive function Cross-sectional | ||||||
| Verhaeghen, 2003 42) | Cross-sectional analysis of Berlin aging study (BASE) in Germany | Locally representative sample predominantly above 70 years old | 516 | 84.9 | 50.0 | NA |
| Elwood, 2002 44) | Cross-sectional analysis of the Caerphilly cohort in South Wales | Representative sample of men | Around 1,700 | Range 55–69 | 0 | NA |
| Lyall, 2017 46) | Cross-sectional analysis of baseline UK Biobank cohort | General population | 478,557 | 56.4 ± 8.1 | 54.7 | NA |
| Case control | ||||||
| Ahto, 1999 45) | Case control study in Lieto , Finland | Residents | 486 (patients with CHD 162, controls 324) | Range 64–85+ | 45.0 | NA |
| Longitudinal | ||||||
| Volonghi, 2013 47) | Longitudinal cohort of Oxford Vascular Study in UK | Population based | 616 (ACS 216, TIA 182, Minor stroke 218) | ACS 68.1 ± 12.4, TIA 72.5 ± 11.7, Minor stroke 71.0 ± 12.5 | ACS 27, TIA 55, Minor stroke 33 | 5 |
| Reijmer, 2011 41) | Hoorn Study, Netherland | Population based | 380 | Range 50–75 | 50.0 | Cognitive function assessed 7 years after the assessment of CAD |
| Arntzen, 2011 43) | Tromsø Study in Norway | Population based | 5,033 | Men 58.8 ± 9.2, Women 58.2 ± 9.7 | 55.8 | Cognitive function assessed 7 years after the assessment of CAD |
| Topic 2: Association with changes of cognitive function over time (longitudinal) | ||||||
| Lipnicki, 2013 49) | Longitudinal cohort of Sydney Memory and Ageing Study (MAS) in Australia | Community based | 889 | 78.6 ± 4.8 | 54.1 | 2 |
| Kalmijn, 1996 51) | Longitudinal cohort of the Zutphen Elderly Study in Netherlands | Men living in Zutphen | 353 | 74.6 ± 4.2 | 0 | 3 |
| Almeida, 2012 48) | Prospective case control, Heart Mind study in the western Australia | Community volunteers | 231 (controls 81, CHD 73, CHF 77) | Controls 69.3 ± 11.3, CHD 67.8 ± 9.5, CHF 68.4 ± 10.2 | Controls 67.9, CHD 33.3, CHF 16.9 | 2 |
| Mielke, 2007 52) | Longitudinal cohort of Cache County Study on Memory, Health, and Aging (CCSMHA), Utah in the United States | Local residents with Alzheimer disease | 135 | 84.2 ± 6.5 | 65.9 | ≥ 1 |
| Bleckwenn, 2017 50) | Longitudinal cohort of Ageing, cognition, and dementia in primary care patients (AgeCoDe) in Germany | Patients with AD from primary health care | 118 | 85.6 ± 3.3 | 74.6 | 3 |
| Topic 3: Association with MCI or dementia Cross-sectional | ||||||
| Wang, 2015 55) | Cross-sectional study in North China | Community based | 3,136 | Range 60–80+ | 59.3 | NA |
| Roberts, 2010 54) | Cross-sectional in Olmsted county, the United States | Residents | 1,969 | 80.4 | 49.1 | NA |
| Zou, 2014 56) | Cross-sectional study in China | Hospital and community based | 597 | Range 60–95 | 56.6 | NA |
| Hai, 2012 26) | Cross-sectional study in China | Residents in Southwest China | 202 | 82.5 ± 2.1 | 25.7 | NA |
| Kuroki, 2018 57) | PROST (Project in Sado for Total Health) study in Japan | Outpatients | 565 | Range 62–79 | 48.7 | NA |
| Stephan, 2017 58) | Cognitive Function and Ageing Study (CFAS) in UK | General population | 2,050 | ∼ 75 (estimated) | ∼ 63 (estimated) | NA |
| Heath, 2014 27) | Cross-sectional study in Scotland | Population based | 616,245 (1,061 cases) | Range 40–64 | 49.5 | NA |
| Ross, 1999 25) | Cross-sectional analysis of a Honolulu Heart Program, Honolulu-Asia aging study in Hawai, the United States | Japanese American men | 3,509 (Vascular dementia 68, stroke no dementia 106, no stroke no dementia 3,335) | Range 71–93 | 0 | NA |
| Deng, 2018 28) | Cross-sectional study in Chongqing, China | Residents | 1,781 | ≥ 60 | 60.5 | NA |
| Dolan, 2010 53) | Cross-sectional analysis of Baltimore Longitudinal Study of Aging (BLSA) Autopsy Program in the United States | Predominantly white | 200 | 87.6 ± 7.1 (age at death) | 33.5 | NA |
| Case control | ||||||
| Jacob, 2017 31) | Retrospective case control of the Disease Analyzer database (IMS Health) in Germany | German primary care patients | 7,208 (3,604 patients with initial diagnosis of MCI; 3,604 controls without MCI) | 75.2 ± 9.1 | 45.3 | 3 years of continuous follow-up prior to the index date of MCI |
| Bursi, 2006 10) | Retrospective case control in Rochester, the United States | Local residents | 1,832 (916 patients with dementia, 916 matched controls) | Median 82 range 38–102 | 72.0 | NA |
| Massaia, 2001 59) | Retrospective case control study in the University of Torino, Italy | Consecutive patients and controls in the Geriatric Institute | 456 (228 patients with AD and 228 cognitively intact controls) | AD patients 74.5 ± 7.0, controls 75.1 ± 7.7 | NR | NA |
| Booker, 2016 29) | Retrospective case control of the Disease Analyzer database (IMS Health) in Germany | German primary care patients | 23,912 (11,956 patients with initial diagnosis of dementia, 11,956 controls without dementia) | 80.4 ± 5.3 | 61.0 | 10 years of continuous follow-up before index data of dementia |
| Takahashi, 2012 30) | Retrospective case control at Mayo Clinic in Olmsted County, USA | Patients living within Olmsted County received care at the Mayo Clinic | 410 (205 cases of vascular dementia and 205 paired controls) | 81.9 ± 7.8 | 59.0 | NA |
| Longitudinal (risk of MCI/dementia) | ||||||
| Newman, 2005 12) | Longitudinal cohort of Cardiovascular Health Study (CHS) in US | Community based | 2,539 | Range 65–97 | 60.0 | 5.4 |
| Rusanen, 2014 11) | Longitudinal cohort of Cardiovascular Risk factors, aging and dementia (CAIDE) study, Finland | Population based | 1,510 | 50.3 ± 6.0 at baseline | 62.4 | 25.5 ± 6.3 from baseline 7.8 ± 1 from first re-examination |
| Hayden, 2006 61) | Cache County Study of Memory Health and Aging (CCSMHA), USA | Residents of Cache Country, Utah, USA | 3,264 | 74.0 ± 6.4 | 58.2 | 3.2 |
| Haring, 2013 13) | Longitudinal cohort of Women's Health Initiative Memory Study (WHIMS) in the United States | Postmenopausal women | 6,445 | Range 65–79 | 100.0 | 8.4 median |
| Ikram, 2008 62) | Longitudinal cohort of Rotterdam Study in Netherland | Residents | 6,347 (No MI 5578, Recognized MI 424, Unrecognized MI 345) | No MI 68.3 ± 8.5, Recognized MI 71.2 ± 8.2, Unrecognized MI 71.8 ± 8.8 | No MI 61.4, Recognized MI 30.0, Unrecognized MI 53.9 | 10 |
| Brayne, 1998 60) | A prevalence and incidence study of dementia in Cambridge city | Participants were from selected group general practices | 376 | ≥ 75 | 63.6 | 2.4 |
| Kivipelto, 2002 33) | Prospective FINMONICA study in Finland | Population based | 1,287 | Range 65–79 | 61.8 | 21.0 ± 4.9 |
| Chen, 2011 32) | Prospective Anhui cohort study in China | Residents | 1,307 | ≥ 65 | NR | 3.9 median |
| Lin, 2017 63) | Taiwan's National Health Insurance Research Database | Population based | 49,955 (Depression 9,991 Nondepression 39,964) | Range 29–51 | 61.2 | ∼7 |
CAD, Coronary Artery Disease; SD, Standard Deviation; ACS, Acute Coronary Syndrome; TIA, Transient Ischemic Attack; NA, Not Applicable; NR: not reported; CHD, Coronary Heart Disease; CHF, Chronic Heart Failure; MCI, Mild Cognitive Impairment; AD, Alzheimer's Disease; MI, Myocardial Infarction.
Supplementary Table 4. Exposure and outcomes of included studies on the association between coronary artery calcium and cognitive function.
| Study, year | CT type | Type of Calcium score | Data type of input variable | Outcomes | Adjustments for confounders | Main results |
|---|---|---|---|---|---|---|
| Topic 1: Association with cognitive function Cross-sectional | ||||||
| Reis, 2013 35) | MDCT | Agatston score | Categorical (0, 1–99, 100–399, ≥ 400) | Cognitive performance assessed with the DSST, Stroop Test, and RAVLT. | Age, sex, race, educational level, study center, BMI, smoking status, alcohol use, dyslipidemia, hypertension, and diabetes | Higher CAC was significantly associated with lower DSST score. |
| Vidal, 2010 16) | MDCT | Agatston score | Quartiles of CAC calculated separately by gender | Prevalence of dementia and cognitive scores of separate cognitive domains | Age, education level presence of depressive symptoms and cardiovascular risk factors including ever smoker, prevalent coronary heart disease, current hypertension and diabetes, and midlife systolic pressure and total cholesterol | Increasing quartile of CAC associated with lower cognitive scores of speed of processing, executive function, and increased percentage of dementia. |
| Suemoto, 2017 34) | MDCT | Agatston score | Binary (CAC < 100: absent or mild atherosclerosis, CAC ≥ 100; moderate to severe atherosclerosis) | Cognitive function was assessed by using DWRT, CFT, TMT by trained examiners | Age, sex, race, marital status, education income, hypertension, diabetes, LDL-cholesterol, HDL cholesterol, smoking, alcohol use, physical activity, BMI, depression and thyroid function status | Participants with CAC ≥ 100 had worse cognitive performance in global cognition, DWRT TMT scores, compared to those with CAC < 100. |
| Longitudinal | ||||||
| Hugenschmidt, 2013 36) | NA | Agatston score | Natural log transformed | Cognitive performance tested with a battery of tests including DSST, RAVLT and Semantic Fluency Task. | Age sex and education | Higher burden of coronary calcification was significantly associated with worse cognitive performance on DSST, RAVLT, and semantic fluency task after a mean of 6.7 years, in participants with T2DM. |
| Rossetti, 2015 37) | EBT | Agatston score | Binary (CAC > 10 Agatston units were defined as positive) | MoCA Scores | NA | Mean of MoCA total score (SD) after about 6 years was 23.69 (3.87) for CAC ≤ 10:, and 22.35 (4.40) for CAC > 10, p-value 0.038. |
| Topic 2: Association with changes of cognitive function over time (longitudinal) | ||||||
| Jacobson, 2011 38) | NA | Agatston score | Binary (0 versus > 0) | Deterioration of cognitive scores on 8 cognitive domains | Sex, baseline age, baseline education level, painful neuropathy reported at follow-up, visual acuity, length of follow-up, the number of interval cognitive tests taken | No evidence supporting the association between presence of coronary calcification and rapid decline of cognitive scores in T1DM patients. |
| Topic 3 : Association with MCI/dementia Longitudinal (risk of MCI/dementia) | ||||||
| Bos, 2015 17) | MDCT | Volume score | Ln(calcification +1.0 mm3) | Incidence of dementia was diagnosed according to DSM-III-R | Age, sex and education level | CAC score was not significantly associated with risk of dementia: HR 1.05 (95% CI 0.80, 1.36), of AD: HR 1.09 (95%CI 0.81, 1.46) censored for stroke. |
| Fujiyoshi, 2017 18) | EBT, MDCT | Agatston score | Log2 (CAC score +1) | Incidence of dementia was diagnosed based on ICD10 codes | Age, sex, race, education level, having health insurance, physical activity, smoking, obesity, hypertension, medications for hypertension or dyslipidemia, systolic blood pressure, non-HDL cholesterol, diabetes, APOE epsilon-4 genotype | log2 (CAC score +1) was significantly associated with risk of dementia (HR 1.18; 95% CI 1.03, 1.36) after multivariable adjustment and exclusion of interim stroke. |
| Kuller, 2016 40) | EBT | Agatston score | Categorical (0, 1–10, 11–100, 101–400, > 400) | Incidence of dementia basis of standard criteria | NA | White women with a CAC score > 400 had around 3 times higher risk of dementia than those with CAC score = 0. |
| Guo, 2019 39) | EBT | Agatston score | Categorical (0, 1–100, 101–300, > 300) | Cognitive impairment defined as having two or more of cognitive test scores ≥ 1.5 SD worse than norms | Education, sex, age, diabetes duration, APOE epsilon-4, ever smoking, BMI, HbA1c, cholesterols (HDL and non-HDL), triglycerides, urinary albumin excretion rate, hypertension, proliferative retinopathy, distal symmetric polyneuropathy, and statin use were offered for model selection. | Compared to CAC score = 0, OR (95%CI) of MCI for CAC score 1–100, 101−300 and > 300 were 1.4 (0.6, 3.6), 2.3 (0.6, 9.7), and 7.9 (1.6, 38.5), respectively. |
CAC, Coronary Artery Calcium ; CT, Computed Tomography; MDCT, Multi-detector Computed Tomography; EBT, Electron Beam Tomography; SD, Standard Deviation; BMI, Body Mass Index; LDL, Low-density Lipoprotein; HDL, High-density Lipoprotein; DSST, Digit Symbol Substitution Task; RAVLT, Rey Auditory-verbal Learning Task; MoCA, Montreal Cognitive Assessment; DWRT, Delayed Word Recall Test; CFT, Category Fluency Test; TMT, Trail Making Test; T2DM, Type 2 Diabetes Mellitus; HR, Hazard Ratio; CI, Confidence Interval; ICD10, International Statistical Classification of Diseases and Related Health Problems 10th version; DSM-III-R, Diagnostic and Statistical Manual of Mental Disorders Version III Revised; AD: Alzheimer's Disease; NA, not available; APOE, apolipoprotein E
Supplementary Table 5. Exposure and outcomes of included studies on the association between coronary artery disease and cognitive function.
| Study, year | Ascertainment of exposure | Outcomes | Adjustments for confounders | Main results |
|---|---|---|---|---|
| Topic 1: Association with cognitive function Cross-sectional | ||||
| Verhaeghen, 2003 42) | In order to ascertain MI and CHD diagnoses, medical history, physical examination, resting ECG results, interview with family doctor were considered | Cognitive performance including perceptual speed, episodic memory, fluency and knowledge was assessed using cognitive test battery separately | Age, sex, social-economic status | Prevalent of CHD associated with worse cognitive performance on fluency. |
| Elwood, 2002 44) | Medical history of IHD including previous MI, ECG ischemia, angina recorded via interviews as well as ECG testing | Tests of cognitive function including AH4, CAMCOG, MMSE and the Choice Reaction Time were performed | Age, education level, mood at the time of testing | Compared with men without disease, decrements in men with IHD was 16% SD for the AH4, 13% SD for the MMSE, 14% SD for the CAMCOG and 17% SD for the CRT. |
| Lyall, 2017 46) | CAD defined as history of physician-diagnosed angina and/or MI. | Cognitive abilities were tested by three tests including verbal-numerical reasoning, visual memory test and reaction time test. | Age, sex, ethnicity, depression, education, Townsend deprivation score, smoking status, alcohol intake, medication use and BMI | Presence of CAD associated with poorer cognitive scores, for reasoning (effect size −0.107, 95%CI −0.135, −0.079), log reaction time (effect size 1.005, 95%CI 1.004, 1.007), log memory error scores (effect size 1.017, 95%CI 1.011, 1.023). |
| Case control | ||||
| Ahto, 1999 45) | MI ascertained by checking medical records or ECG results. AP defined as chest pain on effort fulfilling the Rose questionnaire's criteria. | Cognitive impairment defined as total MMSE scores under 23 tested by 2 trained nurses. | NA | MMSE score (mean ± SD) among Men: CAD 26.5 ± 4.9, Controls 27.0 ± 3.6 (p-value 0.70); among Women: CAD 26.5 ± 3.4 Controls 26.9 ± 3.1 (p-value 0.35). |
| Longitudinal | ||||
| Reijmer, 2011 41) | IHD defined as Minnesota codes on ECG or self-reported history of MI | Neuropsychological test battery was used for cognitive assessment | Age and sex | Presence of IHD significantly associated with lower information processing speed. |
| Arntzen, 2011 43) | Definition of CHD was history of MI and/ or prevalent AP | Cognitive performance assessed by the twelve word memory test, digit-symbol coding test and tapping test. | Age, education, physical activity, smoking systolic blood pressure, total cholesterol, HDL-cholesterol, BMI, diabetes, depression | No evidence supporting CHD correlated with lower cognitive scores |
| Volonghi, 2013 47) | ACS defined as ST elevation and non-ST elevation MI, or unstable angina, according to currently accepted criteria | Cognitive function assessed using MMSE, TICSm, and MoCA by trained research nurses. | NA | ACS patients had worse cognitive function than those with TIA |
| Topic 2: Association with changes of cognitive function over time (longitudinal) | ||||
| Lipnicki, 2013 49) | CAD defined as previous diagnosis of MI or angina. | Cognitive performance assessed by a battery of neuropsychological tests | Age and sex | CAD associated with greater decline in memory. |
| Kalmijn, 1996 51) | History of MI and AP obtained from a standardized questionnaire | Cognitive decline defined as a decrease in MMSE of above 1 SD from 1990 to 1993 | Age, education and baseline MMSE score | History of CAD increased the risk of cognitive decline with OR 1.7 (95%CI 0.8, 3.5). |
| Almeida, 2012 48) | Clinical and biochemical evidence of past MI with normal left ventricular function and no clinical symptoms of congestive heart failure | CAMCOG scores | Age, sex and CAMCOG scores at baseline | No difference between CAD and control groups on changes of CAMCOG. |
| Mielke, 2007 52) | Information on previous MI obtained via proxy and self-report | Decline as reflected by scores of MMSE and CDR | Age, sex, education, dementia duration, APOE epsilon-4, depression, baseline MMSE score | MI and AP predicted cognitive decline (MMSE scores) in patients with AD. |
| Bleckwenn, 2017 50) | Diagnosis of CHD or MI reported by GPs | Decline of cognitive function in patients with AD assessed by MMSE and the CDR | Age, sex, education, and time since dementia diagnosis | Presence of CHD related to rapid decline of cognition in patients with AD. |
| Topic 3: Association with MCI or dementia Cross-sectional | ||||
| Wang, 2015 55) | CHD checked through medical history | Diagnostic criteria of MCI was according to the proposal of Petersen et al. | Not clarified | CHD was not associated with MCI (effect size not reported). |
| Roberts, 2010 54) | Ascertainment and criteria described in reference, in brief the assessment was based on medical history, diagnostic test, and ICD codes. | Prevalent MCI including amnestic and non-amnestic subtypes diagnosed according to published criteria | Age, sex, years of education, diabetes, hypertension, stroke, BMI, depression, dyslipidemia, APOE genotype | OR of MI for MCI was 0.91 (95%CI 0.55, 1.49). |
| Zou, 2014 56) | History of coronary heart disease including silent MI, AP, MI, or ischemic cardiomyopathy, assessed by ECG and color doppler ultrasound | MCI diagnosed according to patients complains and MMSE and CDR scores. | Age, gender and education | OR of CHD for MCI, OR 0.988 (95%CI 0.981, 0.996). |
| Hai, 2012 26) | History of CHD ascertained by interview | MCI diagnosed following the criteria proposed by Petersen et al. | Not clarified | OR of CHD for MCI 6.76 (95%CI 2.74, 16.67). |
| Kuroki, 2018 57) | Medical history of IHD obtained from clinical records | MMSE score < 24 considered to indicate MCI. | Age, sex, smoking and drinking habit, number of teeth | OR of IHD for MCI 2.73 (95% CI 1.32, 5.67) |
| Stephan, 2017 58) | CHD composite variable incorporating the presence of self-reported heart attack (single question asking about the presence or absence of the condition) or angina based on the Rose Diagnostic Scale. | MMSE score < 24 considered to reflect MCI. | Age, sex, years of education, disease comorbidity | OR of CHD for MCI 1.02 (95%CI 0.71–1.40). |
| Heath, 2014 27) | Information of IHD extracted using electronic medical records | Diagnosis of dementia fulfilled DSM-IV | Age, sex, neurodegenerative disorder, learning disability, socioeconomic status | OR of IHD for dementia 1.9 (95%CI 1.5, 2.4) |
| Ross, 1999 25) | Prevalent CHD defined as MI or AP without describing ascertainment method | Vascular dementia diagnosed based on the criteria proposed by California Alzheimer Disease Diagnostic and Treatment Centers | Age | OR of CHD for vascular dementia 2.60 (95%CI 1.59, 4.25). |
| Deng, 2018 28) | CHD ascertained by questionnaire | Dementia diagnosis based on the criteria of the Diagnostic and Statistical Manual of Mental Disorders | Living area, age, marital status, smoking, exercise, social activities, BMI, hypertension, depression | OR of CHD for dementia 1.596 (95%CI 1.017–2.506) |
| Dolan, 2010 53) | Coronary atherosclerosis examined postmortem | Diagnosis of dementia based on DSM-III-R and the pathology assessed by autopsy | Age, sex | No association between coronary atherosclerosis and dementia |
| Case control | ||||
| Jacob, 2017 31) | CHD diagnosed based on ICD codes I20–I25 | MCI diagnosed based on ICD 10 codes by neurologists or psychiatrists | Disorders including, anxiety, depression, hyperlipidemia, obesity, hypertension, diabetes | OR of CHD for MCI 1.17 (95%CI 1.04, 1.32). |
| Bursi, 2006 10) | MI defined based on ICD codes 410–414 | Dementia diagnosed according to DSM-IV | NA | OR of MI for dementia 1.00 (95%CI 0.62, 1.62) |
| Massaia, 2001 59) | History of acute MI | AD diagnosed according to DSM-III and the NINCDS-ADRDA work group criteria | NA | No significant difference between AD and controls regarding a history of acute MI |
| Booker, 2016 29) | CHD diagnosed in primary care based on ICD codes I20–I25 | Dementia diagnosed based on ICD codes. | Diabetes, hypertension, obesity, hyperlipidemia, medications | OR of CHD for dementia 1.07 (95%CI 1.04, 1.14). |
| Takahashi, 2012 30) | History of angina and diagnosis of | Clinically documented diagnosis of definite vascular dementia based upon the NINDS-AIREN criteria | Not adjusted | ORs of angina and previous MI for vascular dementia 1.22 (95%CI 0.79, 1.88) and 1.11 (95%CI 0.66, 1.87) respectively. |
| Longitudinal (risk of MCI/dementia) | ||||
| Newman, 2005 12) | Prevalent MI and AP assessed by self-report and confirmed by medical records, test results, medication use. | Incidence of dementia diagnosed based on clinical criteria | Age, race, education, income, APOE episilon-4, modified MMSE score | HR of MI for dementia 1.3 (95%CI 1.0, 1.9) and HR of AP for incident of dementia 1.3 (95%CI 1.0, 1.7) |
| Rusanen, 2014 11) | CAD based on ICD codes, ascertained by self-reported history of MI or AP | Incidence of dementia diagnosed according to the DSM-IV | Sex, education, systolic blood pressure, cholesterol, BMI, APOE, smoking, physical activity, diabetes or impaired glucose tolerance | HR of CAD diagnosed midlife (at or before baseline) and late-life (at or before first reexamination) for dementia 0.80 (95%CI 0.38, 1.67) and 1.66 (95%CI 0.87, 3.16), respectively |
| Hayden, 2006 61) | History of MI obtained by interview | Dementia diagnosed according to DSM-III-R, the NINCDS-ADRDA criteria was used for AD diagnoses. Vascular dementia was identified with the NINDSAIREN criteria. | Age, sex, education, APOE episilon-4, hypertension, high cholesterol, diabetes, obesity | HR of MI for dementia 1.13, (95%CI 0.59, 2.03), for AD 1.11, (95%CI 0.49, 2.26) and for vascular dementia 1.06, (95%CI 0.34, 2.93) |
| Haring, 2013 13) | MI defined as reported history of clinical MI, or ECG results with evolving Q-wave | Measures for incidence of MCI or probable dementia based on DSM-IV | Age, education, race, smoking, alcohol intake, physical activity diabetes, sleep hours, hypertension, BMI, depression, waist-hip ratio, hypercholesterolemia, aspirin use | HR of MI for dementia 2.18 (95%CI 1.19, 3.99), for MCI 2.56 (95%CI 1.64, 4.01) and for MCI or dementia 2.10 (95%CI 1.40, 3.15) |
| Ikram, 2008 62) | MI including unrecognized, Q-wave and recognized subtypes screened by interview and then confirmed by clinical data and ECG characteristics | Incident dementia ascertained following a 3-step protocol and diagnosed based on DSM-III-R | Age, sex, APOE episilon-4, systolic blood pressure, diastolic blood pressure, BMI, diabetes, atrial fibrillation, current smoking, intima media thickness, total cholesterol and HDL cholesterol | HR of unrecognized MI for dementia 2.23, (95%CI 1.24, 4.01) in men, in women 1.17, (95%CI 0.74, 1.83), and in total 1.35, (95%CI 0.95, 1.92). |
| Brayne, 1998 60) | History of heart attack ascertained from informants | Incidence of dementia ascertained based on the clinical criteria | Age, sex | OR of history of MI for dementia 2.94 (95%CI 1.2, 7.21) |
| Kivipelto, 2002 33) | History of MI assessed with a self-administrated questionnaire | Dementia diagnosed according to the criteria of the DSM-IV | Age, APOE episilon-4, education level, sex, smoking, alcohol consumption | OR of MI for AD or vascular dementia 2.5 (95%CI 1.2, 5.4) |
| Chen, 2011 32) | Doctor-diagnosed angina recorded | Dementia diagnosis based on DSM-III | Age, sex, BMI, urban-rurality, socio-economic status, smoking, social network, psychosocial factors | OR of Angina for dementia 2.58 (95%CI 1.01, 6.59). |
| Lin, 2017 63) | ICD-9-CM used for diagnosis coding, further specified for CHD | Vascular dementia (ICD-9-CM code 290.4x) diagnosed by a psychiatrist or a neurologist | Age, sex, comorbidities like hypertension, diabetes, dyslipidemia; urbanization level, monthly income level, depressive disorder | HR of CHD for vascular dementia in patients with and without depression 2.26 (95%CI 1.07, 4.75) |
CAD, Coronary Artery Disease; IHD, Ischemic Heart Disease; ECG, electrocardiogram; CHD, Coronary Heart Disease; MI, Myocardial Infarction; SD, Standard Deviation; CAMCOG, Cambridge Cognitive Examination of the Elderly Revised; MoCA, Montreal Cognitive Assessment; MMSE, Mini Mental State Examination; AP, Angina Pectoris; ACS, Acute Coronary Syndrome; BMI, Body Mass Index; HDL, High-density Lipoprotein; TICSm, Telephone Interview for Cognitive Status-modified; TIA, Transient Ischemic Attack; OR, Odds Ratio; CI, Confidence Interval; CDR, Clinical Dementia Rating; MCI, Mild Cognitive Impairment; AD, Alzheimer's Disease; GP, General Practitioner; DSM-III-R, Diagnostic and Statistical Manual of Mental Disorders Version III Revised; HR, Hazard Ratio; ICD, International Statistical Classification of Diseases and Related Health Problems NINCDS-ADRDA : National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association; NINDS-AIREN: National Institute of Neurological Disorders and Stroke and Association Internationale pour la Recherche et l'Enseignement en Neurosciences; NA, not available; APOE, apolipoprotein E
Quality assessment
Supplementary Table 6. Quality assessment of cross-sectional studies included in the meta-analysis.
| Selection |
Comparability (maximum two stars) |
Outcome |
|||||
|---|---|---|---|---|---|---|---|
| Study, author | Representativeness of the sample | Sample size | Non-respondents | Ascertainment of the exposure (risk factor) (maximum two stars) | The subjects in different outcome groups are comparable, based on the study design or analysis. Confounding factors are controlled | Assessment of the outcome (maximum two stars) | Statistical test |
| Ross 1999 | ★ | ★ | ★★ | ★★ | ★ | ||
| Roberts 2010 | ★ | ★ | ★ | ★★ | ★★ | ★ | |
| Hai 2012 | ★ | ★ | ★★ | ★ | |||
| Zou 2014 | ★ | ★ | ★ | ★ | ★★ | ||
| Heath 2014 | ★ | ★ | ★★ | ★★ | ★ | ||
| Stephen 2017 | ★ | ★ | ★ | ★★ | ★★ | ★ | |
| Kuroki 2018 | ★ | ★ | ★★ | ★★ | ★ | ||
| Deng 2018 | ★ | ★ | ★★ | ★ | ★ | ||
Star is rewarded for each item when the study presents the feature of quality. Each study can be rewarded a maximum of one star for each item except a maximum of two stars can be given to the items including ascertainment of the exposure (risk factor), comparability and assessment of the outcome. In brief, the quality criteria were defined as follows: representativeness of the sample: all subjects or random sampling; sample size: justified and satisfactory; non-respondents: response rate is satisfactory, characteristics between respondents and non-respondents were comparable ; ascertainment of exposure (risk factor): validated measurement tool = 2 stars, non-validated measurement tool, but the tool is available or described = 1 star; comparability: study controls for most important factor (defined as age and sex) (1 star) and control for any additional factor (defined as cardiovascular risk factors) (1 star); assessment of the outcome: was independent blind assessment or record linkage = 2 stars, selfreport = 1 star; statistical test: clearly described and appropriate, and the measurement of the association is presented including confidence intervals and the p value.
Supplementary Table 7. Quality assessment of case-control studies included in the meta-analysis.
| Selection |
Comparability (maximum two stars) |
Outcome |
||||||
|---|---|---|---|---|---|---|---|---|
| Study, author | Is the case definition adequate? | Representativeness of the cases | Selection of controls | Definition of controls | Comparability of cases and controls on the basis of the design or analysis | Ascertainment of exposure | Same method of ascertainment for cases and controls | Non-response rate |
| Bursi 2006 | ★ | ★ | ★ | ★ | ★ | ★ | ||
| Booker 2016 | ★ | ★ | ★ | |||||
| Takahashi 2012 | ★ | ★ | ★ | ★ | ||||
| Jacob 2017 | ★ | ★ | ★ | |||||
Star is rewarded for each item when the study presents the feature of quality. Each study can be rewarded a maximum of one star for each item except a maximum of two stars can be given to the item comparability. In brief, the quality criteria were defined as follows: is the case definition adequate: yes with independent validation; representativeness of the cases: consecutive or obviously representative series of cases; selection of controls: community controls; definition of controls: no history of disease (endpoint); comparability: study controls for age and sex (1 star) and controls for cardiovascular risk factors (1 star); ascertainment of exposure: secure record or structured interview where blind to case/control status; same method of ascertainment for cases and controls: yes; non-response rate: same rate for both groups.
Supplementary Table 8. Quality assessment of longitudinal cohort studies included in the meta-analysis.
| Selection | Comparability (maximum two stars) | Outcome | ||||||
|---|---|---|---|---|---|---|---|---|
| Study, author | Representativeness of the exposed cohort | Selection of the non-exposed cohort | Ascertainment of exposure | Demonstration that outcome of interest was not present at start of study | Comparability of cohorts on the basis of the design or analysis | Assessment of the outcome | Was follow-up long enough for outcomes to occur | Adequacy of follow up of cohorts |
| Rusanen 2014 | ★ | ★ | ★ | ★★ | ★ | ★ | ||
| Newman 2005 | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | |
| Hayden 2006 | ★ | ★ | ★ | ★ | ★★ | ★ | ||
| Haring 2013 | ★ | ★ | ★ | ★★ | ★ | ★ | ||
| Ikram 2008 | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | ★ |
| Lin 2017 | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | ★ |
| Brayen 2006 | ★ | ★ | ★ | ★★ | ★ | |||
| Chen 2011 | ★ | ★ | ★★ | ★ | ||||
| Kivipelto 2002 | ★ | ★ | ★★ | ★ | ||||
Star is rewarded for each item when the study presents the feature of quality. Each study can be rewarded a maximum of one star for each item except a maximum of two stars can be given to the item comparability. In brief, the quality criteria were defined as follows: representativeness of the exposed cohort: truly or somewhat representative of the population based community; selection of the non-exposed cohort: draw from the same community as the exposed cohort; ascertainment of exposure: secured record or structured interview; demonstration that outcome of interest was not present at start of study : yes; comparability: study controls for age and sex (1 star) and controls for cardiovascular risk factors (1 star); assessment of outcome: independent blind assessment or record linkage; was follow-up long enough for outcomes to occur: yes, at least 5 years; adequacy of follow up cohorts: complete follow up all subjects accounted for or subjects lost to follow up unlikely to introduce bias: small number (5%) lost follow up or description provided of those lost.
Coronary Artery Calcium Score and Cognitive Function
Association with Cognitive Function
Three cross-sectional studies17, 34, 35) and two longitudinal cohort studies36, 37) examined the cross-sectional association between CAC and cognitive scores for different cognitive domains. Increasing severity of CAC was associated with worse performance of episodic memory34, 36), semantic fluency36), executive function17, 34–36), and global cognition34, 37).
Association with Cognitive Decline
One study examined the association between CAC and deterioration of cognitive function over 18 years of follow-up in patients with type 1 diabetes (n = 1,045). There was no difference in mean change of cognitive scores between diabetes patients with and without CAC38).
Association with cognitive impairment or dementia
Four longitudinal studies reported the association between increased CAC at baseline and clinically relevant cognitive impairment or dementia18, 19, 39, 40). The Rotterdam study in the elderly (n = 2,326) showed that increased CAC volume was associated with modestly increased risk of dementia after 6 years of follow-up18), but this result did not reach statistical significance (HR 1.05 per Ln(calcium volume+1.0 mm3), 95%CI 0.80–1.36). On the other hand, in the Multi–Ethnic Study of Atherosclerosis (MESA) study, which includes a population with broader age range and different races/ethnicities (n = 6,293), there was a statistically significant increased risk of dementia after a median of 12.2 years (HR 1.18 per log2 (CAC score +1), 95%CI 1.03–1.36)19). Because these two studies used different units to measure CAC, it was not possible to perform meta-analysis. A smaller study that mostly included women of ≥ 80 years (n = 311) reported that white elderly women with CAC score > 400 had around three times higher risk of dementia after 10+ years, compared with those with CAC score of 0 40). An even smaller study in type 1 diabetes patients (n = 148) found significantly elevated risk of MCI with increasing CAC score categories after 14 years39).
Clinical Coronary Artery Disease and Cognitive Function
Association with Cognitive Function
Seven studies reported the association between clinical CAD and cognitive function41–47). Specifically, three cross-sectional studies (n = 516–478,557) found that presence of CAD was associated with poorer cognitive scores in the domain of fluency42), memory46), and global cognition44). A case–control study (n = 446) reported that the Mini Mental State Examination (MMSE) score of CAD cases was lower than that of controls, but this was not statistically significant45). Furthermore, a prospective study (n = 616) found that CAD patients had worse cognitive scores than had controls at 1- and 5-year follow-up47), and a study with longer follow-up (7 years, n = 380) found that CAD was associated with worse information processing speed41). However, in a larger population-based cohort study in Norway (n = 5,033), no association was found between CAD and cognitive test scores (12 word memory test, digit-symbol coding test, and tapping test)43).
Association with Cognitive Decline
Five longitudinal studies reported the relationship between clinical CAD and change in cognitive function over time48–52). A relatively small study (n = 231) found that the decline of global cognitive function over 2 years in patients with a history of CAD and normal heart function was not worse than that in patients without CAD48). In contrast to this, a larger study in 889 elderly (70–90 years) found that CAD was associated with greater decline in memory over 2 years49). Similarly, a study in elderly men (n = 353) found that CAD increased cognitive decline over 3 years (OR 1.7, 95%CI 0.8–3.5)51). In addition, two other studies in 118 and 135 AD patients showed that CAD accelerated decline on both Clinical Dementia Rating scale and MMSE scores over ≥ 1–3 years50, 52).
Association with Cognitive Impairment or Dementia
Ten cross-sectional studies (n = 200–616,245) reported on the association between CAD and clinically diagnosed MCI (n = 6) or dementia (n = 4)25–28, 53–58). Five studies reported that CAD was significantly associated with MCI/dementia, with ORs varying from 1.60 to 6.76 25–28, 57). Four studies with MCI as outcome did not find an association or reported a tendency to an inverse association54–56, 58). The largest study found an OR of ischemic heart disease for dementia of 1.9 (95%CI 1.5–2.4)27). Meta-analysis was possible for eight studies25–28, 54, 56–58). Pooled OR of CAD for MCI/dementia was 1.66 (95%CI 1.17–2.37), with significant heterogeneity between studies (I2 = 90.5%, p < 0.001) (Fig. 2A). One study investigated coronary atherosclerosis confirmed by autopsy and found that an increased burden of intracranial atherosclerosis, but not coronary atherosclerosis, was associated with dementia53).
Fig. 2.
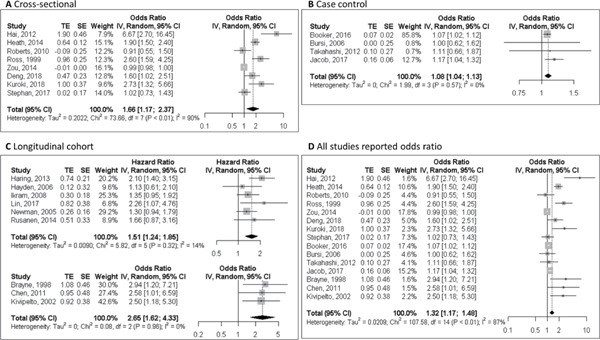
Forest plot of the association between clinical coronary artery disease and mild cognitive impairment or dementia in cross-sectional studies. (A); in case–control studies (B); in cohort studies (C); and in all studies reporting odds ratio (D).
OR, odds ratio; HR, hazard ratio
Five case–control studies (n = 410–23,912) evaluated the association between CAD and MCI/dementia10, 29–31, 59). The largest studies found slight but significant positive associations, of which one focused on MCI (OR 1.17, 95%CI 1.04–1.32)31) and the other on dementia (OR 1.07, 95%CI 1.04–1.14)29). The three smaller studies found no significant association between CAD with dementia10), AD59), or vascular dementia30). Four studies could be included in the meta-analysis with a resulting pooled OR of 1.08 (95%CI 1.04–1.13) and no significant heterogeneity I2 = 0.0%, p = 0.57 (Fig. 2B).
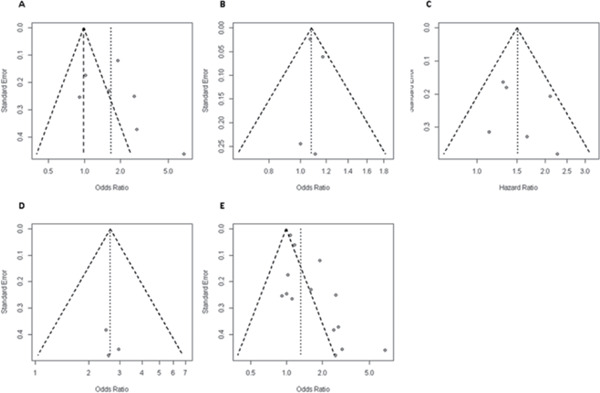
Nine prospective studies (n = 376–49,955) with follow-up from 2.4 to 25.5 years addressed the association between CAD and risk of MCI/dementia11–13, 32, 33, 60–63. Five studies showed that clinical CAD significantly increased the risk of dementia, with HRs of 2.1–2.9 13, 32, 33, 60, 63). In the Rotterdam study, unrecognized MI determined by electrocardiography was not related to dementia overall; however, there was a positive association in men (HR 2.23, 95%CI 1.24–4.01)62). Another two studies found that patients with MI, mostly based on self-report, tended to have a higher risk of dementia (HR 1.1–1.3)12, 61). One study investigated the association of midlife CAD (diagnosed at or before baseline examination) and late-life CAD (diagnosed at or before first re-examination) with dementia and found that midlife CAD was not associated with dementia, whereas participants with late-life CAD tended to have a higher risk of dementia (HR 1.66, 95%CI 0.81–3.16)11). Meta-analysis was conducted in studies that reported HRs11–13, 61–63) and studies that reported ORs32, 33, 60) separately. Pooled HR and pooled OR of CAD for MCI/dementia were 1.51 (95%CI 1.24–1.85, I2 = 14.1%, p = 0.32) and 2.65 (95%CI 1.62–4.33, I2 = 0.0%, p = 0.96), respectively (Fig. 2C). The funnel plot was symmetric (Supplementary Fig. 1C) for the pooled HR, whereas formal statistical testing was not performed because of insufficient number of studies. Sensitivity analysis did not detect any study that was of particular influence on the pooled HR.
Supplementary Fig. 1.
Funnel plots of the association between coronary artery disease and mild cognitive impairment or dementia in cross-sectional studies. (A), in case-control studies (B), in cohort studies that reported hazard ratio (C), in cohort studies that reported odds ratio (D), and in all studies reported odds ratio (E).
Finally, an overall effect size of the association of CAD with MCI/dementia was calculated by including the data from all cross-sectional, case–control, and cohort studies (n = 15). Pooled OR of CAD for MCI/dementia was 1.32 (95%CI 1.17–1.48), with significant heterogeneity between studies (I2 = 87.0%, p < 0.001) (Fig. 2D). The funnel plot (Supplementary Fig. 1E) displayed asymmetry, with Egger's test p value < 0.001. Sensitivity analysis did not detect any study that was of particular influence on the pooled OR.
Discussion
This systematic review, including 46 studies, evaluated the current evidence of the association between (sub)clinical CAD and cognitive function. Prior studies about associations of (sub)clinical CAD with cognitive function and cognitive decline had heterogeneous methodology and inconsistent findings. Two population-based studies investigated the association between CAC and risk of dementia, both finding a tendency toward higher risk of dementia as CAC severity increased. Overall, cross-sectional, case–control, and longitudinal studies showed that clinical CAD was significantly associated with MCI/dementia, but high heterogeneity mainly caused by cross-sectional studies. In clinical CAD, risk of MCI and dementia was increased by 50%. Compared with two prior systematic review articles on the association between CAD and cognitive function64, 65), our study has strengths that include the evaluation of subclinical CAD as assessed by CAC score in relation to dementia and restriction of clinical CAD studies to pre-cardiac intervention results.
Coronary Artery Calcium Score and Cognitive Function
The CAC score, a commonly used non-invasive imaging biomarker for subclinical CAD, is a robust predictor of cardiovascular events66). To our knowledge, this is the first systematic review to assess the predictive value of CAC for cognitive outcomes. Two population-based longitudinal studies, MESA and Rotterdam study, found a tendency toward higher risk of dementia as CAC severity increased18, 19). As they used a different CAC scoring method, meta-analysis could not be performed. Conversely, some studies showed that intracranial artery atherosclerosis, but not coronary atherosclerosis, was associated with MCI and dementia53, 67). It is not irrational to presume that coronary atherosclerosis and intracranial artery atherosclerosis are likely to be concomitant. Another possible explanation is that both coronary atherosclerosis and dementia are age-related diseases sharing risk factors such as smoking, hypercholesterolemia, hypertension, and diabetes6). Although we tried to stratify the studies that used adjustment for risk factors, this was not feasible because considerable heterogeneity existed in the number and type of confounders that were included in the models across studies. However, after adjusting for covariates that may affect the effect estimates, CAC was still significantly associated with risk of dementia in the MESA study. Also, in clinical CAD studies with full adjustment for major cardiovascular risk factors, associations remained significant. These findings suggest a relationship between (sub)clinical CAD and dementia, beyond cardiovascular risk factors. Alternatively, the association between CAC and dementia may be explained by the potential mediating effect of cerebrovascular disease (e.g., stroke), since an association has been found between severity of CAC and risk of stroke68). However, in MESA, after excluding interim stroke, associations between CAC score and risk of dementia remained statistically significant (HR 1.18, 95%CI 1.03–1.36)19), although associations between CAC volume and risk of dementia became statistically nonsignificant in the Rotterdam study (HR 1.05, 95%CI 0.80–1.36)18). Future studies need to clarify whether CAC merely reflects generalized atherosclerosis, confounded by shared risk factors, or whether coronary atherosclerosis is more directly related to MCI/dementia, and to which type of dementia. Even so, since the CAC score is increasingly used in cardiovascular risk stratification in cardiac asymptomatic individuals, our results can raise awareness of the elevated risk of dementia in individuals with increased CAC at a very early stage and thus allow for timely prevention of cognitive decline.
Clinical Coronary Artery Disease and Cognitive Function
By pooling results of all study types (n = 15), we found a significant association between CAD and MCI/dementia, with a pooled OR of 1.32 (95%CI 1.17–1.48); however, substantial heterogeneity exists between studies (I2 = 87.0%, p < 0.001). This heterogeneity likely has multiple causes. Many different study designs and patient samples were included. Also, differences in the definition and assessment of CAD may have contributed. For example, the definition of clinical CAD comprised MI or AP or both. Compared with the use of medical records and tests such as electrocardiography, a self-report strategy used by some studies for the diagnosis of CAD is less objective and may increase information bias. In addition, there was diversity in assessment of cognitive function and there may have been differences in percentage of dementia subtypes. Most studies used the Diagnostic and Statistical Manual of Mental Disorders for diagnosis of dementia without specifying subtypes; only a few studies clearly differentiated dementia subtypes25, 30, 61, 63). Also, differences in duration of the follow-up period may play a role. For example, in the study by Hayden et al., there was no significant association between clinical CAD and dementia after a relatively short follow-up period (about 3 years), whereas associations may only become manifest after a longer time61). Finally, differences in study populations may have contributed to heterogeneity. The majority of the studies in the meta-analysis was population based. The study of Haring et al. is an exception, as it consists of a cohort of postmenopausal women13).
With respect to reporting bias, the number of studies per study design was insufficient to be able to perform a formal statistical test; however, the funnel plots of cohort and case–control studies were visually symmetric, indicating unlikely presence of bias. Nevertheless, pooling cohort and case–control studies together with cross-sectional studies indicated potential presence of bias. It may be due to high heterogeneity among cross-sectional studies, or it is likely that cross-sectional studies with a negative result were not published. This may lead to potential overestimation of the results. To deal with this issue, more longitudinal cohort studies are needed to validate the suggested association. As longitudinal cohort studies are assumed to have a higher level of evidence, we also pooled the results from these high-quality studies only. For these studies (n = 6), we found that CAD is significantly associated with risk of dementia (pooled HR 1.51, 95%CI 1.24–1.85), and this time with minor interstudy heterogeneity (I2 = 14.1%, p = 0.32).
Deckers et al. and Wolters et al.64, 65) also conducted systematic reviews to evaluate the association between CAD and dementia, and both studies found a significant association but with different estimated pooled effects. Deckers et al. performed a meta-analysis in seven longitudinal cohort studies resulting in an OR of 1.55 (95%CI 1.20–2.00), whereas Wolter et al. included nine population-based cohorts resulting in a pooled relative risk of 1.26 (95%CI 1.06–1.49)64, 65). In our systematic review, we also found a significant association between the two diseases, with increased risk estimates very similar to the prior results. The difference in OR between the study of Deckers et al. and our study (1.55 vs 1.32) may be explained by the difference in selection criteria and consequent difference in studies included for the final analysis. The prior systematic reviews did not exclude studies that comprise patients with invasive coronary artery revascularization such as coronary artery bypass grafting before assessment of cognitive function. Invasive coronary interventions themselves may influence the association between CAD and cognitive function, as, for example, hypoperfusion during bypass surgery can impair the washout of microemboli, with potential subsequent brain ischemia and infarction, and increased long-term risk of dementia69). Although there is no study that directly compared invasive coronary interventions with medical management regarding cognitive outcomes, many have reported that cardiac catheterization increases the risk of silent cerebral infarction, which is related to cognitive decline22, 70). Furthermore, other cardiovascular diseases including stroke, atrial fibrillation, and heart failure can contribute to cognitive decline and dementia8, 9, 71). We accounted for the effect of invasive interventions and the latter cardiovascular diseases on the association between clinical CAD and cognitive function in the selection of studies so that the magnitude of association would not be distorted. Only studies with patient groups that had not undergone invasive treatment prior to cognitive function assessment or studies that had adjusted for these factors (invasive intervention and CAD complications) were included. This results in a more accurate estimate of the relationship of CAD itself with cognitive function. Furthermore, we investigated both subclinical and clinical CAD in our study.
Limitations
This systematic review has some limitations. First, although we made strict selection criteria in order to exclude the impact of potential confounders and interactions such as invasive coronary interventions, heart failure, atrial fibrillation, and stroke on cognitive outcomes, many studies did not report complications and/or invasive treatments in patients after developing CAD. This may have led to selection bias and could have impacted the magnitude of the association between CAD and cognition. On the other hand, because we excluded studies that specifically included patients with prior coronary intervention, perhaps the most severe CAD patient group was excluded. Second, although CAC severity was associated with risk of dementia, different CAC units were used. In clinical practice, the Agatston score is the most commonly used method for CAC quantification, although discussion exists whether better markers could be determined72). More data are needed to accurately estimate the effect size of Agatston-based CAC scores in predicting dementia. Furthermore, we did not perform meta-analysis per dementia subtype, since most studies reported syndrome diagnosis of dementia without clearly distinguishing subtypes, and only a limited number of studies focused on one specific dementia subtype, vascular dementia. Accurate differential diagnosis of dementia subtypes is a clinical challenge. In research studies, Alzheimer's dementia and vascular dementia are the most common subtypes of dementia, which are, however, supposedly different in pathophysiology. CAD may be strongly associated to vascular pathology than to neurodegenerative pathology. Nevertheless, as vascular pathology is frequently observed to be co-existent with AD, recently, an integrated approach to diagnosis, treatment, and prevention of dementia was proposed73). More understanding of the association between CAD and dementia subtypes is important.
Conclusion
This systematic review and meta-analysis shows an association between (sub)clinical CAD and cognitive function. Limited evidence suggests the CAC score is associated with risk of dementia. In clinical CAD, risk of MCI and dementia is increased by 50%, as supported by stronger evidence. These findings call for further investigation of whether and how coronary atherosclerosis is involved in the etiology and pathogenesis of cognitive decline and dementia and whether the relationship differs by type of dementia.
Acknowledgments
We gratefully acknowledge the help of the medical information expert, Mrs. Sjoukje van der Werf, in checking the search strategy.
Conflict of Interest
The PhD project of Congying Xia is part of the ImaLife project, which is funded by an institutional research grant from Siemens Healthineers and by the Ministry of Economic Affairs and Climate Policy by means of the PPP Allowance made available by the Top Sector Life Sciences & Health to stimulate public–private partnerships. Matthijs Oudkerk is involved in the company iDNA B.V. There are no other conflicts of interest to disclose.
References
- 1). Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P, American Heart Association Statistics C and Stroke Statistics S : Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation, 2017; 135: e146-e603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2). Prince MJ, Wimo A, Guerchet MM, Ali GC, Wu Y-T, Prina M: World Alzheimer Report 2015 - The Global Impact of Dementia, Alzheimer's Disease International, London, 2015 [Google Scholar]
- 3). Petersen RC, Caracciolo B, Brayne C, Gauthier S, Jelic V, Fratiglioni L: Mild cognitive impairment: a concept in evolution. J Intern Med, 2014; 275: 214-228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4). Kaffashian S, Dugravot A, Elbaz A, Shipley MJ, Sabia S, Kivimaki M, Singh-Manoux A: Predicting cognitive decline: a dementia risk score vs. the Framingham vascular risk scores. Neurology, 2013; 80: 1300-1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5). Kuller LH, Lopez OL, Newman A, Beauchamp NJ, Burke G, Dulberg C, Fitzpatrick A, Fried L, Haan MN: Risk factors for dementia in the cardiovascular health cognition study. Neuroepidemiology, 2003; 22: 13-22 [DOI] [PubMed] [Google Scholar]
- 6). Qiu C: Preventing Alzheimer's disease by targeting vascular risk factors: hope and gap. Journal of Alzheimer's disease: JAD, 2012; 32: 721-731 [DOI] [PubMed] [Google Scholar]
- 7). Attems J, Jellinger KA: The overlap between vascular disease and Alzheimer's disease--lessons from pathology. BMC medicine, 2014; 12: 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8). Loke YK, Hale R, Potter JF, Myint PK, Kwok CS, Loke YK, Hale R, Potter JF, Myint PK: Atrial fibrillation and incidence of dementia: a systematic review and metaanalysis. Neurology, 2011; 76: 914-922 [DOI] [PubMed] [Google Scholar]
- 9). Hajduk AM, Kiefe CI, Person SD, Gore JG, Saczynski JS: Cognitive change in heart failure : A systematic review. Circulation: Cardiovascular Quality and Outcomes, 2013; 6: 451-460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10). Bursi F, Rocca WA, Killian JM, Weston SA, Knopman DS, Jacobsen SJ, Roger VLVL: Heart disease and dementia: A population-based study. American Journal of Epidemiology, 2006; 163: 135-141 [DOI] [PubMed] [Google Scholar]
- 11). Rusanen M, Kivipelto M, Levälahti E, Laatikainen T, Tuomilehto J, Soininen H, Ngandu T, Levalahti E, Laatikainen T, Tuomilehto J, Soininen H, Ngandu T, Levälahti E, Laatikainen T, Tuomilehto J, Soininen H, Ngandu T: Heart diseases and long-term risk of dementia and Alzheimer's disease: a population-based CAIDE study. Journal of Alzheimer's disease: JAD, 2014; 42: 183-191 [DOI] [PubMed] [Google Scholar]
- 12). Newman AB, Fitzpatrick AL, Lopez O, Jackson S, Lyketsos C, Jagust W, Ives D, Dekosky ST, Kuller LH: Dementia and Alzheimer's disease incidence in relationship to cardiovascular disease in the cardiovascular health study cohort. Journal of the American Geriatrics Society, 2005; 53: 1101-1107 [DOI] [PubMed] [Google Scholar]
- 13). Haring B, Leng X, Robinson J, Johnson KC, Jackson RD, Beyth R, Wactawski-Wende J, von Ballmoos MW, Goveas JS, Kuller LH, Wassertheil-Smoller S: Cardiovascular Disease and Cognitive Decline in Postmenopausal Women: Results From the Women's Health Initiative Memory Study. Journal of the American Heart Association, 2013; 2: e000369-e000369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14). Fink HA, Hemmy LS, MacDonald R, Carlyle MH, Olson CM, Dysken MW, McCarten JR, Kane RL, Garcia SA, Rutks IR, Ouellette J, Wilt TJ: Intermediate- and Long-Term Cognitive Outcomes After Cardiovascular Procedures in Older Adults: A Systematic Review. Annals of internal medicine, 2015; 163: 107-117 [DOI] [PubMed] [Google Scholar]
- 15). Elias-Smale SE, Proença RV, Koller MT, Kavousi M, Van Rooij FJAA, Hunink MG, Steyerberg EW, Hofman A, Oudkerk M, Witteman JCMM: Coronary calcium score improves classification of coronary heart disease risk in the elderly: The Rotterdam study. Journal of the American College of Cardiology, 2010; 56: 1407-1414 [DOI] [PubMed] [Google Scholar]
- 16). Vliegenthart R, Oudkerk M, Hofman A, Oei HH, van Dijck W, van Rooij FJ, Witteman JC: Coronary calcification improves cardiovascular risk prediction in the elderly. Circulation, 2005; 112: 572-577 [DOI] [PubMed] [Google Scholar]
- 17). Vidal J-SS, Sigurdsson S, Jonsdottir MK, Eiriksdottir G, Thorgeirsson G, Kjartansson O, Garcia ME, Van Buchem MA, Harris TB, Gudnason V, Launer LJ: Coronary artery calcium, brain function and structure: The AGESReykjavik study. Stroke, 2010; 41: 891-897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18). Bos D, Vernooij MW, de Bruijn RFAG, Koudstaal PJ, Hofman A, Franco OH, van der Lugt A, Ikram MA: Atherosclerotic calcification is related to a higher risk of dementia and cognitive decline. Alzheimer's & dementia: the journal of the Alzheimer's Association, 2015; 11: 639-647.e631 [DOI] [PubMed] [Google Scholar]
- 19). Fujiyoshi A, Jacobs DR, Jr., Fitzpatrick AL, Alonso A, Duprez DA, Sharrett AR, Seeman T, Blaha MJ, Luchsinger JA, Rapp SR: Coronary Artery Calcium and Risk of Dementia in MESA (Multi-Ethnic Study of Atherosclerosis). Circ Cardiovasc Imaging, 2017; 10: e005349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20). Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB: Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA, 2000; 283: 2008-2012 [DOI] [PubMed] [Google Scholar]
- 21). Moher D, Liberati A, Tetzlaff J, Altman DG: Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med, 2009; 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22). Muqtadar H, Testai FD, Gorelick PB: The dementia of cardiac disease. Current cardiology reports, 2012; 14: 732-740 [DOI] [PubMed] [Google Scholar]
- 23). Wells GS, B, Wells GA SB, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P: Ottawa Hospital Research Institute. 2014;
- 24). Herzog R, Álvarez-Pasquin MJ, Díaz C, Del Barrio JL, Estrada JM, Gil Á: Are healthcare workers' intentions to vaccinate related to their knowledge, beliefs and attitudes? a systematic review. BMC Public Health, 2013; 13: 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25). Ross GW, Petrovitch H, White LR, Masaki KH, Li CY, Curb JD, Yano K, Rodriguez BL, Foley DJ, Blanchette PL, Havlik R: Characterization of risk factors for vascular dementia: the Honolulu-Asia Aging Study. Neurology, 1999; 53: 337-343 [DOI] [PubMed] [Google Scholar]
- 26). Hai S, Dong B, Liu Y, Zou Y: Occurrence and risk factors of mild cognitive impairment in the older Chinese population: a 3-year follow-up study. International journal of geriatric psychiatry, 2012; 27: 703-708 [DOI] [PubMed] [Google Scholar]
- 27). Heath CA, Mercer SW, Guthrie B: Vascular comorbidities in younger people with dementia: a cross-sectional population-based study of 616 245 middle-aged people in Scotland. Journal of neurology, neurosurgery, and psychiatry, 2014; 86: jnnp-2014-309033- [DOI] [PubMed] [Google Scholar]
- 28). Deng J, Cao C, Jiang Y, Peng B, Wang T, Yan K, Lian J, Wang Z: Prevalence and effect factors of dementia among the community elderly in Chongqing, China. Psychogeriatrics, 2018; 18: 412-420 [DOI] [PubMed] [Google Scholar]
- 29). Booker A, Jacob LE, Rapp M, Bohlken J, Kostev K: Risk factors for dementia diagnosis in German primary care practices. International psychogeriatrics, 2016; 28: 1059-1065 [DOI] [PubMed] [Google Scholar]
- 30). Takahashi PY, Caldwell CR, Targonski PV: Effect of vascular burden as measured by vascular indexes upon vascular dementia: a matched case-control study. Clinical interventions in aging, 2012; 7: 27-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31). Jacob L, Bohlken J, Kostev K: Risk Factors for Mild Cognitive Impairment in German Primary Care Practices. J Alzheimers Dis, 2017; 56: 379-384 [DOI] [PubMed] [Google Scholar]
- 32). Chen R, Hu Z, Wei L, Ma Y, Liu Z, Copeland JR: Incident Dementia in a Defined Older Chinese Population. PloS one, 2011; 6: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33). Kivipelto M, Helkala E-L, Laakso MP, Hänninen T, Hallikainen M, Alhainen K, Iivonen S, Mannermaa A, Tuomilehto J, Nissinen A, Soininen H: Apolipoprotein E ε4 Allele, Elevated Midlife Total Cholesterol Level, and High Midlife Systolic Blood Pressure Are Independent Risk Factors for Late-Life Alzheimer Disease. Annals of internal medicine, 2002; 137: 149. [DOI] [PubMed] [Google Scholar]
- 34). Suemoto CK, Bittencourt MS, Santos IS, Bensenor IM, Lotufo PA: Coronary artery calcification and cognitive function: cross-sectional results from the ELSA-Brasil study. Int J Geriatr Psychiatry, 2017; 32: e188-e194 [DOI] [PubMed] [Google Scholar]
- 35). Reis JP, Launer LJ, Terry JG, Loria CM, Zeki Al Hazzouri A, Sidney S, Yaffe K, Jacobs DR, Whitlow CT, Zhu N, Carr JJ: Subclinical atherosclerotic calcification and cognitive functioning inmiddle-aged adults: The CARDIA study. Atherosclerosis, 2013; 231: 72-77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36). Hugenschmidt CE, Hsu F-CC, Hayasaka S, Carr JJ, Freedman BI, Nyenhuis DL, Williamson JD, Bowden DW: The influence of subclinical cardiovascular disease and related risk factors on cognition in type 2 diabetes mellitus: The DHS-Mind study. Journal of Diabetes and its Complications, 2013; 27: 422-428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37). Rossetti HC, Weiner M, Hynan LS, Cullum CM, Khera A, Lacritz LH: Subclinical atherosclerosis and subsequent cognitive function. Atherosclerosis, 2015; 241: 36-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38). Jacobson AM, Ryan CM, Cleary PA, Waberski BH, Weinger K, Musen G, Dahms W: Biomedical risk factors for decreased cognitive functioning in type 1 diabetes: an 18 year follow-up of the Diabetes Control and Complications Trial (DCCT) cohort. Diabetologia, 2011; 54: 245-255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39). Guo J, Nunley KA, Costacou T, Miller RG, Rosano C, Edmundowicz D, Orchard TJ: Greater progression of coronary artery calcification is associated with clinically relevant cognitive impairment in type 1 diabetes. Atherosclerosis, 2019; 280: 58-65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40). Kuller LH, Lopez OL, MacKey RH, Rosano C, Edmundowicz D, Becker JT, Newman AB: Subclinical cardiovascular disease and death, dementia, and coronary heart disease in patients 80+ years. Journal of the American College of Cardiology, 2016; 67: 1013-1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41). Reijmer YD, van den Berg E, Dekker JM, Nijpels G, Stehouwer CDA, Kappelle LJ, Biessels GJ: The metabolic syndrome, atherosclerosis and cognitive functioning in a non-demented population: the Hoorn Study. Atherosclerosis, 2011; 219: 839-845 [DOI] [PubMed] [Google Scholar]
- 42). Verhaegen P, Borchelt M, Smith J, Verhaeghen P, Borchelt M, Smith J, Verhaegen P, Borchelt M, Smith J: Relation between cardiovascular and metabolic disease and cognition in very old age: cross-sectional and longitudinal findings from the berlin aging study. Health Psychology, 2003; 22: 559-569 [DOI] [PubMed] [Google Scholar]
- 43). Arntzen KA, Schirmer H, Wilsgaard T, Mathiesen EB: Impact of cardiovascular risk factors on cognitive function: the Tromsø study. European journal of neurology, 2011; 18: 737-743 [DOI] [PubMed] [Google Scholar]
- 44). Elwood PC, Pickering J, Bayer A, Gallacher JEJ: Vascular disease and cognitive function in older men in the Caerphilly cohort. Age and Ageing, 2002; 31: 43-48 [DOI] [PubMed] [Google Scholar]
- 45). Ahto M, Isoaho R, Puolijoki H, Laippala P, Sulkava R, Kivelä SL, Kivela SL: Cognitive impairment among elderly coronary heart disease patients. Gerontology, 1999; 45: 87-95 [DOI] [PubMed] [Google Scholar]
- 46). Lyall DM, Celis-Morales CA, Anderson J, Gill JM, Mackay DF, McIntosh AM, Smith DJ, Deary IJ, Sattar N, Pell JP: Associations between single and multiple cardiometabolic diseases and cognitive abilities in 474 129 UK Biobank participants. Eur Heart J, 2017; 38: 577-583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47). Volonghi I, Pendlebury ST, Welch SJV, Mehta Z, Rothwell PM: Cognitive outcomes after acute coronary syndrome: a population based comparison with transient ischaemic attack and minor stroke. Heart (British Cardiac Society), 2013; 99: 1509-1514 [DOI] [PubMed] [Google Scholar]
- 48). Almeida OP, Beer C, Lautenschlager NT, Arnolda L, Alfonso H, Flicker L: Two-year course of cognitive function and mood in adults with congestive heart failure and coronary artery disease: the Heart-Mind Study. International psychogeriatrics, 2012; 24: 38-47 [DOI] [PubMed] [Google Scholar]
- 49). Lipnicki DM, Sachdev PS, Crawford J, Reppermund S, Kochan NA, Trollor JN, Draper B, Slavin MJ, Kang K, Lux O, Mather KA, Brodaty H: Risk factors for latelife cognitive decline and variation with age and sex in the Sydney Memory and Ageing Study. PloS one, 2013; 8: e65841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50). Bleckwenn M, Kleineidam L, Wagner M, Jessen F, Weyerer S, Werle J, Wiese B, Luhmann D, Posselt T, Konig HH, Brettschneider C, Mosch E, Weeg D, Fuchs A, Pentzek M, Luck T, Riedel-Heller SG, Maier W, Scherer M: Impact of coronary heart disease on cognitive decline in Alzheimer's disease: a prospective longitudinal cohort study in primary care. Br J Gen Pract, 2017; 67: e111-e117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51). Kalmijn S, Feskens EJ, Launer LJ, Kromhout D: Cerebrovascular disease, the apolipoprotein e4 allele, and cognitive decline in a community-based study of elderly men. Stroke, 1996; 27: 2230-2235 [DOI] [PubMed] [Google Scholar]
- 52). Mielke MM, Rosenberg PB, Tschanz J, Cook L, Corcoran C, Hayden KM, Norton M, Rabins PV, Green RC, Welsh-Bohmer KA, Breitner JCS, Munger R, Lyketsos CG: Vascular factors predict rate of progression in Alzheimer disease. Neurology, 2007; 69: 1850-1858 [DOI] [PubMed] [Google Scholar]
- 53). Dolan H, Crain B, Troncoso J, Resnick SM, Zonderman AB, OBrien RJ: Atherosclerosis, dementia, and Alzheimer disease in the Baltimore Longitudinal Study of Aging cohort. Annals of neurology, 2010; 68: 231-240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54). Roberts RO, Knopman DS, Geda YE, Cha RH, Roger VLVL, Petersen RC: Coronary heart disease is associated with non-amnestic mild cognitive impairment. Neurobiology of Aging, 2010; 31: 1894-1902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55). Wang Y, Song M, Yu L, Wang L, An C, Xun S, Zhao X, Gao Y, Wang X: Mild cognitive impairment: vascular risk factors in community elderly in four cities of Hebei Province, China. PloS one, 2015; 10: e0124566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56). Zou Y, Zhu Q, Deng Y, Duan J, Pan L, Tu Q, Dai R, Zhang X, Chu L-W, Lü Y, Lu Y: Vascular risk factors and mild cognitive impairment in the elderly population in Southwest China. American journal of Alzheimer's disease and other dementias, 2014; 29: 242-247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57). Kuroki A, Sugita N, Komatsu S, Wakasugi M, Yokoseki A, Yoshihara A, Kobayashi T, Nakamura K, Momotsu T, Endo N, Sato K, Narita I, Yoshie H: The number of remaining teeth as a risk indicator of cognitive impairment: A cross-sectional clinical study in Sado Island. Clin Exp Dent Res, 2018; 4: 291-296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58). Stephan BCM, Minett T, Muniz-Terrera G, Harrison SL, Matthews FE, Brayne C: Neuropsychological profiles of vascular disease and risk of dementia: implications for defining vascular cognitive impairment no dementia (VCI-ND). Age Ageing, 2017; 46: 755-760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59). Massaia M, Pallavicino Di Ceva A, Bo M, Cappa G, Zannella P, Persico D, Ferrario E, Fabris F: Risk factors for dementia of Alzheimer's type: a case-control, retrospective evaluation. Archives of Gerontology and Geriatrics, 2001; 7: 253-259 [DOI] [PubMed] [Google Scholar]
- 60). Brayne C, Gill C, Huppert FA, Barkley C, Gehlhaar E, Girling DM, O'connor DW, Paykel ES: Vascular Risks and Incident Dementia: Results from a Cohort Study of the Very Old. Original Research Article Dement Geriatr Cogn Disord, 1998; 9: 175-180 [DOI] [PubMed] [Google Scholar]
- 61). Hayden KM, Zandi PP, Lyketsos CG, Khachaturian AS, Bastian LA, Charoonruk G, Tschanz JT, Norton MC, Pieper CF, Munger RG, Breitner JCS, Welsh-Bohmer KA, Cache County I, Investigators CC, Cache County I : Vascular risk factors for incident Alzheimer disease and vascular dementia - The Cache County study. Alzheimer Disease & Associated Disorders, 2006; 20: 93-100 [DOI] [PubMed] [Google Scholar]
- 62). Ikram MA, van Oijen M, de Jong FJ, Kors JA, Koudstaal PJ, Hofman A, Witteman JCM, Breteler MMB: Unrecognized myocardial infarction in relation to risk of dementia and cerebral small vessel disease. Stroke, 2008; 39: 1421-1426 [DOI] [PubMed] [Google Scholar]
- 63). Lin WC, Hu LY, Tsai SJ, Yang AC, Shen CC: Depression and the risk of vascular dementia: a population-based retrospective cohort study. Int J Geriatr Psychiatry, 2017; 32: 556-563 [DOI] [PubMed] [Google Scholar]
- 64). Wolters FJ, Segufa RA, Darweesh SKL, Bos D, Ikram MA, Sabayan B, Hofman A, Sedaghat S: Coronary heart disease, heart failure, and the risk of dementia: A systematic review and meta-analysis. Alzheimer's & dementia: the journal of the Alzheimer's Association, 2018; 14: 1493-1504 [DOI] [PubMed] [Google Scholar]
- 65). Deckers K, Schievink SHJ, Rodriquez MMF, van Oostenbrugge RJ, van Boxtel MPJ, Verhey FRJ, Kohler S: Coronary heart disease and risk for cognitive impairment or dementia: Systematic review and meta-analysis. PloS one, 2017; 12: e0184244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66). Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, Liu K, Shea S, Szklo M, Bluemke DA, O'Leary DH, Tracy R, Watson K, Wong ND, Kronmal RA: Coronary Calcium as a Predictor of Coronary Events in Four Racial or Ethnic Groups. New England Journal of Medicine, 2008; 358: 1336-1345 [DOI] [PubMed] [Google Scholar]
- 67). Dearborn JL, Zhang Y, Qiao Y, Suri MFK, Liu L, Gottesman RF, Rawlings AM, Mosley TH, Alonso A, Knopman DS, Guallar E, Wasserman BA: Intracranial atherosclerosis and dementia: The Atherosclerosis Risk in Communities (ARIC) Study. Neurology, 2017; 88: 1556-1563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68). Vliegenthart R, Hollander M, Breteler MMB, van der Kuip DAM, Hofman A, Oudkerk M, Witteman JCM: Stroke is associated with coronary calcification as detected by electron-beam CT: the Rotterdam Coronary Calcification Study. Stroke, 2002; 33: 462-465 [DOI] [PubMed] [Google Scholar]
- 69). Kuzma E, Airdrie J, Littlejohns TJ, Lourida I, Thompson-Coon J, Lang IA, Scrobotovici M, Thacker EL, Fitzpatrick A, Kuller LH, Lopez OL, Longstreth WT, Jr., Ukoumunne OC, Llewellyn DJ: Coronary Artery Bypass Graft Surgery and Dementia Risk in the Cardiovascular Health Study. Alzheimer disease and associated disorders, 2017; 31: 120-127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70). Fink HA, Hemmy LS, MacDonald R, Carlyle MH, Olson CM, Dysken MW, McCarten JR, Kane RL, Rutks IR, Ouellette J, Wilt TJ: AHRQ Technology Assessments. In: Cognitive Outcomes After Cardiovascular Procedures in Older Adults: A Systematic Review, Agency for Healthcare Research and Quality (US), Rockville (MD), 2014 [PubMed] [Google Scholar]
- 71). Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, Launer LJ, Laurent S, Lopez OL, Nyenhuis D, Petersen RC, Schneider JA, Tzourio C, Arnett DK, Bennett DA, Chui HC, Higashida RT, Lindquist R, Nilsson PM, Roman GC, Sellke FW, Seshadri S, American Heart Association Stroke Council CoEaP, Council on Cardiovascular Nursing, Council on Cardiovascular Radiology and Intervention, and Council on Cardiovascular Surgery and Anesthesia : Vascular contributions to cognitive impairment and dementia: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke, 2011; 42: 2672-2713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72). Willemink MJ, van der Werf NR, Nieman K, Greuter MJW, Koweek LM, Fleischmann D: Coronary artery calcium: A technical argument for a new scoring method. Journal of cardiovascular computed tomography, 2018; [DOI] [PubMed] [Google Scholar]
- 73). Kling MA, Trojanowski JQ, Wolk DA, Lee VM, Arnold SE: Vascular disease and dementias: paradigm shifts to drive research in new directions. Alzheimer's & dementia: the journal of the Alzheimer's Association, 2013; 9: 76-92 [DOI] [PMC free article] [PubMed] [Google Scholar]


